Ciliochoroidal effusion was found in one fifth of eyes with central serous chorioretinopathy using anterior-segment optical coherence tomography. Central serous chorioretinopathy may accompany fluid accumulation in the anterior segment more frequently than previously expected in association with thick sclera.
Key words: anterior-segment optical coherence tomography, central serous chorioretinopathy, choroidal thickness, ciliochoroidal effusion, pachychoroid, scleral thickness, uveal effusion syndrome
Purpose:
To investigate the prevalence of ciliochoroidal effusion (CE) in central serous chorioretinopathy (CSC) using anterior-segment optical coherence tomography and its association with the clinical features of CSC.
Methods:
Overall, 164 eyes of 164 patients with CSC and 51 eyes of 51 age- and sex-matched normal control participants were retrospectively examined. Anterior-segment optical coherence tomography was used to assess patients with CSC and control subjects for CE and scleral thickness. Central serous chorioretinopathy eyes were divided into two groups: eyes with CE (CE group) and eyes without CE (non-CE group). Scleral thickness was measured at the point that was 6 mm posterior to the scleral spur in four directions.
Results:
Among the 164 eyes with CSC, 32 eyes (19.5%) displayed CE, and this proportion was significantly higher than that in control subjects (2.0%) (P = 0.001). Scleral thickness was significantly greater in the CE group compared with the non-CE group at all four directions (P < 0.05 for all). Multivariable analysis revealed that the mean scleral thickness (odds ratio: 1.01; 95% confidence interval: 1.00–1.02; P = 0.007) was significantly associated with the incidence of CE.
Conclusion:
Central serous chorioretinopathy may accompany fluid accumulation in the anterior segment more frequently than previously expected in association with thick sclera.
Central serous chorioretinopathy (CSC), a representative pachychoroid spectrum disorder,1 is characterized by serous retinal detachment (SRD) with or without detachment of retinal pigment epithelium (RPE) in the posterior pole.2 Central serous chorioretinopathy most often affects middle-aged men3 with emmetropic or hyperopic eyes. Many predisposing factors have been reported to exacerbate or induce CSC, such as psychological stress,4 type A personality,5 pregnancy,6 smoking habits,7 steroid use,8 hyperopic refractive error,9 and short axial length.9 However, the precise cause and pathogenesis of CSC remain unclear.
Idiopathic uveal effusion syndrome (UES) is associated with nanophthalmos and scleral abnormalities. It is a relatively rare disease that demonstrates ciliochoroidal effusion (CE) and SRD.10 The proposed pathogenesis of UES is the accumulation of fluid and exudates from choroidal vessels as a result of the compression of vortex veins by scleral thickening11 and impaired transscleral outflow because of decreased scleral permeability.12 Uveal effusion syndrome shares clinical characteristics with CSC, such as the accumulation of subretinal fluid and choroidal vascular abnormalities. Therefore, it is often misdiagnosed as CSC, especially its atypical variants, such as bullous CSC.13 It is characterized by exudative SRD and inferior bullous retinal detachment. However, UES commonly differs from CSC in terms of the following clinical characteristics: 1) UES does not show the typical pattern for one or several leakages at the level of the RPE on fluorescein angiography (FA), which is seen in CSC; and 2) CSC does not show clinically evident CE seen in UES.
Recently, we reported that the axial length was significantly shorter in CSC eyes than in control eyes.9 Furthermore, we demonstrated that scleral thickness was significantly greater in CSC eyes than in control eyes using anterior-segment optical coherence tomography (AS-OCT).14 Based on our previous findings, CSC was found to share anatomical features like a short axial length and thickened sclera with UES, and these features may be partially associated with CSC pathogenesis.
Ciliochoroidal effusion is an accumulation of fluid in the suprachoroidal space located between the ciliary body–choroid complex and sclera. It is one of the most important clinical features of UES. In addition to UES, CE is often seen in a variety of conditions, including hypotony after glaucoma surgery,15 trauma and intraocular surgery,16 primary angle-closure glaucoma,17 Vogt–Koyanagi–Harada disease,18 and scleritis.19 In UES, CE appears to be a result of increased choroidal permeability caused by the compression of vortex veins and impaired transscleral outflow because of scleral thickening. Therefore, similar clinical features and a similar physiopathology may also be observed in eyes with CSC.
