Abstract
It has recently become apparent that inflammatory reactions including nitric oxide (NO) release contribute to the outcome of pulmonary infections. To investigate the effect of NG-monomethyl-l-arginine (L-NMMA), a NO synthase inhibitor, on the pathogenesis of pneumococcal pneumonia, we inoculated CD1 Swiss mice with 107 CFU of Streptococcus pneumoniae. Treatment with two daily subcutaneous injections of 3 mg of L-NMMA per kg of body weight (over a 5-day period) reproducibly delayed mortality, as the number of surviving mice 72, 84, and 96 h after infection was increased by 16.8% (P < 0.05), 25.0% (P < 0.005), and 11.5% (P < 0.05), respectively. In fact, the following chronology of events was noted in L-NMMA-treated infected animals, compared to the untreated infected controls. (i) At 12 to 24 h after infection, larger amounts of leukotriene B4 in bronchoalveolar lavage (BAL) fluid associated with greater neutrophilia in lung tissue and alveolar spaces and more persistent release of tumor necrosis factor alpha, interleukin-1 alpha (IL-1α), and IL-6 were observed. (ii) At 24 to 72 h, there was better preservation of lung ultrastructure, including reduction of edema in the interstitium and protection of alveolar spaces, despite identical bacterial growth in lungs, in L-NMMA-treated infected animals than in untreated animals. (iii) At 72 to 96 h, the death rate was delayed, despite the absence of antibiotic therapy. In our experiment, partial blockade of NO release was achieved. These data indicate that NO plays an important role in the induction of tissue injury and death during pneumococcal pneumonia and that L-NMMA is helpful for host protection.
The fatality rate associated with Streptococcus pneumoniae pneumonia still approximates 23%, despite the use of potent antibiotics and aggressive intensive care support (37). Therapeutic challenges that stand before us include the development of more effective vaccines that protect against colonization by pneumococci, the development of antibiotics that bypass the widespread emergence of multiresistant strains, the strengthening of the immune response in immunosuppressed patients, and the control of overwhelming inflammatory reactions that are associated with tissue injury, shock, and death in immunocompetent hosts. In fact, there is growing evidence that aspects of the immune response greatly contribute to the high mortality rate associated with this threatening infection (reviewed in reference 4). We recently reported the chronology of events that participate in the pathogenesis of fatal pneumococcal pneumonia, which includes the release of tumor necrosis factor alpha (TNF-α), interleukin-1 alpha (IL-1α), IL-6, leukotriene B4 (LTB4), and large amounts of nitric oxide (NO) in lung tissue and alveolar spaces (4).
The physiology, pathology, and clinical relevance of endogenous NO which is formed from the amino acid l-arginine under stimulation of two constitutive NO synthases (cNOS) and one inducible NO synthase (iNOS) have been reviewed (7, 14, 15, 25, 30, 31, 49, 51). NO exerts beneficial vasoactive effects that contribute to maintaining homeostasis and blood flow in normal hosts (49). Its overproduction has been shown to inactivate enzymes that are crucial to mitochondrial respiration and DNA replication, and NO may form highly reactive oxidants capable of damaging target cells (50). Although these mechanisms most likely contribute to the observed killing properties of NO against various microorganisms in animal models of infectious diseases (reviewed in reference 54), NO might worsen pulmonary injury during fatal pneumococcal pneumonia (4). In fact, the beneficial versus detrimental roles of NO during pneumococcal pneumonia have been poorly explored. NO might modulate polymorphonuclear neutrophil (PMN) adhesion (22), regulate cytokine synthesis (24), or influence survival rate (54).
Based on the hypothesis that NO possibly has a multifaceted role during fatal pneumococcal pneumonia, ranging from regulation of vascular tone and leakage to leukocyte activity to tissue cytotoxicity, we investigated the possibility that by reducing NO levels with a competitive inhibitor of NO synthesis, NG-monomethyl-l-arginine (L-NMMA), beneficial immunomodulation may be instituted.
(The results of this work have been presented in part previously [36]).
MATERIALS AND METHODS
Pneumococcal pneumonia model.
Pneumonia was induced to lightly anesthetized female CD1 Swiss mice (20 to 22 g) by intranasal inoculation of 50 μl of phosphate-buffered saline (PBS) containing 107 log-phase CFU of S. pneumoniae serotype 3, a clinical strain isolated by blood culture. To facilitate migration of the inoculum to the alveoli, mice were held in a vertical position for 2 min. They had free access to mouse chow and water throughout the experiment and were exposed to alternate standardized light and dark periods of 14 and 10 h/day, respectively. Control mice received intranasal PBS.
Treatment with L-NMMA.
L-NMMA, monoacetate salt (no. 475886; Calbiochem, La Jolla, Calif.), was prepared daily by dissolving the powder in saline to achieve doses of 3 mg/kg of body weight. Subcutaneous (s.c.) injections were started immediately before the infection (time zero on day 0) and were administered every 12 h over a 5-day period. Control animals received s.c. injections of saline. The treatment regimen with L-NMMA was chosen to achieve submaximal rather than complete inhibition of NO, as minimal amounts of NO undoubtedly are required for maintenance of physiological and immunological functions (20, 29, 41, 53, 54) and as NO might also contribute to restraining bacterial growth in lungs and its dissemination in blood. In fact, preliminary experiments with a low dose (3 mg/kg) and a high dose (30 mg/kg) of L-NMMA suggested that only a low dose is beneficial for the survival rate. In the present experiment, we initiated L-NMMA therapy just before the infection and maintained intermittent injections over a 5-day period, as we had previously observed in our pneumonia model an early secretion and a late secretion of NO after infection with pneumococci (4). The same schedule was used as previously reported by other investigators (54).
Experimental protocol. (i) Survival rate studies.
Four consecutive experiments, involving a total of 110 mice, were performed. In each experiment, all mice were infected with freshly prepared inoculum, as described above; half of the mice were treated with L-NMMA, and the other half received saline. The survival rate was monitored every 12 h during a 5-day period.
(ii) Pathogenesis studies.
Animals were infected at time zero; treatments with L-NMMA were initiated immediately before infection and were maintained every 12 h until sacrifice of the animals. The four groups of animals included uninfected untreated mice (control), uninfected treated mice (L-NMMA), infected untreated mice (infected), and infected treated mice (infected plus L-NMMA). At time zero and 12, 24, 48, and 72 h, six animals per group were sacrificed. Blood, bronchoalveolar lavage (BAL) fluid, and lung tissue were sampled to determine bacterial growth, cellular response, and inflammatory mediator levels. Six additional animals per group were also sacrificed after the fifth injection of L-NMMA (15 min or 2 h after the 48-h injection) to verify the inhibitory effect of L-NMMA on NO levels shortly after the injection of the drug.
