Abstract
Simple Summary
Genotyping of BRCA genes is required for treatment with PARP inhibitors. Purposing to address treatment needs and familial cancer risk, the aim of our study was to introduce the most appropriate testing workflow in epithelial ovarian cancer patients (EOC) using germline and tumor genotyping of BRCA and other hereditary breast and/or ovarian cancer (HBOC) susceptibility genes. In consecutive patients with advanced non-mucinous EOC, who responded to platinum-based chemotherapy, germline and tumor genotyping were performed using Illumina’s next generation sequencing (NGS) panels. Sensitivity of tumor genotyping for detection of germline pathogenic/likely pathogenic variants (PV/LPV) was 96.2% for BRCA and 93.3% for HBOC genes. With germline genotyping-only strategy, 58.8% of HBOC PV/LPV and 68.4% of BRCA PV/LPV were detected. By tumor genotyping-only, 96.1% of HBOC PV/LPV and 97.4% of BRCA PV/LPV were detected. Tumor genotyping first followed by germline genotyping detects nearly all germline and somatic PV/LPV in the shortest time.
Abstract
Detection of germline and somatic pathogenic/likely pathogenic variants (PV/LPV) in BRCA genes is at the moment a prerequisite for use of PARP inhibitors in different treatment settings of different tumors. The aim of our study was to determine the most appropriate testing workflow in epithelial ovarian cancer (EOC) patients using germline and tumor genotyping of BRCA and other hereditary breast and/or ovarian cancer (HBOC) susceptibility genes. Consecutive patients with advanced non-mucinous EOC, who responded to platinum-based chemotherapy, were included in the study. DNA extracted from blood and FFPE tumor tissue were genotyped using NGS panels TruSightCancer/Hereditary and TruSight Tumor 170. Among 170 EOC patients, 21.8% had BRCA germline or somatic PV/LPV, and additionally 6.4% had PV/LPV in other HBOC genes. Sensitivity of tumor genotyping for detection of germline PV/LPV was 96.2% for BRCA genes and 93.3% for HBOC genes. With germline genotyping-only strategy, 58.8% of HBOC PV/LPV and 68.4% of BRCA PV/LPV were detected. By tumor genotyping-only strategy, 96.1% of HBOC PV/LPV and 97.4% of BRCA PV/LPV were detected. Genotyping of tumor first, followed by germline genotyping seems to be a reasonable approach for detection of PV/LPV in breast and/or ovarian cancer susceptibility genes in non-mucinous EOC patients.
Keywords: BRCA, HBOC, ovarian cancer, tumor genotyping, germline genotyping, PARP inhibitors, reverse mutation
1. Introduction
Ovarian cancer (OC) is the sixth leading cause of cancer death among Slovene women, according to the data from the Cancer Registry of Republic of Slovenia. The incidence of OC in Slovenia is slowly declining, with 130 new cases in 2016 [1]. The most important risk factor for ovarian cancer is a family history of breast and ovarian cancer, often in association with inherited variants in BRCA1 and BRCA2 as well as in other breast and/or ovarian cancer susceptibility genes [2].
Different subtypes of OC have diverse origins, genetic alterations, and clinic-pathological features [3]. Among epithelial ovarian cancer (EOC) tumors, high-grade serous carcinomas (HGSC) are highly aggressive, resulting in poor overall prognosis. Genetically, 95% of HGSC tumors harbor TP53 pathogenic/likely pathogenic variants (PV/LPV) and more than 50% display homologous recombination deficiency [4,5]. According to early reports from the Cancer Genome Atlas data (TCGA), 20% of HGSC harbor a PV/LPV in the BRCA1 or BRCA2 gene, of which 17% are germline and 3% are somatic PV/LPV [4]. Some subsequent reports, including the registration study for Olaparib, report up to 50% of BRCA positive tumors in the population of patients with serous EOC [6]. In aforementioned subsequent studies reported percentage of somatic PV/LPV in BRCA genes ranged from 20% to 46% of all BRCA PV/LPV detected in EOC patients [6,7]. Pathogenic/likely pathogenic variants in BRCA1 and BRCA2 genes were also reported in endometroid and clear cell ovarian carcinoma in 15% (4/26) and 10% (2/19), respectively [5,8].
Genetic counseling and testing for germline variants in BRCA1 and BRCA2 and in other hereditary breast and/or ovarian cancer (HBOC) susceptibility genes was established when the significance of hereditary cancer screening and preventive measures was recognized. Furthermore, testing for PV/LPV in BRCA genes has also become important for personalized treatment in patients with epithelial ovarian carcinoma.
According to the 2020 American Society of Clinical Oncology (ASCO) guidelines, testing for germline variants in BRCA and other susceptibility genes should be performed in all patients with EOC regardless of the clinical features or family history of cancer [9]. In addition, in the Recommendation 1.1. of the very same guidelines, it is stated that “Somatic tumor testing for BRCA1 and BRCA2 pathogenic or likely pathogenic variants should be performed in women who do not carry a germline pathogenic or likely pathogenic BRCA1/2 variant” [9].
European Society for Medical Oncology and European Society of Gynecological Oncology (ESMO-ESGO) consensus conference recommendations on ovarian cancer published in 2019 recognize that poly-ADP ribose polymerase (PARP) inhibitors have greatest activity in patients with PV/LPV in BRCA genes, and thus testing for BRCA1/2 PV/LPV is recommended for all non-mucinous ovarian cancer [10]. They also suggest to consider testing for PV/LPV in other homologous recombination (HR) genes (particularly RAD51C, RAD51D, BRIP1, and PALB2), since studies have shown that tumors harboring PV/LPV in those HR genes respond to PARP inhibitors [10,11]. ESMO-ESGO recommendations also emphasize the importance of the detection of germline and somatic PV/LPV in BRCA genes, but give no precise recommendations on the testing strategy. In Slovenia, PARP inhibitors are prescribed as the first line treatment for advanced ovarian cancer (AOC) patients if the tumor harbors germline or somatic BRCA PV/LPV. Therefore, BRCA status should be determined in AOC patients before treatment with PARP inhibitors. When planning the testing strategy, it is important to recognize that 54–80% of all BRCA1/2 PV/LPV in EOC tumors are germline, while the remaining variants are somatic [6,12]. Since patients benefit from treatment regardless whether variants are germline or somatic, it appears reasonable to test tumor tissue first, followed by germline testing from normal tissue (preferable from blood) for the purposes of genetic counseling. Tumor testing itself would theoretically reveal somatic as well as germline PV/LPV. However, the genotyping of tumor samples is challenging, since tumor tissue is most commonly preserved by formalin fixation causing DNA fragmentation, which may affect downstream genomic analyses [13]. In addition, around 50% of EOC have deficient homologous recombination, leading to extensive gains and losses of genomic material, which could additionally impair the detection of PV/LPVs in tumor tissue [4]. The aim of the study was to verify that formalin-fixed paraffin-embedded (FFPE) tumor tissue sequencing could reliably detect germline variants using matched tumor–blood samples. The study compares detected PV/LPV from tumors with the PV/LPV from blood samples of the same patients. It includes an assessment of efficiency of tumor and germline genotyping techniques to detect clinically actionable PV/LPV in BRCA genes and other hereditary breast and/or ovarian cancer susceptibility genes, as well as the most effective patient management pathways especially considering patients’ responsiveness to genetic counseling referral. The final aim was to introduce the most appropriate testing workflow, which considers the medical implications and capacities of genetic counseling services as well as the needs of medical oncologists for personalized treatment decisions.
2. Materials and Methods
2.1. Study Design
In the study, 170 consecutive patients, with either newly diagnosed advanced or recurrent non-mucinous epithelial ovarian cancer (non-mucinous EOC), including fallopian tube cancer and primary peritoneal serous cancers, who responded to platinum-based chemotherapy, were included from January 2019 to April 2020. Borderline EOC were not included in the study. All patients in this study were treated and tested at the Institute of Oncology, Ljubljana, Slovenia. The study was approved by National Ethics Committee (permission no. 100/05/15 on 27 July 2015 and permission no. 0120-303/218/3 on 13 June 2018) and Institutional Ethics Committee of Institute of Oncology Ljubljana (IOL) (permission no. ERID-KESOPKR/97 on 17 September 2015 and permission no. ERID-EK/136 on 21 May 2018), and registered at ISRCTN registry on 24 November 2015 (ISRCTN42408038). Patients’ samples were obtained after appropriate informed consents.
