Abstract
We report our clinical and laboratory experience treating a 50-year-old patient who was critically ill with extensively drug-resistant Acinetobacter baumannii necrotizing pneumonia complicated by empyema in Detroit, Michigan. A precision medicine approach using whole-genome sequencing, susceptibility testing, and synergy analysis guided the selection of rational combination antimicrobial therapy.
Keywords: Acinetobacter baumannii, combination therapy, antimicrobial resistance, pneumonia, sulbactam–durlobactam
Acinetobacter baumannii is an urgent threat and critical pathogen for which new antimicrobials are needed [1, 2]. Its numerous intrinsic and acquired resistance mechanisms often render conventional antimicrobials ineffective, leading to the emergence of extensively drug-resistant (XDR) strains, defined as nonsusceptibility to ≥1 agent in all but ≤2 antibiotic categories [3]. Although infections caused by XDR A. baumannii continue to increase worldwide, there remain sparse evidence-based data detailing effective therapeutic options. Cefiderocol, Food and Drug Administration (FDA) approved in 2020 for hospital-acquired bacterial pneumonia (HABP)/ventilator-associated bacterial pneumonia (VABP) caused by gram-negative susceptible microorganisms including A. baumannii, has demonstrated in vitro activity against multidrug-resistant (MDR) A. baumannii isolates harboring class D β-lactamases, including OXA-23, OXA-24, OXA-40, OXA-51, and OXA-58 [4]. In contrast, eravacycline, FDA approved in 2018 for complicated intraabdominal infections (cIAIs), has not yet earned an indication specifically for A. baumannii, nor has it been assigned a Clinical and Laboratory Standards Institute or FDA breakpoint despite showing in vitro activity against MDR A. baumannii isolates [5]. Recently, the investigational drug durlobactam, a potent inhibitor of Ambler class A, C, and D β-lactamases, when used in combination with sulbactam, restored activity of sulbactam against carbapenem-resistant A. baumannii isolates [6, 7]. As the incidence of XDR A. baumannii isolates increases, health care teams are gaining experience with these agents as monotherapy and in combination with traditional antimicrobials to explore their place in therapy.
CASE PRESENTATION
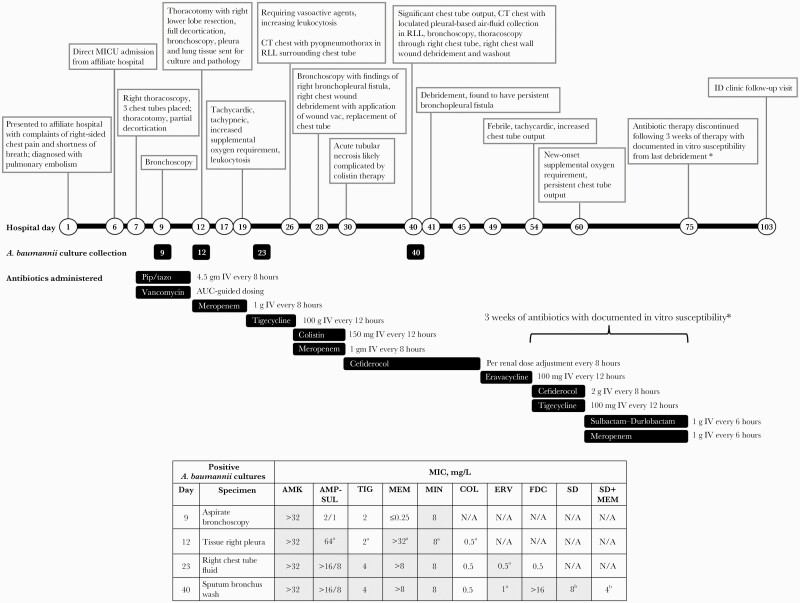
A 50-year-old man presented to an affiliate hospital with chest pain and shortness of breath. Chest computed tomography (CT) showed pulmonary emboli throughout the left lower segmental arteries and right lower lobe (RLL) with progression of previously seen pulmonary infarction. On hospital day 6, the patient transferred in as an intensive care unit direct admission and was intubated for severe respiratory distress. The next day, his repeat CT showed progressive necrosis of the RLL infarction, and he underwent a thoracotomy with partial decortication and right thoracoscopy with 3 chest tubes placed and was started on empiric piperacillin-tazobactam and intravenous (IV) vancomycin (Figure 1). A bronchoalveolar lavage on hospital day 9 resulted positive for meropenem-susceptible A. baumannii on hospital day 12, so antibiotics were switched to 3-hour extended infusion meropenem. Also, on hospital day 12, the patient underwent another thoracotomy with RLL resection and complete decortication due to a new large multiloculated pleural effusion. Pathology of the pleural peel demonstrated acute fibrinopurulent exudate, and the resected RLL demonstrated extensive abscess, necrosis, and hemorrhage. Pleural tissue culture demonstrated XDR A. baumannii on hospital day 18, with intermediate susceptibility