Abstract
At sites of neutrophilic inflammation, tissue injury by neutrophil elastase is favored by phagocyte-induced hypochlorous acid-dependent inactivation of the natural elastase inhibitor α1-antitrypsin. In the present study, cefoperazone prevented α1-antitrypsin inactivation by neutrophils and reduced the recovery of hypochlorous acid from these cells. Moreover, the antibiotic reduced the free elastase activity in a neutrophil suspension supplemented with α1-antitrypsin without affecting the cells’ ability to release elastase. These data suggest that the drug inactivates hypochlorous acid before its reaction with α1-antitrypsin, thereby permitting the antiprotease-mediated blockade of released elastase. In conclusion, cefoperazone appears to have the potential for limiting elastase-antielastase imbalances, attenuating the related tissue injury at sites of inflammation.
Human neutrophils are crucial in protecting the host against infections (10). Nevertheless, they also contribute to the damage of infected tissue sites (18, 24, 32). In this context, much attention has recently been focused on neutrophil-derived proteases, primarily elastase (8, 12, 32), known to have broad proteolytic specificity and, consequently, high potential to cause tissue destruction (8, 32). At sites of neutrophilic inflammation, including processes elicited by microorganism invasion, the elastolytic activity is indeed favored by the ability of phagocytes to inactivate natural antiproteolytic systems, such as the elastase inhibitor α1-antitrypsin (α1-AT) (4, 5, 10, 19, 26). Taking into account this phenomenon, the idea of moderating the protease-antiprotease imbalance at inflamed or infected tissue sites by protecting α1-AT from inactivation looks like an attractive possibility to approach the treatment of tissue injury pharmacologically. In the present study, we provide evidence for the ability of a cephalosporin, cefoperazone, among other antibiotics, to prevent α1-AT inactivation by neutrophils, reducing elastase activity in the pericellular microenvironment.
Human neutrophils were isolated from heparinized venous blood by dextran sedimentation and subsequent centrifugation on a Ficoll-Hypaque density gradient as previously described (6). The inactivation of α1-AT by neutrophils was accomplished by incubating (30 min at 37°C) 2.5 × 106 neutrophils with 125 μg of α1-AT (Calbiochem, San Diego, Calif.) and 10 ng of phorbol myristate acetate (Sigma Chemical Co., St. Louis, Mo.) per ml in a final volume of 0.25 ml (22). At the end of the incubation period, methionine (500 nmol) was added to quench residual oxidants, and then the elastase inhibitory capacity of α1-AT in the supernatant was determined spectrophotometrically (22) by testing its capacity to inhibit porcine pancreatic elastase (PPE). Moreover, hypochlorous acid (HOCl) generated by neutrophils was measured by the taurine-trapping technique (33) as previously described (6), whereas the ability of neutrophils to release elastase was studied by measuring the capacity of cell-free supernatants to cleave the elastase substrate methoxy–Ala–Ala–Pro–Val–p-nitroamilide (19). The capacity of α1-AT to complex with PPE, after being incubated with HOCl (80 μM) in the presence of 100 μg of cefoperazone or cefotaxime per ml, was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as previously described (7). Briefly, 4 μg of α1-AT was incubated with 1.6 μg of PPE for 30 min. The samples were then heated at 100°C for 1.5 min in a solution containing 2% SDS (Bethesda Research Laboratories, Inc., Gaithersburg, Md.), 5% β-mercaptoethanol (Sigma), 0.001% bromophenol blue (Sigma), and 65 mM Tris-HCl (pH 8.8). The samples were then analyzed by slab gel electrophoresis according to the method of Laemmli (16) with a 3% polyacrylamide stacking gel (pH 6.8) and a 7.5% polyacrylamide resolving gel (pH 8.8). After electrophoresis, protein bands were visualized with a silver stain (Bio-Rad, Richmond, Calif.).