Inclusion and exclusion criteria were the same as described previously by our group [14]. Inclusion criteria were as follows: WHO performance status 0–1, histologically non-mucinous EOC, tubal or primary peritoneal cancer, availability of tumor tissue samples, indication for treatment with surgery (primary debulking or interval debulking) and chemotherapy with platinum agents (adjuvant or neoadjuvant or treatment of metastatic disease), normal kidney function (creatinine < 100 µmol/L, endogenous creatinine clearance (ECC) > 75 mL/L), and signed informed consent. Exclusion criteria were as follows: mucinous EOC, unavailability of FFPE tissue samples, WHO performance status ≥2, and surgery and/or platinum-based chemotherapy not indicated.
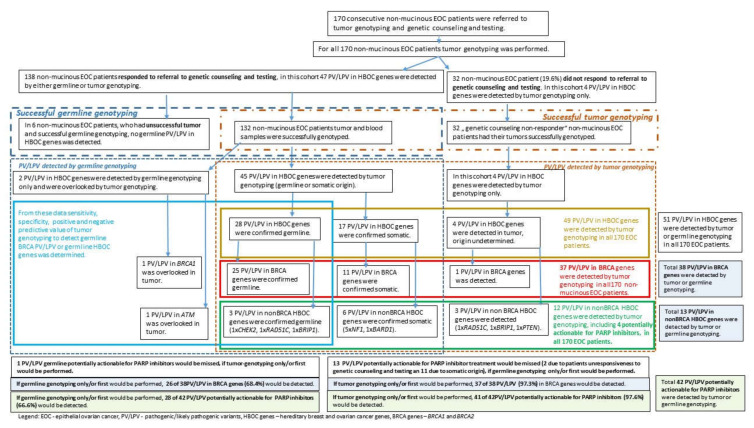
All patients’ tumor samples were genotyped for the presence of BRCA1 and BRCA2 PV/LPV as well as for PV/LPV in other hereditary breast and/or ovarian cancer susceptibility genes. In parallel, all patients were referred to genetic counseling and germline testing for PV/LPV in hereditary breast and/or ovarian cancer susceptibility genes. Until the end of April 2020, 138 out of 170 patients with genotyped tumors underwent genetic counseling and germline genetic testing (group of matched tumor–germline samples) (Scheme 1). Family history of BRCA-related cancers (breast, ovarian, prostate, and pancreatic adenocarcinoma) was evaluated based on a questionnaire and reviewed by a clinical geneticist. Additionally, most diagnoses of cancer were verified in the Cancer Registry of Republic of Slovenia (http://www.slora.si/en, accessed on 15 May 2020), if possible. Genetic counseling was performed at the Department of Clinical Cancer Genetics IOL, while tumor and germline genetic testing was performed at the Department of Molecular Diagnostics IOL.
Scheme 1.
Study workflow.
For this study, a virtual gene panel of 19 hereditary breast and/or ovarian cancer (HBOC) susceptibility genes was used for tumor and germline genotyping: ATM (LRG_135t1), BARD1 (LRG_297t1), BRCA1 (LRG_292t1), BRCA2 (LRG_293t1), BRIP1 (LRG_300t1), CDH1 (LRG_301t1), CHEK2 (LRG_302t1), MLH1 (LRG_216t1), MSH2 (LRG_218t1), MSH6 (LRG_219t1), NF1 (LRG_214t1), NBN (LRG_158t1), PALB2 (LRG_308t1), PMS2 (LRG_161t1), PTEN (LRG_311t1), RAD51C (LRG_314t1), RAD51D (LRG_516t1), STK11 (LRG_319t1), and TP53 (LRG_321t1).
Since data from the literature indicate that majority (95%) of HGSC tumors harbor TP53 PV/LPV, the TP53 gene was excluded from analysis. The narrowed-down targeted gene panel is referred to as “HBOC genes” and it includes all above-described breast and/or ovarian cancer susceptibility genes with the exception of the TP53 gene. In the following content “BRCA genes” include only the BRCA1 and BRCA2 genes.
2.2. Samples
Tumor samples were all formalin fixed paraffin embedded (FFPE), and were evaluated by a pathologist according to the established laboratory protocol at the Department of Pathology at the IOL or at the Clinic of Gynecology and Obstetrics, Ljubljana University Medical Centre, Slovenia. Sample evaluation comprised of histological examination and conformation of the tumor type, determination of the percentage of tumor cells in the sample, and selection of the representative tumor block for DNA isolation. From the selected tumor block 10 μm thick slides were cut; the first and last slide were eosin–hematoxylin stained and used to visualize the presence of tumor cells, and to guide macro-dissection of tumor area from the unstained slides.
Whole blood samples were collected in EDTA tubes.
2.3. DNA Isolation
According to the established laboratory protocol genomic DNA was isolated from a whole blood sample using InnuPREP Master Blood Kit (Analytik Jena, Jena, Germany), and from FFPE-tissue sample using GeneRead DNA FFPE Kit (Qiagen GmbH, Hilden, Germany) or MagMax DNA/RNA kit (ThermoFisher Scientific, Woodward, St. Austin, TX, USA). Extracted DNA was quantified using Qubit dsDNA HS Assay kit and fluorimeter Qubit 3.0. (both ThermoFisher Scientific, Eugene, OR, USA).
2.4. Next Generation Sequencing
2.4.1. Tumor Genotyping
Next generation sequencing (NGS) of tumor DNA samples was performed on NextSeq 550 Sequencing System, using TruSight Tumor 170-DNA (Illumina, San Diego, CA, USA) library preparation kit. Bioinformatical analysis was performed as described previously by our group [15]. This panel includes all previously listed HBOC susceptibility genes, where all protein coding exons are included, with the exception of exon 1 and promotor region of BRCA1 and BRCA2 genes, and NF1 and PMS2 genes, which are only partially covered. Samples were successfully sequenced, if >93.5% of targeted regions were covered >250×. The laboratory sensitivity of the method is: (i) >95% for detection of single nucleotide variants (SNV) with variant allele frequency (VAF) > 5%; (ii) >95% for detection of small (2–11 bp) insertion/deletion variants (indels) with VAF > 10%. The laboratory sensitivity and specificity of the method for detection of indels larger than 10 bp was not assessed. The copy number variations (CNVs) in genes of a virtual gene panel was determined using normalized coverage data from TruSight Tumor 170 Local App (Illumina). For BRCA genes only, an intragenic exonic deletion was considered, if two adjacent exons of BRCA genes had fold change under 0.6.
Variants detected in tumor sample were classified for their clinical importance according to ACMG/AMP/ASCO guidelines [16]. Variants are described according to HGVS v20.05 nomenclature [17].
2.4.2. Genotyping for Germline Alterations/Variants
Next generation sequencing of blood DNA samples was performed on Illumina MiSeqDx Sequencing System using TruSight Cancer Panel or TruSight Hereditary Panel (Illumina, San Diego, CA, USA) to enrich and sequence of all translated exons and ±25 bp flanking intronic regions of all HBOC genes, bioinformatics and copy number analysis were performed as described by our group previously [14,15]. Samples were successfully sequenced if >95% of targeted regions were covered >40×. NGS sequencing has 100% sensitivity and specificity for detection of heterozygous germline SNV and small indels (<10 bp) according to Sanger sequencing.
Germline variants were classified for their clinical importance according to ACMG/AMP guidelines [18,19]. Variants are described according to HGVS v20.05 nomenclature [17].
All PV/LPV germline variants in HBOC genes detected by NGS were additionally confirmed by Multiplex Ligation-dependent Probe Amplification analysis or Sanger sequencing.
