Abstract
Simple Summary
Monoclonal antibodies (mAbs) have the ability to specifically target tumor-cell antigens. This unique property has led to their use in the delivery of radioisotopes to tumor sites (scintigraphic imaging and radioimmunotherapy (RIT)). The choice of the radionuclide depends on its unique physical properties and intended use. Using radiolabeled mAbs with imaging techniques provides critical data that are essential for predicting side effects and determining an optimal antibody dose and treatment schedule. While RIT has been successful in the management of hematological malignancies, the treatment of solid tumors remains challenging. Various strategies are being investigated to improve the efficacy of RIT in solid tumors.
Abstract
Radioimmunoconjugates consist of a monoclonal antibody (mAb) linked to a radionuclide. Radioimmunoconjugates as theranostics tools have been in development with success, particularly in hematological malignancies, leading to approval by the US Food and Drug Administration (FDA) for the treatment of non-Hodgkin’s lymphoma. Radioimmunotherapy (RIT) allows for reduced toxicity compared to conventional radiation therapy and enhances the efficacy of mAbs. In addition, using radiolabeled mAbs with imaging methods provides critical information on the pharmacokinetics and pharmacodynamics of therapeutic agents with direct relevance to the optimization of the dose and dosing schedule, real-time antigen quantitation, antigen heterogeneity, and dynamic antigen changes. All of these parameters are critical in predicting treatment responses and identifying patients who are most likely to benefit from treatment. Historically, RITs have been less effective in solid tumors; however, several strategies are being investigated to improve their therapeutic index, including targeting patients with minimal disease burden; using pre-targeting strategies, newer radionuclides, and improved labeling techniques; and using combined modalities and locoregional application. This review provides an overview of the radiolabeled intact antibodies currently in clinical use and those in development.
Keywords: radioimmunotherapy, radioisotopes, radiolabeled monoclonal antibodies, theranostics
1. Introduction
Since the initial concept of “magic bullets” was proposed over a century ago, through to the discovery of hybridoma technology, monoclonal antibodies (mAbs) are now a vital component in the armamentarium for the management of cancers. The unique ability of mAbs to specifically target a broad variety of tumor-specific antigens has led to their expanded application as antibody-conjugated therapies (ACTs). ACTs combine the specificity of mAbs or antibody fragments, with highly potent payloads often resulting in superior efficacy and/or reduced toxicity [1]. Radioimmunoconjugates (radiolabeled antibodies) are mAb linked to a radionuclide [2]. Radioimmunoconjugates as therapeutic and/or diagnostic agents in the management of cancer have been in development with some success for a few decades now. Significant strides have been made since the first radioimmunoconjugate was developed, leading to improved therapeutic efficacy [3,4]. Mabs and antibody-related therapies can be efficiently labeled with a variety of radionuclides for theranostic purposes. The radionuclides commonly used include actinium-225 (225Ac), astatine-211 (211At), bismuth-213 (213Bi), indium-111 (111In), iodine-123 (123I), iodine-124 (124I), iodine-131 (131I), lead-212 (212Pb), lutetium-177 (177Lu), technetium-99m (99mTc), copper-64 (64Cu), gallium-68 (68Ga), yttrium-86 (86Y), yttrium-90 (90Y), and zirconium-89 (89Zr) [5]. Based on their radiation properties, therapeutic radionuclides can be classified as β-particles, α-particles, or Auger electron emitters. β- particles are negatively charged electrons emitted from the nucleus with a long range and low linear energy transfer (LET). They are the most frequently used emission type for RIT agents and include lutetium-177(177Lu), yttrium-90 (90Y), and iodine-131 (131I). Alpha-particles, in contrast, have significantly higher energies, very short path lengths, and high LET. Alpha particles are emerging as an exciting new class of radionuclides with increased biological killing efficacy and lack of non-specific bystander effects seen with β-particle irradiation on normal tissue. These include astatine-211 (211At), actinium-225 (225Ac), thorium-227 (227Th), and bismuth-213 (213Bi).
This review provides an overview of radiolabeled intact antibodies currently in clinical use for the detection and treatment of hematological cancers and solid tumors, as well as those in development; examples of such clinical trials are shown in Table 1. We do not discuss smaller engineered antibody-based proteins or peptides, as this is beyond the scope of this review.
Table 1.
Examples of clinical trials evaluating radiolabeled antibodies for imaging and therapy.
| Antigen | Radiolabeled Antibody | Application | Tumor | Phase | Trial Status | References |
|---|---|---|---|---|---|---|
| PD-L1 and PD1 | 89Zr-atezolizumab | Diagnostic | Breast cancer | Pilot | Recruiting | NCT04222426 |
| 89Zr-atezolizumab | Diagnostic | Renal cell carcinoma | I | Recruiting | NCT04006522 | |
| 89Zr-durvalumab | Diagnostic | Lymphoma | I | Recruiting | NCT03610061 | |
| 18F-PDL1 | Diagnostic | Lung cancer | Pilot | Recruiting | NCT03564197 | |
| 18F- atezolizumab | Diagnostic | Esophageal and rectal cancer | Pilot | Active, not recruiting | NCT04564482 | |
| 89Zr-envafolimab (KN035) | Diagnostic | PD-L1 positive solid tumors | Pilot | Recruiting | NCT04977128 | |
| 89Zr-atezolizumab | Diagnostic | Lymphoma | Pilot | Recruiting | NCT03850028 | |
| 68Ga-WL12 | Diagnostic | Gastrointestinal tumors | Pilot | Recruiting | NCT04629326 | |
| 89Zr-M7824 | Diagnostic | NSCLC | 1 | Recruiting | NCT04297748 | |
| 89Zr-MPDL3280A | Diagnostic | Solid tumor | Pilot | Recruiting | NCT02453984 | |
| 89Zr-atezolizumab | Diagnostic | RCC | 1 | Recruiting | NCT04006522 | |
| 89Zr-REGN3504 | Diagnostic | HCC and Gastric/GEJ tumors | 1 | Recruiting | NCT03746704 | |
| 89Zr-pembrolizumab | Diagnostic | NSCLC | 2 | Status unknown | NCT03065764 ± | |
| 89Zr-crefmirlimab | Diagnostic | Melanoma, MCC, RCC, NSCLC | 2 | Recruiting | NCT05013099 | |
| 64Cu-pembrolizumab | Diagnostic | Hematological and solid tumors | 1 | Recruiting | NCT04605614 | |
| PSMA | 177Lu-HuJ591 + Ketoconazole | Therapeutic | Prostate cancer | II | Recruiting | NCT00859781 |
| 177Lu-7E11-C5.3 | Therapeutic | Prostate cancer | I | Status unknown | NCT00441571 ± | |
| CAIX/MN | 89Zr-girentuximab | Diagnostic | Urothelial cancers | I/II | Recruiting | NCT05018442 |
| 89Zr-girentuximab | Diagnostic | Clear cell renal cell cancer—(ZIRCON) | III | Recruiting | NCT03849118 | |
| 89Zr-girentuximab | Diagnostic | Non-muscle invasive bladder cancer | I | Recruiting | NCT04897763 | |
| 89Zr-girentuximab | Diagnostic | Urothelial cancers | I | Recruiting | NCT05046665 | |
| 89Zr-girentuximab | Diagnostic | Triple negative breast cancer | II | Recruiting | NCT04758780 | |
| IGF-1R | 225Ac-FPI-1434 | Therapeutic | IGF-1R expressing solid tumors | I/II | Recruiting | NCT03746431 |
| GD2 | 131I-3F8 | Therapeutic | Brain tumors and leptomeningeal disease | II | Active, not recruiting | NCT00445965 |
| EGFR | 125I-425 | Therapeutic | Brain tumors | I | Status unknown | NCT01317888 ± |
| 89Zr-Nimotuzumab | Diagnostic | Lung and colorectal cancers | I/II | Recruiting | NCT04235114 | |
| 89Zr-Panitumumab | Diagnostic | Colorectal cancers | I/II | Recruiting | NCT03764137 | |
| 89Zr-ABT806 | Diagnostic | High grade glioma | Pilot | Status unknown | NCT03058198 | |
| HER2 | 64Cu-Trastuzumab | Diagnostic | Stage III Breast cancer | II | Active, not recruiting | NCT02827877 |
| 64Cu-Trastuzumab | Diagnostic | Breast cancer | Pilot | Active, not recruiting | NCT01093612 | |
| 64Cu-Trastuzumab | Diagnostic | Breast cancer | Piot | Active not recruiting | NCT02226276 | |
| 89Zr-Pertuzumab | Diagnostic | HER2-Positive Solid Tumors | I | Recruiting | NCT04692831 | |
| CD25 | 90Y-basiliximab + BEAM protocol (carmustine etoposide cytarabine melphalan) | Therapeutic | HL | I | Active, not recruiting | NCT01476839 |
| CD33 | 225Ac-Lintuzumab | Therapeutic | MM | I | Status unknown | NCT02998047 |
| 225Ac-Lintuzumab + Venetoclax | Therapeutic | AML | I | Recruiting | NCT03867682 | |
| 225Ac-Lintuzumab + Venetoclax + Azacitidine | Therapeutic | AML | I/II | Not yet recruiting | NCT03932318 | |
| 225Ac-Lintuzumab | Therapeutic | AML (older patients ≥ 60 yrs) | I/II | Active, not recruiting | NCT02575963 | |
| 225Ac-Lintuzumab + Cladribine + Cytarabine + Filgastrim + Mitoxantrone (CLAG-M) | Therapeutic | AML | I | Recruiting | NCT03441048 |
Abbreviations: AML, acute myeloid leukemia; GEJ, gastro-esophageal cancer; HCC, hepatocellular carcinoma; HL, Hodgkin’s lymphoma; IGF-1R, type I insulin-like growth factor receptor; MCC, Merkel cell carcinoma; MM, multiple myeloma; NSCLC, non-small-cell lung cancer; PDL-1, programmed death ligand-1; RCC, renal cell carcinoma. ± Status unknown or withdrawn (no subjects enrolled).
2. Molecular Imaging
The ability of mAbs and antibody-related therapeutics to specifically target tumor-specific antigens allows expanding their application for theranostic approaches. Using radiolabeled mAbs with imaging methods, such as single-photon emission computerized tomography (SPECT) or positron-emission tomography (PET), provides vital insights into the pharmacokinetics and pharmacodynamics of therapeutic agents, heterogeneity, and dynamic changes of antigen expression, antigen engagement and uptake in tumors [6]. All of these parameters are critical for guiding and monitoring therapy responses, predicting toxicity, and identifying patients most likely to benefit from treatment. In addition, they provide critical information with direct relevance to the optimization of dosing and scheduling of mAbs. Zirconium-89 (t1/2, 3.27 days) and indium-111 (t1/2, 2.8 days) are two of the most commonly used radionuclides for PET and SPECT imaging, respectively.
2.1. Epidermal Growth Factor Receptor (EGFR)
Cetuximab, an IgG1 chimeric EGFR targeting mAb and radiolabeled with zirconium-89, has shown to be well tolerated [7] and demonstrated a strong correlation between uptake and response to cetuximab in patients with mCRC [8]. A larger study by the same group, however, showed no relationship between tumor uptake of 89Zr-cetuximab and treatment response [9]. These data suggest, in addition to EGFR expression, that 89Zr-cetuximab tumor uptake is influenced by other pharmacokinetic and dynamic mechanisms [9,10].
Panitumumab is a humanized anti-EGFR mAb. Panitumumab radiolabeled with a variety of radionuclides, including yttrium-86, copper-64, indium-111, and zirconium-89, which have been evaluated as PET probes for imaging EGFR [11]. Compared to 86Y-labeled-cetuximab, 86Y-labeled-panitumumab demonstrated higher tumor uptake and significantly lower liver uptake in a malignant mesothelioma model [12]. While 89Zr-panitumumab uptake highly correlated with EGFR expression, it is unable to detect EGFR mutations and mutations in downstream signaling pathways, such as KRAS and PTEN [11,13]. A first in human dosimetry study of 89Zr-panitumumab in patients with metastatic CRC reported the whole-body effective dose between 0.264 mSv/MBq (0.97 rem/mCi) and 0.330 mSv/MBq (1.22 rem/mCi), with the liver receiving the highest dose [14]. A bio-imaging study evaluating 89Zr-Panitumumab in patients with newly diagnosed colon cancer with lymph node involvement is currently enrolling (NCT03764137).
Nimotuzumab is an affinity-optimized anti-EGFR mAb. Preclinical studies in breast and colorectal carcinoma models demonstrated tumor uptake of 89Zr-nimotuzumab increasing up to 168 h post-injection, and, notably, EGFR expression was clearly visualized as early as 24 h post-injection [15]. 89Zr-nimotuzumab is being evaluated in a phase I/II trial to demonstrate the feasibility of identifying patients with EGFR-expressing tumors most likely to respond to anti-EGFR therapies (NCT04235114).
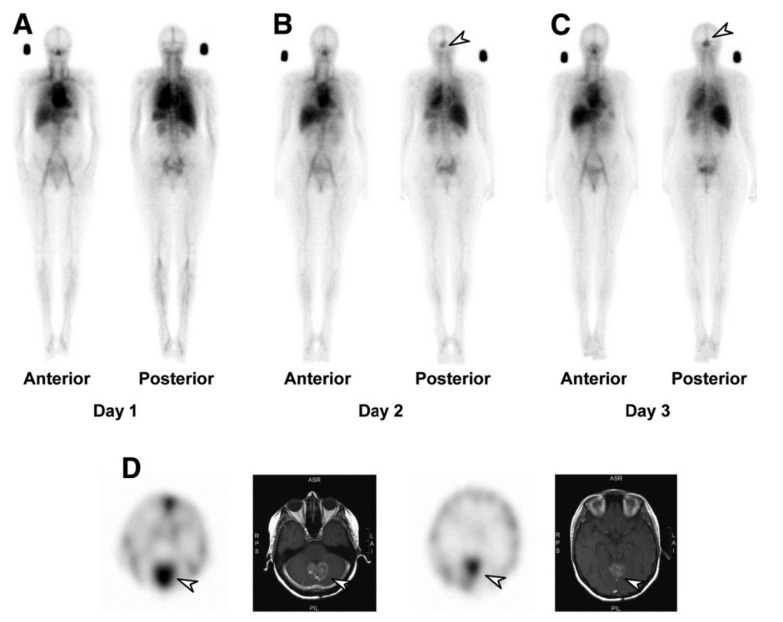
The novel anti-EGFR mAb, 806 (mAb806) targets a tumor-selective epitope of EGFR that is exposed on overexpressed, mutant, or ligand-activated forms of EGFR. Significantly, mAb806 does not bind to EGFR in normal tissues [16,17]. In a phase I study, 111In-labeled ch806, a chimeric form of mAb806, demonstrated excellent tumor uptake in a variety of solid tumors, and with no normal tissue uptake [18,19]. The humanized form of mAb806 (ABT-806) lacks conventional anti-EGFR mAb toxicities and is well tolerated [17]. Furthermore, biodistribution studies of 111In-ABT-806 (ABT-806i) showed high uptake in tumor, and no binding to normal tissue expressed EGFR, thus confirming the tumor-specific nature of mAb806 (Figure 1) [20].
Figure 1.
