Abstract
Encapsulated Klebsiella pneumoniae strains frequently induce fatal nosocomial pneumonia. Cefodizime (CEF) as an antibiotic is suspected to enhance host resistance against various microbial invasions through interactions with bacteria and host cells. To investigate the influence of CEF on the pulmonary response to Klebsiella that does not merely result from direct bacterial clearance by the drug, we inoculated mice with heat-killed fluorescein isothiocyanate-labeled K. pneumoniae. CEF upregulated (P < 0.01) the early Klebsiella-induced secretion of tumor necrosis factor alpha, as well as the number (P < 0.01) and phagocytic efficacy (P < 0.001) of alveolar macrophages. By contrast, the late polymorphonuclear neutrophil recruitment (P < 0.05) and levels of interleukin-1 alpha (IL-1α) (P < 0.05) and IL-6 (P < 0.05) were reduced. The stimulation of an early immune response by CEF followed by late reduction in inflammation may be beneficial against bacterial pneumonia.
Bacterial pneumonia is the leading cause of death among all nosocomial infections (20), and Klebsiella pneumoniae remains one of the main gram-negative causative agents (6). Virulent strains produce large capsules that hamper phagocytosis by resident alveolar macrophages (8), which results in multilobar pneumonia, bacteremia, and high mortality rate. There is also growing evidence that overwhelming inflammatory reactions participate in fatal pneumonia induced by gram-positive and gram-negative strains (reviewed in reference 3). In addition, an increasing percentage of nosocomial K. pneumoniae isolates are becoming resistant to multiple antibiotics commonly used in intensive care units. These observations emphasize the importance of using treatments that optimize host response through beneficial drug-host-bug interactions.
Cefodizime (CEF) is an expanded spectrum cephalosporin that shows good experimental and clinical efficacy against lower respiratory tract infections induced by K. pneumoniae (2, 12, 31, 32). The drug has already been shown to enhance the bactericidal activity of phagocytes against K. pneumoniae in a mouse model of peritonitis (27). This apparent immunoenhancing property resulted from reduction of the thickness of the newly synthesized capsule as bacteria divided and tried to proliferate in the presence of sub-MIC levels of CEF (28). As a consequence, the surface charge density was modified (25), facilitating binding of the complement and therefore promoting phagocytosis (26). The antibiotic was also claimed to display various immunomodulatory effects (4, 11, 13, 15, 16, 21, 22, 24, 36–38) that apparently accounted for its capacity to increase survival of mice infected with CEF-resistant pathogens (12–14, 19). However, the influence of CEF on the inflammatory host response has never been studied in K. pneumoniae pneumonia.
While CEF may interfere with cell division of live bacteria, thus influencing the course of inflammation, we investigated the pulmonary response in mice inoculated with heat-killed bacteria that cannot be disrupted by the drug and whose capsule may not be fragilized through cell division. We labeled K. pneumoniae with fluorescein isothiocyanate (FITC) to follow phagocytosis through flow cytometry techniques (4, 9, 33). This is the first study to monitor the influence of CEF on inflammatory cell recruitment, phagocytosis efficacy, and cytokine release (tumor necrosis factor alpha [TNF-α], interleukin-1 alpha [IL-1α], and IL-6) in lungs of mice infected with K. pneumoniae.
K. pneumoniae ATCC 8047 was cultured for 12 h at 37°C in brain heart infusion broth, inactivated by heating at 60°C for 1.5 h, and labeled with FITC by stirring 108 CFU/ml for 2 h in 0.5 M carbonate-bicarbonate buffer (pH 9.5) containing 0.2 mg of FITC per ml. Bacteria were then washed and resuspended in phosphate-buffered saline (PBS) for inoculation to animals. Lightly anesthetized CD1 Swiss mice (20 to 22 g) received one intranasal dose of 107 heat-killed FITC-labeled K. pneumoniae cells contained in 50 μl of PBS. To facilitate the migration of the inoculum to the alveoli, animals were held in a vertical position for 2 min. This technique with inactivated fluorescent bacteria was shown to stimulate pulmonary inflammation in 100% of the mice without contamination from the upper airways (4, 9).
