Abstract
Background
Younger age of antiretroviral therapy (ART) initiation is associated with smaller viral reservoirs in perinatally acquired HIV-1 infection, but there is wide variability among early-treated infants. Predictors of this variability are not fully described.
Methods
Sixty-three neonates diagnosed with HIV-1 <48 hours after birth in Johannesburg, South Africa, were started on ART as soon as possible. Fifty-nine (94%) infants received nevirapine prophylaxis from birth until ART start. Viably preserved peripheral blood mononuclear cells (PBMCs) collected at regular intervals to 48 weeks, and from mothers at enrollment, were tested using integrase-targeted, semi-nested, real-time quantitative hydrolysis probe (TaqMan) PCR assays to quantify total HIV-1 subtype C viral DNA (vDNA). Predictors were investigated using generalized estimating equation regression.
Results
Thirty-one (49.2%) infants initiated ART <48 hours, 24 (38.1%) <14 days, and 8 (12.7%) >14 days of birth. Three-quarters were infected despite maternal antenatal ART (however, only 9.5% of women had undetectable viral load closest to delivery) and 86% were breastfed. Higher infant CD4+ T-cell percentage and viral load <100 000 copies/mL pre-ART were associated with lower vDNA in the first 48 weeks after ART start. No antenatal maternal ART and breastfeeding were also associated with lower vDNA. Older age at ART initiation had a discernible negative impact when initiated >14 days.
Conclusions
Among very early treated infants, higher CD4+ T-cell percentage and viral load <100 000 copies/mL pre-ART, infection occurring in the absence of maternal antenatal ART, and breastfeeding were associated with lower levels of HIV-1 DNA in the first 48 weeks of treatment.
Clinical Trials Registration. clinicaltrials.gov (NCT02431975).
Keywords: infant, HIV-1, antiretroviral therapy, viral reservoir
Initiation of antiretroviral therapy in the first 2 weeks of life, higher CD4+ T-cell percentage and viral load <100 000 copies/mL pre–antiretroviral therapy (-ART), no maternal antenatal ART, and breastfeeding were associated with smaller persisting viral reservoir in infants.
It is well established that antiretroviral therapy (ART) started at a young age in perinatal human immunodeficiency virus (HIV)-1 infection, or soon after primary infection in adults, leads to the establishment of a smaller viral reservoir [1–7]. In first-generation pediatric studies, young age was defined quite broadly, often including up to 6 months of age. After the provocative findings of a short period of undetectable viremia in the absence of ART in the infant in Mississippi who started ART within hours of birth [8], second-generation pediatric studies were commenced focusing on very early ART within hours or days of birth. Long-term sustained viral control off ART has been reported in rare cases of early-treated children [9, 10]. This is consistent with studies in adults that have observed post-treatment viral control after ART interruption if ART was initiated during primary infection [11, 12].
These second-generation studies of neonates initiating ART very early in life have confirmed the smaller size of the viral reservoir [13–16]. However, despite very early ART initiation, there is heterogeneity in the size of the persisting viral reservoir [16]. Some of this heterogeneity is related to poor adherence to ART, which is not surprising given the many challenges of sustaining adherence with infant ART [17]. Factors associated with this variability are not well understood.
We conducted a study of very early treated infants in Johannesburg, South Africa [18, 19]. Here we report maternal and infant predictors of the size of the persisting viral reservoir in this cohort. Recruitment methods and the profile of our cohort provided a valuable opportunity to investigate effects of the timing of ART initiation across a narrow range of age.
METHODS
Study Population
Neonates with confirmed HIV-1 infection, diagnosed less than 48 hours of birth, at Rahima Moosa Mother and Child Hospital, Johannesburg, South Africa, were started on ART as soon as possible between 1 March 2015 and 30 September 2016 (clinicaltrials.gov #NCT02431975) [18, 19]. Protocols were approved by Institutional Review Boards of the University of the Witwatersrand and Columbia University. Written informed consent was obtained from mothers for their own and their infants’ participation.
Neonates were identified through 2 parallel programs: (1) routine birth testing where blood was sent to a central laboratory and tested using the HIV-1 total nucleic acid (TNA) COBAS TaqMan HIV-1 Qualitative Test version 2.0 (Roche Molecular Systems, Branchburg, NJ) and (2) point-of-care program utilizing on-site Xpert HIV-1 Qual (Cepheid, Sunnyvale, CA) [20]. Point-of-care testing was implemented when staff capacity permitted.
After a positive Xpert result, the residual sample was re-tested. Neonates with 2 positive Xpert results were eligible for immediate ART initiation. Infants with positive or indeterminate results from the routine program were also eligible. These infants were recalled to the site for ART initiation once their results were available. Thus, the study included intrauterine-infected infants diagnosed less than 48 hours of birth. Just over half had been co-tested on-site and had an opportunity to start ART immediately, whereas others were not co-tested and could only start ART once recalled to the site.
The initial ART regimen consisted of nevirapine, lamivudine, and zidovudine if the infant was less than 42 weeks postmenstrual age. Nevirapine was changed to lopinavir-ritonavir no sooner than 42 weeks postmenstrual age, taking into account patient readiness. Drugs were given as liquid formulations twice daily. Either stavudine or abacavir were given in the event of zidovudine toxicity, and abacavir substituted for zidovudine once infants were 3 months of age or older. Daily cotrimoxazole prophylaxis was started at 4 to 6 weeks of age. Routine infant prophylaxis was 1 dose of nevirapine after birth and daily nevirapine for 6 weeks. Infants considered high risk had twice-daily zidovudine added. Prophylaxis was discontinued once ART was initiated.
