Abstract
A total of 24,591 nonhuman salmonella strains isolated in Germany between 1986 and 1998 were examined for their resistance to nalidixic acid by an agar diffusion method. The rate of resistance (inhibition zone, ≤13 mm) ranged from 0.2% in 1986 to a peak of 14.8% in 1990. Between 1991 and 1998 the MICs for nalidixic acid-resistant strains ranged from more than 256 μg/ml for nalidixic acid to between 0.25 and 128 μg/ml for enrofloxacin. In the early 1990s a particularly high incidence of fluoroquinolone resistance (49.5%) was seen among isolates of Salmonella enterica serotype Typhimurium (Salmonella Typhimurium) definitive phage type 204c that mainly originated from cattle. Among isolates from poultry an increase in the incidence of nalidixic acid resistance to a peak of 14.4% was observed in 1994. This peak was due to the presence of specific resistant serotypes, mainly serotypes Hadar, Saintpaul, Paratyphi B (d-tartrate positive; formerly serotype Java) and Newport. Such strains exhibited a decreased susceptibility to enrofloxacin (MIC, 1 μg/ml). Among isolates from pigs the peak incidence of resistance was reached in 1993, with 7.5% of isolates resistant to nalidixic acid and enrofloxacin. The study demonstrates an increase in the incidence of strains that are resistant to nalidixic acid and that have decreased susceptibility to enrofloxacin after the licensing of enrofloxacin. In addition, the number of other serotypes that exhibited nalidixic acid resistance or reduced enrofloxacin susceptibility increased among the total number of isolates investigated between 1992 and 1998.
Fluoroquinolones are antimicrobial agents related to the naphthyridine nalidixic acid. They are valuable for the treatment of infections caused by pathogenic bacteria in humans and animals because of their wide spectra and high levels of antimicrobial activity (38). They act by inhibiting DNA gyrase and topoisomerase IV in susceptible bacteria. The World Health Organization (WHO) estimated the production and usage of quinolones to be about 120 metric tons mainly in the United States, the European Union, Japan, and South Korea and 1,820 metric tons alone in China (38). During 1997, the usage of fluoroquinolones within the European Union was estimated to be 43 metric tons (3). In Germany the fluoroquinolone enrofloxacin was first licensed for use in veterinary medicine in May 1989. In The Netherlands, enrofloxacin had been approved 2 years earlier, and other European countries followed with the introduction of enrofloxacin in the early 1990s. In some instances the use of enrofloxacin was paralleled by a decrease in the susceptibility to quinolones of zoonotic bacteria isolated from food-producing animals and humans in Europe (8, 9, 19, 20). Quinolone and fluoroquinolone resistance in Campylobacter and Salmonella enterica has mainly been due to single point mutations in gyrA (12, 13, 26), which encodes the A subunit of DNA gyrase, and rarely in gyrB, which encodes the B subunit of DNA gyrase (10, 14). Other mechanisms have also been proposed, e.g., mutations in the parC gene (15, 35) and decreased uptake of antimicrobial agents (24, 37).
Salmonellae are known to cause severe disease in humans and animals and are the leading cause of food-borne infections in many countries. Serotyping is performed by the Kauffmann-White scheme (27), and in many countries S. enterica serotype Typhimurium (Salmonella Typhimurium) and serotype Enteritidis (Salmonella Enteritidis) predominate. These serotypes can be further differentiated by phage typing and molecular techniques (2, 23, 33, 36). The most commonly isolated salmonellae are often clonally distributed, for example, the recently predominating clones of Salmonella Typhimurium definitive phage type (DT) 104 (5, 33) or Salmonella Enteritidis phage type 4 (16, 30).
In humans most infections caused by nontyphoidal salmonellae are self-limiting and antibiotic therapy is not indicated. However, in life-threatening situations treatment with fluoroquinolones is recommended, and this is particularly applicable for infections caused by multidrug-resistant salmonellae (1). Quinolone resistance in veterinary salmonella isolates from Germany was first observed in 1988. Strains of multidrug-resistant Salmonella Typhimurium DT 204c highly resistant to fluoroquinolones were isolated from cattle in a defined area near the Dutch border with Germany (11, 17). Such resistance was caused by mutations in the gyrA and gyrB genes (14). Hof et al. (18) reported on a nonfatal case of salmonellosis in an 11-year-old girl who was infected with such a strain presumably by ingestion of contaminated meat. In 1990 the number of isolates of Salmonella Typhimurium DT 204c decreased in Germany. However, in parallel, the number of pentadrug-resistant isolates (resistant to ampicillin, chloramphenicol, streptomycin, sulfonamides, and tetracyclines) of Salmonella Typhimurium DT 104 increased, and the strains soon became the most prevalent salmonella serotype and phage type among veterinary isolates (28). In England and Wales a decrease in susceptibility to fluoroquinolones in isolates of Salmonella Typhimurium DT 104 was observed following the licensing of enrofloxacin for veterinary use in 1993 (31, 34). Recently, in Denmark, a fatal case of quinolone-resistant Salmonella Typhimurium DT 104 was reported in an outbreak associated with the consumption of contaminated pork (4). The outbreak highlighted the transfer of fluoroquinolone-resistant strains from animals to humans and the potential problems associated with the treatment of patients infected with such clones.
