Abstract
Chlamydia pneumoniae is a well-established cause of community-acquired pneumonia and bronchitis in adults and children. Chronic infections with C. pneumoniae have been implicated in the development of atherosclerosis and other diseases in humans. Methods currently used for the culture and propagation of C. pneumoniae are not analogous to the infection as it occurs in vivo. We have established a model of continuous C. pneumoniae infection in vitro. HEp-2 cells inoculated with CM-1 and TW-183 strains have been persistently infected for periods of over 1.5 and 2 years, respectively. The cultures were maintained without centrifugation or the addition of cycloheximide, fresh host cells, or chlamydia. We observed cycles of host cell lysis, detachment, and regrowth with both strains of C. pneumoniae. Continuous C. pneumoniae infections may more closely resemble the actual events as they occur in vivo and, therefore, may be a better model for the in vitro study of C. pneumoniae infection. When we used continuously infected cells to determine the effects of azithromycin and ofloxacin on C. pneumoniae propagation in vitro, we found that both drugs reduced but did not completely eliminate the organism. This may be an important observation, as the failure of antibiotic therapy against C. pneumoniae infection in humans has been described.
Chlamydia pneumoniae, like other members of its genus, is capable of causing chronic and asymptomatic infections (3). Infection with C. pneumoniae has been implicated in the development of atherosclerosis and other chronic diseases in humans (6, 14). Methods currently used for culture and propagation of C. pneumoniae are not analogous to the infection as it occurs in vivo. To further study this problem, we established a model of continuous C. pneumoniae infection in vitro, in which cell growth, lysis, and chlamydia production are balanced. We also investigated the in vitro activities of azithromycin and ofloxacin against C. pneumoniae in this system. Continuous C. pneumoniae infection may more closely resemble the actual events as they occur in vivo and, therefore, may be a better model for the in vitro study of C. pneumoniae infection.
MATERIALS AND METHODS
Continuous C. pneumoniae infection in vitro.
Confluent HEp-2 cell monolayers were inoculated with C. pneumoniae strains TW-183 (ATCC VR-2282) and CM-1 (ATCC VR1360) to achieve almost 100% infection (12). After 3 to 5 days, when lysis and detachment of most of the infected cells were seen, the original medium was replaced with fresh medium with no added cycloheximide. After 1 week, the growth of new cells was observed. When cell monolayers became confluent again, the supernatants were tested for the presence of viable Chlamydia, and cells were split onto new flasks. A small portion of cell suspension was seeded onto several wells of a 96-well microtiter plate and was examined for the presence of inclusions by staining with a fluorescein-conjugated genus-specific monoclonal antibody (Pathfinder Chlamydia Culture Conformation System; Sanofi Diagnostics, Redmond, Wash.) 4 to 72 h after seeding. Cells continuously infected with both strains were split three to five times per month in a ratio not exceeding 1:4, with medium being changed between splits.
Antibiotic activity assay. Primary C. pneumoniae infection.
Confluent HEp-2 monolayers grown on 24-well plates were inoculated in duplicate (one well contained a 12-mm-diameter coverslip) with 103 to 104 inclusion-forming units (IFU)/ml of TW-183 and CM-1. The plates then were centrifugated at 1,700 × g for 1 h. After centrifugation, the cell monolayers were overlaid with medium containing 0.125 μg of azithromycin or 1.0 μg of ofloxacin per ml (the minimum inhibitory concentration at which 90% of the isolates are inhibited [MIC90] for each drug) (4, 5).
Continuous C. pneumoniae infection.
Continuously infected HEp-2 cells were seeded into 24-well plates the day prior to the experiment. The medium was then replaced with one containing either 0.125 μg of azithromycin or 1.0 μg of ofloxacin per ml on the day of experiment.
Additionally, continuously infected cells were seeded onto 12-well plates and were treated with 0.125, 0.25, or 0.5 μg of azithromycin per ml and 1.0, 2.0, or 4.0 μg of ofloxacin per ml. The drug-containing supernatant media of all infected cells were replaced daily with fresh media containing the same drugs at the same concentrations.