Antiretroviral therapy was initiated based on results of the first round of diagnostic testing. To confirm diagnosis, a second blood sample was collected prior to the first ART dose. Qualitative HIV-1 TNA diagnostic polymerase chain reaction (PCR) was repeated and viral load (VL) testing was done (quantitative HIV-1 RNA COBAS AmpliPrep/COBAS TaqMan HIV-1 test, version 2.0; Roche Molecular Systems). If subsequent tests did not confirm the diagnosis, infants were excluded and were managed based on the profile of their results. Antiretroviral therapy was continued indefinitely. A protocol that had intended to interrupt ART for those who met certain eligibility criteria was not implemented [18].
Infants were followed with regular blood draws and this analysis focuses on results through 48 weeks. Viral load testing was done at 4, 8, 12, 16, 24, 36, and 48 weeks. Diagnostic HIV-1 PCR tests were repeated at 24 and 48 weeks. CD4+ T-cell count and percentage (TruCount Method; BD Biosciences, Heidelberg, Germany) was measured at enrollment and 24, and 48 weeks. Viably preserved peripheral blood mononuclear cells (PBMCs) collected at baseline and 4, 12, 24, and 48 weeks were stored.
Maternal blood samples were collected at enrollment and tested for VL and CD4+ T-cell count. Antenatal information, maternal HIV treatment history, and data on neonatal and obstetric characteristics were collected.
Laboratory Methods
This analysis includes 146 longitudinal samples collected from 63 infants, including 13 pre-ART samples and 133 samples collected in the first 48 weeks as well as 62 samples collected from their respective mothers. These numbers reflect visits where 2 or more vials of PBMCs were stored. When only 1 vial was stored, the selected time point was not tested.
Two semi-nested, real-time, quantitative, hydrolysis-probe (TaqMan) PCR assays (sn-qPCR) to quantify total HIV-1 subtype C viral DNA (vDNA) were developed based on methods previously described [21, 22]. The assays target 2 regions of integrase (int) and were designed using all HIV-1 subtype C pol gene sequences available (http://www.hiv.lanl.gov). All samples were assayed with 1 assay and samples that failed to amplify were assayed using the second assay to ensure that failed amplification was not due to mutations in binding regions of primer and/or probe target sequences. The sn-qPCR assays were run as previously described [4]. Sequences of primers and probes are described in the Supplementary Material.
Genomic DNA (gDNA) was extracted from stored PBMCs (Qiagen, Düsseldorf, Germany). An RNase digestion step was incorporated during extraction to ensure no carryover of viral RNA. The gDNA was quantified using both a NanoDrop 2000c spectrophotometer (ThermoFisher Scientific, Waltham, MA) and a Qubit 2.0 fluorometer (ThermoFisher Scientific). For the majority of the samples (>90%), 5 μg gDNA was assayed (1 μg/well) and only 1.4% of samples (n = 3) had less than 3 μg assayed (all these samples had detectable vDNA). Five micrograms of gDNA equates to 7.58 × 105 PBMCs; therefore, measured vDNA copies were multiplied by a factor of 1.32 to report as vDNA copies/106 PBMC equivalents (PEs). The factor was adjusted where less gDNA was assayed.
The number of vDNA copies was determined using a standard curve method (known copies of linearized p8MJ4 plasmid DNA cloned with a HIV-1 subtype C gag-pol gene serially diluted and amplified in a background of HIV-1–negative human gDNA at the same concentrations as experimental wells). To minimize variation between PCR runs, standard curve dilutions were prepared in bulk and dispensed into respective 8-well PCR tube strips and stored at −20°C until utilized, and standard curves were prepared in duplicate for the 105, 104, 103, and 102 copies and in 4-fold for the 101 and 100 copies and run over two 8-well PCR tube strips. Analyses involved choosing curves with slope values as closely matched as possible between different PCR runs to minimize inter-run variation.
Statistical Analysis
Spearman correlations and linear regression were used for analyses of maternal vDNA levels and for analyses at single points in time. Generalized estimating equation (GEE) regression models with exchangeable within-subject covariance structure were used to describe associations and to conduct multivariable analysis when longitudinal measures from infants in the cohort were analyzed. CD4+ T-cell parameters measured in the first month after ART initiation were imputed for baseline values when pre-ART values were missing. Five infants missing any baseline CD4+ T-cell measurement had it imputed based a linear regression prediction from their observed VL. Analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Almost half (49.2%) the neonates started ART less than 48 hours after birth. Of the remaining infants, 38.1% started ART within a median of 7 days (interquartile range [IQR]: 4, 17 days), while 8 (12.7%) started after 14 days of age. Most infants (86%) received only nevirapine prophylaxis from birth and a further 8% received nevirapine and zidovudine. Median CD4+ T-cell percentage was 39.5 and median pre-ART VL was 12 815 copies/mL. Most (75%) infants were infected despite maternal antenatal ART (however, only 9.5% of women had undetectable VL closest to delivery) and 86% were breastfed (Table 1).
Table 1.