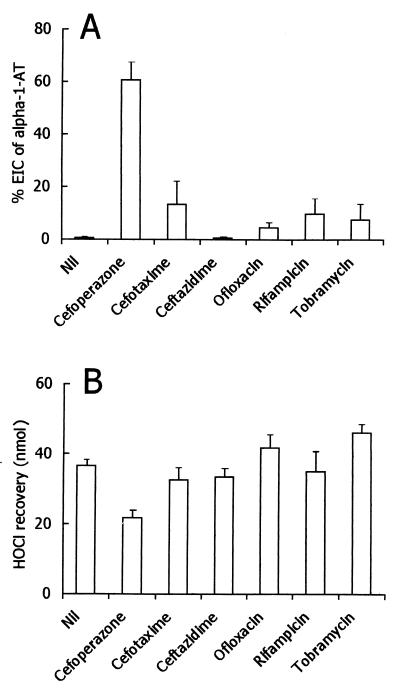
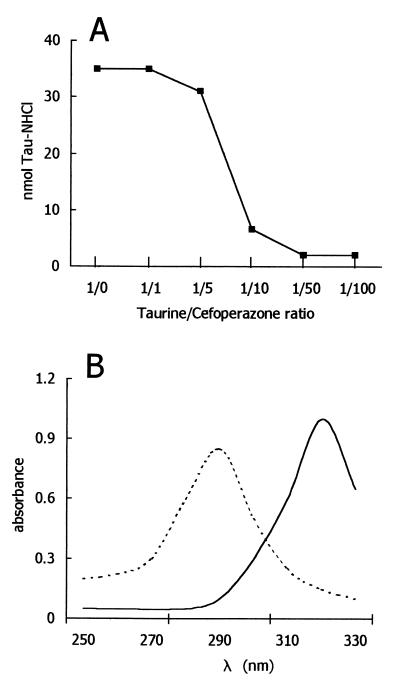
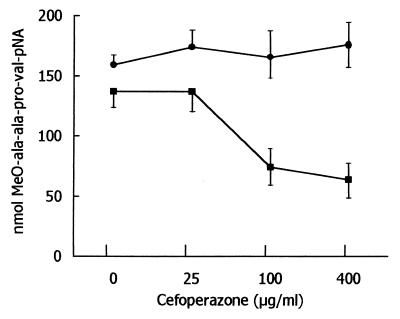
When exposed to 2.5 × 106 stimulated human neutrophils for 30 min, α1-AT (125 μg) was completely inactivated, as determined by its ability to inhibit PPE. As summarized in Fig. 1, of the various antibiotics tested, only cefoperazone prevented α1-AT inactivation efficiently. Taking into account that neutrophils inactivate α1-AT with HOCl or another compound with similar characteristics (22), the effects of antibiotics on the recovery of HOCl from activated neutrophils were tested. At the concentrations used herein, cefoperazone had inhibitory activity, whereas other antibiotics were substantially inactive (Fig. 1). Cefoperazone did not affect neutrophil O2− production and degranulation (data not shown), so interference with cell activation is unlikely. On the contrary, it seems that the drug is able to trap HOCl that is generated by neutrophils. Consistent with this possibility, cefoperazone was capable of competing with taurine for HOCl, thereby preventing the formation of taurine monochloramine (Fig. 2). Moreover, the absorbance spectrum of HOCl was changed by cefoperazone, consistent with the reaction of the antibiotic with the oxidant. One possible explanation of the present results is that HOCl or other neutrophil-derived oxidants directly inactivate neutrophil elastase by a cefoperazone-inhibitable process. In order to test this possibility, the release of elastase from neutrophils was tested in the absence and presence of cefoperazone. As shown in Fig. 3, activated neutrophils were capable of releasing detectable levels of free elastase activity. The levels of detectable enzymatic activity in the cell-free supernatants were unaffected by the presence of cefoperazone, even at a concentration of 400 μg/ml. Thus, the effects of cefoperazone cannot be attributed to a drug’s ability to interfere directly with elastase activity. Moreover, the levels of elastase activity were unaffected by α1-AT (Fig. 3), suggesting that neutrophils inactivate the antiprotease before it can react with released elastase. Finally, cefoperazone caused a dose-dependent reduction of the elastase activity released by neutrophils in the presence of α1-AT (Fig. 3). This means that the antibiotic prevented α1-AT inactivation, thereby allowing an α1-AT-mediated blockade of released elastase.
FIG. 1.