Herein, the prevalence of CE in CSC was investigated using AS-OCT and its association with the clinical features of CSC. Ciliochoroidal effusion has also been described as “uveal effusion,” “choroidal effusion,” “ciliochoroidal detachment,” and “choroidal detachment.” Because we have evaluated the findings based on the cross-sectional OCT images of ciliary body and peripheral choroid, we have used the term “CE” in this study.
Methods
This retrospective, single-center, cross-sectional study was approved by the Ethics Committee of University of the Ryukyus Hospital (approval number: 1503) and was performed in accordance with the tenets set forth in the Declaration of Helsinki. Written informed consent was obtained from all patients before their participation in the study.
This study included 164 eyes of 164 patients (28 women and 136 men) who were initially diagnosed with CSC, at the University of the Ryukyus Hospital, between October 2018 and January 2021. Diagnosis of CSC was based on SRD involving the macula, together with confirmation of leakage from the RPE on FA. Choroidal vascular hyperpermeability (CVH) appeared as hyperfluorescence in the middle phase or late phase of indocyanine green angiography (ICGA). Exclusion criteria were the following: eyes with a history of any other retinal disease, including choroidal neovascularization, uveitis, scleritis, glaucoma, primary angle-closure, trauma, and a history of ocular surgery, including cataract surgery, scleral buckling, or photodynamic therapy. Additionally, patients with a history of systemic corticosteroid medications or a history of related systemic diseases associated with CSC (i.e., collagen vascular disease, current pregnancy, organ transplantation, Cushing syndrome, and renal disease) were excluded from the study.
All patients underwent a comprehensive ocular examination, including measurement of best-corrected visual acuity with Landolt C charts, intraocular pressure, autorefractometry (ARK-1a; NIDEK, Gamagori, Japan), axial length measurement using ocular biometry (IOL Master 700; Carl Zeiss Meditec, La Jolla, CA), slit-lamp examination, color fundus photography, fundus autofluorescence photography (TRC-50DX; Topcon, Tokyo, Japan), FA and ICGA (Spectralis HRA + OCT; Heidelberg Engineering, Heidelberg, Germany), swept-source OCT (DRI-OCT Triton; Topcon, Tokyo, Japan), and AS-OCT (CASIA 2; TOMEY, Tokyo, Japan). To exclude the possibility of secondary choroidal neovascularization, all CSC patients underwent OCT angiography (DRI-OCT Triton; Topcon, Tokyo, Japan [October 2018–March 2019], Zeiss Plex Elite 9000; Carl Zeiss Meditec, Dublin, CA [April 2019–January 2021]).
The spherical equivalent was defined as the sum of the spherical power and half the cylinder power. Subfoveal choroidal thickness (SCT) was measured at the foveal center as the distance between Bruch membrane and the chorioscleral interface using the caliper measurement tool of OCT; data were used from the horizontal line scans.
Scleral thickness measurement using AS-OCT was performed as described in our previous study (Figure 1).14 First, we identified the superior, inferior, lateral, and medial rectus muscles as low reflective bands by gazing in the direction opposite to the targeted muscle; they were differentiated from the sclera that was visualized as a relatively high reflective band. Next, we determined the anterior scleral line based on the four identified rectus muscles and detected the posterior boundaries of the sclera based on the signal intensity from the choroid. The AS-OCT images were obtained by scanning parallel to the four rectus muscles with a diameter of 16 mm. The raster scan mode (consisting of 16 B-scans with a width of 4 mm) was applied to measure the scleral thickness at the center of each of the four rectus muscles. Finally, the measurements of scleral thickness were conducted in four directions: superior, temporal, inferior, and nasal, as the vertical distance between the anterior and posterior borders of the sclera at a point 6 mm posterior to the scleral spur. The presence of CE using AS-OCT was determined by two retina specialists (N.T. and N.I.). The presence or absence of CE was determined using AS-OCT images in the four directions used for scleral thickness measurements. Ciliochoroidal effusion was observed as a clearly hyporeflective area between the posterior boundaries of the sclera and the outer surface of the ciliary body or the choroid. The clinical diagnosis of CE was based on the presence of CE in at least one of the OCT images at four locations (superior, temporal, inferior, and nasal). Two specialists were assigned to evaluate the clinical features; a presence of CE was determined only when both retina specialists made the same judgment. Choroidal vascular hyperpermeability area was measured manually in each of the late-phase ICGA images within a 55 × 55 range, using the draw region tool with the Spectralis HRA + OCT. The detailed method for measuring the CVH areas has been previously described.20
Fig. 1.
