Abstract
Background
Providing incentives to screen close contacts for tuberculosis (TB) is an alternative to household-based contact investigation. We aimed to characterize patients and contexts where this incentive-based strategy might be preferred.
Methods
This is a secondary analysis of a cluster randomized trial of TB contact investigation in Limpopo District, South Africa, conducted between 2016 and 2020. Twenty-eight clinics were randomly allocated to household-based vs incentive-based contact investigation. In the incentive-based arm, index participants and contacts received transport reimbursement and incentives for TB screening and microbiological diagnosis of contacts. We estimated differences in mean number of contacts per index participant with household-based vs incentive-based contact investigation overall and within subgroups of index participants.
Results
A total of 3776 contacts (1903 in the incentive-based and 1873 in the household-based arm) were referred by 2501 index participants. A higher proportion of contacts in the incentive-based than household-based arm were adults (72% vs 59%), reported chronic TB symptoms (25% vs 16%) or ever smoking (23% vs 11%). Index participants who walked or bicycled to a clinic referred 1.03 more contacts per index (95% confidence interval [CI], .48 to 1.57) through incentive-based than household-based investigation. Index participants living with >5 household members referred 0.48 more contacts per index (95% CI, .03 to .94) through household-based than incentive-based investigation.
Conclusions
Relative to household-based investigation, incentive-based investigation identifies contacts likely at higher risk for active TB. Incentive-based investigation may be more appropriate for index participants who can easily access clinics, versus household-based investigation for patients with large households.
Clinical Trials Registration. NCT02808507.
Keywords: contact tracing, incentive, index patient epidemiology, motivation, tuberculosis
Incentive-based contact investigation for tuberculosis is an alternative to household-based investigation that identifies a higher-risk group of contacts. Incentive-based contact investigation may be particularly appropriate for patients who do not require motorized transport to access clinics.
Tuberculosis (TB) is a leading cause of infectious mortality worldwide, estimated to have caused 1.5 million deaths in 2018 [1]. To achieve early TB detection and timely care, contact investigation has been recommended for many years by the World Health Organization [2] and national guidelines [1]. Traditionally, contact investigations have focused on household contacts [3], but household contact investigation has not been implemented widely in resource-limited, high TB burden settings [4]. Barriers to implementation of household contact investigation include difficulty in locating households and contacts at home, constrained numbers of healthcare workers to perform contact investigation, and the cost of transport to visit households [5-7]. Furthermore, household contact investigation may miss household contacts who spend most of their time outside the home (including young adult men, known to bear a disproportionate burden of TB) [8] and close contacts from other settings such as the workplace [3]. Patients who live by themselves also do not qualify for household contact investigation, even though they may have a large number of nonhousehold contacts and likely contribute substantially to TB transmission in the community [9].
Offering monetary incentives for contacts to present to a clinic can be an alternative to traditional household contact investigation. Monetary incentives have been shown to motivate healthcare workers to perform screening in the community [5] and increase patient adherence to TB treatment [10]. Moreover, incentivization can enable expansion of investigations beyond household contacts and can empower contacts to present at times convenient to them, which may help identify contacts who might be disproportionately missed by household contact investigation. Effective implementation of incentive-based contact investigation, however, depends on a clear description of the types of index patients and contacts who participate and subgroups of index patients for whom incentive-based contact investigation might be particularly effective. The purpose of this study was therefore to provide a comprehensive description of the characteristics and effectiveness of incentive-based TB contact investigation in comparison to household-based investigation in 2 districts of South Africa.
METHODS
Study Setting
From July 2016 to January 2020, a cluster-randomized, controlled trial, the Kharitode study, was conducted in a rural district (Vhembe) and a periurban district (Waterberg) of Limpopo Province, South Africa [11]. Limpopo Province is sparsely populated (46.1 people per km2 in 2016) [12, 13] and has the fourth lowest provincial gross domestic product ($22 287 in 2017) [13, 14] in South Africa. TB incidence in Limpopo Province was 301 per 100 000 in 2015 [12, 15, 16], lower than national estimates but higher than most other sub-Saharan African countries [1, 15]. The trial randomized 56 primary health clinics (clusters) to either facility-based case finding or contact investigation. Among the 28 clinics randomized to contact investigation, 14 were subrandomized to household contact tracing and 14 to incentive-based contact tracing. After 18 months of enrollment and a 6-month washout period, the clinics in each contact investigation arm were crossed over, and the alternative contact investigation strategy was performed in each of the 28 clinics for the next 18 months. This evaluation of contact investigation uses data from these 28 clinics over the full 36-month analysis period [11].