111In-ABT-806 (ABT-806i) biodistribution and SPECT/CT images of patient with high-grade glioma on (A) day 1, (B) day 2, and (C) day 3, demonstrating that rapid uptake of ABT-806i in known glioblastoma (arrow) is identified as early as day 2 and increases over time. (D) SPECT/MR images showing high uptake of ABT-806i in glioblastoma (arrow) in a posterior fossa lesion. Visualization of ABT-806i uptake in the anterior venous sinus is due to blood-pool activity. Reprinted with permission: Gan, H.K., et al. A Phase 1 and Biodistribution Study of ABT-806i, an 111In-Radiolabeled Conjugate of the Tumor-Specific Anti-EGFR Antibody ABT-806. J Nucl Med, 2021. 62 (6): p. 787–794 [20].
2.2. HER2
Given the predictive and prognostic value of HER2, in particular, in breast and gastric cancers, and the heterogeneity of expression of HER2 in some tumors, accurate determination of HER2 status is critical [21]. Numerous preclinical studies have shown high receptor saturation and tumor uptake of radiolabeled pertuzumab and trastuzumab, using a variety of radionuclides, including zirconium-89, copper-64, iodine-131, lutetium-177, and indium-111 [22,23,24,25,26,27]. In a biodistribution study, 89Zr-trastuzumab demonstrated high and HER2-specific tumor uptake; furthermore, previously undetected brain metastases were identified [28,29]. Other studies have shown the potential of imaging HER2 with 89Zr-trastuzumab to determine HER2 status and tumor heterogeneity and to predict response to anti-HER2 therapies [30,31,32]. While most of the studies have been performed in breast cancer, a study of 89Zr-trastuzumab in esophagogastric cancer patients demonstrated high HER2-specific tumor uptake [33]. Apart from 89Zr, trastuzumab has also been radiolabeled with various radionuclides; 64Cu-labeled trastuzumab has comparable tumor-to-tissue ratios to 89Zr-trastuzumab and is able to identify primary and metastatic lesions with low uptake seen in normal tissue, with the average tumor to non-tumor ratio for primary tumors and metastatic lesions calculated to be 3.1 and 3.2, respectively [34,35,36]. Similar specific uptake was also reported in a phase I study of 177Lu-trastuzumab in HER2-positive primary and metastatic breast lesions [37]. 89Zr-pertuzumab has similarly shown to successfully identify HER2-positive disease in patients with HER2-positive andHER2-negative metastatic breast cancer [38,39]. Despite their specificity and affinity for HER2, radiolabeled-trastuzumab and pertuzumab reveal high liver uptake due to catabolism of the mAb-chelate which impacts on the detection of liver metastases [34,37].
2.3. CAIX
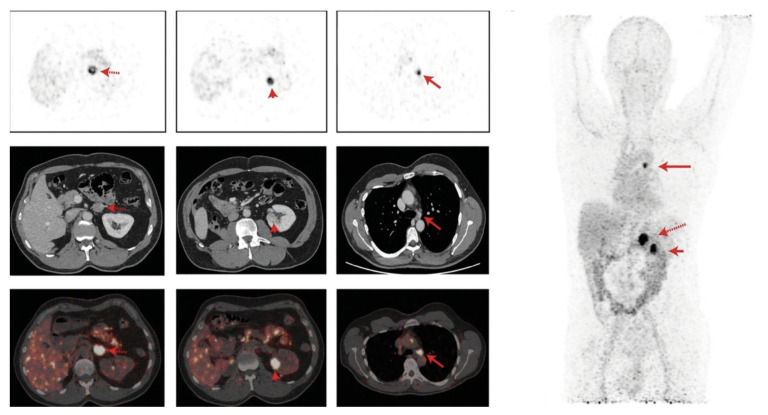
Carbonic anhydrase IX (CAIX) is a hypoxia-induced enzyme expressed on cancer cells and associated with treatment resistance [40]. CAIX is overexpressed in clear-cell renal-cell tumors (ccRCC) due to a mutation in the Von Hippel–Lindau protein [41]. A number of biodistribution studies have evaluated 89Zr-labeled girentuximab, an anti-CAIX monoclonal antibody [42,43,44]. These studies have shown 89Zr-girentuximab was safe and allowed differentiation between ccRCC and non-ccRCC lesions, enabling early detection of tumor recurrence and inform clinical decision making (Figure 2). In a phase III study, the imaging of CAIX with 124I-girentuximab was shown to accurately identify ccRCC lesions with the diagnostic equivalence to biopsy [45]. A phase III study (ZIRCON) is underway, assessing the sensitivity and specificity of 89Zr-girentuximab in detecting ccRCC (NCT03849118).
Figure 2.
89Zr-girentuximab in renal cancer patient. Left panel: top row, axial PET imaging at 168 h post administration (h p.a).; middle row, contrast enhanced CT; bottom row, fused PET/CT imaging showing (from left to right) 89Zr-girentuximab uptake in the adrenal gland (red arrow), left kidney (red arrowhead), and mediastinal lymph node (red arrow). Right panel: the maximum intensity projection (MIP) whole-body PET image at 168 h p.a. Reprinted with permission from Merkx, R.I.J.; Lobeek, D.; Konijnenberg, M.; et al. Phase I study to assess safety, biodistribution, and radiation dosimetry for 89Zr-girentuximab in patients with renal cell carcinoma. Eur J Nucl Med Mol Imaging, 2021. 48, 3277–3285 [43].
2.4. Mesothelin
Mesothelin (MSLN) is a cell-surface glycoprotein that is highly expressed in several tumor types, including pancreatic cancer, ovarian cancer, and malignant pleural mesothelioma [46,47]. The biological function of MSLN is not fully understood. A variety of MSLN-targeting agents, including mAbs (e.g., EPR4509, EPR19025-42, MMOT0530A, and amatuximab), ADCs (anetumab ravtansine), immunotoxin (SSP1), and CAR-T cells have been conjugated to tracers and tested in malignancies overexpressing MSLN to determine mesothelin expression and correlate with tumor uptake and response to treatment [47]. All the tracers showed specific tumor uptake and the potential to predict the response to MSLN-directed therapies [47]. In addition, tumor uptake was MSLN-mediated and correlated with antibody dose and tumor size [48,49]. In a phase I study, the anti-MSLN antibody MMOT0530A radiolabeled with 89Zr- [50] demonstrated maximum tumor uptake four days’ post-injection (mean SUVmax of 13.1 (±7.5)), with uptake observed in at least one tumor lesion in all patients (range 1–8). Heterogeneity of 89Zr-MMOT0530A tumor uptake occurred both intra-patient, with a 2.4-fold mean tumor uptake difference, and inter-patient, with a mean difference of 5.3-fold. The tracer tumor uptake (SUVmax on day 4) correlated with MSLN expression, as determined by immunohistochemistry (IHC) on archival tumor tissue [50].
2.5. Programed Death 1 (PD-1) and Programmed Death-Ligand 1 (PD-L1)
Immune checkpoint inhibitors, in particular, anti-PD1 and anti-PDL1 therapies, have changed the treatment paradigms in a number of tumor types, demonstrating objective and durable responses. PD-L1 expression testing by IHC is the most widely used biomarker of response to immunotherapy, despite being predictive of response in less than a third of cases. Furthermore, multiple studies have shown the efficacy of immunotherapies in patients with PD-L1-negative tumors [51]. A number of factors limit the predictive value of PDL-1 expression as determined by IHC, including the heterogeneity of PD-L1 expression, differences in PD-L1 scoring systems, staining platforms, and the types of cells tested for PD-L1 expression, as well as the dynamic changes in PD-L1 expression over time. Non-invasive molecular imaging of PD-(L)1 expression, using radiolabeled tracers, is being evaluated in clinical trials to overcome these limitations and is supported by a number of preclinical studies [52,53,54,55,56,57,58]. Preclinical studies show tracer specificity for PD-L1 irrespective of the radionuclide (indium-111, copper-64, and zirconium-89), with uptake correlating with levels of PD-L1 expression, allowing for the assessment of inter- and intra-tumoral heterogeneity [59]. Clinical studies have shown variable uptake in tumors that better correlated with clinical responses than IHC or RNA-sequencing-based approaches [60,61]. However, significant tracer uptake heterogeneity between patients and between different lesions within the same patient, high radiotracers accumulation in the spleen and liver, and poor central nervous system (CNS) tracer penetration are significant challenges that still need addressing [61].
3. Radiolabeled Antibodies for Cancer Treatment
When a radiolabeled mAb binds to its target antigen, the emitting radionuclide results in DNA-strand breaks through the generation of free radicals, resulting in apoptosis and programmed necrosis. In contrast to other anticancer therapies, radioimmunoconjugates may not require internalization (with the exception of alpha-particle therapy) and do not target specific signaling pathways to exert their antitumor effects. Compared to naked mAbs, treatment with radiolabeled mAbs can result in high response rates with lower toxicities, particularly when compared to many other systemic cancer treatments [62]. However, toxicity due to red marrow exposure related to the circulating half-life of intact mAbs is generally dose limiting. For therapeutic purposes, most radioimmunoconjugates contain either alpha-particle- or beta-particle-emitting radionuclides. The β-emitters are light energetic electrons that travel over a relatively long path length (0.5–12 mm) and have a low LET (approximately 0.2 keV/μm). They induce the formation of free radicals, causing DNA damage [63]. Examples of β-emitting radionuclides include iodine-131, copper-67, lutetium-177, or yttrium-90; iodine-131 and yttrium-90 have been used in most clinical RIT trials and are considered the current standard to which all other therapeutic radionuclides are compared [64]. In contrast, α emitters are positively charged heavy particles with a short-range path length of (20–100 μm) and high LET (approximately 100 keV/μm) [65].
3.1. Approved Radioimmunoconjugates
131I-tositumomab (Bexxar®; GlaxoSmithKline) and 90Y-ibritumomab tiuxetan (Zevalin®; Biogen Idec) have been approved by the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) in the management of non-Hodgkin B-cell lymphoma (NHL). 131I-chTNT (Cotara®) for the treatment of refractory advanced lung cancer and 131I-Metuximab (Licartin®) for the treatment of hepatocellular carcinoma (HCC) have been approved by the Chinese National Medical Products Administration (NMPA) [66].
90Y-Ibritumomab tiuxetan (Zevalin®; Biogen Idec) comprises the CD20 targeting mAb, ibritumomab, linked to the radionuclide yttrium-90. Patients treated with 90Y-Ibritumomab receive a dose of unlabeled rituximab to block CD-20+ binding sites on B-cells located in the circulation and in the spleen. The dose of 90Y-Ibritumomab is calculated based on the patient’s weight and platelet count. 90Y-Ibritumomab was first approved in 2002 for the treatment of low-grade or follicular lymphoma refractory to rituximab and relapsed or refractory low-grade follicular or transformed lymphoma. Then, in 2009, 90Y-Ibritumomab received expanded approval for treatment of patients with previously untreated follicular NHL who have achieved a partial or complete response to first-line chemotherapy. Several studies have demonstrated the superiority of 90Y-ibritumomab tiuxetan over rituximab. In a phase III trial, treatment with 90Y-ibritumomab tiuxetan resulted in superior overall response rates (ORR) (80% versus 56% for the rituximab group) and a number of complete responses (30% versus 16% in the rituximab group) [67,68,69]. In addition, the combination of 90Y-ibritumomab tiuxetan with chemotherapy resulted in improved progression-free survival (PFS) (2-year PFS 59% versus 39% for the chemotherapy-alone group) and overall survival (OS) (2-year OS 91% versus 62% for the chemotherapy group alone) with no significant added toxicity [70]. In a randomized phase III trial (First-Line Indolent trial), 90Y-ibritumomab tiuxetan was shown to be efficacious as consolidation therapy in patients with advanced-stage follicular lymphoma in first remission. After a median follow-up of 7.3 years, PFS was significantly improved with 90Y-ibritumomab tiuxetan (41% versus 22% for control group), with a prolonged median time to next treatment (8.1 years versus 3.0 years for the control group) [71,72]. Similar benefits of ibritumomab tiuxetan as consolidation therapy have been shown in other studies in patients with intermediate and high-risk follicular lymphoma [73,74,75].
Tositumomab and 131I-tositumomab (Bexxar®; GlaxoSmithKline), an anti-CD20 mAb regimen, received FDA approval in June 2003 for the treatment of CD20-positive relapsed/refractory follicular NHL. Similar to 90Y-Ibritumomab, prior to administration of 131I-tositumomab, unlabeled tositumomab is administered to bind B-cells in circulation and in the spleen. A meta-analysis of heavily pretreated patients (received at least four prior regimens) with indolent lymphoma treated with 131I-tositumomab reported ORR of 68% [76]. Treatment-naive patients treated with chemotherapy, followed by one dose of tositumomab and 131I-tositumomab, had a 100% response rate with the median response duration not reached after a median follow-up of 8.4 years [77]. There was, however, no difference in PFS (2-year PFS 48.6% versus 47.9%, respectively) or OS (2-year OS 65.6% versus 61%, respectively) reported in a phase III trial comparing rituximab plus chemotherapy with 131I-tositumomab and chemotherapy with autologous hematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma [78]. RIT consolidation in patients with follicular lymphoma has shown encouraging results in a meta-analysis; however, further studies comparing the benefits of consolidation RIT to maintenance rituximab are required [79]. Tositumomab and 131I-tositumomab were withdrawn from the market in 2014, due to lack of commercial demand [1].
131I-chTNT has been approved by the NMPA in China for the treatment of refractory advanced lung cancer [66,80]. Tumor necrosis treatment (TNT) differs from other ACTs through the targeting of necrotic regions within the tumor rather than surface antigens [81]. Based on promising efficacy data, 131I-chTNT has received approval from the Chinese State Food and Drug Administration for the treatment of patients with advanced lung cancer who were previously treated with radiotherapy or chemotherapy [82]. In the pivotal study, pretreated patients with advanced lung cancer received intravenous, intra-tumoral or a combination of intravenous and intratumoral 131I-chTNT. The ORR was 34.6%; patients receiving intratumoral injection only (n = 16) had an ORR 56%, and patients treated with a combination of intravenous and intratumoral (n = 5) had an ORR of 40% [80]. Response rates were similar irrespective of route of administration. Hematological toxicity was most commonly reported in patients who received RIT intravenously.
131I-metuximab (Licartin®, Chengdu Huashen Biotechnology) is a radioimmunoconjugate targeting CD147, which is a transmembrane glycoprotein associated with hepatocarcinogenesis, HCC growth, and metastasis [83]. The combination of 131I-metuximab with transcatheter arterial chemoembolization or radiofrequency ablation in patients with intermediate and advanced HCC has shown be safe and resulted in delayed recurrence and improved OS [84,85,86,87]. In addition, in a randomized trial, 131I-metuximab post-orthotopic liver transplantation significantly reduced rates of recurrence rates (by 30.4%) and increased survival (by 20.6%) versus placebo [88]. 131I-metuximab is approved by the China State Food and Drug Administration for the treatment of primary HCC.
3.2. Radioimmunoconjugates in Development
3.2.1. Solid Tumors
In contrast to hematological malignancies, the efficacy of radiolabeled mAbs in solid tumors has been modest, with responses seen mainly in tumors with high antigen expression, and those treated with fractionated RIT protocols or with the combination of RIT with other agents, typically chemotherapy [89]. Efficacy of RIT in solid tumors is limited by factors not present in hematological tumors, such as poor perfusion and heterogeneities in blood flow the presence of tumor stroma, heterogeneous target antigen expression, and inherent resistant to radiation therapy. New approaches, including fractionated RIT, modified pharmacokinetics, alpha-particle labeled antibodies, and combination with radiation sensitizers, may provide enhanced efficacy and are being evaluated in clinical trials.
