Abstract
Simple Summary
The current meta-analysis highlighted that proton pump inhibitors (PPIs) and histamine-2-receptor antagonists (H2RAs) could impact immune checkpoint inhibitors (ICIs) efficacy in NSCLC patients, highlighting the need for a deeper comprehension of factors involved in treatment response or resistance. Since the number of indications and NSCLC patients receiving ICIs is supposed to increase further soon, identifying the impact of these agents on NSCLC immunotherapy represents a compelling and urgent need regarding NSCLC.
Abstract
(1) Background: In recent years, immunotherapy has revolutionized the treatment landscape of non-small cell lung cancer (NSCLC), representing a therapeutic breakthrough in this field. Antacid agents such as proton pump inhibitors (PPIs) and histamine-2-receptor antagonists (H2RAs) are commonly prescribed for extended periods in NSCLC patients, and these drugs have the potential to modify the efficacy of immune checkpoint inhibitors (ICIs). (2) Materials and Methods: Herein, we conducted a systematic review and meta-analysis to investigate the impact of PPIs and H2RAs on progression-free survival (PFS) and overall survival (OS) among patients receiving immunotherapy for metastatic NSCLC. Effect measures for OS were Hazard Ratios (HRs) and 95% Confidence Intervals (CIs), which were extracted from available studies. Forest plots were used to assess HRs to describe the relationship between treatment and OS in the specified cohorts of patients. (3) Results: Six studies were included in the analysis, involving 2267 patients. The pooled HRs for OS and PFS were 1.4 (95% CI, 1.25–1.58) and 1.29 (95% CI, 1.17–1.43), respectively, suggesting that PPIs and H2RAs administration was negatively associated with PFS and OS. (4) Conclusion: Concomitant antacid use could modify the activity of ICIs in NSCLC patients.
Keywords: antiacid, proton pump inhibitors, immunotherapy, non-small cell lung cancer, immune checkpoint inhibitors
1. Introduction
Non-small cell lung cancer (NSCLC) remains one of the most common malignancies worldwide [1]. Several risk factors have been traditionally associated with the onset of NSCLC, including, among others, smoking, air pollution, occupational exposure, radiation, radon, and asbestos [2]. Recent years have witnessed the emergence of a wide range of systemic treatments for NSCLC patients, such as immunotherapy (as monotherapy or in combination with other anticancer agents), and targeted therapies [3]. As regards the former, immune checkpoint inhibitors (ICIs) have revolutionized NSCLC treatment, following the results of several practice-changing phase III clinical trials, and great progress has recently been made in this setting [4]. Firstly, the landmark KEYNOTE-024 phase III study conducted by Reck and colleagues reported the superiority of the PD-1 inhibitor pembrolizumab over standard chemotherapy for NSCLC patients with PD-L1 Tumor Proportion Score (TPS) ≥ 50% [5]. According to the results of this study, ICI monotherapy significantly improved progression-free survival (PFS), overall survival (OS), and overall response rate (ORR) in this patient population, leading to the United States Food and Drug Administration (FDA) approval of this agent for metastatic NSCLC patients without driver gene mutations and PD-L1 expression ≥ 50% [6]. Subsequently, a therapeutic revolution has characterized the NSCLC treatment scenario, as witnessed by the presentation and publication of an impressive number of clinical trials assessing ICIs monotherapy as first- or later-line treatment, as well as immune-based combinations [7]. Among these, the phase III KEYNOTE-189 trial showed a statistically significant and clinically meaningful PFS and OS benefit in non-squamous NSCLC patients treated with first-line pembrolizumab combined with pemetrexed and platinum compared to chemotherapy alone [8]. Similarly, the KEYNOTE-407 reported that a pembrolizumab combination with carboplatin and taxane chemotherapy was superior to chemotherapy alone as front-line therapy in metastatic squamous cell carcinoma [9]. Other immune-based combinations (e.g., chemotherapy plus ICIs and bevacizumab, the double checkpoint blockade with an anti-PD-1 agent and a CLTA-4 inhibitor, etc.) have reported practice-changing results in other trials, including the IMpower130, the CheckMate 227, and the CheckMate 9LA [10,11,12,13,14].
However, if the NSCLC immunotherapy era seems to have come, some questions remain unanswered, including identifying reliable predictors of response and the optimal choice between monotherapy and combination strategies [15,16,17]. Only a part of NSCLC patients seems to benefit from ICIs, and it is fundamental to investigate the underlying mechanisms and factors impairing the efficacy of immunotherapy [18]. For example, recent studies have explored the role of the gut microbiome in affecting physiological immune function, modifying the activity of cancer immunotherapy [19].