On days 1, 2, 3, 4, 5, and 6 after inoculation and addition of antibiotics, the infected cells from primary and continuous cultures were collected and frozen, then defrosted and ultrasonicated. Tenfold dilutions from each well were inoculated onto fresh HEp-2 cells in 96-well plates. The plates were centrifuged at 1,700 × g, incubated for 72 h, fixed, and stained as above. The IFU per milliliter for every isolate at each time point and under the effect of each drug was calculated. Reduction of IFU was calculated relative to controls with no antibiotics added.
RESULTS
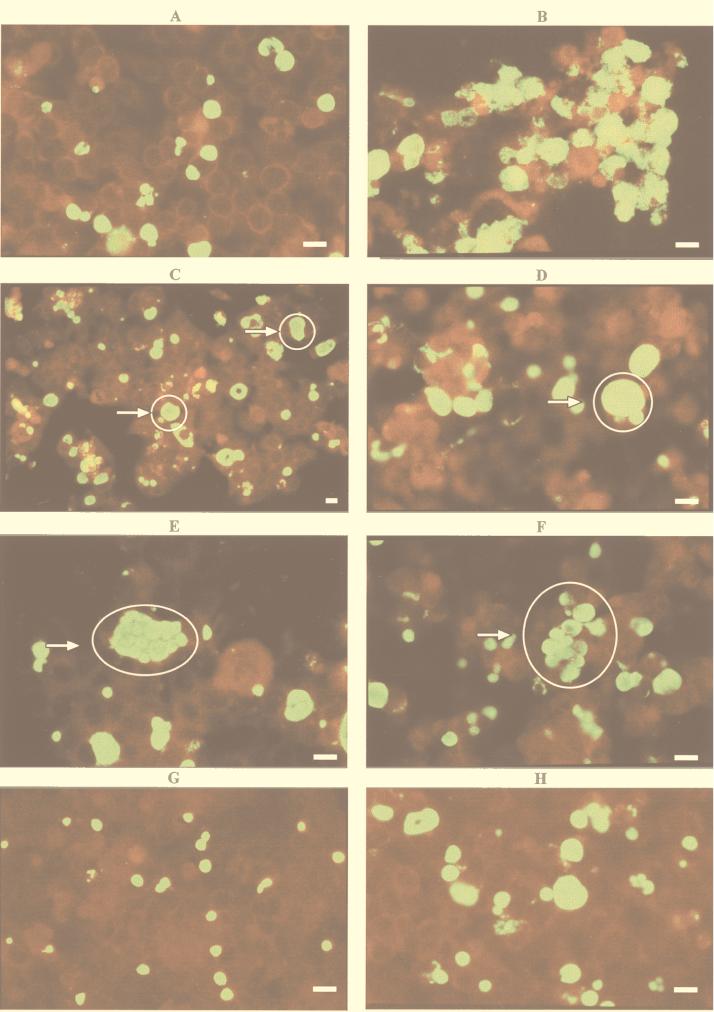
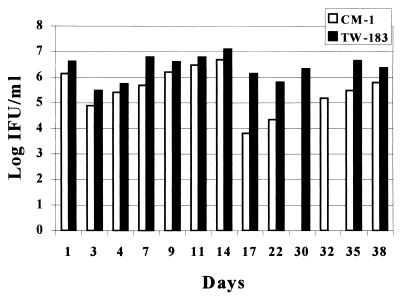
Continuous C. pneumoniae infection was established in vitro with TW-183 and CM-1. The cultures were maintained without centrifugation or the addition of cycloheximide, fresh host cells, or chlamydia for over 2 years for TW-183 and over 18 months for CM-1. We observed cycles of cell lysis and detachment (Fig. 1B) and regrowth (Fig. 1A) with TW-183. The period of time between cycles varied from 7 to 14 days and strongly depended on the frequency of splitting the cells and changing the media. Cycles of lysis and regrowth were also observed with cells infected with CM-1. We often observed multiple (Fig. 1E), clustered (Fig. 1F), and large (greater than or equal to 1.5 to 2 times the size of host cells) inclusions, especially with TW-183 (Fig. 1C and D). Continuous infection with TW-183 produced, on average, 0.5 to 2 log units more infectious progeny than CM-1 (Fig. 2).
