Abstract
The Rockwood Clinical Frailty Scale is a validated rapid assessment of frailty phenotype and predictor of mortality in the geriatric population. Using data from a large tertiary care burn center, we assessed the association between admission frailty in an elderly burn population and inpatient outcomes. This was a retrospective analysis of burn patients ≥65 years from 2015 to 2019. Patients were assigned to frailty subgroups based on comprehensive medical, social work, and therapy assessments. Cox proportional hazards regression was used to estimate associations between admission frailty and 30-day inpatient mortality. Our study included 644 patients (low frailty: 262, moderate frailty: 345, and high frailty: 37). Frailty was associated with higher median TBSA and age at admission. The 30-day cumulative incidence of mortality was 2.3%, 7.0%, and 24.3% among the low, moderate, and high frailty strata, respectively. After adjustment for age, TBSA, and inhalation injury, high frailty was associated with increased 30-day mortality, compared to low (hazard ratio 5.73; 95% confidence interval 1.86, 17.62). Moderate frailty also appeared to increase 30-day mortality, although estimates were imprecise (hazard ratio 2.19; 95% confidence interval 0.87–5.50). High frailty was associated with increased morbidity and healthcare utilization, including need for intensive care stay (68% vs 37% and 21%, P < .001) and rehab or care facility at discharge (41% vs 25% and 6%, P < .001), compared to moderate and low frailty subgroups. Our findings emphasize the need to consider preinjury physiological state and the increased risk of death and morbidity in the elderly burn population.
Frailty is a dynamic status resulting from a decline in physiological reserve and remains a major burden in the aging population. Due to loss of functional homeostasis, frail patients are especially vulnerable to adverse outcomes, including morbidity and death.1–4 Conditions such as thermal injury can cause long-term impairment and result in new or worsening frailty.4 Older age is an independent predictor of frailty; however, younger patients are not immune to its effects. Clinically, this can be seen when physiologic age appears to play a greater role in outcomes than chronologic age.5
Frailty has gained the attention of researchers and clinicians alike given its impact on cost and healthcare utilization to individuals, families, and the healthcare system.2 Even after apparently minor injuries, frailer individuals have been shown to have a disproportionate change in health state, and in some cases, this may lead to a decline from independent to dependent status.3 Early identification of frail individuals allows for early intervention, which may mitigate poor outcomes, especially in the hospitalized population.1 Identifying frailty early in the disease course can influence prognosis and management approaches, resulting in significant impact on quality of life and goals of care decisions.2
Admission frailty assessment has been shown to provide a more complete evaluation of elderly burn patients, providing for enhanced prognostic ability for patient morbidity and mortality in this cohort.6–9 The Rockwood Clinical Frailty Scale version 2.0 (Figure 1) has been validated as both a rapid assessment of frailty phenotype and a predictor of mortality and other clinical outcomes in the geriatric population, even when applied retrospectively.3,4,10 Burn injury results in a severe multisystem physiologic insult and mortality in the elderly burn population is a well-documented disparity5–9; however, there is a lack of burn and critical care literature examining this correlation in a large study population.
Figure 1.
Rockwood Clinical Frailty Scale version 2.0.
Using burn registry data from a large, tertiary care burn facility, we assessed the association between admission frailty in an elderly burn population and inpatient mortality, morbidity, and healthcare utilization. We hypothesized that burn patients with increased frailty would have worse outcomes in terms of mortality, morbidity, and healthcare utilization.
METHODS
Study Design and Population
This was a retrospective cohort study of adults aged 65 years and older admitted with a burn injury (including inhalational injury alone) to our tertiary care facility between 2015 and 2019 (n = 652). Patients were identified using the burn center registry, which includes data for all burn and skin disorder admissions collected for reporting to the National Burn Registry. Only the first hospitalization for a burn injury of a patient during the study period was included. Manual chart reviews were completed for each patient to determine Rockwood Frailty Score and whether the patient was residing at a skilled nursing facility prior to burn injury. This registry was then linked to the UNC Hospital Epidemiology database, which captures all healthcare-associated infections (HAIs) through real-time, comprehensive, hospital-wide surveillance in accordance with the Centers for Disease Control and Prevention (CDC) criteria.11
This study received approval from the Institutional Review Board at the University of North Carolina at Chapel Hill (IRB# 19-1166).