Antacid agents such as proton pump inhibitors (PPIs) and histamine-2-receptor antagonists (H2RAs) are commonly prescribed for extended periods in NSCLC patients, and these drugs have been suggested to modify the activity of anticancer therapies through several mechanisms, including gut microbiome changes [20]. However, available literature reports controversial results, with some studies highlighting a trend towards lower ICI activity and worse clinical outcomes in patients receiving antacids and immunotherapy, and other trials reporting no effect or even longer survival in subjects treated with concomitant PPIs or H2RAs [21,22]. Based on these premises, we performed a systematic review and meta-analysis to investigate the impact of antacids on NSCLC patients treated with ICIs.
2. Materials and Methods
2.1. Search Strategies
All clinical trials published from 10 June 2000 to 15 January 2022, were retrieved. Keywords used for searching on PubMed/Medline, Cochrane Library, and EMBASE were: “immunotherapy” or “nivolumab” or “ipilimumab” or “atezolizumab” or “pembrolizumab” or “durvalumab” or “avelumab” or “immune checkpoint inhibitors” and “metastatic lung cancer” and “lung cancer” or “non-small cell lung cancer” or “NSCLC” AND “proton pump inhibitors” or “PPI” or “omeprazole” or “pantoprazole” or “lansoprazole” or “esomeprazole” or “rabeprazole” or “histamine-2-receptor antagonists” or “ranitidine”. Only articles published in peer-reviewed journals, with available full text, and written in English were considered. Furthermore, proceedings of the main international oncological meetings (American Society of Clinical Oncology, American Association for Cancer Research, European Society of Medical Oncology, European Council of Clinical Oncology) were also searched from 2000 onward for relevant abstracts.
The search and review of the articles were evaluated by 3 authors independently.
2.2. Aims of the Systematic Review and Meta-Analysis
The aims of the systematic review and meta-analysis were:
To evaluate PFS in NSCLC patients receiving concomitant antacids (PPIs and/or H2RAs) and ICIs.
To evaluate OS in NSCLC patients receiving concomitant antacids (PPIs and/or H2RAs) and ICIs.
2.3. Selection Criteria
Studies selected from the first analysis were then restricted to: (1) clinical trials in NSCLC patients; (2) participants treated with ICIs; (3) studies with available data in terms of PPIs and H2RAs use; (4) studies with available data regarding PFS and OS in patients receiving immunotherapy.
2.4. Data Extraction and Quality Assessment
The following data were extracted for each publication: (1) study information (author, carry out country, inclusion criteria, study design); (2) type and dose of ICI; (3) number of patients; (4) type of antacid (PPIs and H2RAs). Three separate authors conducted the search and identification independently. The current analysis was conducted according to Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines (Table A1) [23,24]. We state that the current meta-analysis has not been registered on PROSPERO.
2.5. Statistical Design
All statistical analyses were performed using ProMeta 3 software.
Effect measures for OS were Hazard Ratios (HRs) and 95% Confidence Intervals (CIs), which were extracted from available studies. Forest plots were used to assess HRs to describe the relationship between treatment and OS in the specified cohorts of patients.
Statistical heterogeneity between trials was examined using the Chi-square test and the I2 statistic; substantial heterogeneity was considered to exist when the I2 value was greater than 50% or there was a low p value (<0.10) in the Chi-square test [25]. When no heterogeneity was noted, the fixed effects model was used, while the random-effects model was applied in the presence of significant heterogeneity.
3. Results
3.1. Search Results
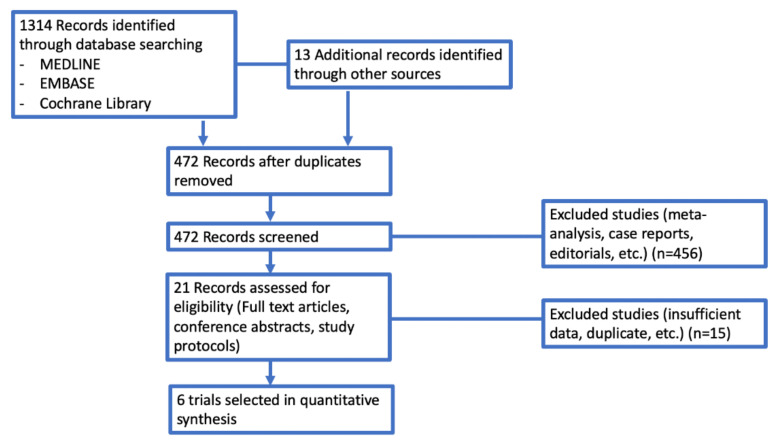
In our search, we found 1327 potentially relevant reports, which were subsequently restricted to 6 [26,27,28,29,30,31]. We excluded 1321 records as non-pertinent reports (pre-clinical studies, meta-analysis and systematic reviews, review articles, editorials, case reports, ongoing trials/trials in progress, no immunotherapy arm trials), as shown in Figure 1.