CEF was dissolved in saline and injected subcutaneously (s.c.) to 30 mice in doses of 30 mg/kg of body weight every 12 h from 4 days before until 8 h after inoculation with K. pneumoniae. Thirty infected control animals received saline. Additional controls included uninfected mice that were treated with CEF or left untreated. The schedule of CEF administration was based upon previously reported in vivo immunomodulatory effects in humans and animals (4, 19, 38). Five animals per group were sacrificed either before infection (time zero) or at 1, 2, 4, 12, or 24 h postinfection. At the time of sacrifice, mice were killed by cervical dislocation and bronchoalveolar lavages (BAL) were performed to follow leukocyte recruitment, phagocytosis efficacy, and cytokine release.
Leukocyte recruitment in alveoli was evaluated by harvesting a total of 3 ml of BAL fluid in cold PBS. After centrifugation at 3,400 × g for 10 min, supernatants were used to detect cytokines, while inflammatory cells in the pellet were fixed in a final solution of PBS–1% paraformaldehyde. TNF-α, IL-1α, and IL-6 were quantified by using commercial enzyme-linked immunosorbent assay kits (TNF-α, no. 80-2802-00; IL-1α, no. 1900-01; IL-6, no. 80-3748-01; Genzyme Corporation, Cambridge, Mass.). Total leukocytes were quantified with a hemacytometer, and macrophages and polymorphonuclear neutrophils (PMNs) were distinguished on Diff-Quick (no. B4132-1; Baxter, Pointe-Claire, Québec, Canada)-stained cytospin preparations. Cell suspensions were also analyzed with an Epics 753 flow cytometer (Coulter Electronics) as already described (4, 9), to quantify both the number of cells actively involved in phagocytosis and the mean intensity of fluorescence per phagocytosing cell, which depends on the number of ingested bacteria per phagocyte. Both parameters in this experiment reflected in vivo rather than ex vivo phagocytosis, as bacteria were exposed to phagocytes before the latter were harvested from lungs.
Statistical analysis of the difference between groups was performed on StatView SE+ Graphics (Abaccus Concepts Inc., Berkeley, Calif.) by analysis of variance, using a least-squares method. If the F test indicated a difference within groups (P < 0.05), group comparisons were performed by using the Fisher PLSD (protected least significant difference) test and a P value of <0.05 was considered significant. Data are presented as means ± standard errors of the mean (SEM).
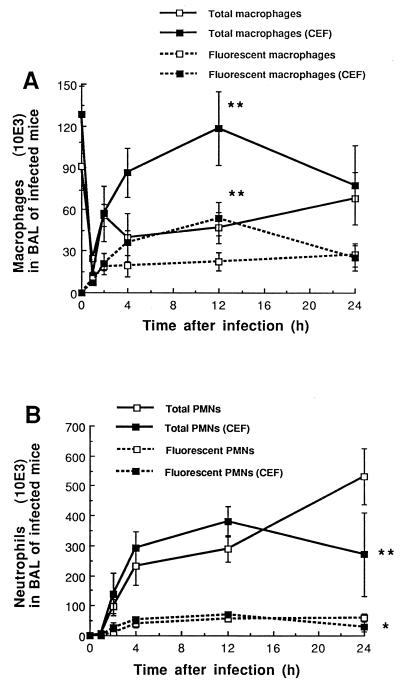
Figure 1A shows the influence of CEF on the total number of macrophages recovered in the BAL fluid of mice inoculated with K. pneumoniae, as well as on the number of macrophages actively involved in phagocytosis. The total number of macrophages sharply decreased shortly (1 h) after infection in both treated and untreated animals. However, significantly better recovery was observed thereafter in the CEF-treated mice, with counts 2.5-fold higher than in the untreated group (P < 0.01 at 12 h). Flow cytometry analysis confirmed the larger amount of phagocytosing cells, as shown by high fluorescence counts in the CEF group at 12 h (P < 0.01). In addition, the mean fluorescence intensity per phagocytosing cell at 1 h (an apparently critical time for macrophage-bacteria interactions) proved to be higher in the CEF group, with 74.9 ± 3.5 versus 59.1 ± 3.2 U for the CEF-treated versus the untreated infected mice, respectively (P < 0.001). CEF in uninfected mice had no influence on any cell population count.