Development of infection.
The bacterial growth in lungs was monitored by using a microbiological assay. The lungs and heart were taken together and weighed before and after blood removal with 20 ml of sterile saline, which was infused through the right ventricle until the effluent was clear. The lungs were then homogenized with a Potter device at a ratio of 1 g/10 ml of a 50 mM concentration of potassium phosphate buffer, pH 6.5; bacteria were quantified in 20 μl of this crude homogenate by plating 10-fold dilutions on blood sheep agar followed by 18 h of incubation at 37°C in an atmosphere of 5% CO2. Hemocultures were done after sampling blood from the retro-orbital sinus of the left eye with a heparinized capillary, followed by plating on blood agar. Twelve infected animals per group (treated with L-NMMA or placebo) were chosen to follow the frequency and time course of bacteremia.
Inflammatory cells.
Recruitment of leukocytes to alveolar spaces was determined by harvesting a total of 3 ml of BAL fluid, as previously described (4). BAL fluids were centrifuged at 3,400 × g for 10 min, and pelleted cells were resuspended in PBS for quantification with a hemacytometer. Cell populations were enumerated from Diff-Quick (no. B4132-1; Baxter, Pointe-Claire, Québec, Canada)-stained cytospin preparations. PMN infiltration in lung tissue was quantified through the measurement of myeloperoxidase (MPO), as previously described (4). Cell populations in blood were also determined after blood sampling with heparinized tubes (no. 17.1523; Sarstedt, Montreal, Québec, Canada). Differentiation of leukocytes was made by counting 100 cells on a smear stained with Wright reagent.
Inflammatory mediators.
TNF-α, IL-1α, and IL-6 levels were detected in the supernatant of BAL fluid, in the supernatant of the lung homogenates, and in sera from the animals. Sera was obtained by centrifuging blood for 10 min at 4°C in a microcentrifuge at maximal speed. Lung homogenates were obtained as described above, then 600 μl of phosphate buffer containing 20 U of aprotinin and 0.2% CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate} were added to 600 μl of homogenate before centrifugation at 3,000 × g for 30 min at 4°C. Cytokines were assayed by using enzyme-linked immunosorbent assay kits (TNF-α, no. 80-2802-00; IL-1α, no. 1900-01; IL-6, no. 80-3748-01; Genzyme Corporation, Cambridge, Mass.). IL-1α, compared to IL-1β and TNF-α, exerts its biologic activity in a membrane-associated form (1). Therefore, cytokines that act mostly through local intercellular contact (IL-1α) or that play mostly a systemic role (TNF-α) were evaluated. LTB4 levels were quantified by using radioimmunoassay kits, according to supplied methodology (no. 8-6020; Cedarlane, Hornby, Ontario, Canada). The release of NO was evaluated through measurement of its oxidized nitrite metabolites, after conversion of nitrates to nitrites, according to the reduction procedure and colorimetric method of Griess (12).
Histology.
Whole left lungs were fixed in 10% neutral buffered formalin, embedded in paraffin, and then processed for light microscopy. Tissue sections of inflamed areas were also fixed in 2.5% glutaraldehyde–0.1 M phosphate buffer (pH 7.4), postfixed in 1% osmium tetroxide, dehydrated, and embedded in Epon according to standard methodology (4).
Statistical analysis.
Statistical analysis of the differences between groups was performed on StatView SE+ Graphics (Abaccus Concepts Inc., Berkeley, Calif.) by analysis of variance, using a least-squares method. If the F test indicated a difference within groups, comparisons were performed by using the Fisher PLSD (protected least significant difference) test. All data are presented as means ± standard errors of the mean (SEM). The survival functions were estimated with the Kaplan-Meier method, which is also known as the product limit estimator. This method can deal with censored data and has been shown to be the nonparametric maximum likelihood estimator. The comparisons between survival curves were made by using the log-rank statistic, also known as the Mantel-Haenszel statistic. This statistic is more sensitive than the Wilcoxon statistic for differences between groups occurring at later time points, as in our experiment. A P value of <0.05 was considered significant.
RESULTS
Survival rate studies.
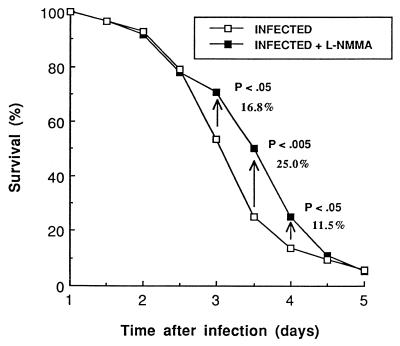
In our model of pneumococcal pneumonia, only 53.8% of untreated infected animals were still living 3 days after infection, 25% survived 3.5 days, 13.5% survived 4 days, and all died within 6 days. L-NMMA delayed mortality consistently, with reproducible increases in the number of surviving mice between 2.5 and 4.5 days postinfection in each of the four consecutive experiments, whose results are summarized in Fig. 1. In fact, there were increases in the survival rates for L-NMMA-treated mice of 16.8% (P < 0.05), 25.0% (P < 0.005), and 11.5% (P < 0.05) at 3.0, 3.5, and 4.0 days, respectively. More specifically, at 3.5 days, there were 5 of 11, 9 of 16, 5 of 17, and 10 of 14 mice still alive in the treated groups compared to 2 of 8, 5 of 13, 1 of 16, and 5 of 15 mice, respectively, in the untreated groups in the consecutive experiments. The comparisons of the overall survival curves from 0 to 96 h by using log-rank statistics reached a P value of <0.05. All animals finally died in the absence of antibiotic therapy.
FIG. 1.
Percent survival of mice infected with 107 CFU of S. pneumoniae and treated with a placebo or L-NMMA (3 mg/kg doses started immediately before infection and maintained twice daily over a 5-day period). The curves represent treated and untreated mice and include 58 and 52 mice, respectively. The survival rate at specific time points was estimated with the Kaplan-Meier method. Comparisons between the whole survival curves were made by using the log-rank statistic, and a P value of <0.005 was reached at 3.5 days.
Pathogenesis studies and development of infection.