Figure 1.
Selection of trials included in the meta-analysis according to PRISMA statement.
We also excluded the study recently published by Hopkins and colleagues since this study included immune-based combinations with chemotherapy and atezolizumab, while our analysis was focused on immunotherapeutic agents used as monotherapy. Table 1. reports a summary of the included studies [26,27,28,29,30,31].
Table 1.
Summary of all the included studies in the present meta-analysis.
| Author Name [Reference] | Year | Region | Number of Patients Receiving Antacids | Number of Patients No Antacids | Type of ICIs | Type of Antiacids |
|---|---|---|---|---|---|---|
| Hakozaki [26] | 2019 | Japan | 47 | 43 | Nivolumab | PPIs or H2RAs |
| Zhao [27] | 2019 | China | 40 | 69 | Nivolumab Pembrolizumab SHR-1210 |
PPIs |
| Chalabi [28] | 2020 | Worldwide | 234 | 523 | Atezolizumab | PPIs |
| Peng [29] | 2021 | United States | 89 | 144 | Nivolumab Pembrolizumab Nivolumab plus ipilimumab |
PPIs |
| Rounis [30] | 2021 | Greece | 23 | 43 | Atezolizumab Nivolumab Pembrolizumab |
PPIs |
| Cortellini [31] | 2021 | Italy | 547 | 465 | Atezolizumab Nivolumab Pembrolizumab |
PPIs or H2RAs |
Abbreviations: H2RAs: histamine-2-receptor antagonists; ICIs: immune checkpoint inhibitors; PPIs: proton pump inhibitors
3.2. Overall Survival
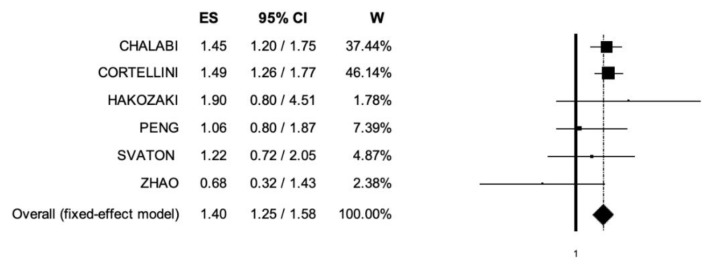
Six trials included data regarding OS [26,27,28,29,30,31]. The pooled HR for OS was 1.4 (95% CI, 1.25–1.58) (Figure 2), suggesting that patients receiving ICIs and PPIs and/or H2RAs presented lower OS compared to patients without antacids administration; the analysis was associated with low heterogeneity (I2 of 0%), and thus, a fixed-effects model was used.
Figure 2.
Forest plot of comparison between non-small cell lung cancer patients receiving immune checkpoint inhibitors with concomitant PPIs and/or H2RAs use or not; the outcome (ES) was Hazard Ratio of Overall Survival. Abbreviations: CI: confidence interval; ES: Effect Size (Hazard Ratio); W: Weight.
3.3. Progression-Free Survival
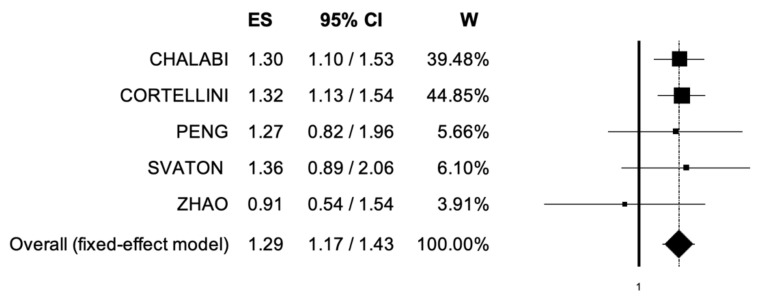
Five trials included data regarding PFS [27,28,29,30,31]. The pooled HR for PFS in the comparison between UC patients receiving immunotherapy with or without concomitant PPIs and/or H2RAs was 1.29 (95% CI, 1.17–1.43) (Figure 3). The analysis showed low heterogeneity, and a fixed-effect model was used (I2 = 0%).
