Abstract
Background
Methotrexate (MTX) is among the most effective disease modifying anti‐rheumatic drugs (DMARDs) in rheumatoid arthritis (RA) with less toxicity and better tolerability.
Objectives
To evaluate the efficacy and toxicity of MTX monotherapy compared to MTX combination with non‐biologic DMARDs in adult with RA.
Search methods
Trials were identified in MEDLINE (1950 to 2009), EMBASE (1980 to 2009), the Cochrane Controlled trials Registry (CENTRAL) (up to 2009), the American and European scientific meeting abstracts 2005‐9, the reference lists of all relevant studies, letters, and review articles.
Selection criteria
Randomized controlled trials comparing MTX monotherapy versus MTX combined with other non‐biologic DMARDs of at least 12 weeks of trial duration in adult RA patients.
Data collection and analysis
Two reviewers independently identified eligible studies,extracted the data, and assessed the risk of bias of relevant studies.The efficacy analysis was stratified into 3 groups based on previous DMARDs use: DMARD naive, MTX inadequate response, and non‐MTX DMARDs inadequate response. The toxicity analysis was stratified by DMARD combination and pooled across trials for each combination. Our prespecified primary analysis was based on total withdrawal rates for efficacy or toxicity.
Main results
A total of 19 trials (2,025 patients) from 6,938 citations were grouped by the type of patients randomised. Trials in DMARD naive patients showed no significant advantage of the MTX combination versus monotherapy; withdrawals for lack of efficacy or toxicity were similar in both groups (risk ratio (RR) 1.16, 95% CI.0.70 to 1.93, absolute risk difference(ARD) 5%, 95%CI‐3% to 13%). Trials in MTX or non‐MTX DMARDs inadequate responder patients also showed no difference in withdrawal rates between the MTX combo versus mono groups with RR 0.86 95% CI 0.49 to1.51, ARD ‐2 %, 95% CI‐13 % to 8 % and RR 0.75 95% CI 0.41 to 1.35, ARD ‐10%, 95% CI ‐31% to 11%, respectively. Significant reductions of pain and improvement in physical function (measured by Health Assessment Questionnaire or HAQ) were found in the MTX combination group, but only in MTX‐inadequate responders (absolute risk difference ‐9.72%, 95%CI ‐14.7% to ‐4.75% for pain and mean difference (MD) ‐0.28, 95%CI ‐0.36 to ‐0.21 (0‐3) for HAQ).
Authors' conclusions
When the balance of efficacy and toxicity is taken into account, the moderate level of evidence from our systematic review showed no statistically significant advantage of the MTX combination versus monotherapy. Trials are needed that compare currently used MTX doses and combination therapies.
Keywords: Adult; Humans; Antirheumatic Agents; Antirheumatic Agents/adverse effects; Antirheumatic Agents/therapeutic use; Arthritis, Rheumatoid; Arthritis, Rheumatoid/drug therapy; Drug Therapy, Combination; Drug Therapy, Combination/methods; Methotrexate; Methotrexate/adverse effects; Methotrexate/therapeutic use; Randomized Controlled Trials as Topic
Plain language summary
Methotrexate alone versus methotrexate in combination with other medications for rheumatoid arthritis
This summary of a Cochrane review presents what we know from research about the effect of methotrexate in combination with other drugs compared to methotrexate alone for rheumatoid arthritis (RA).
What is rheumatoid arthritis and what is methotrexate?
When you have rheumatoid arthritis, your immune system, which normally fights infection, attacks the lining of your joints. This makes your joints swollen, stiff and painful. The small joints of your hands and feet are usually affected first. There is no cure for RA at present, so the treatments aim to relieve pain and stiffness and improve your ability to move. Drugs such as methotrexate also aim to help prevent permanent damage to your joints that can happen if RA is not treated.
Methotrexate is a Disease‐Modifying Anti‐Rheumatic Drug (DMARD). Methotrexate may treat rheumatoid arthritis by decreasing the activity of the immune system. Methotrexate is a common treatment for RA and may be prescribed in combination with other drugs, especially in people who are not improving on methotrexate alone. DMARDs like methotrexate come as tablets, capsules and, in some cases, injections.
What the research says
There is probably little or no difference in symptoms of RA when taking methotrexate in combination with other disease‐modifying anti‐rheumatic drugs (DMARDs) or methotrexate alone.
There may be slightly more side effects when taking methotrexate in combination with other disease‐modifying anti‐rheumatic drugs (DMARDs) than methotrexate alone. Side effects may include stomach problems, liver problems, anaemia or infection.
In people who never took DMARDs before,
16 out of 100 stopped taking methotrexate because of harmful effects or no benefit
19 out of 100 (12 to 32) stopped taking methotrexate in combination with another DMARD
In people who did not improve with methotrexate,
19 out of 100 stopped taking methotrexate because of harmful effects or no benefit
16 out of 100 (9 to 28) stopped taking methotrexate in combination with another DMARD
In people who did not improve with other DMARDs,
35 out of 100 stopped taking methotrexate because of harmful effects or no benefit
26 out of 100 (14 to 47) stopped taking methotrexate in combination with another DMARD
Overall,
9 out of 100 people stopped taking methotrexate because of side effects
14 out of 100 (10 to 18) stopped taking methotrexate in combination with another DMARD
Summary of findings
Summary of findings for the main comparison. MTX combination compared to MTX monotherapy for Rheumatoid arthritis.
| MTX combination compared to MTX monotherapy for Rheumatoid arthritis | ||||||
| Patient or population: patients with Rheumatoid arthritis Settings: Efficacy and toxicity Intervention: MTX combination Comparison: MTX monotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| MTX monotherapy | MTX combination | |||||
| ACR 50 response of DMARDs naive patients Number of responders Follow‐up: 12‐24 months | 291 per 1000 | 512 per 1000 (186 to 1000) | RR 1.76 (0.64 to 4.85) | 127 (2 studies3) | ⊕⊕⊝⊝ low1,2 | Absolute Risk Difference=19% (0%, 40%), Relative Percent Change=76% (‐36%, 385%), NNT=not statistically significant. |
| ACR 50 response of MTX inadequate responders number of responders Follow‐up: 6‐12 months | 61 per 1000 | 277 per 1000 (153 to 500) | RR 4.54 (2.51 to 8.2) | 404 (3 studies7) | ⊕⊕⊕⊝ moderate4,5,6 | Absolute Risk Difference=22% (15%, 28%), Relative Percent Change=354% (151%, 720%), NNT=5 (3,10). |
| ACR 50 response of non‐MTX DMARDs inadequate responders number of responders Follow‐up: 12‐24 months | 154 per 1000 | 259 per 1000 (145 to 460) | RR 1.68 (0.94 to 2.99) | 158 (2 studies10) | ⊕⊕⊕⊝ moderate8,9 | Absolute Risk Difference=11% (‐12%, 34%), Relative Percent Change=68% (‐6%, 199%), NNT=not statistically significant. |
| Withdrawal due to lack of efficacy or toxicity of DMARDs naive patients number of withdrawals Follow‐up: 12‐24 months | 165 per 1000 | 191 per 1000 (115 to 318) | RR 1.16 (0.7 to 1.93) | 405 (5 studies12) | ⊕⊕⊕⊝ moderate1,11 | Absolute Risk Difference=5% (‐3%, 13%), Relative Percent Change=16% (‐30%, 93%), NNT= not statistically significant. |
| Withdrawal due to lack of efficacy or toxicity of MTX inadequate responders number of withdrawals Follow‐up: 6‐12 months | 185 per 1000 | 159 per 1000 (91 to 279) | RR 0.86 (0.49 to 1.51) | 476 (3 studies14) | ⊕⊕⊕⊝ moderate5,6,13 | Absolute Risk Difference=‐2% (‐13%, 8%), Relative Percent Change=‐14% (‐51%, 51%), NNT= Not statistically significant. |
| Withdrawal due to lack of efficacy or toxicity of non‐MTX DMARDs inadequate responders number of withdrawals Follow‐up: 6‐24 months | 345 per 1000 | 259 per 1000 (141 to 466) | RR 0.75 (0.41 to 1.35) | 329 (5 studies18) | ⊕⊝⊝⊝ very low8,9,15,16,17 | Absolute Risk Difference=‐10% (‐31%, 11%), Relative Percent Change=‐25% (‐59%, 35%), NNT=Not statistically significant. |
| Withdrawal due to adverse reactions (Pooled across regimens) number of withdrawals Follow‐up: 6‐60 months | 86 per 1000 | 137 per 1000 (103 to 182) | RR 1.59 (1.2 to 2.12) | 1624 (17 studies19) | ⊕⊝⊝⊝ very low1,4,5,6,8,11,13,15,17 | Absolute Risk Difference=6% (3%, 9%), Relative Percent Change=59% (20%, 112%), NNT= 18 (10, 47). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 In Marchesoni et al 's study, patients were not blinded. 2 In O'Dell 2006' study, blinding of outcome assessors was unclear 3 The studies which provided data are Marchesoni 2003 and O'Dell 1996. 4 In Ogrendik's study, the adequacy of randomisation, allocation concealment, and blinding of outcome assessors were unclear 5 In Kremer's study, blinding of outcome assessors was unclear 6 In Lehman's study, concomitant systemic steroid was higher in MTX group (52% in MTX vs. 18% in MTX+im gold) 7 The studies which provided data are Kremer 2002, Lehman 2005 and Ogrendik 2007. 8 In Ichikawa's study, the adequacy of randomisation, allocation concealment and blinding of the outcome assessors were unclear. Co‐interventions were not similar between the two arms of the trial. 9 In Capell's study, there is no report on concomitant NSAIDS, intra‐articular or intramuscular steroid comparing between both arms. 10 The studies which provided data are Capell 2007 and Ichikawa 2005. 11 In Taschioglu' study, the adequacy of randomisation, blinding patients and physician, and the similarity of co‐interventions were not clear. 12 The studies which provided data are Dougados 1999, Haagsma 1997, Marchesoni 2003, O'Dell 2006 and Taschioglu 2003. 13 In Tugwell's study, oral prednisolone at dose of equal or less than 10 mg/d was permitted; however, there is no report on the number of patients who took prednisolone throughout the trial. 14 The studies which provided data are Kremer 2002, Lehman 2005 and Tugwell 1995. 15 In Hanyu's study, method of randomisation was unclear. It is an open‐labelled study. 16 O'Dell 1996 ' s, Lehman et al' s, Ichikawa's, and Capell's studies favoured MTX combination therapy, while others favoured MTX monotherapy 17 O'Dell 1996 is an outlier, favouring MTX combination, while others favoured MTX monotherapy 18 The studies which provided data are Capell 2007, Ferraz 1994, Hanyu 1999, Ichikawa 2005 and O'Dell 1996. 19 The studies which provided data are Capell 2007, Dougados 1999, Haagsma 1997, Islam 2000, Taschioglu 2003, Ferraz, 1994, O'Dell 1996, Wilkins 1992, Hetland 2006, Marchesoni 2003, Tugwell 1995, Kremer 2002, Lehman 2005, O'Dell 2006, Ogrendik 2007, Ichikawa, 2005 and Hanyu 1999.
Background
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease of unknown etiology. It is the most common form of inflammatory arthritis and affects approximately 0.5 to 1 % of the global population (Heath 1992). It is characterized by the inflammation of the synovial tissue, which if untreated, leads to permanent structural damage and eventual long term disability and impaired quality of life. Early diagnosis and aggressive treatment, therefore, is the fundamental strategy to suppress inflammation before patients develop irreversible damage (van der Heijde1996,Möttönen 1999,Bukhari 2003,Bykerk 2005,Makinen 2007). Diseases modifying anti‐rheumatic drugs (DMARDs) are the mainstay of treatment in RA.
Methotrexate (MTX) is among the most effective disease modifying anti‐rheumatic drugs (DMARDs) in RA with less toxicity and better tolerability. It is an analogue of folic acid and of aminopterin (4‐amino‐pteroyl glutamic acid), a folic acid antagonist. Over the last 20 years, it has been used in the treatment of rheumatoid arthritis (RA) as well as many rheumatic diseases. Many pharmacological mechanisms of MTX action have been suggested including: 1) inhibition of de novo purine synthesis , 2) promotion of adenosine release leading to inhibition of production of pro‐inflammatory cytokines [TNF‐ , interleukin‐6 (IL‐6), and IL‐8] and leukocyte accumulation , 3) Induction of activated T cell apoptosis and clonal deletion, 4) Inhibition of IL‐1 production , and 5) Reduction of IL‐6, IL‐8, soluble TNF receptor, and soluble IL‐2 receptor level However, the mechanism by which low dose MTX modulates inflammation in RA is still unclear.
Unfortunately, MTX alone may not fully control disease activity. Increasingly, MTX is used in combination with other non‐biologic DMARDs (Möttönen 1999,Goekoop YPM 2005, Goekoop YPM 2007). Many MTX and traditional DMARDs combination regimens have been studied (Dougados 1999, Marchesoni 2003,Taschioglu 2003), but several important questions remain unclear. What is the relative benefit and toxicity of MTX mono versus MTX combination with DMARDs? When should the combination DMARD therapy be used: initially or only after a trial of MTX monotherapy? Finally, which is the preferred combination DMARD strategy? These questions are particularly salient as formularies in many countries require the use of MTX mono and MTX combo therapies prior to reimbursing for the more expensive biologic drugs.
Objectives
To evaluate the efficacy and toxicity of MTX monotherapy compared to MTX combination with non‐biologic DMARDs in adult with RA.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) of MTX monotherapy versus MTX combined with other non‐biologic DMARDs of at least 12 weeks of trial duration (open label extensions were excluded as well as studies comparing DMARDs not currently used, e.g., oral gold)
Types of participants
Adult RA patients with age of equal or older than 18 years old
Types of interventions
Intervention group: MTX combined with other non‐biologic DMARDs Control group: MTX alone or MTX plus placebo
Types of outcome measures
1) Efficacy
Outcome measures included in the Outcome Measures in Rheumatoid Arthritis Clinical Trials (OMERACT) 1993 core set (OMERACT 1993) including: number of tender joints, number of swollen joints, pain (Visual Analogue Scale or VAS), patient global assessment (VAS), physician global assessment (VAS), functional status (Health Assessment Questionnaire or HAQ, Arthritis Impact Measurement Scales (AIMS), and Problem Elicitation Technique (PET), and acute phase reactant ‐ erythrocyte sedimentation rate (ESR) and C‐reactive protein (CRP)
American College of Rheumatology (ACR) core set (Felson 1993): ACR 20, 50, or 70 responses (Felson 1995 )and ACR remission (Pinals 1981)
Disease activity score (DAS) (van der Heijde 1992) and Disease activity score 28 (DAS28) (Prevoo 1995)
European League Against Rheumatism (EULAR) response (Van Gestel 1996)
2) Toxicity
Number of patients who experienced total adverse events or individual adverse events such as gastrointestinal (GI) adverse events (any adverse events except liver toxicity), hepatotoxicity (transaminitis), mucositis, haematological adverse events (anaemia, leucopenia, and/or thrombocytopenia), or infection.
3) Withdrawal These were analysed as :
Number of patients who withdrew from lack of efficacy
Number of patients who withdrew due to adverse events
Number of combined patients who withdrew from lack of efficacy or due to adverse events
Our pre‐specified major analysis was based on total withdrawal rates for efficacy or toxicity because this outcome is the simplest criterion of benefit/risk ratio for drug evaluation whether a drug is stopped for inefficacy or adverse events.
Search methods for identification of studies
We performed a search of electronic bibliographic databases including MEDLINE (1950 to 2009), EMBASE (1980 to 2009), and the Cochrane Controlled Trials Register (CENTRAL) (up to 2009) using a search strategy that combined MeSH terms, keywords, and textwords for "rheumatoid arthritis", "methotrexate", and "randomised controlled trials". We also searched the abstracts of the Annual scientific meetings of American College of Rheumatology (ACR) and European League Against Rheumatism (EULAR) from 2005 to 2009, the references lists of all relevant studies, letters, and review articles. All languages were included.
Data collection and analysis
Data abstraction and assessment of risk of bias Two reviewers (WK, JT) independently screened the titles and abstracts of the citations and retrieved relevant articles based on pre‐specified inclusion and exclusion criteria. Two reviewers (WK,VP) independently extracted the data and assessed the quality of relevant studies. If the reviewers found any discrepancy between their information, then a consensus was reached by looking at the original article and discussing with the senior reviewer. We assessed study quality using the 'risk of bias' tool suggested by the Cochrane musculoskeletal group.The following methodological domains were assessed. • Sequence generation ‐ was the method used to generate the allocation sequence appropriate to produce comparable groups? • Allocation sequence concealment ‐ was the method used to conceal the allocation sequence appropriate to prevent the allocation being known in advance of, or during, enrolment? • Blinding of participants, care providers, and outcome assessors ‐ were measures used to blind study participants, care providers, and outcome assessors from knowledge of which intervention a participant received? • Incomplete outcome data ‐ how complete were the outcome data for the primary outcomes? Were drop‐out rates and reasons for withdrawal reported? Were missing data imputed appropriately? We considered an overall completion rate of 80% or higher as a low risk of bias.If completion rates were only provided by group, a less than 80% completion rate in the treatment group was considered a high risk of bias. • Selective outcome reporting ‐ were appropriate outcomes reported and were any key outcomes missing? • Other potential threats to validity (considering external validity, e.g. relevant use of co‐interventions including non‐steroidal anti‐inflammatory drugs, corticosteroid, the similarity of the baseline characteristics that may influence the treatment response,e.g., the presence of rheumatoid factor or anti‐CCP) We explicitly judged each of these criteria using: Yes = low risk of bias; No = high risk of bias; and Unclear = either lack of information or uncertainty over the potential for bias. Data Synthesis The efficacy analysis was stratified into 3 groups based on previous DMARD use. The "DMARD naive, parallel strategy" refers to trials where patients who never received DMARDs (including MTX) were randomised to start MTX alone or MTX plus another DMARD. The "MTX inadequate response, step‐up strategy" refers to trials where patients with inadequate response to MTX were randomised to continue the use of MTX alone or to add a second DMARD. The "Non‐MTX DMARDs inadequate response, step‐up strategy" refers to trials where patients with inadequate response to DMARDs (other than MTX) were randomly switched to MTX alone or MTX plus another DMARD.
The toxicity analysis was stratified by DMARD combination and pooled across trials for each combination.
For continuous measures of efficacy, we used either end‐of‐trial data or change from baseline and pooled them as weighted mean differences (WMD) or standardized mean difference (SMD) as appropriate using a random effects model. For the categorical measures of efficacy and toxicity, the end‐of‐trial results were pooled and estimated using the relative risk (RR) with a random effects model. For efficacy, a RR greater than 1 favours MTX combination therapy: MTX combination therapy increases efficacy, while for toxicity and withdrawals, a RR greater than 1 favours MTX monotherapy: MTX combination therapy increases toxicity or withdrawal.
The heterogeneity of the trials for each pooled analysis was assessed using the Cochran's Q (or chi‐square) test and the I2 test. A value greater than 50% may indicate substantial heterogeneity.
Results
Description of studies
Our search retrieved 6,938 citations. After review of titles and abstracts and removal of duplicates across databases, 39 full‐text articles were retrieved for further evaluation, and 20 articles (from 19 studies) were retained for our analysis (Figure 1).
1.