This paper analyzes the incidence in Germany of quinolone resistance among isolates of S. enterica from animal sources during the last 13 years, with particular reference to the susceptibility to enrofloxacin of veterinary isolates of Salmonella Typhimurium DT 104 obtained in 1997 and 1998.
MATERIALS AND METHODS
Bacterial strains.
In a 13-year period (1986 to 1998), 29,563 nonhuman salmonella isolates from different locations in Germany were received at the National Salmonella Reference Laboratory (NRL-Salm) in Berlin. The majority of strains were from food-producing animals, domestic animals, and other animals, for example, horses, rats, and reptiles. The remaining strains were from food, animal feed, or environmental samples. The isolates were distributed into four categories: bovine, poultry, porcine, and other. The categories bovine, poultry, and porcine refer to isolates from the respective animals or the corresponding food items; the category other contained the remaining isolates and other isolates for which the source was not well indicated. The typing of isolates at NRL-Salm follows a hierarchical protocol, including serotyping, phage typing, and molecular typing. Serotyping was performed by the Kauffmann-White scheme (27), and phage typing was performed by using the scheme of Anderson et al. (2) for Salmonella Typhimurium and the system of Ward et al. (36), as applied in the Central Public Health Laboratory, London, United Kingdom, for Salmonella Enteritidis.
Agar diffusion test.
Susceptibility to the quinolone nalidixic acid was tested by the agar diffusion test in accordance with the guidelines of the German Institute for Standards (DIN 58940, part 3) (6). Briefly, about 106 CFU of salmonella cells was inoculated onto Mueller-Hinton agar plates (diameter, 9 cm), and antibiotic-containing discs (Oxoid Ltd., London, England) were applied. The plates were incubated at 37°C for 20 h, resulting in a semiconfluent growth. The discs contained 30 μg of nalidixic acid. Strains are described as resistant if the diameter of the inhibition zone was ≤13 mm. Control strain Escherichia coli ATCC 25922 was included on each set of plates and exhibited an inhibition zone of 22 to 28 mm (22).
MIC determination.
The MICs of nalidixic acid and enrofloxacin were determined by a broth microdilution method in accordance with the German standard guideline DIN 58940, part 8 (7). Each well contained a final bacterial inoculum of about 105 CFU/ml in Mueller-Hinton broth. For nalidixic acid (Sigma, Deisenhofen, Germany) the range of antibiotic concentrations tested was 0.25 to 256 μg/ml, and for enrofloxacin (Bayer AG, Leverkusen, Germany) the range was 0.0075 to 8 μg/ml. For enrofloxacin susceptibility, strains which grew at a concentration of 8 μg/ml were further tested with between 2 and 256 μg/ml for determination of MICs. All plates were incubated at 37°C for 20 h. The MIC was defined as the lowest concentration that produced no visible growth. Control strain E. coli ATCC 25922 was included on each set of plates. The MICs for the control strain were 2 μg/ml for nalidixic acid and 0.015 μg/ml for enrofloxacin (21). The breakpoints for the interpretation of resistance and susceptibility were those recommended by the German standard guideline DIN 58940. For nalidixic acid-susceptible strains MICs were ≤8 μg/ml, and MICs were ≥16 μg/ml for resistant strains. MICs for enrofloxacin-susceptible strains were ≤0.5 μg/ml, MICs for intermediate strains were 1 μg/ml, and MICs for resistant strains were ≥2 μg/ml.
RESULTS
Quinolone resistance in isolates from various sources.