To investigate the microbiological and inflammatory events that characterized both groups before death, we undertook a series of experiments on the pathogenesis of pneumonia. Bacterial counts recovered from lung homogenates indicated steady growth in untreated infected animals between 24 and 72 h, with counts ranging from 0.7 × 108 CFU/g at 24 h to 1.4 × 108 CFU/g at 48 h to 6.3 × 108 CFU/g at 72 h. Although the counts varied slightly (from 1.9 × 108 to 0.8 × 108 to 12.3 × 108 at 24, 48, and 72 h, respectively) after therapy with L-NMMA, no statistical difference could be demonstrated between both groups, so the drug did not markedly alter bacterial growth. The percentages of positive hemocultures were 42, 92, and 100% for the untreated mice and 58, 92 and 100% for L-NMMA-treated animals at 24, 48, and 72 h postinfection, respectively. The difference at 24 h was not statistically significant.
Inflammatory cells.
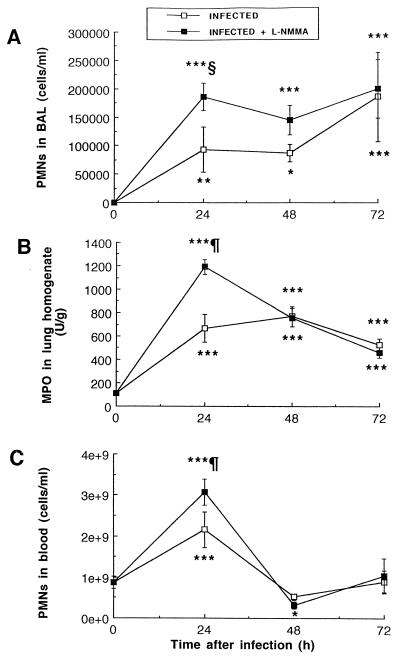
Infection with S. pneumoniae stimulated PMN recruitment from blood vessels to lung tissue to alveoli, as shown in Fig. 2. While negligible amounts of PMNs could be detected in the BAL fluid of uninfected mice (treated with L-NMMA or untreated), infection resulted in enhanced PMN counts in BAL fluid (Fig. 2A) and MPO levels in lung homogenates (Fig. 2B) from 24 h until death. Enhancement in PMN counts was also observed in blood at 24 h, which was followed by a sharp decline thereafter (Fig. 2C). Treatment with L-NMMA resulted in higher PMN counts in the BAL fluid, lung tissue, and blood of mice at 24 h (P < 0.01, P < 0.001, and P < 0.001, respectively) than in those of untreated infected mice. The data did not differ significantly between both groups thereafter, except for a slightly more profound leukopenia condition at 48 h in treated animals. In fact, PMNs sharply declined in blood at 48 h, whereas they remained elevated in the BAL fluid of both groups until death of the animals.
FIG. 2.
Mean (SEM) PMN recruitment in alveolar spaces (A), lung homogenate (B), and blood (C), as evaluated by cell count (A and C) and MPO assay (B). Infection was induced at time zero with 3.5 × 107 CFU of S. pneumoniae/mouse, and treatment with L-NMMA (3 mg/kg doses) was started at time zero and maintained twice daily throughout the experiment. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.001; all compared to preinfection values. §, P < 0.01; ¶, P < 0.001; both between infected and infected plus L-NMMA-treated mice.
An additional cell population was recruited to the alveoli from 48 to 72 h after infection: monocyte counts increased from 24 × 103 to 34 × 103, 90 × 103, and 165 × 103 cells/ml in BAL fluid at 0, 24, 48, and 72 h, respectively, in untreated infected mice (P < 0.01 at 48 h and P < 0.001 at 72 h, compared to preinfection values) and from 24 × 103 to 44 × 103, 113 × 103, and 169 × 103 cells/ml in treated infected animals (P < 0.001 at 48 and 72 h, compared to preinfection values). L-NMMA did not significantly affect monocyte recruitment, as no statistical difference could be demonstrated between both infected groups. No differences in lymphocyte counts could be observed among groups in this experiment.
LTB4 levels.
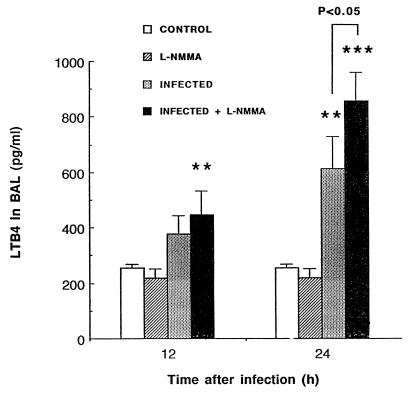
The chemotactic influence of LTB4 on PMN recruitment was investigated early after infection, as these cells were demonstrated to migrate early to alveoli. Figure 3 represents LTB4 secretion in alveoli, as evaluated in BAL fluid. While L-NMMA by itself did not induce LTB4 release, the drug did stimulate the pneumococcus-induced secretion of leukotrienes by responder cells (P < 0.01 between infected and control mice at 24 h and P < 0.05 between infected and infected plus L-NMMA mice). The assay for LTB4 was not performed at times later than 24 h, since differences in the chemotaxis of PMNs were not observed between infected groups beyond that time.
FIG. 3.
Mean (SEM) LTB4 levels in cell-free BAL fluid of mice infected with 3.5 × 107 CFU of S. pneumoniae. Animals that were sacrificed at 12 h had received a single injection of L-NMMA (3 mg/kg) at time zero, while animals sacrificed at 24 h had received a second injection 12 h postinfection. Comparisons indicated by the asterisks (∗∗, P < 0.01, and ∗∗∗, P < 0.001) were made between infected animals and their respective uninfected controls (infected versus control and infected plus L-NMMA versus L-NMMA).
Inflammatory mediators.