Results of the literature search and disposition of the potentially relevant studies
* Number is not equal to the sum of number from each database due to duplication among databases
Abbreviations :‐ CENTRAL = Cochrane Central Register of Controlled Trials, ACR = American College of rheumatology, EULAR =European League Against Rheumatism, RA = Rheumatoid arthritis, MTX =Methotrexate, RCT = Randomised controlled trial
The total number of patients in the trials was 2,025. Most of the trials were 6 or 12 months in duration. The doses of MTX ranged between 5 to 20 mg/wk, but most were between 7‐15 mg/wk. MTX was administered orally in all trials. Three trial strategies were identified according to the DMARDs used before randomisation: a DMARD naive group, a MTX inadequate response group, and a non‐MTX DMARD inadequate response group.
Six trials used a parallel strategy where DMARD naive patients were started either on MTX alone or on a combination of MTX + sulfasalazine (SSZ) (Taschioglu 2003, Dougados 1999, Haagsma 1997) , MTX + cyclosporine (CSA) (Hetland 2006,Marchesoni 2003), and MTX + doxycycline (O'Dell 2006). All included early rheumatoid arthritis patients with less than 1 year of disease duration.
Five trials used a step‐up strategy in MTX inadequate responders where the patients were either continued on MTX plus placebo or a second DMARD was added : MTX + leflunomide (LEF) (Kremer 2002), MTX + CSA (Tugwell 1995), MTX+intramuscular gold (Lehman 2005), MTX + levofloxacin (Ogrendik 2007), and MTX + zolendronic acid (Jarette 2006). The criteria for MTX failure or inadequate response were different across the studies. The "inadequate response" dose of MTX in these studies ranged 7.5 to 20 mg /wk. The duration on MTX before randomisation was 1(Jarette 2006), 3 (Lehman 2005; Tugwell 1995), or 6 (Kremer 2002; Ogrendik 2007) months.
Eight trials used a step‐up strategy in non‐MTX inadequate responders (who had never received MTX before randomisation), they were randomised to MTX alone or to MTX + SSZ (Haagsma 1994, Capell 2007), MTX + azathioprine (AZA) (Willkins 1992; Willkins 1995), MTX + chloroquine (CQ) (Ferraz 1994), MTX + SSZ + hydroxychloroquine (HCQ) (O'Dell 1996), MTX + bucillamine (BUC) (Ichikawa 2005), and MTX + previous DMARDs including: gold, D‐penicillamine , BUC, and SSZ (Hanyu 1999).
The criteria for DMARD failure or inadequate response were different across the studies. Previous DMARD use was not clear in one study (Islam 2000).
Risk of bias in included studies
Figure 2 and Figure 3 provide a graphical summary of the results of risk of bias for the 20 articles from 19 studies.
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Ten studies (Haagsma 1997, Hetland 2006, O'Dell 2006, Kremer 2002, Lehman 2005, Willkins 1992, Willkins 1995, Ferraz 1994, O'Dell 1996, Capell 2007) demonstrated appropriate randomisation, adequate treatment allocation concealment, adequate blinding of intervention in both patients and care providers as well as clearly reported number and reason for withdrawal and drop out. Seven of these Haagsma 1997, Hetland 2006, Kremer 2002, Willkins 1992, Willkins 1995, Ferraz 1994, O'Dell 1996), were high quality (comparison groups had similar baseline characteristics and co‐interventions and acceptable withdrawals and drop outs), but one study (O'Dell 2006) had a high drop out rate (˜59%). In two studies (Lehman 2005, Capell 2007), there was unequal co‐interventions including steroid or NSAIDS between the treatment groups. One of these (Lehman 2005) also had high drop out rate in the MTX +placebo group due to lack of efficacy (52% vs 24% in MTX vs MTX+gold respectively).
In five studies (Dougados 1999, Tugwell 1995, Jarette 2006, Ogrendik 2007, Ichikawa 2005 ), the method of randomisation was not explicitly described; additionally co‐intervention was either unclear (Dougados 1999, Tugwell 1995, Ogrendik 2007) or higher (Jarette 2006, Ichikawa 2005) (NSAIDS or steroid) in the MTX treatment group. Blinding of outcome assessors and treatment allocation concealment were unclear in all these five studies except Tugwell et al's.
Due to their unblinded nature, five studies (Marchesoni 2003, Taschioglu 2003, Haagsma 1994, Hanyu 1999,Islam 2000) were rated lower and in three the method of randomisation was also unclear (Hanyu 1999,Islam 2000, Taschioglu 2003).
There was no selective outcome reporting or dissimilarity of baseline characteristics between groups in all included studies.
For the primary outcome, withdrawal due to either lack of efficacy or toxicity, eight out of 13 studies included in the meta‐analysis were rated well in terms of the adequacy of randomisation and treatment allocation concealment, blinding, addressing incomplete outcome data, and reporting of appropriate outcomes. Two studies (Dougados 1999, Ichikawa 2005) were rated poor because the method of randomisation and allocation concealment were unclear. In addition, co‐interventions was either unclear (Dougados 1999) or dissimilar between groups (Ichikawa 2005). Due to their unblinded method, 3 studies (Marchesoni 2003, Taschioglu 2003, Hanyu 1999) were rated lower.
Effects of interventions
See: Table 1
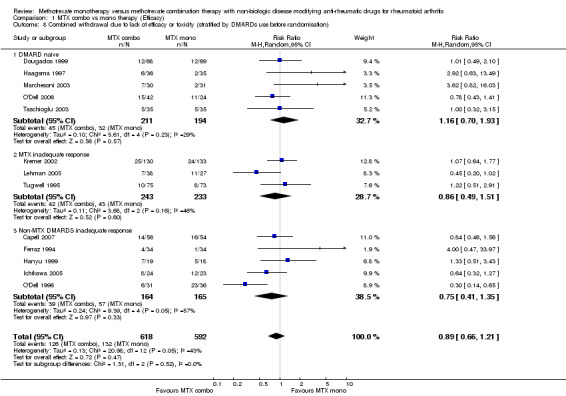
Combined withdrawal due to lack of efficacy and toxicity Our major analysis was based on withdrawals for both efficacy and safety; data were available for 13 of the 19 trials. The results showed no advantage of combination therapy over MTX monotherapy in either pooled data across all trial or subgroups [RR 0.89 (95% confidence interval (CI) 0.66 to 1.21) for all trials, RR 1.16 (95%CI 0.70 to 1.93) for DMARD naive, RR 0.86 (95% CI 0.49 to 1.51) for MTX inadequate response, and RR 0.75 (95% CI 0.41 to 1.35) for non‐MTX DMARD inadequate response]. However, there was statistically significant heterogeneity in non‐MTX DMARD inadequate response group (I2 =57.4%, Chi‐square = 9.39, df = 4, P= 0.05) with one important outlier: the combination of MTX + SSZ + HCQ showed better efficacy/ toxicity ratio over MTX alone with RR of 0.3 (95%CI 0.14 to 0.65).
Efficacy DMARD naive, Parallel design The number of patients who withdrew due to lack of efficacy was available in five of the six trials (405 patients) with combination of MTX + SSZ (Dougados 1999; Taschioglu 2003; Haagsma 1997), MTX + CSA (Marchesoni 2003), and MTX + doxycycline (O'Dell 2006), MTX combination therapy yielded less patient withdrawal than monotherapy, but it was not statistically significant [RR 0.63 (95%CI 0.34 to 1.17)].
The ACR responses were available in three of the six trials that compared MTX monotherapy to MTX combination therapy in MTX naive patients. These trials included a total of 287 patients. Combination arms were MTX + cyclosporine (CSA) (Marchesoni 2003; Hetland 2006) and MTX + doxycycline (O'Dell 2006). The only statistically significant result was for the ACR 70 response in one CSA trial (Marchesoni 2003) with RR of 2.41 (95% CI 1.07 to 5.44) favouring the MTX combination arm.
The EULAR response was available in three trials (368 patients) with combinations of MTX+ Sulfasalazine (SSZ) (Haagsma 1997; Dougados 1999) or MTX + CSA (Hetland 2006). There was no statistically significant difference between the two groups for a "good" or "moderate" EULAR response or remission.
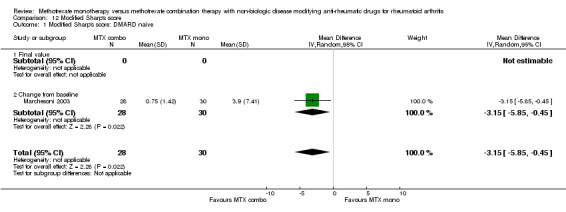
Individual continuous efficacy measures were available in two of the six studies comparing MTX alone to MTX + SSZ. There were no statistically significant differences in responses for the number of the tender joint count [WMD ‐1.7 (95% CI ‐6.11 to 2.71)] (Haagsma 1997), pain [WMD ‐1.36 (95% CI‐5.11 to 2.4)] (Taschioglu 2003) , patient global assessment [WMD 0.7 (95%CI ‐10.24 to 11.64)] (Haagsma 1997), ESR [WMD ‐1.62 (95% CI ‐6.98 to 3.74)] (Haagsma 1997; Taschioglu 2003), and CRP [SMD 0.66 (95% CI ‐2.7 to 4.1)] (Taschioglu 2003). However, the HAQ score response was slightly higher in the combination group of those 2 studies [WMD 0.1 (95% CI 0.09 to 0.11)] (Haagsma 1997; Taschioglu 2003). The radiographic outcome from one study of MTX+CSA (Marchesoni 2003) showed a small but statistically significant reduced progression in the combination therapy [WMD of Modified Sharp's score ‐3.15 (95%CI ‐5.85 to ‐0.45)]. MTX inadequate response, Step‐up design The number of patients who withdrew due to lack of efficacy was available in 3 of the 5 trials (476 patients) with combination of MTX + LEF (Kremer 2002), MTX + CSA (Tugwell 1995), and MTX+ intramuscular (im) gold (Lehman 2005) showing significantly fewer patient withdrawals than in the MTX monotherapy group with RR of 0.42 (95%CI 0.21 to 0.84).
The ACR responses were available in four of five trials (552 patients) that compared MTX monotherapy to MTX combination therapy in MTX inadequate response patients. Combination arms included MTX + leflunomide (LEF) (Kremer 2002), MTX + CSA (Tugwell 1995), MTX + im gold (Lehman 2005), and MTX + levofloxacin (Ogrendik 2007). In this group of trials, combination therapy was significantly more effective than MTX monotherapy with RR of 2.51 (95%CI 1.92 to 3.28) for ACR 20 response, RR of 4.54 (95%CI 2.51 to 8.2) for ACR 50 response, and RR of 5.59 (95%CI 2.08 to 15.01) for ACR 70 response. There was no data on ACR remission and EULAR response.
Individual continuous efficacy measures were also available in four of these five trials. The MTX combination responses were significantly greater than monotherapy for the number of the tender joint count [SMD ‐0.51 (95% CI ‐0.69 to ‐0.33)] (Kremer 2002; Tugwell 1995; Lehman 2005), the number of the swollen joint count [SMD ‐0.45 (95% C I‐0.63 to ‐0.27) ] (Lehman 2005; Kremer 2002; Tugwell 1995), pain [WMD ‐8.15 (95% CI ‐14.52 to ‐1.79)] (Kremer 2002; Tugwell 1995; Lehman 2005; Jarette 2006), patient global assessment [WMD ‐8.15 (95% CI‐14.52 to ‐1.79)] (Kremer 2002; Tugwell 1995; Lehman 2005; Jarette 2006), physician global assessment [WMD ‐10.91 (95%CI ‐18.98, ‐2.84)] (Kremer 2002; Tugwell 1995; Lehman 2005; Jarette 2006), CRP [SMD ‐12.1 (95% CI‐19.84 to ‐4.36)] (Kremer 2002), and HAQ [WMD ‐0.28 (95% CI ‐0.36 to ‐0.21)] (Kremer 2002; Lehman 2005; Tugwell 1995). However, a statistically significant difference was not found for ESR [WMD ‐0.53 (95% CI‐11.47 to 10.41)] (Tugwell 1995; Lehman 2005; Kremer 2002) . The radiographic outcome from one study 105 showed less progression in the combination of MTX+ Zolendronic acid (Jarette 2006), but it was not statistical significance [WMD of Modified Sharp's score ‐1.4 (95%CI ‐2.81 to 0.01)].
Non‐MTX DMARD inadequate response, Step‐up design The number of patients who withdrew due to lack of efficacy was available in 5 of 8 trials (329 patients) with combinations of MTX + Chloroquine (CQ) (Ferraz 1994), MTX + SSZ+ hydroxychloroquine (HCQ) (O'Dell 1996), MTX + SSZ (Capell 2007), MTX + BUC (Ichikawa 2005), and MTX + previous DMARDs (BUC, D‐penicillamine, and im gold) (Hanyu 1999). MTX combination therapy yielded significantly fewer patient withdrawals than monotherapy with RR of 0.37 (95%CI 0.16 to 0.87).
The ACR responses were available in two of eight trials (158 patients) that compared MTX monotherapy to MTX combination therapy in non‐ MTX inadequate responders. Only the pooled ACR 20 showed a statistically significant benefit for the combinations of MTX+SSZ (Capell 2007) and MTX+bucillamine(BUC) (Ichikawa 2005) over monotherapy with RR of 1.85 (95%CI 1.21 to 2.83). There was no data on ACR remission.
The EULAR response criteria was available for one of these two studies (Capell 2007) and showed no statistically significant difference between two groups. Individual continuous efficacy measures were available in five of the eight trials. The MTX combination responses were significantly greater than monotherapy for the number of the tender joint count [WMD ‐4 (95% CI ‐6.82 to ‐1.18)] (O'Dell 1996), the number of the swollen joint count [SMD ‐0.66 (95% CI ‐1.15 to ‐0.17)] (Ferraz 1994; Haagsma 1994; O'Dell 1996), patient global assessment [WMD ‐10 (95% CI ‐19.16 to ‐0.40)] (O'Dell 1996), and physician global assessment [WMD ‐10 (95% CI ‐14.8 to ‐5.2)] (O'Dell 1996). However, statistically significant difference were not found for pain [WMD ‐5.99 (95% CI ‐24.99 to13.02)] (Ferraz 1994; Haagsma 1994), ESR [WMD ‐4.29 (95% CI ‐10.72 to 2.13)] (Ferraz 1994; Haagsma 1994; O'Dell 1996; Hanyu 1999), CRP [WMD ‐1.2 (95% CI ‐2.95 to 0.55)] (Hanyu 1999), and HAQ [WMD ‐0.17 (95% CI ‐0.48 to 0.14)] (Ferraz 1994).
Toxicity The toxicity analysis was stratified and pooled by DMARD combinations.
Total adverse reactions were reported in eight of the nineteen trials (797 patients: 400 in the combination vs. 397 in the monotherapy groups). Overall, the number of adverse events was not increased in the MTX + SSZ [RR 1.13 (95%CI 0.94‐1.35)] (Haagsma 1994; Dougados 1999; Haagsma 1997; Taschioglu 2003) and MTX + LEF combinations [RR 1 (95%CI 0.94‐1.08) (Kremer 2002) versus MTX monotherapy. There was a non‐significant trend for increased adverse events in the MTX + CSA combination [RR 3.62 (95%CI 0.82‐16.30)] (Marchesoni 2003). Both the MTX + AZA (Willkins 1995) and MTX+ im gold (Lehman 2005) combinations increased the risk of total adverse events with RR of 1.67 (95%CI 1.21 to 2.3) and RR of 2.61 (95%CI 1.22 to 5.55), respectively.
Gastrointestinal adverse events Gastrointestinal (GI) related adverse events (excluding liver toxicity reported below) were available for seven trials (692 patients: 351 patients in combination vs. 341 in monotherapy groups). Both MTX + SSZ (Haagsma 1994; Haagsma 1997; Dougados 1999; Taschioglu 2003) and MTX + LEF (Kremer 2002) combination increased the risk of GI adverse events significantly (RR 1.75, 95%CI 1.14 to 2.67 for MTX + SSZ and RR 1.67, 95%CI 1.17 to 2.4 for MTX + LEF). GI adverse events were not increased in MTX + CSA [RR 4.13 (95%CI 0.49‐34.89)] (Marchesoni 2003) and MTX + intramuscular gold combinations [RR 0.71 (95%CI 0.05‐10.87)] (Lehman 2005).
Abnormal liver function was analysed in seven trials (673 patients: 336 in combination vs. 337 in monotherapy groups). MTX + LEF 101 significantly increased the risk of abnormal liver function with RR of 4.3 (95%CI 2.58 to 7.15). While MTX + SSZ (Haagsma 1994; Haagsma 1997; Dougados 1999; Taschioglu 2003), MTX + CSA (Marchesoni 2003), and MTX + BUC (Ichikawa 2005) showed a non‐statistically significant, increased risk of abnormal liver enzymes (RR 1.77, 95%CI 0.29 to 10.78 for MTX + SSZ, RR 3.1, 95%CI 0.13 to 73.19 for MTX + CSA, and RR 3, 95%CI 0.13 to 70.02 for MTX + BUC).
Mucositis was analysed in four trials (229 patients: 123 patients in combination vs. 106 in monotherapy groups). MTX + intramuscular gold increased the risk of mucositis (RR 9.33, 95%CI 0.55 to 158.98) (Lehman 2005), but it was not significant. There was no increased risk in MTX + SSZ [RR 0.62 (95%CI 0.16‐2.34)] (Haagsma 1994; Haagsma 1997; Dougados 1999; Taschioglu 2003).
Hematological adverse events were reported in six trials (415 patients: 215 in combination vs. 200 in monotherapy groups). No difference was demonstrated for the combinations of MTX+SSZ with RR of 2.36 (95%CI 0.66‐8.48) (Haagsma 1994; Haagsma 1997; Dougados 1999; Taschioglu 2003), MTX+ im gold with RR of 1.42 (95%CI 0.14‐14.89) (Lehman 2005), and MTX + BUC (Ichikawa 2005) with RR of 0.32 (95%CI 0.01‐7.48) compared with MTX monotherapy.
Infection was analysed in four trials (454 patients: 231 in combination vs. 223 in monotherapy).The risk of infection slightly increased in MTX + SSZ [RR 1.35 (95%CI 0.6‐3.04) (Haagsma 1997; Taschioglu 2003) and MTX + im gold [RR 1.6 (95%CI 0.82‐3.13)] (Lehman 2005), but it was not statistically significant. MTX + LEF did not increase the risk of infection [RR 0.79 (95%CI 0.6‐1.02) (Kremer 2002). Withdrawal due to adverse reaction In 17 of the 19 trials (1,624 patients: 824 in combination group vs. 800 monotherapy group), combination therapy resulted in more withdrawal due to adverse reactions than monotherapy (RR 1.59, 95% CI 1.2‐2.12), but the differences were statistically significant only for the MTX+CSA (Tugwell 1995; Marchesoni 2003; Hetland 2006) and MTX+AZA combinations (Willkins 1992) with RR of 1.88 (95%CI 1.02 to 3.5) and RR of 5.18 (95%CI 1.58 to 16.95), respectively.
Discussion
Despite the introduction of new biologic therapies, methotrexate alone or in combination with other traditional DMARDs remains the recommended first line therapy for most patients with RA 112. Our systematic review addressed the respective risks and benefits of monotherapy versus combination. Nineteen studies met our inclusion/exclusion criteria. Trials of DMARD combinations used different designs: "DMARD naive, parallel strategy," "MTX inadequate response, step‐up strategy," and "Non‐MTX DMARDs inadequate response, step‐up strategy". These 3 strategies were studied in the different populations (according to previous treatment prior to randomisation) and therefore answered different clinical questions.
The "DMARD naive, parallel strategy" is the only design that addresses the questions of whether a combination DMARD therapy should be used initially or only after a trial of MTX monotherapy. Only ACR70 responses showed a statistically significant improvement for the combination therapies but with increasing risk of withdrawals due to toxicity. Additionally, none of the trials that reported other composite or single outcome measures could demonstrate a benefit of an initial course of combination therapy over MTX monotherapy in DMARD naive patients over 12 to 24 months of follow‐up. Although the pooled RR of the primary outcome, withdrawal due to lack of efficacy and toxicity, showed a trend in favour of MTX monotherapy, the benefit of MTX combination therapy over monotherapy cannot be clearly addressed because the confidence interval was wide and crossed 1.
