Abstract
Simple Summary
The compelling evidence demonstrating the benefits of exercise to cancer survivors is biased towards ‘more well’ patients undertaking exercise interventions in tightly controlled (highly supervised) conditions. The aim of this trial was to evaluate the safety, feasibility, and effect of a 12-week exercise intervention delivered under ‘real-world’ conditions (that is, with low-level supervision, defined as five sessions across 12 weeks) compared with the same exercise intervention delivered under high-level supervision (20 sessions across 12 weeks). The understudied, less well women with breast cancer were the target population; that is, women with stage II or higher disease at diagnosis, and at least one comorbidity or treatment-related side-effect. The results showed that exercise was safe and feasible for this understudied breast cancer subgroup, and, while women who received the exercise intervention with low-level supervision experienced improvements in quality of life and physical function, greater gains in strength and exercise self-efficacy were observed for women who had the exercise intervention delivered via high-level supervision. Future research will determine whether the extra benefit gained through higher supervision levels lead to longer term quality of life and survival benefits.
Abstract
The aim of this comparative, effectiveness trial was to evaluate the safety, feasibility and effect of an exercise intervention delivered via low-level versus high-level supervision. The target population were women who were diagnosed with ≥stage II breast cancer, had ≥ one comorbidity and/or persistent treatment-related side-effects, and were insufficiently physically active. Sixty women (50 ± 9 years) were randomized to the low-supervision group (n = 30) or high-supervision group (n = 30). The low-supervision group participated in a 12-week, individually-tailored exercise intervention supported by five supervised sessions with an exercise professional. The high-supervision group participated in the same exercise intervention but received 20 supervised sessions across the 12-week period. The target weekly dosage of 600 metabolic equivalent minutes of exercise per week (MET-mins/wk) and the session content, such as safety and behaviour change topics, were standardized between the groups. The primary outcomes were intervention safety, defined as the number, type, and severity of exercise-related adverse events (e.g., musculoskeletal injury or exacerbated treatment-related side effects), and feasibility, which was defined as compliance to target exercise dosage. The effect of the intervention on quality of life, physical activity, self-efficacy, fitness, and strength was also assessed (pre- and post-intervention, and at 12-week follow-up). The intervention was safe, with no exercise-related adverse events of grade 3 or above in either group. Both groups reported high compliance to the target exercise dosage (median MET-mins/wk: High = 817; Low = 663), suggesting the exercise intervention was feasible, irrespective of supervision level. Improvements in quality of life, physical activity and fitness were observed post-intervention and maintained at follow-up for both groups (p < 0.05). Only the high-supervision group showed clinically-relevant improvements in strength and self-efficacy at post-intervention (p < 0.05). Individually-targeted exercise delivered under high- or low-levels of supervision is safe, feasible and beneficial for women with stage II+ breast cancer. Future research needs to assess whether the greater gains observed in the group who received higher supervision may contribute to longer term maintenance of physical activity levels and overall health benefits. Australian and New Zealand Clinical Trials Registry: ACTRN12616000547448.
Keywords: exercise, neoplasms, survivorship, safety, patient compliance
1. Introduction
The compelling evidence-base demonstrating the benefit of exercise for cancer survivors supports the continued call for exercise to be formally integrated into care during and following treatment [1]. This evidence has informed internationally-endorsed exercise guidelines [1,2], including specific weekly exercise dosage recommendations to improve physical functioning, health-related quality of life, anxiety, depression, and fatigue [1]. International exercise oncology guidelines recommend exercise dosages that approximate those recommended for healthy adults; that is, 150 min of moderate-intensity exercise per week [1]. However, as highlighted by these guidelines, confidence regarding the safety, feasibility, benefit and generalizability of the extant exercise oncology evidence is limited by (1) a bias towards samples of patients with the most common cancers and better prognoses, and (2) a lack of evaluation of delivery models suited to resource-constrained health care systems [1,2,3,4,5].
Compared with the wider breast cancer population, participants in exercise and breast cancer trials are more likely to have earlier-stage disease, higher pre- and post-diagnosis physical activity (PA) levels, and be otherwise generally well (that is, have few or no comorbidities, such as arthritis or pre-existing cardiovascular disease, and have mild or no persistent treatment-related side-effects) [5,6]. Higher stage of disease at diagnosis has been associated with higher frequency and severity of treatment-related side-effects and lower levels of PA [7]. It therefore seems plausible that those who have the most to gain from exercise are less likely to have participated in exercise oncology research, and concurrently, may be at higher risk of exercise-related adverse events and/or may not be able or willing to participate, although this topic remains understudied [8,9].
The majority (>60%) of published exercise oncology studies are efficacy trials evaluating exercise interventions conducted under highly controlled conditions [10]. These trials involved highly-supervised exercise interventions, with at least one exercise session per week supervised by an exercise professional (ExP) with advanced training in exercise oncology [10]. Additionally, these trials were primarily conducted at university or hospital clinics rather than within community-based settings [10]. In contrast, effectiveness trials, and in particular, comparative effectiveness trials which compare different intervention dosages or delivery modes, are limited [11]. Furthermore, exercise prescribed in effectiveness trials tends to be of lower intensity than the moderate-to-high intensity that is currently recommended in exercise oncology guidelines [12].
While there is growing momentum behind the campaign to provide exercise to cancer survivors, funding models that support cancer rehabilitation services are highly varied across health systems in developed countries [1]. Even when a government or third-party payer funding model supports exercise services, there are likely to be out-of-pocket expenses for the patient [13]. Few countries have health system funding available to support exercise services, with Australia and Germany being the noted exceptions. The United States and the United Kingdom both have large community-based programs (e.g., Livestrong and MoveMore), however funding for these programs relies on community partnerships, leaving the program vulnerable to loss of support [3]. Australia has arguably one of the most universal funding models, with all cancer patients eligible for reimbursement for up to five sessions with an allied health professional (e.g., accredited exercise physiologist or physiotherapist) per year under a government-funded chronic disease management scheme [14]. However, there is no evidence that this real-world level of contact with an exercise professional is safe or effective [15].
As observed in pharmaceutical and surgical oncology trials [4,5], sample bias and interventions that are not representative of clinical practice limit external validity and present as barriers to research translation. Therefore, the aim of the SAFE trial was to evaluate the safety and feasibility (primary outcomes), and effect (secondary outcomes) of an exercise intervention delivered via low-level versus high-level supervision, in an understudied subgroup of women with breast cancer. Women were randomised to receive the exercise intervention delivered via a supervision-model consistent with either,
real-world: representative of the model currently funded by the Australian healthcare system [14]. This involved five supervised sessions across 12 weeks, with all other exercise sessions unsupervised. This group was the “low-supervision group”.
research: consistent with conditions commonly observed in previous exercise oncology trial protocols [10]. This involved 20 supervised sessions across 12 weeks, with all other exercise sessions unsupervised. This group was the “high-supervision group”.
The understudied target population was breast cancer survivors who had a high burden of disease based on disease stage, comorbidities, and persistent treatment-related side-effects, and were insufficiently active. These women are typically excluded from studies or less likely to volunteer [6].