Incentive-based Contact Investigation
In incentive-based clinics, all adults (and guardians of children) with newly diagnosed TB were asked to participate. Consenting patients in clinics allocated to incentive-based contact investigation were provided with 10 vouchers each to distribute to close contacts whom they thought may be at risk of TB. The voucher contained instructions on where and when to present for free TB screening, the monetary amount of the incentive, and the expiration date (2 months after the date of index patient enrollment; Supplementary Figure 1).
Contacts who presented with a voucher to any study clinic received an incentive of 20 South African Rand (ZAR; equivalent to $1.60 US dollars [USD]) [17], regardless of eligibility. If any symptoms of TB were present, contacts were asked to complete a brief face-to-face survey, undergo symptom-based TB screening, and provide a sputum sample for Xpert MTB/RIF (Cepheid, Inc, Sunnyvale, CA) testing. The cartridge for Xpert testing changed from the G4 cartridge to the more sensitive Ultra cartridge on July 2018. Those who tested Xpert-positive received an additional ZAR 40 (approximately USD 3.20) and were referred for free TB treatment. Those whose samples were insufficient, contaminated, or invalid on laboratory testing received ZAR 50 (approximately USD 4.00) for repeat specimen collection. Every travel fare to the clinic in response to the voucher or related to TB diagnosis was reimbursed in addition to these incentives, initially at ZAR 30 (approximately USD 2.40) and later increased to ZAR 50 in May 2019 to reflect local costs of round-trip public transportation. All incentives and reimbursements were provided in cash.
At the end of the 2-month eligibility period, index patients were provided a single cash incentive equal to ZAR 20 per referred contact who returned to a study clinic. The index patient was not provided the identities of contacts who enrolled, only the number thereof. An additional ZAR 100 (approximately USD 8.00) was paid to the index patient if a contact was diagnosed with Xpert-positive TB.
Household-based Contact Investigation
Index patients with TB who consented to participate in the household-based contact investigation provided their primary household address for a household visit. Study staff made up to 3 visits to each household to meet with all eligible household contacts for study enrollment. Each household visit consisted of a household census and a brief survey including demographic and clinical data, symptom-based TB screening, human immunodeficiency virus testing, and sputum collection if any symptoms of TB were present. TB symptoms were defined by self-report and included any current cough, subjective fever, weight loss (>5 kg or sufficient to loosen clothes), or drenching night sweats. Xpert-positive results were delivered directly to the household through a follow-up visit. Contacts who tested positive were immediately referred for treatment at their nearest clinic.
Statistical Analyses
We characterized sociodemographic and behavioral characteristics of index TB cases and contacts using descriptive statistics. We restricted this analysis to those index cases and contacts who consented and completed a study interview at enrollment.
Among enrolled contacts, we estimated the between-arm absolute difference in mean number of contacts per index patient. We calculated this difference in the study group overall and in subgroups of index patients by using interaction (product) terms in linear regression models to identify index patients who referred more contacts for TB screening in the incentive-based than in the household-based arm. The variables considered in regression were first selected based on being readily ascertained by healthcare workers (ie, could potentially be the basis of a targeted strategy). Variables for multivariable analysis were then restricted via least absolute shrinkage and selection operator (lasso) regression, which penalizes the least important features to coefficient values of zero (Supplementary Tables 1, 5, 9). To account for any clustering effect at the level of the index cases and/or facilities, we estimated robust standard errors that adjust for heteroskedasticity.
We computed the estimation of between-arm and within-arm relative differences among subgroups of index participants in zero-inflated negative binomial regression (relative risk) and logistic regression (odds ratio) models. For logistic regression, we dichotomized thresholds of contact numbers in each study arm. We set the binary thresholds based on the distribution of the number of contacts per index patient (0 [mode] vs >0, <2 [closest integer to mean] vs ≥2, and <9 vs ≥9 [near-maximum] contacts). We repeated the analysis using only the first 18 months of data, before intervention crossover.
We computed confidence intervals (CIs) based on robust covariance matrix estimation with statistical significance defined as a 2-sided alpha of 0.05. All analyses were completed using R, version 3.6.1 (R Foundation, Vienna, Austria). We also inflated CIs (to a 99% level) for potential false discovery due to multiple comparisons using a Bonferroni correction (Supplementary Tables 3, 8).
Ethical Consideration
The University of the Witwatersrand in South Africa Human Research Ethics Committee approved the trial, with an institutional review board authorization agreement with the Johns Hopkins Bloomberg School of Public Health and the University of the Witwatersrand. All participants provided written informed consent.