to colistin (minimum inhibitory concentration [MIC], 0.5 mg/L) and a tigecycline MIC of 2 mg/L (Figure 1), which prompted the switch from meropenem to tigecycline 100 mg every 12 hours on hospital day 19 [3]. On hospital day 26, after a week of tigecycline monotherapy, the patient required vasoactive agents and his CT chest demonstrated RLL pyopneumothorax, so tigecycline was switched to colistin 150 mg IV every 12 hours and meropenem infused over 3 hours. Due to persistent purulent chest tube drainage, the patient underwent a bronchoscopy on hospital day 28, which identified a right bronchopleural fistula. Additionally, the patient experienced acute tubular necrosis with serum creatinine increasing 7-fold from baseline, from 1.10 to 7.60, which, although likely multifactorial, may have been complicated by colistin therapy. For these reasons, the infectious diseases (ID) service requested eravacycline and cefiderocol susceptibilities on the chest tube fluid culture from hospital day 23, which resulted susceptible with an MIC of 0.5 mg/L for cefiderocol via broth microdilution and 0.5 mg/L for eravacycline via E-test (no eravacycline breakpoint available for A. baumannii) on hospital day 29, so colistin and meropenem were switched to renally adjusted cefiderocol. Significant chest tube output continued with an average of 225 mL per day. Out of concern for an unresolving infection, bronchial washings were collected for culture on hospital day 40, which resulted positive for XDR A. baumannii on hospital day 45. The A. baumannii isolate was determined to be cefiderocol-resistant (zone diameter ≤11 mm) per disk diffusion testing, so cefiderocol was switched to eravacycline 1 mg/kg on hospital day 49 based on its MIC of 0.5 mg/L from the previous culture. On hospital day 54, the patient was febrile and tachycardic and had increased chest tube output, and the eravacycline E-test MIC increased to 1 mg/L, so eravacycline was discontinued and combination therapy with cefiderocol 2 g every 8 hours and tigecycline 100 mg every 12 hours was initiated based on published in vitro data demonstrating synergy against 6/6 MDR A. baumannii isolates [8]. While the patient received combination therapy, chest tube output remained persistent, so ID requested susceptibility testing for sulbactam–durlobactam (SUL-DUR), which is available through an expanded access program for MDR A. baumannii infections. The SUL-DUR MIC was 8 mg/L, 1 dilution above the preliminary susceptibility breakpoint (4 mg/L); however, the addition of meropenem reduced the MIC to 4 mg/L, as determined by the institution via broth microdilution.
Figure 1.
Timeline of hospital encounter and Acinetobacter baumannii isolate susceptibility data. Nonsusceptible MIC values and MIC values for which there are no established Clinical and Laboratory Standards Institute breakpoints (eg, TIG, ERV) are in shaded table boxes [16, 17]. aDetermined by e-test. bBased on SUL-DUR preliminary susceptibility breakpoint (4 mg/L). *Documented in vitro susceptibility based on current and previous data in XDR A. baumannii isolates [8]. Abbreviations: AMK, amikacin; AMP-SUL, ampicillin-sulbactam; COL, colistin; CT, computed tomography; CXR, chest X-ray; ERV, eravacycline; FDC, cefiderocol; I, intermediate; ID, infectious diseases; IV, intravenous; MEM, meropenem; MICU, medical intensive care unit; MIN, minocycline; N/A, not available; pip/tazo, piperacillin/tazobactam; R, resistant; RLL, right lower lobe; S, sensitive; SD, sulbactam–durlobactam; TIG, tigecycline; U, unknown breakpoint for A. baumannii; XDR, extensively drug-resistant.