The "MTX inadequate response, step‐up strategy" included five trials where the overall combination therapy was significantly more effective than MTX monotherapy. However, when balancing between risk and benefit, the statistically significant benefit of combination therapy was not demonstrated because the confidence interval was wide and crossed 1. This design does not address the question of what is the preferred therapeutic approach when patients fail MTX monotherapy because these trials continue patients who are considered inadequate responders on the same low dose of MTX in both arms. The response of patients in MTX monotherapy arm would be expected to be less than the response of the patients in MTX combination arm. The results, therefore, showed the greater benefit of combination therapy in this group than in DMARD naive. These trials also do not reflect current practice. The dose of MTX (7‐15 mg/wk) is lower than current use, and patients randomised to the MTX monotherapy arm were kept on the same inadequate low dose of MTX. In actual practice, physicians would increase the dose of MTX or change to parenteral administration before adding another DMARD. For all of these reasons, the superiority of the combination therapies in this group of trials does not have clinical credibility. Therefore, the current evidence for patients with inadequate response to MTX is inconclusive pending results from new trials that compare maximum doses of MTX monotherapy with combination therapies. On the other hands, these studies may be useful for patients who cannot tolerate high‐dose MTX because they address the question of which approach is preferred, MTX combination or monotherapy, in patients who could not tolerate high‐dose MTX?
In the "Non‐MTX DMARDs inadequate response, step‐up strategy" 6 out of 7 studies were available for efficacy analysis. This study design answers the question of which approach is preferred, MTX combination or monotherapy, in patients who did not respond to non‐MTX DMARDs. This study design would be useful if patients failed or had inadequate response to a DMARD and then were switched to another DMARD combined with MTX vs. MTX alone. When balancing the risk and benefit, no conclusions can be reached. Although, there was a trend in favour of MTX combination therapy, the confidence interval was wide and crossed 1. In Capell's study, patients who already failed sulfasalazine 2 g/d were randomised to receive MTX alone or MTX+ the "same dose" of sulfasalazine. In fact, this study compared the efficacy of MTX in both arms and did not address this question. This study actually addressed a different clinical question that is in patients who did not achieve sufficient benefit with SSZ, what is the preferred strategy, adding MTX to SSZ or switching to MTX monotherapy. The Ichikawa's and Hanyu's study are trials of bucillamine, which is not commonly used in North America or Europe.
For toxicity analyses, GI and liver adverse events were higher in the sulfasalazine and leflunomide MTX combinations but did not lead to statistically significant differences in withdrawal rates. The total number of adverse events was higher with the gold and azathioprine MTX combinations. Withdrawal rates due to adverse reactions were higher in all the combination therapies, but the differences were statistically significant only for the combinations of MTX + cyclosporine and MTX + azathioprine.
The simplest criterion of benefit/risk ratio for drug evaluation is whether a drug is stopped for inefficacy or adverse events. This data was available for 13 of the 19 trials and therefore represents the most powerful results from our meta‐analysis. Overall, there was no benefit of MTX combination therapy over monotherapy either within the three design strategies or across all trials. However, one study of the combination MTX, SSZ, and HCQ showed better efficacy/ toxicity ratio over MTX alone.
To answer our question of which is the preferred combination DMARD strategy, our study suggests that one trial has a clear benefit/toxicity advantage: MTX + SSZ + HCQ, but this result needs to be confirmed in additional trials; also the combinations of MTX+ CSA and MTX + AZA should be avoided due to their serious toxicities. The combination of MTX with SSZ or LEF should be used cautiously due to increased GI and liver adverse events.
There are important limitations to our findings, mostly stemming from characteristics of the primary studies they are based on. There were diverse regimens of combination therapy. DMARDs have different efficacy and toxicity, and they also have drug interactions when used in combination. We intended to perform subgroup analysis stratified by regimen to demonstrate their efficacy comparing with MTX monotherapy. However, since there were too few trials comparing the same combination regimen, the benefit of specific combinations of therapy can not be addressed. Furthermore, outcome measures were inconsistently reported across the trials. Some studies reported the efficacy as a composite score. The European trials used DAS or DAS 28, while the others reported ACR response measures. Some studies‐especially those prior to 2000 ‐ reported individual variables of clinical and laboratory outcomes. Few studies reported radiographic outcomes and those that did used different methods of assessment. Lastly, the duration of follow up was also different, likely affecting the assessment and interpretation of efficacy as well as toxicity. All of this heterogeneity complicated the pooling of results across studies. In addition, most studies used lower doses of MTX than in current practice, and several studies were done with drugs that are not commonly used (bucillamine, doxycycline, levofloxacin, chloroquine, im gold, and cyclosporine). Lastly, most of the studies included in our review were short‐term trials; drawing firm risk‐benefit conclusions regarding MTX combination therapy is difficult. Nonetheless, this meta‐analysis presents useful information particularly when looking at total withdrawal rates where combination across a number of studies is possible.
Three previous systematic reviews (Verhoeven 1998; Hochberg 2001; Donahue 2008) and 2 meta‐analyses (Felson 1994; Choy 2005) compared DMARD monotherapy with combination therapy. Felson and Verhoeven studied non‐biological DMARDs with or without MTX. Hoehberg, Choy, and Douahue included both biological and non‐biological DMARDs. Felson, et al (1994) and Verhoeven, et al (1998) concluded that combination DMARD therapy does not substantially improve efficacy with an increase in toxicity. This is consistent with our overall results that include more recent trials. Hochberg, et al (2001) included only 4 studies of MTX combined with both biological and non‐biological DMARDs (cyclosporine, leflunomide, etanercept, and infliximab) in MTX inadequate responder studies and found that ACR responses improved significantly when a second DMARD was added. Choy, et al (2005) reached the same conclusion in subgroup of MTX and non‐MTX inadequate responders based on analysis of withdrawals [OR 0.51 (95%CI 0.3 to 0.82)] but added that improved efficacy is associated with an increased risk of adverse events. Donahue, et al (2008) reported only on a small subset of our included trials (mostly of SSZ combinations), and the remainder of his and Choy's data cannot be compared to our study because they included biologics as well as monotherapies with non‐biologic DMARDs other than MTX.
Authors' conclusions
Implications for practice.
When the balance of efficacy and toxicity is taken into account, the evidence from our systematic review showed no statistically significant advantage of the MTX combination versus monotherapy; only one study with the specific combination of MTX + SSZ + HCQ showed a better efficacy/ toxicity ratio over MTX alone. Adding leflunomide to MTX non‐responders improved efficacy but increased the risk of gastrointestinal adverse events and liver toxicity. Withdrawals because of toxicity varied but were most significant with the MTX+ cyclosporine and MTX+azathioprine combinations.
Implications for research.
Long term trials are needed that compare currently used MTX doses and combination therapies.
Feedback
An error in transcription, 18 November 2014
Summary
An error in transcription from an included study should be rectified, since it's the singular recommendation with implications for practice... " To answer our question of which is the preferred combination DMARD strategy, our study suggests that one trial has a clear benefit/toxicity advantage: MTX + SSZ + HCQ". The combo therapy was for 1g of SSZ daily, however reported in this review as 2g. O'Dell '96 trial was also for two years
Reply
Thanks for the comment. We have done the amendments to O'Dell 96.
Contributors
Geoff Akir.
What's new
| Date | Event | Description |
|---|---|---|
| 25 March 2015 | Feedback has been incorporated | Feedback on an error on trial description got corrected |
History
Review first published: Issue 4, 2010
| Date | Event | Description |
|---|---|---|
| 12 March 2010 | Amended | CMSG ID C200‐R |
| 25 August 2009 | Amended | Converted to new review format. |
Acknowledgements
The authors would like to thank Dr. Valalak Srinonprasert, Prof. Maxime Dougados, Prof. Loreta Carmona, Prof. Desiree van der Heijde, Prof. Maarten Boers, Asst. Prof. Vivian Bykerk, Dr. Carine Salliot, Dr. Karen Visser, Dr. Estebaliz Loza, Dr. Juan‐Antonio Martinez, and 3‐e scientific committee for their suggestions and Amy Faulkner for literature search. As well, we would like to thank Dr. Karine Toupin‐April for her assistance with the Summary of Findings table.
Appendices
Appendix 1. MEDLINE search strategy
1. exp arthritis, rheumatoid/
2. ((rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat$ or reumat$ or revmarthrit$) adj3 (arthrit$ or artrit$ or diseas$ or condition$ or nodule$)).tw.
3. (felty$ adj2 syndrome).tw.
4. (caplan$ adj2 syndrome).tw.
5. (sjogren$ adj2 syndrome).tw.
6. (sicca adj2 syndrome).tw.
7. still$ disease.tw.
8. bechterew$ disease.tw.
9. or/1‐8
10. Methotrexate/
11. Methotrexate.tw.
12. amet?opterine.tw.
13. mexate.tw.
14. Abitrexate.tw.
15. A Met?opterine.tw.
16. Antifolan.tw.
17. Emt?exate.tw.
18. Enthexate.tw.
19. Farmitrexate.tw.
20. Folex.tw.
21. Ledertrexate.tw.
22. Methoblastin.tw.
23. Methohexate.tw.
24. Methotrate.tw.
25. Methylaminopterin.tw.
26. Metotrexate.tw.
27. Mtx.tw.
28. Novatrex.tw.
29. Rheumatrex.tw.
30. or/10‐29
31. 9 and 30
32. randomized controlled trial.pt.
33. controlled clinical trial.pt.
34. randomized.ab.
35. placebo.ab.
36. drug therapy.fs.
37. randomly.ab.
38. trial.ab.
39. groups.ab.
40. or/32‐39
41. (animals not (humans and animals)).sh.
42. 40 not 41
43. 31 and 42
Appendix 2. EMBASE search strategy
1. exp arthritis, rheumatoid/
2. ((rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat$ or reumat$ or revmarthrit$) adj3 (arthrit$ or artrit$ or diseas$ or condition$ or nodule$)).tw.
3. (felty$ adj2 syndrome).tw.
4. (caplan$ adj2 syndrome).tw.
5. (sjogren$ adj2 syndrome).tw.
6. (sicca adj2 syndrome).tw.
7. still$ disease.tw.
8. or/1‐7
9. methotrexate/
10. Methotrexate.tw.
11. mexate.tw.
12. Abitrexate.tw.
13. Amet?opterine.tw.
14. mexate.tw.
15. Abitrexate.tw.
16. A Met?opterine.tw.
17. Antifolan.tw.
18. Emt?exate.tw.
19. Enthexate.tw.
20. Farmitrexate.tw.
21. Folex.tw.
22. Ledertrexate.tw.
23. Methoblastin.tw.
24. Methohexate.tw.
25. Methotrate.tw.
26. Methylaminopterin.tw.
27. Metotrexat$.tw.
28. Mtx.tw.
29. Novatrex.tw.
30. Rheumatrex.tw.
31. or/9‐30
32. (random$ or placebo$).ti,ab.
33. ((single$ or double$ or triple$ or treble$) and (blind$ or mask$)).ti,ab.
34. controlled clinical trial$.ti,ab.
35. RETRACTED ARTICLE/
36. or/32‐35
37. (animal$ not human$).sh,hw.
38. 36 not 37
39. 31 and 38
Appendix 3. Cochrane library search strategy
#1 MeSH descriptor Arthritis, Rheumatoid explode all trees
#2 ((rheumatoid or reumatoid or revmatoid or rheumatic or reumatic or revmatic or rheumat* or reumat* or revmarthrit*) near/3 (arthrit* or artrit* or diseas* or condition* or nodule*)):ti,ab
#3 felty* NEAR/2 syndrome:ti,ab
#4 caplan* NEAR/2 syndrome:ti,ab
#5 sjogren* near/2 syndrome:ti,ab
#6 sicca near/2 syndrome:ti,ab
#7 still* next disease:ti,ab
#8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7)
#9 MeSH descriptor Methotrexate explode all trees
#10 Methotrexate:ti,ab
#11 ametopterine:ti,ab
#12 mexate:ti,ab
#13 Abitrexate:ti,ab
#14 "A Met?opterine":ti,ab
#15 Antifolan:ti,ab
#16 Emtexate:ti,ab
#17 Enthexate:ti,ab
#18 Farmitrexate:ti,ab
#19 Folex:ti,ab
#20 Ledertrexate:ti,ab
#21 Methoblastin:ti,ab
#22 Methohexate:ti,ab
#23 Methotrate:ti,ab
#24 Methylaminopterin:ti,ab
#25 Metotrexate:ti,ab
#26 mtx:ti,ab
#27 Novatrex:ti,ab
#28 Rheumatrex:ti,ab
#29 (#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28)
#30 (#8 AND #29)
Data and analyses
Comparison 1. MTX combo vs mono therapy (Efficacy).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ACR response of DMARD naive | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 ACR 20 | 3 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.88, 1.68] |
| 1.2 ACR 50 | 2 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [0.64, 4.85] |
| 1.3 ACR 70 | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [1.07, 5.44] |
| 1.4 ACR remission | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.80, 2.03] |
| 2 ACR response of MTX inadequate response | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 ACR 20 | 4 | 552 | Risk Ratio (M‐H, Random, 95% CI) | 2.51 [1.92, 3.28] |
| 2.2 ACR 50 | 3 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 4.54 [2.51, 8.20] |
| 2.3 ACR 70 | 3 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 5.59 [2.08, 15.01] |
| 3 ACR response of non‐MTX DMARDs inadequate response | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 ACR 20 | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [1.21, 2.83] |
| 3.2 ACR 50 | 2 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.94, 2.99] |
| 3.3 ACR 70 | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [0.18, 20.65] |
| 4 EULAR response of DMARD naive | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Good response | 2 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.69, 1.37] |
| 4.2 Moderate response | 1 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.81, 2.33] |
| 4.3 Remission | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.84, 1.88] |
| 5 EULAR response of non‐MTX DMARDs inadequate response | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Good response | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 3.38 [0.73, 15.53] |
| 5.2 Moderate response | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Remission | 1 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 3.86 [0.45, 33.42] |
| 6 Withdrawal due to lack of efficacy (stratified by DMARDs use before randomisation) | 13 | 1210 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.32, 0.69] |
| 6.1 DMARDs naive | 5 | 405 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.34, 1.17] |
| 6.2 MTX inadequate response | 3 | 476 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.21, 0.84] |
| 6.3 Non‐MTX DMARDS inadequate response | 5 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.16, 0.87] |
| 7 Withdrawal due to lack of efficacy (by regimen) | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 MTX +SSZ | 4 | 388 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.32, 1.94] |
| 7.2 MTX +SSZ +HCQ | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.07, 0.68] |
| 7.3 MTX +CQ | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 71.15] |
| 7.4 MTX +CSA | 2 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.03, 3.05] |
| 7.5 MTX + AZA | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.6 MTX + LEF | 1 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.28, 1.35] |
| 7.7 MTX + im Gold | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.06, 0.70] |
| 7.8 MTX + Antibiotics | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.26, 1.24] |
| 7.9 MTX + Bucillamine | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.05, 0.78] |
| 7.10 MTX + Miscellaneous | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.06, 14.04] |
| 8 Combined withdrawal due to lack of efficacy or toxicity (stratified by DMARDs use before randomisation) | 13 | 1210 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.66, 1.21] |
| 8.1 DMARD naive | 5 | 405 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.70, 1.93] |
| 8.2 MTX inadequate response | 3 | 476 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.49, 1.51] |
| 8.3 Non‐MTX DMARDS inadequate response | 5 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.41, 1.35] |
| 9 Combined withdrawal due to lack of efficacy or toxicity (stratified by regimen) | 13 | 1210 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.66, 1.21] |
| 9.1 MTX + SSZ | 4 | 388 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.66, 1.53] |
| 9.2 MTX +SSZ + HCQ | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.14, 0.65] |
| 9.3 MTX + CQ | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 4.0 [0.47, 33.97] |
| 9.4 MTX + CSA | 2 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.64, 4.89] |
| 9.5 MTX + LEF | 1 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.64, 1.77] |
| 9.6 MTX + intramuscular Gold | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.20, 1.02] |
| 9.7 MTX + Antibiotics | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.43, 1.41] |
| 9.8 MTX + Bucillamine | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.32, 1.27] |
| 9.9 MTX + miscellaneous DMARDs | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.51, 3.43] |
| 10 Combined withdrawal due to lack of efficacy or toxicity at 6 months | 3 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.76, 1.78] |
| 10.1 MTX inadequate response | 2 | 411 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.71, 1.71] |
| 10.2 Non‐MTX DMARDS inadequate response | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 4.0 [0.47, 33.97] |
| 11 Combined withdrawal due to lack of efficacy or toxicity at 12 months | 7 | 581 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.49, 1.49] |
| 11.1 DMARD naive | 4 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.77, 2.61] |
| 11.2 MTX inadequate response | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.20, 1.02] |
| 11.3 Non‐MTX DMARDS inadequate response | 2 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.19, 1.42] |
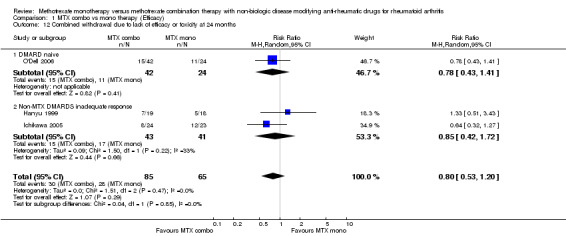
| 12 Combined withdrawal due to lack of efficacy or toxicity at 24 months | 3 | 150 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.53, 1.20] |
| 12.1 DMARD naive | 1 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.43, 1.41] |
| 12.2 Non‐MTX DMARDS inadequate response | 2 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.42, 1.72] |
1.1. Analysis.