FIG. 1.
Influence of CEF on the differential count of macrophages (A) and PMNs (B) recovered in BAL fluid, as well as on the total amount of cells actively involved in phagocytosis (fluorescent cells), after intranasal inoculation of mice with 107 heat-killed FITC-labeled K. pneumoniae cells. CEF was administered s.c. twice daily at 30 mg/kg from 4 days before until 8 h after infection. Data are means ± SEM for five determinations. ∗, P < 0.05; ∗∗, P < 0.01; both between CEF-treated and untreated animals.
Figure 1B shows the profile of PMN recruitment in BAL fluid of mice exposed to K. pneumoniae. PMNs were essentially absent from alveolar spaces before infection and in negligible amounts at 1 h postinfection. Steady growth in PMN recruitment occurred thereafter in untreated animals, reaching peak values of 533 × 103 cells/ml at 24 h, while a significant decrease was observed at the same time in the CEF-treated animals (P < 0.01). The number of phagocytosing PMNs was also decreased by CEF at 24 h (30 ± 16 versus 61 ± 12, P < 0.05). Treatment of control uninfected mice with CEF did not induce cell recruitment, as we had previously published (4).
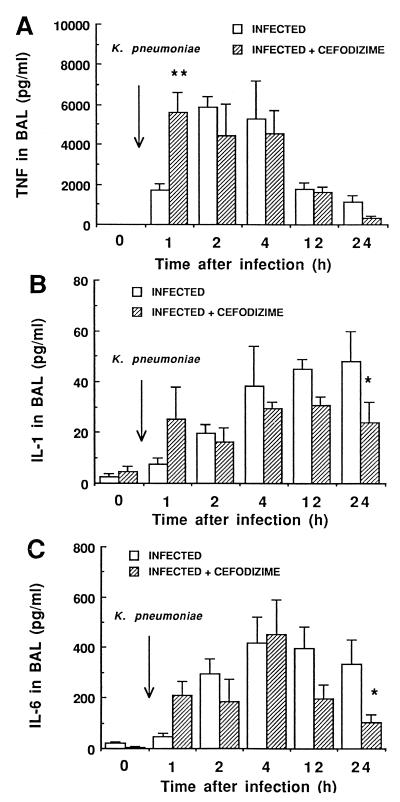
Figure 2 shows TNF-α, IL-1α, and IL-6 release in BAL fluid of mice. All three cytokines were essentially absent in BAL fluid before infection (negligible amounts of IL-1), despite repeated prophylactic administration of CEF. Inoculation of untreated mice infected with K. pneumoniae resulted in an early high TNF secretion (peak level of 5,859 pg/ml at 2 h) (Fig. 2A), but the cytokine secretion rapidly decreased thereafter. Treatment of mice with CEF triggered the early TNF release (significantly higher levels at 1 h; P < 0.01). IL-1 levels in untreated infected animals (Fig. 2B) remained low throughout the experiment (0 to 40 pg/ml), although a steady increase was observed from time zero to 24 h. By contrast, CEF contributed to decreasing the late secretion of this proinflammatory cytokine (P < 0.05 at 24 h). As for IL-6, the same inhibitory effect by CEF characterized the host response at 24 h (P < 0.05).
FIG. 2.
Influence of CEF on TNF-α, IL-1α, and IL-6 levels detected in the supernatant of BAL fluid from CD1 Swiss mice inoculated with 107 heat-killed FITC-labeled K. pneumoniae cells. CEF was given s.c. twice daily at 30 mg/kg from 96 h before to 8 h after infection. Data shown are means ± SEM for five determinations. ∗, P < 0.05, ∗∗, P < 0.01; both between CEF-treated and untreated animals.