2. Methods
2.1. Design and Participants
The SAFE trial (ANZ Clinical Trials Registry: ACTRN12616000547448; 2016-18) was a randomized, comparative effectiveness trial. Ethical approval was obtained from Human Research Ethics Committees at the Queensland University of Technology and participating hospitals, and reporting and conduct adhered to the Consolidating Standards of Reporting Clinical Trials (CONSORT) guidelines [16]. Due to recruitment challenges and financial constraints, modifications were made to the protocol after trial registration but prior to enrolling participants. Details of these changes and justifications are summarized in Table S1.
Study inclusion and exclusion criteria are outlined in Table 1. Potentially eligible women were referred by breast care nurses at one public and two private hospitals, either during routine appointments or via mail-out. Women could also self-refer through study advertisements or the clinical trials registry. All interested women were screened for eligibility. Following written consent (from the participant and treating doctor) and baseline assessment, participants were randomized into either the low-supervision (5 supervised sessions) or high-supervision (20 supervised sessions) intervention groups. Participants were randomized using computer-generated block randomization (blocks of four), stratified by treatment status at baseline (current versus completed treatment, excluding hormone therapy). Allocations were stored in sequentially-numbered envelopes which were given to participants after baseline testing. Staff involved in data collection (but not delivery of the intervention) were blinded to group allocation.
Table 1.
Participant Eligibility Criteria.
| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Must Meet ALL Criteria | Must Meet ≥ 1 Criterion | Meets ANY Criterion |
|
|
|
a Engages in <150 min of self-reported structured exercise per week.
2.2. Exercise Intervention
All participants, regardless of group, received an individualised, progressive 12-week exercise prescription, delivered via an ExP during supervised sessions. This exercise intervention was completed during both supervised sessions and independently during unsupervised sessions. The two groups differed only according to the number of sessions supervised by an ExP across the 12-week intervention. Key differences and similarities between the groups are summarized in online Table S2.
2.3. Low-Supervision Group
The low-supervision group were allocated five supervised sessions across the 12-week intervention period. The first session was scheduled during week 1 and scheduling of subsequent sessions was determined jointly by the ExP and the participant. Participants completed exercise during supervised sessions, as well as independently during unsupervised sessions, as prescribed by the ExP.
2.4. High-Supervision Group
Participants in the high-supervision group were allocated 20 sessions. In weeks 1–8, participants had two supervised sessions per week and in weeks 9–12 participants had one supervised session per week. As with the low-supervision group, prescribed exercise was to be completed during both supervised and unsupervised sessions.
2.5. Supervised Sessions
Supervised sessions involved discussion of weekly exercise prescription, and provision of exercise supervision (ensuring correct technique, monitoring intensity) and exercise counselling (behaviour change and support on overcoming barriers to participation). The ExP used a patient-centered approach during all sessions by following the Chronic Disease Self-Management Intervention Model [17]. This model enables collaborative discussions and consideration of individual circumstances. The ExPs delivering the intervention were all tertiary-trained exercise physiologists with additional study-specific training in exercise oncology. Participants were provided with written exercise prescriptions with details of all exercise prescribed which was to be completed outside of supervised sessions (e.g., type, frequency, intensity, and duration of unsupervised exercise).
2.6. Exercise Prescription
The target weekly exercise dosage was based on Australian guidelines at the time of study commencement [18,19] that recommend the equivalent of 150 min of mixed-mode (i.e., aerobic exercise and at least two resistance exercise sessions per week), moderate-intensity exercise (i.e., 600 metabolic-equivalent minutes, MET-mins). In line with the guidelines, there was flexibility in the specific mode of aerobic and resistance exercises included in individual prescriptions. The goal of the exercise program was to support each participant to reach the target exercise dosage each week through exercises that were judged by the ExP as safe and specific to the participant’s goals, while also accommodating the participant’s exercise mode preferences. Participant characteristics and exercise tolerance/capacity, alongside clinical judgement, were used to determine the most appropriate exercise types. The exercise dosage starting point, weekly volume, exercise mode and progression rate for each participant were also considered (for example sessions see Table 2). Exercise intensity was prescribed and monitored using the Rating of Perceived Exertion (RPE, 6–20 scale [20]). Further details on exercise prescription in line with the Consensus on Exercise Reporting Template [21] are in Table S3.
Table 2.
Example exercise sessions including prescription parameters.
| Exercise Prescription Parameter | Aerobic Exercise Prescription | |
|---|---|---|
| Average Participant | Deconditioned Participant; or Participant Experiencing Period of Activity-Limiting Pain or Nausea |
|
| Mode of Exercise a | Walk, cycle (stationary or bicycle), swim a | Walking (flat road, treadmill, shopping center) or stationary cycling a |
| Frequency, sessions b per week | 3–4 | 6 |
| Intensity | Moderate | Moderate |
| RPE c, 6–20 Borg Scale | 12–14 | 11–13 |
| Duration, minutes | ||
| Individual session | 20–40 | 20 (broken into shorter bouts, as needed, throughout the day, e.g., 4 × 5 min) |
| Total weekly | 110 | 120 |
| Eliciting progressing overload | ||
| Recommendations | Increase speed, load, or incline to maintain RPE | Increase duration of bouts until able to complete 20 min continuously |
| Example | Increase pace, include hills or inclines, intervals of higher speed to maintain overall intensity target across session | Use “talk-test” to identify threshold for moderate-intensity. Symptoms (e.g., fatigue, pain) may influence RPE more than cardiovascular response |
| Exercise Prescription Parameter | Resistance Exercise Prescription | |
| Average Participant | Deconditioned Participant; or Participant Experiencing Period of Activity-Limiting Pain or Nausea | |
| Frequency, sessions b per week | 2 | 2 |
| Intensity | Moderate | Moderate |
| RPE c, 6–20 Borg Scale | 12–14 | 11–13 |
| Repetitions in reserve | Aim for 2–3 repetitions in reserve at the end of each set | Aim for 3–4 repetitions in reserve at the end of each set |
| Duration, minutes | ||
| Individual Session | 20 | 15 |
| Total Weekly | 40 | 30 |
| Session components | ||
| Focus | Muscular strength | Muscular endurance |
| Repetition range | 8–12 | 15–20+ |
| Set range | 2–3 | 2 |
| Example home-based resistance exercises a | ||
| Lower body | Squat Calf-raise on step including dorsiflexion Lateral banded walk Resistance band deadlift |
Sit-to-stand Supine bridge Side-lying hip abduction |
| Upper body | Bent-over row (single-arm, dumbbell) | Resistance band row Resistance band chest press |
| Exercise Recommendations | 4–5 major muscle group exercises, 1 targeted exercise (functional or injury-specific where required) | |
| Eliciting progressive overload | Increase reps or sets, increase resistance or weight, alter exercise tempo | |
a This is not an exhaustive list; if a participant wanted to engage in other aerobic or resistance-based exercises the exercise professional would include the activity in the prescription if it was deemed safe and appropriate to the participant’s goals (e.g., dragon boat training, gym classes, boxing, machine-based resistance exercises). b These exercise sessions may have been completed unsupervised or incorporated as part of one of the supervised sessions. c RPE: Rating of Perceived Exertion.
2.7. Data Collection
Personal, diagnostic, and treatment- and health-related characteristics (Table 3) were collected at enrolment via self-report over the telephone.
Table 3.