Frailty at Admission
Incorporating measures of function, morbidity, and central nervous system impairments, the Rockwood Clinical Frailty Scale ranks a patient’s frailty based on a nine-point continuous scale.10 With accompanying pictographs and descriptions, the scale ranks as follows: category 1: very fit, category 2: well, category 3: managing well, category 4: vulnerable, category 5: mildly frail, category 6: moderately frail, category 7: severely frail, category 8: very severely frail, category 9: terminally ill. Permission to use and print version 2.0 of this clinical frailty scale (Figure 1) was obtained through Dr. Rockwood and colleagues. Patients were assigned to frailty subgroups based on their Rockwood Frailty Score at admission: low (1–3), medium (4–6), or high (7–9). Frailty scoring was based on comprehensive social work, physical and occupational therapy assessments. Patients who did not have complete psychosocial and/or therapy assessments that would allow for appropriate frailty assessment and Rockwood scale assignments were excluded (n = 8).
Outcomes
The primary outcome was 30-day inpatient mortality. Secondary outcomes included length of stay, intensive care unit (ICU) stay, mechanical ventilation days, number of trips to the operating room, hospital costs, discharge disposition, and HAIs. HAIs were identified through the UNC Hospital Epidemiology database and healthcare utilization and outcomes data (including mortality) were extracted from the burn registry.
Covariates
Patient demographics (age, gender, race, insurance status), burn characteristics (etiology, TBSA %, degree of burn, presence of inhalation injury, revised Baux score), location on admission (ICU, stepdown unit, or floor), and comorbidities were ascertained using data from the registry. Hospital costs include all inpatient charges, including hospital room, medications, operational and procedural fees. These data are located to the electronic medical record and downloaded into the burn registry.
Statistical Analysis
We described the distribution of demographics, burn characteristics, and comorbidities for the overall study population and stratified by frailty at admission using frequency distributions for categorical variables, medians, and interquartile ranges for continuous variables. Associations between frailty at admission, age, and TBSA were estimated using Jonckheere’s trend test. Individuals were followed from admission until death, hospital discharge, or the end of study follow-up (30 days), whichever occurred earliest. Thirty-day inpatient mortality was described using Kaplan–Meier estimators, stratified by frailty at admission. Individuals who were discharged alive or who remained alive until the end of study follow-up were censored. A Cox proportional hazards regression model was used to estimate associations between Rockwood Frailty Score at admission and 30-day inpatient mortality, adjusting for age at admission, TBSA (modeled as linear spline),12 and inhalation injury.
Secondary outcomes were described using frequency distributions for categorical variables and medians and interquartile ranges for continuous variables. Cumulative incidence of HAIs, stratified by frailty at admission, was estimated using the Aalen–Johansen estimator, treating inpatient mortality as a competing risk. Associations between frailty at admission and time to first HAI were estimated using a Fine and Gray subdistribution hazards regression model, adjusting for age at admission, TBSA, and inhalation injury.
RESULTS
Patient and Injury Characteristics
Our study included 644 patients, with 262 (40%) in the low, 345 (54%) in the moderate, and 37 (6%) in the high frailty subgroups. Demographics and burn characteristics are described in Table 1. Median age was similar amongst frailty strata, ranging 70 to 74 years. The largest group was 65 to 69 years of age (29%), followed by 70 to 74 years (17%). Fewer patients were in the 80 to 84 years (5%) and 80 to 89 years of age (3%) strata. The majority of our patient population was male (64%) and White (65%). The most common mechanism of burn injury was flame (59%), followed by scald (28%). Frailty was associated with a slightly higher median TBSA burn (low 2.0%; moderate 3.0%; high 3.0%; P = .01). The majority of cutaneous injuries were characterized as second degree; however individuals in the high frailty strata had a higher prevalence of third-degree burns (61% vs 31% and 38%, P = .004), compared to moderate and low frailty subgroups. Patients in the high frailty strata had a higher median revised Baux score. A higher percentage of patients in the high frailty subgroup were living in a skilled nursing facility before hospitalization (14% vs 3% moderate and 0% low frailty groups, P < .001) and were placed in the ICU on admission (68% vs 37% and 21%, P < .001). No meaningful differences were seen across insurance status or comorbidities with the exception of diabetes mellitus, which was more prevalent in the high frailty subgroup (41% vs 33% and 29%, P = .12).
Table 1.