RESULTS
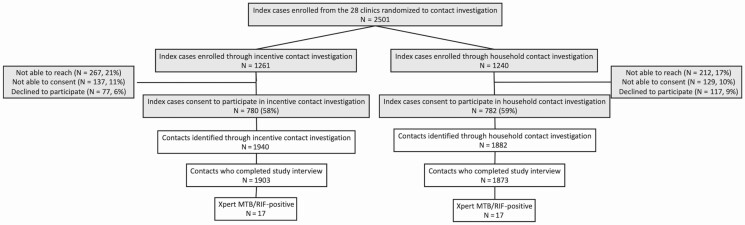
Among 2501 TB index patients (1261 in the incentive-based arm, 1240 in the household-based arm), 1562 (62%) consented to participate in contact investigation (Figure 1). A total of 3776 contacts (a mean of 2.42 contacts per index patient) completed the study interview. The mean number of contacts was 2.43 per index patient in the incentive-based arm (1903 contacts from 780 cases; median, 0; interquartile range [IQR], 0–4) and 2.33 per case in the household-based arm (1873 contacts from 782 cases; median, 1; IQR, 0–4; Figure 2).
Figure 1.
Study flow diagrams. The left side of the figure shows the population in the incentive-based contact investigation arm, and the right side shows the population in the household contact investigation arm. Numbers differ slightly from those in the primary report of intervention effectiveness (Hanrahan et al, under review) in that only those contacts who completed the study interview were included in the current analysis. Abbreviation: MTB/RIF, Mycobacterium tuberculosis/rifampin.
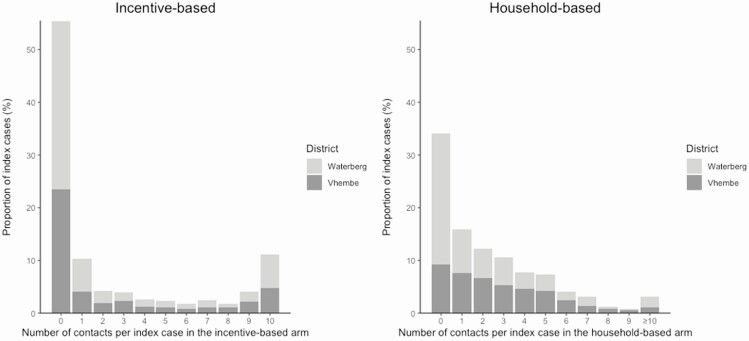
Figure 2.
Distribution of number of contacts among participants diagnosed with tuberculosis (“index participants”). The left panel shows the distribution of the number of contacts referred by each index participant in the incentive-based contact investigation arm, while the right panel shows the corresponding distribution in the household contact investigation arm. The distribution is split by districts—Waterberg (periurban) in light grey and Vhembe (rural) in dark grey. The overall mean number of contacts referred per case was similar between the 2 arms: 2.43 (95% confidence interval [95% CI], 2.18 to 2.69) in the incentive-based arm vs 2.33 (95% CI, 2.15 to 2.51) in the household-based arm. However, the proportion of index participants referring either zero (55% vs 34%) or ≥10 contacts (11% vs 3%) was higher in the incentive-based arm. By district, the proportion of index participants referring either zero or ≥10 contacts was higher in Waterberg than in Vhembe for both arms.
In the incentive-based arm, 55% of index participants referred zero contacts and 11% referred the maximum number of 10 contacts, whereas 87% of index participants in the household-based arm referred between 1 and 5 contacts. (The median number of household members was 5.) The number of contacts referred did not show a temporal trend (Supplementary Figure 2).
Demographic and behavioral characteristics of index patients were similar between the 2 arms, reflecting the initial randomization (Table 1). However, contacts in the incentive-based arm were more likely than those in the household-based arm to be adults aged ≥15 years (72% vs 58%; difference, 15%; 95% CI for difference, 11% to 17%), experience chronic TB symptoms (25% vs 16%; difference, 9%; 95% CI, 6% to 12%), and have a lifetime history of tobacco smoking (23% vs 11%; difference, 12%; 95% CI, 10% to 15%). Among contacts identified and enrolled in the incentive-based arm, 57% were household members and 77% had known the index participant for at least 5 years. The median time from enrollment of the index case to testing of the contact in the incentive-based arm was 8 days (IQR, 3–19).
Table 1.