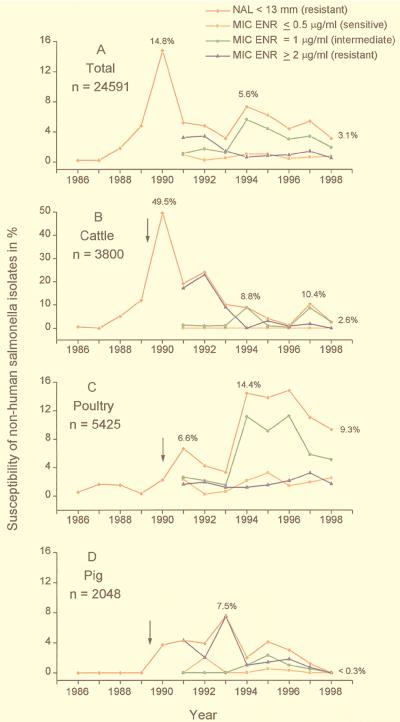
A total of 24,591 salmonella strains isolated between 1986 and 1998 were investigated by the agar diffusion test for their nalidixic acid susceptibility. Of these, 1,198 (4.9%) were resistant to nalidixic acid. Resistant strains isolated between 1991 and 1998 were investigated in more detail. With one exception, the nalidixic acid MIC for all strains was in excess of 256 μg/ml; the single exceptional strain required only 128 μg/ml for growth inhibition. The MICs of enrofloxacin ranged from 0.5 to 128 μg/ml. The prevalence of isolates resistant to enrofloxacin (MIC, ≥2 μg/ml) in relation to the total number of strains investigated varied between 3.4% in 1992 and 0.5% in 1998 (Fig. 1A). The incidence of intermediate strains (MIC, 1 μg/ml) ranged from 1.1% in 1991 to 5.6% in 1994, and the incidence of susceptible strains (MIC, ≤0.5 μg/ml) ranged from 0.2% in 1992 to 1% in 1994. In contrast, the proportion of isolates resistant to nalidixic acid ranged from 0.2% in 1986 to 14.8% in 1990 (Fig. 1A). The percentage of resistant strains varied considerably among strains from pigs, cattle, or poultry, but in all animals the proportion of isolates resistant to nalidixic acid increased in those years which followed the first use of enrofloxacin (Fig. 1A to C).
FIG. 1.
Percent resistance to nalidixic acid (NAL; inhibition zone, ≤13 mm) and relative MICs of enrofloxacin (ENR; susceptible, intermediate, resistant) for nalidixic acid-resistant, nonhuman salmonella strains isolated in Germany between 1986 and 1998 (received between January and October 1998). (A) Total rate for all isolates. (B) Isolates from cattle. (C) Isolates from poultry. (D) Isolates from pigs. The arrows indicate the dates of licensing of enrofloxacin for veterinary use in Germany (for pigs and cattle, May 1989; for poultry, January 1990). N is the number of isolates investigated.
In cattle, the incidence of strains with quinolone resistance peaked in 1990, when almost 50% of isolates were resistant (Fig. 1B); the average prevalence of resistance between 1990 and 1992 was nearly 31%. Phage typing revealed that the high incidence was mainly caused by the predominance of isolates of high-level fluoroquinolone-resistant Salmonella Typhimurium DT 204c (quinolone MIC range, 32 to 640 μg/ml) (11, 17). This DT 204c clone was also defined by a multidrug-resistance profile and by the possession of a distinct plasmid profile (11). Since 1992 the percentage of nalidixic acid-resistant isolates has decreased, and in 1998 only 2.6% of isolates were resistant (Fig. 1B). In 1994 and 1997 nalidixic acid-resistant strains from cattle belonged mainly to Salmonella Hadar and for the most part originated from a distinct region of Germany (Niedersachsen). The MICs of enrofloxacin for these Salmonella Hadar strains were considerably lower than those for the Salmonella Typhimurium DT 204c isolates and ranged from 1 to 2 μg/ml. Since 1996 almost all quinolone resistance has been found in isolates of Salmonella Typhimurium DT 104 (see below).
In poultry, the percentage of isolates resistant to nalidixic acid increased from 0.3% in 1989 to 14.4% in 1994 and plateaued until 1996 (Fig. 1C). Most of these nalidixic acid-resistant isolates belonged to Salmonella Hadar and displayed a reduced susceptibility to enrofloxacin (MICs, 1 μg/ml).
In pigs, the incidence of resistance was, in general, lower than that in cattle or poultry (Fig. 1D). The first two resistant strains appeared in 1990. These were highly fluoroquinolone resistant and were identified as Salmonella Typhimurium DT 204c. In 1993 the incidence of resistance reached a maximum of 7.5% and was also exclusively among Salmonella Typhimurium DT 204c strains. Resistance to nalidixic acid and decreased susceptibility to enrofloxacin (MICs, 1 μg/ml) were also observed in single isolates of Salmonella Saintpaul, Hadar, and Derby and non-phage-typeable Salmonella Typhimurium.
Susceptibilities of selected serotypes to quinolones.
Higher prevalences of resistance to nalidixic acid were mainly detected in specific serotypes, for example, Salmonella Hadar, Paratyphi B (d-tartrate positive), and Saintpaul (Table 1). These serotypes are primarily found in poultry. In the last 5 years the prevalence of nalidixic acid-resistant poultry isolates was 57% for Salmonella Hadar, 69% for Salmonella Paratyphi B (d-tartrate positive), and 67% for Salmonella Saintpaul.