Patient demographics and burn characteristics
| Characteristic | Rockwood Frailty Score | |||||
|---|---|---|---|---|---|---|
| 1–3 | 4–6 | 7–9 | ||||
| 262 (40%) | 345 (54%) | 37 (6%) | ||||
| Age, median (IQR) | 70.0 | (67, 75) | 71.0 | (68, 77) | 74.0 | (70, 79) |
| Age category, n (%) | 116 | (44.3) | 124 | (35.9) | 7 | (18.9) |
| 65–69 | 74 | (28.2) | 100 | (29.0) | 13 | (35.1) |
| 70–74 | 45 | (17.2) | 57 | (16.5) | 8 | (21.6) |
| 75–79 | 17 | (6.5) | 32 | (9.3) | 4 | (10.8) |
| 80–84 | 9 | (3.4) | 15 | (4.3) | 4 | (10.8) |
| 80–89 | 1 | (0.4) | 17 | (4.9) | 1 | (2.7) |
| Gender, n (%) | ||||||
| Male | 197 | (75.2) | 191 | (55.4) | 23 | (62.2) |
| Female | 65 | (24.8) | 154 | (44.6) | 14 | (37.8) |
| Race, n (%) | ||||||
| White | 175 | (67.6) | 210 | (61.8) | 30 | (81.1) |
| Black | 69 | (26.6) | 118 | (34.7) | 4 | (10.8) |
| Other | 15 | (5.8) | 12 | (3.5) | 3 | (8.1) |
| Living in SNF, n (%) | 0 | (0.0) | 10 | (2.9) | 5 | (13.5) |
| BMI, median (IQR) | 27.9 | (24, 32) | 27.4 | (23, 32) | 25.3 | (21, 30) |
| Admission year, n (%) | ||||||
| 2015 | 50 | (19.1) | 63 | (18.3) | 9 | (24.3) |
| 2016 | 53 | (20.2) | 54 | (15.7) | 7 | (18.9) |
| 2017 | 56 | (21.4) | 74 | (21.4) | 6 | (16.2) |
| 2018 | 42 | (16.0) | 97 | (28.1) | 6 | (16.2) |
| 2019 | 61 | (23.3) | 57 | (16.5) | 9 | (24.3) |
| Burn etiology, n (%) | ||||||
| Fire | 157 | (59.9) | 198 | (57.4) | 22 | (59.5) |
| Scald | 72 | (27.5) | 102 | (29.6) | 7 | (18.9) |
| Contact | 19 | (7.3) | 34 | (9.9) | 6 | (16.2) |
| Chemical | 7 | (2.7) | 5 | (1.4) | 1 | (2.7) |
| Electrical | 4 | (1.5) | 2 | (0.6) | 0 | (0.0) |
| Other | 3 | (1.1) | 4 | (1.2) | 1 | (2.7) |
| TBSA, median (IQR) | 2.0 | (1, 5) | 3.0 | (1, 7) | 3.0 | (2, 8) |
| TBSA category, n (%) | ||||||
| <5% | 193 | (73.7) | 233 | (67.5) | 22 | (59.5) |
| 5 to <10% | 42 | (16.0) | 54 | (15.7) | 9 | (24.3) |
| 10 to <20% | 19 | (7.3) | 35 | (10.1) | 4 | (10.8) |
| ≥20% | 8 | (3.1) | 23 | (6.7) | 2 | (5.4) |
| Burn degree, n (%) | ||||||
| First degree | 2 | (0.8) | 0 | (0.0) | 0 | (0.0) |
| Second degree | 167 | (67.9) | 204 | (61.6) | 13 | (39.4) |
| Third degree | 77 | (31.3) | 127 | (38.4) | 20 | (60.6) |
| Missing | 16 | 14 | 4 | |||
| Inhalation injury, n (%) | 9 | (3.4) | 19 | (5.5) | 5 | (13.5) |
| Revised Baux score, median (IQR) | 74.1 | (70, 80) | 77.0 | (71, 87) | 83.5 | (76, 86) |
| Location on admission, n (%) | ||||||
| ICU | 55 | (21.1) | 128 | (37.1) | 25 | (67.6) |
| Stepdown | 68 | (26.1) | 104 | (30.1) | 8 | (21.6) |
| Floor | 138 | (52.9) | 113 | (32.8) | 4 | (10.8) |
| Insurance type, n (%) | ||||||
| Medicare | 223 | (85.1) | 312 | (90.4) | 29 | (78.4) |
| Private | 21 | (8.0) | 19 | (5.5) | 6 | (16.2) |
| Military/campus | 7 | (2.7) | 2 | (0.6) | 2 | (5.4) |
| Medicaid | 1 | (0.4) | 3 | (0.9) | 0 | (0.0) |
| Self-pay | 4 | (1.5) | 5 | (1.4) | 0 | (0.0) |
| Other | 6 | (2.3) | 4 | (1.2) | 0 | (0.0) |
| Smoker, n (%) | 46 | (17.6) | 106 | (30.7) | 11 | (29.7) |
| Comorbidities, n (%) | ||||||
| Asthma | 7 | (2.7) | 9 | (2.6) | 4 | (10.8) |
| Cancer | 5 | (1.9) | 19 | (5.5) | 3 | (8.1) |
| CAD/MI | 37 | (14.1) | 66 | (19.1) | 3 | (8.1) |
| COPD | 27 | (10.3) | 102 | (29.6) | 17 | (45.9) |
| CHF | 9 | (3.4) | 34 | (9.9) | 3 | (8.1) |
| CVA | 10 | (3.8) | 41 | (11.9) | 8 | (21.6) |
| Diabetes | 75 | (28.6) | 114 | (33.0) | 15 | (40.5) |
| Renal disease | 3 | (1.1) | 14 | (4.1) | 2 | (5.4) |
BMI, body mass index; CAD/MI, coronary artery disease/myocardial infarction; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; ICU, intensive care unit; IQR, interquartile range; SNF, skilled nursing facility.