Baseline Characteristics of 63 Neonates With HIV Initiating Early Antiretroviral Therapy and Their Mothers at Rahima Moosa Mother and Child Hospital, Johannesburg, South Africa, Between 1 March 2015 and 30 September 2016
| Total (N = 63) | Initiate ART <48 Hours of Birth (n = 31) | Initiate ART 48 Hours to 14 Days (n = 24) | Initiate ART >14 Days of Birth (n = 8) | P | |
|---|---|---|---|---|---|
| Maternal characteristics | |||||
| Maternal HIV RNA closest to birth, median (IQR), copies/mL | 18 031 (530, 84 000) | 13 296 (1002, 81 517) | 23 000 (373, 96 752) | 3779 (484, 285 380) | .996 |
| Maternal HIV RNA closest to birth, n (%) | |||||
| <50 copies/mL | 6 (9.5) | 3 (9.7) | 2 (8.3) | 1 (12.5) | .395 |
| 50 to <1000 copies/mL | 14 (22.2) | 4 (12.9) | 8 (33.3) | 2 (25.0) | |
| ≥1000 copies/mL | 43 (68.3) | 24 (77.4) | 14 (58.3) | 5 (62.5) | |
| Maternal CD4 count closest to birth, cells/mm3 | 298 | 366 | 255 | 387 | .079 |
| Median (IQR) | (223, 596) | (226, 725) | (131, 472) | (274, 545) | |
| Maternal CD4 count closest to birth, n (%) | |||||
| <200 cells/mm3 | 11 (17.5) | 4 (12.9) | 7 (29.2) | 0 | .306 |
| 200–349 cells/mm3 | 23 (36.5) | 11 (35.5) | 9 (37.5) | 3 (37.5) | |
| 350–499 cells/mm3 | 10 (15.9) | 4 (12.9) | 3 (12.5) | 3 (37.5) | |
| ≥500 cells/mm3 | 19 (30.2) | 12 (38.7) | 5 (20.8) | 2 (25.0) | |
| Maternal ART, n (%) | |||||
| ART started before pregnancy and continued | 8 (12.7) | 3 (9.7) | 5 (20.8) | 0 | .509 |
| ART started during pregnancy | 39 (61.9) | 20 (64.5) | 14 (58.4) | 5 (62.5) | |
| No ART up until delivery | 16 (25.4) | 8 (25.8) | 5 (20.8) | 3 (37.5) | |
| Infant characteristics | |||||
| Sex, n (%) | |||||
| Male | 28 (44.4) | 17 (54.8) | 9 (37.5) | 2 (25.0) | .253 |
| Female | 35 (55.6) | 14 (45.2) | 15 (62.5) | 6 (75.0) | |
| Age at ART start in days of life | |||||
| Median (IQR) | 2 (1, 7) | 1 (1) | 6 (4, 8) | 69 (32, 102) | NA |
| Prophylaxis before ART start, n (%) | |||||
| Nevirapine only | 54 (85.7) | 30 (96.8) | 19 (79.2) | 5 (62.5) | .006 |
| Nevirapine + zidovudine | 5 (7.9) | 0 | 2 (8.3) | 3 (37.5) | |
| None | 4 (6.4) | 1 (3.2) | 3 (12.5) | 0 | |
| Initial regimen, n (%) | |||||
| Nevirapine/lamivudine/zidovudine | 55 (87.3) | 31 (100.0) | 22 (91.7) | 2 (25.0) | <.01 |
| Nevirapine/lamivudine/stavudine | 1 (1.6) | 0 | 1 (4.2) | 0 | |
| Ritonavir-lopinavir/lamivudine/ zidovudine | 1 (1.6) | 0 | 1 (4.2) | 0 | |
| Ritonavir-lopinavir/lamivudine/abacavir | 6 (9.5) | 0 | 0 | 6 (75.0) | |
| Birth weight, median (IQR), g | 2935 (2510, 3160) | 2,980 (2845, 3300) | 2,558 (1980, 3013) | 2925 (2600, 3068) | .011 |
| Gestational age by Ballard, n (%) | |||||
| ≥37 weeks (term) | 53 (84.1) | 30 (96.8) | 15 (62.5) | 8 (100.0) | .001 |
| <37 weeks (preterm) | 10 (15.9) | 1 (3.2) | 9 (37.5) | 0 | |
| Mode of delivery, n (%) | |||||
| Vaginal | 47 (74.6) | 27 (87.1) | 15 (62.5) | 5 (62.5) | .071 |
| Cesarean | 16 (25.4) | 4 (12.9) | 9 (37.5) | 3 (37.5) | |
| Pretreatment HIV RNA, median (IQR), copies/mL | 12 815 (2020, 224 515) | 25 091 (5355, 224 515) | 2225 (694, 86 935) | 37 891 (6373, 4 905 810) | .171 |
| Pretreatment viral load, n (%) | |||||
| <100 copies/mL | 1 (1.6) | 1 (3.2) | 0 | 0 | .570 |
| 100 to <1000 copies/mL | 10 (15.9) | 2 (6.5) | 7 (29.2) | 1 (12.5) | |
| 1000–10 000 copies/mL | 16 (25.4) | 8 (25.8) | 6 (25.0) | 2 (25.0) | |
| 10 000–100 000 copies/mL | 17 (27.0) | 10 (32.3) | 5 (20.8) | 2 (25.0) | |
| ≥100 000 copies/mL | 19 (30.2) | 10 (32.3) | 6 (25.0) | 3 (37.5) | |
| Pretreatment CD4 percentage, median (IQR), % | 39.5 (32.7, 50.7) | 40.8 (32.7, 48.9) | 44.8 (35.8, 52.6) | 26.3 (18.6, 33.6) | .223 |
| Pretreatment CD4 percentage, n (%) | |||||
| <25% (severe) | 9 (15.5) | 2 (6.9) | 3 (14.3) | 4 (50.0) | .004 |
| 25–30% (advanced) | 5 (8.6) | 4 (13.8) | 0 | 1 (12.5) | |
| 30–35% (mild) | 7 (12.1) | 4 (13.8) | 1 (4.8) | 2 (25.0) | |
| >35% (none or not significant) | 37 (63.8) | 19 (65.5) | 17 (81.0) | 1 (12.5) | |
| Ever breastfed, n (%) | |||||
| Yes | 54 (85.7) | 29 (93.6) | 17 (70.8) | 8 (100.0) | .040 |
| No | 9 (14.3) | 2 (6.4) | 7 (29.2) | 0 |
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; IQR, interquartile range.