Our results suggest that CEF in this model contributed to stimulating the early pulmonary response to K. pneumoniae, including TNF release and the recruitment and phagocytic efficacy of macrophages. These early events most likely contributed to downmodulating the late host response, including PMN recruitment and IL-1 and IL-6 levels, as the infection subsided. The model of infection that we used did not allow bacteria to proliferate, and clearance strictly resulted from phagocytosis rather than from disruption provoked by the drug. Our observations confirm that, besides the usual bactericidal properties of CEF which obviously contribute to modulate pathogenesis and inflammation during infection with live bacteria, CEF can also influence the host response through additional drug-host-bug interactions.
In fact, CEF was already shown to exert positive effects on chemotaxis and/or phagocytosis of macrophages or PMNs in some in vitro, ex vivo, or in vivo experiments (10–14, 17, 19, 21, 23, 29, 37, 38). Although the immunomodulating properties of the drug were attributed to the thio-thiazolyl side chain localized at position 3 of the cephem nucleus, few satisfactory mechanisms of action were evoked, except for its potential affinity for surface components, such as membrane glycoproteins (10). CEF in our experiment may have interacted with binding sites specific to the complement or with epitopes or receptors that trigger cytokine or chemokine release. Although it seems unlikely that binding of CEF to penicillin binding proteins would have altered peptidoglycan or the thickness of the outer capsule of inactivated K. pneumoniae, we do not exclude the possibility that leukocytes were activated upon contact with CEF-exposed bacteria. We believe that independently of the mechanism involved, the influence of CEF is beneficial both through its early activation of the immune response that was shown to be necessary for bacterial clearance in various animal models (1, 5, 18, 34, 35) and through the late inhibition of inflammatory reactions that are associated with tissue damage and death during the evolution of pneumonia (reviewed in reference 3).
We are the first to report an increased intrapulmonary response of alveolar macrophages to K. pneumoniae in animals treated with CEF. While a high TNF secretion coincided with the elevated activity of phagocytosing macrophages in the CEF group (intense fluorescence), other cytokines, such as granulocyte-macrophage colony-stimulating factor (30), and chemokines may have participated in macrophage recruitment. In fact, the macrophage kinetics that were observed in alveoli (BAL fluid) during the first 4 h after bacterial inoculation were quite interesting. While the initial sharp decrease over the first hour may be interpreted in terms of death among phagocytosing macrophages that faced the inoculum, the quick replacement over the subsequent 3 h possibly reflects recruitment of interstitial macrophages from lung tissue rather than monocyte recruitment from the bloodstream, as the latter occurs late in the course of pneumococcal infection (3). It seems unlikely, however, that macrophage disruption would have contributed to further enhancing TNF levels in CEF-treated animals, as identical cell counts were observed at 1 h postinfection in both treated infected and untreated infected groups.
The late reduction in inflammation that we observed in this experiment corroborates and enlightens our previously reported findings with mice exposed to repeated inoculations of heat-killed Streptococcus pneumoniae that were also treated with CEF (4). By stimulating the early immune response, CEF apparently contributes to accelerating healing, thus explaining the low PMN counts and cytokine levels recovered in BAL fluid at later times in the pathogenesis process. In the present experiment (unique bacterial inoculation), IL-1 and IL-6 levels were reduced by CEF, while the TNF level was already low in untreated mice 24 h after infection. Although PMNs are capable of secreting IL-1 and IL-6 (7) and macrophages were mostly associated with TNF in our experiment, localization of the cellular sources affected by CEF remains to be done. In addition, CEF deserves further investigation when therapy is initiated after inhalation of either living or inactivated bacteria by animals. Gamma interferon could also be quantified as an additional marker of inflammation in pneumonia.