Mortality
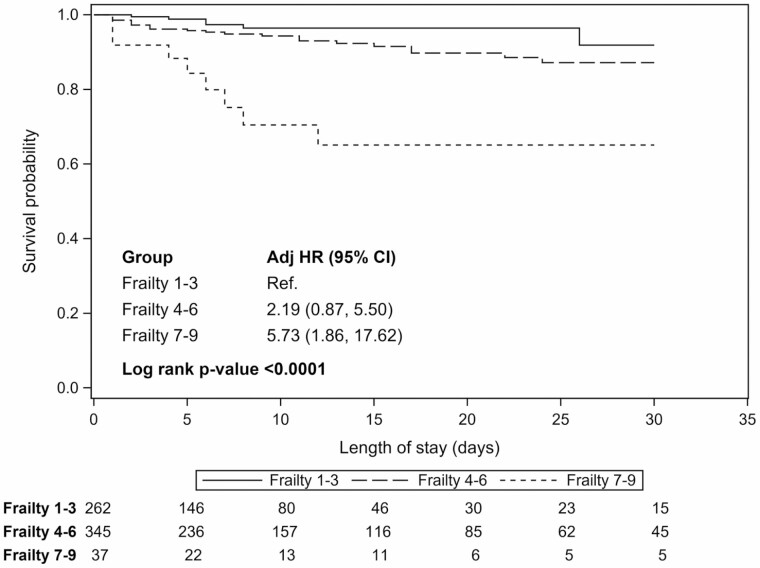
The 30-day cumulative incidence of mortality was 2.3%, 7.0%, and 24.3% among the low, moderate, and high frailty strata, respectively. Frailty was associated with higher median age (low: 70.0 years, moderate: 71.0 years, and high: 74.0 years; P < .001). After adjustment for age, TBSA, and inhalation injury, high frailty was still associated with increased 30-day mortality, compared to low frailty (hazard ratio 5.73; 95% confidence interval 1.86, 17.62) (Figure 2). Moderate frailty also appeared to increase 30-day mortality compared to low frailty, although estimates were imprecise (hazard ratio 2.19; 95% confidence interval 0.87–5.50).
Figure 2.
Time to mortality. 1) Survival probabilities, stratified by Rockwood Frailty Score at admission, were estimated using the Kaplan–Meier estimator. Individuals were followed from admission until death, hospital discharge, or the end of study follow-up (30 days), whichever occurred earliest. Individuals who were discharged alive or who remained alive until the end of study follow-up were censored. 2) Hazard ratios (HRs) were estimated using a Cox proportional hazards regression model. Individuals were followed from admission until death, hospital discharge, or the end of study follow-up (30 days), whichever occurred earliest. Individuals who were discharged alive or who remained alive until the end of study follow-up were censored. The model adjusted for age at admission, TBSA (modeled using linear splines), and inhalation injuries (yes/no).