Comparison 1 MTX combo vs mono therapy (Efficacy), Outcome 1 ACR response of DMARD naive.
1.2. Analysis.

Comparison 1 MTX combo vs mono therapy (Efficacy), Outcome 2 ACR response of MTX inadequate response.
1.3. Analysis.

Comparison 1 MTX combo vs mono therapy (Efficacy), Outcome 3 ACR response of non‐MTX DMARDs inadequate response.
1.4. Analysis.

Comparison 1 MTX combo vs mono therapy (Efficacy), Outcome 4 EULAR response of DMARD naive.
1.5. Analysis.

Comparison 1 MTX combo vs mono therapy (Efficacy), Outcome 5 EULAR response of non‐MTX DMARDs inadequate response.
1.6. Analysis.

Comparison 1 MTX combo vs mono therapy (Efficacy), Outcome 6 Withdrawal due to lack of efficacy (stratified by DMARDs use before randomisation).
1.7. Analysis.

Comparison 1 MTX combo vs mono therapy (Efficacy), Outcome 7 Withdrawal due to lack of efficacy (by regimen).
1.8. Analysis.
Comparison 1 MTX combo vs mono therapy (Efficacy), Outcome 8 Combined withdrawal due to lack of efficacy or toxicity (stratified by DMARDs use before randomisation).
1.9. Analysis.

Comparison 1 MTX combo vs mono therapy (Efficacy), Outcome 9 Combined withdrawal due to lack of efficacy or toxicity (stratified by regimen).
1.10. Analysis.

Comparison 1 MTX combo vs mono therapy (Efficacy), Outcome 10 Combined withdrawal due to lack of efficacy or toxicity at 6 months.
1.11. Analysis.

Comparison 1 MTX combo vs mono therapy (Efficacy), Outcome 11 Combined withdrawal due to lack of efficacy or toxicity at 12 months.
1.12. Analysis.
Comparison 1 MTX combo vs mono therapy (Efficacy), Outcome 12 Combined withdrawal due to lack of efficacy or toxicity at 24 months.
Comparison 2. MTX combo vs MTX monotherapy (Toxicity).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total adverse events | 8 | 797 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.99, 1.50] |
| 1.1 MTX+SSZ | 4 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.94, 1.35] |
| 1.2 MTX+LEF | 1 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.92, 1.08] |
| 1.3 MTX+CSA | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 3.62 [0.82, 16.03] |
| 1.4 MTX+AZA | 1 | 105 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [1.21, 2.30] |
| 1.5 MTX+intramuscular gold | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 2.61 [1.22, 5.55] |
| 2 GI side effects | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 MTX+SSZ | 4 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 1.75 [1.14, 2.67] |
| 2.2 MTX+LEF | 1 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [1.17, 2.40] |
| 2.3 MTX+CSA | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 4.13 [0.49, 34.89] |
| 2.4 MTX+intramuscular gold | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.05, 10.87] |
| 3 Abnormal liver function (Transaminitis) | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 MTX+SSZ | 4 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.29, 10.78] |
| 3.2 MTX+LEF | 1 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 4.30 [2.58, 7.15] |
| 3.3 MTX+CSA | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 3.10 [0.13, 73.16] |
| 3.4 MTX+Bucillamine | 1 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.13, 70.02] |
| 4 Mucositis | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 MTX+SSZ | 3 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.16, 2.34] |
| 4.2 MTX+ intramuscular gold | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 9.33 [0.55, 158.98] |
| 5 Haematological side effects | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 MTX+SSZ | 4 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 2.36 [0.66, 8.48] |
| 5.2 MTX+ intramuscular gold | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.14, 14.89] |
| 5.3 MTX+Bucillamine | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.48] |
| 6 Infection | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 MTX+SSZ | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.60, 3.04] |
| 6.2 MTX+LEF | 1 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.60, 1.02] |
| 6.3 MTX+ intramuscular gold | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.82, 3.13] |
| 7 Withdrawal due to adverse reaction (by regimen) | 17 | 1624 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.20, 2.12] |
| 7.1 MTX+SSZ | 5 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.73, 1.92] |
| 7.2 MTX+CQ | 1 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.33, 27.42] |
| 7.3 MTX+SSZ+HCQ | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.14, 1.76] |
| 7.4 MTX+AZA | 1 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 5.18 [1.58, 16.96] |
| 7.5 MTX+CSA | 3 | 369 | Risk Ratio (M‐H, Random, 95% CI) | 1.88 [1.02, 3.50] |
| 7.6 MTX+LEF | 1 | 263 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.83, 3.97] |
| 7.7 MTX+intramuscular gold | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 2.84 [0.34, 24.04] |
| 7.8 MTX+ antibiotics | 2 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 1.90 [0.48, 7.49] |
| 7.9 MTX+Bucillamine | 1 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 2.88 [0.64, 12.82] |
| 7.10 MTX+ miscellaneous DMARDS | 1 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.48, 4.22] |
| 8 Withdrawal due to adverse events (stratified by DMARDs use before randomisation) | 16 | 1582 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [1.17, 2.09] |
| 8.1 DMARD naive | 6 | 565 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [1.04, 2.83] |
| 8.2 MTX inadequate response | 4 | 552 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [1.05, 3.41] |
| 8.3 Non‐MTX inadequate response | 6 | 465 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.74, 3.18] |
2.1. Analysis.

Comparison 2 MTX combo vs MTX monotherapy (Toxicity), Outcome 1 Total adverse events.
2.2. Analysis.

Comparison 2 MTX combo vs MTX monotherapy (Toxicity), Outcome 2 GI side effects.
2.3. Analysis.

Comparison 2 MTX combo vs MTX monotherapy (Toxicity), Outcome 3 Abnormal liver function (Transaminitis).
2.4. Analysis.

Comparison 2 MTX combo vs MTX monotherapy (Toxicity), Outcome 4 Mucositis.
2.5. Analysis.

Comparison 2 MTX combo vs MTX monotherapy (Toxicity), Outcome 5 Haematological side effects.
2.6. Analysis.

Comparison 2 MTX combo vs MTX monotherapy (Toxicity), Outcome 6 Infection.
2.7. Analysis.

Comparison 2 MTX combo vs MTX monotherapy (Toxicity), Outcome 7 Withdrawal due to adverse reaction (by regimen).
2.8. Analysis.

Comparison 2 MTX combo vs MTX monotherapy (Toxicity), Outcome 8 Withdrawal due to adverse events (stratified by DMARDs use before randomisation).
Comparison 3. Swollen joint count.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Swollen joint count: MTX inadequate response | 3 | 476 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.63, ‐0.27] |
| 1.1 Final value | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Change from baseline | 3 | 476 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.63, ‐0.27] |
| 2 Swollen joint count: Non‐MTX DMARDs inadequate response | 2 | 135 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.15, ‐0.17] |
| 2.1 Final value | 2 | 135 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.15, ‐0.17] |
| 2.2 Change from baseline | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.

Comparison 3 Swollen joint count, Outcome 1 Swollen joint count: MTX inadequate response.
3.2. Analysis.

Comparison 3 Swollen joint count, Outcome 2 Swollen joint count: Non‐MTX DMARDs inadequate response.
Comparison 4. Tender joint count.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Tender joint count: DMARD naive | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐6.11, 2.71] |
| 1.1 Final value | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Change from baseline | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐1.70 [‐6.11, 2.71] |
| 2 Tender joint count: MTX inadequate response | 3 | 476 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.69, ‐0.33] |
| 2.1 Final value | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Change from baseline | 3 | 476 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐0.69, ‐0.33] |
| 3 Tender joint count: Non‐MTX DMARDs inadequate response | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐6.82, ‐1.18] |
| 3.1 Final value | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐4.0 [‐6.82, ‐1.18] |
| 3.2 Change from baseline | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.

Comparison 4 Tender joint count, Outcome 1 Tender joint count: DMARD naive.
4.2. Analysis.

Comparison 4 Tender joint count, Outcome 2 Tender joint count: MTX inadequate response.
4.3. Analysis.

Comparison 4 Tender joint count, Outcome 3 Tender joint count: Non‐MTX DMARDs inadequate response.
Comparison 5. Pain.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain: DMARD naive | 2 | 140 | Mean Difference (IV, Random, 95% CI) | ‐1.36 [‐5.12, 2.40] |
| 1.1 Final value | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐5.52, 2.46] |
| 1.2 Change from baseline | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐11.24, 11.24] |
| 2 Pain: MTX inadequate response | 4 | 515 | Mean Difference (IV, Random, 95% CI) | ‐9.72 [‐14.70, ‐4.75] |
| 2.1 Final value | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Change from baseline | 4 | 515 | Mean Difference (IV, Random, 95% CI) | ‐9.72 [‐14.70, ‐4.75] |
| 3 Pain: Non‐MTX DMARDs inadequate response | 2 | 108 | Mean Difference (IV, Random, 95% CI) | ‐5.99 [‐24.99, 13.02] |
| 3.1 Final value | 1 | 68 | Mean Difference (IV, Random, 95% CI) | 3.40 [‐9.12, 15.92] |
| 3.2 Change from baseline | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐16.0 [‐30.26, ‐1.74] |
5.1. Analysis.

Comparison 5 Pain, Outcome 1 Pain: DMARD naive.
5.2. Analysis.

Comparison 5 Pain, Outcome 2 Pain: MTX inadequate response.
5.3. Analysis.

Comparison 5 Pain, Outcome 3 Pain: Non‐MTX DMARDs inadequate response.
Comparison 6. Patient global assessment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patient global assessment: DMARD naive | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐10.24, 11.64] |
| 1.1 Final value | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Change from baseline | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.70 [‐10.24, 11.64] |
| 2 Patient global assessment: MTX inadequate response | 4 | 520 | Mean Difference (IV, Random, 95% CI) | ‐8.15 [‐14.52, ‐1.79] |
| 2.1 Final value | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Change from baseline | 4 | 520 | Mean Difference (IV, Random, 95% CI) | ‐8.15 [‐14.52, ‐1.79] |
| 3 Patient global assessment: Non‐MTX DMARDs inadequate response | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐10.0 [‐19.60, ‐0.40] |
| 3.1 Final value | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐10.0 [‐19.60, ‐0.40] |
| 3.2 Change from baseline | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.

Comparison 6 Patient global assessment, Outcome 1 Patient global assessment: DMARD naive.
6.2. Analysis.

Comparison 6 Patient global assessment, Outcome 2 Patient global assessment: MTX inadequate response.
6.3. Analysis.

Comparison 6 Patient global assessment, Outcome 3 Patient global assessment: Non‐MTX DMARDs inadequate response.
Comparison 7. Physician global assessment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Physician global asessment: MTX inadequate response | 4 | 520 | Mean Difference (IV, Random, 95% CI) | ‐10.91 [‐18.98, ‐2.84] |
| 2 Physician global assessment: Non‐MTX inadequate response | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐10.0 [‐14.80, ‐5.20] |
7.1. Analysis.

Comparison 7 Physician global assessment, Outcome 1 Physician global asessment: MTX inadequate response.
7.2. Analysis.