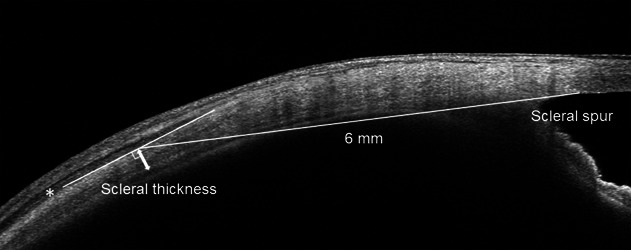
Scleral thickness measurement using anterior-segment optical coherence tomography. The anterior scleral line was determined based on the low reflective bands of the rectus muscles (asterisk). The posterior scleral line was determined by the difference in reflection intensity between the choroid and sclera. Scleral thickness measurements (double-headed arrow) were conducted vertically at the point 6 mm posterior to the scleral spur in the superior, temporal, inferior, and nasal directions.
Age, sex, spherical equivalent, axial length, presence of CE, SCT, and the scleral thickness of CSC patients, and those of the control participants were compared. The control participants visited our hospital during the same period and were selected at random from a database of patients without ocular disease or a history of eye surgery; they were matched for age, sex, and axial length with patients having CSC. Patients were divided into two groups, as follows: patients with CE (CE group) and those without CE (non-CE group). The CE and non-CE groups were compared in regard to age, sex, spherical equivalent, axial length, SCT, scleral thickness, and CVH areas. Additionally, we analyzed the relationship between the presence of CE and clinical features such as age, sex, SCT, and the mean scleral thickness value in four directions using multivariable analysis. In bilateral CSC and control cases, the right eye was selected.
Statistical Analysis
All data were analyzed using the Statistical Analysis System software (Version 9.4; SAS, SAS Inc, Cary, NC). Categorical data were assessed using Fisher exact test and the Mann–Whitney U test was used to compare the quantitative variables between the two independent groups. Associations among age, sex, SCT, the mean value of scleral thickness in four directions, and the presence of CE in CSC eyes were assessed using odds ratios and their 95% confidence intervals from the multivariable analysis. All values are expressed as the mean ± SD. Statistical significance was set at P < 0.05.
Results
In this study, 164 patients with CSC (CSC group) and 51 control patients (control group) were included. The mean age of the CSC group was 51.6 ± 12.1 years and that of the control group was 51.7 ± 10.0 years. Table 1 shows the demographic and clinical characteristics of the CSC and control groups included in this study. The presence of CE was significantly higher in the CSC group (32 eyes, 19.5%) compared with the control group (1 eye, 2.0%) (P = 0.001). Of the 32 eyes in the CSC group, 5, 2, 11, and 14 eyes had CE in 4, 3, 2, and 1 directions, respectively. In terms of locational difference, CE was observed at the superior, temporal, inferior, and nasal points in 21, 20, 11, and 10 eyes, respectively. One eye in the control group had CE at the superior and temporal points. Subfoveal choroidal thickness was significantly greater in the CSC group than in the control group (P < 0.001). Scleral thickness was significantly greater in the CSC group compared with the control group at the superior (P = 0.041), temporal (P < 0.001), inferior (P = 0.036), and nasal (P = 0.035) points. No statistically significant differences were observed between the two groups with regard to age, sex, spherical equivalent, or axial length.
Table 1.