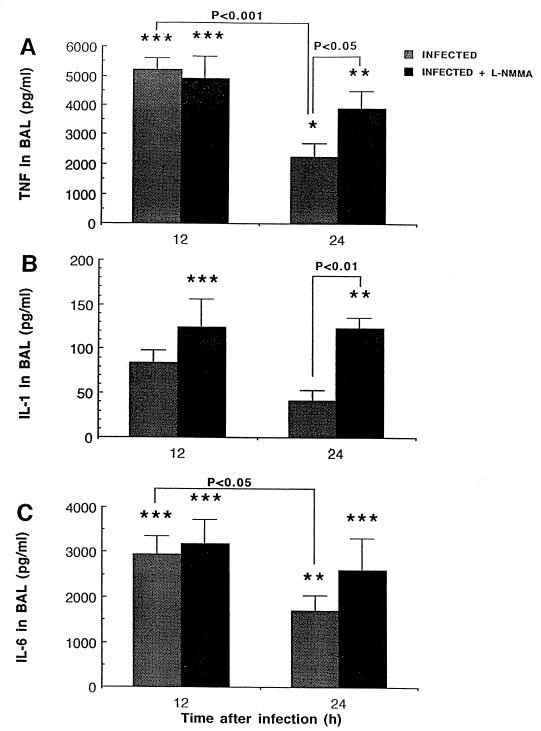
Cytokine levels were also quantified in our model. Infection with S. pneumoniae resulted in early high secretions of TNF-α, IL-6, and, to a certain extent, IL-1α in BAL fluid (Fig. 4). Levels of all three cytokines rapidly declined thereafter in untreated infected mice (P < 0.001 and P < 0.05 between 12 and 24 h for TNF-α and IL-6, respectively). Although L-NMMA did not induce cytokine release by itself and control uninfected mice had no cytokine detectable in BAL fluid, treatment of infected animals with L-NMMA significantly prevented downregulation of proinflammatory cytokines from 12 to 24 h (P < 0.05 and P < 0.01 between infected and infected plus L-NMMA animals at 24 h for TNF-α and IL-1α, respectively). Nevertheless, all three cytokines decreased to very low levels in BAL fluid at 48 h, so that no detectable difference could be seen thereafter.
FIG. 4.
Mean (SEM) TNF-α, IL-1α, and IL-6 levels in cell-free BAL fluid 12 and 24 h after intranasal inoculation of mice with 3.5 × 107 CFU of S. pneumoniae. Animals that were sacrificed at 12 h received a single injection of L-NMMA (3 mg/kg s.c.) at time zero, while animals that were sacrificed at 24 h received a second injection at 12 h. Comparisons indicated by the asterisks (∗, P < 0.05; ∗∗, P < 0.01; and ∗∗∗, P < 0.001) were made between infected animals and their respective uninfected controls (infected versus control and infected plus L-NMMA versus L-NMMA). Negligible amounts of cytokines could be detected in uninfected mice.
NO release.
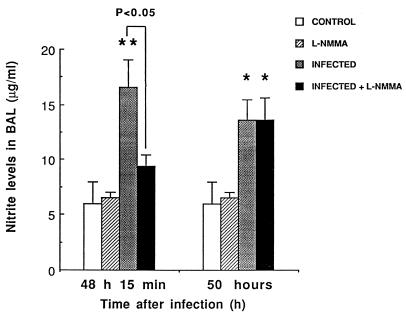
The time point that was chosen to check NO inhibition by L-NMMA was based on previously reported data on NO in our model (4). Triggering of NO secretion after S. pneumoniae was still evidenced 48 to 50 h postinfection (P < 0.05 between control and infected mice) (Fig. 5). As expected, L-NMMA achieved transient inhibition of NO secretion. Fifteen minutes after the 48-h injection (fifth injection) of L-NMMA, NO levels in infected treated mice were similar to those in uninfected controls (P < 0.05). The inhibition could not be evidenced for periods longer than 2 h, and repeated inoculations of L-NMMA in our experiment ensured partial NO blockade.
FIG. 5.
NO release, as evaluated by the measurement of nitrites (mean + SEM) in cell-free BAL fluid 15 min and 2 h after the 5th injection of L-NMMA. Infection was induced by intranasal inoculation of 3.5 × 107 CFU of S. pneumoniae/mouse at time zero, and L-NMMA was injected s.c. at doses of 3 mg/kg every 12 h, initiated just before the infection. Comparisons indicated by the asterisks (∗, P < 0.05, and ∗∗, P < 0.01) were made between infected animals and their respective controls (infected versus control and infected plus L-NMMA versus L-NMMA).
Histopathology.
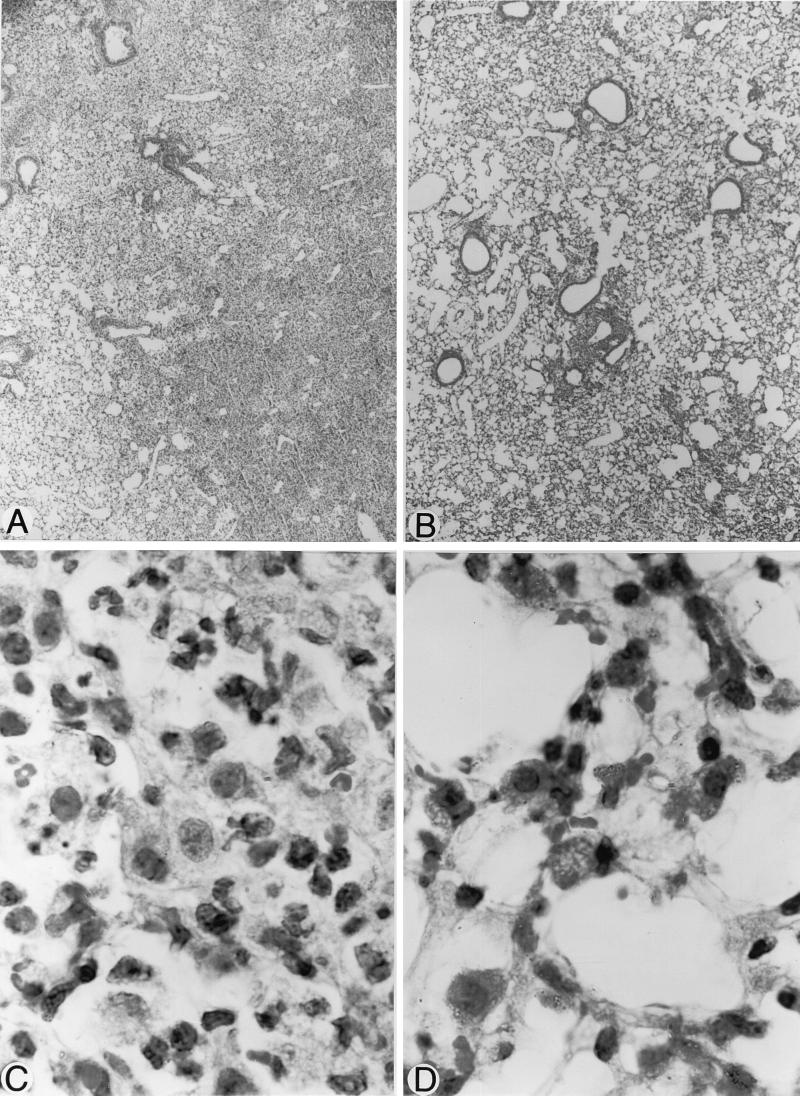
Histological examination of the lungs by light microscopy showed better preservation of alveolar architecture, larger alveolar spaces, and less edema in the tissue interstitium in infected treated animals (Fig. 6B and D) than in untreated infected mice (Fig. 6A and C). Involvement of the whole lung surface was noted in untreated infected mice as inflammation progressed: the initial foci of inflammation were restricted to perivascular areas localized close to infected bronchioles, which contained numerous inflammatory cells; progressive parenchymal involvement included edema and deterioration of the alveolar and interstitium ultrastructures. Protection against injury induced by inflammation occurred at 24, 48, and 72 h postinfection in L-NMMA-treated animals, although it was better evidenced at 48 h (Fig. 6), a time when the alveoli remained particularly wide open, compared to the alveoli in untreated infected controls.