Comparison 7 Physician global assessment, Outcome 2 Physician global assessment: Non‐MTX inadequate response.
Comparison 8. HAQ.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HAQ: DMARD naive | 2 | 141 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.09, 0.11] |
| 1.1 Final value | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.09, 0.11] |
| 1.2 Change from baseline | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.37, 0.27] |
| 2 HAQ: MTX inadequate response | 3 | 476 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.36, ‐0.21] |
| 2.1 Final value | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Change from baseline | 3 | 476 | Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.36, ‐0.21] |
| 3 HAQ: Non‐MTX DMARDs inadequate response | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.48, 0.14] |
| 3.1 Final value | 1 | 68 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.48, 0.14] |
| 3.2 Change from baseline | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
8.1. Analysis.

Comparison 8 HAQ, Outcome 1 HAQ: DMARD naive.
8.2. Analysis.

Comparison 8 HAQ, Outcome 2 HAQ: MTX inadequate response.
8.3. Analysis.

Comparison 8 HAQ, Outcome 3 HAQ: Non‐MTX DMARDs inadequate response.
Comparison 9. ESR.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ESR: DMARD naive | 2 | 141 | Mean Difference (IV, Random, 95% CI) | ‐1.62 [‐6.98, 3.74] |
| 1.1 Final value | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐3.58, 3.12] |
| 1.2 Change from baseline | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐7.0 [‐17.72, 3.72] |
| 2 ESR: MTX inadequate response | 3 | 476 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐11.47, 10.41] |
| 2.1 Final value | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Change from baseline | 3 | 476 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐11.47, 10.41] |
| 3 ESR: Non‐MTX DMARDs inadequate response | 4 | 212 | Mean Difference (IV, Random, 95% CI) | ‐4.29 [‐10.72, 2.13] |
| 3.1 Final value | 3 | 172 | Mean Difference (IV, Random, 95% CI) | ‐2.94 [‐11.11, 5.24] |
| 3.2 Change from baseline | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐9.10 [‐19.13, 0.93] |
9.1. Analysis.

Comparison 9 ESR, Outcome 1 ESR: DMARD naive.
9.2. Analysis.

Comparison 9 ESR, Outcome 2 ESR: MTX inadequate response.
9.3. Analysis.

Comparison 9 ESR, Outcome 3 ESR: Non‐MTX DMARDs inadequate response.
Comparison 10. CRP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CRP: DMARD naive | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 0.66 [‐2.78, 4.10] |
| 1.1 Final value | 1 | 70 | Mean Difference (IV, Random, 95% CI) | 0.66 [‐2.78, 4.10] |
| 1.2 Change from baseline | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 CRP: MTX inadequate response | 1 | 263 | Mean Difference (IV, Random, 95% CI) | ‐12.1 [‐19.84, ‐4.36] |
| 2.1 Final value | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Change from baseline | 1 | 263 | Mean Difference (IV, Random, 95% CI) | ‐12.1 [‐19.84, ‐4.36] |
| 3 CRP: Non‐MTX DMARDs inadequate response | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.95, 0.55] |
| 3.1 Final value | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐2.95, 0.55] |
| 3.2 Change from baseline | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
10.1. Analysis.

Comparison 10 CRP, Outcome 1 CRP: DMARD naive.
10.2. Analysis.

Comparison 10 CRP, Outcome 2 CRP: MTX inadequate response.
10.3. Analysis.

Comparison 10 CRP, Outcome 3 CRP: Non‐MTX DMARDs inadequate response.
Comparison 11. DAS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 DAS: DMARD naive | 2 | 208 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.77, 0.12] |
| 1.1 Final value | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Change from baseline | 2 | 208 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.77, 0.12] |
| 2 DAS: Non‐MTX DMARDs inadequate response | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.3 [‐1.74, ‐0.86] |
| 2.1 Final value | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Change from baseline | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.3 [‐1.74, ‐0.86] |
11.1. Analysis.

Comparison 11 DAS, Outcome 1 DAS: DMARD naive.
11.2. Analysis.

Comparison 11 DAS, Outcome 2 DAS: Non‐MTX DMARDs inadequate response.
Comparison 12. Modified Sharp's score.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Modified Sharp's score: DMARD naive | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐3.15 [‐5.85, ‐0.45] |
| 1.1 Final value | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Change from baseline | 1 | 58 | Mean Difference (IV, Random, 95% CI) | ‐3.15 [‐5.85, ‐0.45] |
| 2 Modified Sharp's score: MTX inadequate response | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐1.4 [‐2.81, 0.01] |
| 2.1 Final value | 0 | 0 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Change from baseline | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐1.4 [‐2.81, 0.01] |
12.1. Analysis.
Comparison 12 Modified Sharp's score, Outcome 1 Modified Sharp's score: DMARD naive.
12.2. Analysis.