Renal Cell Carcinoma
In a phase I study of 177Lu-girentuximab in patients with metastatic ccRCC [90] myelotoxicity was dose limiting and 17/23 (74%) patients showed stable disease three months after the first treatment, with just over a half (54%) receiving a second dose. In a follow-up phase II study of 14 patients, eight patients (57%) were found to have stable disease and one (7%) had a partial regression after the first treatment. Myelotoxicity was observed in most patients, and it prevented retreatment in some patients [91].
Prostate Cancer
Prostate-specific membrane antigen (PSMA) is a transmembrane protein that is overexpressed in castrate-resistant tumors, making it an attractive theranostic target. In an early phase trial, CYT-356, a PSMA-targeting mAb, radiolabeled with 90Y, had no therapeutic effect, with myelosuppression being the dose-limiting toxicity (DLT) [92]. A phase II study was stopped early, due to futility [93].
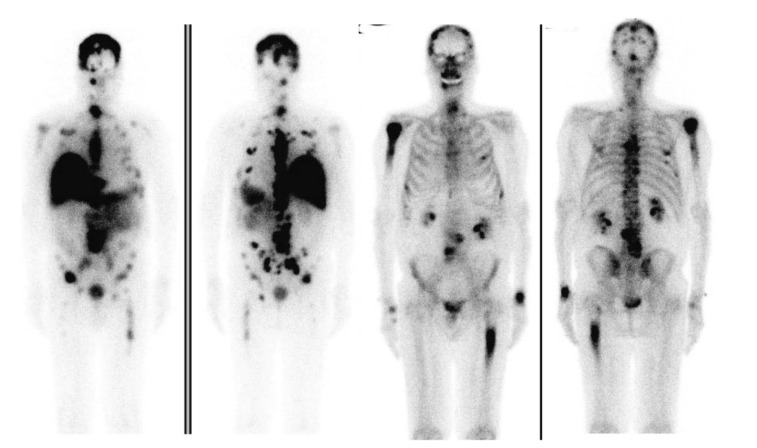
Hu-J591 (rosopatamab) mAb targeting PSMA radiolabeled with yttrium-90 and lutetium-177 via a DOTA chelate was studied in two phase I studies [94,95]. J591 demonstrated good tumor targeting in nearly all patients (42/43), and dose related antitumor activity and PSA decline were reported in both trials. A phase II study of 177Lu-J591 in heavily pretreated patients with prostate cancer was undertaken, confirming the safety, efficacy, and tumor-targeting ability [96]. A phase I study combining docetaxel for its radiosensitizing properties with 177Lu-J591, which was administered in fractionated dosing, showed that the combination was well tolerated; however, grade 4 myelosuppression was reported in 30% (5/15) of patients (Figure 3) [97]. 177Lu-labeled J591 therapy is now under investigation in the salvage setting (NCT00859781), as well as in the second line setting for the treatment of PSMA-expressing castrate-resistant metastatic prostate cancer (PROSTACT; NCT04876651).
Figure 3.
Left to right: 177Lu-J591 anterior and posterior whole-body images 7 days after 177Lu-J591 administration, and Technetium 99m-methyl diphosphonate (99mTc-MDP) bone scan anterior and posterior whole-body images, showing 177Lu-J591 targeting of extensive bone and soft-tissue metastases. Reprinted with permission from: Tagawa S.T, et al. Phase 1/2 Study of Fractionated Dose Lutetium-177–Labeled Anti–Prostate-Specific Membrane Antigen Monoclonal Antibody J591 (177Lu-J591) for Metastatic Castration-Resistant Prostate Cancer. Cancer, 2019 Aug 1; 125 (15): 2561–2569 [97].
PSMA-targeting radiolabeled small molecules, such 177Lu-PSMA-617 and 225Ac-PSMA-617, have demonstrated clinical and biochemical responses with low toxicity in chemotherapy-naive patients, as well as patients who have progressed on standard therapeutic options, respectively [98,99,100]. The success of these treatments has further increased interest in PSMA-directed RIT.
Colon Cancer
Carcinoembryonic antigen (CEA) is a glycoprotein produced by >90% of colonic epithelial cells. CEA plays an important role as a ligand involved in cancer dissemination [101]. In a phase II study, patients with metastatic colorectal cancer who received a single dose of the radiolabeled humanized anti-CEA mAb 131I-labetuzumab, following R0 resection of liver metastases, had superior OS compared to a similar contemporaneous group of control patients that did not receive RIT [102,103]. The main toxicities reported were hematological. A larger phase II study using 131I-labetuzumab as “adjuvant” treatment in mCRC patients, post-metastectomy, resulted in a median time to progression and OS of 16 and 55 months, respectively; however, significant grade 4 hematological toxicity was seen, including myelodysplastic syndrome in 5/63 patients enrolled [104].
The A33 cell surface antigen is expressed in >95% of human colon cancers, and in normal colonic and small bowel epithelium. It is, however, absent in other epithelial tissues [105]. RIT trials of iodinated murine A33 mAb (131I-muA33 and 125I-muA33) showed safety, tolerability, and specific tumor-targeting in patients with advanced colorectal cancer, with modest antitumor activity [106,107]. A humanized IgG1 huA33 antibody has high affinity for A33, and in clinical trials, it has been shown to have an acceptable toxicity profile [108]. A biodistribution study of 131I and 125I labeled huA33 showed excellent tumor uptake and penetrated to the center of large necrotic metastatic lesions. No dose-limiting toxicities were reported [109]. In a phase I trial of 131I-huA33 in pretreated patients with mCRC, hematological toxicity was seen to be dose dependent and dose limiting. Excellent tumor localization was seen, along with some antitumor effects [110]. 131I-huA33 in combination with capecitabine resulted in greater tumor responses than those observed with 131I-huA33 alone with no significant added toxicity [111].
In a dose-escalation study, the 125I-radiolabeled chimeric antibody, 17-1A, directed against epithelial cell-adhesion molecule (Ep-CAM), no dose-limiting toxicities were observed, albeit no tumor responses [112].
Panitumumab conjugated to α-emitter, 212Pb, resulted in improved survival in a preclinical CRC model, with enhanced benefit observed when combined with chemotherapy [113].
Ovarian Cancer
RIT in ovarian cancer is typically administered intraperitoneally rather than by intravenous administration. Responses typically correlate to tumor volume, with higher responses observed in patients with minimal residual disease or with low disease burden [114].
MUC1, a member of the mucin family, is a glycosylated protein that is overexpressed in a variety of epithelial cancers. MUC1 plays a crucial role in tumorigenesis, cancer progression, and treatment resistance [115]. In a phase II trial, patients with ovarian cancer in remission after chemotherapy treated with 90Y labeled anti-MUC1 antibody, HMFG-1, had a significant improvement in five-year survival compared to the controls (80% vs. 55%, respectively) [116]. In a randomized phase III multicenter trial, the addition of RIT resulted in a decrease in intraperitoneal relapse rates, but this did not translate to an improvement in OS when compared with standard care [117,118].
Tumor-associated glycoprotein 72 (TAG-72) is overexpressed in a range of solid tumors. CC49, an anti-TAG-72 antibody, has shown excellent tumor targeting, albeit minimal antitumor activity as an unlabeled antibody [119]. A number of anti-TAG-72 intact antibodies radiolabeled with iodine-131, yttrium-90, and lutetium-177 have been evaluated clinically [120,121,122]. In an early phase study, patients with treatment-resistant recurrent/persistent ovarian cancer received 90Y-CC49 in combination with intraperitoneal paclitaxel and interferon. Of the patients with measurable disease, two had PRs lasting 2 and 4 months, while 11 patients with non-measurable disease had a median time to progression of 6 months [122]. CC49 radiolabeled with alpha-emitters have also been tested in preclinical ovarian cancer models with promising results [123].
A phase I multicenter trial of 225Ac-FPI-1434 (anti-IGF-1R mAb) is currently being conducted in patients with solid tumors that show uptake of 111In-FPI-1547 (NCT03746431). Several other potential targets and radiolabeled antibodies have been evaluated in patients with solid tumors, showing acceptable safety profiles but modest antitumor activity [124].
Pancreatic Ductal Adenocarcinoma (PDAC) Cancer
Clinical trials investigating RIT in PDAC have predominantly used β-emitting radionuclides, and, to our knowledge, α RIT has not been tested in a clinical trial in PDAC. The most common target investigated is mucin 1 (MUC1), which is overexpressed in >60% of PDAC. However, the exact role of MUC1 in PDAC is still unknown [125]. In a phase I trial, 90Y-clivatuzumab tetraxetan was well tolerated with manageable hematologic toxicity [126]. The addition of low-dose gemcitabine resulted in improved disease control and OS [127,128]. However, a phase III study (PANCRIT®-1) investigating the combination was prematurely terminated due to futility after an interim analysis (NCT 01956812).
Over 90% of pancreatic cancers overexpress CEA [129]. In a phase I/II study KAb201, an anti-CEA mAb that was radiolabeled with isodine-131 was well tolerated, but it had only modest efficacy. 131I-KAb201 was administered either intravenously or intra-arterially, with no significant difference in therapeutic efficacy seen between the administration routes [130].
Primary Brain Tumors
Tenascin is a multi-domain extracellular matrix glycoprotein that is highly expressed in several cancers, including in high-grade gliomas [131]. 131I-labeled mAb 81C6, a tenascin-targeting mAb, demonstrated high specificity in biodistribution studies [132]. These studies also showed limited intra-tumoral penetration following intravenous or intra-arterial administration, and subsequent studies have used intracranial administration [133]. A number of dosimetry and phase I/II studies have investigated 131I-81C6 in patients with newly diagnosed malignant high-grade gliomas. These studies used a single dosing regimen and demonstrated improvements in survival rates compared to historical controls and significantly minimal hematologic and neurological toxicity, including lower rates of radionecrosis. A correlation between the radiation-absorbed dose and tumor recurrence or radionecrosis was also reported [134,135,136,137,138,139]. Other studies demonstrated improvements in survival rates for patients with newly diagnosed and recurrent glioblastoma treated with repeated doses of 131I-labeled anti-tenascin murine antibodies, BC-2 and BC-4 [140,141,142]. Moreover, 81C6 mAb has also been radiolabeled with astatine-211, an alpha emitter, and tested in patients with recurrent gliomas [133]. No dose-limiting toxicities were reported, and there was an improvement in median survival times (54 weeks versus 23–31 weeks for patients treated with conventional therapies).
EGFR aberrations, including amplification, deletion, or mutation, are detected in ~60% of glioblastoma [143]. Two large phase II studies evaluated an EGFR targeting mAb radiolabeled with iodine-125, 125I-mAb 425, in patients with high-grade gliomas. These studies demonstrated that RIT was well tolerated and resulted in a survival benefit compared to a contemporaneous control group that did not receive RIT [144,145]. Another EGFR RIT-targeting modality, nimotuzumab radiolabeled with rhenium-188, was evaluated in a phase 1 study in patients with high-grade glioma as a single-dose intracavitary injection. Durable responses were observed in three patients, lasting more than one year; however, early severe neurological symptoms and late-onset radionecrosis were observed with the higher dose of 188Re [146]. Other potential RIT targets showing promise in gliomas in preclinical studies include carbonic anhydrase, cadherin 5, and Integrin αVβ3 [147].
3.3. Hematological Cancers
3.3.1. Lymphoma
Several RIT agents targeting populations of cells expressing CD21, CD22, CD37, and the human leukocyte antigen DR (HLA-DR) are being investigated.
CD22 is a transmembrane glycoprotein that is widely expressed across malignant B-cell histologies [148]. In contrast to Zevalin® and Bexxar®, the anti-CD22 90Y-epratuzumab tetraxetan can be administered without a loading dose of cold antibody. A phase I/II trial assessed 90Y-epratuzumab in patients with relapsing B lymphoma [149]. Patients had imaging studies one week prior to treatment with 111In-epratuzumab. The trial enrolled patients in two cohorts: patients who had prior high-dose chemotherapy (Group 2) and patients who did not have a prior stem-cell transplantation (SCT) (Group 1) [149]. Irrespective of tumor targeting and tumor size, antitumor responses were seen in both indolent and aggressive NHL. Similarly, antitumor responses have also been seen from using fractionated dosing of 90Y-epratuzumab with toxicities that were primarily hematological and dose dependent [150,151]. The efficacy of fractionated 90Y-epratuzumab as consolidation therapy after rituximab-based therapy was investigated in elderly (age>60 years) patients presenting with stage I/II bulky or stage III/IV diffuse large B-cell lymphoma (DLBCL). The estimated 2-year event-free survival was 75%, and grade 3/4 thrombocytopenia and neutropenia were seen in 84% and 79% patients, respectively. One patient each developed myelodysplastic syndrome and acute myeloid leukemia post-RIT [152,153].
BAY 1862864 is a CD22-targeting antibody radiolabeled to the α-particle emitting radionuclide thorium-227. BAY1862864 was evaluated in a first-in-human study in patients with CD22-positive relapsed/refractory B cell NHL [154]. In this study, the DLTs were febrile neutropenia and thrombocytopenia, with nearly half the patients (48%) developing grade ≥3 treatment-emergent adverse events, with the most common being myelotoxicity. The MTD was not reached. The ORR reported was 25%, with responses higher in patients with relapsed low-grade versus high-grade lymphomas (30% vs. 11%, respectively).
Lym-1, a monoclonal antibody which preferentially targets malignant B-lymphocytes, when labeled with iodine-131, has been shown to induce durable remissions in patients with NHL resistant to chemotherapy [155,156]. Dose-limiting toxicity was thrombocytopenia, and non-hematologic toxicities were typically of low grade. Dose-limiting toxicity of 90Y-Lym-1 was also thrombocytopenia and more than half of the patients enrolled onto the study had a partial response or stabilization of chemotherapy-refractory NHL after RIT [157].
Other antigens have been investigated as targets for RIT in lymphoma in early phase studies, including CD21 [158,159] and CD37 [160,161,162].
3.3.2. Multiple Myeloma
Numerous cell-surface antigens expressed on multiple myeloma (MM) cells have been targeted by mAbs, including anti-CD20 rituximab, anti-CD33, anti-CD38, anti-CD54, anti-CD74, anti-CD317, and anti-CD319 [2]. MM cells display inherent sensitivity to radiation, providing strong rationale to extend the use of mAbs to RIT.
CD33 antigen is expressed on MM plasmocytes in 20–35% of MM patients and is associated with unfavorable cytogenetics and a poor prognosis [163,164]. 225Ac-lintuzumab is a CD33-directed RIT. A phase I study of 225Ac-lintuzumab in patients with MM that have failed standard therapy is underway (NCT02998047) [165].