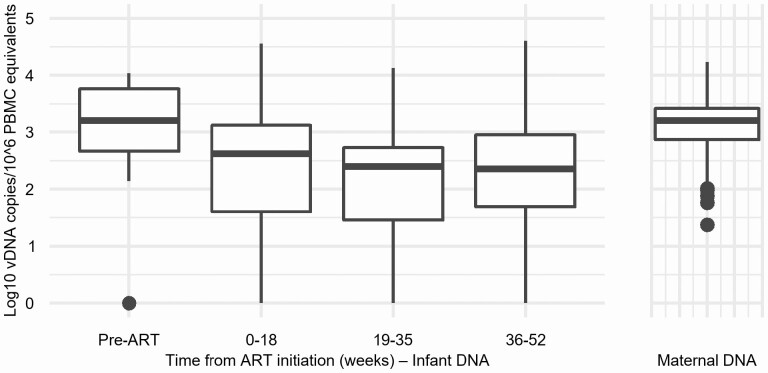
Pre-ART, the median infant vDNA was 3.21 log10 copies/106 PEs (IQR: 2.67, 3.76), similar to that observed in their mothers (median, 3.20 log10 copies/106 PBMCs; IQR: 2.87, 3.42), and declined to 2.62 log10 copies/106 PEs (IQR: 1.58, 3.13) after a median of 7.4 weeks of ART and more slowly thereafter (Figure 1). The average decline was −.045 log copies/106 PEs/month (95% confidence interval [CI]: −.072, −.018).
Figure 1.
Box plots of maternal HIV-1 DNA levels and infant HIV-1 DNA levels by time in weeks from initiation of ART. Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; PBMC, peripheral blood mononuclear cell; vDNA, viral DNA.
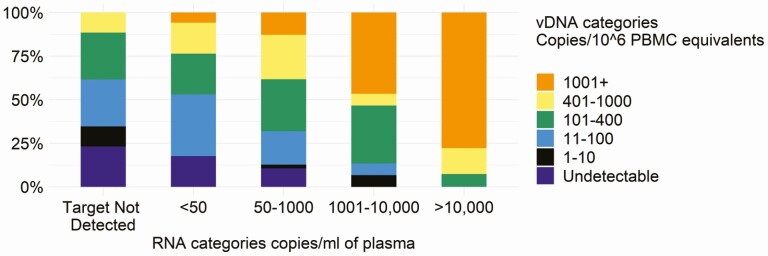
Infant vDNA post-ART was positively correlated with concurrent VL (Figure 2): for example, vDNA levels were 10 or fewer copies/106 PEs in 34.6% of VL measurements that resulted as “target not detected” but in 6.7% where VL was more than 1000 copies/mL (Figure 2).
Figure 2.
Cross-tabulation between infant cell-associated HIV-1 DNA and infant HIV-1 RNA in plasma (viral load) in categories over the first 48 weeks of antiretroviral therapy. Abbreviations: HIV, human immunodeficiency virus; PBMC, peripheral blood mononuclear cell; vDNA, viral DNA.
There was no discernible decline in infant vDNA after ART initiation if adjusting for concurrent plasma VL (−.02 [95% CI: −.05, .02] log copies/106 PEs/month).
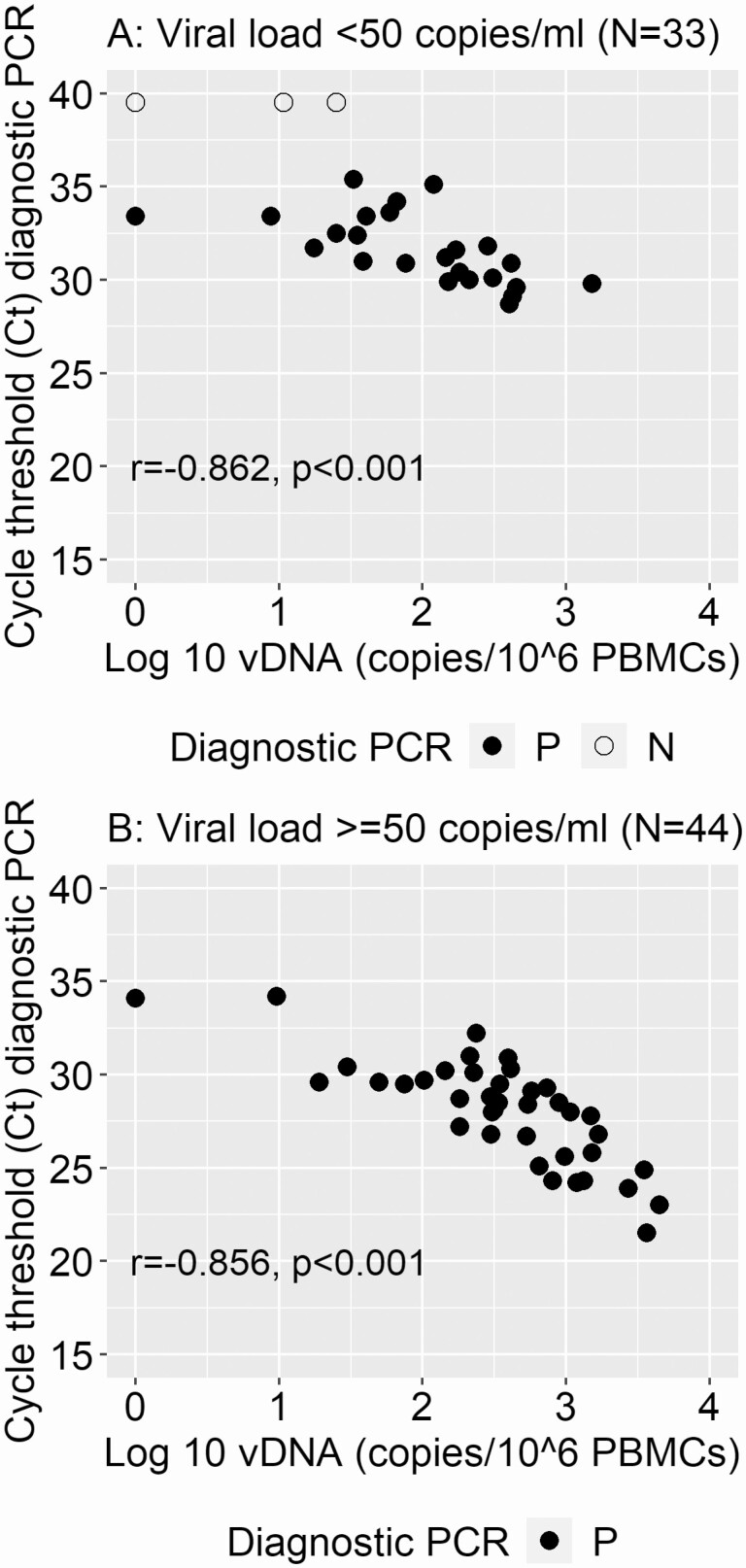
Infant vDNA was negatively correlated with cycle threshold (Ct) values from the diagnostic PCR at 24 and 48 weeks. The correlation was consistent when stratified for plasma VL (Figure 3). Similarly to vDNA results, the shift to higher Ct values at 24 and 48 weeks was most marked in those with a VL less than 50 copies/mL (Supplementary Figure 1).