TABLE 1.
Range of MICs of enrofloxacin for prevalent nalidixic acid-resistant serotypes
| Year |
Salmonella Typhimurium
|
Salmonella Hadar
|
Salmonella Paratyphi B (d-tartrate positive)
|
Salmonella Saintpaul
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | No. (%) NALra | ENRb MIC range (μg/ml) | No. | No. (%) NALr | ENR MIC range (μg/ml) | No. | No. (%) NALr | ENR MIC range (μg/ml) | No. | No. (%) NALr | ENR MIC range (μg/ml) | |
| 1986 | 463 | 2 (0.4) | NDc | 14 | 0 | 17 | 0 | 56 | 0 | |||
| 1987 | 467 | 1 (0.2) | ND | 36 | 0 | 6 | 0 | 42 | 2 (4.8) | ND | ||
| 1988 | 931 | 28 (3.0) | 0.5–128 | 24 | 0 | 9 | 0 | 36 | 0 | |||
| 1989 | 1,156 | 113 (9.8) | 0.5–128 | 39 | 0 | 4 | 0 | 20 | 1 (5.0) | ND | ||
| 1990 | 906 | 338 (37.3) | 0.5–128 | 37 | 3 (8.1) | ND | 3 | 0 | 25 | 1 (4.0) | ND | |
| 1991 | 220 | 28 (12.7) | 0.5–128 | 45 | 3 (6.7) | 0.5–1 | 0 | 0 | 14 | 3 (21.4) | 0.5 | |
| 1992 | 189 | 33 (17.5) | 0.5–128 | 34 | 6 (17.6) | 0.5–2 | 2 | 0 | 17 | 2 (11.8) | 0.5–1 | |
| 1993 | 285 | 17 (6.0) | 1–128 | 22 | 5 (22.7) | 1–2 | 2 | 0 | 25 | 5 (20.0) | 0.5–1 | |
| 1994 | 200 | 2 (1.0) | 1–128 | 72 | 61 (84.7) | 0.5–4 | 3 | 1 (33.3) | 0.5 | 13 | 5 (38.5) | 0.5–1 |
| 1995 | 675 | 13 (1.9) | 0.5–128 | 95 | 64 (67.4) | 1–4 | 6 | 2 (33.3) | 0.5–1 | 35 | 14 (40.0) | 0.5–1 |
| 1996 | 670 | 16 (2.4) | 0.5–128 | 54 | 41 (75.9) | 1–4 | 15 | 11 (73.3) | 1–2 | 14 | 5 (35.7) | 0.5–1 |
| 1997 | 569 | 3 (0.5) | 1–2 | 83 | 52 (62.7) | 1–8 | 42 | 24 (57.1) | 0.5–4 | 68 | 9 (13.2) | 0.5–2 |
| 1998d | 772 | 8 (1.0) | 0.5–1 | 74 | 18 (24.3) | 1–2 | 20 | 11 (55.0) | 1–4 | 20 | 6 (30.0) | 0.5–2 |
NALr, nalidixic acid resistant.
ENR, enrofloxacin.
ND, not determined.
Only strains received from January 1998 to October 1998 were investigated.
The incidence of quinolone-resistant isolates of Salmonella Typhimurium peaked in 1990, at 37.3%. This was due to the prevalence of fluoroquinolone-resistant strains of DT 204c for which enrofloxacin MICs ranged from 64 to 128 μg/ml (Table 1). Since 1990 the prevalence of phage type DT 204c isolates has declined dramatically, to less than 0.5% in 1997. The MICs of enrofloxacin for nalidixic acid-resistant Salmonella Typhimurium strains not belonging to phage type DT 204c has ranged from 0.5 to 4 μg/ml; since 1996 such strains have mainly been DT 104.
Nalidixic acid-resistant Salmonella Hadar was first observed in 1990, when 3 of 37 isolates (8.1%) were found to be resistant to this antimicrobial agent. Since then, the prevalence of nalidixic acid-resistant strains of this serotype increased continuously to 84.7% in 1994. Between 1995 and 1997 the incidence of nalidixic acid resistance varied between 62.6 and 75.9% but dropped to 24.3% in 1998. The MICs of enrofloxacin for nalidixic acid-resistant Salmonella Hadar strains ranged from 0.5 to 8 μg/ml (Table 1). Enrofloxacin MICs for the majority of the nalidixic acid-resistant Salmonella Hadar strains isolated between 1994 and 1998 (average, 86%) were 1 μg/ml. The first observed Salmonella Hadar strain for which the MIC was 2 μg/ml was isolated in 1992, and subsequently, the prevalence of strains for which MICs were 2 μg/ml increased to 25% in 1997. The first strain for which the MIC was 4 μg/ml was isolated in 1994, and the incidence of such strains increased to 7.7% in 1997. For one Salmonella Hadar strain isolated from poultry in 1997 the MIC was 8 μg/ml. These data show that the rate of quinolone resistance has increased among Salmonella Hadar strains, and simultaneously, sensitivity to enrofloxacin has decreased. A similar situation was observed in Salmonella Paratyphi B (d-tartrate positive) and Salmonella Saintpaul (Table 1).