Effects of various antibiotics on inactivation of α1-AT by neutrophils and on HOCl recovery from activated cells. Each antibiotic was tested at 100 μg/ml, i.e., at micromolar concentrations of 155 (cefoperazone), 219 (cefotaxime), 183 (ceftazidime), 277 (ofloxacin), 122 (rifampin), and 214 (tobramycin). (A) Inactivation of α1-AT in the presence of various antibiotics. Results are expressed as means ± 1 standard error of the mean of four or five determinations, depending on the antibiotic. Nil (no antibiotic) versus cefoperazone, P < 0.01; Nil versus other compounds, P > 0.05 (Kruskal-Wallis nonparametric analysis-of-variance test followed by Dunn’s multiple comparisons). EIC, elastase inhibitory capacity. (B) Effects of various antibiotics on HOCl recovery from 106 neutrophils. Results are expressed as means ± 1 standard error of the mean of three to seven determinations, depending on the antibiotic. Nil versus cefoperazone, P < 0.05; Nil versus other antibiotics, P > 0.05 (Kruskal-Wallis nonparametric analysis-of-variance test followed by Dunn’s multiple comparisons).
FIG. 2.
Cell-free interactions between HOCl and cefoperazone. (A) Effects of different doses of cefoperazone on the recovery of taurine monochloramine (Tau-NHCl) from a mixture of HOCl and taurine. The experiments were carried out by adding ∼35 nmol of HOCl to mixtures of taurine plus cefoperazone (final volume of 1 ml). The taurine concentration was 100 μM (constant). (B) Absorbance spectrum of HOCl alone (- - - ) and in presence of cefoperazone (—). HOCl and cefoperazone concentrations were 1 mM in phosphate-buffered saline, pH 7.4; final solution, pH 7.4.
FIG. 3.
Effects of various doses of cefoperazone on elastase activity detectable in supernatants of neutrophils incubated in the presence (■) or absence (●) of α1-AT (3.5 μg) under the following conditions: neutrophils, 2 × 105; phorbol myristate acetate, 10 ng/ml; final volume, 175 μl; and incubation time, 60 min. Results are expressed as nanomoles of substrate cleaved per hour by supernatants of neutrophils (mean ± 1 standard error of the mean, n – 4).
These results raise several considerations. In fact, at sites of neutrophil inflammation, the tissue-protective α1-AT screen can be overcome by excessive waves of extravascular neutrophil recruitment and, more easily, by the capacity of neutrophils to inactivate α1-AT (19, 27, 32). Taking into account this neutrophil ability, we can imagine that neutrophil elastase can easily digest major connective components, such as basal membrane proteins, fibronectin, elastin, collagens, and proteoglycans (10, 32). Consistent with this view, inactivated α1-AT as well as free and active elastase has been found in fluids from inflamed tissues (1, 29, 34). In this context, some anti-inflammatory drugs, such as 5-aminosalicylic acid and sulfanilamide derivatives (primarily nimesulide and dapsone), have been shown to prevent the inactivation of α1-AT by neutrophils (21, 22), raising the possibility that they protect the antiprotease shield from inactivation. Here, we present evidence that the antibiotic cefoperazone was capable of trapping HOCl so efficiently that it protected α1-AT from neutrophil-mediated oxidation. Moreover, and probably more interestingly, this antibiotic was also able to down-regulate the expression of free elastase activity in a neutrophil suspension supplemented with α1-AT, i.e., under conditions simulating the microenvironment at sites of inflammation. Thus, it appears that cefoperazone can actually inactivate HOCl before its reaction with α1-AT, thereby allowing α1-AT interaction with and formation of a blockade for released elastase. Therefore, owing to its potential to control elastase activity by rescuing α1-AT, this antibiotic may alter the protease-antiprotease balance, facilitating protection of infected tissue sites. This seems to be particularly interesting in that the concentrations of cefoperazone found to be effective herein can be achieved in serum and in lung tissue after in vivo administration (13, 23, 30).