With data demonstrating in vitro susceptibility to SUL-DUR plus meropenem, cefiderocol and tigecycline combination therapy was discontinued, and 1 g sulbactam/1 g durlobactam every 6 hours plus meropenem 1 g every 6 hours was started per the manufacturer’s protocol on hospital day 62 due to the patient’s increased supplemental oxygen requirement and persistent chest tube output. On hospital day 75, after 13 days of SUL-DUR and meropenem and resolution of chest tube output, the patient completed 3 weeks of antibiotic therapy with documented in vitro susceptibility from the last debridement, with no reported adverse drug effects [8]. Antibiotics were discontinued, and 2 days later he was cleared for discharge. The patient followed up with ID as an outpatient and 4 weeks later was at his prehospital baseline.
For all XDR A. baumannii isolates, chromosomal DNA extraction, whole-genome sequencing (WGS), and genomic content analysis were performed at Entasis Therapeutics. Full methods and accession numbers are provided in the Supplementary Data. Isolates underwent sequence analysis of known antibiotic resistance genes. All 3 tested patient isolates (collected on hospital days 12, 23, 40) encoded resistance genes for aminoglycosides, fluoroquinolones, macrolides, sulfonamides, and tetracyclines, plus 2 Class D and 1 Class C carbapenemase genes: OXA-23, OXA-66, and Acinetobacter-derived cephalosporinase (ADC)-30. The OXA-23 carbapenemase is a type of acquired resistance, whereas OXA-66 and AmpC β-lactamases are intrinsic mechanisms that confer carbapenem and cephalosporin resistance, respectively [9]. The cefiderocol-resistant isolate revealed a mutation in the TonB-dependent siderophore receptor (A1S_0980) [K628], which was likely the source of cefiderocol resistance. Full WGS results are provided in the Supplementary Data.
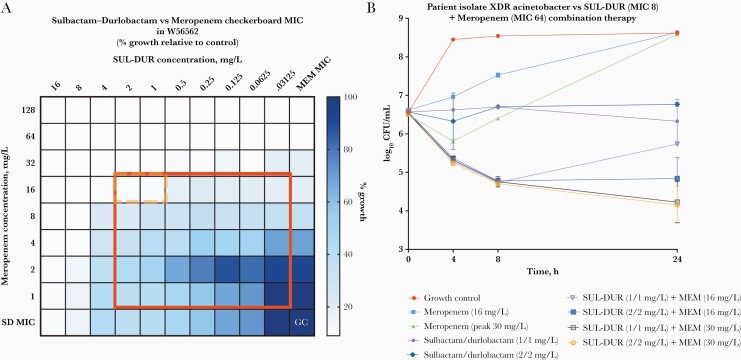
Checkerboard assays, combination MICs, and time-kill analyses (TKA) were used to explore potential synergistic effects of SUL-DUR in combination with meropenem for A. baumannii against the cefiderocol-resistant isolate. Meropenem powder was purchased commercially from Sigma Chemical Company (St. Louis, MO, USA), and SUL-DUR powder was provided by the manufacturer, Entasis Therapeutics (Waltham, MA, USA). All in vitro analyses were performed following Clinical Laboratory Standards Institute (CLSI) guidelines [10]. Combination MIC testing with SUL-DUR (durlobactam 4 mg/L kept constant) in the presence of subinhibitory amounts of meropenem (0.5 × MIC of meropenem) was performed, as was meropenem in the presence of subinhibitory amounts of SUL-DUR (0.5 × MIC of SUL-DUR) [11]. Combination MIC testing revealed a 4-fold meropenem MIC reduction in the presence of SUL-DUR (MIC 64 to 16) and a 2-fold SUL-DUR MIC reduction in the presence of meropenem (MIC 8 to 4).
The isolate was further evaluated for SUL-DUR and meropenem synergy by checkerboard analysis in duplicate (using durlobactam 4 mg/L kept constant). Synergy was defined as a fractional inhibitory concentration (FIC) index ≤0.5 [12]. The FIC index considers the combination of antibiotics that produces the greatest change from the individual MIC. The checkerboard assay revealed SUL-DUR and meropenem synergy with FIC = 0.375 (Figure 2A).
Figure 2.