Susceptibility of Salmonella Typhimurium phage type DT 104 isolates to enrofloxacin.
DT 104 has become the predominant Salmonella Typhimurium phage type among German veterinary isolates since 1994 (28). Of all Salmonella Typhimurium strains studied, 8.7% in 1996, 14.4% in 1997, and 12.8% in the first 9 months of 1998 were of phage type DT 104. These strains originated most frequently from cattle or pigs (Table 2). All DT 104 strains isolated in 1997 and 1998 were investigated for their susceptibility to enrofloxacin by measuring the MICs for the strains (Table 2). The range of enrofloxacin MICs was 0.015 to 2 μg/ml in 1997 and 0.03 to 1 μg/ml in 1998. In 1997 and 1998 the prevalences of DT 104 isolates for which the enrofloxacin MIC was 0.06 μg/ml were 76 and 83%, respectively. In 1997, the MIC was 0.25 μg/ml for 10% of strains; in contrast, in 1998 only one strain for which the MIC was 0.25 μg/ml was observed. The high prevalence in 1997 of strains for which MICs were >0.25 μg/ml was caused by an outbreak of DT 104 in cattle in Berlin, in which enrofloxacin was frequently used for therapy. In contrast, in 1998 the seven DT 104 isolates for which MICs were 1 μg/ml were isolated in different regions of Germany. These data show that, according to the recommended breakpoint for enrofloxacin (≥2 μg/ml), only one strain isolated in 1997 can be regarded as resistant.
TABLE 2.
Source and number of Salmonella Typhimurium DT 104 isolated in 1997 and 1998 and their MICs of enrofloxacin
| Year | Source | No. (%) of isolates | No. of NALra isolates | No. of DT 104 isolates for which the enrofloxacin MIC (μg/ml) was as follows:
|
MIC (μg/ml)b
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.015 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 50% | 90% | ||||
| 1997 | Total | 320 (100) | 3 | 4 | 5 | 244 | 32 | 32 | 0 | 2 | 1 | 0.06 | 0.25 |
| Pig | 167 (52.2) | 0 | 2 | 3 | 140 | 22 | 0 | 0 | 0 | 0 | 0.06 | 0.125 | |
| Cattle | 74 (23.0) | 2 | 0 | 1 | 34 | 6 | 31 | 0 | 1 | 1 | 0.125 | 0.25 | |
| Poultry | 6 (1.9) | 0 | 2 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0.06 | 0.06 | |
| Other | 73 (22.8) | 1 | 0 | 1 | 66 | 4 | 1 | 0 | 1 | 0 | 0.06 | 0.06 | |
| 1998c | Total | 319 (100) | 8 | 0 | 2 | 266 | 42 | 1 | 1 | 7 | 0 | 0.06 | 0.125 |
| Pig | 163 (51.1) | 0 | 0 | 0 | 135 | 27 | 1 | 0 | 0 | 0 | 0.06 | 0.125 | |
| Cattle | 57 (17.9) | 5 | 0 | 2 | 47 | 3 | 0 | 0 | 5 | 0 | 0.06 | 0.125 | |
| Poultry | 20 (6.3) | 2 | 0 | 0 | 15 | 3 | 0 | 0 | 2 | 0 | 0.06 | 0.125 | |
| Other | 79 (24.8) | 1 | 0 | 0 | 69 | 9 | 0 | 1 | 0 | 0 | 0.06 | 0.125 | |
NALr, nalidixic acid resistant.
50% and 90%, MICs at which 50 and 90% of isolates, respectively, are inhibited.
Only strains received from January 1998 to October 1998 were investigated.
DISCUSSION
In the present study the prevalence of resistance to quinolones among 24,591 German veterinary salmonella isolates obtained between 1986 and 1998 was examined. The results show that in three food-producing animal species, cattle, poultry, and pigs, the incidence of quinolone-resistant strains increased substantially in the years following the licensing of quinolones. A high prevalence of resistant strains was especially detected in isolates that originated from cattle, and such isolates were mainly to Salmonella Typhimurium DT 204c (nalidixic acid MIC range, 128 to >256 μg/ml). The MICs of enrofloxacin for these strains ranged from 0.25 to 128 μg/ml.