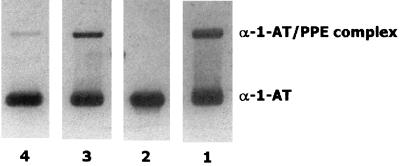
It is known that HOCl is a strong oxidant able to react rapidly with various chemical groups, such as primary amines, thiols, and thioethers (22, 28). All the cephalosporins have at least one thioether group. Moreover, the cephalosporins tested here have one additional thioether group outside the cephalosporin ring. Consistent with these structural characteristics, cephalosporins, including those found to be ineffective herein, have been reported to scavenge HOCl efficiently (2, 11, 17, 20). Nevertheless, these studies regarding the ability of cephalosporins to react with HOCl were carried out with relatively high drug concentrations and/or by preincubating the drugs with HOCl before the addition of the HOCl substrate (2, 17, 20). However, when the drugs were incubated with HOCl and the substrate simultaneously, no scavenging activity was observed at the concentrations used herein (17). In this regard, Wasil and coworkers observed a similar phenomenon with nonsteroidal anti-inflammatory agents (31). Finally, compared with previous findings of some of the authors of this paper (20), a 20-fold reduction in the concentration of taurine used to trap HOCl appears to increase the sensitivity of the assay, thereby uncovering a particular affinity of cefoperazone for HOCl. In order to further test this particular behavior of cefoperazone, HOCl was added to a mixture of cefoperazone and α1-AT, and then the ability of α1-AT to complex with elastase was tested by SDS-PAGE. In a parallel experiment, cefoperazone was replaced with cefotaxime. As shown in Fig. 4, cefoperazone displayed more potent α1-AT-protecting activity than cefotaxime. This is in agreement with the results of testing the elastase inhibitory capacity, shown in Fig. 1. Although the different efficiencies of the various cephalosporins tested herein remain to be fully understood, the activity of cefoperazone might be related to its ability to react with HOCl faster than other drugs react. This seems to be in agreement with the concepts initially proposed by Wasil et al. (31).
FIG. 4.
Analysis of the interaction of α1-AT incubated with HOCl in the presence of cefoperazone or cefotaxime. The experiments were carried out by adding HOCl (80 μM) to mixtures of α1-AT (50 μg/ml) plus cefoperazone or cefotaxime (100 μg/ml). After incubation (15 min), the preparations were treated with methionine, and aliquots containing 4 μg of α1-AT were incubated with 1.6 μg of PPE for 30 min and analyzed by SDS-PAGE. Lane 1, α1-AT plus PPE; lane 2, HOCl plus α1-AT and PPE; lane 3, HOCl plus cefoperazone, α1-AT, and PPE; lane 4, HOCl plus cefotaxime, α1-AT, and PPE.
Finally, as far as cefoperazone is concerned, it is unlikely that its antioxidant activity can exert negative effects on the microbicidal function of neutrophils. In fact, cephalosporins are not cell-penetrating agents (25); therefore, very low levels of these drugs can be expected to reach phagolysosomes, where bacterial killing takes place. In conclusion, numerous studies have dealt with the interplay between neutrophils and antimicrobial agents in the attempt to identify antibiotics able to act synergistically with the bacterial killing systems of neutrophils (25). More recently, the possibility of modulating exaggerated inflammatory responses to microbial invasion in tissues has gained interest. In particular, it has been suggested that certain antimicrobial agents have antioxidant properties (11, 15, 17, 20), thereby limiting the ability of neutrophils to lyse surrounding target cells (2, 3, 20), i.e., by inference, limiting the ability of neutrophils recruited at sites of inflammation to damage local parenchymal cells. On the other hand, in the attempt to design strategies to control connective tissue damage at sites of neutrophilic inflammation, it has been found that certain modified cephalosporins act as potent inhibitors of neutrophil elastase activity (9, 14). Although the best therapeutic approach to tissue injury in infectious neutrophilic inflammatory reactions must be etiology driven, i.e., must use appropriate antimicrobial treatments, the present study provides a rational basis for encouraging the development of antibiotics also having antiprotease-related histoprotective potential.
Acknowledgments
This work was supported by grants to F.D. from Italian MURST.