Characteristics of Individuals Enrolled in a Trial of Contact Investigation for Tuberculosis in Rural South Africa
| Characteristic | Incentive-based Arm | Household-based Arm | ||
|---|---|---|---|---|
| Index Participants | Contacts | Index Participants | Contacts | |
| (N = 780) | (N = 1903) | (N = 782) | (N = 1873) | |
| Age,a median (IQR), y | 38 (28–49) | 25 (13–42) | 39 (29–48) | 19 (8–36) |
| <15 | 37 (5%) | 523 (28%) | 26 (3%) | 759 (41%) |
| 15–34 | 284 (36%) | 703 (37%) | 274 (35%) | 586 (31%) |
| 35–54 | 330 (42%) | 425 (22%) | 362 (46%) | 254 (14%) |
| ≥55 | 124 (16%) | 236 (12%) | 117 (15%) | 242 (13%) |
| Male sex | 448 (57%) | 788 (41%) | 457 (58%) | 719 (38%) |
| District | ||||
| Vhembe | 343 (44%) | 886 (47%) | 342 (44%) | 976 (52%) |
| Waterberg | 437 (56%) | 1018 (54%) | 440 (56%) | 897 (48%) |
| Self-reported TB symptoms | ||||
| Cough | 635 (81%) | 577 (30%) | 623 (80%) | 414 (22%) |
| Fever | 385 (49%) | 169 (9%) | 361 (46%) | 123 (7%) |
| Weight loss | 561 (72%) | 199 (11%) | 538 (69%) | 105 (6%) |
| Night sweats | 518 (66%) | 295 (16%) | 508 (65%) | 158 (8%) |
| Chronic TB symptoms (≥2weeks) | 462 (59%) | 470 (25%) | 476 (61%) | 295 (16%) |
| Human immunodeficiency virus statusa | ||||
| Negative | 307 (39%) | 1304 (69%) | 306 (39%) | 1296 (69%) |
| Positive, not on treatment | 49 (6%) | 51 (3%) | 68 (9%) | 36 (2%) |
| Positive, on treatment | 397 (51%) | 195 (10%) | 384 (49%) | 107 (6%) |
| Mode of transport to clinica | ||||
| Public motorized | 59 (8%) | 74 (4%) | 71 (9%) | ... |
| Private motorized | 442 (57%) | 601 (32%) | 421 (54%) | ... |
| Nonmotorized | 276 (35%) | 1229 (65%) | 289 (37%) | ... |
| Travel time to clinic, median (IQR), minutes | 30 (20–60) | 30 (20–60) | 30 (15–60) | ... |
| Cost of one-way trip transport to clinic (South African Rand)b, median (IQR) | 10 (0–18) | 6 (0–10) | 10 (0–20) | ... |
| Educationa,c | ||||
| Foundation phase | 108 (14%) | 524 (28%) | 111 (14%) | 723 (39%) |
| Intermediate phase | 68 (9%) | 248 (13%) | 81 (10%) | 247 (13%) |
| Senior phase | 171 (22%) | 380 (20%) | 182 (23%) | 335 (18%) |
| Further education | 428 (55%) | 742 (39%) | 401 (51%) | 554 (30%) |
| Employmenta | ||||
| Employed | 137 (18%) | 159 (8%) | 126 (16%) | 130 (7%) |
| Student/occasional work | 164 (21%) | 780 (41%) | 154 (20%) | 744 (40%) |
| Unemployed/Retired | 447 (57%) | 740 (39%) | 488 (62%) | 640 (34%) |
| Lifetime history of smokinga | 340 (44%) | 443 (23%) | 358 (46%) | 205 (11%) |
| Number of household members, median (IQR) | 5 (3–7) | ... | 5 (3–7) | ... |
| Relationship to the index case | ||||
| Family members in household | ... | 877 (46%) | ... | 1816 (97%) |
| Nonfamily members in household | ... | 213 (11%) | ... | 56 (3%) |
| Family member outside household | ... | 424 (22%) | ... | ... |
| Friend/acquaintance/work colleague | ... | 376 (20%) | ... | ... |
| Do not know each other | ... | 14 (1%) | ... | ... |
| Long-term (>5 y) relationship with index case | 1457 (77%) | 1252 (67%) | ||
Abbreviations: IQR, interquartile range; TB, tuberculosis.
aMissing values were not included. Numbers missing in the incentive-based and household-based arms were as follows: age, 5 (0.6%) and 3 (0.4%); human immunodeficiency virus, 27 (3.5%) and 24 (3.1%); transportation, 3 (0.4%) and 1 (0.1%); education, 5 (0.6%) and 7(0.9%); employment, 32 (4.1%) and 14 (1.8%); and smoking, 5 (0.6%) and 1 (0.1%).
bAt the time of the study, 20 South African Rand was equal to approximately USD 1.16.
cThe South Africa General Education and Training stage is divided into the foundation (grades 1–3), intermediate (grades 4–6), senior phase (grades 7–9), and further education and training (includes grades 10–12).