Among all isolates, the incidence of nalidixic acid-resistant strains varied between 0.2 and 14.8%. However, isolates from different animals showed considerable differences in the prevalence of nalidixic acid resistance per year. While in 1990 nearly 50% of isolates from cattle were highly resistant to nalidixic acid and enrofloxacin, for isolates from poultry an increase in the incidence of resistance has only occurred since 1994. Among isolates from pigs the incidence of resistance was, in general, low.
The data presented here have shown that the use of quinolones in food-producing animals leads, sometimes even before official approval of use of the drugs, to an initial increase in the prevalence of resistance. In subsequent years the levels of resistance have decreased, and in some cases they have reached low levels. Why this decrease has happened is speculative. One explanation could be that increasing levels of resistance lead to changes in prescription habits, resulting in a decline in the rate of use of such drugs. In order to show that this is the case, the incidence of resistance needs to be correlated to the consumption of the compounds in the individual animal species. Unfortunately, such data are not available for Germany.
Direct comparison of the data presented here with those from other reports is difficult because of differences in the breakpoints, fluoroquinolones, and methods used for the determination of resistance. However, reports from many countries have indicated that zoonotic bacteria including Salmonella spp. have shown decreased susceptibility to fluoroquinolones (9, 13, 20, 25, 39). This study shows that in Germany only specific serovars are responsible for the increasing prevalence of resistance and decreased susceptibility to fluoroquinolones. The selection of Salmonella strains resistant to fluoroquinolones has occurred in a stepwise fashion over several years. An increasing prevalence of quinolone-resistant Salmonella Hadar in animals and humans has been documented (29, 32), possibly indicating the epidemic spread of a particular clone which might have a greater potential for the development of resistance.
This seems to be true for Salmonella Typhimurium DT 104. Until 1995 no nalidixic acid-resistant isolates were identified. In 1996, 2.3% of isolates were nalidixic acid resistant. This number dropped to 1% in 1997 but increased to 2.5% in 1998. The range of enrofloxacin MICs for these strains was 0.5 to 4 μg/ml. It is possible, that in the coming years the relative proportion of quinolone-resistant DT 104 will increase if the selection pressure persists and the enrofloxacin MIC distribution will be shifted toward higher values, as has been observed in England and Wales (31, 32).
On the basis of the data presented above, it is important that the prudent use of fluoroquinolones in the veterinary field be encouraged and, in particular, that their application maximize the therapeutic effect and minimize the emergence of resistance (38). Global resistance-monitoring programs should be established and the consumption of fluoroquinolones should be registered. If resistance increases to dramatic levels, the veterinary use of fluoroquinolones must be curtailed in order to lower the selective pressure.
ACKNOWLEDGMENTS
We thank F. Pirro from Bayer AG (Germany, Monheim) for the supply of enrofloxacin. We are grateful to Gabriele Berendonk, Cornelia Bunge, Bernhard Hoog, Frank Rösel, and Antje Steinbeck for expert technical assistance. Special thanks go to John Threlfall and Clifford Wray for correcting the manuscript.
REFERENCES
- 1.Alam M N, Haq S A, Das K K, Baral P K, Mazid M N, Siddique R U, Rahman K M, Hasan Z, Khan M A S, Dutta P. Efficacy of ciprofloxacin in enteric fever: comparison of treatment duration in sensitive and multidrug-resistant Salmonella. Am J Trop Med Hyg. 1995;53:306–311. doi: 10.4269/ajtmh.1995.53.306. [DOI] [PubMed] [Google Scholar]
- 2.Anderson E S, Ward L R, de Saxe M J, de Sa J D H. Bacteriophage-typing designations of Salmonella typhimurium. J Hyg Camb. 1977;78:297–300. doi: 10.1017/s0022172400056187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anonymous. Verbrauch von Antibiotika. Deutsches Tierärztebl. 1998;46:1095. [Google Scholar]