Figure 3.
Associations between infant HIV-1 DNA levels and Ct values from diagnostic PCR tests conducted at 24 and 48 weeks after antiretroviral therapy initiation. Panel A displays those children with concurrent viral load results of <50 copies/mL of plasma and panel B those with ≥50 copies/mL. Solid dots indicate where the diagnostic PCR resulted as positive and open circles indicate where the diagnostic PCR resulted as negative. Abbreviations: Ct, cycle threshold; HIV, human immunodeficiency virus; N, negative; P, positive; PBMC, peripheral blood mononuclear cell; PCR, polymerase chain reaction; vDNA, viral DNA.
We examined maternal and infant predictors of infant vDNA post-ART adjusting for time on therapy. Lower maternal CD4+ T-cell count (<350 cells/mm3) and maternal ART during pregnancy were associated with higher infant vDNA (Table 2). Maternal VL and maternal vDNA levels were not significantly associated with infant vDNA levels. Lower maternal CD4+ T-cell counts and higher maternal VL were correlated with higher maternal vDNA levels, but receipt of antenatal ART was not associated with maternal vDNA levels (Supplementary Figure 2).
Table 2.
Univariable and Multivariable Associations Between Maternal and Infant Characteristics and Infant HIV-1 DNA in the First 48 Weeks of Antiretroviral Therapy Adjusted for Time After Treatment Started (59 Infants, 133 Measurements)
| Univariable Modela | Multivariable Modelb | |||||
|---|---|---|---|---|---|---|
| Parameter Coefficient | 95% Confidence Interval | P | Parameter Coefficient | 95% Confidence Interval | P | |
| Maternal characteristics | ||||||
| Maternal vDNA (log10 copies/106 PBMC equivalents) | .3070 | −.0992, .7133 | .1385 | … | … | … |
| Maternal VL (log10 copies/mL) | .0728 | −.0881, .2336 | .3751 | … | … | … |
| Maternal CD4 count ≤350 cells/mm3 (ref: >350) | .5553 | .0418, 1.0688 | .0340 | … | … | … |
| Mother had no ART prior to delivery (ref: had ART) | −.9914 | −1.6080, −.3748 | .0016 | −.9802 | −1.3676, −.5928 | <.0001 |
| Infant characteristics | ||||||
| Male sex (ref: female) | .0828 | −.4402, .6058 | .7563 | … | … | … |
| Start ART <48 hours after birth (ref: >14 days) | −.9087 | −1.5650, −.2524 | .0067 | −.4016 | −.9273, .1241 | .1343 |
| Start ART 49 hours to 14 days (ref: >14 days) | −.7467 | −1.4509, −.0426 | .0377 | −.3773 | −.9155, .1608 | .1693 |
| Low birth weight (ref: >2500 g) | −.1936 | −.8319, .4448 | .5523 | … | … | … |
| Preterm (ref: term) | −.5240 | −1.1509, .1029 | .1014 | … | … | … |
| Delivery mode cesarean (ref: vaginal) | −.1783 | −.8042, .4477 | .5767 | … | … | … |
| Pre-ART infant disease severity markers | ||||||
| Infant diagnostic PCR Ct value | −.1006 | −.1895, −.0117 | .0266 | … | … | … |
| Infant pre-ART VL (ref: <3 log10 copies/mL) | ||||||
| 3 to <4 log10 | .0489 | −.6541, .7520 | .8915 | … | … | … |
| 4 to <5 log10 | −.0602 | −.7543, .6340 | .8651 | … | … | … |
| ≥5 log10 | .7040 | .0026, 1.4054 | .0492 | .4071 | .0417, .7726 | .0290 |
| Infant pre-ART CD4 percent | −.0460 | −.0593, −.0327 | <.0001 | −.0418 | −.0556, −.0279 | <.0001 |
| Postnatal | ||||||
| Ever breastfed (ref: never breastfed) | −.2610 | −1.0331, .5110 | .5076 | −.5539 | −.9673, −.1406 | .0086 |
| Time in months after ART start | −.0449 | −.0720, −.0177 | .0012 | −.0338 | −.0584, −.0091 | .0073 |
Generalized estimating equation (GEE) analysis predicting post-ART vDNA in log copies/106 PBMC equivalents.