Baseline Characteristics.
| Total n = 60 |
High Group n = 30 |
Low Group n = 30 |
|
|---|---|---|---|
| Personal Characteristics | Mean (SD) or n (%) | ||
| Age (years), mean (SD) | 50.1 (9.0) | 51.0 (9.5) | 49.2 (8.5) |
| <50 years | 29 (48%) | 16 (53%) | 13 (43%) |
| ≥50 years | 31 (52%) | 14 (47%) | 17 (57%) |
| Household income | |||
| Lower income (bottom 40th percentiles) a | 24 (40%) | 12 (40%) | 12 (40%) |
| Marital status | |||
| Married/de-factor | 42 (70%) | 22 (73%) | 20 (67%) |
| Private Health Insurance (Yes) | 48 (80%) | 25 (83%) | 23 (77%) |
| Body-mass index (kg/m2) | 28.9 (6.2) | 29.2 (6.7) | 28.6 (5.7) |
| Body-mass index (n, %) | |||
| Healthy or underweight | 19 (32%) | 9 (30%) | 10 (33%) |
| Overweight | 18 (30%) | 9 (30%) | 9 (30%) |
| Obese | 23 (38%) | 12 (40%) | 11 (37%) |
| Minutes of structured exercise/week, mean (SD) | 42.0 (57.6) | 41.0 (55.8) | 41.0 (60.1) |
| Diagnostic Characteristics | Median (range) or n (%) | ||
| Breast Cancer stage | |||
| Stage II | 28 (47%) | 12 (40%) | 16 (54%) |
| Stage III | 20 (33%) | 14 (47%) | 6 (20%) |
| Stage IV | 7 (12%) | 3 (10%) | 4 (13%) |
| Unsure or unknown b | 5 (8%) | 1 (3%) | 4 (13%) |
| Months since diagnosis c | 18 (2–243) | 16 (2–215) | 24 (2–243) |
| Side of breast cancer | |||
| Dominant side | 27 (45%) | 12 (40%) | 15 (50%) |
| Non-dominant side | 30 (50%) | 17 (57%) | 13 (43%) |
| Bilateral | 3 (5%) | 1 (3%) | 2 (7%) |
| Treatment Characteristics | n (%) | ||
| Most extensive surgery | |||
| Mastectomy | 43 (72%) | 19 (64%) | 24 (80%) |
| Lumpectomy | 16 (27%) | 10 (33%) | 6 (20%) |
| No surgery | 1 (2%) | 1 (3%) | 0 (0%) |
| No. of nodes removed | |||
| 0 | 0 (0%) | 0 (0%) | 0 (0%) |
| 1–4 | 17 (28%) | 7 (23%) | 10 (33%) |
| 5–9 | 6 (10%) | 2 (7%) | 4 (13%) |
| 10+ | 25 (42%) | 13 (43%) | 12 (40%) |
| Unsure or unknown | 12 (20%) | 8 (27%) | 4 (13%) |
| Treatment status | |||
| Currently receiving treatment c, d | 22 (37%) | 10 (33%) | 12 (40%) |
| Treatments received (current or past) | |||
| Chemotherapy (yes) | 55 (92%) | 25 (83%) | 30 (100%) |
| Radiation therapy (yes) | 45 (75%) | 25 (83%) | 20 (67%) |
| Hormone therapy (yes) | 32 (53%) | 18 (60%) | 14 (47%) |
| Health Characteristics | n (%) | ||
| Number of comorbidities | 1 (0–6) | 1 (0–6) | 1 (0–5) |
| 0 comorbidities | 21 (35%) | 9 (30%) | 12 (40%) |
| 1–2 comorbidities | 29 (48%) | 15 (50%) | 14 (47%) |
| 3–4 comorbidities | 7 (12%) | 4 (13%) | 3 (10%) |
| 5–6 comorbidities | 3 (5%) | 2 (7%) | 1 (3%) |
| Number of side effects | 4.4 (2.1) | 4.7 (2.1) | 4.1 (2.1) |
| 0–2 side effects | 11 (19%) | 5 (17%) | 6 (20%) |
| 3–4 side effects | 24 (40%) | 10 (33%) | 14 (46%) |
| 5–6 side effects | 15 (25%) | 10 (33%) | 5 (16%) |
| 7+ side effects | 10 (17%) | 5 (17%) | 5 (16%) |
| Number of side effects (≥moderate severity) | 2.8 (1.9) | 3.1 (1.9) | 2.5 (1.8) |
| 0–2 moderate+ side effects | 28 (47%) | 12 (40%) | 16 (54%) |
| 3–4 moderate+ side effects | 21 (35%) | 11 (37%) | 10 (33%) |
| 5–6 moderate+ side effects | 9 (15%) | 6 (20%) | 3 (10%) |
| 7+ moderate+ side effects | 2 (3%) | 1 (3%) | 1 (3%) |
a Based on cut-off for lower-income households, i.e., lowest 40th percentiles of gross household income (ABS Survey of Income and Housing, 2015–2016 and 2017–2018). Data missing for n = 10; b Confirmed stage II or above based on referral by medical team; c Not including hormone therapy (i.e., women undergoing just hormone therapy were classified as having completed treatment); d Stratification factor (currently undergoing treatment: yes, no).
2.8. Primary Outcomes
Safety and feasibility data were collected systematically throughout the intervention. Participants were asked to record the details of all exercise completed (e.g., duration, intensity, mode) and any adverse events (AEs) in a study-specific logbook on a daily basis; these data were then recorded in case management folders by the ExP at each session. Study records of recruitment, retention and delivery were also extracted from the case management folders for feasibility analysis. Objectively-assessed and patient-reported outcomes were assessed at baseline (pre-intervention) and post-intervention (12 weeks post-baseline), with patient-reported outcomes also assessed at 12 weeks post-intervention.
AEs (safety outcome) were defined in accordance with the Good Clinical Practice Guidelines [22] as ‘any unfavorable and unintended sign, symptom or disease that occurs in a participant whether it is considered to be study- or non-study-related’. AEs were deemed to be exercise-related AEs (ExAEs) if they occurred during or within two hours of supervised or unsupervised exercise or had a clear mechanism relating the AE to exercise (as determined by treating medical team or senior ExP). Participants were asked at each supervised session if they had experienced any AEs since the previous session. AE descriptions and severity ratings were based on the Common Terminology Criteria for Adverse Events, Version 4 [23]. Exercise-related AEs could include injuries (including falls), medical events (e.g., unstable angina), and/or exacerbations of treatment-related side effects. Adverse events were classified as grade 3 if they led to hospitalisation and/or led to limitations in self-care activities of daily living [23]. The a priori acceptable threshold for safety was no grade 3 or above ExAEs.
Feasibility was defined as the median volume of weekly exercise completed during the intervention compared with the weekly intervention target (600 MET-mins), similar to the method used by Scott et al. [24]. The intervention was to be deemed feasible if the feasibility rate for the group was ≥75%. MET-mins for each session were calculated as minutes of exercise multiplied by the MET-value equivalent to the reported RPE (conversion of RPE to MET-value was extrapolated from Norton et al. [25]).
2.9. Secondary Outcomes
Quality of life, exercise self-efficacy and total weekly PA were assessed via self-report questionnaire using the Patient Reported Outcomes Measurement Information System (PROMIS®) Global Health Scale [26], a cancer-specific exercise-barriers self-efficacy scale [27], and Active Australia Survey [28], respectively. Aerobic fitness, upper-body strength and lower-body strength were measured using the 6-min walk test (following the protocol of the American Thoracic Society [29]), the YMCA bench press [30] with a modified weight of 10 kg (to accommodate potential upper-limb post-surgical limitations) and the 30-s sit-to-stand [31], respectively. Clinically-relevant changes were determined a priori based on thresholds identified from previous studies in similar populations or based on distribution of baseline values (½ standard deviation (SD)) when minimally-important differences had not previously been assessed [32,33,34,35].