CD38 is highly expressed on MM plasmocytes’ cell surface (>90%) and has immunomodulatory effects [166]. 211At-OKT10-B10 (211At-CD38) is an anti-CD38 mAb radiolabeled with the alpha-emitting radionuclide astatine-211 [167]. Preclinical studies showed excellent tumor-to-normal-organ ratios, and only modest responses were seen in a bulky xenograft model of MM. However, in a disseminated disease model designed to reflect low-burden minimal residual disease, RIT produced sustained remission [167]. A phase I clinical trial evaluating 211At-OKT10-B10 in combination with an ASCT-conditioning regimen in MM patients is underway (NCT04466475). Another CD38 targeting radioimmunoconjugate demonstrated prolonged tumor retention and potent antitumor effects compared to the naked antibody, without any significant toxicity [168].
CD20 is heterogeneously expressed on myeloma cells with the presence of clonogenic CD20-positive precursor B cells in MM seen in 13–22% of patients [164,169]. In a single center study, 90Y-Ibritumomab tiuxetan was evaluated in combination with high-dose melphalan prior to ASCT in MM patients [170]. Despite reporting encouraging results, a subsequent phase II trial was prematurely terminated due to modest response rates and increase in peri-ASCT bacterial infections [171].
Syndecan-1 (CD138), a transmembrane proteoglycan, is highly expressed on MM cells [172]. In a phase I/II bio-imaging trial, a single therapeutic dose of 131I-B-B4 showed a high uptake in the bone marrow and liver, with all patients experiencing transient grade 3 or higher hematologic toxicities. No objective responses were observed [173].
3.3.3. Acute Myeloid Leukemia
Acute Myeloid Leukemia (AML) cells are characterized by aberrant expression of myeloid and lymphoid lineage markers. CD45 is a transmembrane glycoprotein highly expressed on all hematopoietic cells and most AML cells; therefore, it is ideal to target the bone marrow [174]. In an early phase study, 131I-BC8, a radiolabeled anti-CD45 mAb, in combination with cyclophosphamide and 12-Gy total body irradiation (TBI) in patients with refractory AML/ALL demonstrated favorable biodistribution, with a higher estimated radiation absorbed dose to marrow and spleen than to normal organs. The overall leukemia-free survival (LFS) was 29% [175]. Subsequently, two phase II studies were undertaken in patients with AML in a first remission (CR1) and in patients with advanced AML [176,177]. Patients with advanced AML, matched related (MRD) and unrelated (MUD) donors, were treated with TBI 12Gy with chemotherapy and 131I-BC8. The LFS was 42% with outcomes identical in the MRD and the MUD groups [176]. Patients with AML in CR1 with intermediate-risk or high-risk cytogenetic features received RIT in combination with chemotherapy, using family donors. Overall LFS was 60% with the LFS higher for intermediate-risk vs. high-risk patients (68 vs. 40%, respectively) [177].
CD33 is only expressed on myeloid cells and lymphocytes and frequently expressed on AML cells [178]. Lintuzumab (SGN-33 and HuM195) is CDR-grafted IgG1 humanized mAb directed against CD33 (179). In a phase I trial, Lintuzumab showed similar pharmacology to murine M195 and was well-tolerated without significant immunogenicity [179]. 131I-Lintuzumab in combination with busulfan and cyclophosphamide as a conditioning regimen for allogeneic bone-marrow transplantation was well tolerated and resulted in prolonged myelosuppression. A fifth of patients with relapsed/refractory AML in this study experienced significant improvements in long-term survival [180]. In an attempt to avoid the myelosuppression experienced with β-emitting constructs, the α-emitters bismuth-213 and actinium-225 were conjugated to lintuzumab. Both radioconjugates have demonstrated encouraging results in phase I/II in AML patients [181,182]. Based on this, a number of trials are evaluating 225Ac-lintuzumab in AML patients (NCT03867682, NCT03932318, NCT02575963, and NCT03441048) and in patients with CD33-positive multiple myeloma (NCT02998047).
4. Conclusions
Radioimmunoconjugates have been shown to be useful for both the detection of tumors and for therapy, and can be combined with conventional therapies to enhance the therapeutic efficacy of mAbs [114]. In addition, the use of mAb radioconjugates as imaging theranostics allows for real-time antigen quantitation, detect heterogeneity, and dynamic changes in antigen expression, all of which can be vital for guiding and monitoring therapy responses, as well as in drug development, providing important information concerning pharmacokinetics of mAbs and patient selection and drug dose required for therapeutic efficacy. Despite RIT demonstrating therapeutic efficacy in hematological malignancies, the same benefit has yet to be seen in solid tumors and represents the principal challenge of the future. Several strategies have been proposed to improve their therapeutic index and are being evaluated, including targeting patients with minimal disease burden, using pre-targeting strategies, newer radionuclides (including alpha-particle isotopes), and using combined therapeutic modalities (such as DNA sensitizers) and locoregional application [114]. Furthermore, improvements in radiochemistry and scale of production may help to reduce the high production costs and allow for the wider use of these therapies. The opportunities for RIT are expanding, and further trials will demonstrate the potential of this approach in treating cancer patients.
Acknowledgments
S.P is the recipient of a Fellowship from the Victorian Government Department of Health and Human Services acting through the Victorian Cancer Agency. A.M.S is the recipient of an NHRMC Investigator Fellowship No. 1177837.
Author Contributions
S.P., S.T.L., H.K.G. and A.M.S. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
H.K.G has received Institutional support for trials from AbbVie. A.M.S is an inventor of patents relating to mAb806 and has received Institutional support for trials from AbbVie, Astra Zeneca, Merck, Telix, Fusion.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Parakh S., Parslow A., Gan H.K., Scott A. Antibody-mediated delivery of therapeutics for cancer therapy. Expert Opin. Drug Deliv. 2016;13:401–419. doi: 10.1517/17425247.2016.1124854. [DOI] [PubMed] [Google Scholar]
- 2.Bruins W.S.C., Zweegman S., Mutis T., Van De Donk N.W. Targeted therapy with immunoconjugates for multiple myeloma. Front. Immunol. 2020;11:1155. doi: 10.3389/fimmu.2020.01155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barbet J., Bardiès M., Bourgeois M., Chatal J.-F., Chérel M., Davodeau F., Faivre-Chauvet A., Gestin J.-F., Kraeber-Bodéré F. Radiolabeled antibodies for cancer imaging and therapy. Antib. Eng. 2012;907:681–697. doi: 10.1007/978-1-61779-974-7_38. [DOI] [PubMed] [Google Scholar]
- 4.Kraeber-Bodéré F., Barbet J., Chatal J.-F. Radioimmunotherapy: From current clinical success to future industrial breakthrough? J. Nucl. Med. 2016;57:329–331. doi: 10.2967/jnumed.115.167247. [DOI] [PubMed] [Google Scholar]
- 5.Harsini S., Rezaei N. Cancer Immunology. Springer; Cham, Switzerland: 2020. Cancer imaging with radiolabeled monoclonal antibodies; pp. 739–760. [Google Scholar]
- 6.Burvenich I.J.G., Parakh S., Lee F.-T., Guo N., Liu Z., Gan H.K., Rigopoulos A., O’Keefe G.J., Gong S.Y., Goh Y.W., et al. Molecular imaging of T cell co-regulator factor B7-H3 with 89Zr-DS-5573a. Theranostics. 2018;8:4199. doi: 10.7150/thno.25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Loon J., Even A.J., Aerts H.J., Öllers M., Hoebers F., van Elmpt W., Dubois L., Dingemans A.-M.C., Lalisang R.I., Kempers P., et al. PET imaging of zirconium-89 labelled cetuximab: A phase I trial in patients with head and neck and lung cancer. Radiother. Oncol. 2017;122:267–273. doi: 10.1016/j.radonc.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Menke-Van Der Houven C.W., McGeoch A., Bergstrom M., McSherry I., Smith D.A., Cleveland M., Al-Azzam W., Chen L., Verheul H., Hoekstra O.S., et al. Immuno-PET imaging to assess target engagement: Experience from 89Zr-anti-HER3 mAb (GSK2849330) in patients with solid tumors. J. Nucl. Med. 2019;60:902–909. doi: 10.2967/jnumed.118.214726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Helden E., Elias S., Gerritse S., van Es S., Boon E., Huisman M., van Grieken N.C.T., Dekker H., van Dongen G.A.M.S., Vugts D.J., et al. [89Zr] Zr-cetuximab PET/CT as biomarker for cetuximab monotherapy in patients with RAS wild-type advanced colorectal cancer. Eur. J. Nucl. Med. Mol. Imaging. 2020;47:849–859. doi: 10.1007/s00259-019-04555-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aerts H.J., Dubois L., Perk L., Vermaelen P., van Dongen G.A., Wouters B.G., Lambin P. Disparity between in vivo EGFR expression and 89Zr-labeled cetuximab uptake assessed with PET. J. Nucl. Med. 2009;50:123–131. doi: 10.2967/jnumed.108.054312. [DOI] [PubMed] [Google Scholar]
- 11.Chang A.J., De Silva A.R., Lapi E.S. Development and characterization of 89Zr-labeled panitumumab for immuno–positron emission tomographic imaging of the epidermal growth factor receptor. Mol. Imaging. 2013;12:17. [PMC free article] [PubMed] [Google Scholar]
- 12.Nayak T.K., Garmestani K., Milenic D.E., Baidoo K.E., Brechbiel M.W. HER1-targeted 86Y-panitumumab possesses superior targeting characteristics than 86Y-cetuximab for PET imaging of human malignant mesothelioma tumors xenografts. PLoS ONE. 2011;6:e18198. doi: 10.1371/journal.pone.0018198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sartore-Bianchi A., Bencardino K., Cassingena A., Venturini F., Funaioli C., Cipani T., Amatu A., Pietrogiovanna L., Schiavo R., Di Nicolantonio F., et al. Therapeutic implications of resistance to molecular therapies in metastatic colorectal cancer. Cancer Treat. Rev. 2010;36:S1–S5. doi: 10.1016/S0305-7372(10)70012-8. [DOI] [PubMed] [Google Scholar]
- 14.Lindenberg L., Adler S., Turkbey I.B., Mertan F., Ton A., Do K., Kummar S., Gonzalez E.M., Bhattacharyya S., Jacobs P.M., et al. Dosimetry and first human experience with 89Zr-panitumumab. Am. J. Nucl. Med. Mol. Imaging. 2017;7:195. [PMC free article] [PubMed] [Google Scholar]
- 15.Chekol R., Bernhard W., Viswas R.S., Alizadeh E., Hartimath S., Barreto K., Geyer R.C., Fonge H. 89Zr-nimotuzumab for potential clinical translation as an anti-EGFR immunoPET agent. J. Nucl. Med. 2017;58:688. [Google Scholar]
- 16.Johns T.G., Adams T.E., Cochran J.R., Hall N.E., Hoyne P.A., Olsen M.J., Kim Y.-S., Rothacker J., Nice E.C., Walker F., et al. Identification of the epitope for the epidermal growth factor receptor-specific monoclonal antibody 806 reveals that it preferentially recognizes an untethered form of the receptor. J. Biol. Chem. 2004;279:30375–30384. doi: 10.1074/jbc.M401218200. [DOI] [PubMed] [Google Scholar]
- 17.Gan H.K., Burgess A.W., Clayton A.H.A., Scott A. Targeting of a conformationally exposed, tumor-specific epitope of EGFR as a strategy for cancer therapy. Cancer Res. 2012;72:2924–2930. doi: 10.1158/0008-5472.CAN-11-3898. [DOI] [PubMed] [Google Scholar]
- 18.Panousis C.G., Rayzman V., Johns T., Renner C., Liu Z., Cartwright G., Lee F.-T., Wang D.-N., Gan H., Cao D., et al. Engineering and characterisation of chimeric monoclonal antibody 806 (ch806) for targeted immunotherapy of tumours expressing de2-7 EGFR or amplified EGFR. Br. J. Cancer. 2005;92:1069–1077. doi: 10.1038/sj.bjc.6602470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scott A.M., Lee F.-T., Tebbutt N., Herbertson R., Gill S.S., Liu Z., Skrinos E., Murone C., Saunder T., Chappell B., et al. A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc. Natl. Acad. Sci. USA. 2007;104:4071–4076. doi: 10.1073/pnas.0611693104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gan H.K., Burge M., Solomon B., Lee S.T., Holen K.D., Zhang Y., Ciprotti M., Lee F., Munasinghe W., Fischer J., et al. A Phase 1 and Biodistribution Study of ABT-806i, an 111In-Radiolabeled Conjugate of the Tumor-Specific Anti-EGFR Antibody ABT-806. J. Nucl. Med. 2021;62:787–794. doi: 10.2967/jnumed.120.253146. [DOI] [PubMed] [Google Scholar]
- 21.Parakh S., Gan H.K., Parslow A.C., Burvenich I., Burgess A.W., Scott A.M. Evolution of anti-HER2 therapies for cancer treatment. Cancer Treat. Rev. 2017;59:1–21. doi: 10.1016/j.ctrv.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Marquez B.V., Ikotun O.F., Wright B., Zheleznyak A., Richard P., Lapi S.E. PET imaging of 89Zr-labeled pertuzumab in HER2-positive breast cancer xenografts. Cancer Res. 2014;74:109. [Google Scholar]
- 23.Capala J., Bouchelouche K. Molecular imaging of HER2-positive breast cancer-a step toward an individualized “Image and Treat” strategy. Curr. Opin. Oncol. 2010;22:559. doi: 10.1097/CCO.0b013e32833f8c3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kameswaran M., Gota V., Ambade R., Gupta S., Dash A. Preparation and preclinical evaluation of 131I-trastuzumab for breast cancer. J. Label. Compd. Radiopharm. 2017;60:12–19. doi: 10.1002/jlcr.3465. [DOI] [PubMed] [Google Scholar]