Demographic and Clinical Characteristics of CSC Group and Control Group
| CSC Group (n = 164) | Control Group (n = 51) | P | |
| Age (years), mean ± SD | 51.6 ± 12.1 | 51.7 ± 10.0 | 0.738 |
| Female, n (%) | 28 (17.1%) | 15 (29.4%) | 0.071 |
| Spherical equivalent (diopters), mean ± SD | −0.73 ± 2.17 | −0.52 ± 1.97 | 0.942 |
| Axial length (mm), mean ± SD | 23.73 ± 0.97 | 23.99 ± 1.16 | 0.161 |
| CE, n (%) | 32 (19.5%) | 1 (2.0%) | 0.001* |
| SCT (μm), mean ± SD | 410.7 ± 106.7 | 287.0 ± 97.1 | <0.001* |
| Scleral thickness (μm), mean ± SD | |||
| Superior | 412.0 ± 61.1 | 389.1 ± 53.7 | 0.041* |
| Temporal | 432.4 ± 55.0 | 401.0 ± 51.6 | <0.001* |
| Inferior | 447.8 ± 58.9 | 431.7 ± 39.9 | 0.036* |
| Nasal | 432.8 ± 60.6 | 414.5 ± 40.7 | 0.035* |
P < 0.05, considered to be statistically significant.
Of the 164 CSC eyes, 32 were categorized into the CE group (Figure 2), and 132 were categorized into the non-CE group (Figure 3). The demographic and clinical characteristics of the CE and non-CE groups are shown in Table 2. In the univariable analysis, we observed no significant differences in age, sex, spherical equivalent, axial length, SCT, or area of CVH between the CE and non-CE groups. Scleral thickness was significantly greater in the CE group compared with the non-CE group at the superior (P = 0.005), temporal (P = 0.003), inferior (P = 0.011), and nasal (P = 0.009) points. The multivariable analysis of the factors contributing to the incidence of CE is summarized in Table 3. Variables, such as age, sex, SCT, and the mean value of scleral thickness, in four directions were included in the multivariable analysis. The mean value of scleral thickness in four directions (odds ratio, 1.01; 95% confidence interval, 1.003–1.020; P = 0.007) was the sole variable significantly associated with CE in CSC in the multivariable logistic regression analysis.
Fig. 2.
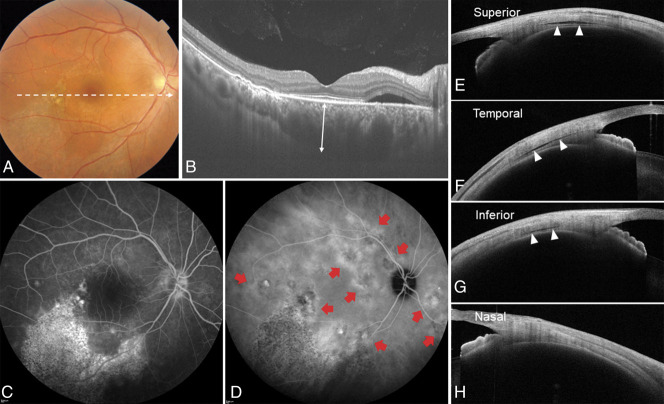
A representative case of the right eye of a 42-year-old man with central serous chorioretinopathy with ciliochoroidal effusion. The spherical equivalent refractive error was +1.500 diopter, and the axial length was 24.19 mm. A. Color fundus photograph showed a SRD and a descending tract with alteration of the retinal pigment epithelium in the macular area. B. Optical coherence tomography along the white dotted line in the color fundus photograph revealed SRD and pachychoroid with markedly dilated choroidal vessels. The subfoveal choroidal thickness (double-headed arrow) was 537 µm. C. Fluorescein angiography demonstrated several leakages in the macular area. Descending tracts corresponded to hyperfluorescent areas with window defects. D. Indocyanine green angiography revealed multifocal areas of CVH (red arrows). The total area of CVH was 14.24 mm2. E–H. Anterior-segment OCT demonstrates cross-sectional images of the anterior sclera in four directions. Ciliochoroidal effusion (arrowheads) was evident as a clearly hyporeflective area between the sclera and the ciliary body or the choroid at the superior, temporal, and inferior points. Scleral thickness at the superior, temporal, inferior, and nasal points was 472 µm, 534 µm, 539 µm, and 493 µm, respectively.
Fig. 3.