2.5. Multiplex Ligation-Dependent Probe Amplification (MLPA) and Sanger Sequencing
MLPA was performed to confirm the presence of copy number variations (CNVs) in blood DNA samples—using SALSA P045-BRCA2/CHEK2 Probemix and/or SALSA P002-BRCA1 (both MRC-Holland, Amsterdam, The Netherlands). Direct Sanger sequencing was performed to confirm the presence of SNV and small indels in blood DNA samples. Sanger sequencing and MLPA were performed as described by our group previously [15].
2.6. Statistical Analysis
To validate whether tumor genotyping strategy can successfully detect germline PV/LPV in HBOC genes, matched tumor–blood samples with sufficient genotyping quality were included in the analysis. Blood genotyping was taken as the reference method for germline PV/LPV detection. Since germline as well as tumor genotyping give binary results (positive or negative for PV/LPV), the sensitivity, specificity, positive, and negative predictive values for tumor genotyping to detect germline PV/LPV or patients with germline PV/LPV were calculated.
Sensitivity, specificity and predictive values of positive and negative tumor genotyping result were calculated as follows. Sensitivity of tumor genotyping for detection of patient with germline PV/LPV = a/(a + c). Specificity of tumor genotyping for detection of patient with germline PV/LPV = d/(b + d). Predictive value of a positive tumor genotyping result to predict positive germline genotyping result = a/(a + b). Predictive value of a negative tumor genotyping result to predict negative germline genotyping result = d/(c + d). The meaning of symbols are; a—true positives for germline variants (cases that were positive by germline and tumor genotyping); b—false positives for germline variants (cases that were negative by germline genotyping and positive by tumor genotyping); c—false negatives for germline variants (cases that were positive by germline genotyping and negative by tumor genotyping); d—true negatives for germline variants (cases that were negative by germline and tumor genotyping).
We also performed an assessment of workloads associated with three different approaches to genetic testing in ovarian cancer patients, using descriptive statistics.
3. Results
In the current study, a total of 170 FFPE tumor specimens from consecutive patients with advanced non-mucinous EOC were genotyped for the presence of PV/LPV in BRCA genes and other pre-specified genes. At the time of data analysis, 81.2% (138/170) patients opted for genetic counseling and germline testing; 32 patients whose tumors were successfully genotyped did not undergo genetic counseling and germline testing. Since in six tumor samples, NGS sequencing did not meet the quality criteria threshold, the final number of patients with successfully tested matched tumor-blood samples was 132. Germline NGS testing was performed in 127 (out of 132 blood samples), while 5 blood samples were genotyped using Sanger sequencing only for BRCA PV/LPV detected in tumor (Scheme 1). In our pair-matched cohort 75% (99/132) had confirmed somatic PV/LPV in the TP53 gene.
3.1. Frequencies of Germline and Somatic PV/LPV
Our entire study group was divided in three subgroups—1. subgroup of patients with unsuccessful tumor genotyping; 2. subgroup of patients with successfully matched tumor and germline genotyping; and 3. subgroup of non-responders to referral to genetic counseling and testing. In Table 1, Table 2 and Table 3, cumulative genotyping results are presented. Among all 170 patients included in the study, 51 PV/LPV in HBOC genes were found in 27.1% of patients either by tumor or germline genotyping (Table 1). Germline PV/LPV in HBOC genes were found in 17.1% of patients, while somatic PV/LPV were found in 8.8%. In 2.3% of patients, the origin of PV/LPV was unknown, since these patients did not attend genetic counseling and confirmatory germline testing (subgroup of non-responders). Two germline PV/LPV, found in two different patients (1.2% of 170), were missed by tumor genotyping (one patient with BRCA1 large deletion and another patient with SNV in ATM) (Scheme 1, Table 1).
Table 1.
Pathogenic/likely pathogenic variants (PV/LPV) in HBOC genes among all 170 consecutive advanced non-mucinous EOC patients.
| Group/Subgroup No. of Patients |
PV/LPV (No.) | Patients with PV/LPV (No., %) |
Germline PV/LPV (No.) |
Patients with Germline PV/LPV (No., %) |
Somatic PV/LPV (No.) |
Patients with Somatic PV/LPV (No., %) |
PV/LPV with Unknown Origin * (No.) |
Patients with PV/LPV with Unknown Origin * (No., %) |
|---|---|---|---|---|---|---|---|---|
| Entire study group 170 |
51 | 46 (27.1%) | 30 *** | 29 (17.1%) | 17 | 15 (8.8%) | 4 | 4 (2.3%) |
| 1. patients with unsuccessful tumor genotyping ** 6 |
0 | 0 | 0 | 0 | NA | NA | NA | NA |
| 2. patient with successful tumor and germline genotyping 132 |
47 | 42 (31.8%) | 30 *** | 29 (21.9%) | 17 | 15 (11.3%) | 0 | 0 |
| 3. non-responders 32 |
4 | 4 | NA | NA | NA | NA | 4 | 4 (12.5%) |
%—percent of patients within the group/sub-group; PV/LPV—pathogenic/likely pathogenic variants; *—PV/LPV with unknown origin were found in patients that did not refer to genetic counseling and confirmatory germline testing; **—among 6 patients with unsuccessful tumor genotyping, no PV/LPV was found by germline genotyping (Scheme 1); ***—only 28 germline PV/LPV were detected by tumor genotyping; NA—not applicable.
Table 2.
Pathogenic/likely pathogenic variants (PV/LPV) in BRCA genes among all 170 consecutive advanced non-mucinous EOC patients.
| Group/Subgroup No. of Patients |
PV/LPV (No.) | Patients with PV/LPV (No., %) |
Germline PV/LPV (No.) |
Patients with Germline PV/LPV (No., %) |
Somatic PV/LPV (No.) |
Patients with Somatic PV/LPV (No.,%) |
PV/LPV with Unknown Origin * (No.) |
Patients with PV/LPV with Unknown Origin * (No., %) |
|---|---|---|---|---|---|---|---|---|
| Entire study group 170 |
38 | 37 (21.8%) | 26 | 26 (15.3%) *** | 11 | 11 (6.5%) *** | 1 | 1 (0.6%) |
| 1. patients with unsuccessful tumor genotyping ** 6 |
0 | 0 | 0 | 0 | NA | NA | NA | NA |
| 2. patient with successful tumor and germline genotyping 132 |
37 | 36 (27.2) | 26 | 26 (19.6%) *** | 11 | 11 (8.3%) *** | 0 | 0 |
| 3. non-responders 32 |
1 | 1 (3.1%) | NA | NA | NA | NA | 1 | 1 (3.1%) |
%—percent of patients within the group/sub-group; PV/LPV—pathogenic/likely pathogenic variants; *—PV/LPV with unknown origin were found in patients, that did not refer to genetic counseling and confirmatory germline testing; **—among 6 patients with unsuccessful tumor genotyping, no PV/LPV was found by germline genotyping (Scheme 1); ***—one patient is a carrier of somatic and germline PV/LPV in BRCA2, and one patient is carrier of somatic PV/LPV in BRCA2 and germline PV/LPV in CHEK2. There are 10 patients carrying only somatic BRCA PV/LPV (without germline BRCA PV/LPV); NA—not applicable.
Table 3.
Pathogenic/likely pathogenic variants (PV/LPV) in nonBRCA HBOC genes among all 170 consecutive advanced non-mucinous EOC patients.
| Group/Subgroup No. of Patients |
PV/LPV (No.) |
Patients with PV/LPV (No., %) |
Germline PV/LPV (No.) |
Patients with Germline PV/LPV (No., %) |
Somatic PV/LPV (No.) |
Patients with Somatic PV/LPV (No., %) |
PV/LPV with Unknown Origin * (No.) |
Patients with PV/LPV with Unknown Origin * (No., %) |
|---|---|---|---|---|---|---|---|---|
| Entire study group. 170 |
13 | 11 (6.4%) | 4 *** | 3 (1.7%) | 6 **** | 5 (2.9%) | 3 ***** | 3 (1.7%) |
| 1. patients with unsuccessful tumor genotyping ** 6 |
0 | 0 | 0 | 0 | NA | NA | NA | NA |
| 2. patient with successful tumor and germline genotyping 132 |
10 | 8 (6.0%) | 4 *** | 3 (2.2%) | 6 **** | 5 (3.8%) | 0 | 0 |
| 3. non-responders 32 |
3 | 3 (9.3%) | NA | NA | NA | NA | 3 ***** | 3 (9.3%) |
%—percent of patients within the group/sub-group: PV/LPV—pathogenic/likely pathogenic variants; *—PV/LPV with unknown origin were found in patients that did not refer to genetic counseling and confirmatory germline testing; **—among 6 patients with unsuccessful tumor genotyping, no PV/LPV was found by germline genotyping (Scheme 1); ***—germline PV/LPV in RAD51C, ATM, BRIP1, and CHEK2; ****—somatic PV/LPV in NF1 (5 variants), and in BARD1 (1 variant), one somatic PV/LPV in the NF1 gene was found in patient harboring at the same time germline PV/LPV in the BRCA1 gene and two somatic PV/LPV in NF1 were found in one patient (Tables S1 and S2); *****—one in RAD51C, one in BRIP1 and one in PTEN found in non-responders to genetic counseling/testing (Scheme 1); NA—not applicable.