- 25.Jiang D., Im H.-J., Sun H., Valdovinos H., England C.G., Ehlerding E.B., Nickles R.J., Lee D.S., Cho S.Y., Huang P., et al. Radiolabeled pertuzumab for imaging of human epidermal growth factor receptor 2 expression in ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging. 2017;44:1296–1305. doi: 10.1007/s00259-017-3663-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marquez B.V., Ikotun O.F., Zheleznyak A., Wright B., Hari-Raj A., Pierce R.A., Lapi S.E. Evaluation of 89Zr-pertuzumab in breast cancer xenografts. Mol. Pharm. 2014;11:3988–3995. doi: 10.1021/mp500323d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Persson M., Gedda L., Lundqvist H., Tolmachev V., Nordgren H., Malmström P.-U., Carlsson J. [177Lu] Pertuzumab: Experimental Therapy of HER-2–Expressing Xenografts. Cancer Res. 2007;67:326–331. doi: 10.1158/0008-5472.CAN-06-2363. [DOI] [PubMed] [Google Scholar]
- 28.Dijkers E.C., Oude Munnink T.H., Kosterink J.G., Brouwers A.H., Jager P.L., De Jong J.R., Van Dongen G.A., Schroder C.P., Lub-de Hooge M.N., de Vries E.G. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharmacol. Ther. 2010;87:586–592. doi: 10.1038/clpt.2010.12. [DOI] [PubMed] [Google Scholar]
- 29.Dijkers E.C., Kosterink J.G., Rademaker A.P., Perk L.R., van Dongen G.A., Bart J., de Jong J.R., de Vries E.G.E., Lun-de Hooge M.N. Development and characterization of clinical-grade 89Zr-trastuzumab for HER2/neu immunoPET imaging. J. Nucl. Med. 2009;50:974–981. doi: 10.2967/jnumed.108.060392. [DOI] [PubMed] [Google Scholar]
- 30.Gebhart G., Lamberts L.E., Wimana Z., Garcia C., Emonts P., Ameye L., Stroobants S., Huizing M., Aftimos P., Tol J., et al. Molecular imaging as a tool to investigate heterogeneity of advanced HER2-positive breast cancer and to predict patient outcome under trastuzumab emtansine (T-DM1): The ZEPHIR trial. Ann. Oncol. 2016;27:619–624. doi: 10.1093/annonc/mdv577. [DOI] [PubMed] [Google Scholar]
- 31.Bensch F., Brouwers A.H., Hooge M.N.L.-D., de Jong J.R., van der Vegt B., Sleijfer S., de Vries E.G.E., Schröder C.P. 89Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:2300–2306. doi: 10.1007/s00259-018-4099-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ulaner G.A., Hyman D.M., Lyashchenko S.K., Lewis J.S., Carrasquillo J. 89Zr-trastuzumab PET/CT for detection of human epidermal growth factor receptor 2–positive metastases in patients with human epidermal growth factor receptor 2–negative primary breast cancer. Clin. Nucl. Med. 2017;42:912–917. doi: 10.1097/RLU.0000000000001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Donoghue J.A., Lewis J.S., Pandit-Taskar N., Fleming S.E., Schöder H., Larson S.M., Beylergil V., Ruan S., Lyashchenko S.K., Zanzonico P.B., et al. Pharmacokinetics, biodistribution, and radiation dosimetry for 89Zr-trastuzumab in patients with esophagogastric cancer. J. Nucl. Med. 2018;59:161–166. doi: 10.2967/jnumed.117.194555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamura K., Kurihara H., Yonemori K., Tsuda H., Suzuki J., Kono Y., Honda N., Kodaira M., Yamamoto H., Yunokawa M., et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J. Nucl. Med. 2013;54:1869–1875. doi: 10.2967/jnumed.112.118612. [DOI] [PubMed] [Google Scholar]
- 35.Mortimer J.E., Bading J.R., Park J.M., Frankel P.H., Carroll M.I., Tran T.T., Poku E.K., Rockne R.C., Raubitschek A.A., Shively J.E., et al. Tumor uptake of 64Cu-DOTA-trastuzumab in patients with metastatic breast cancer. J. Nucl. Med. 2018;59:38–43. doi: 10.2967/jnumed.117.193888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kurihara H., Hamada A., Yoshida M., Shimma S., Hashimoto J., Yonemori K., Tani H., Miyakita Y., Kanayama Y., Wada Y., et al. 64Cu-DOTA-trastuzumab PET imaging and HER2 specificity of brain metastases in HER2-positive breast cancer patients. EJNMMI Res. 2015;5:8. doi: 10.1186/s13550-015-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhusari P., Vatsa R., Singh G., Parmar M., Bal A., Dhawan D.K., Mittal B.R., Shukla J. Development of Lu-177-trastuzumab for radioimmunotherapy of HER2 expressing breast cancer and its feasibility assessment in breast cancer patients. Int. J. Cancer. 2017;140:938–947. doi: 10.1002/ijc.30500. [DOI] [PubMed] [Google Scholar]
- 38.Ulaner G.A., Lyashchenko S.K., Riedl C., Ruan S., Zanzonico P.B., Lake D., Jhaveri K., Zeglis B., Lewis J.S., O’Donoghue J.A. First-in-human human epidermal growth factor receptor 2–targeted imaging using 89Zr-Pertuzumab PET/CT: Dosimetry and clinical application in patients with breast cancer. J. Nucl. Med. 2018;59:900–906. doi: 10.2967/jnumed.117.202010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ulaner G.A., Carrasquillo J.A., Riedl C.C., Yeh R., Hatzoglou V., Ross D.S., Jhaveri K., Chandarlapaty S., Hyman D.M., Zeglis B.M., et al. Identification of HER2-positive metastases in patients with HER2-negative primary breast cancer by using HER2-targeted 89Zr-pertuzumab PET/CT. Radiology. 2020;296:370–378. doi: 10.1148/radiol.2020192828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huizing F.J., Garousi J., Lok J., Franssen G., Hoeben B.A.W., Frejd F.Y., Boerman O.C., Bussink J., Tolmachev V., Heskamp S. CAIX-targeting radiotracers for hypoxia imaging in head and neck cancer models. Sci. Rep. 2019;9:18898. doi: 10.1038/s41598-019-54824-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oosterwijk-Wakka J.C., Boerman O.C., Mulders P.F.A., Oosterwijk E. Application of monoclonal antibody G250 recognizing carbonic anhydrase IX in renal cell carcinoma. Int. J. Mol. Sci. 2013;14:11402–11423. doi: 10.3390/ijms140611402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verhoeff S.R., van Es S.C., Boon E., van Helden E., Angus L., Elias S.G., Oosting S.F., Aarntzen E.H., Brouwers A.H., Kwee T.C., et al. Lesion detection by [89Zr] Zr-DFO-girentuximab and [18F] FDG-PET/CT in patients with newly diagnosed metastatic renal cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:1931–1939. doi: 10.1007/s00259-019-04358-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merkx R.I.J., Lobeek D., Konijnenberg M., Jiménez-Franco L.D., Kluge A., Oosterwijk E., Mulders P.F., Rijpkema M. Phase I study to assess safety, biodistribution and radiation dosimetry for 89Zr-girentuximab in patients with renal cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:3277–3285. doi: 10.1007/s00259-021-05271-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hekman M.C.H., Rijpkema M., Aarntzen E.H., Mulder S.F., Langenhuijsen J.F., Oosterwijk E., Boerman O.C., Oyen W.J.G., Mulders P.F.A. Positron emission tomography/computed tomography with 89Zr-girentuximab can aid in diagnostic dilemmas of clear cell renal cell carcinoma suspicion. Eur. Urol. 2018;74:257–260. doi: 10.1016/j.eururo.2018.04.026. [DOI] [PubMed] [Google Scholar]
- 45.Divgi C.R., Uzzo R.G., Gatsonis C., Bartz R., Treutner S., Yu J.Q., Chen D., Carrasquillo J., Larson S., Bevan P., et al. Positron emission tomography/computed tomography identification of clear cell renal cell carcinoma: Results from the REDECT trial. J. Clin. Oncol. 2013;31:187. doi: 10.1200/JCO.2011.41.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carmon K.S., Azhdarinia A. Application of Immuno-PET in antibody–drug conjugate development. Mol. Imaging. 2018;17:1536012118801223. doi: 10.1177/1536012118801223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conte M., Frantellizzi V., Matto A., De Vincentis G. New insight and future perspective of mesothelin-targeted agents in nuclear medicine. Clin. Transl. Imaging. 2020;8:265–278. doi: 10.1007/s40336-020-00379-9. [DOI] [Google Scholar]
- 48.Shin I.S., Lee S.-M., Kim H.S., Yao Z., Regino C., Sato N., Cheng K.T., Hassan R., Campo M.F., Albone E.F., et al. Effect of chelator conjugation level and injection dose on tumor and organ uptake of 111In-labeled MORAb-009, an anti-mesothelin antibody. Nucl. Med. Biol. 2011;38:1119–1127. doi: 10.1016/j.nucmedbio.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Montemagno C., Cassim S., Trichanh D., Savary C., Pouyssegur J., Fagret D., Broisat A., Ghezzi C. 99mTc-A1 as a novel imaging agent targeting mesothelin-expressing pancreatic ductal adenocarcinoma. Cancers. 2019;11:1531. doi: 10.3390/cancers11101531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lamberts L.E., Menke-van der Houven C.W., ter Weele E.J., Bensch F., Smeenk M.M., Voortman J., Hoekstra O.S., Williams S.P., Fine B.M., Maslyar D., et al. ImmunoPET with anti-mesothelin antibody in patients with pancreatic and ovarian cancer before anti-mesothelin antibody–drug conjugate treatment. Clin. Cancer Res. 2016;22:1642–1652. doi: 10.1158/1078-0432.CCR-15-1272. [DOI] [PubMed] [Google Scholar]
- 51.Davis A.A., Patel V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer. 2019;7:278. doi: 10.1186/s40425-019-0768-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heskamp S., Hobo W., Molkenboer-Kuenen J.D., Olive D., Oyen W.J., Dolstra H., Boerman O.C. Noninvasive imaging of tumor PD-L1 expression using radiolabeled anti–PD-L1 antibodies. Cancer Res. 2015;75:2928–2936. doi: 10.1158/0008-5472.CAN-14-3477. [DOI] [PubMed] [Google Scholar]
- 53.Chatterjee S., Lesniak W.G., Gabrielson M., Lisok A., Wharram B., Sysa-Shah P., Azad B.B., Pomper M.G., Nimmagadda S. A humanized antibody for imaging immune checkpoint ligand PD-L1 expression in tumors. Oncotarget. 2016;7:10215. doi: 10.18632/oncotarget.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hettich M., Braun F., Bartholomä M.D., Schirmbeck R., Niedermann G. High-resolution PET imaging with therapeutic antibody-based PD-1/PD-L1 checkpoint tracers. Theranostics. 2016;6:1629. doi: 10.7150/thno.15253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lesniak W.G., Chatterjee S., Gabrielson M., Lisok A., Wharram B., Pomper M.G., Nimmagadda S. PD-L1 detection in tumors using [64Cu] atezolizumab with PET. Bioconjugate Chem. 2016;27:2103–2110. doi: 10.1021/acs.bioconjchem.6b00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.England C.G., Ehlerding E.B., Hernandez R., Rekoske B.T., Graves S.A., Sun H., Liu G., McNeel D.G., Barnhart T.E., Cai W. Preclinical pharmacokinetics and biodistribution studies of 89Zr-labeled pembrolizumab. J. Nucl. Med. 2017;58:162–168. doi: 10.2967/jnumed.116.177857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.England C.G., Jiang D., Ehlerding E.B., Rekoske B.T., Ellison P.A., Hernandez R., Barnhart T.E., McNeel D.G., Huang P., Cai W. 89Zr-labeled nivolumab for imaging of T-cell infiltration in a humanized murine model of lung cancer. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:110–120. doi: 10.1007/s00259-017-3803-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Veen E.L., Giesen D., Pot-de Jong L., Jorritsma-Smit A., De Vries E.G., Lub-de Hooge M.N. 89Zr-pembrolizumab biodistribution is influenced by PD-1-mediated uptake in lymphoid organs. J. Immunother. Cancer. 2020;8:e000938. doi: 10.1136/jitc-2020-000938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Broos K., Lecocq Q., Raes G., Devoogdt N., Keyaerts M., Breckpot K. Noninvasive imaging of the PD-1: PD-L1 immune checkpoint: Embracing nuclear medicine for the benefit of personalized immunotherapy. Theranostics. 2018;8:3559. doi: 10.7150/thno.24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bensch F., Van der veen E.L., Lub-de Hooge M.N., Jorritsma-Smit A., Boellaard R., Kok I.C., Oosting S.F., Schröder C.P., Hiltermann T.J.N., Van Der Wekken A.J., et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 2018;24:1852–1858. doi: 10.1038/s41591-018-0255-8. [DOI] [PubMed] [Google Scholar]
- 61.Niemeijer A.N., Leung D., Huisman M.C., Bahce I., Hoekstra O.S., van Dongen G.A.M.S., Boellaard R., Du S., Hayes W., Smith R., et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat. Commun. 2018;9:4664. doi: 10.1038/s41467-018-07131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gill M., Falzone N., Du Y., Vallis A.K. Targeted radionuclide therapy in combined-modality regimens. Lancet Oncol. 2017;18:e414–e423. doi: 10.1016/S1470-2045(17)30379-0. [DOI] [PubMed] [Google Scholar]
- 63.Pouget J.-P., Navarro-Teulon I., Bardies M., Chouin N., Cartron G., Pèlegrin A., Azria D. Clinical radioimmunotherapy—the role of radiobiology. Nat. Rev. Clin. Oncol. 2011;8:720–734. doi: 10.1038/nrclinonc.2011.160. [DOI] [PubMed] [Google Scholar]
- 64.Larson S.M., Carrasquillo J.A., Cheung N.-K.V., Press O.W. Radioimmunotherapy of human tumours. Nat. Rev. Cancer. 2015;15:347–360. doi: 10.1038/nrc3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sgouros G. Alpha-particles for targeted therapy. Adv. Drug Deliv. Rev. 2008;60:1402–1406. doi: 10.1016/j.addr.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 66.Huang G. Nuclear Medicine in Oncology. Springer; Singapore: 2019. [Google Scholar]
- 67.Wiseman G.A., Gordon L.I., Multani P.S., Witzig T.E., Spies S., Bartlett N.L., Schilder R.J., Murray J.L., Saleh M., Allen R.S., et al. Ibritumomab tiuxetan radioimmunotherapy for patients with relapsed or refractory non-Hodgkin lymphoma and mild thrombocytopenia: A phase II multicenter trial. Blood J. Am. Soc. Hematol. 2002;99:4336–4342. doi: 10.1182/blood.V99.12.4336. [DOI] [PubMed] [Google Scholar]