2.10. Statistical Analysis
Safety and feasibility were reported as number (%) and group medians (minimum, maximum) and a Mann-Whitney U-test was used to test significance for differences between groups [24]. Generalised estimating equations were used to determine time, group, (high-supervision versus low-supervision) and time by group effects [36,37,38]. Estimated means, 95% confidence intervals (CI) and p-values are reported for each estimate and mean differences. CI and p-values from pairwise results are presented in exploration of significant time effects and group by time interactions. The sample size for this study was based on estimating 80% compliance to the exercise target with a 95% confidence interval of ±10%. A group size of 30 participants allowed for 5% withdrawals and 5% loss-to-follow-up across the 24 weeks of the intervention and follow-up period (sample size formula based on Hooper, 2019 [39]). Intention-to-treat principles were applied during data analyses. All analyses were undertaken using IBM SPSS Statistics for Windows, version 25.0, IBM: New York, USA.
3. Results
The two exercise groups had similar personal, treatment and behavioural characteristics at baseline (Table 3). Two-thirds of women (n = 39) reported at least one comorbidity and 98% (n = 59) reported one or more persistent treatment-related side-effects, with an average of 4.3 (SD 2.3) comorbidities and/or side-effects per participant.
3.1. Primary Outcome: Safety
There were no grade 3 or above ExAEs (Table 4), and there was no difference in AE rate, type, or severity between groups (Table S4). The majority of ExAEs were mild (grade 1 n = 86/126 ExAEs); 20% required an interruption or modification to the intervention. Two ExAEs occurred during and following baseline testing (n = 1, chest pain during the 6-min walk test; n = 1, ‘severe’ upper-body delayed-onset muscle soreness in days following testing, likely caused by YMCA bench press test). These were not included in the analysis of intervention safety. The participant with exercise-induced chest pain was referred to her medical team for clearance prior to commencing the intervention.
Table 4.
Safety of exercise: Adverse events during the SAFE intervention.
| All Women | High Group | Low Group | |
|---|---|---|---|
| n = 59 | n = 30 | n = 29 | |
| Primary Outcome: | |||
| Number of ≥ grade 3 exercise-related AE | 0 | 0 | 0 |
| Adverse Events | |||
| Number of AEs (total) | 177 | 136 | 41 |
| Number of women reporting AEs | 41 (69%) | 23 (77%) | 18 (62%) |
| Median (range) number of AEs per participant | 2 (0–19) | 4 (0–19) | 1 (0–7) |
| Exercise-related Adverse Events | |||
| Number of exercise-related AEs (total) | 126 | 103 | 23 |
| Number of women reporting exercise-related AEs | 34 (58%) | 23 (77%) | 11 (38%) |
| Median (range) number of exercise-related AEs per participants | 1 (0–14) | 3 (0–14) | 0 (0–6) |
| Number of women reporting exercise-related AEs | |||
| 0 AEs | 26 (44%) | 7 (23%) | 19 (66%) |
| 1–2 AEs | 16 (27%) | 8 (27%) | 8 (28%) |
| 3–4 AEs | 8 (14%) | 6 (20%) | 2 (7%) |
| 5–10 AEs | 8 (14%) | 7 (23%) | 1 (3%) |
| >10 AEs | 2 (3%) | 2 (7%) | 0 (0%) |
AE: Adverse Event.
3.2. Primary Outcome: Feasibility
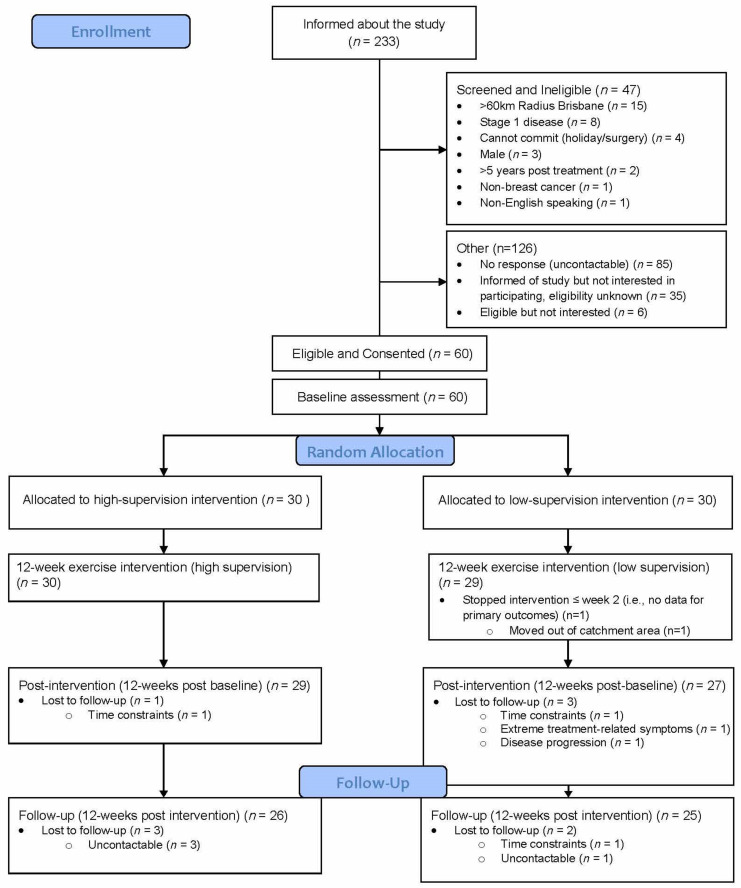
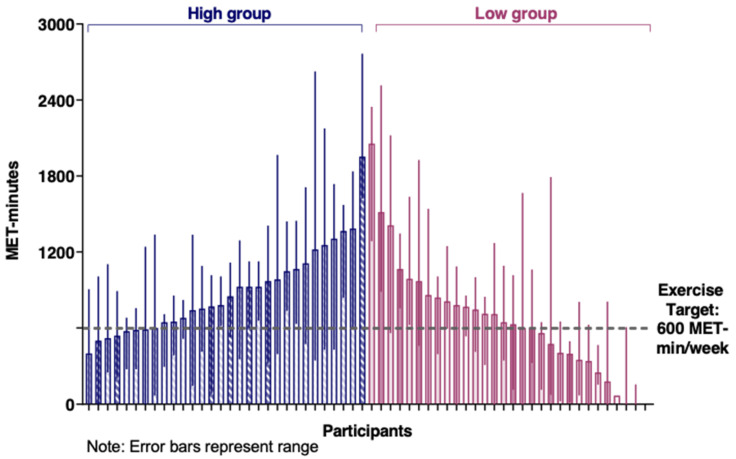
The intervention completion rate was 98% (59/60 women; Figure 1: Flow diagram of recruitment and retention of participants). The median percentage of attended scheduled ExP sessions was 100% (high: median 20 [range 4–20]; low: median 5 [range 4–5]). Median weekly MET-mins for both groups exceeded the feasibility threshold of 75% (i.e., 450 MET-mins) and the weekly goal of 600 MET-mins, although this was higher in the high-supervision group (817 MET-mins [min–max: 446–2103]) compared to the low-supervision group (663 MET-mins [min–max: 30–1924]; p = 0.047). There was wide variation within groups in weekly MET-mins observed across the intervention (see Figure 2). Figure 3 provides a graphical representation of the variation in weekly MET-mins between individuals within each group, as well as within each individual. A median of two resistance sessions per week were completed by women in both groups (min–max: High = 0–7; Low = 0–5).