Figure 3.
Forest plot of comparison between non-small cell lung cancer patients receiving immune checkpoint inhibitors with concomitant PPIs and/or H2RAs use or not; the outcome (ES) was Hazard Ratio of Progression-Free Survival. Abbreviations: CI: confidence interval; ES: Effect Size (Hazard Ratio); W: Weight.
3.4. Publication Bias
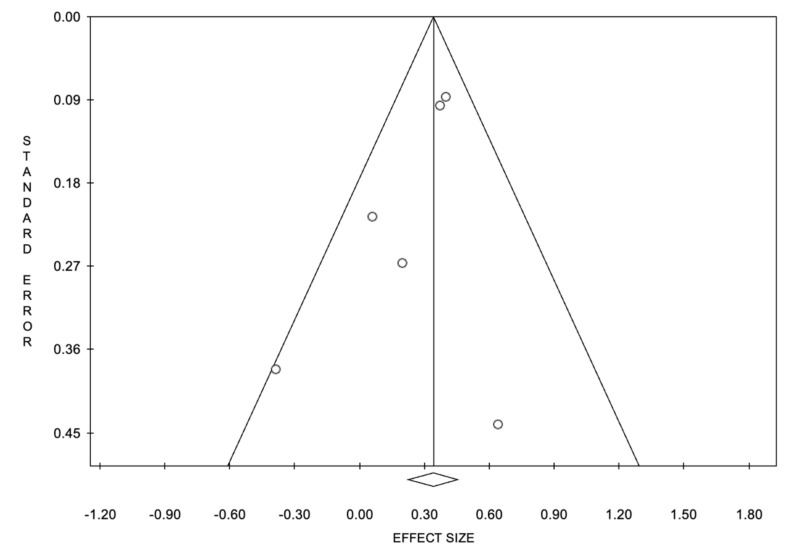
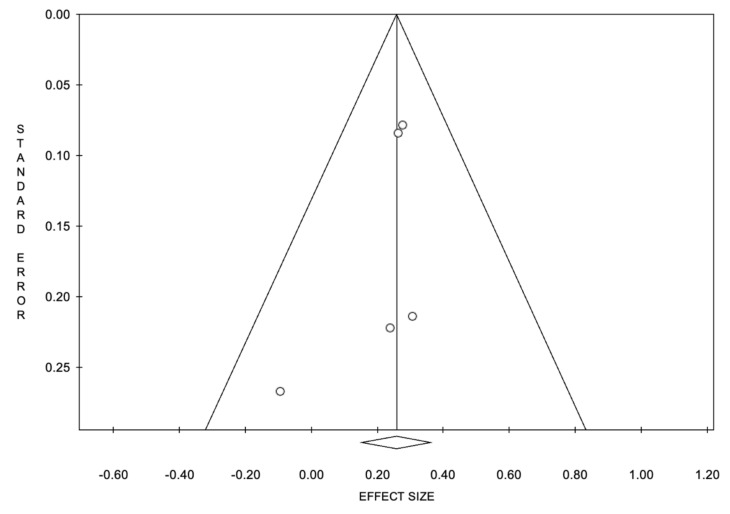
The funnel plots of OS and PFS showed basic symmetry, suggesting no publication bias (Figure 4 and Figure 5).
Figure 4.
Funnel plot of overall survival.
Figure 5.
Funnel plot of progression-free survival.
4. Discussion
Several phase I to III clinical trials have established the role of immunotherapy in metastatic NSCLC, producing unprecedented paradigm shifts in a relatively short period [32,33]. ICIs, as monotherapy or in combination with other anticancer agents, have revolutionized previous NSCLC treatment algorithms, prompting researchers and clinicians to consider the expansion of the role of immunotherapy in other settings, including the earlier stage of the disease (e.g., as neoadjuvant and adjuvant therapy) [34]. To the best of the authors’ knowledge, the current study represents the most comprehensive and updated meta-analysis in literature systematically assessing the impact of concomitant PPIs and/or H2RAs on ICI efficacy in NSCLC. According to our results, the analysis highlighted shorter median OS and median PFS in the case of concomitant antacid administration.