Abbreviations: ART, antiretroviral therapy; Ct, cycle threshold; HIV, human immunodeficiency virus; PBMC, peripheral blood mononuclear cell; PCR, polymerase chain reaction; ref, reference; vDNA, viral DNA; VL, viral load.
aUnivariable model is for each characteristic individually adjusted for time after ART start.
bMultivariable model is for all characteristics shown in the table including time after ART start.
In terms of infant characteristics, starting ART at less than 48 hours and starting ART later than 48 hours but within 14 days were associated with lower vDNA levels post-ART than starting ART at more than 14 days of age. Infant sex, birth weight, preterm birth, and mode of delivery were not associated with post-ART infant vDNA levels (Table 2).
With regard to markers of disease severity pre-ART, higher Ct values from the initial diagnostic PCR predicted lower levels of post-ART infant vDNA. Pre-ART VL displayed discontinuity of association across the range of values, with only VL greater than 100 000 copies/mL associating with higher post-ART infant vDNA. Higher pre-ART CD4+ T-cell percentage was associated with lower post-ART vDNA with a consistent gradient (Table 2).
In multivariable analysis, lack of maternal ART during pregnancy, higher infant CD4+ T-cell percentage, and VL less than 100 000 copies/mL at baseline remained significantly associated with lower post-ART infant vDNA levels, adjusting for time on ART (Table 2). Once adjusting for these parameters, any breastfeeding was associated with approximately half a log10 lower post-ART vDNA than no breastfeeding.
In an analysis restricting to post-ART vDNA measurements taken when VL was less than 50 copies/mL, lack of maternal ART during pregnancy, higher infant CD4+ T- cell percentage, and breastfeeding were associated with lower vDNA levels post-ART (Supplementary Table 1).
Median vDNA levels over the first 48 weeks of ART were similar in those starting ART at less than 48 hours versus starting after 48 hours but less than 14 days but were higher in those starting ART later than 14 days of age. The rate of decline was similar across the groups (Table 3).
Table 3.
Timing of Infant Antiretroviral Therapy Initiation and Infant HIV-1 DNA in the First 48 Weeks of Antiretroviral Therapy
| Age of the Infant When ART Was Started | |||
|---|---|---|---|
| <48 Hours | 49 Hours–14 Days | >14 Days | |
| No. of infants | 28 | 23 | 8 |
| No. of samples | 70 | 46 | 17 |
| vDNA copies/PBMC equivalents, median (IQR) | 2.42 (1.46, 2.87) | 2.39 (1.53, 2.95) | 3.17 (2.54, 3.74) |
| Slope (95% CI) of vDNA decline after ART start per month in each group | −.0410 (−.084, −.002), P = .062 | −.0493 (−.902, −.0083), P = .0183 | −.0299 (−.0603, .0005), P = .0535 |
| Univariable associations between age at ART start and vDNA in the first 48 weeks adjusting for time after ART start | |||
| ART started >14 days is referent group, B-coefficient (95% CI), P value | −.9087 (−1.5650, −.2524), P = .0067 | −.7467 (−1.4509, −.0426), P = .0377 | Referent |
| ART started <48 hours is referent group, B-coefficient (95% CI), P value | Referent | .1620 (−.3944, 0.7183), P = .5682 | .9087 (.2524, 1.5650), P = .0067 |
| Multivariable associations between age at ART start and vDNA in the first 48 weeks adjusting for time after ART start | |||
| ART started >14 days is referent group, B-coefficient (95% CI), P value, adjusted for maternal ART status, baseline viral load, breastfeeding and time after ART start (not CD4+ T-cell percentage) | −.9890 (−1.6299, −.3481), P = .0025 | −.9777 (−1.5824, −.3730), P = .0015 | Referent |
| ART started >14 days is referent group, B-coefficient (95% CI), P value, adjusted for maternal ART status, baseline viral load, breastfeeding, time after ART start, and baseline infant CD4+ T-cell percentage | −.4016 (−.9273, 0.1241), P = .1343 | −.3773 (−.9155, .1608), P = .1693 | Referent |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus; IQR, interquartile range; PBMC, peripheral blood mononuclear cell; vDNA, viral DNA.
Infants starting ART at less than 48 hours and those starting ART after 48 hours but within 14 days had lower post-ART vDNA levels relative to those starting after 14 days of age. If, however, the reference group was shifted, there were no significant differences in vDNA levels between infants who started at less than 48 hours compared with those who started after 48 hours but within 14 days (Table 3). In multivariable analysis adjusting for maternal ART, baseline infant VL, and breastfeeding, the 2 groups who started within 14 days of age had lower vDNA over the first 48 weeks of treatment than those who started after 14 days. However, if baseline CD4+ T-cell percentage was included as a covariate, this association was attenuated (Table 3). We also conducted sensitivity analyses excluding imputed CD4+ T-cell data and re-running multivariable analyses. Results were essentially unchanged.
DISCUSSION
Cell-associated HIV-1 DNA in infants declined rapidly in the first 24 weeks after very early ART but decline was slower in the 24- to 48-week period. This is consistent with results reported by others [2, 6, 14]. Total vDNA includes integrated and unintegrated HIV-1 genomes and overestimates the size of the viral reservoir. However, this marker tracks strongly with other measures of replication-competent virus and with clinically meaningful endpoints, including time to rebound after ART interruption [23]. We also observed strong correlations between vDNA levels and plasma VL, consistent with ART effects [6].
There was a strong negative correlation between vDNA measured by the qPCR research assay and Ct values from a commercially available, diagnostic assay. We have previously reported the clinical utility of the routine diagnostic assay as an approximation for research assays quantifying the viral reservoir [24]. This is encouraging as quantitation of vDNA burden adds useful information beyond what is available from VL.