Comparison 12 Modified Sharp's score, Outcome 2 Modified Sharp's score: MTX inadequate response.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Capell 2007.
| Methods | Randomized controlled trial, double‐blind Design :‐ Step‐up Sample size :‐ 687 in phase I and 56 (MTX+SSZ), 55(SSZ+placebo) and 54(MTX+placebo) in phase II Trial duration:‐ 18 months | |
| Participants | RA with less than 10 years of disease duration
Active RA ( defined by DAS>2.4)
Mean age(range) :‐ 56(30‐78) yrs MTX+SSZ, 53(34‐79) yrs MTX
Feamle :‐ 75% MTX+SSZ, 79% MTX
Disease duration(range) :‐ 1(1‐9) yrs MTX+SSZ, 1(1‐9) yrs MTX
Rheumatoid factor + :‐ 68% MTX+SSZ, 65% MTX
Median dose of MTX:‐ 12.5 mg/wk MTX+SSZ, 15 mg/wk MTX Concomittant non‐steroidal anti‐inflammatory drugs and other drugs were continued. Intra‐articular or intra‐muscular steroid was permitted but not within 1 months of assessment period |
|
| Interventions | Phase I:‐ All patients received SSZ 40 mg/kg/d or 4 g/d maximum for the initial 6 months and then patients who had DAS equal or more than 2.4 were enrolled into phase II Phase II:‐ Patients were randomly assigned to one of three groups including MTX 25 mg/wk+SSZ 2g/d MTX 7.5‐25 mg/wk + placebo SSZ 40 mg/kg/d or 4 g/d maximum+placebo | |
| Outcomes | ACR, EULAR response , DAS, RAI, SJC, pain, PGA, EGA, ERS, CRP, HAQ, Sharp/vDH's score, | |
| Notes | Use data only in MTX+placebo and MTX+SSZ group in phase II at 6‐18 months Intention‐to‐treat, Last observation carried forward for missing data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were assigned to one of the three groups by an independent off‐site administrator using randomisation software". |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate, Quote: "Patients were assigned to one of the three groups by an independent off‐site administrator using randomisation software". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It was clearly stated the numbers and reasons of incomplete follow‐up in both arms and both phase I and II in Table 2 and figure 1 |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported |
| Other bias | High risk | Co‐interventions were not similar. Quote: "Concomitant non‐steroidal anti‐inflammatory drugs and other drugs were continued. Intra‐articular or intramuscular corticosteroid was permitted". However, there was no report on the use of these medications comparing between both arms |
| Blinding (patient assessed)? | Low risk | Quote: "Double‐blind three treatment groups" and "Placebo SASP at the previously achieved number of tablets by 6 months........" |
| Blinding (Physician assessed)? | Low risk | Quote: "Double‐blind three treatment groups" and "Placebo SASP at the previously achieved number of tablets by 6 months........" |
| Blinding (Outcome assessor assessed)? | Low risk | Quote: "Double‐blind three treatment groups" and "Placebo SASP at the previously achieved number of tablets by 6 months........" |
Dougados 1999.
| Methods | Randomized controlled trial, double blind Design :‐ Paralell Sample size :‐ 68 (MTX+SSZ), 69 (MTX+placebo) Trial duration:‐ 52 weeks | |
| Participants | RA with less than 1 year of disease duration Active disease (DAS>3) DMARDS and steroid naive Mean age(SEM) :‐ 52(2) yrs MTX+SSZ, 50(2) yrs MTX Feamle :‐ 77% MTX+SSZ, 74% MTX Disease duration(SEM) :‐ 10.6(1) months‐ MTX+SSZ, 18.4(5.2) months‐ MTX Rheumatoid factor + :‐ 71% MTX+SSZ, 62% MTX Concomittant treatment ‐ no data | |
| Interventions | SSZ 3 g/d+MTX 15 mg/wk vs. MTX, SSZ 3 g/d+placebo | |
| Outcomes | ACR, EULAR,TJC, SJC,Pain score, PGA,EGA, HAQ, Sharp's score | |
| Notes | Intention‐to‐treat, Last observation carried forward for missing data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not explicitly described. |
| Allocation concealment (selection bias) | Unclear risk | The method of randomisation was not explicitly described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Figure 1 presented the numbers and reasons for withdrawal in 3 groups. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported |
| Other bias | Unclear risk | Although it was stated that "Previous drug treatment for RA other than analgesics and NSAIDs was not allowed—that is, all the patients were corticosteroid and disease modifying drug naive", it is not clear regarding concomitant NSAIDS and analgesic comparing between groups during the trial. |
| Blinding (patient assessed)? | Low risk | Quote: "double blind, double dummy controlled........" |
| Blinding (Physician assessed)? | Low risk | Quote: "double blind, double dummy controlled........" |
| Blinding (Outcome assessor assessed)? | Unclear risk | We were not clear if the outcome assessor and the care provider were the same person. |
Ferraz 1994.
| Methods | Randomized controlled trial, double blind Design :‐ Paralell Sample size :‐ 41 (MTX+CQ), 41 (MTX+Placebo) Trial duration:‐ 6 months | |
| Participants | Active disease RA and had failed to respond to NSAIDS and at least one DMARD had not used any DMARDS in the past 2 months , were on stable dose (up to 7.5 mg/d) of prednisolone and NSAIDS for at least 4 weeks Mean age(SD) :‐ 49.7(13.9) yrs MTX+CQ, 43.6(11.9) yrs MTX Feamle :‐ 82% MTX+CQ, 85% MTX Disease duration(SD) :‐ 9.24(7.94) yrs‐ MTX+CQ, 6.19(4.74) yrs‐ MTX Rheumatoid factor + :‐24% MTX+CQ, 26% MTX Mean dose of MTX:‐ 7.5 mg/wk both groups Concomittant treatment ‐ systemic steroid 24% MTX+CQ, 23% MTX | |
| Interventions | MTX 7.5 mg/week + CQ 250 mg/day vs MTX 7.5 mg/week + placebo | |
| Outcomes | TJC, SJC, pain, HAQ, ESR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomised by each centre, in block of 4, to one of the treatment, using random number table" |
| Allocation concealment (selection bias) | Low risk | Quote: "Eligible patients were randomised by each centre, in block of 4, to one of the treatment, using random number table" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop out rate and reasons were summarized in table 2 |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported. |
| Other bias | Low risk | Quote: "NSAIDS and corticosteroid were kept in a stable dose throughout the study period" |
| Blinding (patient assessed)? | Low risk | Quote: "Patients and rheumatologists were blinded to the treatment groups" and "Chloroquine and placebo were indistinguishable in terms of shape and colour" |
| Blinding (Physician assessed)? | Low risk | Quote: "Patients and rheumatologists were blinded to the treatment groups" and "Chloroquine and placebo were indistinguishable in terms of shape and colour" |
| Blinding (Outcome assessor assessed)? | Low risk | Quote: "Patients and rheumatologists were blinded to the treatment groups" and "Chloroquine and placebo were indistinguishable in terms of shape and colour" |
Haagsma 1994.
| Methods | Randomized controlled trial, open label Design :‐ Step‐up Sample size :‐ 22 (MTX+SSZ), 18 (MTX) Trial duration:‐ 24 weeks | |
| Participants | RA who had an insufficient response to SSZ according to their treating physician were consider for selection. Mean age(SD) :‐ 59.3(12.3) yrs MTX+SSZ, 51.8(13.9) yrs MTX Feamle :‐ 82% MTX+SSZ, 78% MTX Disease duration(SD) :‐ 4.7(4.2) yrs‐ MTX+SSZ, 5.3(4.2) yrs‐ MTX Rheumatoid factor + :‐77% MTX+SSZ, 72% MTX Mean dose of MTX:‐ 7.9 mg/wk MTX+SSZ, 8.3 mg/wk MTX Concomittant treatment ‐ no data | |
| Interventions | MTX 15 mg/week + SSZ 2 g/day vs. MTX 15 mg/week | |
| Outcomes | RAI, SJC, pain, ESR, DAS | |
| Notes | Intention‐to‐treat, Last observation carried forward for missing data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "... were randomised using the balanced allocation method." |
| Allocation concealment (selection bias) | High risk | Quote : This study was a single‐observer 24‐wk randomised parallel open clinical trial......." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Two patients, both receiving the combination treatment dropped out, one because of a cardiac operation and sequelae unrelated to therapy and one because of the development of and 'overlap' syndrome with..." |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported. |
| Other bias | Low risk | Quote : "NSAIDS were given in a stable dose.", " No systemic corticosteroid was allowed.", and "One local injection of corticosteroid was permitted... but was excluded from the analysis" |
| Blinding (patient assessed)? | High risk | Quote : This study was a single‐observer 24‐wk randomised parallel open clinical trial......." |
| Blinding (Physician assessed)? | High risk | Quote : This study was a single‐observer 24‐wk randomised parallel open clinical trial......." |
| Blinding (Outcome assessor assessed)? | Unclear risk | We were not clear if the outcome assessor and the care provider were the same person. |
Haagsma 1997.
| Methods | Randomized controlled trial, double blind Design:‐ Paralell Sample size :‐ 36 (MTX+SSZ), 35 (MTX+placebo), 34(SSZ) Trial duration:‐ 52 weeks | |
| Participants | RA with disease duration less than 1 year Active RA defined as DAS more than 3 DMARDS naive Mean age(SD) :‐ 57(12.2) yrs MTX+SSZ, 59.4(13.2) yrs MTX Feamle :‐ 67% MTX+SSZ, 66% MTX Disease duration(SD) :‐ 2.6(1.4) months‐ MTX+SSZ, 3(2.3) months‐ MTX Rheumatoid factor + :‐94% MTX+SSZ, 94% MTX Concomittant treatment ‐ no data Mean dose of MTX:‐ 7.5 mg/wk MTX+SSZ, 15 mg/wk MTX | |
| Interventions | SSZ 3g/d+MTX 7.5 mg/wk vs. MTX 15 mg/wk+placebo vs. SSZ 3 g/d +placebo | |
| Outcomes | TJC, RAI, SJC, pain, PGA,EGA,HAQ,ESR | |
| Notes | Use data of MTX+SSZ and MTX +placebo only Intention‐to‐treat, Last observation carried forward for missing data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised in blocks of six..." |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomised in blocks of six..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 20 patients withdrew prematurely (before week 52) from the
trial. Three patients in the SSZ group and one patient in the COMBI group were withdrawn before the end of their follow‐up because of inefficacy. For reasons
of toxicity, nine patients in the SSZ group, two in the MTX group and five in the COMBI group." In addition, Figure 4 also listed the reason for withdrawal due to toxicity. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported. |
| Other bias | Low risk | Quote: "All patients had a concomitant NSAID in a dose which was preferably not altered during the study period. No systemically administered corticosteroids were permitted. and "Intra‐articular injections of corticosteroids were sparingly and evenly administered (four injections in SSZ, three in MTX,five in COMBI)." |
| Blinding (patient assessed)? | Low risk | Quote : ".. MTX‐matching placebo...." and ".... SSZ‐matching placebo" and "This was a randomised, controlled, double‐blind, 52 week trial with one observer" |
| Blinding (Physician assessed)? | Low risk | Quote: "This was a randomised, controlled, double‐blind, 52 week trial with one observer" |
| Blinding (Outcome assessor assessed)? | Low risk | Quote: "This was a randomised, controlled, double‐blind, 52 week trial with one observer" |
Hanyu 1999.
| Methods | Randomized controlled trial, open label Design :‐ Step‐up Sample size :‐ 19 (MTX+previous DMARDS), 18 (MTX) Trial duration:‐ 5 years | |
| Participants | Active RA and insufficient response to treatment with gold, D‐penicillamine, bucillamine or sulfasalazine. Mean age(SD) :‐ 55.6(2.2) yrs MTX+ previous DMARDS, 54(2.9) yrs MTX Feamle :‐ 79% MTX+previous DMARDS, 83% MTX Disease duration(SD) :‐ 13.1(2.1) yrs‐ MTX+previous DMARDS, 13.8(1.5) yrs‐ MTX Rheumatoid factor + :‐89% MTX+previous DMARDS, 94% MTX Mean dose of MTX:‐ 5 mg/wk MTX combo, 7.5 mg/wk MTX Concomittant treatment ‐ systemic steroid 74% MTX+previous DMARDS, 78% MTX | |
| Interventions | MTX 5 mg/wk+ previous DMARDS(SSZ, Penicillamine,Bucillamine, Gold) vs. MTX 7.5 mg/wk | |
| Outcomes | Joint score, Lansbury index, grip strength, morning stiffness, ESR, CRP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated in the article only "Patients were randomly assigned to.." . The method of randomisation was not explicitly described. |
| Allocation concealment (selection bias) | High risk | It was stated in the article only "Patients were randomly assigned to.." . The method of randomisation was not explicitly described. This study is an open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number and reasons of withdrawal were presented in Table 4 |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported. |
| Other bias | Low risk | Concomittant systemic steroid use were similar in both arms (74% in MTX+previous DMARDS group vs.78% in MTX group) |
| Blinding (patient assessed)? | High risk | This study is an open‐label trial. |
| Blinding (Physician assessed)? | High risk | This study is an open‐label trial. |
| Blinding (Outcome assessor assessed)? | High risk | This study is an open‐label trial. |
Hetland 2006.
| Methods | Randomized controlled trial, double blind Design :‐ Paralell Sample size :‐ 80 (MTX+CSA), 80 (MTX+placebo) Trial duration:‐ 52 weeks | |
| Participants | Active RA with less than 6 months' duration DMARDS naive Median age(IQR) :‐ 53.2(44.5‐62.4) yrs MTX+CSA, 51(39.5‐62.5) yrs MTX Feamle :‐ 64% MTX+CSA, 70% MTX Disease duration,median (IQR) :‐ 3.2(2.4‐4.6) months MTX+CSA, 3.9(2.8‐4.6) months MTX Rheumatoid factor + :‐ 70% MTX+CSA, 59% MTX Mean dosage of MTX :‐ 12.5 mg/wk MTX+CSA, 15 mg/wk MTX Concomittant non‐steroidal anti‐inflammatory drugs 60% MTX+CSA, 66% MTX. | |
| Interventions | MTX 20 mg/wk+CSA 2.5 mg/kg/d vs. MTX 20 mg/wk Intra‐articular injection of betamethasone was given in all swollen joints (maximum 4 joints or 4 ml per visit) at weeks 0,2, 4, 6, 8 and every 4 weeks thereafter up to week 52 . | |
| Outcomes | ACR,DAS28,HAQ,Larsen's score | |
| Notes | Intention‐to‐treat, Last observation carried forward for missing data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised in blocks of 4 from a computer‐generated list of study numbers." |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate. Quote: "...The code was kept locked up, and the study numbers were assigned centrally by the good clinical practice monitor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Reasons for withdrawal from the study are shown in Figure 1." |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported. |
| Other bias | High risk | Co‐interventions were not similar. Quote: "The cumulative dose of betamethasone from week 12 onward was higher in the monotherapy group (median 5.8 ml [IQR 2.5–11.1 ml]) than in the combination therapy group (3.3 ml [IQR 1.0–9.1 ml])" |
| Blinding (patient assessed)? | Low risk | Quote: "...double‐blind,parallel‐group, placebo‐controlled trial" |
| Blinding (Physician assessed)? | Low risk | Quote: "...double‐blind,parallel‐group, placebo‐controlled trial" |
| Blinding (Outcome assessor assessed)? | Unclear risk | We were not clear if the outcome assessor and the care provider were the same person. |
Ichikawa 2005.
| Methods | Randomized controlled trial, double blind Design :‐ Paralell Sample size :‐ 24 (MTX+Bucillamine), 41 (MTX+Placebo) Trial duration:‐ 96 weeks | |
| Participants | Active RA, MTX or Bucillamine(BUC) naive, never received prednisolone more than 7.5 mg/day Mean age(SD) :‐ 49.2(13.9) yrs MTX+BUC, 52.7(9.3) yrs MTX Feamle :‐ 83.3% MTX+BUC, 69.6% MTX Disease duration(SD) :‐ 10.6(6.6) yrs‐ MTX+BUC 8.2(4.8) yrs‐ MTX Rheumatoid factor + :‐96% MTX+BUC, 83% MTX Mean dose of MTX:‐ 8 mg/wk both groups Concomittant treatment ‐ systemic steroid 17% MTX+BUC, 35% MTX | |
| Interventions | MTX 8 mg/wk +Bucillamine 200 mg/d vs MTX 8 mg/wk | |
| Outcomes | TJC, SJC,Pain, PGA, EGA, HAQ, ESR, CRP | |
| Notes | Intention‐to‐treat | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "One of the test drugs was assigned randomly according to the usage number for the test drug distributed beforehand..." |
| Allocation concealment (selection bias) | Unclear risk | "One of the test drugs was assigned randomly according to the usage number for the test drug distributed beforehand..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for withdrawal were clearly described. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported. |
| Other bias | High risk | Co‐interventions were not similar. The use of corticosteroid was higher in MTX arm compared to MTX combination arm. |
| Blinding (patient assessed)? | Low risk | Quote: " A placebo indistinguishable in appearance from the drug was prepared for MTX and BUC for a double dummy medication technique" |
| Blinding (Physician assessed)? | Low risk | Quote: " A placebo indistinguishable in appearance from the drug was prepared for MTX and BUC for a double dummy medication technique" |
| Blinding (Outcome assessor assessed)? | Unclear risk | We were not clear if the outcome assessor and the care provider were the same person. |
Islam 2000.
| Methods | Randomized controlled trial, open label Design :‐ Paralell Sample size :‐ 27 (MTX+SSZ), 27 (MTX) analysed only completers 19 (MTX+SSZ), 23 (MTX) Trial duration:‐ 6 months | |
| Participants | RA with less than 3 months of disease duration Mean age(SD) :‐ 39.74(11.08) yrs MTX+SSZ, 32.35(14.79) yrs MTX Feamle :‐ 79% MTX+SSZ, 83% MTX Mean dose of MTX:‐ no data Concomittant treatment ‐ no data | |
| Interventions | SSZ 2 g/d+MTX 7.5‐15 mg/wk vs. MTX 7.5‐15 mg/wk | |
| Outcomes | TJC,SJC, PGA,EGA,Functional class(I‐IV),ESR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: It was stated in the article only "The subjects were randomly assigned to.." . The method of randomisation was not explicitly described . |
| Allocation concealment (selection bias) | Unclear risk | Quote: It was stated in the article only "The subjects were randomly assigned to.." . The method of randomisation was not explicitly described . |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for withdrawal were clearly described. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported. |
| Other bias | Unclear risk | Co‐interventions were not mentioned. |
| Blinding (patient assessed)? | High risk | This study is an open‐label trial. |
| Blinding (Physician assessed)? | High risk | This study is an open‐label trial. |
| Blinding (Outcome assessor assessed)? | High risk | This study is an open‐label trial. |
Jarette 2006.
| Methods | Randomized controlled trial, double blind Design :‐ Step‐up Sample size :‐ 18 (MTX+Zoledronic acid), 21 (MTX+placebo) Trial duration:‐ 26 weeks | |
| Participants | RA symptom for less than 2 years active disease and clinical synovitis in at least the wrist or hand joints were on MTX 4 weeks before randomisation Mean age(range) :‐ 50.2(30‐76) yrs MTX+Zoledronic acid, 53.5(33‐72) yrs MTX+placebo Feamle :‐ 55.6% MTX+Zolindronic acid, 57.1% MTX+placebo Disease duration(range) :‐ 5.6 (0.4‐20.6) months MTX+Zolindronic acid, 5.5 (0.4‐14.2) months MTX Rheumatoid factor + :‐ 83% MTX+Zolindronic acid, 71% MTX Mean dosage of MTX :‐ 14 mg/wk MTX+ Zolindronic acid , 11.9 mg/wk MTX The use of concomitant intraarticular/intramuscular corticosteroid was higher in MTX+Zolindronic acid group (72% of patients in MTX+Zolindronica acid group vs. 43% in MTX group) | |
| Interventions | Zoledronic acid 5 mg infusion at baseline and 13 week +MTX 7.5‐20 mg/wk vs. MTX +placebo infusion | |
| Outcomes | TJC,SJC,Pain, PGA, EGA, MRI of the second to fifth proximal interphalangeal and metacarpophalangeal joints and of wrist | |
| Notes | Intention‐to‐treat | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was not stated in detail on the method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | It was not stated in detail on the method of randomisation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no drop‐out in both arms. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported. |
| Other bias | High risk | Co‐interventions were not similar. The use of concomitant intraarticular/intramuscular corticosteroid was higher in MTX+Zolindronic acid group (72% of patients in MTX+Zolindronica acid group vs. 43% in MTX group) |
| Blinding (patient assessed)? | Low risk | Quote: "This single‐centre, double‐blind, randomised, placebo‐controlled trial was conducted at........" and "The study treatment was packaged in a blinded manner. Both placebo and zoledronic acid infusions were prepared by an independent pharmacist and were identical in appearance." |