- 68.Witzig T.E., Flinn I.W., Gordon L.I., Emmanouilides C., Czuczman M.S., Saleh M.N., Cripe L., Wiseman G., Olejnik T., Multani P.S., et al. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin’s lymphoma. J. Clin. Oncol. 2002;20:3262–3269. doi: 10.1200/JCO.2002.11.017. [DOI] [PubMed] [Google Scholar]
- 69.Witzig T.E., Molina A., Gordon L., Emmanouilides C., Schilder R.J., Flinn I., Darif M., Macklis R., Vo K., Wiseman G.A. Long-term responses in patients with recurring or refractory B-cell non-Hodgkin lymphoma treated with yttrium 90 ibritumomab tiuxetan. Cancer. 2007;109:1804–1810. doi: 10.1002/cncr.22617. [DOI] [PubMed] [Google Scholar]
- 70.Shimoni A., Avivi I., Rowe J.M., Yeshurun M., Levi I., Or R., Patachenko P., Avigdor A., Zwas T., Nagler A. A randomized study comparing yttrium-90 ibritumomab tiuxetan (Zevalin) and high-dose BEAM chemotherapy versus BEAM alone as the conditioning regimen before autologous stem cell transplantation in patients with aggressive lymphoma. Cancer. 2012;118:4706–4714. doi: 10.1002/cncr.27418. [DOI] [PubMed] [Google Scholar]
- 71.Morschhauser F., Radford J., Van Hoof A., Botto B., Rohatiner A.Z.S., Salles G., Soubeyran P.-L., Tilly H., Bischof-Delaloye A., Van Putten W.L.J., et al. 90Yttrium-ibritumomab tiuxetan consolidation of first remission in advanced-stage follicular non-Hodgkin lymphoma: Updated results after a median follow-up of 7.3 years from the international, randomized, phase III first-line indolent trial. J. Clin. Oncol. 2013;31:1977–1983. doi: 10.1200/JCO.2012.45.6400. [DOI] [PubMed] [Google Scholar]
- 72.Morschhauser F., Radford J., Van Hoof A., Vitolo U., Soubeyran P.-L., Tilly H., Huijgens P.C., Kolstad A., D’Amore F., Diaz M.G., et al. Phase III trial of consolidation therapy with yttrium-90–ibritumomab tiuxetan compared with no additional therapy after first remission in advanced follicular lymphoma. J. Clin. Oncol. 2008;26:5156–5164. doi: 10.1200/JCO.2008.17.2015. [DOI] [PubMed] [Google Scholar]
- 73.Hainsworth J.D., Spigel D.R., Markus T.M., Shipley D., Thompson D., Rotman R., Dannaher C., Greco F.A. Rituximab plus short-duration chemotherapy followed by Yttrium-90 Ibritumomab tiuxetan as first-line treatment for patients with follicular non-Hodgkin lymphoma: A phase II trial of the Sarah Cannon Oncology Research Consortium. Clin. Lymphoma Myeloma. 2009;9:223–228. doi: 10.3816/CLM.2009.n.044. [DOI] [PubMed] [Google Scholar]
- 74.Jacobs S.A., Swerdlow S.H., Kant J., Foon K.A., Jankowitz R., Land S.R., DeMonaco N., Joyce J., Osborn J.L., Evans T.L., et al. Phase II trial of short-course CHOP-R followed by 90Y-ibritumomab tiuxetan and extended rituximab in previously untreated follicular lymphoma. Clin. Cancer Res. 2008;14:7088–7094. doi: 10.1158/1078-0432.CCR-08-0529. [DOI] [PubMed] [Google Scholar]
- 75.Provencio M., Cruz Mora M.Á., Gómez-Codina J., Quero Blanco C., Llanos M., García-Arroyo F.R., de la Cruz L., Gumá J., Delgado J.R., Álvarez R., et al. Consolidation treatment with Yttrium-90 ibritumomab tiuxetan after new induction regimen in patients with intermediate-and high-risk follicular lymphoma according to the follicular lymphoma international prognostic index: A multicenter, prospective phase II trial of the Spanish Lymphoma Oncology Group. Leuk. Lymphoma. 2014;55:51–55. doi: 10.3109/10428194.2013.790544. [DOI] [PubMed] [Google Scholar]
- 76.Fisher R.I., Kaminski M.S., Wahl R.L., Knox S.J., Zelenetz A.D., Vose J.M., Leonard J.P., Kroll S., Goldsmith S.J., Coleman M. Tositumomab and iodine-131 tositumomab produces durable complete remissions in a subset of heavily pretreated patients with low-grade and transformed non-Hodgkin’s lymphomas. J. Clin. Oncol. 2005;23:7565–7573. doi: 10.1200/JCO.2004.00.9217. [DOI] [PubMed] [Google Scholar]
- 77.Link B.K., Martin P., Kaminski M.S., Goldsmith S.J., Coleman M., Leonard J.P. Cyclophosphamide, vincristine, and prednisone followed by tositumomab and Iodine-131–tositumomab in patients with untreated low-grade follicular lymphoma: Eight-year follow-up of a multicenter phase II study. J. Clin. Oncol. 2010;28:3035–3041. doi: 10.1200/JCO.2009.27.8325. [DOI] [PubMed] [Google Scholar]
- 78.Vose J.M., Carter S., Burns L.J., Ayala E., Press O.W., Moskowitz C.H., Stadtmauer E.A., Mineshi S., Ambinder R., Fenske T., et al. Phase III randomized study of rituximab/carmustine, etoposide, cytarabine, and melphalan (BEAM) compared with iodine-131 tositumomab/BEAM with autologous hematopoietic cell transplantation for relapsed diffuse large B-cell lymphoma: Results from the BMT CTN 0401 trial. J. Clin. Oncol. 2013;31:1662. doi: 10.1200/JCO.2012.45.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rose A.C., Shenoy P.J., Garrett G., Seward M., Kucuk R.A., Doksansky H., Nastoupil L.J., Flowers C.R. A systematic literature review and meta-analysis of radioimmunotherapy consolidation for patients with untreated follicular lymphoma. Clin. Lymphoma Myeloma Leuk. 2012;12:393–399. doi: 10.1016/j.clml.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 80.Chen S., Yu L., Jiang C., Zhao Y., Sun D., Li S., Liao G., Chen Y., Fu Q., Tao Q., et al. Pivotal study of iodine-131–labeled chimeric tumor necrosis treatment radioimmunotherapy in patients with advanced lung cancer. J. Clin. Oncol. 2005;23:1538–1547. doi: 10.1200/JCO.2005.06.108. [DOI] [PubMed] [Google Scholar]
- 81.Steel G. Cell loss as a factor in the growth rate of human tumours. Eur. J. Cancer. 1967;3:381–387. doi: 10.1016/0014-2964(67)90022-9. [DOI] [PubMed] [Google Scholar]
- 82.Yu L., Ju D.W., Chen W., Li T., Xu Z., Jiang C., Chen S., Tao Q., Ye D., Hu P., et al. 131I-chTNT radioimmunotherapy of 43 patients with advanced lung cancer. Cancer Biother. Radiopharm. 2006;21:5–14. doi: 10.1089/cbr.2006.21.5. [DOI] [PubMed] [Google Scholar]
- 83.Xiong L., Edwards C.K., Zhou L. The biological function and clinical utilization of CD147 in human diseases: A review of the current scientific literature. Int. J. Mol. Sci. 2014;15:17411–17441. doi: 10.3390/ijms151017411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.He Q., Lu W.-S., Liu Y., Guan Y.-S., Kuang A.-R. 131I-labeled metuximab combined with chemoembolization for unresectable hepatocellular carcinoma. World J. Gastroenterol. 2013;19:9104. doi: 10.3748/wjg.v19.i47.9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu L., Yang Y.-F., Ge N.-J., Shen S.-Q., Liang J., Wang Y., Zhou W.-P., Shen F., Wu M.-C. Hepatic Arterial Iodine-131–Labeled Metuximab Injection Combined with Chemoembolization for Unresectable Hepatocellular Carcinoma: Interim Safety and Survival Data from 110 Patients. Cancer Biother. Radiopharm. 2010;25:657–663. doi: 10.1089/cbr.2010.0801. [DOI] [PubMed] [Google Scholar]
- 86.Zhu Z.-X., Liao M.-H., Wang X.-X., Huang J.-W. Transcatheter arterial chemoembolization plus 131I-labelled metuximab versus transcatheter arterial chemoembolization alone in intermediate/advanced stage hepatocellular carcinoma: A systematic review and meta-analysis. Korean J. Radiol. 2016;17:882–892. doi: 10.3348/kjr.2016.17.6.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bian H., Zheng J.-S., Nan G., Li R., Chen C., Hu C.-X., Zhang Y., Sun B., Wang X.-L., Cui S.-C., et al. Randomized trial of [131I] metuximab in treatment of hepatocellular carcinoma after percutaneous radiofrequency ablation. J. Natl. Cancer Inst. 2014;106:dju239. doi: 10.1093/jnci/dju239. [DOI] [PubMed] [Google Scholar]
- 88.Xu J., Shen Z.-Y., Chen X.-G., Zhang Q., Bian H.-J., Zhu P., Xu H.-Y., Song F., Yang X.-M., Mi L., et al. A randomized controlled trial of Licartin for preventing hepatoma recurrence after liver transplantation. Hepatology. 2007;45:269–276. doi: 10.1002/hep.21465. [DOI] [PubMed] [Google Scholar]
- 89.Kraeber-Bodéré F., Bodet-Milin C., Rousseau C., Eugène T., Pallardy A., Frampas E., Carlier T., Ferrer L., Gaschet J., Davodeau F., et al. Radioimmunoconjugates for the treatment of cancer. Semin. Oncol. 2014;41:613–622. doi: 10.1053/j.seminoncol.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 90.Stillebroer A.B., Boerman O.C., Desar I.M., Boers-Sonderen M.J., van Herpen C.M., Langenhuijsen J.F., Smith-Jones P.M., Oosterwijk E., Oyen W.J., Mulders P.F. Phase 1 Radioimmunotherapy Study with Lutetium 177–labeled Anti-Carbonic Anhydrase IX Monoclonal Antibody Girentuximab in Patients with Advanced Renal Cell Carcinoma. Eur. Urol. 2013;64:478–485. doi: 10.1016/j.eururo.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 91.Muselaers C.H., Boers-Sonderen M.J., van Oostenbrugge T.J., Boerman O.C., Desar I.M., Stillebroer A.B., Mulder S.F., Herpen C.M.L., Langenhuijsen J.F., Oosterwijk E., et al. Phase 2 study of lutetium 177–labeled anti–carbonic anhydrase IX monoclonal antibody girentuximab in patients with advanced renal cell carcinoma. Eur. Urol. 2016;69:767–770. doi: 10.1016/j.eururo.2015.11.033. [DOI] [PubMed] [Google Scholar]
- 92.Deb N., Goris M., Trisler K., Fowler S., Saal J., Ning S., Becker M., Marquez C., Knox S. Treatment of hormone-refractory prostate cancer with 90Y-CYT-356 monoclonal antibody. Clin. Cancer Res. 1996;2:1289–1297. [PubMed] [Google Scholar]
- 93.O’Donnell R.T., DeNardo S.J., Miers L.A., Lamborn K.R., Kukis D.L., DeNardo G.L., Meyers F.J. Combined modality radioimmunotherapy for human prostate cancer xenografts with taxanes and 90yttrium-DOTA-peptide-ChL6. Prostate. 2002;50:27–37. doi: 10.1002/pros.10029. [DOI] [PubMed] [Google Scholar]
- 94.Milowsky M.I., Nanus D.M., Kostakoglu L., Vallabhajosula S., Goldsmith S.J., Bander N.H. Phase I trial of yttrium-90—labeled anti—prostate-specific membrane antigen monoclonal antibody J591 for androgen-independent prostate cancer. J. Clin. Oncol. 2004;22:2522–2531. doi: 10.1200/JCO.2004.09.154. [DOI] [PubMed] [Google Scholar]
- 95.Bander N.H., Milowsky M.I., Nanus D.M., Kostakoglu L., Vallabhajosula S., Goldsmith S.J. Phase I trial of 177lutetium-labeled J591, a monoclonal antibody to prostate-specific membrane antigen, in patients with androgen-independent prostate cancer. J. Clin. Oncol. 2005;23:4591–4601. doi: 10.1200/JCO.2005.05.160. [DOI] [PubMed] [Google Scholar]
- 96.Tagawa S.T., Milowsky M.I., Morris M.J., Vallabhajosula S., Christos P., Akhtar N.H., Osborne J., Goldsmith S.J., Larson S., Taskar N.P., et al. Phase II study of lutetium-177–labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2013;19:5182–5191. doi: 10.1158/1078-0432.CCR-13-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tagawa S.T., Vallabhajosula S., Christos P.J., Jhanwar Y.S., Batra J.S., Lam L., Osborne J., Beltran H., Molina A.M., Goldsmith S.J., et al. Phase 1/2 study of fractionated dose lutetium-177–labeled anti–prostate-specific membrane antigen monoclonal antibody J591 (177Lu-J591) for metastatic castration-resistant prostate cancer. Cancer. 2019;125:2561–2569. doi: 10.1002/cncr.32072. [DOI] [PubMed] [Google Scholar]
- 98.Hofman M.S., Emmett L., Sandhu S., Iravani A., Joshua A.M., Goh J.C., Pattison D.A., Tan T.H., Kirkwood I.D., Ng S., et al. [177Lu] Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804. doi: 10.1016/S0140-6736(21)00237-3. [DOI] [PubMed] [Google Scholar]
- 99.Sartor O., de Bono J., Chi K.N., Fizazi K., Herrmann K., Rahbar K., Tagawa S.T., Nordquist L.T., Vaishampayan N., El-Haddad G., et al. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021;385:1091–1103. doi: 10.1056/NEJMoa2107322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sathekge M., Bruchertseifer F., Knoesen O., Reyneke F., Lawal I., Lengana T., Davis C., Mahapane J., Corbett C., Vorster M., et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: A pilot study. Eur. J. Nucl. Med. Mol. Imaging. 2019;46:129–138. doi: 10.1007/s00259-018-4167-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thomas D., Fourkala E.-O., Apostolidou S., Gunu R., Ryan A., Jacobs I., Menon U., Alderton W., Gentry-Maharaj A., Timms J.F. Evaluation of serum CEA, CYFRA21-1 and CA125 for the early detection of colorectal cancer using longitudinal preclinical samples. Br. J. Cancer. 2015;113:268–274. doi: 10.1038/bjc.2015.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liersch T., Meller J., Kulle B., Behr T.M., Markus P., Langer C., Ghadimi B.M., Wegener W.A., Kovacs J., Horak I.D., et al. Phase II trial of carcinoembryonic antigen radioimmunotherapy with 131I-labetuzumab after salvage resection of colorectal metastases in the liver: Five-year safety and efficacy results. J. Clin. Oncol. 2005;23:6763–6770. doi: 10.1200/JCO.2005.18.622. [DOI] [PubMed] [Google Scholar]
- 103.Liersch T., Meller J., Bittrich M., Kulle B., Becker H., Goldenberg D.M. Update of carcinoembryonic antigen radioimmunotherapy with 131I-labetuzumab after salvage resection of colorectal liver metastases: Comparison of outcome to a contemporaneous control group. Ann. Surg. Oncol. 2007;14:2577–2590. doi: 10.1245/s10434-006-9328-x. [DOI] [PubMed] [Google Scholar]