Multiple studies in children have reported smaller viral reservoirs when ART is started at younger ages [1–7]. However, it is only in recent cohorts that ART has been started very early within days of birth [14, 16]. In these cohorts, sample size and study design have limited the investigation of whether ART needs to be started at less than 48 hours of birth, or whether starting only slightly later is adequate. Given the logistic challenges, having a slightly more liberal window would facilitate clinical practice. Our data indicate that, if initiated within the first 14 days after birth, there is no discernible impact of earlier ART initiation on cell-associated HIV-1 DNA in the first year of treatment. Nevertheless, our finding that adjustment for baseline CD4+ T-cell percentage attenuated the benefit of starting within 14 days reinforces the importance of timely ART initiation to avoid inevitable disease progression.
Higher baseline infant CD4+ T-cell percentage was a predictor of lower levels of vDNA in the first year of treatment. Pre-ART VL also predicted higher vDNA levels but displayed a clear threshold, with higher vDNA levels observed only once VL exceeded 100 000 copies/mL. This is similar to observations in adults [25]. We have previously reported that maternal antenatal ART influences baseline infant VL [19]. This, combined with infant prophylaxis, may render baseline infant VL a less informative prognostic marker. Infant CD4+ T-cell parameters, less susceptible to these short-term antiretroviral effects, retain their prognostic value. In univariable analysis, the Ct value from the diagnostic PCR, an indirect marker of baseline viral reservoir size, also predicted post-ART vDNA levels, but this association was attenuated after adjustment for baseline CD4+ T-cell percentage.
An unexpected finding was that infants who acquired infection despite maternal antenatal ART had higher post-ART vDNA levels than infants whose mothers had not received ART during pregnancy. We speculate that this may be due to an enrichment of select immunogenetic risk factors in infants who acquire infection despite maternal ART and/or immunomodulatory effects of maternal ART on seeding of the viral reservoir. Alternatively, maternal ART could lead to a larger representation of infants who acquired infection earlier during the pregnancy, potentially before ART was initiated, and hence have had a longer time to progress. Our data are too limited to disentangle these pathways and further investigation is required.
Most, but not all, infants were breastfed in our cohort, allowing comparison of vDNA levels by breastfeeding status. Breastfeeding was associated with lower vDNA levels after adjusting for baseline characteristics. One possible explanation may be antiretroviral drug penetrance into breast milk, which may have increased dosages to which infants were exposed and potentially made up for adherence lapses. Most mothers were receiving regimens containing efavirenz, which is known to have breast-milk penetrance [26]. Whether the magnitude of drug penetrance into breast milk is sufficient to lead to this effect is unclear. We have previously reported 2 cases of children who were initially considered infected at birth but who then experienced periods of testing negative off ART on diagnostic tests while breastfeeding continued. Their status reverted to positive with breastfeeding cessation [27]. In the pre-ART era, breastfeeding was associated with reduced mortality in infants with HIV-1 infection [28], consistent with its known benefits to protect against severe disease and death in HIV-1–exposed, uninfected, and unexposed children [29]. Breastfeeding protects infant health through multiple overlapping immune pathways [30]. Our results point to the need for further investigation of these pathways in the context of very early treatment.
There are several limitations of our study. Prior studies have found pre-ART reservoir size to associate with subsequent reservoir size [25] and cautious selection of precious PBMC samples for this investigation led to limited numbers of pre-ART samples and incomplete longitudinal sampling. With the goal of understanding predictors of the size of the viral reservoir, we developed a total vDNA quantitation assay most suitable for epidemiologic studies with small sample volumes. This assay overestimates the size of the reservoir in peripheral blood and does not distinguish functional from nonfunctional HIV-1 genomes. Peripheral quantitation of the viral reservoir may not be a proxy for the viral reservoir size in tissue compartments. Our comparisons are observational, not randomized, and vulnerable to potential confounding by unmeasured factors. Antiretroviral therapy adherence plays a critical role in treatment outcome [17] and nonadherence can obscure the role of biological factors. Results pertain only to HIV-1 infection that is acquired intrauterine.
In conclusion, we observed that infant pre-ART characteristics, including CD4+ T-cell percentage and VL, predict vDNA on ART. We also identified intriguing associations between breastfeeding and lack of maternal antenatal ART and lower cell-associated vDNA in very early treated infants. Our results support the benefit of very early ART initiation in infants, suggesting that, even if the window of less than 48 hours is missed, a week longer delay does not convey discernible disadvantage.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. The work was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institute of Allergy and Infectious Disease, National Institutes of Health (grant number U01HD080441), United States Agency for International Development (USAID)/PEPfAR, the South African National HIV Programme, and South African Research Chairs Initiative of the Department of Science and Innovation and National Research Foundation of South Africa.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Ananworanich J, Puthanakit T, Suntarattiwong P, et al. ; HIV-NAT 194 Study Group . Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS 2014; 28:1015–20. [DOI] [PubMed] [Google Scholar]