The influence of antibiotics on inflammation is an expanding field of research, especially as bacterial resistance to traditional drugs is rapidly increasing and people seek to optimize host response to infectious agents with immunomodulator drugs. Antibiotics that enhance host resistance and protect the host through direct or indirect effects on inflammation, in addition to their bactericidal properties, hopefully will contribute to improving therapy of threatening infections. For each new antibiotic investigated, in vitro MICs and in vivo pharmacokinetic data, as well as immunology determinations, should be provided.
Acknowledgments
This work was supported by a grant from Hoechst Marion Roussel, Romainville, France.
We thank Maurice Dufour for flow cytometry analysis.
REFERENCES
- 1.Amura C R, Fontan P A, Sanjuan N, Sordelli D O. The effect of treatment with interleukin-1 and tumor necrosis factor on Pseudomonas aeruginosa lung infection in a granulocytopenic mouse model. Clin Immunol Immunopathol. 1994;73:261–266. doi: 10.1006/clin.1994.1196. [DOI] [PubMed] [Google Scholar]
- 2.Barré J. Pharmacokinetics of cefodizime: a review of the data on file. J Antimicrob Chemother. 1990;26(Suppl. C):95–101. doi: 10.1093/jac/26.suppl_c.95. [DOI] [PubMed] [Google Scholar]
- 3.Bergeron Y, Ouellet N, Deslauriers A M, Simard M, Olivier M, Bergeron M G. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect Immun. 1998;66:912–922. doi: 10.1128/iai.66.3.912-922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergeron Y, Ouellet N, Deslauriers A M, Simard M, Olivier M, Bergeron M G. Reduction by cefodizime of the pulmonary inflammatory response induced by heat-killed Streptococcus pneumoniae in mice. Antimicrob Agents Chemother. 1998;42:2527–2533. doi: 10.1128/aac.42.10.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broug-Holub E, Toews G B, Van Iwaarden J F, Strieter R M, Kunkel S L, Paine R, Standiford T J. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumonia: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect Immun. 1997;65:1139–1146. doi: 10.1128/iai.65.4.1139-1146.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter J L. Klebsiella pulmonary infections: occurrence at one medical center and review. Rev Infect Dis. 1990;12:672–682. doi: 10.1093/clinids/12.4.672. [DOI] [PubMed] [Google Scholar]
- 7.Cassatella M A. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 8.Domenico P, Straus D C. Extracellular polysaccharide production by Klebsiella pneumoniae and its relation to virulence. Can J Microbiol. 1985;32:472–478. doi: 10.1139/m85-088. [DOI] [PubMed] [Google Scholar]
- 9.Duong M, Simard M, Bergeron Y, Ouellet N, Côté-Richer M, Bergeron M G. Immunomodulating effects of HMR 3004 on pulmonary inflammation caused by heat-killed Streptococcus pneumoniae in mice. Antimicrob Agents Chemother. 1998;42:3309–3312. doi: 10.1128/aac.42.12.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fietta A, Bersani C, Bertoletti R, Grassi F M, Grassi G G. In vitro and ex vivo enhancement of nonspecific phagocytosis by cefodizime. Chemotherapy. 1988;34:430–434. doi: 10.1159/000238603. [DOI] [PubMed] [Google Scholar]
- 11.Gialdroni-Grassi G, Shah P M. Cefodizime host-defence enhancement: considerations of dose-response relationships in healthy volunteers. Infection. 1992;20(Suppl. 1):S51–S53. doi: 10.1007/BF01709953. [DOI] [PubMed] [Google Scholar]