- 104.Sahlmann C.O., Homayounfar K., Niessner M., Dyczkowski J., Conradi L.C., Braulke F., Meller B., Beißbarth T., Michael Ghadimi B., Meller J., et al. Repeated adjuvant anti-CEA radioimmunotherapy after resection of colorectal liver metastases: Safety, feasibility, and long-term efficacy results of a prospective phase 2 study. Cancer. 2017;123:638–649. doi: 10.1002/cncr.30390. [DOI] [PubMed] [Google Scholar]
- 105.Garinchesa P., Sakamoto J., Welt S., Real F., Rettig W., Old L. Organ-specific expression of the colon cancer antigen A33, a cell surface target for antibody-based therapy. Int. J. Oncol. 1996;9:465–471. doi: 10.3892/ijo.9.3.465. [DOI] [PubMed] [Google Scholar]
- 106.Welt S., Scott A.M., Divgi C.R., Kemeny N.E., Finn R.D., Daghighian F., Germain J.S., Richards E.C., Larson S.M., Old L. J Phase I/II study of iodine 125-labeled monoclonal antibody A33 in patients with advanced colon cancer. J. Clin. Oncol. 1996;14:1787–1797. doi: 10.1200/JCO.1996.14.6.1787. [DOI] [PubMed] [Google Scholar]
- 107.Welt S., Divgi C.R., Kemeny N., Finn R.D., Scott A.M., Graham M., Germain J.S., Richards E.C., Larson S.M., Oettgen H.F., et al. Phase I/II study of iodine 131-labeled monoclonal antibody A33 in patients with advanced colon cancer. J. Clin. Oncol. 1994;12:1561–1571. doi: 10.1200/JCO.1994.12.8.1561. [DOI] [PubMed] [Google Scholar]
- 108.Welt S., Ritter G., Williams C., Cohen L.S., John M., Jungbluth A., Richards E.A., Old L.J., Kemeny N.E. Phase I study of anticolon cancer humanized antibody A33. Clin. Cancer Res. 2003;9:1338–1346. [PubMed] [Google Scholar]
- 109.Scott A.M., Lee F.-T., Jones R., Hopkins W., MacGregor D., Cebon J.S., Hannah A., Chong G., Paul U., Papenfuss A., et al. A phase I trial of humanized monoclonal antibody A33 in patients with colorectal carcinoma: Biodistribution, pharmacokinetics, and quantitative tumor uptake. Clin. Cancer Res. 2005;11:4810–4817. doi: 10.1158/1078-0432.CCR-04-2329. [DOI] [PubMed] [Google Scholar]
- 110.Chong G., Lee F.T., Hopkins W., Tebbutt N., Cebon J.S., Mountain A.J., Chappell B., Papenfuss A., Schleyer P., U P., et al. Phase I trial of 131I-huA33 in patients with advanced colorectal carcinoma. Clin. Cancer Res. 2005;11:4818–4826. doi: 10.1158/1078-0432.CCR-04-2330. [DOI] [PubMed] [Google Scholar]
- 111.Herbertson R.A., Tebbutt N.C., Lee F.-T., Gill S., Chappell B., Cavicchiolo T., Saunder T., O’Keefe G.J., Poon A., Lee S.T., et al. Targeted chemoradiation in metastatic colorectal cancer: A phase I trial of 131I-huA33 with concurrent capecitabine. J. Nucl. Med. 2014;55:534–539. doi: 10.2967/jnumed.113.132761. [DOI] [PubMed] [Google Scholar]
- 112.Meredith R.F., Khazaeli M., Plott W.E., Spencer S.A., Wheeler R.H., Brady L.W., Woo D.V., LoBuglio A.F. Initial Clinical Evaluation of Iodine-125-Labeled Chimeric 17-lA for Metastatic Colon Cancer. J. Nucl. Med. 1995;36:2229–2233. [PubMed] [Google Scholar]
- 113.Milenic D.E., Baidoo K.E., Kim Y.-S., Barkley R., Brechbiel M.W. Targeted α-particle radiation therapy of HER1-positive disseminated intraperitoneal disease: An investigation of the human anti-EGFR monoclonal antibody, panitumumab. Transl. Oncol. 2017;10:535–545. doi: 10.1016/j.tranon.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bethge W.A., Sandmaier B.M. Targeted cancer therapy using radiolabeled monoclonal antibodies. Technol. Cancer Res. Treat. 2005;4:393–405. doi: 10.1177/153303460500400407. [DOI] [PubMed] [Google Scholar]
- 115.Lau S.K., Weiss L.M., Chu P.G. Differential expression of MUC1, MUC2, and MUC5AC in carcinomas of various sites: An immunohistochemical study. Am. J. Clin. Pathol. 2004;122:61–69. doi: 10.1309/9R6673QEC06D86Y4. [DOI] [PubMed] [Google Scholar]
- 116.Epenetos A.A., Hird V., Lambert H., Mason P., Coulter C. Long term survival of patients with advanced ovarian cancer treated with intraperitoneal radioimmunotherapy. Int. J. Gynecol. Cancer. 2000;10:44–46. doi: 10.1046/j.1525-1438.2000.99510.x. [DOI] [PubMed] [Google Scholar]
- 117.Verheijen R.H., Massuger L.F., Benigno B.B., Epenetos A.A., Lopes A., Soper J.T., Markowska J., Vyzula R., Jobling T., Stamp G., et al. Phase III trial of intraperitoneal therapy with yttrium-90-labeled HMFG1 murine monoclonal antibody in patients with epithelial ovarian cancer after a surgically defined complete remission. J. Clin. Oncol. 2006;24:571–578. doi: 10.1200/JCO.2005.02.5973. [DOI] [PubMed] [Google Scholar]
- 118.Oei A.L., Verheijen R.H., Seiden M.V., Benigno B.B., Lopes A.D.B., Soper J.T., Epenetos A.A., Massuger L.F. Decreased intraperitoneal disease recurrence in epithelial ovarian cancer patients receiving intraperitoneal consolidation treatment with yttrium-90-labeled murine HMFG1 without improvement in overall survival. Int. J. Cancer. 2007;120:2710–2714. doi: 10.1002/ijc.22663. [DOI] [PubMed] [Google Scholar]
- 119.Bohdiewicz P.J., Scott G.C., Juni J.E., Fink-Bennett D., Wilner F., Nagle C., Dworkin H.J. Indium-111 OncoScint CR/OV and F-18 FDG in colorectal and ovarian carcinoma recurrences. Early observations. Clin. Nucl. Med. 1995;20:230–236. doi: 10.1097/00003072-199503000-00009. [DOI] [PubMed] [Google Scholar]
- 120.Murray J.L., Macey D.J., Kasi L.P., Rieger P., Cunningham J., Bhadkamkar V., Zhang H.-Z., Schlom J., Rosenblum M.G., Podoloff D.A. Phase II radioimmunotherapy trial with 131I-CC49 in colorectal cancer. Cancer. 1994;73:1057–1066. doi: 10.1002/1097-0142(19940201)73:3+<1057::AID-CNCR2820731345>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 121.Alvarez R.D., Partridge E.E., Khazaeli M.B., Plott G., Austin M., Kilgore L., Russell C.D., Liu T., Grizzle W.E., Schlom J., et al. Intraperitoneal radioimmunotherapy of ovarian cancer with 177Lu-CC49: A phase I/II study. Gynecol. Oncol. 1997;65:94–101. doi: 10.1006/gyno.1996.4577. [DOI] [PubMed] [Google Scholar]
- 122.Alvarez R.D., Huh W.K., Khazaeli M.B., Meredith R.F., Partridge E.E., Kilgore L.C., Grizzle E.W., Shen S., Austin J.M., Barnes M.N., et al. A phase I study of combined modality 90Yttrium-CC49 intraperitoneal radioimmunotherapy for ovarian cancer. Clin. Cancer Res. 2002;8:2806–2811. [PubMed] [Google Scholar]
- 123.Minnix M., Li L., Yazaki P.J., Miller A.D., Chea J., Poku E., Liu A., Wong J.Y.C., Rockne R.C., Colcher D., et al. TAG-72–Targeted α-Radionuclide Therapy of Ovarian Cancer Using 225Ac-Labeled DOTAylated-huCC49 Antibody. J. Nucl. Med. 2021;62:55–61. doi: 10.2967/jnumed.120.243394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tomblyn M.B., Katin M.J., Wallner P.E. The new golden era for radioimmunotherapy: Not just for lymphomas anymore. Cancer Control. 2013;20:60–71. doi: 10.1177/107327481302000109. [DOI] [PubMed] [Google Scholar]
- 125.Suh H., Pillai K., Morris D.L. Mucins in pancreatic cancer: Biological role, implications in carcinogenesis and applications in diagnosis and therapy. Am. J. Cancer Res. 2017;7:1372. [PMC free article] [PubMed] [Google Scholar]
- 126.Gulec S.A., Cohen S.J., Pennington K.L., Zuckier L.S., Hauke R.J., Horne H., Wegener W.A., Teoh N., Gold D.V., Sharkey R.M., et al. Treatment of advanced pancreatic carcinoma with 90Y-Clivatuzumab Tetraxetan: A phase I single-dose escalation trial. Clin. Cancer Res. 2011;17:4091–4100. doi: 10.1158/1078-0432.CCR-10-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Picozzi V.J., Ramanathan R.K., Lowery M.A., Ocean A.J., Mitchell E.P., O’Neil B.H., Guarino M.J., Conkling P.R., Cohen S.J., Bahary N., et al. Feasibility and results of a randomized phase Ιb study of fractionated 90Y-clivatuzumab tetraxetan in patients with metastatic pancreatic cancer having two or more prior therapies. J. Clin. Oncol. 2014;32:4026. doi: 10.1200/jco.2014.32.15_suppl.4026. [DOI] [Google Scholar]
- 128.Ocean A.J., Pennington K.L., Guarino M.J., Sheikh A., Bekaii-Saab T., Serafini A.N., Lee D., Sung M.W., Gulec S.A., Goldsmith S.J., et al. Fractionated radioimmunotherapy with 90Y-clivatuzumab tetraxetan and low-dose gemcitabine is active in advanced pancreatic cancer: A phase 1 trial. Cancer. 2012;118:5497–5506. doi: 10.1002/cncr.27592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yamaguchi K., Enjoji M., Tsuneyoshi M. Pancreatoduodenal carcinoma: A clinicopathologic study of 304 patients and immunohistochemical observation for CEA and CA19-9. J. Surg. Oncol. 1991;47:148–154. doi: 10.1002/jso.2930470303. [DOI] [PubMed] [Google Scholar]
- 130.Sultana A., Shore S., Raraty M.G., Vinjamuri S., Evans J.E., Smith C.T., Lane S., Chauhan S., Bosonnet L., Garvey C., et al. Randomised Phase I/II trial assessing the safety and efficacy of radiolabelled anti-carcinoembryonic antigen I131KAb201 antibodies given intra-arterially or intravenously in patients with unresectable pancreatic adenocarcinoma. BMC Cancer. 2009;9:66. doi: 10.1186/1471-2407-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ventimiglia J.B., Wikstrand C.J., Ostrowski L.E., Bourdon M.A., Lightner V.A., Bigner D.D. Tenascin expression in human glioma cell lines and normal tissues. J. Neuroimmunol. 1992;36:41–55. doi: 10.1016/0165-5728(92)90029-K. [DOI] [PubMed] [Google Scholar]
- 132.Colapinto E.V., Lee Y., Humphrey P.A., Zalutsky M.R., Friedman H.S., Bullard D.E., Bigner D.D. The Localisation of Radiolabeled Murine Monoclonal Antibody 81C6 and its Fab Fragment in Human Glioma Xenografts in Athymic Mice. Br. J. Neurosurg. 1988;2:179–191. doi: 10.3109/02688698808992668. [DOI] [PubMed] [Google Scholar]
- 133.Zalutsky M.R., Moseley R.P., Coakham H.B., Coleman E.R., Bigner D.D. Pharmacokinetics and tumor localization of 131I-labeled anti-tenascin monoclonal antibody 81C6 in patients with gliomas and other intracranial malignancies. Cancer Res. 1989;49:2807–2813. [PubMed] [Google Scholar]
- 134.Bigner D.D., Brown M., Coleman R.E., Friedman A.H., Friedman H.S., McLendon R.E., Bigner S.H., Zhao X.-G., Wikstrand C.J., Pegram C.N., et al. Phase I studies of treatment of malignant gliomas and neoplastic meningitis with131I-radiolabeled monoclonal antibodies anti-tenascin 81C6 and anti-chondroitin proteoglycan sulfate Me1-14 F (ab′)2-a preliminary report. J. Neuro-Oncol. 1995;24:109–122. doi: 10.1007/BF01052668. [DOI] [PubMed] [Google Scholar]
- 135.Cokgor I., Akabani G., Kuan C.-T., Friedman H.S., Friedman A.H., Coleman R.E., McLendon R.E., Bigner S.H., Zhao X.-G., Garcia-Turner A.M., et al. Phase I trial results of iodine-131–labeled antitenascin monoclonal antibody 81C6 treatment of patients with newly diagnosed malignant gliomas. J. Clin. Oncol. 2000;18:3862–3872. doi: 10.1200/JCO.2000.18.22.3862. [DOI] [PubMed] [Google Scholar]
- 136.Reardon D.A., Akabani G., Edward Coleman R., Friedman A.H., Friedman H.S., Herndon J.E., Cokgor I., McLendon R.E., Pegram C.N., Provenzale J.M., et al. Phase II trial of murine 131I-labeled antitenascin monoclonal antibody 81C6 administered into surgically created resection cavities of patients with newly diagnosed malignant gliomas. J. Clin. Oncol. 2002;20:1389–1397. doi: 10.1200/JCO.2002.20.5.1389. [DOI] [PubMed] [Google Scholar]
- 137.Akabani G., Reardon A.D., Coleman R.E., Wong T.Z., Metzler S.D., Bowsher E.J., Barboriak D.P., Provenzale J.M., Greer K.L., Delong D., et al. Dosimetry and radiographic analysis of 131I-labeled anti− tenascin 81C6 murine monoclonal antibody in newly diagnosed patients with malignant gliomas: A Phase II study. J. Nucl. Med. 2005;46:1042–1051. [PubMed] [Google Scholar]
- 138.Reardon D.A., Zalutsky M.R., Akabani G., Coleman R.E., Friedman A.H., Herndon J.E., McLendon R.E., Pegram C.N., Quinn J.A., Rich J.N., et al. A pilot study: 131I-antitenascin monoclonal antibody 81c6 to deliver a 44-Gy resection cavity boost. Neuro Oncol. 2008;10:182–189. doi: 10.1215/15228517-2007-053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reulen H.-J., Poepperl G., Goetz C., Gildehaus F.J., Schmidt M., Tatsch K., Pietsch T., Kraus T., Rachinger W. Long-term outcome of patients with WHO Grade III and IV gliomas treated by fractionated intracavitary radioimmunotherapy. J. Neurosurg. 2015;123:760–770. doi: 10.3171/2014.12.JNS142168. [DOI] [PubMed] [Google Scholar]
- 140.Riva P., Arista A., Sturiale C., Tison V., Lazzari S., Franceschi G., Spinelli A., Casi M., Sarti G., Campori F., et al. Glioblastoma therapy by direct intralesional administration of I-131 radioiodine labeled antitenascin antibodies. Cell Biophys. 1994;24:37–43. doi: 10.1007/BF02789213. [DOI] [PubMed] [Google Scholar]
- 141.Riva P., Franceschi G., Arista A., Frattarelli M., Riva N., Cremonini A.M., Giuliani G., Casi M. Local application of radiolabeled monoclonal antibodies in the treatment of high grade malignant gliomas: A six-year clinical experience. Cancer Interdiscip. Int. J. Am. Cancer Soc. 1997;80:2733–2742. doi: 10.1002/(SICI)1097-0142(19971215)80:12+<2733::AID-CNCR53>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 142.Riva P., Franceschi G., Frattarelli M., Riva N., Guiducci G., Cremonini A.M., Giuliani G., Casi M., Gentile R., Jekunen A.A., et al. 131I radioconjugated antibodies for the locoregional radioimmunotherapy of high-grade malignant glioma: Phase I and II study. Acta Oncol. 1999;38:351–359. doi: 10.1080/028418699431438. [DOI] [PubMed] [Google Scholar]