| Blinding (Physician assessed)? | Low risk | Quot: "This single‐centre, double‐blind, randomised, placebo‐controlled trial was conducted at........" and "The study treatment was packaged in a blinded manner. Both placebo and zoledronic acid infusions were prepared by an independent pharmacist and were identical in appearance." |
| Blinding (Outcome assessor assessed)? | Unclear risk | We were not clear if the outcome assessor and the care provider were the same person. |
Kremer 2002.
| Methods | Randomized, double‐blind, placebo‐controlled Design :‐ Step‐up Sample size at entry : MTX+Placebo ‐133, MTX+Leflunimide ‐133 Trial duration : 24 wk | |
| Participants | Active RA despite taking stable dose of MTX 10‐20 mg/wk for at least 6 months Mean age(SD) :‐ 55.6(11.7) LEF, 56.6(11.37) placebo Female:‐ 99(76.2%) LEF , 107(81%) placebo Disease duration :‐ 10.5(8.35) y LEF, 12.7(9.56) y placebo Positive rheumatoid factors :‐ 99(79%) LEF, 113(88%) placebo Mean dose of MTX(SD):‐ 16.8(2.7) MTX+LEF, 16.1(2.9) MTX Concomittent systemic steroid :‐ 77(59.2%) LEF, 86(64.7%) placebo | |
| Interventions | LEF 20 mg/d + previous dose of MTX vs. Placebo + previous dose of MTX | |
| Outcomes | TJC,SJC,Pain, PGA, EGA, HAQ, ESR, CRP | |
| Notes | Intention‐to‐treat | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done by using the Aventis standard random‐code generator." and "A set of 500 random numbers was generated, with treatment groups randomly assigned in a balanced manner (1:1 ratio) within each block of four consecutive random numbers (block size, 4)." |
| Allocation concealment (selection bias) | Low risk | Quote: " A randomisation schedule, generated by and stored with Quintiles, Inc., Kansas City, Missouri, was used to assign sequential numbers to randomly allocated treatment codes." and "The randomisation code used was concealed from investigators and patients throughout the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number and reasons of withdrawal were described in figure 1. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported. |
| Other bias | Low risk | The use of concomitant NSAIDS [85 (65%) LEF vs. 97 (73%) MTX+placebo] and steroid [77(59%) LEF+MTX, 86(65%) MTX+placebo] were comparable between groups. |
| Blinding (patient assessed)? | Low risk | Quote: "The randomisation code used was concealed from investigators and patients throughout the study." and "Patients were randomly assigned to receive leflunomide, 100 mg/d, for 2 days followed by 10 mg/d or matching placebo." |
| Blinding (Physician assessed)? | Low risk | Quote: "The randomisation code used was concealed from investigators and patients throughout the study." and "Patients were randomly assigned to receive leflunomide, 100 mg/d, for 2 days followed by 10 mg/d or matching placebo." |
| Blinding (Outcome assessor assessed)? | Unclear risk | We were not clear if the outcome assessor and the care provider were the same person. |
Lehman 2005.
| Methods | Randomized controlled trial, double blind Design :‐ Step‐up Sample size :‐ 38 (MTX+ intramuscular gold), 27 (MTX+placebo) Trial duration:‐ 48 weeks | |
| Participants | RA with disease duration more than 4 months and less than 10 years sub‐optimal response at least 12 wk of MTX equal or more than 15 mg/wk unless toxicity necessitated a lower dosage (defined as equal or more than 4 swollen joints, equal or more than 5 tender joints and ESR equal or more than 25 mm/hr or morning stiffness more than 30 minutes. MTX, folic acid and prednisolone (up to 10 mg/d) had to have been received at a stable dosage for 4 weeks Mean age(SD) :‐ 51(11) yrs MTX+gold, 54(13) yrs MTX+Placebo Feamle :‐ 84% MTX+gold, 78% MTX Disease duration(SD) :‐ 3.4(2.5) yrs MTX+gold, 2.8(2.7) yrs MTX Rheumatoid factor + :‐ 67% MTX+gold, 63% MTX Mean dosage of MTX(SD) :‐ 18(5.1) mg/wk MTX+ gold, 18(6.5) mg/wk MTX Concomittant non‐steroidal anti‐inflammatory drugs:‐ 92% MTX+gold, 89% MTX Concomittent systemic steroid:‐ 18% MTX+gold, 52% MTX | |
| Interventions | stable dose of previous of MTX+Gold 10‐50 mg/wk or as tolerated vs. Previous dose of MTX +placebo | |
| Outcomes | TJC, SJC,Pain, PGA, EGA, HAQ, ACR | |
| Notes | Intention‐to‐treat | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Block randomisation, stratified by study centre, was generated with a random number table with variable‐sized blocks." |
| Allocation concealment (selection bias) | Low risk | Quote: "Center investigators received unique randomisation codes in sequentially numbered sealed opaque envelopes." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients withdrew due to toxicity. This was included in our major analysis. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported. |
| Other bias | High risk | Concomittent systemic steroid was higher in MTX group (18% MTX+gold, 52% MTX) |
| Blinding (patient assessed)? | Low risk | Quote: "The gold or placebo was administered using graduated 1‐ml amber‐coloured glass syringes" |
| Blinding (Physician assessed)? | Low risk | Quote: "The gold or placebo was administered using graduated 1‐ml amber‐coloured glass syringes" |
| Blinding (Outcome assessor assessed)? | Low risk | Quote:"....double‐observer assessment technique was employed. A blinded assessor other than the centre investigator or study nurse, who was kept uninformed of patient treatment and treatment response, assessed the key variables for the primary outcome..." and "At the start of the study, the centre nurse instructed patients not to discuss any aspect of their care or condition with the blinded assessor,...." |
Marchesoni 2003.
| Methods | Randomized controlled trial, single blind (the clinical investigator) Design :‐ Paralell Sample size :‐ 30 (MTX+CSA), 30 (MTX) Trial duration:‐ 12 months | |
| Participants | Active RA, DMARDS naive Mean age(SD) :‐ 46.6(10.5) yrs MTX+CSA, 49.3(10.2) yrs MTX Feamle :‐ 93% MTX+CSA, 90% MTX Disease duration(SD) :‐ 0.9(0.7) yrs‐ MTX+CSA, 0.9(0.7) yrs‐ MTX Rheumatoid factor + 36% MTX+CSA, 74% MTX Mean dose of MTX(SD):‐ 9.5 (1.7) mg/wk in CSA+MTX, 11.2(3.4) mg/wk in MTX Mean dose of CSA(SD):‐ 2.5 (0.6) mg/kg/d Concomittant treatment ‐ no more than 10 mg/d of prednisolone and NSAIDS was permitted | |
| Interventions | CSA up to 4 mg/kg/d+MTX up to 20mg/wk im vs.MTX up to 20 mg/wk | |
| Outcomes | ACR , HAQ,Sharp/vDH | |
| Notes | Intention‐to‐treat, Last observation carried forward for missing data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote:"The patients were allocated to one of the two treatment arms according to a randomisation list,......". It is not clear how this list was generated and assigned to patients. |
| Allocation concealment (selection bias) | Low risk | Quote:"..... a randomised list, using the sealed envelope procedure" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/30 patient in CSA+MTX arm failed to come to the schedules visits and 7/30 discontinued due to toxicity. In MTX group, 2/31 patients discontinued due to toxicity. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported. |
| Other bias | Low risk | Quote:"Local corticosteroid injection were not allowed in the joints of the hands and feet used to score the radiographic changes" |
| Blinding (patient assessed)? | High risk | Quote: "This was a 12‐month, controlled, randomised single‐blind (The clinical investigator was blinded to the treatment)." |
| Blinding (Physician assessed)? | Low risk | Quote: "This was a 12‐month, controlled, randomised single‐blind (The clinical investigator was blinded to the treatment)." |
| Blinding (Outcome assessor assessed)? | High risk | Quote: "This was a 12‐month, controlled, randomised single‐blind (The clinical investigator was blinded to the treatment)." |
O'Dell 1996.
| Methods | Randomised controlled trial, double blind Design:‐ Paralell Sample size :‐ 31(MTX+SSZ+HCQ), 36 (MTX+placebo) Trial duration:‐ 24 months | |
| Participants | Active RA with more than 6 months of disease duration
Poor response to treatment with at least one of the following :gold,HCQ,Penicillamine, SSZ,MTX
Mean age(range) :‐ 50(27‐67) yrs MTX+SSZ+HCQ, 50(21‐69) yrs MTX+placebo
Female :‐ 66% MTX+SSZ+HCQ, 70% MTX
Disease duration(SD) :‐ 10(10) months‐ MTX+SSZ+HCQ, 10(8) months‐ MTX
Rheumatoid factor + :‐ 84% MTX+SSZ+HCQ, 89% MTX
Mean dose of MTX:‐ no data Concomittant treatment ‐ systemic steroid of equal or less than 10 mg/d 52% in MTX+SSZ+HCQ and 53% in MTX and NSAIDS were permitted |
|
| Interventions | MTX 17.5mg/wk+SSZ 1g/d+ HCQ 400mg/d vs.MTX 17.5mg/wk+placebo | |
| Outcomes | TJC, SJC, PGA,EGA,ESR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"The pharmacy performed the randomisation; equal numbers of cards with each group assignment were mixed, drawn, and placed in sequentially numbered envelopes that were opened as the patients were enrolled." |
| Allocation concealment (selection bias) | Low risk | Quote:"The pharmacy performed the randomisation; equal numbers of cards with each group assignment were mixed, drawn, and placed in sequentially numbered envelopes that were opened as the patients were enrolled." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote:"Thirteen patients discontinued the study because of drug toxicity, and 37 patients did so because of lack of efficacy. Two patients were withdrawn from the study for protocol violations". |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported. |
| Other bias | Low risk | Concomittant use of systemic steroid of equal or less than 10 mg/d were comparable between 2 groups (52% in MTX+SSZ+HCQ and 53% in MTX and NSAIDS ) |
| Blinding (patient assessed)? | Low risk | Quote:"We enrolled 102 patients in this two‐year, double‐blind, randomised, controlled study" |
| Blinding (Physician assessed)? | Low risk | Quote:"We enrolled 102 patients in this two‐year, double‐blind, randomised, controlled study" |
| Blinding (Outcome assessor assessed)? | Unclear risk | We were not clear if the outcome assessor and the care provider were the same person. |
O'Dell 2006.
| Methods | Randomized controlled trial, double blind Design :‐ Paralell Sample size :‐ 18 (Doxycycline 20 mg twice daily+MTX) , 24 Doxycycline 100 mg twice daily+MTX and 24 MTX+placebo Trial duration:‐ 2 years | |
| Participants | Active RA with 6 weeks to less than 1 year of disease duration and had positive rheumatoid factor DMARD naive Mean age(range) :‐ 49.5(35‐47) yrs doxy 100 mg+MTX, 49.9(27‐74) yrs doxy 20 mg+MTX, 55.7(41‐47) yrs MTX+placebo Feamle :‐ 67% doxy 100 mg+MTX, 89% doxy 20 mg+MTX and 70% MTX Disease duration(SD) :‐ 5(3.1) months‐doxy 100 mg+MTX , 5.4(2.9) doxy 20 mg+MTX, 4.8(2.7) months‐ MTX Rheumatoid factor + :‐ 100% all three groups Mean dose of MTX:‐ no data Concomittant treatment ‐ NSAIDS and prednisolone less than 7.5 mg/d were permitted. A total 2 intra‐articular steroid injection were allowed but not within 6 weeks prior to the evaluation period | |
| Interventions | Doxycycline 20 mg twice daily+MTX 7.5‐17.5 mg/wk Doxycycline 100 mg twice daily+MTX 7.5‐17.5 mg/wk Placebo +MTX 7.5‐17.5 mg/wk | |
| Outcomes | TJC, SJC, PGA, EGA, HAQ, ESR | |
| Notes | Intention‐to‐treat | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote :"The pharmacy handled the randomisation; equal numbers of cards with each group assignment were mixed, drawn, and placed in sequentially numbered envelopes that were opened as the patients were enrolled." |
| Allocation concealment (selection bias) | Low risk | Quote :"The pharmacy handled the randomisation; ........... sequentially numbered envelopes that were opened as the patients were enrolled." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for discontinuation were shown in figure 1 |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported. |
| Other bias | Low risk | Previuos use of NSAIDs and systemic steroid were allowed at stable dose throughout the study. |
| Blinding (patient assessed)? | Low risk | Quote:"We enrolled 66 patients in this 2‐year, double‐blind, randomised, controlled trial." |
| Blinding (Physician assessed)? | Low risk | Quote:"We enrolled 66 patients in this 2‐year, double‐blind, randomised, controlled trial." |
| Blinding (Outcome assessor assessed)? | Unclear risk | We were not clear if the outcome assessor and the care provider were the same person. |
Ogrendik 2007.
| Methods | Randomized controlled trial, double blind Design:‐ Step‐up Sample size :‐ 38 (MTX+levofloxacin), 38 (MTX+placebo) Trial duration:‐ 6 months | |
| Participants | Active RA defined as equal or more than 10 swollen joints, equal or more than 12 tender joints and ESR equal or more than 28 mm/hr or CRP equal or more than 2 mg/dl Before receiving the study drugs, all patients had been taking MTX 15‐25 mg/wk for at least 6 months Mean age(SD) :‐ 51(9) yrs MTX+levofloxacin, 49(10) yrs MTX+Placebo Feamle :‐ 71% MTX+ levofloxacin, 74% MTX Disease duration(SD) :‐ 13(9) yrs MTX+ levofloxacin, 12(8) yrs MTX Rheumatoid factor + :‐ 84% MTX+ levofloxacin, 74% MTX Concomittent systemic steroid:‐ 63% MTX+ levofloxacin, 68% MTX Mean dosage of MTX(SD) :‐ 15(8.3) mg/wk MTX+ levofloxacin, 17.5(9.1) mg/wk MTX | |
| Interventions | Levofloxacin 500 mg/d + previous dose of MTX vs. Placebo + previous dose of MTX | |
| Outcomes | TJC,SJC,Pain, PGA,EGA,HAQ, ESR,CRP | |
| Notes | Intention‐to‐treat, Last observation carried forward for missing data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not described. |
| Allocation concealment (selection bias) | Unclear risk | The method of randomisation was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote:"Among the patients receiving levofloxacin plus methotrexate, the proportions of patients who withdrew because of inadequate symptom control were 5%; among the patients receiving placebo plus methotrexate, it was 11%" |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported. |
| Other bias | Unclear risk | Although patients continued NSAIDS and prednisone (at 10 mg daily or less) at a stable dose during the study period, the use of analgesics such as acetaminophen, codeine, or pain killers were permitted. There was no report on the number of patients who took analgesics comparing between both arms. |
| Blinding (patient assessed)? | Low risk | Quote:"This was a 6‐month, mono‐centre, randomised, double‐blind, placebo‐controlled study." |
| Blinding (Physician assessed)? | Low risk | Quote:"This was a 6‐month, mono‐centre, randomised, double‐blind, placebo‐controlled study." |
| Blinding (Outcome assessor assessed)? | Unclear risk | We were not clear if the outcome assessor and the care provider were the same person. |
Taschioglu 2003.
| Methods | Randomized controlled trial, open label Design:‐ Paralell Sample size :‐ 35 (MTX+SSZ), 35 (MTX) analysed only completers:‐ 27(MTX+SSZ) + 28 (MTX) Trial duration:‐ 12 months | |
| Participants | RA with less than 1 year of disease duration DMARDS naive Active disease (DAS>3) DMARDS and steroid naive Mean age(SD) :‐ 45.88(6.46) yrs MTX+SSZ, 45.16(5.93) yrs MTX Feamle :‐ 81% MTX+SSZ, 86% MTX Disease duration(SD) :‐ 6.9(6.35) months‐ MTX+SSZ, 7.48(4.03) months‐ MTX Rheumatoid factor + :‐ 85% MTX+SSZ, 88% MTX Mean dose of MTX:‐ 7.5 mg/wk both groups Concomittant treatment ‐ NSAIDS were allowed during the study period | |
| Interventions | MTX 7.5 mg/kg+SSZ 2g/d vs.MTX 7.5 mg/wk | |
| Outcomes | RAI,SJC,Pain,HAQ,ESR,CRP | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not described. |
| Allocation concealment (selection bias) | High risk | The method of randomisation was not described, and this was open label trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers and reasons for withdrawal were explicitly described. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported. |
| Other bias | Low risk | Alhtough NSAIDS and analgesics were allowed, the dose had to be stable throughout the trial. No systemic and intra‐articular steroid were permitted. |
| Blinding (patient assessed)? | High risk | This study was open‐label trial. |
| Blinding (Physician assessed)? | High risk | This study was open‐label trial. |
| Blinding (Outcome assessor assessed)? | Low risk | Quote:" Each patients was evaluated by the same observers who blinded to study protocol." |
Tugwell 1995.
| Methods | Randomized controlled trial, double blind Design:‐ Step‐up Sample size :‐ 75 (MTX+CSA), 73 (MTX+placebo) Trial duration:‐ 6 months | |
| Participants | RA who had partial response to MTX 15 mg/wk at stable dose for at least 3 months Mean age(SD) :‐ 55.4(12.9) yrs MTX+CSA, 54.3(14.5) yrs MTX Feamle :‐ 72% MTX+CSA, 73% MTX Mean dose of MTX(SD):‐ equal or less than 15 mg/wk Mean dose of CSA(SD):‐ 2.97(1.02) mg/kg/d Disease duration(SD) :‐ 11.2(8.3) months‐ MTX+CSA, 9.4(7.8) months‐ MTX Concomittant treatment ‐ no more than 10 mg/d of prednisolone was permitted | |
| Interventions | CSA 2.5 mg/kg/d ‐ 5mg/kg/d+ previous dose of MTX vs. previous dose of MTX | |
| Outcomes | ACR, TJC, SJC,Pain, PGA, EGA, HAQ, ESR | |
| Notes | Intention‐to‐treat, Last observation carried forward for missing data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not described. |
| Allocation concealment (selection bias) | Low risk | Quote:"A separate randomisation schedule was generated at each centre. Gelatin capsules of cyclosporine and placebo were prepared by Sandoz (Basel, Switzerland); they were identical in taste and appearance." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number and reasons for withdrawal were shown in table 2. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported. |
| Other bias | Unclear risk | No more than 10 mg/d of prednisolone was permitted; however, there is no report on the number of patients who were taken prednisolone throughout the trial. |
| Blinding (patient assessed)? | Low risk | Quote:"Capsule of cyclosporine and placebo were prepared by Sandoz (Basel, Switzerland); they were identical in taste and appearance." |
| Blinding (Physician assessed)? | Low risk | Quote:"Capsule of cyclosporine and placebo were prepared by Sandoz (Basel, Switzerland); they were identical in taste and appearance." |
| Blinding (Outcome assessor assessed)? | Low risk | Quote:"The patients, primary care physicians, and study investigators were unaware of the study‐group assignments group." |
Willkins 1992.
| Methods | Randomized controlled trial, double‐blind Design:‐ Step‐up Sample size :‐ 69( MTX+AZA), 67(MTX+placebo) Trial duration:‐ 24 weeks | |
| Participants | RA with inadequate disease control or toxic response to treatment with injectable gold (at least 750 mg total dose), auranofin(6 months of 6 mg daily dose), penicillamine (500 mg daily dose for 3 months) Mean age(range) :‐ 56(29‐79) yrs MTX+AZA, 54(21‐85) yrs MTX Feamle :‐ 82% MTX+AZA, 74% MTX Disease duration(range) :‐ 8(1‐54) yrs‐ MTX+AZA, 10(1‐40) yrs‐ MTX Median dose of MTX and AZA:‐ no data Concomittant treatment ‐ no data | |
| Interventions | Level I :‐ MTX 5 mg/week + AZA 50 mg/day vs MTX 5 mg/week Level II:‐ MTX 7.5 mg/week + AZA 100 mg/day vs MTX 7.5 mg/week Level III:‐ MTX 15 mg/week + AZA 150 mg/day vs MTX 15 mg/week All subjects entered the study at dosage level I and dosage increase were instituted at week 6, 12 and or 18 in non‐responsive patients. | |
| Outcomes | TJC, SJC, PGA, EGA, HAQ The primary response was a clinical response of more than 30% improvement from baseline in at least 3 of these parameter at week 24. | |
| Notes | Intention‐to‐treat | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote:"Permuted‐blocks design was used to put medication assignments in random order." |
| Allocation concealment (selection bias) | Low risk | Quote:"Permuted‐blocks design was used to put medication assignments in random order." and "The “blindness” was maintained by the addition of placebo tablets, such that at each dosage level, the same number of identical tablets were dispensed." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: " In 212 patients, 3 failed to initiate treatment and were excluded from analysis.". The rest of patients continued throughout the trial and were include in the analysis |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported. |
| Other bias | Low risk | Quote:"iIntraarticular injections of steroid into a single joint were permitted, but the injected joint was not considered in the evaluations." |
| Blinding (patient assessed)? | Low risk | Quote:"This study was double blind" and.....and "The “blindness” was maintained by the addition of placebo tablets, such that at each dosage level, the same number of identical tablets were dispensed." |