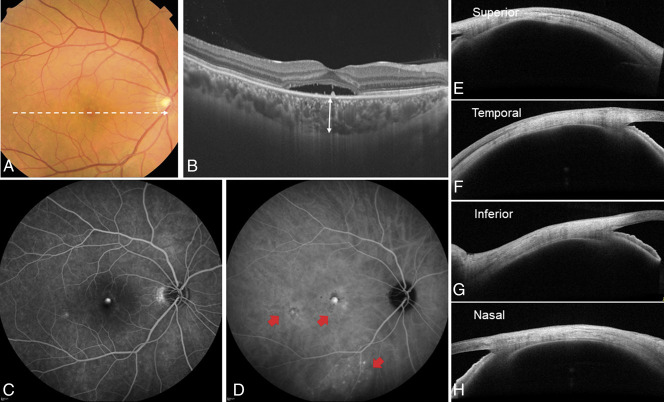
A representative case of the right eye of a 43-year-old man with central serous chorioretinopathy without ciliochoroidal effusion. The spherical equivalent refractive error was +1.125 diopters, and the axial length was 23.21 mm. A. Color fundus photograph showed a SRD in the macular area. B. Optical coherence tomography along the white dotted line in the color fundus photograph revealed SRD and pachychoroid with dilated choroidal vessels. The subfoveal choroidal thickness (double-headed arrow) was 360 µm. C. Fluorescein angiography demonstrated typical leakage in the central macula. D. Indocyanine green angiography revealed multifocal areas of CVH (red arrows). The total area of CVH was 7.22 mm2. E–H. Anterior-segment OCT demonstrates cross-sectional images of the anterior sclera in four directions. The scleral thickness at the superior, temporal, inferior, and nasal directions was 403 µm, 370 µm, 415 µm, and 405 µm, respectively.
Table 2.
Demographic and Clinical Characteristics of Patients With or Without CE in CSC
| CE Group (n = 32) | Non-CE Group (n = 132) | P | |
| Age (years), mean ± SD | 53.6 ± 13.0 | 51.1 ± 11.9 | 0.462 |
| Female, n (%) | 4 (12.5%) | 24 (18.2%) | 0.603 |
| Spherical equivalent (diopters), mean ± SD | −0.60 ± 2.19 | −0.77 ± 2.17 | 0.510 |
| Axial length (mm), mean ± SD | 23.86 ± 0.85 | 23.70 ± 1.00 | 0.319 |
| SCT (μm), mean ± SD | 433.5 ± 109.2 | 405.2 ± 105.8 | 0.145 |
| CVH (mm2), mean ± SD | 16.6 ± 15.8 | 16.8 ± 14.5 | 0.578 |
| Scleral thickness (μm), mean ± SD | |||
| Superior | 438.9 ± 66.8 | 405.5 ± 58.0 | 0.005* |
| Temporal | 456.3 ± 54.7 | 426.6 ± 53.6 | 0.003* |
| Inferior | 472.7 ± 63.0 | 441.8 ± 56.5 | 0.011* |
| Nasal | 462.9 ± 68.2 | 425.5 ± 56.5 | 0.009* |
P < 0.05, considered to be statistically significant.
Table 3.
Multivariable Analysis of the Factors Contributing to the Incidence of CE in CSC
| Odds Ratio | 95% Confidence Interval | P | |
| Age (years) | 1.02 | 0.983–1.059 | 0.284 |
| Male (vs. female) | 0.93 | 0.277–3.109 | 0.903 |
| SCT (μm) | 1.00 | 0.999–1.007 | 0.195 |
| Scleral thickness* (μm) | 1.01 | 1.003–1.020 | 0.007† |
Mean value of scleral thicknesses in four directions (superior, temporal, inferior, nasal).
P < 0.05, considered to be statistically significant.
Discussion
To the best of our knowledge, this is the first study to investigate the prevalence of CE in eyes with CSC using AS-OCT and its association with the clinical characteristics of CSC in a large cohort. The following results are noteworthy: 1) CE was observed in 19.5% of CSC eyes, and the frequency was significantly higher in the CSC group than in the control group; 2) in CSC eyes, anterior scleral thickness at the superior, temporal, inferior, and nasal points in the CE group was significantly greater than that in the non-CE group; and 3) the mean value of anterior scleral thickness was significantly associated with the presence of CE in the multivariable logistic regression analysis. These results suggest that a thick sclera may be involved in the development of CE in CSC.