Recent reports have observed the significant importance of gut microbiome in modifying immunotherapy efficacy [35]. Identifying reliable predictors of response to ICIs is needed to better modulate the therapeutic process. Not only PD-L1, TMB, MSI, but also novel, emerging focuses of research are under development, including human microbiota [36,37,38]. In particular, the gut microbiome could be the key to enhancing anticancer immune response and, finally, to improve the prognosis of cancer patients receiving ICIs [39]. In addition, several multicenter, retrospective trials have explored the impact of concomitant medications, including metformin, aspirin, antibiotics, and antacids, on immunotherapy efficacy, reporting conflicting results [40]. Interestingly, the immunomodulatory effect produced by agents such as PPIs could impair the activity of ICIs, modifying gut microbiota—a well-known regulator of homeostasis [41]. From a biological point of view, antacids like PPIs and H2RAs may alter the gut microbiome through several mechanisms, including the decrease of bacterial richness, changes in gastric pH, transformations of bacterial species, and intestinal barrier dysfunctions [42,43]. In addition, some pre-clinical reports have also suggested that PPIs and H2RAs could impair the physiological function of polymorphonuclear neutrophils, natural killer cells, and cytotoxic T-lymphocytes, with all these elements being involved in ICIs efficacy [44,45]. Moreover, growing evidence suggests that gut microbiota dysbiosis may reduce the activity of ICIs, as also suggested in recently published studies in other settings and malignancies, including urothelial carcinoma [46]. For example, a pooled analysis from individual-participant data from IMvigor210 and IMvigor211 has highlighted that PPI use may represent a negative prognostic marker in advanced urothelial carcinoma treated with ICI therapy.
The current meta-analysis presents some strengths and limitations to be noticed. Among the strengths, the study includes an overall large number of metastatic NSCLC patients (n = 2267) receiving immunotherapy and represents the most updated meta-analysis on this topic. At the same time, some limitations should be acknowledged. Among these, the meta-analysis was based on aggregate data and not on individual-patient data; secondly, the included studies assessed different ICIs and antacids (PPIs and H2RAs), with these trials reporting also notable differences in terms of study design, sample size, and patient population (e.g., studies conducted in different geographical areas, an element representing a possible source of heterogeneity). Since ICIs such as atezolizumab, nivolumab, and pembrolizumab share some features but do not have superimposable mechanisms of action, this element could have introduced some bias. Lastly, no data regarding the impact of antacids on ICIs toxicity were available, and thus, we did not include this assessment in our meta-analysis. In addition, since it is likely that NSCLC patients included in our analysis were taking not only PPIs or H2RAs but also other medications—and since it is impossible to fully account for these effects—this bias cannot be excluded and avoided. Lastly, since our analysis was focused on ICIs monotherapy, we did not include the recently published study conducted by Hopkins and colleagues since this trial assessed immune-based combinations and not single-agent treatments [32]. Moreover, we did not include patients receiving chemotherapy in our analysis, and there is no control arm with chemotherapy-based regimens.
The current study suggests that concomitant antacids administration could be associated with shorter clinical outcomes in NSCLC patients treated with immunotherapy, confirming the results of recently published retrospective studies. However, most studies are small and underpowered; larger, prospective clinical trials are greatly needed to address this unmet need and identify whether specific medications such as PPIs or H2RAs could affect ICIs efficacy. Although our findings should be interpreted with caution, we believe that the current study has the merit of supporting the exploration of the role of PPIs and H2RAs in NSCLC immunotherapy due to the potentially meaningful clinical impact of these drugs in clinical practice. Further studies are warranted to clarify the relationship between ICIs, PPIs, H2RAs, and gut microbiota.
5. Conclusions
The current meta-analysis highlighted that PPIs and H2RAs could impact ICIs efficacy in NSCLC patients, highlighting the need for a deeper comprehension of factors involved in treatment response or resistance. Our results should be interpreted cautiously, and the available evidence is not sufficient to demonstrate a close link between worse clinical outcomes and PPIs and H2RAs use in NSCLC patients treated with ICIs. Since the number of indications and NSCLC patients receiving ICIs is supposed to increase further soon, identifying the impact of these agents on NSCLC immunotherapy represents a compelling and urgent need regarding this aggressive malignancy.
Appendix A
Table A1.
PRISMA 2009 Checklist.
| Section/Topic | # | Checklist Item | Reported on Page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 1 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 1, 2 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 1, 2 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 2, 3 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 2, 3 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 2, 3 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 2, 3 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 2, 3 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 2, 3 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 2, 3 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 2, 3 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 2, 3 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 2, 3 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | 2, 3 |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 4, 5, 6 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 4, 5, 6 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 4, 5, 6 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 4, 5, 6 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | 4, 5, 6 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | 4, 5, 6 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 7 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 7 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 7, 8 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 8 |
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097. For more information, visit: www.prisma-statement.org.