- 143.Brennan C.W., Verhaak R.G., McKenna A., Campos B., Noushmehr H., Salama S.R., Zheng S., Chakravarty D., Sanborn J.Z., Berman S.H., et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155:462–477. doi: 10.1016/j.cell.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Emrich J.G., Brady L.W., Quang T.S., Class R., Miyamoto C., Black P., Rodeck U. Radioiodinated (I-125) monoclonal antibody 425 in the treatment of high grade glioma patients: Ten-year synopsis of a novel treatment. Am. J. Clin. Oncol. 2002;25:541–546. doi: 10.1097/01.COC.0000041009.06780.E5. [DOI] [PubMed] [Google Scholar]
- 145.Li L., Quang T.S., Gracely E.J., Kim J.H., Emrich J.G., Yaeger T.E., Jenrette J.M., Cohen S.C., Black P., Brady L.W. A phase II study of anti–epidermal growth factor receptor radioimmunotherapy in the treatment of glioblastoma multiforme. J. Neurosurg. 2010;113:192–198. doi: 10.3171/2010.2.JNS091211. [DOI] [PubMed] [Google Scholar]
- 146.Casaco A., López G., García I., Rodríguez J.A., Fernández R., Figueredo J., Torres L., Pintado A.P., Batista J., Leyva R., et al. Phase I single-dose study of intracavitary-administered Nimotuzumab labeled with 188-Re in adult recurrent high-grade glioma. Cancer Biol. Ther. 2008;7:333–339. doi: 10.4161/cbt.7.3.5414. [DOI] [PubMed] [Google Scholar]
- 147.Bailly C., Vidal A., Bonnemaire C., Kraeber-Bodéré F., Chérel M., Pallardy A., Rousseau C., Garcion E., Lacoeuille F., Hindré F., et al. Potential for nuclear medicine therapy for glioblastoma treatment. Front. Pharmacol. 2019;10:772. doi: 10.3389/fphar.2019.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jovanovic D., Djurdjevic P., Andjelkovic N., Zivic L. Possible role of CD22, CD79b and CD20 expression in distinguishing small lymphocytic lymphoma from chronic lymphocytic leukemia. Contemp. Oncol. 2014;18:29–33. doi: 10.5114/wo.2013.38570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sharkey R.M., Brenner A., Burton J., Hajjar G., Toder S.P., Alavi A., Matthies A., Tsai D.E., Schuster S.J., Stadtmauer E.A., et al. Radioimmunotherapy of non-Hodgkin’s lymphoma with 90Y-DOTA humanized anti-CD22 IgG (90Y-Epratuzumab): Do tumor targeting and dosimetry predict therapeutic response? J. Nucl. Med. 2003;44:2000–2018. [PubMed] [Google Scholar]
- 150.Lindén O., Hindorf C., Cavallin-Ståhl E., Wegener W.A., Goldenberg D.M., Horne H., Ohlsson T., Stenberg L., Strand S.-E., Tennvall J. Dose-fractionated radioimmunotherapy in non-Hodgkin’s lymphoma using DOTA-conjugated, 90Y-radiolabeled, humanized anti-CD22 monoclonal antibody, epratuzumab. Clin. Cancer Res. 2005;11:5215–5222. doi: 10.1158/1078-0432.CCR-05-0172. [DOI] [PubMed] [Google Scholar]
- 151.Morschhauser F., Kraeber-Bodéré F., Wegener W.A., Harousseau J.-L., Petillon M.-O., Huglo D., Trümper L.H., Meller J., Pfreundschuh M., Kirsch C.-M., et al. High rates of durable responses with anti-CD22 fractionated radioimmunotherapy: Results of a multicenter, phase I/II study in non-Hodgkin’s lymphoma. J. Clin. Oncol. 2010;28:3709–3716. doi: 10.1200/JCO.2009.27.7863. [DOI] [PubMed] [Google Scholar]
- 152.Kraeber-Bodere F., Pallardy A., Le Gouill S., Maisonneuve H., Lamy T., Bouabdallah K., Le Gouill S., Tournilhac O., Daguindau E., Jardel H., et al. Consolidation anti-CD22 fractionated radioimmunotherapy with 90y-epratuzumab tetraxetan following R-CHOP in elderly DLBCL patients: A lysa phase II prospective trial. Blood. 2012;120:906. doi: 10.1182/blood.V120.21.906.906. [DOI] [Google Scholar]
- 153.Kraeber-Bodere F., Pallardy A., Maisonneuve H., Campion L., Moreau A., Soubeyran I., Le Gouill S., Tournilhac O., Daguindau E., Jardel H., et al. Consolidation anti-CD22 fractionated radioimmunotherapy with 90Y-epratuzumab tetraxetan following R-CHOP in elderly patients with diffuse large B-cell lymphoma: A prospective, single group, phase 2 trial. Lancet Haematol. 2017;4:e35–e45. doi: 10.1016/S2352-3026(16)30168-5. [DOI] [PubMed] [Google Scholar]
- 154.Lindén O., Bates A.T., Cunningham D., Hindorf C., Larsson E., Cleton A., Pinkert J., Huang F., Bladt F., Hennekes H., et al. 227Th-Labeled Anti-CD22 Antibody (BAY 1862864) in Relapsed/Refractory CD22-Positive Non-Hodgkin Lymphoma: A First-in-Human, Phase I Study. Cancer Biother. Radiopharm. 2021;36:672–681. doi: 10.1089/cbr.2020.4653. [DOI] [PubMed] [Google Scholar]
- 155.DeNardo S.J., DeNardo G.L., O’Grady L.F., Macey D.J., Mills S.L., Epstein A.L., Peng J.-S., McGahan J.P. Treatment of a patient with B cell lymphoma by 1-131 LYM-1 monoclonal antibodies. Int. J. Biol. Markers. 1987;2:49–53. doi: 10.1177/172460088700200107. [DOI] [PubMed] [Google Scholar]
- 156.DeNardo G.L., DeNardo S.J., Goldstein D.S., Kroger L.A., Lamborn K.R., Levy N.B., McGahan J.P., Salako Q., Shen S., Lewis J.P. Maximum-tolerated dose, toxicity, and efficacy of (131) I-Lym-1 antibody for fractionated radioimmunotherapy of non-Hodgkin’s lymphoma. J. Clin. Oncol. 1998;16:3246–3256. doi: 10.1200/JCO.1998.16.10.3246. [DOI] [PubMed] [Google Scholar]
- 157.O’Donnell R.T., Shen S., Denardo S.J., Wun T., Kukis D.L., Goldstein D.S., Denardo G.L. A phase I study of 90Y-2IT-BAD-Lym-1 in patients with non-Hodgkin’s lymphoma. Anticancer. Res. 2000;20:3647–3655. [PubMed] [Google Scholar]
- 158.Czuczman M.S., Straus D.J., Divgi C.R., Graham M., Garin-Chesa P., Finn R., Myers J., Old L.J., Larson S.M., Scheinberg D.A. Phase I dose-escalation trial of iodine 131-labeled monoclonal antibody OKB7 in patients with non-Hodgkin’s lymphoma. J. Clin. Oncol. 1993;11:2021–2029. doi: 10.1200/JCO.1993.11.10.2021. [DOI] [PubMed] [Google Scholar]
- 159.Scheinberg D.A., Straus D.J., Yeh S.D., Divgi C., Garin-Chesa P., Graham M., Pentlow K., Coit D., Oettgen H.F., Old L.J. A phase I toxicity, pharmacology, and dosimetry trial of monoclonal antibody OKB7 in patients with non-Hodgkin’s lymphoma: Effects of tumor burden and antigen expression. J. Clin. Oncol. 1990;8:792–803. doi: 10.1200/JCO.1990.8.5.792. [DOI] [PubMed] [Google Scholar]
- 160.Press O.W., Eary J.F., Badger C.C., Martin P.J., Appelbaum F.R., Levy R., Miller R., Brown S., Nelp W.B., Krohn K.A., et al. Treatment of refractory non-Hodgkin’s lymphoma with radiolabeled MB-1 (anti-CD37) antibody. J. Clin. Oncol. 1989;7:1027–1038. doi: 10.1200/JCO.1989.7.8.1027. [DOI] [PubMed] [Google Scholar]
- 161.Kaminski M.S., Fig L.M., Zasadny K.R., Koral K.F., DelRosario R.B., Francis I.R., Hanson A.C., Normolle D.P., Mudgett E., Liu C.P. Imaging, dosimetry, and radioimmunotherapy with iodine 131-labeled anti-CD37 antibody in B-cell lymphoma. J. Clin. Oncol. 1992;10:1696–1711. doi: 10.1200/JCO.1992.10.11.1696. [DOI] [PubMed] [Google Scholar]
- 162.Kolstad A., Illidge T., Bolstad N., Spetalen S., Madsbu U., Stokke C., Blakkisrud J., Løndalen A., O’Rourke N., Beasley M., et al. Phase 1/2a study of 177Lu-lilotomab satetraxetan in relapsed/refractory indolent non-Hodgkin lymphoma. Blood Adv. 2020;4:4091–4101. doi: 10.1182/bloodadvances.2020002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee K.H., Seo H.S., Sohn J.Y., Lee E., Lee H., Eom H.-S., Kong S.-Y. Aberrant expression of CD33 is associated with poor prognosis in patients with multiple myeloma and tumor progression. Cancer Res. 2016;76:3123. [Google Scholar]
- 164.Robillard N., Wuillème S., Lodé L., Magrangeas F., Minvielle S., Avet-Loiseau H. CD33 is expressed on plasma cells of a significant number of myeloma patients, and may represent a therapeutic target. Leukemia. 2005;19:2021–2022. doi: 10.1038/sj.leu.2403948. [DOI] [PubMed] [Google Scholar]
- 165.Levy M.Y., Cicic D., Bergonio G., Berger M. Trial in progress: Phase I study of actinium-225 (225Ac)-lintuzumab in patients with refractory multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2017;17:S329–S330. doi: 10.1016/j.clml.2017.07.141. [DOI] [Google Scholar]
- 166.van de Donk N.W., Richardson P.G., Malavasi F. CD38 antibodies in multiple myeloma: Back to the future. Blood J. Am. Soc. Hematol. 2018;131:13–29. doi: 10.1182/blood-2017-06-740944. [DOI] [PubMed] [Google Scholar]
- 167.O’Steen S., Comstock M.L., Orozco J.J., Hamlin D.K., Wilbur D.S., Jones J.C., Kenoyer A., Nartea M.E., Lin Y., Miller B.W., et al. The α-emitter astatine-211 targeted to CD38 can eradicate multiple myeloma in a disseminated disease model. Blood J. Am. Soc. Hematol. 2019;134:1247–1256. doi: 10.1182/blood.2019001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dawicki W., Allen K.J., Jiao R., Malo M.E., Helal M., Berger M.S., Ludwig D.L., Dadachova E. Daratumumab-225Actinium conjugate demonstrates greatly enhanced antitumor activity against experimental multiple myeloma tumors. Oncoimmunology. 2019;8:1607673. doi: 10.1080/2162402X.2019.1607673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kapoor P., Greipp P.T., Morice W.G., Rajkumar S.V., Witzig T.E., Greipp P.R. Anti-CD20 monoclonal antibody therapy in multiple myeloma. Br. J. Haematol. 2008;141:135–148. doi: 10.1111/j.1365-2141.2008.07024.x. [DOI] [PubMed] [Google Scholar]
- 170.Dispenzieri A., D’Souza A., Gertz A.M., Laumann K., Wiseman G., Lacy M.Q., LaPlant B., Buadi F., Hayman S.R., Kumar S.K., et al. A phase 1 trial of 90Y-Zevalin radioimmunotherapy with autologous stem cell transplant for multiple myeloma. Bone Marrow Transplant. 2017;52:1372–1377. doi: 10.1038/bmt.2017.164. [DOI] [PubMed] [Google Scholar]
- 171.Gao L., Sprague K.A., Nikpoor N., Klingemann H.G., Miller K.B., Comenzo R., Klein A.K. A phase II, safety and efficacy study of fixed dose radioimmunotherapy (Zevalin, yttrium-90 ibritumomab tiuxetan) for patients with incomplete response to chemotherapy prior to autologous stem cell transplant (ASCT) for multiple myeloma. Biol. Blood Marrow Transplant. 2015;21:S199. doi: 10.1016/j.bbmt.2014.11.304. [DOI] [Google Scholar]
- 172.Yang Y., MacLeod V., Dai Y., Khotskaya-Sample Y., Shriver Z., Venkataraman G., Sasisekharan R., Naggi A., Torri G., Casu B., et al. The syndecan-1 heparan sulfate proteoglycan is a viable target for myeloma therapy. Blood J. Am. Soc. Hematol. 2007;110:2041–2048. doi: 10.1182/blood-2007-04-082495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rousseau C., Ferrer L., Supiot S., Bardies M., Davodeau F., Faivre-Chauvet A., Baumgartner P., Wijdenes J., Lacombe M., Barbet J., et al. Dosimetry results suggest feasibility of radioimmunotherapy using anti-CD138 (B-B4) antibody in multiple myeloma patients. Tumor Biol. 2012;33:679–688. doi: 10.1007/s13277-012-0362-y. [DOI] [PubMed] [Google Scholar]
- 174.Thomas M.L. The leukocyte common antigen family. Annu. Rev. Immunol. 1989;7:339–369. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- 175.Matthews D.C., Appelbaum F.R., Eary J.F., Fisher D.R., Durack L.D., Hui T.E., Martin P.J., Mitchell D., Press O.W., Storb R., et al. Phase I study of 131I-anti-CD45 antibody plus cyclophosphamide and total body irradiation for advanced acute leukemia and myelodysplastic syndrome. Blood J. Am. Soc. Hematol. 1999;94:1237–1247. [PubMed] [Google Scholar]
- 176.Kotzerke J., Bunjes D., Scheinberg A.D. Radioimmunoconjugates in acute leukemia treatment: The future is radiant. Bone Marrow Transplant. 2005;36:1021–1026. doi: 10.1038/sj.bmt.1705182. [DOI] [PubMed] [Google Scholar]
- 177.Pagel J.M., Appelbaum F.R., Eary J.F., Rajendran J., Fisher D.R., Gooley T., Ruffner K., Nemecek E., Sickle E., Durack L., et al. 131I–anti-CD45 antibody plus busulfan and cyclophosphamide before allogeneic hematopoietic cell transplantation for treatment of acute myeloid leukemia in first remission. Blood. 2006;107:2184–2191. doi: 10.1182/blood-2005-06-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hoyer J.D., Grogg K.L., Hanson C.A., Gamez J.D., Dogan A. CD33 detection by immunohistochemistry in paraffin-embedded tissues: A new antibody shows excellent specificity and sensitivity for cells of myelomonocytic lineage. Am. J. Clin. Pathol. 2008;129:316–323. doi: 10.1309/E36008Y2H08Q1AYY. [DOI] [PubMed] [Google Scholar]
- 179.Caron P.C., Jurcic J.G., Scott A.M., Finn R.D., Divgi C.R., Graham M.C., Jureidini I.M., Sgouros G., Tyson D., Old L.J., et al. A phase 1B trial of humanized monoclonal antibody M195 (anti-CD33) in myeloid leukemia: Specific targeting without immunogenicity. Blood. 1994;83:1760–1768. doi: 10.1182/blood.V83.7.1760.1760. [DOI] [PubMed] [Google Scholar]
- 180.Burke J.M., Caron P.C., Papadopoulos E.B., Divgi C.R., Sgouros G., Panageas K.S., Finn R.D., Larson S.M., O’Reilly R.J., Scheinberg A.D., et al. Cytoreduction with iodine-131-anti-CD33 antibodies before bone marrow transplantation for advanced myeloid leukemias. Bone Marrow Transplant. 2003;32:549–556. doi: 10.1038/sj.bmt.1704201. [DOI] [PubMed] [Google Scholar]
- 181.Mastren T. Targeted Alpha Therapy. Rare Earth Elements and Actinides: Progress in Computational Science Applications. ACS Publications; Washington, DC, USA: 2021. pp. 277–283. [Google Scholar]
- 182.Jurcic J.G., Larson S.M., Sgouros G., McDevitt M.R., Finn R.D., Divgi C.R., Ballangrud Å.M., Hamacher K.A., Ma D., Humm J.L., et al. Targeted α particle immunotherapy for myeloid leukemia. Blood. J. Am. Soc. Hematol. 2002;100:1233–1239. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.