FIG. 6.
Histopathology of lungs 48 h after infection of mice with 3.5 × 107 CFU of S. pneumoniae and treatment with placebo (A and C) or L-NMMA (B and D), 3 mg/kg given every 12 h since initial infection. Better preservation of interstitium and alveolar structures was noted after treatment with L-NMMA, as alveoli were kept open and edema was reduced, compared to those in untreated lungs. Magnifications, ×45 (A and B) and ×375 (C and D).
DISCUSSION
Various NO synthase (NOS) inhibitors, including L-NMMA, NG-nitro-l-arginine methyl ester (L-NAME), aminoguanidine, and l-canavanine, are being investigated for the treatment of infectious diseases. It has already been demonstrated that such inhibitors can prevent the systemic hypotension associated with the high mortality rate in animal models of septic shock; however, conflicting reports have been published regarding the therapeutic efficacy of these drugs against human and animal septic conditions (5, 8, 17, 21, 29, 33, 38–40, 44). In the present study, we investigated L-NMMA in a murine model of pneumococcal pneumonia, which presents both presepticemic and septicemic phases of infection (4). The following chronology of events was observed in L-NMMA-treated infected animals, compared to the untreated infected controls. (i) At 12 to 24 h after infection, larger amounts of LTB4 in BAL fluid associated with greater neutrophilia in lung tissue and alveolar spaces and more persistent release of TNF-α, IL-1α, and IL-6 were observed. (ii) At 24 to 72 h, there was better preservation of lung ultrastructure, including reduction of edema in the interstitium and protection of alveolar spaces, despite identical bacterial growth, in L-NMMA-treated infected mice than in untreated animals. (iii) At 72 to 96 h, the death rate was reproducibly delayed, being characterized by 16.8, 25.0, and 11.5% increases in the survival rate at 72, 84, and 96 h postinfection, despite the absence of antibiotic therapy. To our knowledge, this is the first study to demonstrate an in vivo beneficial effect of L-NMMA on histopathology and the death rate during pneumococcal pneumonia.
Our results differ from the reported decreased survival rate of Klebsiella pneumoniae-challenged mice after therapy with L-NAME (54). Those authors found a 10- to 46-fold increase in bacterial growth associated with a 50% reduction in NO release after L-NAME administration, suggesting that NO participates in the killing of K. pneumoniae, which in turn influences the survival rate. In our experiment, we used doses of L-NMMA fivefold lower than those of Tsai et al. (54), and the protection afforded by L-NMMA appeared unrelated to bacterial clearance. Although several models have been used to demonstrate a role for NO as an effector molecule for the killing of bacteria, parasites and fungi (reviewed in references 11, 27, and 54), we could not demonstrate a link between NO levels, pneumococcal killing, and survival of mice. Modifications in NO levels and PMN counts after L-NMMA may have counterbalanced each other’s effects on bacterial growth by reducing extracellular killing through NO inhibition and by increasing bacterial clearance through enhanced PMN recruitment, thus resulting in apparently unaffected bacterial growth. However, it is conceivable that NO did not indeed participate in the killing of S. pneumoniae, as it is not involved in the clearance of all microorganisms (8, 47). Conversely, enhanced PMN recruitment does not equate with improved phagocytosis, as NO inhibition was also shown to reduce intracellular killing by immune cells (54). We conclude from our data that L-NMMA protected lung tissue and delayed mortality regardless of bacterial growth. This finding appears to be important in view of the fact that studies with patients failed to show a role for NO as a direct microbicidal mechanism against fungus, bacteria, or parasites (27). Alternatively, a number of studies that demonstrated NO synthesis in humans (27, 34, 49) provide support for a signaling role of NO through the second messenger cyclic GMP (a chemical with diverse functions), which might influence the course of infection and inflammation.
In fact, NO possibly has a multifaceted role in pneumonia, ranging from vasodilatation of lung capillaries and the formation of edema to modulation of leukocyte activity to tissue cytotoxicity (3, 30, 31, 49). In our murine model, a time-dependent expression of NO occurs during the course of S. pneumoniae pneumonia (4). In the present experiment, infection was still shown to induce significant NO production, compared to that in uninfected controls. NO is mostly secreted by alveolar and interstitial macrophages during infection (35, 52, 57), but type II pneumocytes, endothelial cells, fibroblasts, and lymphocytes were also shown to secrete NO (16, 28, 30, 42, 56). cNOS isoforms are responsible for the maintenance of physiological functions (vasodilatation, blood flow, and leukocyte-endothelial interactions), while an iNOS mediates additional pathological consequences of inflammation. L-NMMA, which competitively interferes with all three NOS isoforms, significantly inhibited NO. Intermittent injections were preferred over continuous infusion to achieve transient blockade rather than complete inhibition, as partial NO secretion was estimated beneficial for the maintenance of homeostasis and the host response (20, 29, 41, 53, 54).
To unravel the mechanisms whereby L-NMMA protected mice, we examined leukocyte trafficking, inflammatory mediator release, and their relationships to tissue injury. The chronology of events that we previously reported in untreated mice (4) could still be corroborated. After inhalation of pneumococci, maximum proinflammatory cytokine release in BAL fluid occurred at 12 h and then gradually decreased, despite sustained stimulation; massive PMN recruitment followed at 24 h in parallel with LTB4 secretion; tissue injury, including edema to the interstitium and alterations of the alveolar structure, occurred from 24 to 72 h; and monocyte recruitment, high NO release, and bacteremia preceded death. In the present experiment, L-NMMA preserved tissues and delayed mortality, despite, or through, increases in cytokine levels and strong PMN recruitment. In fact, NO was already shown to exert differential regulation of cytokine synthesis (13, 18, 24). Cytokines and chemokines were increased after treatment of Klebsiella-related pneumonia with L-NAME (54). Partial blockade of NO by L-NMMA in our experiment contributed to an increase in TNF and IL-1 secretion which, in turn, may have influenced PMN recruitment through enhanced expression of adhesion molecules (10, 27). As NO upregulates the production of prostaglandins from membrane fatty acids while downregulating leukotriene synthesis from the same source, L-NMMA appears to contribute to the pneumococcus-induced PMN recruitment through a shift from the synthesis of prostaglandins to the synthesis of the chemotactic LTB4 (6, 9, 32, 45, 46, 48, 52).