FIG. 1.
(A to F) Continuous TW-183 infection in HEp-2 cells: A, monolayer (400×); B, lysis (400×); C and D, large inclusions (200 and 400×); E, multiple inclusions (400×); F, clustered inclusions (400×). (G and H) CM-1 infection in HEp-2 cells on day 3: G, primary CM-1 infection (400×); H, continuous CM-1 infection (400×). C. pneumoniae-infected cells were stained with the Pathfinder Chlamydia Culture Confirmation System (Sanofi Diagnostics), which contains fluorescein-conjugated monoclonal antibody and Evans Blue as a counterstain. The chlamydial inclusions stain apple green and the host cells stain dark red when observed under UV light. Bars represent an average host cell size.
FIG. 2.
Chlamydia concentrations during continuous TW-183 and CM-1 infections in vitro.
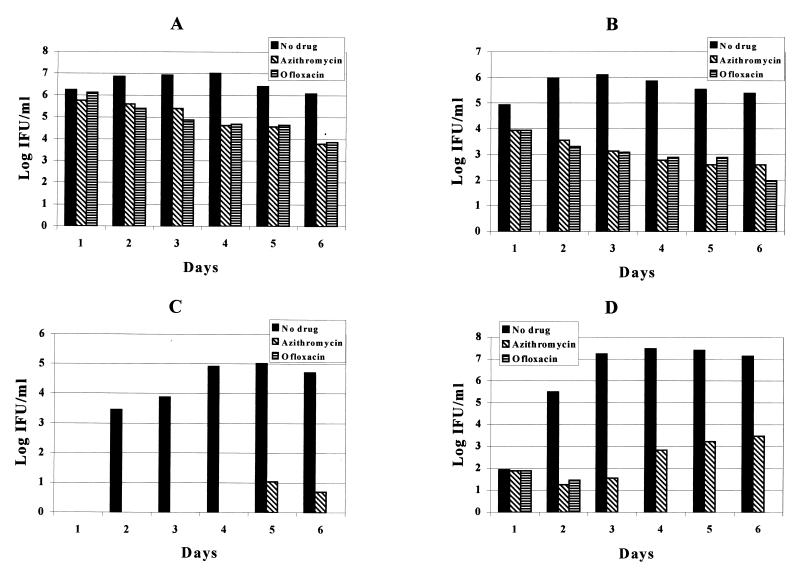
The results of in vitro susceptibility testing of azithromycin and ofloxacin in the primary and continuously infected cells are shown in Fig. 3. Unlike the primarily infected cells, where a 100% reduction in IFU for TW-183 and a 5-log-unit reduction in IFU for CM-1 was observed at day 3 after treatment with both drugs, the reduction in IFU observed with the continuously infected cells ranged from 94.6 to 99.9%. The log reduction of IFU in the primary infection was, on average, 1.8 log units greater for azithromycin and 2.2 log units greater for ofloxacin than for the continuously infected cells.
FIG. 3.
Effects of azithromycin (0.125 μg/ml) and ofloxacin (1.0 μg/ml) on C. pneumoniae infection in vitro. A, continuous TW-183 infection; B, continuous CM-1 infection; C, primary TW-183 infection; D, primary CM-1 infection.