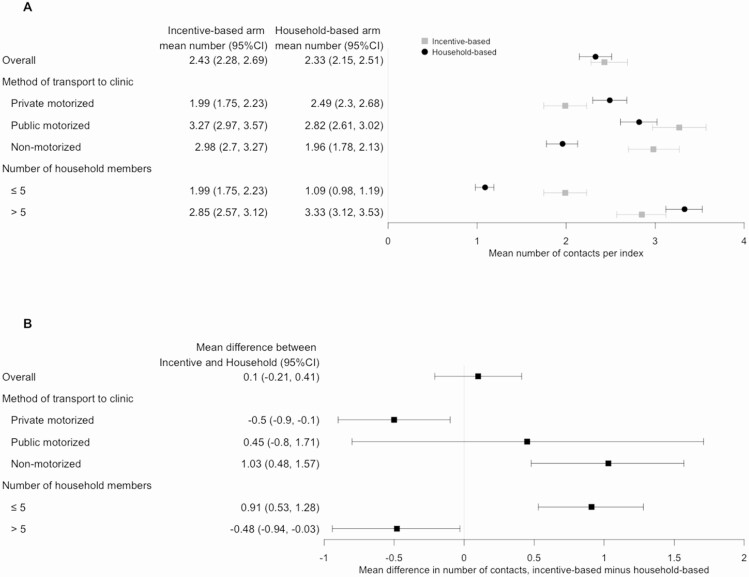
The mean number of contacts enrolled per index patient was similar across the 2 arms overall (mean difference, incentive-based minus household-based arm, 0.10; 95% CI, –.21 to .41; Figure 3). Important differences emerged within specific subgroups of index participants. Specifically, index participants in the incentive-based arm who used nonmotorized transport (eg, walking or bicycle) to attend a clinic referred, on average, 1.03 more contacts per index patient (95% CI, .48 to 1.57) than those in the household contact investigation arm, whereas index patients who used private motorized transport referred 0.50 (95% CI, .10 to .90) more contacts through household-based than through incentive-based investigation (Figure 3, Table 2). Household income of index patients who used nonmotorized transport was lower (median, 1790 ZAR; IQR, 695–3580) than of those who used private motorized transport (median, 2100 ZAR; IQR, 1065–3690; P = .02 for difference). Similarly, index participants who reported living with more than 5 household members referred, on average, 0.48 (95% CI, .03 to .94) more contacts through household-based than incentive-based investigation.
Figure 3.
Number of contacts identified per index participant according to index characteristics. A, Lightly shaded squares (bars) show the point estimate (95% confidence interval [95% CI]) for the number of contacts identified per index participant in the incentive-based arm, whereas darkly shaded circles show corresponding values in the household-based arm. Only the subgroups whose mean difference in the mean number of contacts per index case between arms (in at least 1 category) was greater than 0.50 are shown in this figure. Another figure showing this difference according to all measured index participant characteristics is in the Supplementary Materials. B, The difference in mean number of contacts per index participant (ie, value of lightly shaded squares minus darkly shaded circles). In this figure, numbers larger than zero indicate that the mean number of contacts per index participant among participants of a given subgroup is higher in the incentive-based arm than in the household-based arm.
Table 2.