Morbidity and Healthcare Utilization
Morbidity and healthcare utilization outcomes are reported in Table 2. Median length of stay was similar between frailty subgroups (median 6–9 days). High frailty was associated with a higher prevalence needing an ICU stay during the hospitalization (68% vs 37% and 21%, P < .001) and need for mechanical ventilation (27% vs 19% and 8%, P < .001), compared to moderate and low frailty. The moderate frailty group had the highest median hospitalization cost per day ($5950), followed by the high ($5867), and low frailty strata ($5500). Less than half of our patient population underwent operative intervention (43%). Subgroup analysis revealed that only 22% of patients in the highest frailty strata had a trip to the operating room, compared to 51% and 41% in the low and moderate frailty strata, respectively.
Table 2.
Morbidity and healthcare utilization outcomes
| Rockwood Frailty Score | ||||||
|---|---|---|---|---|---|---|
| 1–3 | 4–6 | 7–9 | ||||
| 262 (40%) | 345 (54%) | 37 (6%) | ||||
| Length of stay, median (IQR) | 6.0 | (1, 11) | 9.0 | (3, 19) | 6.0 | (3, 16) |
| Any visit to ICU, n (%) | 55 | (21.0) | 127 | (36.8) | 25 | (67.6) |
| ICU length of stay,* median (IQR) | 0.0 | (0, 0) | 0.0 | (0, 2) | 1.0 | (0, 4) |
| Any ventilator days, n (%) | 20 | (7.6) | 67 | (19.4) | 10 | (27.0) |
| Number of ventilator days,† median (IQR) | 0.0 | (0, 0) | 0.0 | (0, 0) | 0.0 | (0, 1) |
| Number of trips to operating room, n (%) | ||||||
| 0 | 128 | (48.9) | 204 | (59.1) | 29 | (78.4) |
| 1 | 111 | (42.4) | 101 | (29.3) | 7 | (18.9) |
| 2 | 16 | (6.1) | 25 | (7.2) | 0 | (0.0) |
| 3+ | 7 | (2.7) | 15 | (4.3) | 1 | (2.7) |
| Number of trips to the OR per week among those with OR trips,‡ median (IQR) | 0.8 | (0.4, 0.9) | 0.5 | (0.4, 0.9) | 0.4 | (0.4, 0.9) |
| Discharge status, n (%) | ||||||
| Home | 208 | (79.4) | 163 | (47.2) | 10 | (27.0) |
| Home with service | 30 | (11.5) | 62 | (18.0) | 2 | (5.4) |
| Skilled nursing facility | 9 | (3.4) | 60 | (17.4) | 7 | (18.9) |
| Morgue/died | 8 | (3.1) | 32 | (9.3) | 9 | (24.3) |
| Hospice | 1 | (0.4) | 8 | (2.3) | 3 | (8.1) |
| Rehab | 2 | (0.8) | 9 | (2.6) | 1 | (2.7) |
| Long-term care | 3 | (1.1) | 3 | (0.9) | 3 | (8.1) |
| Acute care facility | 0 | (0.0) | 5 | (1.4) | 1 | (2.7) |
| Discharged AMA | 1 | (0.4) | 0 | (0.0) | 1 | (2.7) |
| Hospital cost per admission, median (IQR) | $36,866 | (5906, 71,301) | $52,922 | (16,677, 114,949) | $43,419 | (17,600, 90,568) |
| Hospital cost per day, median (IQR) | $5500 | (3753, 7298) | $5950 | (4246, 8100) | $5867 | (4758, 10,524) |
| Any HAI, n (%) | 4 | (1.5) | 23 | (6.7) | 1 | (2.7) |
| Bloodstream | 2 | (50.0) | 9 | (42.9) | 0 | (0.0) |
| Pneumonia | 2 | (50.0) | 4 | (19.0) | 1 | (100.0) |
| Skin and soft tissue | 2 | (50.0) | 2 | (9.5) | 1 | (100.0) |
| Gastrointestinal | 1 | (25.0) | 4 | (19.0) | 0 | (0.0) |
| Urinary tract | 1 | (25.0) | 2 | (9.5) | 0 | (0.0) |
| Ear, eye, nose, and throat | 0 | (0.0) | 1 | (4.8) | 0 | (0.0) |
| Unknown | 2 | 2 | 2 | |||
| Organism,§,‖ n (%) | ||||||
| Enterococcus | 2 | (50.0) | 5 | (23.8) | 0 | (0.0) |
| Pseudomonas aeruginosa | 1 | (25.0) | 6 | (28.6) | 0 | (0.0) |
| Candida | 2 | (50.0) | 3 | (14.3) | 0 | (0.0) |
| Staphylococcus | 2 | (50.0) | 2 | (9.5) | 1 | (100.0) |
| Clostridium difficile | 1 | (25.0) | 4 | (19.0) | 0 | (0.0) |
| Escherichia coli | 1 | (25.0) | 1 | (4.8) | 0 | (0.0) |
| Enterobacter | 0 | (0.0) | 2 | (9.5) | 0 | (0.0) |
| Serratia marcescens | 0 | (0.0) | 2 | (9.5) | 0 | (0.0) |
| Actinomyces | 1 | (25.0) | 0 | (0.0) | 0 | (0.0) |
| Klebsiella pneumoniae | 0 | (0.0) | 1 | (4.8) | 0 | (0.0) |
| Acinetobacter baumannii | 0 | (0.0) | 1 | (4.8) | 0 | (0.0) |
| Lactobacillus | 0 | (0.0) | 1 | (4.8) | 0 | (0.0) |
| Bacillus | 0 | (0.0) | 0 | (0.0) | 1 | (100.0) |
| Unknown | 2 | 2 | 2 | |||
AMA, against medical advice; HAI, healthcare-associated infection; ICU, intensive care unit; IQR, interquartile range; OR, operating room.