Five patients had more than one PV/LPV in HBOC genes detected in tumor samples. One patient had a germline CHEK2 PV/LPV and a somatic PV/LPV in the BRCA2 gene, one patient had one germline and one somatic PV/LPV in BRCA2, one patient had one germline PV/LPV in BRCA1 and one somatic PV/LPV in the NF1 gene (Table S1), one had two germline PV/LPV detected, in the ATM and RAD51C genes, and one patient had two somatic PV/LPV in the NF1 gene (Table S2).
Among aforementioned PV/LPV detected in 170 tested patients in the study, PV/LPV in BRCA genes were found in 21.8% of patients (Table 2). Germline PV/LPV in BRCA genes were found in 15.3% of patients, while somatic PV/LPV were found in 6.5% of patients. In one patient somatic as well as germline PV/LPV in the BRCA2 gene were found, while in one patient the origin of PV/LPV in the BRCA1 gene was unknown (Table 2, Scheme 1, and Table S1).
In nonBRCA, HBOC genes PV/LPV were found in 6.4% of patients. (Table 3). Germline PV/LPV in nonBRCA HBOC genes were found in 1.7% of patients (in RAD51C, ATM, BRIP1, and CHEK2) and somatic PV/LPV in 2.9% of patients (five variants in NF1, and one variant in BARD1). In 1.7% of patients, the origin of PV/LPV in nonBRCA HBOC genes (in RAD51C, BRIP1, and PTEN) was unknown since these patients did not attend genetic counseling and confirmatory germline testing (subgroup of non-responders) (Scheme 1 and Table S2).
Retrospective analysis of the subgroup of “non-responders” revealed that 6 of them died less than 4 months after referral to genetic counselling and 13 of them visited genetic counselor after our study’s observation period of 4 months.
3.2. Proportion of Detected PV/LPV in All 170 Consecutive Advanced Non-Mucinous EOC Patients Depending on the Approach to Testing: Tumor Genotyping Only vs. Germline Genotyping Only
In total, 51 PV/LPV in HBOC genes were detected by tumor or germline genotyping in 170 patients. By tumor genotyping only, 49 of 51 (96.1%) PV/LPV would have been detected in 44 patients. Due to patients’ unresponsiveness to genetic counseling/testing (32 patients) and somatic origin of 17 PV/LPV, with germline genotyping only 30 of 51 (58.8%) PV/LPV in HBOC genes would have been detected in 29 patients (Scheme 1, Tables S1 and S2).
In BRCA genes, a total of 38 PV/LPV were detected by tumor or germline genotyping in a cohort of 170 patients. By tumor genotyping only, 37 of 38 (97.4%) PV/LPV would have been detected in 37 patients. With germline genotyping only, 26 of 38 (68.4%) PV/LPV would have been detected in 26 patients (Tables S1 and S2).
If PV/LPV in RAD51C and BRIP1 genes, besides BRCA genes, are also considered as potentially actionable for treatment with PARP inhibitors, then a total of 42 potentially actionable PV/LPV were detected by tumor or germline genotyping. By tumor genotyping only, 41 of 42 (97.6%) potentially actionable PV/LPV would have been detected in 41 patients. With the germline genotyping only, 28 of 42 (66.6%) potentially actionable PV/LPV would have been detected in 28 patients (Scheme 1, Tables S1 and S2).
3.3. Frequencies of Germline and Somatic PV/LPV in HBOC Genes among Subgroup of 132 Advanced Non-Mucinous EOC Patients with Matched Successful Tumor and Germline Genotyping
In the subgroup of 132 patients with matched successful tumor and germline genotyping, 47 PV/LPV in HBOC genes were found in 31.8% of patients either by tumor or germline genotyping (Table 1). Germline PV/LPV in HBOC genes were found in 21.9% of patients, while somatic PV/LPV were found in 11.3% (Table 1). Two PV/LPV were found only by germline genotyping (Scheme 1, Tables S1 and S2). As mentioned previously, in three patients, both somatic and germline PV/LPV in HBOC genes were detected. Two germline PV/LPV were found in two patients, but were missed by tumor genotyping (one patient with BRCA1 large deletion and another patient with SNV in ATM) (Scheme 1, Table 1, Tables S1 and S2). Among the aforementioned PV/LPV detected in 132 non-mucinous EOC patients with matched tumor–blood samples, PV/LPV in BRCA genes were found in 27.2% of patients (Table 2). Germline PV/LPV in BRCA genes were found in 19.5% of patients, while somatic PV/LPV were found in 8.3% of patients. As mentioned before, one of those patients had somatic and germline PV/LPV in BRCA2 and the other somatic PV/LPV in BRCA2 and germline PV/LPV in CHEK2. Ten patients had only somatic PV/LPV in BRCA genes. BRCA1 germline PV/LPV were present in 77.0% of germline BRCA positive patients (20/26), while BRCA2 germline PV/LPV were present in 23.0% of germline BRCA positive patients (6/26) (Table S1). Conversely, somatic PV/LPV variants in the BRCA2 gene were present in 54.5% of somatic BRCA positive patients (6/11) while PV/LPV in the BRCA1 gene were present in 45.4% (5/11) (Table S1, Scheme 1).
In the group of 132 EOC patients, 10 PV/LPV in nonBRCA HBOC genes were found in 6.0% of patients (Table 3). As mentioned before, four germline PV/LPV in nonBRCA HBOC genes were found in three patients (2.2%) (in RAD51C, ATM, BRIP1, and CHEK2). Six somatic PV/LPV in nonBRCA HBOC genes were found in five patients (3.8%) (five in NF1, and one in BARD1). One somatic PV/LPV in the NF1 gene was found in patient harboring at the same time germline PV/LPV in BRCA1. In one patient two somatic PV/LPV in NF1 were detected (Scheme 1, Tables S1 and S2).
3.4. Efficiency of Tumor Genotyping for Detection of Germline Variants
The efficiency of tumor genotyping to detect germline variants from tumor tissue regarding the type of variant was assessed in the group of 132 patients with successful paired tumor and germline genotyping.
Germline genotyping revealed 22 small PV/LPV variants (SNV and small 1–7 bp indels) and 4 large intragenic PV/LPV deletions in BRCA genes (Table S1). Additionally, four PV/LPV variants in nonBRCA genes were detected (Tables S1–S3).
Comparing tumor to germline genotyping, all germline small variants in BRCA genes were also detected in tumors, while one small variant in the ATM gene was missed (ATM: c.5932G > T p.(Glu1978*). Among germline large intragenic deletions in BRCA genes, three out of four variants were detected by tumor genotyping (Table S3). Altogether, 2 PV/LPV out of 30 germline PV/LPV in HBOC genes were not detected, using tumor testing (6.7%).