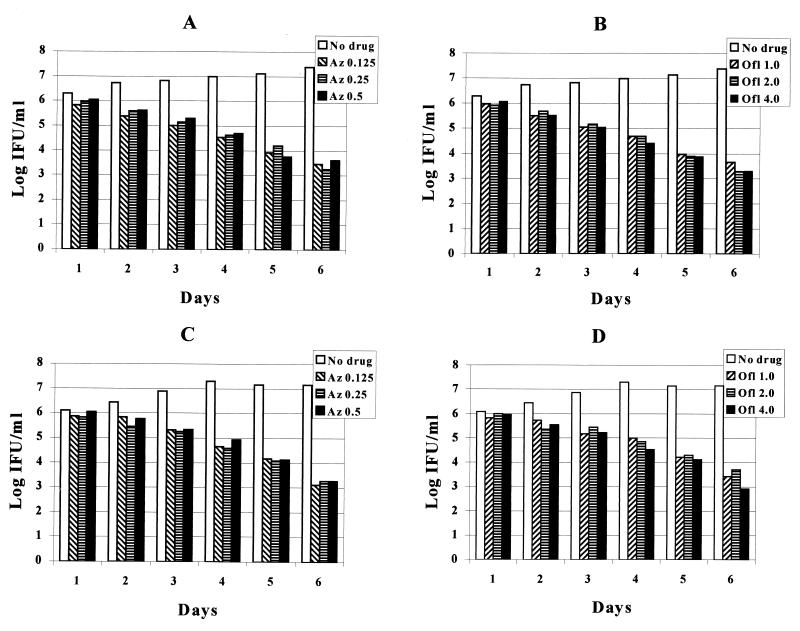
The results of treatment of continuously infected cells with different concentrations of drugs and medium changes every day are shown in Fig. 4. A gradual decrease, but not elimination, of chlamydia IFU was seen with both azithromycin (0.125, 0.25, and 0.5 μg/ml) and ofloxacin (1.0, 2.0, and 4.0 μg/ml). These results are similar to those seen utilizing one dose of the MIC of each drug.
FIG. 4.
Effect of different doses of azithromycin and ofloxacin on continuous C. pneumoniae in vitro. A, TW-183 (azithromycin); B, TW-183 (ofloxacin); C, CM-1 (azithromycin); D, CM-1 (ofloxacin).
DISCUSSION
The ability of chlamydia to establish a long-term association with host cells in vitro has been known since chlamydia was perceived as a virus. This type of in vitro infection was described as persistent or chronic. Continuous Chlamydia psittaci (2, 9–11) and Chlamydia trachomatis (1, 7, 8) infections in various cell lines have been described. Out of the four recognized chlamydial species, C. pneumoniae is considered to be the most difficult to cultivate. However, we were able to establish continuous C. pneumoniae infection in vitro. HEp-2 cells inoculated with CM-1 and TW-183 strains have been persistently infected for over 1.5 and 2 years, respectively. We observed stages of lysis and regrowth in host cells infected with both strains. Similar cycles of cell destruction and proliferation were previously reported for chronic C. psittaci and C. trachomatis infections in vitro by other investigators (8, 11). TW-183 initially appeared to be more infectious, producing significant lysis and more infectious progeny than CM-1. This phenomenon may be secondary to the previous extensive passage of TW-183 in tissue culture. CM-1 was isolated more recently from an adult with pneumonia and may not be as adapted to cell culture as TW-183. These cycles of infection may reflect a process of mutual adaptation of both chlamydia and host cells leading to selection of more infectious strains of chlamydia and inducing cell destruction followed by the selection of more-resistant cells (7, 11). Despite the high concentration of chlamydia (up to 107 IFU/ml) in the supernatant media, some cells contained no inclusions. Preliminary data have shown higher levels of various cytokines in continuously infected cultures than in primary infections, which may indicate increased host cell defenses (13). Benes (1) suggested that direct, cell-to-cell transmission of chlamydia in overcrowded cell layers may help in the evasion of host cell defenses and may lead to a new productive cycle. Our findings with regard to the induction and maintenance of continuous chlamydial infection were similar to those of others (8, 11). Contributing factors needed to establish continuous or chronic infection appeared to be the high initial inoculation dose, the balance of time necessary for chlamydial propagation and host cell regrowth, and the ability of some cells to remain uninfected.
This continuous-culture system may be more analogous than previous systems to the actual events in vivo and may serve as a better model for in vitro studies of C. pneumoniae. When we used continuously infected cells to determine the effect of azithromycin and ofloxacin on C. pneumoniae in vitro, we found that both drugs, at concentrations of up to four times the MIC90s, reduced, but did not completely eliminate, the organism. Our results were similar to those obtained by Galasso and Manire (2), who studied HeLa cells chronically infected with C. psittaci. They found that 7-day treatments with up to 50 μg of tetracycline per ml of medium decreased chlamydia production until it was rendered undetectable, but chlamydia propagation resumed soon after removal of the drug. Only prolonged (15-day) treatment was sufficient to eliminate chlamydia from continuously infected cells. This may be an important observation, as the failure of antibiotic therapy against C. pneumoniae infection in humans has been described (3).