*Calculated among those with any visit to ICU.
†Calculated among those with any ventilator days.
‡Individuals with >3 trips to the OR were assumed to have 3 trips.
§Percentages calculated among those with hospital-acquired infections only.
‖Not mutually exclusive.
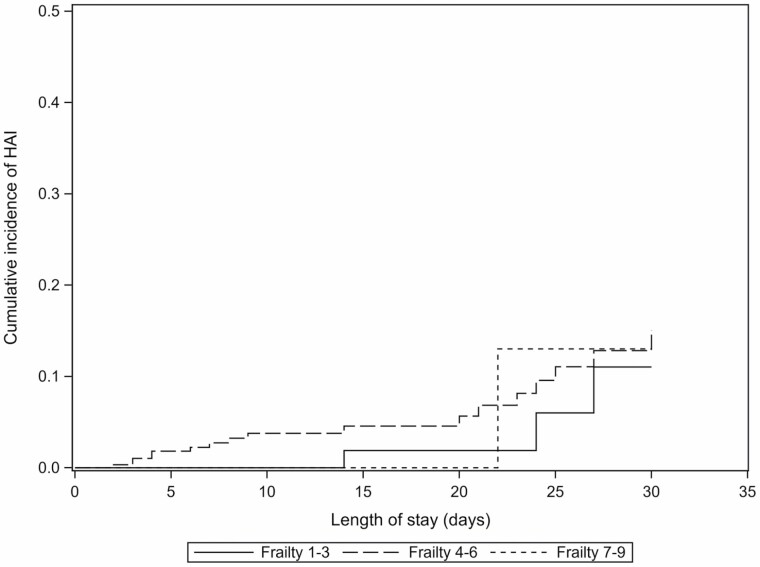
HAIs were infrequent in all frailty subgroups. Bloodstream infection was the most common, followed by pneumonia. When identified, Enterococcus and Pseudomonas aeruginosa were found to be the most prevalent offending organisms. Time to HAI is reported in Figure 3.
Figure 3.
Time to HAI. HAI, healthcare-associated infection. 1) Cumulative incidence of healthcare-associated infections, stratified by Rockwood Frailty Score at admission, was estimated using the Aalen–Johansen estimator. Individuals were followed from admission until their first hospital-associated infection, death, hospital discharge, or the end of study follow-up (30 days), whichever occurred earliest. Individuals who were discharged alive or who remained alive until the end of study follow-up were censored and death was treated as a competing risk.
The proportion of patients discharged to hospice, rehab, long- and short-term care facilities (including skilled nursing facilities) was highest in the high frailty subgroup (41% vs 25% and 6%, P < .001), compared to moderate and low frailty strata, respectively. Those in the moderate and low subgroups were more likely to be discharged home or home with services.
DISCUSSION
To our knowledge, this is the largest study to date examining frailty and mortality. Our results validate previous findings5–9 that high admission frailty is associated with an increased 30-day mortality. Despite a strong correlation between frailty at admission and age, associations persisted after adjustment for age in our Cox proportional hazards model. This suggests that the association observed in our study cannot be explained by differences in age distribution of patients alone. This study highlights the importance of accounting for frailty at admission and its correlation to healthcare utilization and patient morbidity.