In the current study, scleral thickness at all four directions was significantly greater in the CSC group compared with the control group, and the mean value of the scleral thickness at the four directions was 431.3 ± 51.4 µm in the CSC group and 409.1 ± 35.9 µm in the control group, although there were no significant differences in the spherical equivalent or axial length. Despite the increase in sample size, the results for scleral thickness were similar to those in our previous report.14
Ciliochoroidal effusion can have multiple cause other than UES, such as inflammation or hydrostatic movement. Furthermore, the attachment between the sclera and choroid is known to be weak in the peripheral region,21 and excessive fluid tends to pool in the suprachoroidal space. Therefore, all other possible causes of CE apart from UES were excluded from this study.22 In this study, CE was found in 19.5% of eyes with CSC, and the frequency of CE was significantly higher in the CSC group than in the control group. All CEs observed in the CSC eyes were localized in the periphery, and none of them extended to the equatorial and preequatorial regions, which is commonly observed in UES. Notably, CE could only be detected on AS-OCT images. There are a limited number of case reports describing the relationship between CE and CSC. All case reports have described atypical CSC cases, especially those with inferior bullous retinal detachment23,24 or those acquiring CSC after intraocular surgery.25 Ciliochoroidal effusion with CSC observed in the current study was more limited or milder than that in UES. However, CE was observed in 19.5% of CSC cases, whereas it was rarely observed in normal eyes. Based on these results, CE can be considered as a CSC-specific morphological feature, and we believe that CSC and UES partially share similar mechanisms that are involved in the development of CE.
Spaide and Ryan26 reported CSC cases with an accumulation of fluid between the sclera and choroid in the posterior pole using enhanced depth imaging OCT and en-face OCT, and they termed this feature as “loculation of fluid.” They speculated that excessive fluid accumulates in the outer choroid and suprachoroidal space as a result of the saturation of fluid in the choroidal stroma beyond a certain limit. The authors estimated that the cutoff value of SCT for the development of loculation of fluid was 403 µm.26 In the current study, the mean SCT was 433.5 µm in CSC eyes with CE (i.e., greater than 403 µm). Although the loculation of fluid in the posterior pole and CE are features observed in different locations, they are both common findings that can be observed between the sclera and the choroid, suggesting that there seems to be an association between the two features in CSC pathology.27
Uveal effusion syndrome is a rare disease characterized by choroidal protrusion, subretinal fluid, and secondary changes in the RPE. The pathogenesis of UES is implicated in nanophthalmos with scleral abnormalities. There are two main hypotheses regarding the causes of UES. First, Brockhurst11 proposed that the thickened sclera may compress the vortex veins, causing choroidal vascular congestion. Second, Gass12 suggested scleral abnormalities, such as a thickened sclera, rigidity of the scleral stroma, and reduced scleral permeability. Excessive fluid accumulates in the suprachoroidal and subretinal spaces with either mechanism. Recently, we found that CSC eyes had significantly greater scleral thickness than the control eyes, suggesting that a thickened sclera may play a role in the pathological mechanisms of CSC.14 Therefore, we postulated that a thickened sclera, similar to the UES pathologic assessment, possibly disturbs the choroidal venous outflow and reduces the transscleral fluid outflow.14 Uveal effusion syndrome eyes share many characteristics with CSC eyes, such as a short axial length,9 scleral thickening,14 choroidal thickening,28 SRD, and CE,23 although to different degrees. The current study showed that anterior scleral thickness of the CE group was significantly greater than that of the non-CE group at the superior, temporal, inferior, and nasal points. Additionally, using multivariable analysis, we found that a thickened sclera might be the sole factor related to the incidence of CE in CSC cases. Collectively, the CE associated with a thickened sclera was found not only in eyes with UES but also in eyes with CSC, supporting the hypothesis that UES and CSC partly share the same pathophysiology, such as impaired transscleral outflow or the congestion of vortex veins.