Crude Difference in Mean Number of Contacts Referred Comparing Individuals Diagnosed With Tuberculosis (“Index Participants”) With Different Characteristics
| Index Participant Characteristica | Incentive-based | Household-based | Crude Difference in Differencesb Comparing Mean Number of Contacts per Index Participant in Incentive-based vs Household-based Contact Investigation (95% Confidence Interval)c |
|---|---|---|---|
| Age, y | |||
| <25 | 1.12 (0.33 to 1.91) | 0.33 (–0.36 to 1.02) | 0.79 (–0.16 to 1.75) |
| 25–44 | 0.38 (–0.17 to 0.92) | 0.34 (–0.06 to 0.75) | 0.03 (–0.65 to 0.71) |
| ≥45 | Reference | Reference | Reference |
| Female sex (vs male) | 0.61 (0.09 to 1.13) | 0.65 (0.28 to 1.02) | –0.04 (–0.68 to 0.60) |
| Vhembe District (vs Waterberg District) | 0.25 (–0.26 to 0.76) | 0.84 (0.48 to 1.2) | –0.59 (–1.22 to 0.04) |
| Living with HIV (vs not living with HIV) | –0.30 (–0.83 to 0.23) | 0.15 (–0.22 to 0.52) | –0.45 (–1.09 to 0.20) |
| Mode of transport to clinic | |||
| Private motorized | Reference | Reference | Reference |
| Public motorized | 1.28 (0.19 to 2.37) | 0.33 (–0.36 to 1.02) | 0.96 (–0.34 to 2.25) |
| Nonmotorized | 0.99 (0.44 to 1.55) | –0.54 (–0.91 to –0.16) | 1.53 (0.86 to 2.20) |
| Travel time to clinic, minutes | |||
| <30 | Reference | Reference | Reference |
| 30–60 | 0.06 (–0.55 to 0.68) | 0.28 (–0.12 to 0.69) | –0.22 (–0.96 to 0.52) |
| >60 | 0.37 (–0.28 to 1.03) | 0.81 (0.36 to 1.27) | –0.44 (–1.24 to 0.35) |
| Employment | |||
| Regular work | Reference | Reference | Reference |
| Student/Irregular work | 1.01 (0.21 to 1.81) | 0.56 (–0.04 to 1.17) | 0.45 (–0.55 to 1.45) |
| Unemployed/Retired | 0.71 (0.06 to 1.35) | 0.51 (0.03 to 1.00) | 0.20 (–0.61 to 1.00) |
| Number of tuberculosis symptomsd | |||
| 0 | Reference | Reference | Reference |
| 1 | 0.93 (–0.17 to 2.04) | 0.43 (–0.32 to 1.17) | 0.51 (–0.83 to 1.84) |
| 2 | 0.30 (–0.73 to 1.32) | 0.67 (–0.05 to 1.40) | –0.38 (–1.63 to 0.88) |
| 3 | 0.83 (–0.19 to 1.85) | 0.72 (0.02 to 1.41) | 0.11 (–1.12 to 1.34) |
| 4 | 1.17 (0.17 to 2.17) | 0.56 (–0.12 to 1.23) | 0.61 (–0.59 to 1.81) |
| ≥5 household members (vs <5 members) | 0.85 (0.35 to 1.36) | 2.24 (1.93 to 2.55) | –1.39 (–1.98 to –0.80) |
Abbreviation: HIV, human immunodeficiency virus.
aAll characteristics that we considered in the model were selected using lasso penalized regression and presented in this table.
bThese differences correspond to the crude differences shown in Figure 3.
cThe 95% confidence interval is based on robust covariance matrix estimation.
dTuberculosis symptoms include self-reported cough, fever, weight loss, and/or night sweats at the time of the interview.
These differences, whereby incentive-based investigation resulted in more contacts identified among index participants who walked or used a bicycle to attend clinic and household-based contact investigation identified more contacts among index participants with larger households, persisted after multivariable adjustment (Supplementary Table 2), using actual numbers of contacts for cases with ≥10 contacts (rather than truncating at 10, as in the primary analysis; Supplementary Table 4), considering zero-inflated distribution and binary cutoffs at different thresholds of contacts identified per index participant (Supplementary Tables 6, 7, 10–13) or restricting to the first phase of the study (Supplementary Tables 14, 15).
DISCUSSION
Although contact investigation for TB has traditionally focused on household contacts, other methods might be used to identify contacts for further investigation (including testing for active TB and providing preventive therapy). Here, we used data from a randomized trial of 1562 index participants who referred 3776 contacts to compare the yield of a novel incentive-based contact investigation strategy to household contact investigation. Considered as a whole, neither strategy was superior in terms of contacts diagnosed with, and starting treatment for, confirmed TB (unpublished data). However, this analysis highlights that even when performed in the same clinics, different approaches to contact investigation may identify contacts with different characteristics and result in more contacts identified for different types of index patients. For example, patients with large numbers of household members may be more appropriate for household contact investigation, whereas those who can present to a clinic without using motorized transport may be more effectively evaluated using an incentive-based strategy. These findings highlight the importance of studying the feasibility and implementation of alternative contact investigation strategies such that the most effective and least costly contact tracing strategies can be used for different types of patients when they are diagnosed with TB.