- 4.Anonymous. Outbreak of quinolone-resistant, multiresistant Salmonella typhimurium DT 104, Denmark. Weekly Epidemiol Rec. 1998;42:327–328. [PubMed] [Google Scholar]
- 5.Baggesen D L, Aarestrup F M. Characterisation of recently emerged multiple antibiotic-resistant Salmonella enterica serovar typhimurium DT 104 and other multiresistant phage types from Danish pig herds. Vet Rec. 1998;143:95–97. doi: 10.1136/vr.143.4.95. [DOI] [PubMed] [Google Scholar]
- 6.Deutsches Institut für Normung e.V. Methods for the determination of susceptibility of pathogens (except mycobacteria) to antimicrobial agents; agar diffusion test DIN 58940 part 3. Berlin, Germany: Deutsches Institut für Normung e.V.; 1989. [Google Scholar]
- 7.Deutsches Institut für Normung e.V. Methods for the determination of susceptibility of pathogens (except mycobacteria) to antimicrobial agents; microdilution DIN 58940 part 8. Berlin, Germany: Deutsches Institut für Normung e.V.; 1990. [Google Scholar]
- 8.Endtz H P, Ruijs G J, van Kungeren B, Jansen W H, van der Reyden T, Mouton R P. Quinolone resistance in campylobacter isolated from man and poultry following the introduction of fluoroquinolones in veterinary medicine. J Antimicrob Chemother. 1991;27:199–208. doi: 10.1093/jac/27.2.199. [DOI] [PubMed] [Google Scholar]
- 9.Frost J A, Kelleher A, Rowe B. Increasing ciprofloxacin resistance in salmonellas in England and Wales 1991–1994. J Antimicrob Chemother. 1996;37:85–91. doi: 10.1093/jac/37.1.85. [DOI] [PubMed] [Google Scholar]
- 10.Gensberg K, Jin Y F, Piddock L J V. A novel gyrB mutation in a fluoroquinolone-resistant clinical isolate of Salmonella typhimurium. FEMS Microbiol Lett. 1995;132:57–60. doi: 10.1111/j.1574-6968.1995.tb07810.x. [DOI] [PubMed] [Google Scholar]
- 11.Graeber I, Montenegro M A, Bunge C, Boettcher U, Tobias H, Heinemeyer E A, Helmuth R. Molecular marker analysis of Salmonella typhimurium from surface waters, humans, and animals. Eur J Epidemiol. 1995;11:325–331. doi: 10.1007/BF01719438. [DOI] [PubMed] [Google Scholar]
- 12.Griggs D J, Gensberg K, Piddock L J V. Mutations in gyrA gene of quinolone-resistant Salmonella serotypes isolated from humans and animals. Antimicrob Agents Chemother. 1996;40:1009–1013. doi: 10.1128/aac.40.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griggs D J, Hall M C, Jin Y F, Piddock L J V. Quinolone resistance in veterinary isolates of salmonella. J Antimicrob Chemother. 1994;33:1173–1189. doi: 10.1093/jac/33.6.1173. [DOI] [PubMed] [Google Scholar]
- 14.Heisig P. High-level fluoroquinolone resistance in a Salmonella typhimurium isolate due to alterations in both gyrA and gyrB genes. J Antimicrob Chemother. 1993;32:367–377. doi: 10.1093/jac/32.3.367. [DOI] [PubMed] [Google Scholar]
- 15.Heisig P. Genetic evidence for a role of parC mutations in development of high-level fluoroquinolone resistance in Escherichia coli. Antimicrob Agents Chemother. 1996;40:879–885. doi: 10.1128/aac.40.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helmuth R, Schroeter A. Molecular typing methods for S. enteritidis. Int J Food Microbiol. 1994;21:69–77. doi: 10.1016/0168-1605(94)90201-1. [DOI] [PubMed] [Google Scholar]
- 17.Helmuth R, Protz D. How to modify conditions limiting resistance in bacteria in animals and other reservoirs. Clin Infect Dis. 1997;24:8136–8138. doi: 10.1093/clinids/24.supplement_1.s136. [DOI] [PubMed] [Google Scholar]
- 18.Hof H, Ehrhard I, Tschäpe H. Presence of quinolone resistance in a strain of Salmonella typhimurium. Eur J Clin Microbiol Infect Dis. 1991;10:747–749. doi: 10.1007/BF01972501. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs-Reitsma W F, Koenraad P M F J, Bolder N M, Mulder R W A W. In vitro susceptibility of campylobacter and salmonella isolates from broilers to quinolones, ampicillin, tetracycline, and erythromycin. Vet Q. 1994;16:206–208. doi: 10.1080/01652176.1994.9694450. [DOI] [PubMed] [Google Scholar]
- 20.Kresken M, Hafner D, Mittermayer H, Verbist E, Bergogne-Bérézin E, Giamarellou H, Esposito S, van Klingeren B, Kayser F H, Reeves D S, Wiedemann B Study Group ’Bacterial Resistance’ of the Paul-Ehrlich-Society for Chemotherapy. Prevalence of fluoroquinolone resistance in Europe. Infection. 1994;22:S90–S98. doi: 10.1007/BF01793572. [DOI] [PubMed] [Google Scholar]