REFERENCES
- 1.Abbink J J, Kamp A M, Nuijens J H, Swaak T J G, Hack E C. Proteolytic inactivation of α1-antitrypsin and α1-antichymotrypsin by neutrophils in arthritic joints. Arthritis Rheum. 1993;36:168–180. doi: 10.1002/art.1780360206. [DOI] [PubMed] [Google Scholar]
- 2.Cantin A, Woods D E. Protection by antibiotics against myeloperoxidase-dependent cytotoxicity to lung epithelial cells in vitro. J Clin Investig. 1993;91:38–45. doi: 10.1172/JCI116196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carreer R, Deby-Dupont G, Deby C, Jadoul L, Mathy M. Oxidant-scavenging activities of beta-lactam agents. Eur J Clin Microbiol Infect Dis. 1998;17:43–46. doi: 10.1007/BF01584363. [DOI] [PubMed] [Google Scholar]
- 4.Carrell R W. Alpha-1-antitrypsin: molecular pathology, leukocytes and tissue damage. J Clin Investig. 1986;78:1427–1431. doi: 10.1172/JCI112731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark R A, Stone P J, Hag A E, Calore J D, Franzblau C. Myeloperoxidase-catalyzed inactivation of α1-protease inhibitor by human neutrophils. J Biol Chem. 1981;256:3348–3353. [PubMed] [Google Scholar]
- 6.Dallegri F, Ballestrero A, Frumento G, Patrone F. Augmentation of neutrophil-mediated erythrocyte lysis by cells derived in vitro from human monocytes. Blood. 1987;70:1743–1749. [PubMed] [Google Scholar]
- 7.Dallegri F, Ottonello L, Dapino P, Sacchetti C. Effect of nonsteroidal antiinflammatory drugs on the neutrophil promoted inactivation of alpha-1-proteinase inhibitor. J Rheumatol. 1992;19:419–423. [PubMed] [Google Scholar]
- 8.Dallegri F, Ottonello L. Tissue injury in neutrophilic inflammation. Inflamm Res. 1997;46:383–391. doi: 10.1007/s000110050208. [DOI] [PubMed] [Google Scholar]
- 9.Doherty J B, Ashe B M, Argenbright L W, Barker P L, Bonney R J, Chandler G O, Dalghren M E, Dorn C P, Jr, Finke P E, Firestone R A, Fletcher D, Hagmann W K, Munford R, O’Grady L, Maycock A L, Pisano J M, Shah S K, Thompson K R, Zimmerman M. Cephalosporin antibiotics can be modified to inhibit human leukocyte elastase. Nature. 1986;322:192–194. doi: 10.1038/322192a0. [DOI] [PubMed] [Google Scholar]
- 10.Gallin J I, Goldstein I M, Snyderman R. Inflammation. Basic principles and clinical correlates. 2nd ed. New York, N.Y: Raven Press; 1992. [Google Scholar]
- 11.Gunther M R, Mao J, Cohen M S. Oxidant-scavenging activities of ampicillin and sulbactam and their effects on neutrophil functions. Antimicrob Agents Chemother. 1993;37:950–956. doi: 10.1128/aac.37.5.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henson P M, Johnston R B., Jr Tissue injury in inflammation: oxidants, proteinases, and cationic proteins. J Clin Investig. 1987;79:669–674. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kemmerich B, Lode H, Belmega G, Jendroschek T, Borner K, Koeppe P. Comparative pharmacokinetics of cefoperazone, cefotaxime, and moxalactam. Antimicrob Agents Chemother. 1983;23:429–434. doi: 10.1128/aac.23.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knight W B, Maycock A L, Green B G, Ashe B M, Gale P, Weston H, Finke P E, Hagmann W K, Shah S K, Doherty J B. Mechanism of inhibition of human leukocyte elastase by two cephalosporin derivatives. Biochemistry. 1992;31:4980–4986. doi: 10.1021/bi00136a010. [DOI] [PubMed] [Google Scholar]
- 15.Labro M T, El Benna J, Charlier N, Abdelghaffar H, Hakin J. Cefdinir (CI-983), a new oral amino-2-thiazolyl cephalosporin, inhibits human neutrophil myeloperoxidase in the extracellular medium but not the phagolysosome. J Immunol. 1994;152:2447–2455. [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Lapenna D, Cellini L, De Gioia S, Mezzetti A, Ciofani G, Festi D, Cuccurullo F. Cephalosporins are scavengers of hypochlorous acid. Biochem Pharmacol. 1995;49:1249–1254. doi: 10.1016/0006-2952(95)00044-z. [DOI] [PubMed] [Google Scholar]