Although early secretion of TNF, IL-1, and LTB4 and high PMN recruitment have been shown to protect against various infectious diseases through enhanced phagocytosis (reviewed in reference 4), TNF- and IL-1-mediated epithelial cell toxicities and PMN-induced tissue injury were also associated with impaired cell functions, tissue injury, and a fatal outcome of pneumonia (4, 26, 55, 58). Therefore, since high levels of these components in the presence of L-NMMA did not improve bacterial clearance, one might have expected that such high levels at 24 h postinfection would have contributed to worsening the inflammatory damage to tissues. We hypothesize that the protection afforded by L-NMMA therefore resulted from a reduction in direct cytotoxicity induced by NO or its derivatives and that NO-mediated toxicity plays a greater role than cytokine-induced cytotoxicity in our model. In fact, reactive oxygen (superoxide radicals) and nitrogen (NO) species act in concert through the formation of cytotoxic peroxynitrites (2), which induce lipid peroxidation, alter membrane permeability, inactivate key metabolic enzymes, and injure cells (15, 43). NO can also affect membrane phospholipid catabolism, signal transduction (cyclic GMP), energy production, and DNA synthesis (49). Thus, L-NMMA may be of potential value for preserving various biochemical functions.
NO can also affect cell activity by increasing vascular leakage (19, 23), thus contributing to edema. cNOS from vascular endothelial cells were already shown to participate in the early phase of edema, while iNOS from local tissues and infiltrating PMNs played a dominant role during the late phase of edema (after exposure of mice to chemicals) (27). In fact, both beneficial and detrimental effects of NOS inhibitors against edema have been reported, depending on the time of administration (23). Our results suggest that both cNOS and iNOS isoforms contributed to the pathogenesis of pneumonia as L-NMMA administered throughout the evolution of infection protected cells partly by controlling capillary leakage and fluid distribution within tissues.
In conclusion, our results suggest that NO contributes to pulmonary injury and death in pneumococcal pneumonia and that NOS inhibitors might prove useful in the treatment of such threatening infections. Considering that a 100% lethal dose inoculum was used, the modulation of host defenses by NOS inhibitors might even prove more effective against a less lethal inoculum. The addition of antibiotics that will ensure a certain percentage of survival rate by destroying bacteria should be investigated in combination with NOS inhibitors that appear to protect tissues so that the potential additive efficacy of the combination might be observed. However, one should be cautious with these products, because potential harmful effects might also be seen, depending on the dose and time of administration of the inhibitor. Delaying the initiation of treatment most likely modifies the outcome of pneumonia in ways that still need to be investigated. Also, animals and humans differ substantially in the evolution of the disease, so the delay that was observed in the survival rate in our model could be reduced or prolonged in humans. The slower development of pneumonia in humans might allow such inhibitors to be more effective. The use of various immunomodulator drugs should be based on a prior deepening in our understanding of the pathogenic steps of fatal pneumonia.
ACKNOWLEDGMENTS
We thank Michel Duong for his kind participation in the project.
Yves Bergeron and Nathalie Ouellet contributed equally to the work.
REFERENCES
- 1.Abbas A K, Lichtman A H, Pober J S. Effector mechanisms of immune responses. Cytokines. In: Wonsiewicz M J, editor. Cellular and molecular immunology. W. B. Philadelphia, Pa: Saunders Company; 1991. pp. 232–235. [Google Scholar]
- 2.Akaike T, Noguchi Y, Ijiri S, Setoguchi K, Suga M, Zheng Y M, Dietzschold B, Maeda H. Pathogenesis of influenza virus-induced pneumonia: involvement of both nitric oxide and oxygen radicals. Proc Natl Acad Sci USA. 1996;93:2448–2453. doi: 10.1073/pnas.93.6.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antonini J M, Van Dyke K, DiMatteo M, Reasor M J. Attenuation of acute inflammatory effects of silica in rat lung by 21-aminosteroid, U74389G. Inflammation. 1995;19:9–21. doi: 10.1007/BF01534376. [DOI] [PubMed] [Google Scholar]
- 4.Bergeron Y, Ouellet N, Deslauriers A M, Simard M, Olivier M, Bergeron M G. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect Immun. 1998;66:912–922. doi: 10.1128/iai.66.3.912-922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booke M, Meyer J, Lingnau W, Hinder F, Traber L D, Traber D L. Use of nitric oxide synthase inhibitors in animal models of sepsis. New Horiz. 1995;3:123–138. [PubMed] [Google Scholar]
- 6.Busse W W. Pathogenesis and sequelae of respiratory infections. Rev Infect Dis. 1991;13(Suppl. 6):S477–S485. doi: 10.1093/clinids/13.supplement_6.s477. [DOI] [PubMed] [Google Scholar]
- 7.Culotta E, Koshland D E. NO news is good news. Science. 1992;258:1861–1865. doi: 10.1126/science.1361684. [DOI] [PubMed] [Google Scholar]
- 8.Evans T, Carpenter A, Silva A, Cohen J. Inhibition of nitric oxide synthase in experimental gram-negative sepsis. J Infect Dis. 1994;169:343–349. doi: 10.1093/infdis/169.2.343. [DOI] [PubMed] [Google Scholar]
- 9.Folkerts G, Van-der-Linde H, Van-de-Loo P G, Engels F, Nijkamp F P. Leukotrienes mediate tracheal hyperresponsiveness after nitric oxide synthesis inhibition. Eur J Pharmacol. 1995;285:R1–R2. doi: 10.1016/0014-2999(95)00580-e. [DOI] [PubMed] [Google Scholar]