- 12.Klesel N, Limbert M, Seibert G, Winkler I, Schrinner E. Cefodizime, an aminothiazolyl cephalosporin. III. Therapeutic activity against experimentally induced pneumonia in mice. J Antibiot. 1984;37:1712–1718. doi: 10.7164/antibiotics.37.1712. [DOI] [PubMed] [Google Scholar]
- 13.Labro M T. Cefodizime as a biological response modifier: a review of its in vivo, ex vivo and in vitro immunomodulatory properties. J Antimicrob Chemother. 1990;26(Suppl. C):37–47. doi: 10.1093/jac/26.suppl_c.37. [DOI] [PubMed] [Google Scholar]
- 14.Labro M T. Immunological evaluation of cefodizime: a unique molecule among cephalosporins. Infection. 1992;20(Suppl. 1):S45–S47. doi: 10.1007/BF01709951. [DOI] [PubMed] [Google Scholar]
- 15.Labro M T. Experimental evaluation of antibiotics as immunomodulators. J Chemother. 1994;6(Suppl. 3):11–15. [PubMed] [Google Scholar]
- 16.Labro M T. The prohost effect of antimicrobial agents as a predictor of clinical outcome. J Chemother. 1997;9:100–108. [PubMed] [Google Scholar]
- 17.Labro M T, Amit N, Babin-Chevaye C, Hakim J. Cefodizime (HR 221) potentiation of human neutrophil oxygen-independent bactericidal activity. J Antimicrob Chemother. 1987;19:331–341. doi: 10.1093/jac/19.3.331. [DOI] [PubMed] [Google Scholar]
- 18.Laichalk L L, Kunkel S L, Strieter R M, Danforth J M, Bailie M B, Standiford T J. Tumor necrosis factor mediates lung antibacterial host defense in murine Klebsiella pneumonia. Infect Immun. 1996;64:5211–5218. doi: 10.1128/iai.64.12.5211-5218.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Limbert M, Bartlett R R, Dickneite G, Klesel N, Schorlemmer H U, Seibert G, Winkler I, Schrinner E. Cefodizime, an aminothiazolyl cephalosporin. IV. Influence on the immune system. J Antibiot. 1984;37:1719–1726. doi: 10.7164/antibiotics.37.1719. [DOI] [PubMed] [Google Scholar]
- 20.Maloney S B, Williams W J. Epidemic nosocomial pneumonia in the intensive care unit. Clin Chest Med. 1995;16:209–223. [PubMed] [Google Scholar]
- 21.McCafferty A C, McGregor E, Jones M, Henderson J S, Cree I A. The effect of cefodizime on phagocyte function in non-patient volunteers and patients with chronic renal failure. In vitro and ex vivo studies. Int J Clin Lab Res. 1996;26:229–235. doi: 10.1007/BF02602954. [DOI] [PubMed] [Google Scholar]
- 22.Meloni F, Ballabio P, Bianchi L, Grassi F A, Gialdroni-Grassi G G. Cefodizime modulates in vitro tumor necrosis factor-alpha, interleukin-6 and interleukin-8 release from human peripheral monocytes. Chemotherapy. 1995;41:289–295. doi: 10.1159/000239358. [DOI] [PubMed] [Google Scholar]
- 23.Meroni P L, Capsoni F, Borghi M O, Barcellini W, Minonzio F, et al. Immunopharmacological activity of cefodizime in young and elderly subjects: in vitro and ex vivo studies. Infection. 1992;20(Suppl. 1):S61–S63. doi: 10.1007/BF01709956. [DOI] [PubMed] [Google Scholar]
- 24.Morikawa K, Watabe H, Araake M, Morikawa S. Modulatory effect of antibiotics on cytokine production by human monocytes in vitro. Antimicrob Agents Chemother. 1996;40:1366–1370. doi: 10.1128/aac.40.6.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muratsugu M, Miyake Y, Ishida N, Hyodo A, Terayama K. Decrease in surface charge density of Klebsiella pneumoniae treated with cefodizime and enhancement of the phagocytic function of human polymorphonuclear leukocytes stimulated by the drug-treated bacteria. Biol Pharm Bull. 1995;18:1259–1263. doi: 10.1248/bpb.18.1259. [DOI] [PubMed] [Google Scholar]