Figure 1.
Flow diagram of recruitment and retention of participants.
Figure 2.
Median weekly exercise volume undertaken per participant in the high-supervision and low-supervision groups.
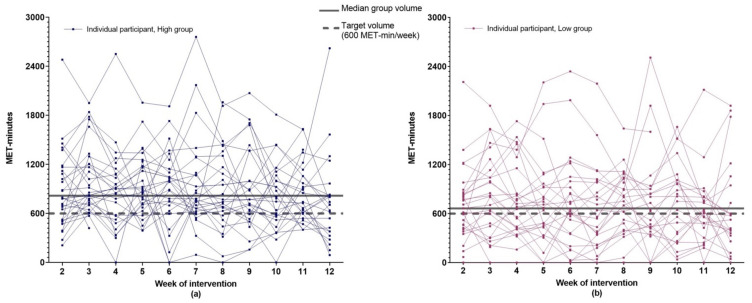
Figure 3.
Weekly exercise volume over the 12-week intervention for each participant. (a) Data from participants in the high-supervision group; (b) Data from participants in the low-supervision group.
3.3. Secondary Outcomes: Effect
Clinically-relevant improvements between baseline and post-intervention for quality of life, PA and fitness were observed for both groups (p < 0.05; Table 5). Quality of life improvements were maintained at the 12-week follow-up. Despite a reduction in minutes of PA between post-intervention and follow-up, PA levels at the 12-week follow-up remained higher than baseline (p < 0.05).
Table 5.
Efficacy of exercise: Mean and 95% confidence intervals of health outcomes at baseline, post-intervention and follow-up assessments.
| Baseline (T1) | Post-Intervention (T2) | Follow-Up (T3) | Δ T1 to T2 a | Δ T2 to T3 a | Δ T1 to T3 a | ||||
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mdiff (95% CI) | |||
| PROMIS Global Physical Health | 5.91 (4.2–7.6) c,d |
0.10 (−1.6–1.7) |
6.01 (3.9–8.0) c,d |
||||||
| High | 40.4 | (38.4–42.5) | 46.7 | (44.2–49.3) | 47.1 | (44.0–50.2) | |||
| Low | 40.6 | (37.6–43.5) | 46.1 | (43.6–48.5) | 45.8 | (42.9–48.8) | |||
| GEE b: Group p = 0.71; Time p < 0.01; Group × Time p = 0.79 | |||||||||
| PROMIS Global Mental Health | 5.4 (3.6–7.0) c,d |
−0.8 (−3.0–1.4) |
4.5 (2.0–7.0) c |
||||||
| High | 41.6 | (39.0–44.3) | 48.4 | (45.8–51.1) | 47.0 | (44.0–50.1) | |||
| Low | 41.9 | (39.3–44.5) | 45.8 | (43.1–48.5) | 45.5 | (43.0–48.0) | |||
| GEE b: Group p = 0.37; Time p < 0.01; Group × Time p = 0.25 | |||||||||
| Exercise-barrier self-efficacy | |||||||||
| High | 35.7 | (29.1–42.2) | 47.0 | (41.0–53.0) | 49.2 | (40.9–57.5) | 11.3 (4.5–18.1) c,d | 2.2 (−5.1–9.6) | 13.5 (3.9–23.3) c,d |
| Low | 32.1 | (25.7–38.5) | 33.7 | (27.5–40.0) | 29.8 | (22.5–37.2) | 1.6 (−4.4–7.7) | −3.9 (−9.3–1.5) | −2.3 (−8.4–3.9) |
| GEE b: Group p = 0.002; Time p = 0.02; Group × Time p = 0.02 | |||||||||
| Physical Activity e | 244.1 (172.7–315.5) c,d |
−31.4 (−124.0–61.3) d |
212.7 (120.1–305.4) c,d |
||||||
| High | 93.1 | (70.0–116.2) | 381.8 | (289.5–474.1) | 349.2 | (224.2–473.5) | |||
| Low | 140.7 | (86.1–195.3) | 340.2 | (249.2–431.1) | 310.0 | (190.6–429.4) | |||
| GEE b: Group p = 0.79; Time p < 0.01; Group × Time p = 0.42 | |||||||||
| 6-min walk test | 53.6 (35.7–71.4) c,d |
- f | - f | ||||||
| High | 494.0 | (454.0–530.0) | 547.0 | (518.0–576.0) | - f | ||||
| Low | 510.0 | (479.0–541.0) | 563.0 | (541.0–585.0) | - f | ||||
| GEE b: Group p = 0.39; Time p < 0.01; Group × Time p = 0.90 | |||||||||
| Modified-YMCA bench press | - f | - f | |||||||
| High | 27.3 | (18.0–36.7) | 43.5 | (32.6–54.4) | - f | 17.9 (5.5–30.3) c,d | |||
| Low | 25.6 | (19.6–31.6) | 29.1 | (21.6–36.7) | - f | 3.6 (−0.3–7.4) | |||
| GEE b: Group p = 0.18; Time p < 0.01; Group × Time p < 0.01 | |||||||||
| 30-s sit to stand | - f | - f | |||||||
| High | 11.6 | (10.0–13.1) | 15.0 | (13.2–16.8) | - f | 3.4 (1.7–5.1) c,d | |||
| Low | 11.3 | (10.1–12.5) | 12.6 | (11.4–13.8) | - f | 1.3 (0.2–2.5) c | |||
| GEE b: Group p = 0.14; Time p < 0.01; Group × Time p = 0.05 | |||||||||
T1: Baseline (pre-intervention); T2: Post-intervention (12 weeks post-baseline); T3: 12-week follow-up (12 weeks post-intervention). a Change scores are reported for whole cohort if no significant group by time interaction (Generalized Estimating Equations (GEE) group × time p > 0.05); reported by group if significant group by time interaction (GEE group × time p ≤ 0.05); b p-value derived from Generalised-estimating equation model; c Statistically significant change between time points p < 0.05; d Clinically relevant/minimally important difference. Defined as a change of: ≥50 m walked in the 6-min walk test [32], ≥20 min of total physical activity per week [33], ≥five units in the PROMIS global physical and mental health scales [34], ≥two repetitions of the sit-to-stand [35], and a change of nine units and 11 repetitions for exercise-barriers self-efficacy and bench press, respectively.; e Physical activity: Total physical activity as measured by Active Australia Survey (self-report minutes walking + moderate physical activity+ [2 × vigorous physical activity]); f 6-min walk test, Bench press and Sit-to-stand were measured at T1 and T2 only.
Group by time interactions were observed for exercise-barriers self-efficacy and upper-body strength (p < 0.05; Table 5), with the high-supervision group showing improvements at post-intervention (gains of 11.3 points and 17.9 repetitions, respectively), which were maintained at follow-up. In contrast, self-efficacy and upper-body strength scores for the low-supervision group remained unchanged over time (1.6 point and 3.6 repetition change between time 1 and 2, respectively, which is below the a priori defined clinically relevant threshold). Sit-to-stand scores for both groups improved between baseline and post-intervention, although only improvement in the High-supervision group met the clinically-relevant threshold.