- 21.Marshall S A, Jones R N, Wanger A, Washington J A, Doern G V, Leber A L, Haugen T H. Proposed MIC quality control guidelines for National Committee for Clinical Laboratory Standards susceptibility tests using seven veterinary antimicrobial agents: ceftiofur, enrofloxacin, florfenicol, penicillin G-novobiocin, pirlimycin, premafloxacin, and spectinomycin. J Clin Microbiol. 1996;34:2027–2029. doi: 10.1128/jcm.34.8.2027-2029.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disc susceptibility tests. M2-A6. 1997. Approved standard. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 23.Olsen J E, Brown D J, Skov M N, Christensen J P. Bacterial typing methods suitable for epidemiological analysis. Applications in investigations of salmonellosis among livestock. Vet Q. 1993;15:125–135. doi: 10.1080/01652176.1993.9694390. [DOI] [PubMed] [Google Scholar]
- 24.Piddock L J V. Mechanisms of resistance to fluoroquinolones: state-of-the-art 1992–1994. Drugs. 1995;49:29–35. doi: 10.2165/00003495-199500492-00006. [DOI] [PubMed] [Google Scholar]
- 25.Piddock L J V. Antimicrobial resistance. WHO Report WHO/EMC/ZOO/97.4. Berlin, Germany: World Health Organization; 1997. Quinolone resistance and campylobacter; pp. 191–198. [Google Scholar]
- 26.Piddock L J V, Ricci V, McLaren I, Griggs D J. Role of mutation in the gyrA and parC genes of nalidixic-acid-resistant Salmonella serotypes isolated from animals in the United Kingdom. J Antimicrob Chemother. 1998;41:635–641. doi: 10.1093/jac/41.6.635. [DOI] [PubMed] [Google Scholar]
- 27.Popoff M Y, Minor L L. Antigenic formulas of the Salmonella serovars. WHO Collaborating Centre for Reference and Research on Salmonella. Paris, France: Institut Pasteur; 1997. [Google Scholar]
- 28.Rabsch W, Schroeter A, Helmuth R. Prevalence of Salmonella enterica subsp. enterica serovar Typhimurium DT 104 in Germany. Newsl. Community Ref. Lab. Salmonella. 1997;3:10–11. [Google Scholar]
- 29.Reilly W J, Munro D S. Antibiotic resistance. Vet Rec. 1997;141:26. [PubMed] [Google Scholar]
- 30.Stanley J, Jones C S, Threlfall E J. Evolutionary lines among Salmonella enteritidis phage types are identified by insertion sequence IS200 distribution. FEMS Microbiol Lett. 1992;82:83–90. doi: 10.1016/0378-1097(91)90425-a. [DOI] [PubMed] [Google Scholar]
- 31.Threlfall E J, Angulo F J, Wall P G. Ciprofloxacin-resistant Salmonella typhimurium DT 104. Vet Rec. 1998;142:255. [PubMed] [Google Scholar]
- 32.Threlfall E J, Graham A, Cheasty T, Ward L R, Rowe B. Resistance to ciprofloxacin in pathogenic Enterobacteriaceae in England and Wales in 1996. J Clin Pathol. 1997;50:1027–1028. doi: 10.1136/jcp.50.12.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Threlfall E J, Hampton M D, Schofield S L, Ward L R, Frost J A, Rowe B. Epidemiological application of differentiating multiresistant Salmonella typhimurium DT 104 by plasmid profile. Commun Dis Rep. 1996;6:R155–R159. [PubMed] [Google Scholar]
- 34.Threlfall E J, Ward L R, Rowe B. Increasing incidence of resistance to trimethoprim and ciprofloxacin in epidemic Salmonella typhimurium DT 104 in England and Wales. Eurosurveillance. 1997;2:81–84. doi: 10.2807/esm.02.11.00187-en. [DOI] [PubMed] [Google Scholar]
- 35.Vila I, Ruiz J, Goni P, Jimenez de Anta M T. Detection of mutations in parC in quinolone-resistant clinical isolates of Escherichia coli. Antimicrob Agents Chemother. 1996;40:491–493. doi: 10.1128/aac.40.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward L R, de Sa J D H, Rowe B. A phage-typing scheme for Salmonella enteritidis. Epidemiol Infect. 1987;99:291–294. doi: 10.1017/s0950268800067765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiedemann B, Heisig P. Mechanisms of quinolone resistance. Infection. 1994;22:73–79. doi: 10.1007/BF01793570. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. Use of quinolones in food animals and potential impact on human health. Report of a WHO meeting. Report WHO/EMC/ZDI/98.10. Geneva, Switzerland: World Health Organization; 1998. [Google Scholar]
- 39.Wray C, McLaren I M, Beedell Y E. Bacterial resistance monitoring of Salmonellas isolated from animals, national experience of surveillance schemes in the United Kingdom. Vet Microbiol. 1993;35:313–319. doi: 10.1016/0378-1135(93)90156-2. [DOI] [PubMed] [Google Scholar]