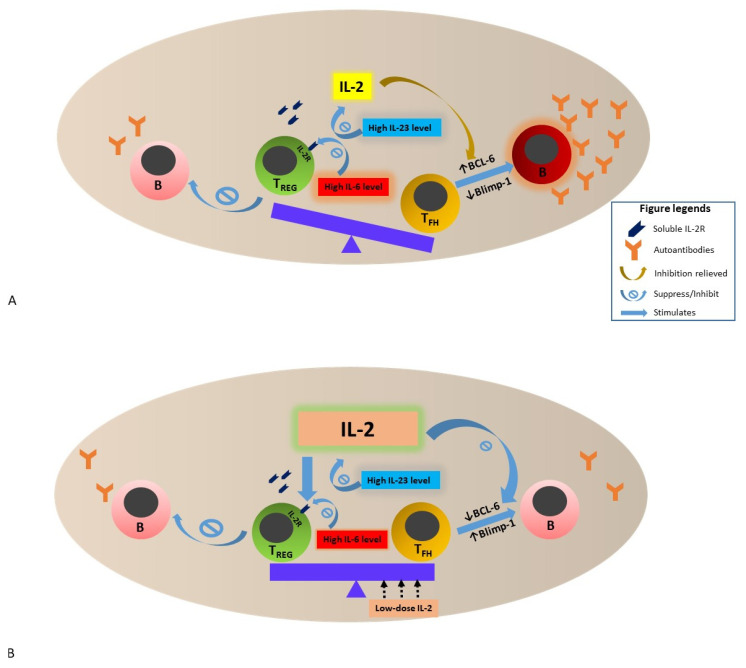
Figure 1.
Schematic presentation of the interactions among TFH, TREG, IL-2, IL-2R and B cells in a lymph node (or a second lymphoid organ) in the setting of SLE. (A). The reasons for low IL-2 environment in SLE are multifactorial—reduction of IL-2 transcription in T cells, increase in soluble IL-2R that bind to IL-2 (see text) and increase in IL-23 expressions are among some of the mechanisms proposed. Furthermore, high IL-6 expression in active SLE reduces the expression of IL-2 receptors expressed on TREG, leading to reduction of TREG regulatory function—for example, suppression of B cells to produce autoantibodies. These alterations impact peripheral immune tolerance by enhancing the development and differentiation of TFH while suppressing TREG function. Coupled with the lifted inhibition of BCL-6 expression and suppression of Blimp-1 expression by reduced IL-2 signaling, proliferation and function of TFH are fueled, leading to activation of B cells and antibody-forming cells and subsequent increased production of high-affinity autoreactive antibodies in SLE. (B). Low-dose IL-2 therapy restores the physiologically balanced activity between TREG and TFH by reversing the low IL-2 environment and stimulating TREG activity via direct binding of IL-2 to IL-2R of TREG. The higher IL-2 microenvironment also restores the balance between BCL-6 and Blimp-1 expression, leading to reduction in autoantibody formation. The impact of low-dose IL-2 therapy on IL-6 and IL-23 in SLE is still unclear. Abbreviations: SLE, systemic lupus erythematosus; IL, interleukin; TREG, regulatory T cell; TFH, follicular T-helper cell; IL-2R, IL-2 receptor; B, B cells; BCL-6; B-cell lymphoma 6 protein; Blimp-1, B lymphocyte-induced maturation protein-1.