4. Discussion
The SAFE trial was an individualised, 12-week exercise intervention with a weekly target exercise dosage of 600 MET-mins. We hypothesized that despite recruiting a potentially higher-risk cohort, the exercise intervention would be safe and feasible (primary outcomes) and beneficial for both the low-supervision and high-supervision groups. Our findings indicate that this exercise is safe and feasible and improves quality of life, PA, and fitness in breast cancer survivors with additional comorbidities and persistent treatment-related side-effects. These findings were consistent, irrespective of whether the intervention was delivered via 20 or five supervised sessions with an ExP. Nonetheless, clinically-relevant improvements in strength outcomes and self-efficacy were observed between pre- and post-intervention for those in the high-supervision group and not for those in the low-supervision group. Results of this comparative effectiveness trial suggest that the current Australian funding for provision of exercise services for those with cancer provides a valuable foundation for improving the lives of breast cancer survivors. However, additional supervision may contribute to greater and potentially more sustainable benefit.
4.1. Safety and Feasibility
No grade 3 or above ExAEs were observed during the intervention period. High average compliance, which exceeded the target exercise dosage, was reported irrespective of group. These findings support individually-prescribed exercise meeting weekly exercise targets recommended to the wider cancer population as safe and feasible for women with more advanced breast cancer, and with additional comorbidities and/or persistent, treatment-related side-effects. Nonetheless, these findings need to be placed in the context from which they are drawn. Specifically, exercise prescriptions were individually tailored by ExPs with tertiary qualifications and oncology experience. All participants (irrespective of group allocation) were routinely educated and advised on exercise safety and on using symptom response to guide subsequent exercise prescription. Although rare, there were reports of breast cancer specific concerns, including lymphoedema, cording and shoulder pain, presenting during the intervention. These are neither unique to SAFE [8] nor do they represent contraindications to exercise participation [2]. However, these issues required sensitivity and consideration in subsequent exercise prescription, highlighting the need for qualified ExPs with oncology-specific training. Half of the AEs reported in SAFE informed a purposeful modification of the subsequent exercise prescription (either mode, frequency, duration, or intensity), and one-quarter of the AEs required referral to other allied health professionals or the treating team. As such, while the SAFE intervention, including when delivered under low-level supervision conditions, was deemed safe, trained ExPs implemented the intervention with input when needed from the wider cancer care team or other allied health professionals.
SAFE AE rates were higher than the AE rates reported following a meta-analysis of studies evaluating exercise involving women with breast cancer [8]. The differences in rates are likely reflective of data collection procedures rather than safety; the majority of trials included in the meta-analysis had poor AE assessment and reporting procedures (34% did not report safety at all). Lack of evidence demonstrating patient safety contributes to clinicians not encouraging exercise participation [40]. While findings from this work can be used to reassure clinicians that exercise is safe, there remains a clear need to improve safety reporting protocols within exercise oncology trials more broadly [41].
Average weekly exercise reported by both SAFE groups exceeded the intervention target. This is consistent with the high feasibility rates reported in exercise studies involving women with stage II or above breast cancer [8] but is notably higher when compared to a study that only included women with stage IV breast cancer undertaking chemotherapy [24]. Specifically, SAFE participants completed an average of 123% of the target exercise dosage, whereas participants in the metastatic breast cancer study only completed 61% of planned MET-hours of exercise. The higher feasibility rates observed in SAFE may be due to the small portion of women with stage IV disease (12%), most (63%) having had completed treatment during study participation, and location of the exercise intervention (SAFE was primarily home-based and not supervised). Nonetheless, the group averages fail to fully reflect individual levels and fluctuations over the intervention duration. For example, most participants in SAFE had at least two weeks in which they completed less than the weekly exercise target (see Figure 3). The impact of non-compliant weeks on patient outcomes and whether a week of low-volume or no exercise can be balanced by a week that exceeds the exercise target without changing outcomes is yet to be determined. Regardless, the individual feasibility results reinforce the need for flexible exercise prescriptions that recognize the likelihood of breast cancer survivors experiencing ‘good’ and ‘bad’ weeks [2]. Conversely, there were a subgroup of women (23% in high, 17% in low) who met the target every week, even in the presence of barriers. It is plausible, but at this point untested, that at least for some of these women, fewer sessions may have reduced participant burden without undue adverse impact on short- or longer-term effect on outcomes, including exercise levels.
4.2. Effect Outcomes
The supervision level of the high-supervision group was designed to represent that commonly observed in published breast cancer and exercise trials (i.e., one to two supervised sessions/week) [10,42,43]. In line with previously published findings [10,43], improvements in quality of life and function were observed for those in the high-supervision group immediately at post-intervention and at 12 weeks post-intervention. The similar improvements observed in the low-supervision group for quality of life, PA, and fitness immediately post- and 12-weeks post-intervention indicate that the Australian funding model for allied health services (five sessions) may be an effective platform for exercise therapy delivery following breast cancer, at least when all five sessions are provided over a 12-week period. However, while similar improvements in quality of life and fitness were observed between the high-supervision and low-supervision groups immediately post-intervention, clinically-relevant improvements in strength were observed only in the high-supervision group. Furthermore, the high-supervision group also showed improvements in self-efficacy (the ability to overcome barriers to exercise), whereas those in the low-supervision group did not, and PA levels were higher throughout and beyond the intervention period for those in the high- versus the low-supervision group. Epidemiological evidence which shows that higher levels of lean tissue (which is directly associated with strength), and PA are independently associated with improved survival post-breast cancer [33,44,45] may suggest that the differences between the high- and low-supervision groups are worthy of attention. Furthermore, the potential benefit of exercise is unclear if delivered via a maximum of five sessions over a 12-month period or when the five sessions are shared among other allied health services typically required by breast cancer survivors including physiotherapy, podiatry, and dietetics, which is the intent of the current Australian funding model.
4.3. Strengths and Limitations
Strengths of the SAFE trial include the successful recruitment of an understudied sample of breast cancer survivors, comprehensive reporting of safety and feasibility data, and evaluation of a real-world delivery model. Limitations of SAFE include potential reporting bias of AE data (e.g., higher recall bias for those in the low-supervision group than the high-supervision group; although likely small for grade 3 or higher AE over a 12-week period), and a relatively short follow-up (12-weeks post-intervention). Furthermore, most of the sample had a history of at least irregular exercise and, while not sufficiently active on trial commencement, they were also not sedentary. The potential impact of this bias on safety, feasibility and efficacy findings is unclear.
4.4. Clinical Implications and Future Research
The results of this trial suggest that an exercise intervention delivered under real-world conditions is appropriate for implementation in a representative cohort of breast cancer survivors. However, future research is needed to better understand whether the greater gains in health outcomes that come with higher levels of supervision translate into longer term quality of life and survival benefits and sustained behaviour change. Future planned research related to this work includes analysis of the cost-effectiveness of the high- versus low-supervision group and exploration of the impact of compliance on patient outcomes, with subsequent findings aiding the translation of these results into clinical practice.