- 2. Uprety P, Chadwick EG, Rainwater-Lovett K, et al. Cell-associated HIV-1 DNA and RNA decay dynamics during early combination antiretroviral therapy in HIV-1-infected infants. Clin Infect Dis 2015; 61:1862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Luzuriaga K, Tabak B, Garber M, et al. HIV type 1 (HIV-1) proviral reservoirs decay continuously under sustained virologic control in HIV-1-infected children who received early treatment. J Infect Dis 2014; 210:1529–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuhn L, Paximadis M, Da Costa Dias B, et al. Age at antiretroviral therapy initiation and cell-associated HIV-1 DNA levels in HIV-1-infected children. PLoS One 2018; 13:e0195514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tagarro A, Chan M, Zangari P, et al. Early and highly suppressive ART are main factors associated with low viral reservoir in European perinatally HIV infected children. J Acquir Immune Defic Syndr 2018; 79:269–76. doi:10.1097/QAI.0000000000001789. PMID: 30211778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bitnun A, Ransy DG, Brophy J, et al. ; Early Pediatric Initiation Canada Child Cure Cohort (EPIC4) Research Group . Clinical correlates of human immunodeficiency virus-1 (HIV-1) DNA and inducible HIV-1 RNA reservoirs in peripheral blood in children with perinatally acquired HIV-1 infection with sustained virologic suppression for at least 5 years. Clin Infect Dis 2020; 70:859–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Massanella M, Puthanakit T, Leyre L, et al. Continuous prophylactic ARV/ART since birth reduces seeding and persistence of the viral reservoir in vertically HIV-infected children. Clin Infect Dis 2021; 73:427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med 2013; 369:1828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Violari A, Cotton MF, Kuhn L, et al. A child with perinatal HIV infection and long-term sustained virological control following antiretroviral treatment cessation. Nat Commun 2019; 10:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frange P, Faye A, Avettand-Fenoël V, et al. ; ANRS EPF-CO10 Pediatric Cohort and the ANRS EP47 VISCONTI study group . HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV 2016; 3:e49–54. [DOI] [PubMed] [Google Scholar]
- 11. Sáez-Cirión A, Bacchus C, Hocqueloux L, et al. ; ANRS VISCONTI Study Group . Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI study. PLoS Pathog 2013; 9:e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Namazi G, Fajnzylber JM, Aga E, et al. The Control of HIV After Antiretroviral Medication Pause (CHAMP) study: posttreatment controllers identified from 14 clinical studies. J Infect Dis 2018; 218:1954–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Zyl GU, Bedison MA, van Rensburg AJ, Laughton B, Cotton MF, Mellors JW. Early antiretroviral therapy in South African children reduces HIV-1-infected cells and cell-associated HIV-1 RNA in blood mononuclear cells. J Infect Dis 2015; 212:39–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Veldsman KA, Janse van Rensburg A, Isaacs S, et al. HIV-1 DNA decay is faster in children who initiate ART shortly after birth than later. J Int AIDS Soc 2019; 22:e25368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maswabi K, Ajibola G, Bennett K, et al. Safety and efficacy of starting antiretroviral therapy in the first week of life. Clin Infect Dis 2020; 72:388–93. doi:10.1093/cid/ciaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garcia-Broncano P, Maddali S, Einkauf KB, et al. Early antiretroviral therapy in neonates with HIV-1 infection restricts viral reservoir size and induces a distinct innate immune profile. Sci Transl Med 2019; 11: eaax7350. doi:10.1126/scitranslmed.aax7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Millar JR, Bengu N, Fillis R, et al. ; Ucwaningo Lwabantwana Consortium . High-frequency failure of combination antiretroviral therapy in paediatric HIV infection is associated with unmet maternal needs causing maternal non-adherence. EClinicalMedicine 2020; 22:100344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kuhn L, Strehlau R, Shiau S, et al. ; LEOPARD Study Team . Early antiretroviral treatment of infants to attain HIV remission. EClinicalMedicine 2020; 18:100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patel F, Shiau S, Strehlau R, et al. Low pretreatment viral loads in infants with HIV in an era of high-maternal antiretroviral therapy coverage. Pediatr Infect Dis J 2021; 40:55–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Technau KG, Kuhn L, Coovadia A, Murnane PM, Sherman G. Xpert HIV-1 point-of-care test for neonatal diagnosis of HIV in the birth testing programme of a maternity hospital: a field evaluation study. Lancet HIV 2017; 4:e442–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pasternak AO, Adema KW, Bakker M, et al. Highly sensitive methods based on seminested real-time reverse transcription-PCR for quantitation of human immunodeficiency virus type 1 unspliced and multiply spliced RNA and proviral DNA. J Clin Microbiol 2008; 46:2206–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kiselinova M, Pasternak AO, De Spiegelaere W, Vogelaers D, Berkhout B, Vandekerckhove L. Comparison of droplet digital PCR and seminested real-time PCR for quantification of cell-associated HIV-1 RNA. PLoS One 2014; 9:e85999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Avettand-Fènoël V, Hocqueloux L, Ghosn J, et al. Total HIV-1 DNA, a marker of viral reservoir dynamics with clinical implications. Clin Microbiol Rev 2016; 29:859–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel F, Thurman C, Liberty A, et al. Negative diagnostic PCR tests in school-aged, HIV-infected children on antiretroviral therapy since early life in Johannesburg, South Africa. J Acquir Immune Defic Syndr 2020; 83:381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gandhi RT, McMahon DK, Bosch RJ, et al. ; ACTG A5321 Team . Levels of HIV-1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PLoS Pathog 2017; 13:e1006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alassane OA, Carlos PM, Mamoudou M, Sounkalo D, Etienne C, Peggy G. Estimation of drug pharmacokinetics from breast feeding: a simple method based on meta-analysis. J Adv Med Pharm Sci 2019; 21:50249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Strehlau R, Paximadis M, Patel F, et al. ; LEOPARD Study Team . HIV diagnostic challenges in breast-fed infants of mothers on antiretroviral therapy. AIDS 2019; 33:1751–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuhn L, Aldrovandi GM, Sinkala M, et al. ; Zambia Exclusive Breastfeeding Study . Effects of early, abrupt weaning on HIV-free survival of children in Zambia. N Engl J Med 2008; 359:130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bulterys M, Ellington S, Kourtis AP. HIV-1 and breastfeeding: biology of transmission and advances in prevention. Clin Perinatol 2010; 37:807–24, ix–x. [DOI] [PubMed] [Google Scholar]
- 30. Labbok MH, Clark D, Goldman AS. Breastfeeding: maintaining an irreplaceable immunological resource. Nat Rev Immunol 2004; 4:565–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.