Sensitivity of the method used for genotyping of ovarian tumor FFPE samples was 100% for small germline PV/LPV in BRCA genes (all of 22 small variants were detected), but when taking into account all HBOC genes the sensitivity for small variant detection was 96.2% (25 cases of 26). The sensitivity of NGS method used for tumor genotyping for detection of germline large intragenic deletions in BRCA genes was 75.0% (three of four large deletions were detected). No large intragenic deletion was found in other nonBRCA HBOC genes. Sensitivity of FFPE ovarian tumor genotyping for detection of all kinds of germline PV/LPV (including SNV, small indels, and large intragenic deletions) was 96.2% (25/26) for BRCA genes and 93.3% (28/30) for all HBOC genes (Tables S1–S3). Identical sensitivity of FFPE ovarian tumor genotyping for detection/recognition of patients with germline PV/LPV in BRCA genes was determined: 96.2% (25/26) (Table 4, Table S1). However, in one patient with two germline PV/LPV in HBOC genes (in RAD51C and ATM) only one PV/LPV (in RAD51C) was detected. For the purposes of our analysis, we considered this patient as not having been recognized as a carrier of germline PV/LPV based on tumor genotyping. Consequently, the sensitivity of FFPE ovarian tumor genotyping for detection/recognition of patients with germline PV/LPV in all HBOC genes was determined to be 93.1% (27/29) (Table 5, Table S2).
Table 4.
Comparison of tumor and germline genotyping results, for germline PV/LPV in BRCA genes, of matched tumor blood samples from the cohort of 132 advanced non-mucinous EOC patients, who had successfully genotyped tumors and blood samples.
| Matched Tumor Samples | Matched Blood Samples | ||
|---|---|---|---|
| No. of patients–carriers of germline PV/LPV in BRCA genes | No. of patients with germline wild type BRCA genes | No. of all patients with genotyped matched blood sample | |
| No. patients with PV/LPV in BRCA genes detected in matched tumor sample | 25 | 10 | 35 |
| No. patients with wild type BRCA genes in matched tumor sample | 1 * | 96 | 97 |
| No. of all patients with genotyped matched tumor samples | 26 | 106 | 132 |
PV/LPV—pathogenic/likely pathogenic variants; * patient with undetected large deletion in the BRCA1 gene (exon 1–2 deletion).
Table 5.
Comparison of tumor and germline genotyping results, for germline PV/LPV in HBOC genes, of matched tumor–blood samples from the cohort of 132 advanced non-mucinous EOC patients, who had successfully genotyped tumors and blood samples.
| Matched Tumor Samples | Matched Blood Samples | ||
|---|---|---|---|
| No. of patients–carriers of germline PV/LPV in HBOC genes | No. of patients with germline wild type HBOC genes | No. of all patients with genotyped matched blood samples | |
| No. patients with PV/LPV in HBOC genes detected in matched tumor samples | 27 | 13 ** | 40 |
| No. patients with wild type HBOC genes in matched tumor sample | 2 * | 90 | 92 |
| No. of all patients with genotyped matched tumor samples | 29 | 103 | 132 |
* Two non-mucinous EOC patients with germline pathogenic/likely pathogenic variants (PV/LPV) were not detected by tumor genotyping—one patient with BRCA1 large deletion and another patient with SNV PV/LPV in ATM (indeed this patient was carrier of two germline mutations, another one in RAD51C, which was detected also by tumor genotyping); ** in one patient two somatic PV/LPV in NF1 were detected. Somatic PV/LPV were detected also in three patients with germline PV/LPV in HBOC genes.
Specificity of tumor genotyping for detection of patients with germline PV/LPV in BRCA genes is 90.6% (96/106) (Table 4), and for detection of patients with germline PV/LPV in HBOC genes 87.3% (90/103) (Table 5).
Positive predictive value of tumor genotyping for detection of patients with germline PV/LPV in BRCA genes was 71.4% (25/35) and for detection of patients with germline PV/LPV in HBOC genes 67.5% (27/40) (Table 4 and Table 5). Meaning that there is a 71.4% probability that PV/LPV in BRCA genes detected in the tumor are of germline origin, and a 67.5% probability that PV/LPV in HBOC genes detected in the tumor are of germline origin.
Negative predictive value of tumor genotyping for detection of patients with germline PV/LPV in BRCA genes was 98.9% (96/97) and for detection of patients with germline PV/LPV in HBOC genes 97.8% (90/92) (Table 4 and Table 5). Meaning, that there is a 98.9% probability that a patient who is negative for PV/LPV in BRCA genes by tumor genotyping will be negative also by germline genotyping, and a 97.8% probability that patients negative for PV/LPV in HBOC genes by tumor genotyping will be negative also by germline genotyping.
3.5. Family History of BRCA-Related Cancers among Matched Advanced Non-Mucinous EOC Patients
Family history of BRCA-related cancers was reported by the referring physician in 45 of 132 patients (34.1%). Thirty germline PV/LPV in at least one of the HBOC genes were detected in 29 of 132 patients (22.0%). Family history of HBOC was seen in 14 of 29 patients with germline PV/LPV (48.3%) (Tables S1 and S2).
Among 103 patients without germline or somatic PV/LPV in HBOC genes, a positive family history was reported in 27 cases (26.0%).
Somatic PV/LPV in BRCA genes were detected in tumors of 11 patients. One of them was also a carrier of germline PV/LPV in BRCA2 and the other was also a carrier of germline PV/LPV in CHEK2. Therefore, there were 10 patients with only somatic BRCA PV/LPV, which represents 7.5% (10/132) of all matched advanced non-mucinous EOC patients. From this cohort of 10 patients, family history of HBOC was reported in 3 patients (33.3%). In other words, in a cohort of 132 matched advanced non-mucinous EOC patients 3 patients (2.3%) with only somatic BRCA PV/LPV also had a family history of HBOC, with one of them carrying a germline PV in the CHEK2 gene.
Two advanced non-mucinous EOC patients, who carried germline PV/LPV (deletion of exons 1 and 2 in BRCA1 and c.5932G > T in ATM), which were not detected in tumor testing, had a diagnosis of high grade serous ovarian cancer and no family history of breast and ovarian cancer. One patient, where a BRCA1 PV/LPV was missed on tumor testing, had a family history of prostate cancer in a second-degree relative. The other patient, who had a germline PV/LPV in RAD51C (detected also in the tumor) and in the ATM gene (not detected in tumor), had no family history of cancer.
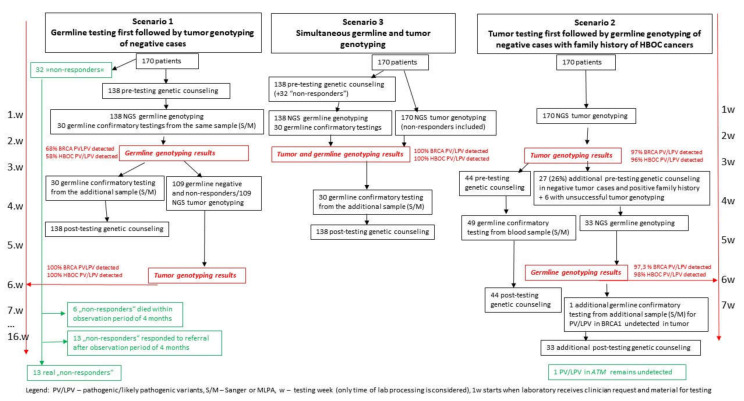
3.6. Analysis of Three Different Approaches to Germline and Tumor Testing
Additionally, we investigated three different approaches to genetic testing of ovarian cancer patients previously reported in the literature and assessed the workloads involved for the diagnostic laboratory and clinical genetics service. Data collected in our study were used to illustrate the detection rates and workloads in two possible hypothetical approaches, Scenario 1 and Scenario 2. Scenario 1 would involve testing all patients who opt for NGS germline testing from blood first. Those who received negative results and those who chose not to undergo germline testing within a 2–3-week timeframe would then be tested using NGS from tumor tissue (Scheme 2). In Scenario 2, all patients would be tested using NGS from tumor tissue first, and those with a positive result would have confirmatory non-NGS testing from blood. Those with negative tumor tissue results and a positive family or personal history indicative of HBOC would receive NGS germline testing. These two approaches were compared to Scenario 3, which involves simultaneous NGS testing from blood and tumor tissue, as performed in this study (Scheme 2). The results are presented in Table S4. Scenario 3 would result in all germline and somatic variants being detected within 2–3 weeks, but would entail 138 NGS germline tests and 170 NGS tumor tissue tests, with 138 pre-test genetic counselling appointments and additional posttest counseling for all positive responders. It would therefore be labor intensive and costly. Scenario 1 would reduce the number of tumor tissue NGS tests to 109, but due to the sequential nature of this approach (blood first, then tumor, if needed), only 68% of all PV/LPV would be detected within the 2–3-week timeframe. Scenario 2 would reduce the number of NGS tests from blood to 33, and the number of pre-test appointments to 77, thus reducing the workload and cost of testing, 97% of all PV/LPV would be detected within 2–3 weeks. The comparison of Scenario 1 and Scenario 2 is presented in Table S5.