One notable finding, as described above, is that incentive-based contact investigation was more effective in identifying contacts from index participants who walked or used a bicycle to attend a clinic. This may reflect the burden on participants, in that those who can walk or bike to clinic may need to spend less time and money to attend a clinic and thus may be more amenable to relatively small incentives offered for clinic-based screening as opposed to being screened in their homes or having to pay for transport to get to a clinic. It may also reflect different cultural preferences among individuals who live closer to clinics (ie, more likely to live in cities and towns) vs those who live further away (and are more likely to live in rural villages) or the relative value of a flat incentive to individuals who may not have the financial resources to afford motorized transport. Further evaluation of incentive-based contact investigation in other sociocultural contexts (eg, in larger cities, in settings of higher or lower prevailing socioeconomic status) may clarify the settings in which incentive-based contact investigation may be an effective alternative to traditional household visits.
Another potential benefit of incentive-based contact investigation is the potential to reach a contact population that is different from household contacts. Specifically, a higher proportion of incentive-based contacts were aged 15–54 years, had a lifetime smoking history, and had more TB symptoms for longer duration than contacts identified through household contact investigation. Most Xpert-positive contacts in the incentive-based arm also attended clinics within a week after being referred and were long-term friends or colleagues rather than household contacts of the index patient. This finding is aligned with previous reports about community-wide transmission outside of the household [18, 19]. Our findings suggest that household-based and incentive-based contact investigation may, in fact, be complementary rather than competing approaches to find close contacts with TB. Further exploration of strategies to identify high-risk contacts of patients diagnosed with TB, including working adults not frequently found in the household and nonhousehold close contacts, are warranted.
Offering monetary incentives, while not frequently used for TB contact investigation, has been successfully implemented in other contexts. For example, financial incentives have improved TB treatment adherence among specific populations such as people who use drugs, who experience homelessness, and who have recently been incarcerated [10]. Performance-based incentives for healthcare workers and facilities have also improved case detection, quality of care, and treatment completion rates [20]. The yield of sexual partners through partner notification at sexually transmitted infection clinics in resource-limited settings was also enhanced by monetary incentives [21]. These previous studies, conducted in low- and middle-income settings, suggest that incentive-based interventions may be effective in motivating engagement. However, it is important to consider the feasibility of implementation in other settings (ie, external validity) before scaling up programs based on monetary incentives to patients in other contexts. Specifically, thought should be given to whether index patients can likely refer contacts with known TB (eg, diagnosed at other clinics) for financial gain. This may be of greater concern in a more urban area, for example, where multiple clinics are in close proximity or if larger financial incentives are deemed necessary to achieve the desired effectiveness. Other important considerations include whether incentives are offered to all referred contacts or conditionally to contacts who test positive, whether cash incentives are appropriate (eg, vs baskets of goods, vouchers, or items such as T-shirts and mugs), how much is an appropriate amount of incentive to provide in each local context, when incentives should be delivered, whether incentive-based intervention is cost-effective, and whether contacts’ confidentiality can be protected [22].
Since contact investigation is an important gateway to preventive therapy, it must also be considered whether nonhousehold contacts identified through incentive-based contact investigation would also be eligible for TB preventive therapy. Our findings of equal and high TB positivity in both arms (unpublished data) would argue that all contacts should be eligible for preventive therapy, regardless of the contact investigation strategy used.
Our results should be interpreted in the context of key limitations. First, fewer than 60% of index participants participated in contact investigation in either arm. While such losses to follow-up likely reflect levels of participation in actual practice, our results may not be based on a representative full underlying population of index participants. Second, while we were able to describe factors associated with the total number of contacts enrolled (and potentially eligible for preventive therapy), our ability to investigate factors associated with Xpert-positive contacts (ie, those eligible for treatment of active TB) was limited for this study with few (17) Xpert-positive contacts in each arm. Third, while we enrolled participants from across 28 primary clinics, our findings may not generalize to other cultural and economic contexts. Future implementation studies of incentive-based TB contact investigation in different settings are therefore needed to evaluate the feasibility of implementing this strategy.
In conclusion, incentive-based contact investigation may be synergistic with household contact investigation, in that incentive-based investigation identified contacts with a unique demographic profile and was more successful among index participants who used nonmotorized transport to attend a clinic. It also facilitated screening of contacts outside the household. Research to explore how best to implement incentive-based investigation as an alternative or addition to household investigation may help to inform a more comprehensive and patient-centered approach to identifying infectious contacts of individuals diagnosed with TB in high-burden settings.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank the study participants, the clinic staff, Vhembe and Waterberg district departments of health, Limpopo Department of Health, and the National Health Laboratory Services.