- 26.Nomura S, Kuroiwa A, Murata K, Nagayama A. Binding of complement C3 to Klebsiella pneumoniae treated with sub-MIC cefodizime and chemiluminescence response of human polymorphonuclear leukocytes. Chemotherapy. 1997;43:132–136. doi: 10.1159/000239547. [DOI] [PubMed] [Google Scholar]
- 27.Nomura S, Nagayama A. In vitro and in vivo enhancement of bactericidal activity of phagocytes against Klebsiella pneumoniae treated with subminimal inhibitory concentrations of cefodizime. Chemotherapy. 1995;41:178–186. doi: 10.1159/000239341. [DOI] [PubMed] [Google Scholar]
- 28.Nomura S, Nagayama A. Mechanism of enhancement of bactericidal activity of phagocytes against Klebsiella pneumoniae treated with subminimal inhibitory concentrations of cefodizime. Chemotherapy. 1995;41:267–275. doi: 10.1159/000239355. [DOI] [PubMed] [Google Scholar]
- 29.Oishi K, Matsumoto K, Yamamoto M, Morito T, Yoshida T. Stimulatory effect of cefodizime on macrophage-mediated phagocytosis. J Antibiot. 1989;42:989–992. doi: 10.7164/antibiotics.42.989. [DOI] [PubMed] [Google Scholar]
- 30.Pacheco Y, Hosni R, Dagrosa E E, Gormand F, Guibert B, Chabannes B, Lagarde M, Perrin-Fayolle M. Antibiotics and production of granulocyte-macrophage colony-stimulating factor by human bronchial epithelial cells in vitro. A comparison of cefodizime and ceftriaxone. Drug Res. 1994;44:559–563. [PubMed] [Google Scholar]
- 31.Pauwels R A. Review of effectiveness of cefodizime in the treatment of lower respiratory tract infections with parenchymal involvement. Infection. 1992;20(Suppl. 1):S26–S30. doi: 10.1007/BF01709947. [DOI] [PubMed] [Google Scholar]
- 32.Piovano C F, Palombini B C, Dagrosa E E, Mendoza F, Facco E B. Cefodizime once daily in the treatment of lower respiratory tract infections. Arzneim-Forsch. 1997;47:674–677. [PubMed] [Google Scholar]
- 33.Simpson S Q, Singh R, Bice D E. Heat-killed pneumococci and pneumococcal capsular polysaccharides stimulate tumor necrosis factor-alpha production by murine macrophages. Am J Respir Cell Mol Biol. 1994;10:284–289. doi: 10.1165/ajrcmb.10.3.8117447. [DOI] [PubMed] [Google Scholar]
- 34.Takashima K, Tateda K, Matsumoto T, Ilzawa Y, Nakao M, Yamaguchi K. Role of tumor necrosis factor alpha in pathogenesis of pneumococcal pneumonia in mice. Infect Immun. 1997;65:257–260. doi: 10.1128/iai.65.1.257-260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai W C, Strieter R M, Zisman D A, Wilkowski J M, Bucknell K A, Chen G H, Standiford T J. Nitric oxide is required for effective innate immunity against Klebsiella pneumoniae. Infect Immun. 1997;65:1870–1875. doi: 10.1128/iai.65.5.1870-1875.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Vlem B, Vanholder R, De Paepe P, Vogelaers D, Ringoir S. Immunomodulating effects of antibiotics: literature review. Infection. 1996;24:275–291. doi: 10.1007/BF01743360. [DOI] [PubMed] [Google Scholar]
- 37.Wenisch C, Bartunek A, Zedtwitz-Liebenstein K, Hiesmayr M, Parschalk B, Pernerstorfer T. Prospective randomized comparison of cefodizime versus cefuroxime for perioperative prophylaxis in patients undergoing coronary artery bypass grafting. Antimicrob Agents Chemother. 1997;41:1584–1588. doi: 10.1128/aac.41.7.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wenisch C, Parschalk B, Hasenhundl M, Wiesinger E, Graninger W. Effect of cefodizime and ceftriaxone on phagocytic function in patients with severe infections. Antimicrob Agents Chemother. 1995;39:672–676. doi: 10.1128/AAC.39.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]