Author Contributions
Conceptualization, A.R.; methodology, A.R.; software, A.C., F.G., S.A., L.R., A.M., E.S.M., V.U., M.L., G.P.; validation, A.C., F.G., S.A., L.R., A.M., E.S.M., V.U., M.L., G.P.; formal analysis, A.R.; investigation, A.R.; resources, A.C., F.G., S.A., L.R., A.M., E.S.M., V.U., M.L., G.P.; data curation, A.C., F.G., S.A., L.R., A.M., E.S.M., V.U., M.L., G.P.; writing—original draft preparation, A.R.; writing—review and editing, A.C., F.G., S.A., L.R., A.M., E.S.M., V.U., M.L., G.P.; visualization, A.C., F.G., S.A., L.R., A.M., E.S.M., V.U., M.L., G.P.; supervision, A.C., F.G., S.A., L.R., A.M., E.S.M., V.U., M.L., G.P.; project administration, A.C., F.G., S.A., L.R., A.M., E.S.M., V.U., M.L., G.P.; funding acquisition, A.C., F.G., S.A., L.R., A.M., E.S.M., V.U., M.L., G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Thai A.A., Solomon B.J., Sequist L.V., Gainor J.F., Heist R.S. Lung cancer. Lancet. 2021;398:535–554. doi: 10.1016/S0140-6736(21)00312-3. [DOI] [PubMed] [Google Scholar]
- 2.Behrend S.J., Giotopoulou G.A., Spella M., Stathopoulos G.T. A role for club cells in smoking-associated lung adenocarcinoma. Eur. Respir. Rev. 2021;30:210122. doi: 10.1183/16000617.0122-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamberti G., Andrini E., Sisi M., Rizzo A., Parisi C., Di Federico A., Gelsomino F., Ardizzoni A. Beyond EGFR, ALK and ROS1: Current evidence and future perspectives on newly targetable oncogenic drivers in lung adenocarcinoma. Crit. Rev. Oncol. Hematol. 2020;156:103119. doi: 10.1016/j.critrevonc.2020.103119. [DOI] [PubMed] [Google Scholar]
- 4.Khanna P., Blais N., Gaudreau P.-O., Corrales-Rodriguez L. Immunotherapy Comes of Age in Lung Cancer. Clin. Lung Cancer. 2017;18:13–22. doi: 10.1016/j.cllc.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Reck M., Rodríguez–Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non–Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019;37:537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 6.Reck M. Pembrolizumab as first-line therapy for metastatic non-small-cell lung cancer. Immunotherapy. 2018;10:93–105. doi: 10.2217/imt-2017-0121. [DOI] [PubMed] [Google Scholar]
- 7.Gadgeel S., Rodríguez-Abreu D., Speranza G., Esteban E., Felip E., Dómine M., Hui R., Hochmair M.J., Clingan P., Powell S.F., et al. Updated Analysis From KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2020;38:1505–1517. doi: 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi L., Rodríguez-Abreu D., Gadgeel S., Esteban E., Felip E., De Angelis F., Domine M., Clingan P., Hochmair M.J., Powell S.F., et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 9.Paz-Ares L., Luft A., Vicente D., Tafreshi A., Gümüş M., Mazières J., Hermes B., Çay Şenler F., Csőszi T., Fülöp A., et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018;379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 10.West H., McCleod M., Hussein M., Morabito A., Rittmeyer A., Conter H.J., Kopp H.-G., Daniel D., McCune S., Mekhail T., et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka I., Morise M. Current Immunotherapeutic Strategies Targeting the PD-1/PD-L1 Axis in Non-Small Cell Lung Cancer with Oncogenic Driver Mutations. Int. J. Mol. Sci. 2021;23:245. doi: 10.3390/ijms23010245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steven A., Fisher S.A., Robinson B.W., Fong K.M., Van Zandwijk N. Immunotherapy for lung cancer. Respirology. 2016;21:821–833. doi: 10.1111/resp.12789. [DOI] [PubMed] [Google Scholar]
- 13.Hellmann M.D., Paz-Ares L., Bernabe Caro R., Zurawski B., Kim S.-W., Carcereny Costa E., Park K., Alexandru A., Lupinacci L., de la Mora Jimenez E., et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019;381:2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 14.Paz-Ares L., Ciuleanu T.-E., Cobo M., Schenker M., Zurawski B., Menezes J., Richardet E., Bennouna J., Felip E., Juan-Vidal O., et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:198–211. doi: 10.1016/S1470-2045(20)30641-0. Erratum in Lancet Oncol. 2021, 22, e92. [DOI] [PubMed] [Google Scholar]