Financial support. This work was funded by the National Institutes of Health/National Institute of Allergy and Infectious Disease (R01AI116787 and R01AI147681 to D. W. D.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.World Health Organization. Global tuberculosis report 2019. Geneva, Switzerland: World Health Organization, 2019. [Google Scholar]
- 2.World Health Organization. Recommendations for investigating contacts of persons with infectious tuberculosis in low- and middle-income countries. Geneva, Switzerland: World Health Organization, 2012. [PubMed] [Google Scholar]
- 3. Fox GJ, Barry SE, Britton WJ, Marks GB. Contact investigation for tuberculosis: a systematic review and meta-analysis. Eur Respir J 2013; 41:140-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. MacPherson P, Webb EL, Variava E, et al. Intensified household contact tracing, prevention and treatment support versus enhanced standard of care for contacts of tuberculosis cases in South Africa: study protocol for a household cluster-randomised trial. BMC Infect Dis 2019; 19:839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ayakaka I, Ackerman S, Ggita JM, et al. Identifying barriers to and facilitators of tuberculosis contact investigation in Kampala, Uganda: a behavioral approach. Implement Sci 2017; 12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alsdurf H, Hill PC, Matteelli A, Getahun H, Menzies D. The cascade of care in diagnosis and treatment of latent tuberculosis infection: a systematic review and meta-analysis. Lancet Infect Dis 2016; 16:1269-78. [DOI] [PubMed] [Google Scholar]
- 7. Fox GJ, Loan LP, Nhung NV, et al. Barriers to adherence with tuberculosis contact investigation in six provinces of Vietnam: a nested case–control study. BMC Infect Dis 2015; 15:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horton KC, MacPherson P, Houben RMGJ, White RG, Corbett EL. Sex differences in tuberculosis burden and notifications in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med 2016; 13:e1002119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wood R, Racow K, Bekker L-G, et al. Indoor social networks in a South African township: potential contribution of location to tuberculosis transmission. PLoS One 2012; 7:e39246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lutge EE, Wiysonge CS, Knight SE, Sinclair D, Volmink J. Incentives and enablers to improve adherence in tuberculosis. Cochrane Database Syst Rev 2015:2-15. http://doi.wiley.com/10.1002/14651858.CD007952.pub3. Accessed 2 February 2021. [DOI] [PMC free article] [PubMed]
- 11. Hanrahan CF, Nonyane BAS, Mmolawa L, et al. Contact tracing versus facility-based screening for active TB case finding in rural South Africa: a pragmatic cluster-randomized trial (Kharitode TB). PLoS Med 2019; 16:e1002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Trust HS. District health barometer. 2020. https://www.hst.org.za/publications/DistrictHealth%20Barometers/District+Health+Barometer+2018-19+Web.pdf. Accessed February 2020.
- 13. Africa SS. Provincial Profile Limpopo, Community Survey 2016, Report number: 03-01-15. 2018. http://www.statssa.gov.za/publications/Report%2003-01-15/Report%2003-01-152016.pdf. Accessed February 2020.
- 14. Africa SS. Four factors about our provincial economies. 2019. http://www.statssa.gov.za/?p=12056. Accessed February 2020.
- 15. TB Statistics South Africa, National, incidence, provincial. https://tbfacts.org/tb-statistics-south-africa/.
- 16. SANAC. South Africa’s national strategic plan for HIV, TB and STIs 2017-2022. 2017. https://sanac.org.za//wp-content/uploads/2017/06/NSP_FullDocument_FINAL.pdf. Accessed February 2020.
- 17. Xe Currency Converter, Convert 1 South African Rand to US Dollar. https://www.xe.com/currencyconverter/convert/?Amount=1&From=ZAR&To=USD. Accessed February 2020.
- 18. Verver S, Warren RM, Munch Z, et al. Proportion of tuberculosis transmission that takes place in households in a high-incidence area. Lancet 2004; 363:212-4. [DOI] [PubMed] [Google Scholar]
- 19. Auld SC, Shah NS, Cohen T, Martinson NA, Gandhi NR. Where is tuberculosis transmission happening? Insights from the literature, new tools to study transmission and implications for the elimination of tuberculosis. Respirology 2018. doi:10.1111/resp.13333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beith A, Eichler R, Weil D. Performance-based incentives for health: a way to improve tuberculosis detection and treatment completion? Cent Glob Dev Work Pap 2007:9-15. [Google Scholar]
- 21. Matoga M, Mmodzi P, Massa C, Bula A, Hosseinipour M, Chasela C. Health system factors influencing partner notification for STIs and HIV in Lilongwe Malawi. A pre-intervention phase assessment for a quality improvement project. J Infect Dis Med 2018; 3:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rice T. The behavioral economics of health and health care. Annu Rev Public Health 2013; 34:431-47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.