Scheme 2.
Comparison of testing scenarios—germline genotyping first vs. tumor genotyping first.
4. Discussion
According to National Comprehensive Cancer Network (NCCN) Guidelines as well as ESMO-ESGO recommendations, all EOC patients, including patients with fallopian tube cancer and primary peritoneal cancer, should undergo germline and somatic tumor testing. Germline testing is required to identify patients with hereditary cancer predisposition and cascade testing. Since the introduction of PARP inhibitors, the BRCA1/2 status (germline or somatic) became important also for personalized treatment decisions in these patients [20]. In addition to BRCA1/2 testing, NCCN and ESMO-ESGO recommend considering somatic tumor testing for PV/LPV in other homologous deficiency (HR) genes as well as the evaluation of HR deficiency when deciding on PARP inhibitor therapy.
As mentioned in the Introduction, there are some remarkable clinical studies researching different angles related to treatment with PARP inhibitors in EOC patients [21]. There are also recommendations (NCCN, ESMO-ESGO) on which genes and what other parameters (genomic scars) should be determined to identify patients who will have significant benefit from PARP inhibitor therapy [10,22]. Unfortunately, neither of these studies, nor the NCCN and ESMO-ESGO guidelines, provide precise guidance on how to approach NGS testing to achieve optimal results. Consequently, different European countries have different NGS strategies for BRCA testing in ovarian cancer. According to accessible literature, in Spain, testing for BRCA germline PV/LPV is offered to all patients with high-grade non-mucinous ovarian carcinomas, followed by somatic testing if negative for germline PV/LPV [5]. Other countries only list which patients are tested, without giving information on whether they are testing first for germinal or somatic alterations [13,23].
To improve the workflow of NGS germline and somatic testing processes in terms of speed, efficiency, and optimal sampling, several questions should be addressed: should tumor and germline genotyping be performed in parallel or one after the other; what would be the optimal testing strategy for identifying the highest proportion of germline and somatic PV/LPV; and, finally, how to perform the required testing within optimal turnaround time frame? Thus, the objective of this article is to provide real world data on germline and tumor testing in advanced non-mucinous EOC patients, collected from January 2019 to April 2020. The final aim is to implement the most appropriate testing workflow covering the needs of genetic counseling as well as the needs of clinicians for treatment decisions.
Given the fact that germline testing for BRCA PV/LPV in individuals from HBOC syndrome families has been in use for a long time for genetic counseling purposes, it would be reasonable to offer germline testing as the first step. Since germline predisposition testing would only detect germline variants, it should be complemented with tumor testing of BRCA negative cases to detect somatic variants. The whole procedure of genetic counseling and germline predisposition testing followed by tumor testing is time consuming, and is based on the presumption that all non-mucinous EOC patients will show up for genetic counseling and testing. As showed in our study as well as in studies of other researchers, a certain proportion of patients do not respond to this referral during the observation period of 4 months [24].
On the other hand, NGS tumor testing would theoretically reveal somatic as well as germline BRCA PV/LPV, but the laboratory should be aware that non-mucinous EOC patients are also candidates for germline testing of PV/LPV in several genes other than BRCA. Based on the results of our study, we propose the optimal strategy is testing the tumor first using an NGS panel that includes all HBOC genes. NGS tumor testing should be able to detect all types of PV/LPV in HBOC genes, from SNV, and small indels to large intragenic deletions or duplications. In positive cases, tumor testing should be followed by conformational germline testing to clarify whether the variant is of germline or somatic origin.
In our study, 96.5% of tumor samples were successfully sequenced, which correlates very well with previous reports in Dutch EOC patient’s cohort (96.8%) and in Italian EOC patient’s cohort (99.0%) [7,25].
In our cohort of matched advanced non-mucinous EOC patients, 36 out of 132 patients (27.2%) were found by germline and tumor testing to have PV/LPV in BRCA genes. Most of the BRCA positive patients carried a germline BRCA PV/LPV (26; 69.4%) while 10 patients (29.7%) had only somatic BRCA PV/LPV (Table 2, Table S1, Scheme 1). Regardless of variant’s origin, PV/LPV in BRCA1 (60.0%) were more prevalent than PV/LPV in BRCA2 (40.0%). Our results agree with the reports of other authors who reported BRCA PV/LPV in 16.7–28.1% of non-mucinous EOC patients [6,7,8,12,25,26,27,28]. The percentage of BRCA positive patients in our study is closest to the results of Italian research group who reported BRCA PV/LPV in 28.1% of 221 non-mucinous non-borderline EOC cases. Among BRCA positive cases there were 78.3% of germline and 21.7% of somatic origin [25]. The lowest ratio of BRCA PV/LPV was reported by the Dutch research group who detected BRCA PV/LPV in 16.7% (germline in 56.8%, and somatic in 43.2%) of non-borderline EOC tumors [7].
The sensitivity of the used NGS method for detection of germline BRCA PV/LPV in FFPE tumor tissue varied between 100% (for small variants) and 75% (for large intragenic deletions). Actually, three of four large intragenic variants were detected by tumor genotyping and the only variant that was not identified, was the deletion of promotor region and exons 1 and 2 of the BRCA1 gene (Table S1). The promoter region and exon 1 are not included in TruSight Tumor 170 library preparation kit; therefore, variants in these two regions cannot be detected. The detection of large intragenic exon copy number variants represents one of the major limitations of FFPE tumor genotyping of non-mucinous EOC tumors due to extensive tumor copy number aberrations caused by homologous recombination deficiency [29]. The other variant that was missed was not in BRCA1/2 genes but in the ATM gene. In the tumor bearing the pathogenic variant ATM: c.5932G > T p. (Glu1978*), loss of heterozygosity (LOH) (enriching wild type allele) occurred and as a consequence, the variant frequency was below the limit of detection used in tumor genotyping (Table S2). However, it is worth mentioning the high negative predictive values of tumor genotyping for detection of germline PV/LPV in BRCA genes and other nonTP53-HBOC genes. Testing the tumor first could therefore reduce the number of germline tests for PV/LPV in these genes.
It is interesting that in the majority of patients carrying the germline BRCA PV/LPV (22 of 26 cases), the variant frequency of the PV/LPV was above 60.0% indicating that there was partial LOH (Supplementary Table S1). In one tumor sample (no. 13), two PV/LPV (one germline and one somatic) in the BRCA2 gene were detected. Somatic PV/LPV was probably the second hit causing deactivation of the wild-type allele. Another patient from our cohort (sample no. 4) acquired a reverse PV in the BRCA1 gene where the nucleotide change occurred at the same codon (at the same allele) as germline nonsense variant c.1687C > T in the BRCA1 gene restoring the reading frame and causing an amino acid change (Table S1).
Based on the results of our study, with germline genotyping only, 15.3% of all patients included in the study (26/170) would qualify for PARP inhibitor therapy (patients with BRCA PV/LPV) in first line setting of ovarian cancer treatment (Table 2). With tumor genotyping, 21.8% of all patients included in the study (37/170) had BRCA PV/LPV (Table 2). Therefore, the number of eligible patients for therapy with PARP inhibitors in first line ovarian cancer treatment setting was increased by substantial 42.0% (11/26) when tumor genotyping was applied. British Gynecological Cancer Society and British association of Gynecological Pathologists came to a similar conclusion and reported that tumor genotyping identified 50% more patients with BRCA mutations than germline genotyping only, while germline genotyping identified 10% more BRCA mutations (large duplications and deletions), which were undetectable by tumor genotyping. Based on this, their recommendation was that germline and tumor genotyping should be performed in parallel [30]. Our analysis of possible approaches to testing indicates that offering tumor tissue testing first, followed by germline testing for PV/LPV detected in the tumor or additional NGS germline testing in cases of positive personal or family history for HBOC, would enable us to detect nearly all PV/LPV as quickly as with parallel blood/tumor testing whilst greatly reducing the cost and workload involved.