Distinct patterns of burn injury and pertinent comorbidities were noted in our patients with highest frailty. Specifically, the frailest patients were more likely to have a higher TBSA of burn and comorbid diabetes mellitus.9 It has been well established that patients with diabetes often exhibit diminished protective sensation due to neuropathy and may endure greater depth of burn injury.13,14 Our frailest patients demonstrated a higher prevalence of third-degree burns and inhalational injury, including higher need for mechanical ventilation. There is conflicting data in the literature regarding the correlation between frailty and inhalation injury, with some studies finding a statistically significant relationship6 and others not.7 We speculate that individuals with higher preadmission frailty would have increased vulnerability to both thermal and inhalation injury, as well as poor ability to self-rescue, due to reduced mobility and dependence on others for personal care.
Thought-provoking observations regarding healthcare resource utilization and cost were found in the moderate and high frailty groups. At present, there is a paucity of literature describing hospitalization costs in the elderly burn population. Given the frailest patients were more likely to require stay in the ICU and require mechanical ventilation, we expected these cases to have the highest median cost per day. However, we found that those with moderate frailty had the highest median total admission costs. We believe this stems from the longer hospital stays seen in the moderately frail group, likely due to increased practice and procedural-based expenses. The highest frailty group may incur lower overall admission costs because these patients either do not live long enough to undergo surgical intervention or because their conditions mandate nonoperative management, which may be performed outside of an acute hospital setting. This has been postulated by other studies,5,8 finding that individuals with low frailty scores were more likely to be fit for surgical intervention. Previous studies have also demonstrated that frail patients, if scored on admission and at discharge, often have an observed increase in frailty scoring at the end of a hospitalization15 and high frailty to be an independent risk factor for discharge to a skilled nursing facility.6,7 Preadmission frailty, in combination with hospital-associated deconditioning, likely contributed to the high proportion of posthospitalization care and rehabilitation required by our frailest patients.
There was a low frequency of HAIs observed in our patient population and we were unable to detect any differences in HAI rates. Infection in the burn population has been shown to result in both high mortality and morbidity, with the most frequent being wound infection, bloodstream infection, urinary tract infection and pneumonia.16,17 Our population demonstrated similar patterns with bloodstream infections and pneumonia as the most common. The most common pathogens vary in the literature, however Pseudomonas, Staphylcoccus aureus, and Actinebacter are among the most commonly identified.18 Our findings highlight that burn patients 65 years of age or older, regardless of frailty status, are susceptible to infection. Early detection and treatment of infection is critical, and clinicians should be well versed in their center’s antibiotic resistance patterns and expedite wound healing to decrease infection risk.16–18
Regarding the eight patients who were excluded from the study analysis, these patients presented with large TBSA burns and died within 24 hours of admission. Palliative care and comfort measures were enacted early, and these cases were not assessed by case management or the therapy teams. These patients had an average revised Baux score of 153.4 (range 107–179). This suggests that in elderly patients whose R-Baux score is above 150, preexisting frailty assessment may be less relevant than severity of burn injury as a prognostic tool.
Our analysis has some limitations. First, this is a retrospective single-institution review and results may not generalize to other burn centers. Second, despite being the largest cohort, our data contained relatively few patients in the high frailty group and many outcomes were rare. This limited our ability to perform several adjusted analyses and many of our estimates were imprecise. By design, this study only looks at prevalence, correlation, and factors predictive of other factors. We could not make statements about why high frailty differs from moderate frailty, but we can make statements on what is associated with each group of frailty class. We also acknowledge the subjectivity of retrospective patient assignment of frailty scores. To limit this bias, we performed a validity assessment to ensure all chart reviewers were consistent in Rockwood Frailty scoring.
Frailty status provides an overview of impairment and can be utilized to make informed management decisions and coordinate interdisciplinary care.2,3 With the use of a rapid assessment tool, detection of frailty is not resource intensive and may lead to improved outcomes in burn patients. By introducing the use of a frailty assessment into clinical practice, clinicians may consider early consultation to nonsurgical services including palliative care, geriatric medicine, and clinical case management to enhance multidisciplinary decision making and treatment plans. Ultimately, we hope that these results will be used to construct a predictive nomogram including frailty for long-term outcomes and mortality for burn patients at admission. Such a tool could optimize family–provider communication regarding patient prognosis and goals of care decisions. In future studies, we intend to use this nomogram within our own burn center population to determine internal validity and applicability of this model.
CONCLUSIONS
High admission frailty is associated with an increased 30-day mortality, even after accounting for age and burn severity. Higher frailty also appears to correlate with increased morbidity and healthcare utilization. Our findings emphasize the need to consider preinjury physiological state and the increased risk of death and morbidity in the elderly burn population.