- 15.Wojas-Krawczyk K., Kubiatowski T. Imperfect Predictors for Lung Cancer Immunotherapy—A Field for Further Research. Front. Oncol. 2020;10:568174. doi: 10.3389/fonc.2020.568174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yarchoan M., Hopkins A., Jaffee E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017;377:2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodman A.M., Kato S., Bazhenova L., Patel S.P., Frampton G.M., Miller V., Stephens P.J., Daniels G.A., Kurzrock R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017;16:2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He B., Dong D., She Y., Zhou C., Fang M., Zhu Y., Zhang H., Huang Z., Jiang T., Tian J., et al. Predicting response to immunotherapy in advanced non-small-cell lung cancer using tumor mutational burden radiomic biomarker. J. Immunother. Cancer. 2020;8:e000550. doi: 10.1136/jitc-2020-000550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Routy B., le Chatelier E., DeRosa L., Duong C.P.M., Alou M.T., Daillère R., Fluckiger A., Messaoudene M., Rauber C., Roberti M.P., et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 20.Weersma R.K., Zhernakova A., Fu J. Interaction between drugs and the gut microbiome. Gut. 2020;69:1510–1519. doi: 10.1136/gutjnl-2019-320204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diao X. Antibiotics and proton pump inhibitors suppress the efficacy of immunotherapy against non-small cell lung cancer. Thorac. Cancer. 2020;11:1763–1764. doi: 10.1111/1759-7714.13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giordan Q., Salleron J., Vallance C., Moriana C., Clement-Duchene C. Impact of Antibiotics and Proton Pump Inhibitors on Efficacy and Tolerance of Anti-PD-1 Immune Checkpoint Inhibitors. Front. Immunol. 2021;12:716317. doi: 10.3389/fimmu.2021.716317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D., Liberati A., Tetzlaff J., Altman D.G., the PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo C.K.-L., Mertz D., Loeb M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med Res. Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petitti D.B. Approaches to heterogeneity in meta-analysis. Stat. Med. 2001;20:3625–3633. doi: 10.1002/sim.1091. [DOI] [PubMed] [Google Scholar]
- 26.Hakozaki T., Okuma Y., Omori M., Hosomi Y. Impact of prior antibiotic use on the efficacy of nivolumab for non-small cell lung cancer. Oncol. Lett. 2019;17:2946–2952. doi: 10.3892/ol.2019.9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao S., Gao G., Li W., Li X., Zhao C., Jiang T., Jia Y., He Y., Li A., Su C., et al. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer. 2019;130:10–17. doi: 10.1016/j.lungcan.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Chalabi M., Cardona A., Nagarkar D., Scala A.D., Gandara D., Rittmeyer A., Albert M., Powles T., Kok M., Herrera F.G. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: Pooled post hoc analyses of the OAK and POPLAR trials. Ann. Oncol. 2020;31:525–531. doi: 10.1016/j.annonc.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Peng K., Chen K., Teply B.A., Yee G.C., Farazi P.A., Lyden E.R. Impact of Proton Pump Inhibitor Use on the Effectiveness of Immune Checkpoint Inhibitors in Advanced Cancer Patients. Ann. Pharmacother. 2021 doi: 10.1177/10600280211033938. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Rounis K., Makrakis D., Papadaki C., Monastirioti A., Vamvakas L., Kalbakis K., Gourlia K., Xanthopoulos I., Tsamardinos I., Mavroudis D., et al. Prediction of outcome in patients with non-small cell lung cancer treated with second line PD-1/PDL-1 inhibitors based on clinical parameters: Results from a prospective, single institution study. PLoS ONE. 2021;16:e0252537. doi: 10.1371/journal.pone.0252537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cortellini A., Tucci M., Adamo V., Stucci L.S., Russo A., Tanda E.T., Spagnolo F., Rastelli F., Bisonni R., Santini D., et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J. Immunother. Cancer. 2020;8:e001361. doi: 10.1136/jitc-2020-001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hopkins A.M., Kichenadasse G., McKinnon R.A., Abuhelwa A.Y., Logan J.M., Badaoui S., Karapetis C.S., Rowland A., Sorich M.J. Efficacy of first-line atezolizumab combination therapy in patients with non-small cell lung cancer receiving proton pump inhibitors: Post hoc analysis of IMpower150. Br. J. Cancer. 2021;126:42–47. doi: 10.1038/s41416-021-01606-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uprety D., Mandrekar S.J., Wigle D., Roden A.C., Adjei A.A. Neoadjuvant Immunotherapy for NSCLC: Current Concepts and Future Approaches. J. Thorac. Oncol. 2020;15:1281–1297. doi: 10.1016/j.jtho.2020.05.020. [DOI] [PubMed] [Google Scholar]