- 10.Gaboury J, Woodman R C, Granger D N, Reinhardt P, Kubes P. Nitric oxide prevents leukocyte adherence: role of superoxide. Am J Physiol. 1993;265:H862–H867. doi: 10.1152/ajpheart.1993.265.3.H862. [DOI] [PubMed] [Google Scholar]
- 11.Gosselin D, DeSanctis J, Boule M, Skamene E, Matouk C, Radzioch D. Role of tumor necrosis factor alpha in innate resistance to mouse pulmonary infection with Pseudomonas aeruginosa. Infect Immun. 1995;63:3272–3278. doi: 10.1128/iai.63.9.3272-3278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green L C, Tannenbaum S R, Goldman P. Nitrate synthesis in the germfree and conventional rat. Science. 1981;212:56–58. doi: 10.1126/science.6451927. [DOI] [PubMed] [Google Scholar]
- 13.Greenberger M J, Streiter R M, Kunkel S L, Danforth J M, Laichalk L L, McGillicudy D C, Standiford T J. Neutralization of MIP-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J Infect Dis. 1996;173:159–163. doi: 10.1093/infdis/173.1.159. [DOI] [PubMed] [Google Scholar]
- 14.Griffith O W, Stuehr D J. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- 15.Gross S S, Wolin M S. Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez H H, Pitt B R, Schwarz M, Watkins S C, Lowenstein C, Caniggia I, Chumley P, Freeman B A. Pulmonary alveolar epithelial inducible NO synthase gene expression: regulation by inflammatory mediators. Am J Physiol. 1995;268:L501–L508. doi: 10.1152/ajplung.1995.268.3.L501. [DOI] [PubMed] [Google Scholar]
- 17.Hock C E, Yin K, Yue G, Wong P Y. Effects of inhibition of nitric oxide synthase by aminoguanidine in acute endotoxemia. Am J Physiol. 1997;272:H843–H850. doi: 10.1152/ajpheart.1997.272.2.H843. [DOI] [PubMed] [Google Scholar]
- 18.Joshi P C, Grogan J B, Thomae K R. Effect of aminoguanidine on in vivo expression of cytokines and inducible nitric oxide synthase in the lungs of endotoxemic rats. Res Commun Mol Pathol Pharmacol. 1996;91:339–346. [PubMed] [Google Scholar]
- 19.Kageyama N, Miura M, Ichinose M, Tomaki M, Ishikawa J, Ohuchi Y, Endoh N, Shirato K. Role of endogenous nitric oxide in airway microvascular leakage induced by inflammatory mediators. Eur Respir J. 1997;10:13–19. doi: 10.1183/09031936.97.10010013. [DOI] [PubMed] [Google Scholar]
- 20.Kavanagh B P, Mouchawar A, Goldsmith J, Pearl R G. Effects of inhaled NO and inhibition of endogenous NO synthesis in oxidant-induced acute lung injury. J Appl Physiol. 1994;76:1324–1329. doi: 10.1152/jappl.1994.76.3.1324. [DOI] [PubMed] [Google Scholar]
- 21.Kengatharan K M, De-Kimpe S J, Thiemermann C. Role of nitric oxide in the circulatory failure and organ injury in a rodent model of gram-positive shock. Br J Pharmacol. 1996;119:1411–1421. doi: 10.1111/j.1476-5381.1996.tb16053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubes P, Suzuki M, Granger D N. Nitric oxide: an endogeneous modulator of leukocyte adhesion. Proc Natl Acad Sci USA. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laszlo F, Whittle B J, Evans S M, Moncada S. Association of microvascular leakage with induction of nitric oxide synthase: effects of nitric oxide synthase inhibitors in various organs. Eur J Pharmacol. 1995;283:47–53. doi: 10.1016/0014-2999(95)00281-o. [DOI] [PubMed] [Google Scholar]
- 24.Liew F Y. Interactions between cytokines and nitric oxide. Adv Neuroimmunol. 1995;5:201–209. doi: 10.1016/0960-5428(95)00009-q. [DOI] [PubMed] [Google Scholar]
- 25.Lowenstein C J, Dinerman J L, Snyder S H. Nitric oxide: a physiologic messenger. Ann Intern Med. 1994;120:227–237. doi: 10.7326/0003-4819-120-3-199402010-00009. [DOI] [PubMed] [Google Scholar]
- 26.Lukacs N W, Ward P A. Inflammatory mediators, cytokines, and adhesion molecules in pulmonary inflammation and injury. Adv Immunol. 1996;62:257–291. doi: 10.1016/s0065-2776(08)60432-0. [DOI] [PubMed] [Google Scholar]
- 27.Lyons C R. The role of NO in inflammation. Adv Immunol. 1995;60:323–355. doi: 10.1016/s0065-2776(08)60589-1. [DOI] [PubMed] [Google Scholar]
- 28.Miles P R, Bowman L, Huffman L. Nitric oxide alters metabolism in isolated alveolar type II cells. Am J Physiol. 1996;271:L23–L30. doi: 10.1152/ajplung.1996.271.1.L23. [DOI] [PubMed] [Google Scholar]
- 29.Minnard E A, Shou J, Naama H, Cech A, Gallagher H, Daly J M. Inhibition of nitric oxide synthesis is detrimental during endotoxemia. Arch Surg. 1994;129:142–147. doi: 10.1001/archsurg.1994.01420260038004. [DOI] [PubMed] [Google Scholar]
- 30.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 31.Moncada S, Higgs E A. Endogenous nitric oxide: physiology, pathology and clinical relevance. Eur J Clin Investig. 1991;21:361–374. doi: 10.1111/j.1365-2362.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- 32.Nakatsuka M, Osawa Y. Selective inhibition of the 12-lipoxygenase pathway of arachidonic acid metabolism by L-arginine or sodium nitroprusside in intact human platelets. Biochem Biophys Res Commun. 1994;200:1630–1634. doi: 10.1006/bbrc.1994.1638. [DOI] [PubMed] [Google Scholar]
- 33.Nava E, Palmer R M J, Moncada S. Inhibition of nitric oxide synthesis in septic shock: how much is beneficial? Lancet. 1991;338:1555–1557. doi: 10.1016/0140-6736(91)92375-c. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson S, Bonecini-Almeuda M G, Lapa-Silva J R, Nathan C, Xie Q W, et al. Inducible nitric oxide synthase in pulmonary alveolar macrophages from patients with tuberculosis. J Exp Med. 1996;183:2293–2302. doi: 10.1084/jem.183.5.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orman K L, Shenep J L, English B K. Pneumococci stimulate the production of the inducible nitric oxide synthase and nitric oxide by murine macrophages. J Infect Dis. 1998;178:1649–1657. doi: 10.1086/314526. [DOI] [PubMed] [Google Scholar]