5. Conclusions
SAFE provides evidence that exercise prescribed alongside education and support in exercise safety and behaviour change via either five or 20 sessions supervised by an ExP is safe, feasible (primary outcomes) and beneficial (secondary outcomes) for women with stage II+ breast cancer. Individually-targeted exercise is appropriate for previously insufficiently active women with breast cancer, even in the presence of chronic side effects and/or comorbidities. Five sessions with an ExP over a 12-week period led to improvements in quality of life and physical function. However, superior gains were observed for women who had 20 sessions with an ExP over the same duration.
Acknowledgments
Study investigators would like to sincerely thank those who agreed to participate in this study. Thank you also to the Breast Care Nurses and the Exercise Professionals who worked on this project and to Sheree Rye for invaluable contributions to the preparation of this manuscript.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14061528/s1, Table S1: Changes to study protocol following trial registration; Table S2. Key Differences Between Exercise Intervention Groups; Table S3: Principles of exercise prescription and reporting used in the SAFE exercise intervention (based on Consensus on Exercise Reporting Template [13]); Table S4: Frequency of grade 1–2 exercise-related adverse events.
Author Contributions
Study conception and design was led by S.C.H., E.E., D.V., R.R.S. and C.P. Material preparation was led by R.R.S., E.E. and S.C.H. Intervention delivery and data collection was supervised by R.R.S., C.X.S. and S.C.H. and performed by B.S. and J.T. Analysis was supervised by S.C.H. and D.V. and performed by R.R.S. and C.X.S. The first draft of the manuscript was written by R.R.S. and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
Funding
The research positions of S.C.H. and E.E. were supported via a Cancer Council Queensland Fellowship and an NHMRC Senior Research Fellowship (#1041789), respectively.
Institutional Review Board Statement
The institutional review boards of the Queensland University of Technology, St Andrew’s Hospital (HREC 15/19) and Mater Health Services approved the study (HREC/14/MHS/155). All participants provided informed consent and were treated in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
De-identified data, dependent variables and participant characteristics may be available for reasonable research purposes via individual request to the authors.
Conflicts of Interest
The authors declare that they have no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Campbell K.L., Winters-Stone K.M., Wiskemann J., May A.M., Schwartz A.L., Courneya K.S., Zucker D.S., Matthews C.E., Ligibel J.A., Gerber L.H., et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019;51:2375–2390. doi: 10.1249/MSS.0000000000002116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayes S.C., Newton R.U., Spence R.R., Galvao D.A. The Exercise and Sports Science Australia position statement: Exercise medicine in cancer management. J. Sci. Med. Sport. 2019;22:1175–1199. doi: 10.1016/j.jsams.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz K.H., Campbell A.M., Stuiver M.M., Pinto B.M., Schwartz A.L., Morris G.S., Ligibel J.A., Cheville A., Galvao D.A., Alfano C.M., et al. Exercise is medicine in oncology: Engaging clinicians to help patients move through cancer. CA Cancer J. Clin. 2019;69:468–484. doi: 10.3322/caac.21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothwell P.M. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet. 2005;365:82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 5.Dirix P., Wyld L., Paluch-Shimon S., Poortmans P. Time for More Inclusive Cancer Trials. J. Natl. Compr. Canc. Netw. 2020;18:1431–1434. doi: 10.6004/jnccn.2020.7652. [DOI] [PubMed] [Google Scholar]
- 6.Spence R., DiSipio T., Schmitz K., Hayes S. Is unsupervised exercise following breast cancer safe for all women? Int. J. Phys. Med. Rehabil. 2014;2:1971-8. doi: 10.4172/2329-9096.1000197. [DOI] [Google Scholar]
- 7.Bluethmann S.M., Foo W., Winkels R.M., Mama S.K., Schmitz K.H. Physical Activity in Older Cancer Survivors: What Role Do Multimorbidity and Perceived Disability Play? J. Aging Phys. Act. 2020;28:311–319. doi: 10.1123/japa.2019-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh B., Spence R.R., Steele M.L., Sandler C.X., Peake J., Hayes S.C. A systematic review and meta-analysis of the safety, feasibility and effect of exercise in women with stage II+ breast cancer. Arch. Phys. Med. Rehabil. 2018;99:2621–2636. doi: 10.1016/j.apmr.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Singh B., Spence R., Steele M.L., Hayes S., Toohey K. Exercise for Individuals With Lung Cancer: A Systematic Review and Meta-Analysis of Adverse Events, Feasibility, and Effectiveness. Semin. Oncol. Nurs. 2020;36:151076. doi: 10.1016/j.soncn.2020.151076. [DOI] [PubMed] [Google Scholar]
- 10.Buffart L.M., Kalter J., Sweegers M.G., Courneya K.S., Newton R.U., Aaronson N.K., Jacobsen P.B., May A.M., Galvao D.A., Chinapaw M.J., et al. Effects and moderators of exercise on quality of life and physical function in patients with cancer: An individual patient data meta-analysis of 34 RCTs. Cancer Treat Rev. 2017;52:91–104. doi: 10.1016/j.ctrv.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Covington K.R., Hidde M.C., Pergolotti M., Leach H.J. Community-based exercise programs for cancer survivors: A scoping review of practice-based evidence. Support Care Cancer. 2019;27:4435–4450. doi: 10.1007/s00520-019-05022-6. [DOI] [PubMed] [Google Scholar]
- 12.Sweegers M.G., Altenburg T.M., Chinapaw M.J., Kalter J., Verdonck-de Leeuw I.M., Courneya K.S., Newton R.U., Aaronson N.K., Jacobsen P.B., Brug J., et al. Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? A systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 2018;52:505–513. doi: 10.1136/bjsports-2017-097891. [DOI] [PubMed] [Google Scholar]
- 13.Hardcastle S.J., Cohen P.A. Effective Physical Activity Promotion to Survivors of Cancer Is Likely to Be Home Based and to Require Oncologist Participation. J. Clin. Oncol. 2017;35:3635–3637. doi: 10.1200/JCO.2017.74.6032. [DOI] [PubMed] [Google Scholar]
- 14.Australian Government Department of Health Chronic Disease Management (Formerly Enhanced Primary Care or EPC)—GP Services. [(accessed on 19 October 2021)]; Available online: https://www1.health.gov.au/internet/main/publishing.nsf/Content/mbsprimarycare-chronicdiseasemanagement.