The real-world data from Slovene advanced non-mucinous EOC patients show that all of them had tumor genotyping and were simultaneously referred for genetic counselling and germline testing. However, 32 of 170 (18.8%) advanced non-mucinous EOC patients did not contact Cancer Genetic Clinic and, consequently, did not undergo germline testing within our study’s observation period. Four of these patients (11.8%) carried a possible germline PV/LPV detected in the BRCA1, RAD51C, BRIP1, or PTEN gene in the tumor sample. If germline genotyping only was performed, 58.8% (30/51) PV/LPV in HBOC genes would be detected. In this case, 4 PV/LPV in HBOC genes could be missed due to patients’ unresponsiveness to genetic counseling/testing together with 17 somatic PV/LPV in HBOC genes. If tumor genotyping only was performed, 96.1% (49/51) PV/LPV in HBOC genes would be detected while 2 germline HBOC PV/LPV would be missed (1 × BRCA1, 1 × ATM) (Scheme 1 and Scheme 2). Similar conclusions were reached when only actionable PV/LPV for PARP inhibitor therapy were considered. With germline genotyping only, 68.4% (26/38) PV/LPV in BRCA genes would be detected, while with tumor genotyping only, 97.4% (37/38) PV/LPV in BRCA genes would be detected (Tables S1 and S2). Since we have detected potentially actionable PV/LPV for PARP inhibitor therapy in BRIP1 and RAD51C genes, the final percentage of actionable detected PV/LPV with germline genotyping was 66.6% (28/42), and with tumor genotyping 97.6% (41/42) (Scheme 1 and Scheme 2).
5. Conclusions
Considering the facts that tumor testing enables detection of somatic and most germline PV/LPV, and that not all patients attend genetic counseling and testing, testing the tumor first seems to be a reasonable approach for detection of PV/LPV in HBOC genes in non-mucinous EOC patients. According to our findings, there is a high probability that non-mucinous EOC patients without PV/LPV in these genes in tumors would be negative also after germline testing. However, one should not forget that false negative results for germline PV/LPV are possible when performing genotyping of tumor tissue from FFPE samples, particularly in the case of large intragenic deletions.
For the purpose of accurate detection of germline and clinically actionable somatic PV/LPV in non-mucinous EOC patients, our proposed workflow would be as follows: NGS genotyping of tumor samples first, followed in tumor positive cases by NGS or Sanger sequencing for determination of origin of PV/LPV (germline or somatic) and genetic counseling. In negative cases, the decision whether the patient is referred to genetic counseling and testing should be based on additional criteria such as personal and family history, histological type of tumor, and its molecular characteristics. Such an approach would enable a timely identification of nearly all germline and somatic PV/LPV, whilst reducing the workload and costs associated with simultaneous testing of blood and tumor samples.
Acknowledgments
We would like to acknowledge all patients and their family members for their participation. We would like to express our gratitude to Anja Zagožen Klasinc, Alenka Bombač, Vesna Vogrič, and Simona Traven, for expert technical assistance. Additionally, we would like to thank Natalija Klopčič, Tina Kerševan, Barbara Babuder, and Svetlana Novak for their help in organizing germline testing and patient management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14061434/s1, Table S1: Detected pathogenic/likely pathogenic variants (PV/LPV) in HBOC genes in BRCA positive non-mucinous EOC patients (from the cohort all 170 patients included in the study). Results of tumor and blood (germline) genotyping, Table S2: Detected pathogenic/likely pathogenic variants (PV/LPV) in HBOC genes in BRCA negative non-mucinous EOC patients (from the cohort all 170 patients included in the study). Results of tumor and blood (germline) genotyping, Table S3: Number of germline pathogenic/likely pathogenic variants (PV/LPV) in BRCA and other HBOC genes detected in tumors of 132 non-mucinous EOC patients (from cohort with matched blood-tumor samples) according to variant type (SNV, small indels, large intragenic deletions), Table S4: Number of procedures performed in different testing approaches; germline testing first (scenario 1) and tumor genotyping first (scenario 2) compared to simultaneous tumor and germline genotyping (scenario 3), Table S5: Summary of two testing approaches: germline testing first (scenario 1) and tumor genotyping first (scenario 2).
Author Contributions
Conceptualization, V.S., A.B. and S.N.; data curation, V.S., A.B. and P.Š.; validation, V.S. and S.N.; visualization, V.S. and S.N.; formal analysis: V.S., V.Š.D., P.Š., G.K., A.B., M.K., K.S., M.B., E.Š., B.G., J.Ž., M.R. and S.N.; investigation, V.S., V.Š.D., P.Š., G.K., A.B., M.K., K.S., M.B., E.Š., B.G., J.Ž., M.R. and S.N.; resources, V.S., V.Š.D., P.Š., G.K., A.B., M.K., K.S., M.B., E.Š., B.G., M.R. and S.N.; writing—original draft preparation, V.S. and A.B.; writing—review and editing, V.S., A.B., G.K., V.Š.D., P.Š., M.K., K.S., M.B., E.Š., B.G., M.R., J.Ž. and S.N.; supervision, S.N.; funding acquisition, S.N. and J.Ž. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the Slovenian research agency, program number: P3-0352.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by National Ethics Committee (permission no. 100/05/15 on 27 July 2015 and permission no. 0120-303/218/3 on 13 June 2018) and Institutional Ethics Committee of Institute of Oncology Ljubljana (IOL) (permission no. ERID-KESOPKR/97 on 17 September 2015 and permission no. ERID-EK/136 on 21 May 2018), and registered at ISRCTN registry on 24 November 2015 (ISRCTN42408038).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting reported results are available upon request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zadnik V., Primic Zakelj M., Lokar K., Jarm K., Ivanus U., Zagar T. Cancer burden in slovenia with the time trends analysis. Radiol. Oncol. 2017;51:47–55. doi: 10.1515/raon-2017-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daly M.B., Pal T., Berry M.P., Buys S.S., Dickson P., Domchek S.M., Elkhanany A., Friedman S., Goggins M., Hutton M.L., et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021;19:77–102. doi: 10.6004/jnccn.2021.0001. [DOI] [PubMed] [Google Scholar]
- 3.Prat J. Ovarian carcinomas: Five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012;460:237–249. doi: 10.1007/s00428-012-1203-5. [DOI] [PubMed] [Google Scholar]