Supplementary Material
ACKNOWLEDGEMENTS
This study would not have been possible without the diligent work and meticulous documentation provided by our medical, clinical care management and therapy teams.
Funding: Paula Strassle is supported by the Division of Intramural Research, National Institute on Minority Health and Health Disparities, National Institutes of Health. The contents and views in this manuscript are those of the authors and should not be construed to represent the views of the National Institutes of Health.
Conflict of interest statement. None declared.
REFERENCES
- 1. Lang PO, Michel JP, Zekry D. Frailty syndrome: a transitional state in a dynamic process. Gerontology 2009;55:539–49. [DOI] [PubMed] [Google Scholar]
- 2. Cicutto LC. Frailty: is this a new vital sign? Chest 2018;154:1–2. [DOI] [PubMed] [Google Scholar]
- 3. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet 2013;381:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ritt M, Schwarz C, Kronawitter Vet al. Analysis of Rockwood et al’s Clinical Frailty Scale and Fried et al’s frailty phenotype as predictors of mortality and other clinical outcomes in older patients who were admitted to a geriatric ward. J Nutr Health Aging 2015;19:1043–8. [DOI] [PubMed] [Google Scholar]
- 5. Masud D, Norton S, Smailes S, Shelley O, Philp B, Dziewulski P. The use of a frailty scoring system for burns in the elderly. Burns 2013;39:30–6. [DOI] [PubMed] [Google Scholar]
- 6. Romanowski KS, Curtis E, Palmieri TL, Greenhalgh DG, Sen S. Frailty is associated with mortality in patients aged 50 years and older. J Burn Care Res 2018;39:703–7. [DOI] [PubMed] [Google Scholar]
- 7. Romanowski KS, Barsun A, Pamlieri TL, Greenhalgh DG, Sen S. Frailty score on admission predicts outcomes in elderly burn injury. J Burn Care Res 2015;36:1–6. [DOI] [PubMed] [Google Scholar]
- 8. Ward J, Phillips G, Radotra Iet al. Frailty: an independent predictor of burns mortality following in-patient admission. Burns 2018;44:1895–902. [DOI] [PubMed] [Google Scholar]
- 9. Maxwell D, Rhee P, Drake M, Hodge J, Ingram W, Williams R. Development of the Burn Frailty Index: a prognostication index for elderly patients sustaining burn injuries. Am J Surg 2019;218:87–94. [DOI] [PubMed] [Google Scholar]
- 10. Rockwood K, Theou O. Using the Clinical Frailty Scale in allocating scarce health care resources. Can Geriatr J 2020;23:210–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weber DJ, Sickbert-Bennett EE, Brown V, Rutala WA. Completeness of surveillance data reported by the National Healthcare Safety Network: an analysis of healthcare-associated infections ascertained in a tertiary care hospital, 2010. Infect Control Hosp Epidemiol 2012;33:94–6. [DOI] [PubMed] [Google Scholar]
- 12. Strassle PD, Williams FN, Napravnik Set al. Improved survival of patients with extensive burns: trends in patient characteristics and mortality among burn patients in a tertiary care burn facility, 2004–2013. J Burn Care Res 2017;38:187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nizamani R, Heisler S, Chrisco L, Campbell H, Jones SW, Williams FN. Osteomyelitis increases the rate of amputation in patients with type 2 diabetes and lower extremity burns. J Burn Care Res 2020;41:981–5. [DOI] [PubMed] [Google Scholar]
- 14. Duke JM, Randall SM, Fear MW, Boyd JH, Rea S, Wood FM. Diabetes mellitus after injury in burn and non-burned patients: a population based retrospective cohort study. Burns 2018;44:566–72. [DOI] [PubMed] [Google Scholar]
- 15. Romanowski K, Curtis E, Barsun A, Palmieri T, Greenhalgh D, Sen S. The frailty tipping point: determining which patients are targets for intervention in a burn population. Burns 2019;45:1051–6. [DOI] [PubMed] [Google Scholar]
- 16. Ramirez-Blanco CE, Ramirez-Rivero CE, Diaz-Martinez LA, Sosa-Avila LM. Infection in burn patients in a referral center in Colombia. Burns 2017;43:642–53. [DOI] [PubMed] [Google Scholar]
- 17. Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev 2006;19:403–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Latifi NA, Karimi H. Correlation of occurrence of infection in burn patients. Ann Burns Fire Disasters 2017;30:172–6. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.