A, Checkerboard analysis for synergy. Columns 1–10 contain 2-fold serial dilutions of SUL-DUR, and rows 1 to 8 contain 2-fold serial dilutions of MEM. The results are used to calculate the FIC value and then assessed for synergism, additive/indifference, or antagonism. In this illustration, “no growth” is represented by white squares and “growth” is represented by blue squares, with increasing darkness representative of higher CFU/mL. The red outline represents the area for potential synergy (FIC ≤0.5). The orange dotted outline represents the area of a demonstrated synergistic effect (FIC ≤0.5) between SUL-DUR and meropenem. B, Time-kill analysis. Planktonic time kill analyses for XDR A. baumannii patient isolate against combination therapy with sulbactam-durlobactam plus meropenem. The addition of meropenem (30 mg/L) to SUL-DUR (1 mg/L and 2 mg/L) demonstrates a synergistic effect. Abbreviations: CFU, colony-forming units; GC, growth control; MEM, meropenem; MIC, minimum inhibitory concentration; SUL-DUR, sulbactam–durlobactam; XDR, extensively-drug resistant.
TKAs were performed using inocula obtained from stationary phase cultures and 2 replicates obtained at each time point, according to CLSI standards [13]. Antimicrobials were tested at their respective synergistic concentrations as determined by checkerboard analysis or maximum concentration of free drug in serum (fCmax) [14, 15]. Sulbactam and durlobactam concentrations were kept at a 1:1 ratio (ie, 2/2 mg/L) to mimic the proposed dosing regimen. Synergy was defined as a >2 log10 CFU/mL reduction over the most potent single agent. Against the XDR A. baumannii, SUL-DUR plus meropenem was synergistic (Figure 2B), which may have contributed to the successful treatment of this patient.
DISCUSSION
The treatment of XDR A. baumannii remains challenging due to its propensity to confer multiple resistance mechanisms and sparse clinical data evaluating potentially effective therapeutic options, including the novel agents eravacycline, cefiderocol, and sulbactam–durlobactam, as monotherapy or in combination with conventional antimicrobials.
While phase 3 trials have demonstrated the efficacy of eravacycline in cIAIs caused by Enterobacterales, limited information exists for A. baumannii infections. Pharmacodynamic/pharmacokinetic (PK/PD) evaluations of eravacycline in Enterobacterales have identified fAUC/MIC targets for stasis and 1-log-kill end points of 27.97 ± 8.29 and 32.60 ± 10.85, respectively [16]. However, the European Medicines Agency (EMA) assessment report for eravacycline suggests that fAUC/MIC targets would not be achieved in Enterobacterales with MICs >0.12 [17]. Therefore, it is especially difficult to interpret the patient isolate eravacycline MICs given the lack of efficacy outcome data and PK/PD analyses for A. baumannii infections. Eravacycline MICs are, in general, 2-fold lower than those of tigecycline against carbapenem-resistant A. baumannii isolates. Additionally, eravacycline remains active against isolates harboring tetracycline efflux pump genes and is reliable against OXA carbapenemases and colistin-resistant isolates [18]. However, clinical trial data evaluating the efficacy of eravacycline against MDR A. baumannii isolates are limited to data from phase 3 cIAI and complicated urinary tract infection (cUTI) trials [19, 20]. For cIAIs, eravacycline was noninferior to ertapenem and meropenem, but only 3% and 2% of those patients had A. baummannii infections, respectively. For cUTI, eravacycline was inferior to levofloxacin and meropenem.
Cefiderocol has demonstrated in vitro activity against >95% of meropenem-nonsusceptible A. baumannii isolates harboring class D β-lactamases per surveillance data using CLSI susceptibility criteria ≤4 mg/L [21]. However, similar to eravacycline, clinical data supporting its use for infections caused by MDR A. baumannii are limited to 2 phase 3 trials. CREDIBLE-CR enrolled 54 patients with infections due to carbapenem-resistant gram-negative bacteria. While clinical cure rates were similar between cefiderocol and the best available therapy (mostly composed of polymyxin-based regimens), patients who received cefiderocol had increased all-cause mortality, which was driven by higher mortality in patients with A. baumannii infections [22]. APEKS-NP enrolled 300 critically ill patients with nosocomial pneumonia and identified no difference in 14-day all-cause mortality between cefiderocol and optimized meropenem (2 g IV every 8 hours, 3-hour extended infusion). Notably, 16% of those enrolled were infected with A. baumannii, of whom 66% were carbapenem-resistant [23].