- 4.Bell D., Berchuck A., Birrer M., Chien J., Cramer D.W., Dao F., Dhir R., DiSaia P., Gabra H., Glenn P., et al. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palacios J., de la Hoya M., Bellosillo B., de Juan I., Matías-Guiu X., Lázaro C., Palanca S., Osorio A., Rojo F., Rosa-Rosa J.M., et al. Mutational Screening of BRCA1/2 Genes as a Predictive Factor for Therapeutic Response in Epithelial Ovarian Cancer: A Consensus Guide from the Spanish Society of Pathology (SEAP-IAP) and the Spanish Society of Human Genetics (AEGH) Virchows Arch. 2020;476:195–207. doi: 10.1007/s00428-019-02709-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ledermann J., Harter P., Gourley C., Friedlander M., Vergote I., Rustin G., Scott C.L., Meier W., Shapira-Frommer R., Safra T., et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–861. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 7.Vos J.R., Fakkert I.E., de Hullu J.A., van Altena A.M., Sie A.S., Ouchene H., Willems R.W., Nagtegaal I.D., Jongmans M.C.J., Mensenkamp A.R., et al. Universal Tumor DNA BRCA1/2 Testing of Ovarian Cancer: Prescreening PARPi Treatment and Genetic Predisposition. JNCI J. Natl. Cancer Inst. 2020;112:161–169. doi: 10.1093/jnci/djz080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pennington K.P., Walsh T., Harrell M.I., Lee M.K., Pennil C.C., Rendi M.H., Thornton A., Norquist B.M., Casadei S., Nord A.S., et al. Germline and Somatic Mutations in Homologous Recombination Genes Predict Platinum Response and Survival in Ovarian, Fallopian Tube, and Peritoneal Carcinomas. Clin. Cancer Res. 2014;20:764–775. doi: 10.1158/1078-0432.CCR-13-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konstantinopoulos P.A., Norquist B., Lacchetti C., Armstrong D., Grisham R.N., Goodfellow P.J., Kohn E.C., Levine D.A., Liu J.F., Lu K.H., et al. Germline and Somatic Tumor Testing in Epithelial Ovarian Cancer: ASCO Guideline. J. Clin. Oncol. 2020;38:1222–1245. doi: 10.1200/JCO.19.02960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colombo N., Sessa C., du Bois A., Ledermann J., McCluggage W.G., McNeish I., Morice P., Pignata S., Ray-Coquard I., Vergote I., et al. ESMO–ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019;30:672–705. doi: 10.1093/annonc/mdz062. [DOI] [PubMed] [Google Scholar]
- 11.Swisher E.M., Lin K.K., Oza A.M., Scott C.L., Giordano H., Sun J., Konecny G.E., Coleman R.L., Tinker A.V., O’Malley D.M., et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): An international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017;18:75–87. doi: 10.1016/S1470-2045(16)30559-9. [DOI] [PubMed] [Google Scholar]
- 12.Ledermann J.A., Harter P., Gourley C., Friedlander M., Vergote I., Rustin G., Scott C., Meier W., Shapira-Frommer R., Safra T., et al. Overall survival in patients with platinum-sensitive recurrent serous ovarian cancer receiving olaparib maintenance monotherapy: An updated analysis from a randomised, placebo-controlled, double-blind, phase 2 trial. Lancet Oncol. 2016;17:1579–1589. doi: 10.1016/S1470-2045(16)30376-X. [DOI] [PubMed] [Google Scholar]
- 13.Capoluongo E., Ellison G., López-Guerrero J.A., Penault-Llorca F., Ligtenberg M.J.L., Banerjee S., Singer C., Friedman E., Markiefka B., Schirmacher P., et al. Guidance Statement On BRCA1/2 Tumor Testing in Ovarian Cancer Patients. Semin. Oncol. 2017;44:187–197. doi: 10.1053/j.seminoncol.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Gornjec A., Novakovic S., Stegel V., Hocevar M., Pohar Marinsek Z., Gazic B., Krajc M., Skof E. Cytology material is equivalent to tumor tissue in determining mutations of BRCA 1/2 genes in patients with tubo-ovarian high grade serous carcinoma. BMC Cancer. 2019;19:296. doi: 10.1186/s12885-019-5535-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klančar G., Blatnik A., Šetrajčič Dragoš V., Vogrič V., Stegel V., Blatnik O., Drev P., Gazič B., Krajc M., Novaković S. A Novel Germline MLH1 In-Frame Deletion in a Slovenian Lynch Syndrome Family Associated with Uncommon Isolated PMS2 Loss in Tumor Tissue. Genes. 2020;11:325. doi: 10.3390/genes11030325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li M.M., Datto M., Duncavage E.J., Kulkarni S., Lindeman N.I., Roy S., Tsimberidou A.M., Vnencak-Jones C.L., Wolff D.J., Younes A., et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J. Mol. Diagn. 2017;19:4–23. doi: 10.1016/j.jmoldx.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Den Dunnen J.T., Dalgleish R., Maglott D.R., Hart R.K., Greenblatt M.S., McGowan-Jordan J., Roux A.-F., Smith T., Antonarakis S.E., Taschner P.E.M. HGVS Recommendations for the Description of Sequence Variants: 2016 Update. Hum. Mutat. 2016;37:564–569. doi: 10.1002/humu.22981. [DOI] [PubMed] [Google Scholar]
- 18.Plon S.E., Eccles D.M., Easton D., Foulkes W.D., Genuardi M., Greenblatt M.S., Hogervorst F.B.L., Hoogerbrugge N., Spurdle A.B., Tavtigian S.V., et al. Sequence variant classification and reporting: Recommendations for improving the interpretation of cancer susceptibility genetic test results. Hum. Mutat. 2008;29:1282–1291. doi: 10.1002/humu.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–423. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer (Version 1.2021) [(accessed on 27 April 2021)]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf.
- 21.Mirza M.R., Coleman R.L., González-Martín A., Moore K.N., Colombo N., Ray-Coquard I., Pignata S. The forefront of ovarian cancer therapy: Update on PARP inhibitors. Ann. Oncol. 2020;31:1148–1159. doi: 10.1016/j.annonc.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Miller R.E., Leary A., Scott C.L., Serra V., Lord C.J., Bowtell D., Chang D.K., Garsed D.W., Jonkers J., Ledermann J.A., et al. ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer. Ann. Oncol. 2020;31:1606–1622. doi: 10.1016/j.annonc.2020.08.2102. [DOI] [PubMed] [Google Scholar]
- 23.Singer C.F., Tan Y.Y., Muhr D., Rappaport C., Gschwantler-Kaulich D., Grimm C., Polterauer S., Pfeiler G., Berger A., Tea M.M. Association between family history, mutation locations, and prevalence of BRCA1 or 2 mutations in ovarian cancer patients. Cancer Med. 2019;8:1875–1881. doi: 10.1002/cam4.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolnick S.J., Rahm A.K., Jackson J.M., Nekhlyudov L., Goddard K.A.B., Field T., McCarty C., Nakasato C., Roblin D., Anderson C.P., et al. Barriers in Identification and Referral to Genetic Counseling for Familial Cancer Risk: The Perspective of Genetic Service Providers. J. Genet. Couns. 2011;20:314–322. doi: 10.1007/s10897-011-9351-3. [DOI] [PubMed] [Google Scholar]
- 25.Fumagalli C., Tomao F., Betella I., Rappa A., Calvello M., Bonanni B., Bernard L., Peccatori F., Colombo N., Viale G., et al. Tumor BRCA Test for Patients with Epithelial Ovarian Cancer: The Role of Molecular Pathology in the Era of PARP Inhibitor Therapy. Cancers. 2019;11:1641. doi: 10.3390/cancers11111641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hennessy B.T.J., Timms K.M., Carey M.S., Gutin A., Meyer L.A., Flake D.D., Abkevich V., Potter J., Pruss D., Glenn P., et al. Somatic Mutations in BRCA1 and BRCA2 Could Expand the Number of Patients That Benefit From Poly (ADP Ribose) Polymerase Inhibitors in Ovarian Cancer. J. Clin. Oncol. 2010;28:3570–3576. doi: 10.1200/JCO.2009.27.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Jonge M.M., Ruano D., van Eijk R., van der Stoep N., Nielsen M., Wijnen J.T., ter Haar N.T., Baalbergen A., Bos M.E.M.M., Kagie M.J., et al. Validation and Implementation of BRCA1/2 Variant Screening in Ovarian Tumor Tissue. J. Mol. Diagn. 2018;20:600–611. doi: 10.1016/j.jmoldx.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Bekos C., Grimm C., Kranawetter M., Polterauer S., Oberndorfer F., Tan Y., Müllauer L., Singer C.F. Reliability of Tumor Testing Compared to Germline Testing for Detecting BRCA1 and BRCA2 Mutations in Patients with Epithelial Ovarian Cancer. J. Pers. Med. 2021;11:593. doi: 10.3390/jpm11070593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watkins J.A., Irshad S., Grigoriadis A., Tutt A.N. Genomic scars as biomarkers of homologous recombination deficiency and drug response in breast and ovarian cancers. Breast Cancer Res. 2014;16:211. doi: 10.1186/bcr3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sundar S., Manchanda R., Gourley C., George A., Wallace A., Balega J., Williams S., Wallis Y., Edmondson R., Nicum S., et al. British Gynaecological Cancer Society/British Association of Gynaecological Pathology consensus for germline and tumor testing for BRCA1/2 variants in ovarian cancer in the United Kingdom. Int. J. Gynecol. Cancer. 2021;31:272–278. doi: 10.1136/ijgc-2020-002112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting reported results are available upon request.