The pathogenesis of CSC remains unclear; however, choroidal abnormalities are known to play an important role. Several recent studies have suggested that choroidal circulation may be impaired in eyes with CSC. Pang et al29 showed dilated outer choroidal vessels and engorged vortex vein ampullas in patients with CSC using ultra-widefield ICGA. Moreover, Hiroe and Kishi30 showed that asymmetric dilated vortex veins were common in eyes with CSC using en-face OCT. Further studies should be conducted to clarify the association between scleral factors and choroidal circulation.
The current study has several limitations. First, because it was a single-center study conducted at a university hospital, there may have been a selection bias with regard to the patients with CSC. Second, the imaging protocol used for determining CE presence was not standardized; we intend to address this issue in a future study. Third, the presence of CE was determined at only four locations, and the prevalence might have been underestimated in both the CSC and control eyes. Fourth, as our method using AS-OCT only evaluates scleral thickness, scleral stiffness that may play a role in CE occurrence could not be evaluated. Finally, it was impossible to evaluate scleral thickness in the posterior pole, even with high-penetration SS-OCT. Although CSC is a disease characterized by posterior pole lesions, the morphological characteristics of the sclera in the posterior pole are still unknown.
In conclusion, the prevalence of CE was much higher in CSC eyes than in control eyes. However, CSC may accompany fluid accumulation in the anterior segment more frequently than previously expected. Moreover, scleral thickness was significantly greater in CSC eyes with CE than in those without CE, suggesting an association between CE and scleral thickness. It is likely that, similar to the pathogenesis of UES, a thickened sclera is closely involved in the pathogenesis of CSC.
Acknowledgments
The authors thank Editage (www.editage.com) for the English language editing.
Footnotes
Supported by JSPS KAKENHI Grant Number JP20K18349.
None of the authors has any conflicting interests to disclose.
References
- 1.Cheung CMG, Lee WK, Koizumi H, et al. Pachychoroid disease. Eye (Lond) 2019;33:14–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. Am J Ophthalmol 1967;63:139. [PubMed] [Google Scholar]
- 3.Kitzmann AS, Pulido JS, Diehl NN, et al. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980-2002. Ophthalmology 2008;115:169–173. [DOI] [PubMed] [Google Scholar]
- 4.Gelber GS, Schatz H. Loss of vision due to central serous chorioretinopathy following psychological stress. Am J Psychiatry 1987;144:46–50. [DOI] [PubMed] [Google Scholar]
- 5.Yannuzzi LA. Type-A behavior and central serous chorioretinopathy. Retina 1987;7:111–131. [DOI] [PubMed] [Google Scholar]
- 6.Quillen DA, Gass DM, Brod RD, et al. Central serous chorioretinopathy in women. Ophthalmology 1996;103:72–79. [DOI] [PubMed] [Google Scholar]
- 7.Chatziralli I, Kabanarou SA, Parikakis E, et al. Risk factors for central serous chorioretinopathy: multivariate approach in a case-control study. Curr Eye Res 2017;42:1069–1073. [DOI] [PubMed] [Google Scholar]
- 8.Tittl MK, Spaide RF, Wong D, et al. Systemic findings associated with central serous chorioretinopathy. Am J Ophthalmol 1999;128:63–68. [DOI] [PubMed] [Google Scholar]
- 9.Terao N, Koizumi H, Kojima K, et al. Short axial length and hyperopic refractive error are risk factors of central serous chorioretinopathy. Br J Ophthalmol 2020;104:1260–1265. [DOI] [PubMed] [Google Scholar]
- 10.Uyama M, Takahashi K, Kozaki J, et al. Uveal effusion syndrome: clinical features, surgical treatment, histologic examination of the sclera, and pathophysiology. Ophthalmology 2000;107:441–449. [DOI] [PubMed] [Google Scholar]