Durlobactam has demonstrated in vitro activity against Ambler class A, C, and D β-lactamases [24]. One study reported the in vitro activity of SUL-DUR against 1722 clinical isolates of the ABC complex (A. baumannii, Acinetobacter calcoaceticus, Acinetobacter nosocomialis, and Acinetobacter pittii) collected globally in 2016 and 2017 [7]. Of the isolates tested, 97.7% had an SUL-DUR MIC of ≤4 μg/mL, the proposed SUL-DUR breakpoint, which is based on preclinical and clinical modeling of joint PK/PD target attainment analysis for sulbactam and durlobactam [25–27]. Among the SUL-DUR-nonsusceptible isolates (2.3%), most encoded either the NDM-1 metallo β-lactamase, which is not inhibited by durlobactam, or amino acid changes in PBP3, the target of sulbactam [7]. Given this information, it is reasonable to conclude that for A. baumannii isolates with MIC >4 μg/mL, the addition of a carbapenem to SUL-DUR therapy may be an alternative therapeutic choice, although additional studies are warranted.
Results were recently released from ATTACK, a global phase 3 registration trial evaluating the safety and efficacy of SUL-DUR for the treatment of carbapenem-resistant A. baumannii HABP, VABP, or bacteremia. In the study, patients were randomized to receive either SUL-DUR (dosed 1 g/1 g infused over 3 hours) or colistin (2.5 mg/kg infused over 30 minutes every 12 hours), both in combination with imipenem/cilastatin. In total, 207 patients were enrolled from 95 clinical sites across 17 countries. SUL-DUR met the primary end point of 28-day all-cause mortality showing noninferiority compared with colistin in a microbiologically modified intent-to-treat population, with a statistical trend toward lower mortality among patients who received SUL-DUR vs colistin (19% [12/63] vs 32.3% [20/62], respectively). Additionally, clinical response at test-of-cure once again favored SUL-DUR with 61.9% compared with 40.3% in the colistin arm. The study’s primary safety objective was also met with a significant reduction in nephrotoxicity among patients who received at least 1 dose of SUL-DUR or colistin (13.2% [12/91] vs/ 37.6% [32/85], respectively; ClinicalTrials.gov NCT03894046: http://clinicaltrials.gov/ct2/show/NCT03894046 [28]. While the use of combination therapy in this study aligns with the newly published Infectious Diseases Society of America guidance for the treatment of moderate to severe infections caused by carbapenem-resistant A. baumannii, it leaves in question the optimal combination agents to be used with SUL-DUR [29].
To our knowledge, this is the first case report documenting SUL-DUR plus meropenem combination therapy as an adjuvant to surgical management for necrotizing XDR A. baumannii pneumonia complicated by empyema. The addition of meropenem to SUL-DUR in this case instead of imipenem–cilastatin was secondary to institutional formulary restrictions. Further in vitro analyses revealed synergistic effects with SUL-DUR plus meropenem against the third XDR patient isolate, which may have contributed to the patient’s resolution of chest tube output. However, without safety and efficacy data from clinical trials, it is difficult to assess the role of meropenem as a combination agent with SUL-DUR in the successful treatment of this patient. The positive impact of this combination may also in part be due to durlobactam’s unique ability to restore sulbactam activity against MDR A. baumannii. A similar case report described combination SUL-DUR and cefiderocol therapy for the treatment of XDR A. baumannii in a patient with severe COVID-19 and septic shock secondary to HABP [30].
CONCLUSIONS
This case describes the clinical use of combination 1 g sulbactam/1 g durlobactam every 6 hours as a 3-hour infusion with meropenem 1 g every 6 hours administered via 30-minute infusion for necrotizing XDR A. baumannii pneumonia/empyema. The resultant in vitro synergy of this combination may have contributed to the successful treatment of this patient. Thus, in patients with XDR A. baumannii demonstrating in vitro resistance to SUL-DUR, combination therapy of SUL-DUR with meropenem may be an appropriate therapy option. Additional clinical trials are necessary to inform use of SUL-DUR both as monotherapy and in combination with conventional antimicrobial therapies pending its approval for use in Acinetobacter infections.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We extend a thank you to Entasis Therapeutics for supplying sulbactam–durlobactam through their expanded access program and to Dr. Samir Moussa of Entasis Therapeutics, who performed the initial sulbactam–durlobactam MIC testing and whole-genome sequencing of the A. baumannii clinical isolates.
Funding. None.