- 18.Malech H L, Gallin J I. Neutrophils in human diseases. N Engl J Med. 1987;317:687–694. doi: 10.1056/NEJM198709103171107. [DOI] [PubMed] [Google Scholar]
- 19.Ossanna P J, Test S T, Matheson N R, Regiani S, Weiss S. Oxidative regulation of neutrophil elastase-alpha-1-proteinase inhibitor interaction. J Clin Investig. 1986;77:1939–1951. doi: 10.1172/JCI112523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ottonello L, Dallegri F, Dapino P, Pastorino G, Sacchetti C. Cytoprotection against neutrophil-delivered oxidant attack by antibiotics. Biochem Pharmacol. 1991;42:2317–2321. doi: 10.1016/0006-2952(91)90236-x. [DOI] [PubMed] [Google Scholar]
- 21.Ottonello L, Dapino P, Pastorino G, Vitale E, Dallegri F. The drug 5-aminosalicylic acid rescues α1-proteinase inhibitor from the neutrophil oxidative inactivation. Digestion. 1992;51:140–145. doi: 10.1159/000200889. [DOI] [PubMed] [Google Scholar]
- 22.Ottonello L, Dapino P, Scirocco M C, Balbi A, Bevilacqua M, Dallegri F. Sulphonamides as anti-inflammatory agents: old drugs for new therapeutic strategies in neutrophilic inflammation? Clin Sci. 1994;88:331–336. doi: 10.1042/cs0880331. [DOI] [PubMed] [Google Scholar]
- 23.Srinivasan S, Francke E L, Neu H C. Comparative pharmacokinetics of cefoperazone and cefamandole. Antimicrob Agents Chemother. 1981;19:298–301. doi: 10.1128/aac.19.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Styrt B. The role of phagocytes in non-infectious diseases. In: Klempner M S, Styrt B, Ho J, editors. Phagocytes and disease. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1989. pp. 145–169. [Google Scholar]
- 25.Styrt B, Ho J, Klempner M S. Interactions between antimicrobial agents and phagocytes. In: Klempner M S, Styrt B, Ho J, editors. Phagocytes and disease. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1989. pp. 119–144. [Google Scholar]
- 26.Swaim M W, Pizzo S V. Methionine sulfoxide and the oxidative regulation of plasma proteinase inhibitors. J Leukoc Biol. 1988;43:365–379. doi: 10.1002/jlb.43.4.365. [DOI] [PubMed] [Google Scholar]
- 27.Test S T, Weiss S J. The generation and utilization of chlorinated oxidants by human neutrophils. Adv Free Radic Biol Med. 1986;2:91–116. [Google Scholar]
- 28.Thomas E L, Jefferson M M, Grisham M B. Myeloperoxidase-catalyzed incorporation of amines into proteins: role of hypochlorous acid and dichloramines. Biochemistry. 1982;21:6299–6308. doi: 10.1021/bi00267a040. [DOI] [PubMed] [Google Scholar]
- 29.Wallaert B, Gresseir B, Marquette C H, Gosset P, Remy-Jardin M, Mizon J, Tonnel A B. Inactivation of α1-proteinase inhibitor by alveolar inflammatory cells from smoking patients with or without emphysema. Am Rev Respir Dis. 1993;147:1537–1543. doi: 10.1164/ajrccm/147.6_Pt_1.1537. [DOI] [PubMed] [Google Scholar]
- 30.Wartenberg K, Tonak J, Knapp W. Lung tissue concentrations of cefoperazone. Infection. 1983;11:280–282. doi: 10.1007/BF01641263. [DOI] [PubMed] [Google Scholar]
- 31.Wasil M, Halliwell B, Moorhouse C P, Duncan C, Huthinson S, Baum H. Biologically-significant scavenging of the myeloperoxidase-derived oxidant hypochlorous acid by some anti-inflammatory drugs. Biochem Pharmacol. 1987;36:3847–3850. doi: 10.1016/0006-2952(87)90448-5. [DOI] [PubMed] [Google Scholar]
- 32.Weiss S J. Tissue destruction by neutrophils. N Engl J Med. 1989;320:565–576. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 33.Weiss S J, Klein R, Slivka A, Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Investig. 1982;70:588–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong P S, Travis J. Isolation and properties of oxidized alpha-1-proteinase inhibitor from human rheumatoid synovial fluid. Biochem Biophys Res Commun. 1980;96:1449–1454. doi: 10.1016/0006-291x(80)90113-8. [DOI] [PubMed] [Google Scholar]