- 11.Brockhurst RJ. Vortex vein decompression for nanophthalmic uveal effusion. Arch Ophthalmol 1980;98:1987–1990. [DOI] [PubMed] [Google Scholar]
- 12.Gass JD. Uveal effusion syndrome. A new hypothesis concerning pathogenesis and technique of surgical treatment. Retina 1983;3:159–163. [PubMed] [Google Scholar]
- 13.Balaratnasingam C, Freund KB, Tan AM, et al. Bullous variant of central serous chorioretinopathy: expansion of phenotypic features using multimethod imaging. Ophthalmology 2016;123:1541–1552. [DOI] [PubMed] [Google Scholar]
- 14.Imanaga N, Terao N, Nakamine S, et al. Scleral thickness in central serous chorioretinopathy. Ophthalmol Retina 2021;5:285–291. [DOI] [PubMed] [Google Scholar]
- 15.Martínez-Belló C, Capeáns C, Sánchez-Salorio M. Ultrasound biomicroscopy in the diagnosis of supraciliochoroidal fluid after trabeculectomy. Am J Ophthalmol 1999;128:372–375. [DOI] [PubMed] [Google Scholar]
- 16.Pavlin CJ, Rutnin SS, Devenyi R, et al. Supraciliary effusions and ciliary body thickening after scleral buckling procedures. Ophthalmology 1997;104:433–438. [DOI] [PubMed] [Google Scholar]
- 17.Sakai H, Morine-Shinjyo S, Shinzato M, et al. Uveal effusion in primary angle-closure glaucoma. Ophthalmology 2005;112:413–419. [DOI] [PubMed] [Google Scholar]
- 18.Kawano Y, Tawara A, Nishioka Y, et al. Ultrasound biomicroscopic analysis of transient shallow anterior chamber in Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol 1996;121:720–723. [DOI] [PubMed] [Google Scholar]
- 19.Ikeda N, Ikeda T, Nomura C, Mimura O. Ciliochoroidal effusion syndrome associated with posterior scleritis. Jpn J Ophthalmol 2007;51:49–52. [DOI] [PubMed] [Google Scholar]
- 20.Terao N, Koizumi H, Kojima K, et al. Association of upregulated angiogenic cytokines with choroidal abnormalities in chronic central serous chorioretinopathy. Invest Ophthalmol Vis Sci 2018;59:5924–5931. [DOI] [PubMed] [Google Scholar]
- 21.Moses RA. Detachment of ciliary body—anatomical and physical considerations. Invest Ophthalmol 1965;4:935–941. [PubMed] [Google Scholar]
- 22.Elagouz M, Stanescu-Segall D, Jackson TL. Uveal effusion syndrome. Surv Ophthalmol 2010;55:134–145. [DOI] [PubMed] [Google Scholar]
- 23.Manayath GJ, Kuthirummal N, Ranjan R, et al. Atypical central serous chorioretinopathy with choroidal detachment: a case report. Retin Cases Brief Rep. 2020. doi: 10.1097/ICB.0000000000001065. [DOI] [PubMed] [Google Scholar]
- 24.Boulanger E, Bonnin S, Delahaye-Mazza C, et al. Central serous chorioretinopathy mimicking idiopathic uveal effusion syndrome. Retin Cases Brief Rep 2021. doi: 10.1097/ICB.0000000000001170. [DOI] [PubMed] [Google Scholar]
- 25.Chang R, Du Y, Peng Z, et al. Acute uveal effusion during phacoemulsification with preoperative central serous chorioretinopathy: a case report. BMC Ophthalmol 2017;17:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spaide RF, Ryan EH, Jr. Loculation of fluid in the posterior choroid in eyes with central serous chorioretinopathy. Am J Ophthalmol 2015;160:1211–1216. [DOI] [PubMed] [Google Scholar]
- 27.Imanaga N, Terao N, Sawaguchi S, et al. Clinical factors related to loculation of fluid in central serous chorioretinopathy. Am J Ophthalmol 2022;235:197–203. [DOI] [PubMed] [Google Scholar]
- 28.Harada T, Machida S, Fujiwara T, et al. Choroidal findings in idiopathic uveal effusion syndrome. Clin Ophthalmol 2011;5:1599–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pang CE, Shah VP, Sarraf D, Freund KB. Ultra-widefield imaging with autofluorescence and indocyanine green angiography in central serous chorioretinopathy. Am J Ophthalmol 2014;158:362–371.e2. [DOI] [PubMed] [Google Scholar]
- 30.Hiroe T, Kishi S. Dilatation of asymmetric vortex vein in central serous chorioretinopathy. Ophthalmol Retina 2018;2:152–161. [DOI] [PubMed] [Google Scholar]