Potential conflicts of interest. M.J.R. has received research support from, has consulted for, or has spoken on behalf of Merck, Shionogi, Paratek, Spero, Tetraphase, and Entasis. All other authors have no conflicts of interest to disclose. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. D.H. and A.J.K.C. constructed the manuscript. J.J.Z., A.S., and H.S. provided clinical care for the patients and revised the manuscript. M.J.R. provided support, conceptual advice, and review at all stages of manuscript preparation. All authors read and approved the final manuscript.
Patient consent. This study does not include factors necessitating patient consent.
Data availability. Data are available at GenBank accession numbers JAJKGU000000000, JAJKGT000000000, and JAJKGS000000000. Full WGS methods and results are provided in the Supplementary Data.
References
- 1. World Health Organization. WHO publishes list of bacteria for which new antibiotics are urgently needed. Available at: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed. Accessed 11 January 2021.
- 2. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2019. US Department of Health and Human Services, Centers for Disease Control and Prevention; 2019. [Google Scholar]
- 3. Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–81. [DOI] [PubMed] [Google Scholar]
- 4. Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF.. In vitro activity of the siderophore cephalosporin, cefiderocol, against carbapenem-nonsusceptible and multidrug-resistant isolates of gram-negative bacilli collected worldwide in 2014 to 2016. Antimicrob Agents Chemother 2018; 62:e01968–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morrissey I, Olesky M, Hawser S, et al. In vitro activity of eravacycline against gram-negative bacilli isolated in clinical laboratories worldwide from 2013 to 2017. Antimicrob Agents Chemother 2020; 64:e01699–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miller A, McLeod S, Mathur T, Morrissey I.. 694. In vitro antibacterial activity of sulbactam–durlobactam (ETX2514SUL) against 121 recent Acinetobacter baumannii isolated from patients in India. Open Forum Infect Dis 2019; 6:XXX–XX. [Google Scholar]
- 7. McLeod SM, Moussa SH, Hackel MA, Miller AA.. In vitro activity of sulbactam-durlobactam against Acinetobacter baumannii -calcoaceticus complex isolates collected globally in 2016 and 2017. Antimicrob Agents Chemother 2020; 64:e02534–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abdul-Mutakabbir JC, Nguyen L, Maassen PT, et al. In vitro antibacterial activity of cefiderocol against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2021; 65:e0264620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hujer AM, Hujer KM, Leonard DA, et al. A comprehensive and contemporary “snapshot” of β-lactamases in carbapenem resistant Acinetobacter baumannii. Diagn Microbiol Infect Dis 2021; 99:115242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; 30th Informational Supplement. CLSI Document MS100. Clinical and Laboratory Standards Institute;2020. [Google Scholar]
- 11. Xhemali X, Smith JR, Kebriaei R, et al. Evaluation of dalbavancin alone and in combination with β-lactam antibiotics against resistant phenotypes of Staphylococcus aureus. J Antimicrob Chemother 2019; 74:82–6. [DOI] [PubMed] [Google Scholar]
- 12. Doern CD. When does 2 plus 2 equal 5? a review of antimicrobial synergy testing. J Clin Microbiol 2014; 52:4124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. National Committee for Clinical Laboratory Standards. Methods for Determining Bactericidal Activity of Antimicrobial Agents. Approved Guideline M26-A. National Committee for Clinical Laboratory Standards;1998. [Google Scholar]
- 14. Pankuch GA, Lin G, Seifert H, Appelbaum PC.. Activity of meropenem with and without ciprofloxacin and colistin against Pseudomonas aeruginosa and Acinetobacter baumannii. Antimicrob Agents Chemother 2008; 52:333–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petersen PJ, Labthavikul P, Jones CH, Bradford PA.. In vitro antibacterial activities of tigecycline in combination with other antimicrobial agents determined by chequerboard and time-kill kinetic analysis. J Antimicrob Chemother 2006; 57:573–6. [DOI] [PubMed] [Google Scholar]
- 16. Zhao M, Lepak AJ, Marchillo K, VanHecker J, Andes DR.. In vivo pharmacodynamic target assessment of eravacycline against Escherichia coli in a murine thigh infection model. Antimicrob Agents Chemother 2017; 61:e00250–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. European Medicine Agency. Assessment report: Xerava. Available at: https://www.ema.europa.eu/en/documents/assessment-report/xerava-epar-public-assessment-report_en.pdf. Accessed 10 January 2022.