- 36.Ouellet N, Bergeron Y, Simard M, Duong M, Olivier M, Beauchamp D, Bergeron M G. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Beneficial immunomodulation of pneumococcal pulmonary infection with NG-monomethyl-L-arginine, abstr. G-56; p. 203. [Google Scholar]
- 37.Pallares R, Linares J, Vadillo M, Cabellos C, Manresa F, Viladrich P F, Martin R, Gudiol F. Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N Engl J Med. 1995;333:474–486. doi: 10.1056/NEJM199508243330802. [DOI] [PubMed] [Google Scholar]
- 38.Parratt J R. Nitric oxide in sepsis and endotoxemia. J Antimicrob Chemother. 1998;41(Suppl. A):31–39. doi: 10.1093/jac/41.suppl_1.31. [DOI] [PubMed] [Google Scholar]
- 39.Petros A, Bennett D, Vallance P. Effect of nitric oxide synthase inhibitors on hypotension in patients with septic shock. Lancet. 1991;338:1557–1558. doi: 10.1016/0140-6736(91)92376-d. [DOI] [PubMed] [Google Scholar]
- 40.Petros A, Lamb G, Leone A, Moncada S, Bennett D, Vallance P. Effects of a nitric oxide synthase inhibitor in humans with septic shock. Cardiovasc Res. 1994;28:34–39. doi: 10.1093/cvr/28.1.34. [DOI] [PubMed] [Google Scholar]
- 41.Pheng L H, Francoeur C, Denis M. The involvement of nitric oxide in a mouse model of adult respiratory distress syndrome. Inflammation. 1995;19:599–610. doi: 10.1007/BF01539139. [DOI] [PubMed] [Google Scholar]
- 42.Punjabi C J, Laskin J D, Pendino K J, Goller N L, Durham S K, Laskin D L. Production of nitric oxide by rat type II pneumocytes: increased expression of inducible nitric oxide synthase following inhalation of a pulmonary irritant. Am J Respir Cell Mol Biol. 1994;11:165–172. doi: 10.1165/ajrcmb.11.2.7519435. [DOI] [PubMed] [Google Scholar]
- 43.Robbins C G, Davis J M, Merritt T A, Amirkhanian J D, Sahgal N, Morin F, Horowitz S. Combined effects of nitric oxide and hyperoxia on surfactant function and pulmonary inflammation. Am J Physiol. 1995;269:L545–L550. doi: 10.1152/ajplung.1995.269.4.L545. [DOI] [PubMed] [Google Scholar]
- 44.Robertson F M, Offner P J, Ciceri D P, Becker W K, Pruitt B A. Detrimental hemodynamic effects of nitric oxide synthase inhibition in septic shock. Arch Surg. 1994;129:149–156. doi: 10.1001/archsurg.1994.01420260045005. [DOI] [PubMed] [Google Scholar]
- 45.Salvemini D, Manning P T, Zweifel B S, Seibert K, Connor J, Currie M G, Needleman P, Masferrer J L. Dual inhibition of nitric oxide and prostaglandin production contributes to the anti-inflammatory properties of nitric oxide synthase inhibitors. J Clin Investig. 1995;96:301–308. doi: 10.1172/JCI118035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sautebin L, Di-Rosa M. Nitric oxide modulates prostacyclin biosynthesis in the lung of endotoxin-treated rats. Eur J Pharmacol. 1994;262:193–196. doi: 10.1016/0014-2999(94)90047-7. [DOI] [PubMed] [Google Scholar]
- 47.Shellito J E, Kolls J K, Olariu R, Beck J M. Nitric oxide and host defense against Pneumocystis carinii infection in a mouse model. J Infect Dis. 1996;173:432–439. doi: 10.1093/infdis/173.2.432. [DOI] [PubMed] [Google Scholar]
- 48.Skerrett S J. Host defenses against respiratory infection. In: Niederman M S, editor. The medical clinics of North America. Pneumonia: pathogenesis, diagnosis and management. W. B. Montreal, Canada: Saunders Company; 1994. pp. 941–966. [DOI] [PubMed] [Google Scholar]
- 49.Snyder S H, Bredt D S. Biological roles of nitric oxide. Sci Am. 1992;266:68–77. doi: 10.1038/scientificamerican0592-68. [DOI] [PubMed] [Google Scholar]
- 50.Stamler J S, Singel D J, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 51.Stuehr D J, Griffith O W. Mammalian nitric oxide synthases. Adv Enzymol Relat Areas Mol Biol. 1992;65:287–346. doi: 10.1002/9780470123119.ch8. [DOI] [PubMed] [Google Scholar]
- 52.Su T H, Martin W J. Pathogenesis and host response in Pneumocystis carinii pneumonia. Annu Rev Med. 1994;45:261–272. doi: 10.1146/annurev.med.45.1.261. [DOI] [PubMed] [Google Scholar]
- 53.Su W Y, Day B J, Kang B H, Crapo J D, Huang Y C, Chang L Y. Lung epithelial cell-released nitric oxide protects against PMN-mediated cell injury. Am J Physiol. 1996;271:L581–L586. doi: 10.1152/ajplung.1996.271.4.L581. [DOI] [PubMed] [Google Scholar]
- 54.Tsai W C, Strieter R M, Zisman D A, Wilkowski J M, Bucknell K A, Chen G H, Standiford T J. Nitric oxide is required for effective innate immunity against Klebsiella pneumoniae. Infect Immun. 1997;65:1870–1875. doi: 10.1128/iai.65.5.1870-1875.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weiss S J. Tissue destruction by neutrophils. N Engl J Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 56.Willis R A, Nussler A K, Fries K M, Geller D A, Phipps R P. Induction of nitric oxide synthase in subsets of murine pulmonary fibroblasts: effect on fibroblast interleukin-6 production. Clin Immunol Immunopathol. 1994;71:231–239. doi: 10.1006/clin.1994.1077. [DOI] [PubMed] [Google Scholar]
- 57.Wizemann T M, Laskin D L. Effects of acute endotoxemia on production of cytokines and nitric oxide by pulmonary alveolar and interstitial macrophages. Ann N Y Acad Sci. 1994;730:336–337. doi: 10.1111/j.1749-6632.1994.tb44284.x. [DOI] [PubMed] [Google Scholar]
- 58.Worthen G S, Haslett C, Rees A J, Gumbay R S, Henson J E, Henson P M. Neutrophil-mediated pulmonary vascular injury. Am Rev Respir Dis. 1987;136:19–28. doi: 10.1164/ajrccm/136.1.19. [DOI] [PubMed] [Google Scholar]