- 15.Singh B., Spence R.R., Eakin E., Hayes S.C. Can five sessions per year with an AEP make a difference to the lives of those with a chronic disease. MOVE ESSA Magazine. 2017;10 [Google Scholar]
- 16.Eldridge S.M., Chan C.L., Campbell M.J., Bond C.M., Hopewell S., Thabane L., Lancaster G.A., PAFS Consensus Group CONSORT 2010 statement: Extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239. doi: 10.1136/bmj.i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiore M., Bailey W., Cohen S., Dorfman S., Goldstein M. Treating Tobacco Use and Dependence: A Clinical Practice Guideline. US Department of Health and Human Services, Public Health Services; Rockville, MD, USA: 2000. [Google Scholar]
- 18.Hayes S.C., Spence R.R., Galvao D.A., Newton R.U. Australian Association for Exercise and Sport Science position stand: Optimising cancer outcomes through exercise. J. Sci. Med. Sport. 2009;12:428–434. doi: 10.1016/j.jsams.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Brown W., Bauman A., Bull F., Burton N. Development of Evidence-Based Physical Activity Recommendations for Adults (18–64 Years) Australian Government Department of Health; Canberra, Australia: 2012. [Google Scholar]
- 20.Borg G. Perceived exertion as an indicator of somatic stress. Scand J. Rehabil. Med. 1970;2:92–98. [PubMed] [Google Scholar]
- 21.Slade S.C., Dionne C.E., Underwood M., Buchbinder R. Consensus on Exercise Reporting Template (CERT): Explanation and Elaboration Statement. Br. J. Sports Med. 2016;50:1428–1437. doi: 10.1136/bjsports-2016-096651. [DOI] [PubMed] [Google Scholar]
- 22.Australian Government Department of Health and Ageing The Australian Clinical Trial Handbook . A Simple, Practical Guide to the to the Conduct of Clinical Trials to International Standards of Good Clinical Practice (GCP) in the Australian Context. Commonwealth of Australia; Canberra, Australia: 2006. [Google Scholar]
- 23.National Institutes of Health. National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE), Version 4.0. U.S. Department of Health and Human Services; Bethesda, MD, USA: 2009. [Google Scholar]
- 24.Scott J.M., Iyengar N.M., Nilsen T.S., Michalski M., Thomas S.M., Herndon J., 2nd, Sasso J., Yu A., Chandarlapaty S., Dang C.T., et al. Feasibility, safety, and efficacy of aerobic training in pretreated patients with metastatic breast cancer: A randomized controlled trial. Cancer. 2018;124:2552–2560. doi: 10.1002/cncr.31368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norton K., Norton L. Pre-Exercise Screening: Guide to the Australian Adult Pre-Exercise Screening System. Exercise and Sport Science Australia, Fitness Australia and Sports Medicine Australia; Queensland, Australia: 2011. [Google Scholar]
- 26.Cella D., Riley W., Stone A., Rothrock N., Reeve B., Yount S., Amtmann D., Bode R., Buysse D., Choi S., et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J. Clin. Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers L.Q., Courneya K.S., Verhulst S., Markwell S., Lanzotti V., Shah P. Exercise barrier and task self-efficacy in breast cancer patients during treatment. Support Care Cancer. 2006;14:84–90. doi: 10.1007/s00520-005-0851-2. [DOI] [PubMed] [Google Scholar]
- 28.Australian Institute of Health and Welfare The Active Australia Survey: A Guide and Manual for Implementation, Analysis and Reporting. Australian Institute of Health and Welfare, Australian Govenment; Canberra, Australia: 2004. [Google Scholar]
- 29.American Thoracic Society, American Thoracic Society statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 30.Golding S. YMCA Fitness Testing and Assessment Manual. Human Kinetics; Champaign, IL, USA: 1989. [Google Scholar]
- 31.Jones C.J., Rikli R.E., Beam W.C. A 30-s chair-stand test as a measure of lower body strength in community-residing older adults. Res. Q. Exerc. Sport. 1999;70:113–119. doi: 10.1080/02701367.1999.10608028. [DOI] [PubMed] [Google Scholar]
- 32.Perera S., Mody S.H., Woodman R.C., Studenski S.A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 33.Li T., Wei S., Shi Y., Pang S., Qin Q., Yin J., Deng Y., Chen Q., Wei S., Nie S., et al. The dose–response effect of physical activity on cancer mortality: Findings from 71 prospective cohort studies. Br. J. Sports. 2016;50:339–345. doi: 10.1136/bjsports-2015-094927. [DOI] [PubMed] [Google Scholar]
- 34.Norman G.R., Sloan J.A., Wyrwich K.W. The truly remarkable universality of half a standard deviation: Confirmation through another look. Expert Rev. Pharmacoecon. Outcomes Res. 2004;4:581–585. doi: 10.1586/14737167.4.5.581. [DOI] [PubMed] [Google Scholar]
- 35.Le Berre M., Apap D., Babcock J., Bray S., Gareau E., Chasse K., Levesque N., Robbins S.M. The Psychometric Properties of a Modified Sit-to-Stand Test With Use of the Upper Extremities in Institutionalized Older Adults. Percept Mot Skills. 2016;123:138–152. doi: 10.1177/0031512516653388. [DOI] [PubMed] [Google Scholar]
- 36.Feng Z., Diehr P., Peterson A., McLerran D. Selected statistical issues in group randomized trials. Annu. Rev. Public Health. 2001;22:167–187. doi: 10.1146/annurev.publhealth.22.1.167. [DOI] [PubMed] [Google Scholar]
- 37.Hayes S.C., Rye S., Disipio T., Yates P., Bashford J., Pyke C., Saunders C., Battistutta D., Eakin E. Exercise for health: A randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment-related side effects following breast cancer. Breast Cancer Res. Treat. 2013;137:175–186. doi: 10.1007/s10549-012-2331-y. [DOI] [PubMed] [Google Scholar]
- 38.Chen H.M., Tsai C.M., Wu Y.C., Lin K.C., Lin C.C. Randomised controlled trial on the effectiveness of home-based walking exercise on anxiety, depression and cancer-related symptoms in patients with lung cancer. Br. J. Cancer. 2015;112:438–445. doi: 10.1038/bjc.2014.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hooper R. Justify Sample Size for a Feasibility Study. National Institute for Health Research; London, UK: 2019. [Google Scholar]
- 40.Brown J.C., Schmitz K.H. The prescription or proscription of exercise in colorectal cancer care. Med. Sci. Sports Exerc. 2014;46:2202–2209. doi: 10.1249/MSS.0000000000000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jones L.W. Evidence-based risk assessment and recommendations for physical activity clearance: Cancer. Appl. Physiol. Nutr. Metab. 2011;36((Suppl. S1)):S101–S112. doi: 10.1139/h11-043. [DOI] [PubMed] [Google Scholar]
- 42.Nock N.L., Owusu C., Flocke S., Krejci S.A., Kullman E.L., Austin K., Bennett B., Cerne S., Harmon C., Moore H., et al. A Community-Based Exercise and Support Group Program Improves Quality of Life in African-American Breast Cancer Survivors: A Quantitative and Qualitative Analysis. Int. J. Sports. Exerc. Med. 2015;1:020. doi: 10.23937/2469-5718/1510020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuller J.T., Hartland M.C., Maloney L.T., Davison K. Therapeutic effects of aerobic and resistance exercises for cancer survivors: A systematic review of meta-analyses of clinical trials. Br. J. Sports Med. 2018;52:1311. doi: 10.1136/bjsports-2017-098285. [DOI] [PubMed] [Google Scholar]
- 44.Padilha C.S., Testa M.T., Marinello P.C., Cella P.S., Voltarelli F.A., Frajacomo F.T., Cechini R., Duarte J.A.R., Guarnier F.A., Deminice R. Resistance Exercise Counteracts Tumor Growth in Two Carcinoma Rodent Models. Med. Sci. Sports Exerc. 2019;51:2003–2011. doi: 10.1249/MSS.0000000000002009. [DOI] [PubMed] [Google Scholar]
- 45.Wirtz P., Baumann F.T. Physical Activity, Exercise and Breast Cancer—What Is the Evidence for Rehabilitation, Aftercare, and Survival? A Review. Breast Care. 2018;13:93–101. doi: 10.1159/000488717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data, dependent variables and participant characteristics may be available for reasonable research purposes via individual request to the authors.