- 34.Broderick S.R. Adjuvant and Neoadjuvant Immunotherapy in Non–small Cell Lung Cancer. Thorac. Surg. Clin. 2020;30:215–220. doi: 10.1016/j.thorsurg.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Lee K.A., Shaw H.M., Bataille V., Nathan P., Spector T.D. Role of the Gut Microbiome for Cancer Patients Receiving Immunotherapy: Dietary and Treatment Implications. Eur. J. Cancer. 2020;138:149–155. doi: 10.1016/j.ejca.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 36.Rizzo A., Brandi G. Biochemical predictors of response to immune checkpoint inhibitors in unresectable hepatocellular carcinoma. Cancer Treat. Res. Commun. 2021;27:100328. doi: 10.1016/j.ctarc.2021.100328. [DOI] [PubMed] [Google Scholar]
- 37.Petrelli F., Iaculli A., Signorelli D., Ghidini A., Dottorini L., Perego G., Ghidini M., Zaniboni A., Gori S., Inno A. Survival of Patients Treated with Antibiotics and Immunotherapy for Cancer: A Systematic Review and Meta-Analysis. J. Clin. Med. 2020;9:1458. doi: 10.3390/jcm9051458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y., Chen H., Chen S., Li Z., Chen J., Li W. The effect of concomitant use of statins, NSAIDs, low-dose aspirin, metformin and beta-blockers on outcomes in patients receiving immune checkpoint inhibitors: A systematic review and meta-analysis. OncoImmunology. 2021;10:1957605. doi: 10.1080/2162402X.2021.1957605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maier L., Pruteanu M., Kuhn M., Zeller G., Telzerow A., Anderson E.E., Brochado A.R., Fernandez K.C., Dose H., Mori H., et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buti S., Bersanelli M., Perrone F., Bracarda S., Di Maio M., Giusti R., Nigro O., Cortinovis D.L., Aerts J.G.J.V., Guaitoli G., et al. Predictive Ability of a Drug-Based Score in Patients with Advanced Non-Small-Cell Lung Cancer Receiving First-Line Immunotherapy. Eur. J. Cancer. 2021;150:224–231. doi: 10.1016/j.ejca.2021.03.041. [DOI] [PubMed] [Google Scholar]
- 41.Li W., Deng Y., Chu Q., Zhang P. Gut microbiome and cancer immunotherapy. Cancer Lett. 2019;447:41–47. doi: 10.1016/j.canlet.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 42.Bruno G., Zaccari P., Rocco G., Scalese G., Panetta C., Porowska B., Pontone S., Severi C. Proton pump inhibitors and dysbiosis: Current knowledge and aspects to be clarified. World J. Gastroenterol. 2019;25:2706–2719. doi: 10.3748/wjg.v25.i22.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laheij R.J.F., Sturkenboom M.C.J.M., Hassing R.-J., Dieleman J., Stricker B.H.C., Jansen J.B.M.J. Risk of Community-Acquired Pneumonia and Use of Gastric Acid–Suppressive Drugs. JAMA. 2004;292:1955–1960. doi: 10.1001/jama.292.16.1955. [DOI] [PubMed] [Google Scholar]
- 44.Aybay C., Imir T., Okur H. The effect of omeprazole on human natural killer cell activity. Gen. Pharmacol. Vasc. Syst. 1995;26:1413–1418. doi: 10.1016/0306-3623(94)00301-3. [DOI] [PubMed] [Google Scholar]
- 45.Zedtwitz-Liebenstein K., Wenisch C., Patruta S., Parschalk B., Daxböck F., Graninger W. Omeprazole treatment diminishes intra- and extracellular neutrophil reactive oxygen production and bactericidal activity. Crit. Care Med. 2002;30:1118–1122. doi: 10.1097/00003246-200205000-00026. [DOI] [PubMed] [Google Scholar]
- 46.Hopkins A.M., Kichenadasse G., Karapetis C.S., Rowland A., Sorich M.J. Concomitant Proton Pump Inhibitor Use and Survival in Urothelial Carcinoma Treated with Atezolizumab. Clin. Cancer Res. 2020;26:5487–5493. doi: 10.1158/1078-0432.CCR-20-1876. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.