REFERENCES
- 1.Benes S. Spread and persistence of infection with a trachoma biovar strain of Chlamydia trachomatis in multiplying and nonmultiplying McCoy cells. Sex Transm Dis. 1990;17:1–6. [PubMed] [Google Scholar]
- 2.Galasso G J, Manire G P. Effect of antiserum and antibiotics on persistent infection of HeLa cells with meningopneumonitis virus. J Immunol. 1961;86:382–385. [PubMed] [Google Scholar]
- 3.Hammerschlag M R, Chirgwin K, Roblin P M, Gelling M, Dumornay W, Mandel L, Smith P, Schachter J. Persistent infection with Chlamydia pneumoniae following acute respiratory illness. Clin Infect Dis. 1992;14:178–182. doi: 10.1093/clinids/14.1.178. [DOI] [PubMed] [Google Scholar]
- 4.Hammerschlag M R, Hyman C L, Roblin P M. In vitro activities of five quinolones against Chlamydia pneumoniae. Antimicrob Agents Chemother. 1992;36:682–683. doi: 10.1128/aac.36.3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammerschlag M R, Qumei K K, Roblin P M. In vitro activities of azithromycin, clarithromycin, l-ofloxacin, and other antibiotics against Chlamydia pneumoniae. Antimicrob Agents Chemother. 1992;36:1573–1574. doi: 10.1128/aac.36.7.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuo C, Shor A, Campbell L A, Fukushi H, Patton D L, Grayston J T. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis. 1993;167:841–849. doi: 10.1093/infdis/167.4.841. [DOI] [PubMed] [Google Scholar]
- 7.Lee C K. Factors affecting the rate at which a trachoma strain of Chlamydia trachomatis establishes persistent infections in mouse fibroblasts (McCoy cells) Infect Immun. 1981;33:954–957. doi: 10.1128/iai.33.3.954-957.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee C K, Moulder J W. Persistent infection of mouse fibroblasts (McCoy cells) with a trachoma strain of Chlamydia trachomatis. Infect Immun. 1981;32:822–829. doi: 10.1128/iai.32.2.822-829.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manire G P, Galasso G J. Persistent infection of HeLa cells with meningopneumonitis virus. J Immunol. 1959;83:529–533. [PubMed] [Google Scholar]
- 10.Moulder J W, Levy N J, Shulman R P. Persistent infection of mouse fibroblasts (L cells) with Chlamydia psittaci: evidence for a cryptic chlamydial form. Infect Immun. 1980;30:874–883. doi: 10.1128/iai.30.3.874-883.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Officer J E, Brown A. Serial changes in virus and cells in cultures chronically infected with psittacosis virus. Virology. 1961;14:88–99. doi: 10.1016/0042-6822(61)90136-2. [DOI] [PubMed] [Google Scholar]
- 12.Roblin P M, Dumornay W, Hammerschlag M R. Use of HEp-2 cells for improved isolation and passage of Chlamydia pneumoniae. J Clin Microbiol. 1992;30:1968–1971. doi: 10.1128/jcm.30.8.1968-1971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roblin P M, Kutlin A, Hammerschlag M R. Chlamydia pneumoniae stimulates production of IL-4 and IL-6 in HEp-2 cells. Pediatr Res. 1997;41:129A. [Google Scholar]
- 14.Saikku P, Leinonen M, Tenkanen L, Linnanmaki E, Ekman M R, Manninen V, Manttari M, Frick M H, Huttunen J K. Chronic Chlamydia pneumoniae infection as a risk factor for coronary heart disease in the Helsinki Heart Study. Ann Intern Med. 1992;116:273–278. doi: 10.7326/0003-4819-116-4-273. [DOI] [PubMed] [Google Scholar]