| Blinding (Physician assessed)? | Low risk | Quote:"This study was double blind" and.....and "The “blindness” was maintained by the addition of placebo tablets, such that at each dosage level, the same number of identical tablets were dispensed." |
| Blinding (Outcome assessor assessed)? | Unclear risk | We were not clear if the outcome assessor and the care provider were the same person. |
Willkins 1995.
| Methods | Continue to the Willikins 1992's study and cross over design at week 24 and were followed up in an open protocol for the ensuring 24 weeks. For those patients who continued with the initial regimen, were followed up in double‐blind manner. | |
| Participants | non‐responders from Willkins 1992's study were invited to cross to one of the other treatment regimens (same protocol) Median dose of MTX:‐ 7.5 mg/wk in both groups at week 48 Median dose of AZA:‐ 75 mg/d MTX+AZA | |
| Interventions | Same as Willikins 1992 | |
| Outcomes | Same as Willikins 1992 and radiograph of hands and wrists | |
| Notes | Intention‐to‐treat analysis and patients in whom therapy was terminated at any time because of toxicity or lack of efficacy and those who crossed over at week 24 were considered non responders at week 48 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It was not described in detail in this article but referred to previous publication (Willkin et al 1992) |
| Allocation concealment (selection bias) | Low risk | It was not described in detail in this article but referred to previous publication (Willkin et al 1992) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of withdrawal and reasons for withdrawal were clearly described. |
| Selective reporting (reporting bias) | Low risk | All primary and secondary outcomes stated in the methods were reported. |
| Other bias | Unclear risk | It was not clear if NSAIDS and steroid were permitted |
| Blinding (patient assessed)? | Low risk | Quote:"For those patients who continued with the initial treatment regimen, both the patient and the physician remained blinded to the treatment." |
| Blinding (Physician assessed)? | Low risk | Quote:"For those patients who continued with the initial treatment regimen, both the patient and the physician remained blinded to the treatment." |
| Blinding (Outcome assessor assessed)? | Unclear risk | We were not clear if the outcome assessor and the care provider were the same person. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Calguneri 1999 | no data of methotrexate monotherapy arm alone (data combined with sulfasalazine and Hydroxychloroquine monotherapy) |
| Clegg 1997 | no outcome of interest |
| Haagsma 1995 | synopsis of Haagsma et al. British Journal of Rheumatology 1994;33:1049‐55(included in this review) |
| Kremer 2004 | open‐label extension of randomised controlled trial |
| Maillefert 2003 | open‐label extension of randomised controlled trial |
| Matucci‐Cerinic 2003 | synopsis of Kremer et al. Annal of Internal Medicine 2002;137:726‐33 9 (included in this review) |
| Mottaghi 2005 | no outcome of interest |
| Mroczkowski 1999 | open‐label extension of randomised controlled trial |
| Nagashima 2006 | non randomised controlled trial (cohort study) |
| Nisar 1994 | non randomised controlled trial (cohort study) |
| O'Dell 1996 | open‐label extension of randomised controlled trial |
| Rou 1998 | non randomised controlled trial (cohort study) |
| Stein 1997 | open‐label extension of randomised controlled trial |
| Trnavsky 1993 | no methotrexate monotherapy arm |
| Willkins 1996 | published in the journal supplements and the key data had been reported in Willikn 1995(included in this review) |
Contributions of authors
WK drafted the protocol, CB provided comments
Review of abstracts:‐ WK, JT, and VP
Data abstraction :‐ WK, VP
Assessment of study quality:‐ WK, VP
Review draft :‐ WK, CB
Draft revision and update:‐ WK, CB
Sources of support
Internal sources
No sources of support supplied
External sources
-
Abbott international, USA.
Unrestricted educational grant
Declarations of interest
None at present.
The original article published in the Annals for Rheumatic Diseases was supported by Abbott with an unrestricted educational grant. Abbott had no role in the study design, literature search, data collection, data analysis, data interpretation or writing of the report.
This current review was updated without the support of any industry sponsor.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Capell 2007 {published data only}
- Capell HA, Madhok R, Porter DR, et al. Combiantion therapy with sulfasalazine and methotrexate is more effective than either drug alone in patients with rheumatoid arthritis with suboptimal response to sulfasalazine:results from double‐blind placebo‐controlled MASCOT study. Ann Rheum Dis 2007;66:235‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dougados 1999 {published data only}
- Dougados M, Combe B, Cantagrel A, et al. Combination therapy in early rheumatoid arthritis:a randomised, controlled, double blind 52 week clinical trial of sulfasalazine and methotrexate compared with single components. Ann Rheum Dis 1999;58:220‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferraz 1994 {published data only}
- Ferraz MB, Pinheiro GR, Helfenstein M, et al. Combination therapy with methotrexate and chloroquine in rheumatoid arthritis. A multicenter randomized placebo‐controlled trial. Scand J Rheum 1994;23(5):231‐6. [DOI] [PubMed] [Google Scholar]
Haagsma 1994 {published data only}
- Haagsma CJ, Van RielP, Rooij D, et al. Combination of methotrexate and sulphasalazine vs methotrexate alone: A randomized open clinical trial in rheumatoid arthritis patients resistant to sulphasalazine therapy. Br J Rheumatol 1994;33(11):1049‐51. [DOI] [PubMed] [Google Scholar]
Haagsma 1997 {published data only}
- Haagsma CJ, Riel PL, Jong A.J, et al. Combination of sulphasalazine and methotrexate versus the single components in early rheumatoid arthritis: a randomized, controlled, double‐blind, 52 week clinical trial. Br J Rheumatol 1997;36(10):1082‐8. [DOI] [PubMed] [Google Scholar]
Hanyu 1999 {published data only}
- Hanyu T, Arai K, Ichikawa H, et al. Long‐term methotrexate (MTX) combination therapy versus MTX alone for active rheumatoid arthritis. Japanease J Rheumatol 1999;9(1):31‐44. [Google Scholar]
Hetland 2006 {published data only}
- Hetland ML, Stengaard‐Pedersen K, Junker P, et al. Combination treatment with methotrexate, cyclosporine, and intraarticular betamethasone compared with methotrexate and intraarticular betamethasone in early active rheumatoid arthritis: An investigator‐initiated, multicenter, randomized, double‐blind, parallel‐group, placebo‐controlled study. Arthritis Rheum 2006;54(5):1401‐9. [DOI] [PubMed] [Google Scholar]
Ichikawa 2005 {published data only}
- Ichikawa Y, Saito T, Yamanaka H at al. Therapeutic effects of the combination of methotrexate and bucillamine in early rheumatoid arthritis: A multicenter, double‐blind, randomized controlled study. Mod Rheumatol 2005;15:323‐8. [DOI] [PubMed] [Google Scholar]
Islam 2000 {published data only}
- Islam MN, Alam MN, Haq SA, et al. Efficacy of sulphasalazine plus methotrexate in rheumatoid arthritis. Bangladesh Med Res Counc Bull 2000;25(1):1‐7. [PubMed] [Google Scholar]
Jarette 2006 {published data only}
- Jarrett SJ, Conaghan PG, Sloan VS, et al. Preliminary evidence for a structural benefit of the new bisphosphonate zoledronic acid in early rheumatoid arthritis. Arthritis Rheum 2006;54(5):1410‐4. [DOI] [PubMed] [Google Scholar]
Kremer 2002 {published data only}
- Kremer JM, Genovese MC, Cannon GW, et al. Concomitant leflunomide therapy in patients with active rheumatoid arthritis despite stable doses of methotrexate: A randomized, double‐blind, placebo‐controlled trial. Ann Int Med 2002;137:726‐33. [DOI] [PubMed] [Google Scholar]
Lehman 2005 {published data only}
- Lehman AJ, Esdaile JM, Kinkhoff AV, et al. A 48‐week, randomized, double‐blind, double‐observer, placebo‐controlled multicenter trial of combination methotrexate and intramuscular gold therapy in rheumatoid arthritis: Results of the METGO study. Arthritis Rheum 2005;52(5):1360‐70. [DOI] [PubMed] [Google Scholar]
Marchesoni 2003 {published data only}
- Marchesoni A, Battafarano N, Arreghini M, et al. Radiographic progression in early rheumatoid arthritis: a 12‐month randomized controlled study comparing the combination of cyclosporin and methotrexate with methotrexate alone. Rheumatology 2003;42:1545‐9. [DOI] [PubMed] [Google Scholar]
O'Dell 1996 {published data only}
- O'Dell JR, Haire C, Erikson N, et al. Treatment of rheumatoid arthritis with methotrexate alone, sulfasalazine and hydroxychloroquine, or a combination of all three medications. N Engl J Med 1996;334(20):1287‐91. [DOI] [PubMed] [Google Scholar]
O'Dell 2006 {published data only}
- O'Dell JR, Elliott JR, Mallek JA, et al. Treatment of early seropositive rheumatoid arthritis: doxycycline plus methotrexate versus methotrexate alone. Arthritis Rheum 2006;54(2):621‐7. [DOI] [PubMed] [Google Scholar]
Ogrendik 2007 {published data only}
- Ogrendik M. Levofloxacin treatment in patients with rheumatoid arthritis receiving methotrexate. Southern Med J 2007;100(2):135‐9. [DOI] [PubMed] [Google Scholar]
Taschioglu 2003 {published data only}
- Tascioglu F, Oner C, Armagan O. Comparison of low dose methotrexate and combination therapy with methotrexate and sulphasalazine in the treatment of early rheumatoid arthritis. J Rheum Med Rehab 2003;14(3):142‐9. [Google Scholar]
Tugwell 1995 {published data only}
- Tugwell P, Pincus T, Yocum D, et al. Combination therapy with cyclosporine and methotrexate in severe rheumatoid arthritis. The Methotrexate‐Cyclosporine Combination Study Group.. N Engl J Med 1995;333(3):137‐41. [DOI] [PubMed] [Google Scholar]
Willkins 1992 {published data only}
- Williams HJ, Ward JR, Reading J, et al. Comparison of auranofin, methotrexate, and the combination of both in the treatment of rheumatoid arthritis. A controlled clinical trial. Arthritis Rheum 1992;35(8):849‐56. [DOI] [PubMed] [Google Scholar]
Willkins 1995 {published data only}
- Willkens RF, Sharp JT, Stablein D, et al. Comparison of azathioprine, methotrexate, and the combination of the two in the treatment of rheumatoid arthritis. A forty‐eight‐week controlled clinical trial with radiologic outcome assessment. Arthritis Rheum 1995;38(12):1799‐806. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Calguneri 1999 {published data only}
- Calguneri M, Pay S, Calikaner Z. Combination therapy versus monotherapy for the treatment of patients with rheumatoid arthritis. Clin Exp Rheum 1999;17:699‐704. [PubMed] [Google Scholar]
Clegg 1997 {published data only}
- Clegg DO, Frederick D, Duffy J, et al. Safety and efficacy of hydroxychloroquine as maintenance therapy for rheumatoid arthritis after combination therapy with methotrexate and hydroxychloroquine. J Rheum 1997;24(10):1896‐902. [PubMed] [Google Scholar]
Haagsma 1995 {published data only}
- Haagsma CJ, Riel PLCM, Putte LBA. Combination sulfasalazine and methotrexate in rheumatoid arthritis:early clinical impressions. Br J Rheum 1995;34(suppl 2):104‐8. [PubMed] [Google Scholar]
Kremer 2004 {published data only}
- Kremer J, Genovese M, Cannon GW. Combination leflunomide and methotrexate (MTX) therapy for patients with active rheumatoid arthritis failing MTX monotherapy: open‐label extension of a randomised, double‐blind, placebo controlled trial. J Rheum 2004;31:1521‐31. [PubMed] [Google Scholar]
Maillefert 2003 {published data only}
- Maillefert JF, Combe B, Goupille P. Long term structural effects of combination therapy in patients with early rheumatoid arthritis: five year follow up of a prospective double blind controlled study. Ann Rheum Dis 2003;62:764‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matucci‐Cerinic 2003 {published data only}
- Matucci‐Cerinic M. Leflunomide improves the clinical response in patients iwth active rheumatoid arthritis treated with methotrexate. Clin Exp Rheum 2003;21(6):695‐6. [PubMed] [Google Scholar]
Mottaghi 2005 {published data only}
- Mottaghi P, Karimzade H. Does chloroquine decrease liver enzyme abnormalities induced by methotrexate in patients with rheumatoid arthritis?. J Res Med Science 2005;10(3):135‐8. [Google Scholar]
Mroczkowski 1999 {published data only}
- Mroczkowski PJ, Weinblatt ME, Kremer JM. Methotrexate and leflunomide combination therapy for patients with active rheumatoid arthritis. Clin Exp Rheum 1999;17(suppl18):S66‐8. [PubMed] [Google Scholar]
Nagashima 2006 {published data only}
- Nagashima M, Matsuoka T, Saitoh K. Treatment continuation rate in relation to efficacy and toxicity in long‐term therapy with low‐dose methotrexate, sulfasalazine, and bucillamine in 1,358 Japanese patients with rheumatoid arthritis. Clin Exp Rheum 2006;24:260‐7. [PubMed] [Google Scholar]
Nisar 1994 {published data only}
- Nisar M, Carlisle L, Amos RS. Methotrexate and sulfasalazine as combination therapy in rheumatoid arthritis. Br J Rheum 1994;33:651‐4. [DOI] [PubMed] [Google Scholar]
O'Dell 1996 {published data only}
- O'Dell JR, Haire C, Erison N, et al. Efficacy of triple DMARD therapy in patients with RA with suboptimal response to methotrexate. J Rheum 1996;23(suppl44):72‐4. [PubMed] [Google Scholar]
Rou 1998 {published data only}
- Rau R, Schleusser B, Herborn G. Longterm combination therapy of refractory and destructive rheumatoid arthritis with methotrexate (MTX) and intramuscular gold or other disease modifying antirheumatic drugs compared to MTX monotherapy. J Rheum 1998;25(8):1485‐92. [PubMed] [Google Scholar]
Stein 1997 {published data only}
- Stein MC, Pincus T, Yocum D. Combination treatment of severe rheumatoid arthritis with cyclosporin and methotrexate for forty‐eight weeks: an open‐label extension study. Arthritis Rheum 1997;40(10):1843‐51. [DOI] [PubMed] [Google Scholar]
Trnavsky 1993 {published data only}
- Trnavsky K, Batterova J, Linduskova M, et al. Combination therapy with hydroxychloroquine and methotrexate in rheumatoid arthritis. Z Rheum 1993;52:292‐6. [PubMed] [Google Scholar]
Willkins 1996 {published data only}
- Willkens RF, Stablein D. Combination treatment of rheumatoid arthritis using azathioprine and methotrexate: a 48 week controlled clinical trial. J Rheum 1996;44(suppl):64‐8. [PubMed] [Google Scholar]
Additional references
Bukhari 2003
- Bukhari M, Wiles M, Lunt M, et al. Influence of disease‐modifying therapy on radiographic outcome in inflammatory polyarthritis at five years: Results from a large observational inception study [Influence of disease‐modifying therapy on radiographic outcome in inflammatory polyarthritis at five years: Results from a large observational inception study]. Arthritis Rheum 2003;48(1):46‐53. [DOI] [PubMed] [Google Scholar]
Bykerk 2005
- Bykerk V, Keystone E. What are the goals and principles of management in the early treatment of rheumatoid arthritis? [What are the goals and principles of management in the early treatment of rheumatoid arthritis?]. Best Pract Res Clin Rheumatol 2005;19(1):147‐61. [DOI] [PubMed] [Google Scholar]
Choy 2005
- Choy EHS, Smith C, Dore CJ, Scott DL. A meta‐analysis of the efficacy and toxicity of combining disease‐modifying anti‐rheumatic drugs in rheumatoid arthritis based on patient withdrawal. Rheumatology 2005;44(11):1414‐21. [DOI] [PubMed] [Google Scholar]
Donahue 2008
- Donahue KE, Gartlehner G, Jonas DE, Lux LJ, Thieda P, Jonas BL, et al. Systematic Review: Comparative Effectiveness and Harms of Disease‐Modifying Medications for Rheumatoid Arthritis.. Ann Intern Med 2008;148(2):124‐34. [DOI] [PubMed] [Google Scholar]
Felson 1993
- Felson D, Anderson JJ, Boers M, Bombardier C, et al. The American college of rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials [The American college of rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials]. Arthritis Rheum 1993;36(6):729‐40. [DOI] [PubMed] [Google Scholar]
Felson 1994
- Felson DT, Anderson JJ, Meenan RF. The efficacy and toxicity of combination therapy in rheumatoid arthritis: A meta‐analysis. Arthritis Rheum 1994;37(10):1487‐91. [DOI] [PubMed] [Google Scholar]
Felson 1995
- Felson D, Anderson J, Boers M, Bombardier C, Furst D, Goldsmith C, et al. American college of rheumatology preliminary definition of improvement in rheumatoid arthritis [American college of rheumatology preliminary definition of improvement in rheumatoid arthritis]. Arthritis Rheum 1995;38(6):727‐35. [DOI] [PubMed] [Google Scholar]
Goekoop YPM 2005
- Goekoop‐Ruiterman YPM, Vries‐Bouwstra J, Allaart C, Zeben D, Kerstens PJSM, Hazes J, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): A randomised, controlled trial.. Arthritis Rheum 2005;52(11):3381‐90. [DOI] [PubMed] [Google Scholar]
Goekoop YPM 2007
- Goekoop‐Ruiterman YPM, Vries‐Bouwstra J, Allaart C, Zeben D, Kerstens PJSM, Hazes J, et al. Comparison of treatment strategies in early rheumatoid arthritis: A randomised Trial. Ann Intern Med 2007;146(6):406‐15. [DOI] [PubMed] [Google Scholar]
Heath 1992
- Heath CJ, Fortin P. Epidemiologic studies of rheumatoid arthritis: future directions [Epidemiologic studies of rheumatoid arthritis: future directions]. J Rheum 1992;32(Suppl):74‐7. [PubMed] [Google Scholar]
Hochberg 2001
- Hochberg MC, Tracy JK, Flores RH. 'Stepping‐up' from methotrexate: A systematic review of randomised placebo controlled trials in patients with rheumatoid arthritis with an incomplete response to methotrexate. Ann Rheum Dis 2001;60(SUPPL. 3):iii51‐iii4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Makinen 2007
- Makinen H, Kautiainen H, Hannonen P, et al. Sustained Remission and Reduced Radiographic Progression with Combination Disease Modifying Antirheumatic Drugs in Early Rheumatoid Arthritis [Sustained Remission and Reduced Radiographic Progression with Combination Disease Modifying Antirheumatic Drugs in Early Rheumatoid Arthritis]. J Rheumatology 2007;34(2):316‐21. [PubMed] [Google Scholar]
Möttönen 1999
- Möttönen T, Hannonen P, Leirisalo‐RepoM, Nissilä M, Kautiainen H, Korpela M. Comparison of combination therapy with single‐drug therapy in early rheumatoid arthritis: a randomised trial. Lancet 1999;353(9164):1568‐73. [DOI] [PubMed] [Google Scholar]
OMERACT 1993
- OMERACT. Outcome measures in rheumatoid arthritis clinical trials. J Rheumatol 1993 1993;20(3):526‐91. [PubMed] [Google Scholar]
Pinals 1981
- Pinals R, Alfonse T, Larsen R. Preliminary criteria for clinical remission in rheumatoid arthritis. Arthritis Rheum 1981;24(10):1308‐15. [DOI] [PubMed] [Google Scholar]
Prevoo 1995
- Prevoo MLL, Van't Hof MA, Kuper HH, Leeuwen MA, Putte LB, Riel PLCM. Modified disease activity scores that include twenty‐eight‐joint counts development and validation in a prospective longitudinal study of patients with rheumatoid arthritis.. Arthritis Rheum 1995;38(1):44‐8. [DOI] [PubMed] [Google Scholar]
van der Heijde 1992
- Heijde DM, van't Hof MA, Riel PL, Leeuwen MA, Rijswijk MH, Putte LB. Validity of single variables and composite indices for measuring disease activity in rheumatoid arthritis. Ann Rheum Dis 1992;51(2):177‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Heijde1996
- Heijde A, Jacobs JWG, Bijlsma JWJ. The Effectiveness of Early Treatment with "Second‐Line" Antirheumatic Drugs: A Randomized, Controlled Trial. Ann Intern Med 1996;124(8):699‐707. [DOI] [PubMed] [Google Scholar]
Van Gestel 1996
- Gestel AM, Prevoo MLL, Van't Hof MA, Rijswijk MH, Putte LBA, Riel PLCM. Development and validation of the European league against rheumatism response criteria for rheumatoid arthritis: Comparison with the preliminary American College of Rheumatology and the world health organization/international league against rheumatism criteria. ArthritisRheum 1996;39(1):34‐40. [DOI] [PubMed] [Google Scholar]
Verhoeven 1998
- Verhoeven AC, Boers M, Tugwell P. Combination therapy in rheumatoid arthritis: updated systematic review. Br Rheumatol 1998;37(6):612‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Katchamart 2009
- Katchamart W, Trudeau J, Phumethum V, Bombardier C. The efficacy and toxicity of methotrexate (MTX) monotherapy vs. MTX combination therapy with non‐biologic disease‐modifying anti‐rheumatic drugs in rheumatoid arthritis: A systematic review and meta‐analysis. Ann Rheum Dis 2009;68(7):1105‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]