- 18. Livermore DM, Mushtaq S, Warner M, Woodford N.. In vitro activity of eravacycline against carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii. Antimicrob Agents Chemother 2016; 60:3840–3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Solomkin JS, Gardovskis J, Lawrence K, et al. IGNITE4: results of a phase 3, randomized, multicenter, prospective trial of eravacycline vs meropenem in the treatment of complicated intraabdominal infections. Clin Infect Dis 2019; 69:921–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Solomkin J, Evans D, Slepavicius A, et al. Assessing the efficacy and safety of eravacycline vs ertapenem in complicated intra-abdominal infections in the investigating gram-negative infections treated with eravacycline (IGNITE 1) trial: a randomized clinical trial. JAMA Surg 2017; 152:224–32. [DOI] [PubMed] [Google Scholar]
- 21. Hackel MA, Tsuji M, Yamano Y, Echols R, Karlowsky JA, Sahm DF.. In vitro activity of the siderophore cephalosporin, cefiderocol, against a recent collection of clinically relevant gram-negative bacilli from North America and Europe, including carbapenem-nonsusceptible isolates (SIDERO-WT-2014 Study). Antimicrob Agents Chemother 2017; 61:e00093–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fetroja. Package Insert. Shionogi Inc.; 2020. [Google Scholar]
- 23. Wunderink RG, Matsunaga Y, Ariyasu M, et al. Cefiderocol versus high-dose, extended-infusion meropenem for the treatment of gram-negative nosocomial pneumonia (APEKS-NP): a randomised, double-blind, phase 3, non-inferiority trial. Lancet Infect Dis 2021; 21:213–25. [DOI] [PubMed] [Google Scholar]
- 24. Durand-Réville TF, Guler S, Comita-Prevoir J, et al. ETX2514 is a broad-spectrum β-lactamase inhibitor for the treatment of drug-resistant gram-negative bacteria including Acinetobacter baumannii. Nat Microbiol 2017; 2:17104. [DOI] [PubMed] [Google Scholar]
- 25. O’Donnell J, Scoy BV, Rubino C, et al. Pharmacokinetics /pharmacodynamics and phase 3 dose projection for the novel β-lactamase inhibitor ETX2514 in combination with sulbactam against Acinetobacter baumannii-calcoaceticus complex (ABC). Paper presented at: ASM Microbe; 23 June 2019: San Francisco, CA. [Google Scholar]
- 26. Rodvold KA, Gotfried MH, Isaacs RD, O’Donnell JP, Stone E.. Plasma and 330 intrapulmonary concentrations of ETX2514 and sulbactam following intravenous administration 331 of ETX2514SUL to healthy adult subjects. Antimicrob Agents Chemother 2018; 62:e01089–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Onufrak NJ, Rubino CM, Ambrose PG, Isaacs R, Srinivasan S, O’Donnell J.. Population pharmacokinetic and pharmacokinetic-pharmacodynamic target attainment analyses of ETX2514SUL to support dosing regimens in patients with varying renal function. Paper presented at: ECCMID 2019; April 13–16, 2019; Amsterdam, the Netherlands. [Google Scholar]
- 28. Entasis Therapeutics. A randomized, active-controlled study to evaluate the efficacy and safety of intravenous sulbactam-ETX2514 in the treatment of patients with infections caused by Acinetobacter baumannii-calcoaceticus complex. ClinicalTrials.gov NCT03894046. Available at: https://clinicaltrials.gov/ct2/show/NCT03894046. Accessed 8 December 2021.
- 29. Tamma PD, Aitken SL, Bonomo RA, Mathers AJ, van Duin D, Clancy CJ.. Infectious Diseases Society of America guidance on the treatment of AmpC β-lactamase-producing Enterobacterales, carbapenem-resistant Acinetobacter baumannii, and Stenotrophomonas maltophilia infections. Version 2.0. Available at: https://www.idsociety.org/practice-guideline/amr-guidance-2.0/. Accessed 9 December 2021. [DOI] [PubMed]
- 30. Zaidan N, Hornak JP, Reynoso D.. Extensively drug-resistant Acinetobacter baumannii nosocomial pneumonia successfully treated with a novel antibiotic combination. Antimicrob Agents Chemother 2021; 0:AAC.00924–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.