Abstract
For patients with Mantle Cell Lymphoma (MCL), there is no recognized standard of care for relapsed/refractory (R/R) disease after treatment with a Bruton’s tyrosine kinase inhibitor (BTKi). Brexucabtagene autoleucel (brexu-cel) represents a promising new treatment modality in MCL. We explored whether brexu-cel was cost-effective for the treatment of R/R MCL. We developed a partitioned survival mixture cure approach to model the costs and outcomes over a lifetime horizon. The clinical data were derived from the ZUMA-2 clinical trial. The costs were estimated from the publicly available Canadian databases, published oncology literature, and pan-Canadian Oncology Drug Review economic guidance reports. The health state utilities were sourced from the ibrutinib submission to the National Institute for Health and Care Excellence for R/R MCL and supplemented with values from the published oncology literature. In the base case over a lifetime horizon, brexu-cel generated an incremental 9.56 life-years and an additional 7.03 quality-adjusted life-years compared to BSC, while associated with CAD 621,933 in additional costs. The resultant incremental cost-utility ratio was CAD 88,503 per QALY gained compared with BSC. Based on this analysis, we found brexu-cel to be a cost-effective use of healthcare resources relative to BSC for treatment of adult patients with R/R MCL previously treated with a BTKi in Canada, though additional research is needed to confirm these results using longer follow-up data.
Keywords: chimeric antigen receptor T cell therapy, gene therapy, cost-effectiveness
1. Introduction
Mantle cell lymphoma is a frequently aggressive subtype of non-Hodgkin lymphoma (NHL), estimated to account for 2–6% of newly diagnosed cases of NHL [1,2,3,4]; in Canada, there are approximately 1500 prevalent cases of MCL, and around 400 new cases diagnosed each year [5].
Patients with MCL typically present with generalized lymphadenopathy and extranodal involvement of the blood, bone marrow, and spleen [2]. Patients are often diagnosed with advanced disease (Stage III or IV), which is characterized by an aggressive clinical course and a poor prognosis [2,6,7]. The 5-year relative survival in MCL is estimated at 30–60% and is the lowest among the different NHL subtypes [6,8]. Although the response rates to frontline chemoimmunotherapy treatments are high, most patients eventually relapse and thus need additional therapy [9,10]. In addition, a proportion of patients have disease which is refractory to initial treatment [9,10,11,12,13,14]. Collectively, these patients are referred to as having relapsed or refractory (R/R) MCL. For patients who are R/R to frontline treatments, treatment options include further chemoimmunotherapy, a Bruton’s tyrosine kinase inhibitor (BTKi) or an allogeneic stem cell transplant (allo-SCT) in rare cases where possible and appropriate; the choice of treatment is influenced by the response duration to frontline therapy, comorbidities, patient age, and overall risk–benefit evaluations [2]. Most patients in Canada will receive a BTKi. However, there are no recognized standard of care options for R/R MCL patients after treatment with a BTKi. Poor outcomes have been observed in R/R MCL patients experiencing disease progression following treatment with BTKi therapy; even with subsequent treatment, the survival outcomes are poor, with a median overall survival (OS) of 1 year or less [9,10,11,12,13,14].
The absence of effective therapies for use after BTKi therapy is reflected in the current treatment guidelines, which do not include clear recommendations for treatment beyond second-line therapy [2,3,15]. There is thus a need for a novel therapeutic for patients previously treated with a BTKi. Brexucabtagene autoleucel (brexu-cel) is an autologous chimeric antigen receptor T cell (CAR T) therapy that may offer an effective treatment option for these patients. The clinical efficacy of brexu-cel was demonstrated in the phase II ZUMA-2 clinical trial. In the primary efficacy analysis, 57% of patients who received a single infusion of brexu-cel were in remission, and median OS and median PFS were not reached at a median follow-up of 12.3 months [16]. Brexu-cel received a Notice of Compliance from Health Canada in 2021 and is approved by the Food and Drug Administration (FDA); conditional authorization has also been granted by the European Medicines Agency [17,18]. In addition, brexu-cel has recently received positive reimbursement recommendations in both Canada and the UK [19,20].
Using data from the ZUMA-2 trial supplemented with the published oncology literature, this analysis explores, from a Canadian health care system perspective, the cost-effectiveness of brexu-cel versus best supportive care (BSC) for the treatment of adult patients with R/R MCL previously treated with a BTKi.
2. Materials and Methods
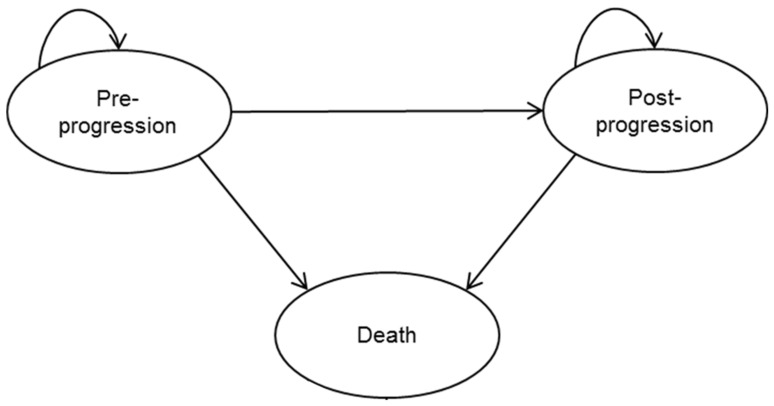
In order to evaluate the cost-effectiveness of brexu-cel versus BSC for adult patients with R/R MCL over a lifetime horizon from a Canadian healthcare system perspective, an economic model was developed in Microsoft Excel (Microsoft Inc., Redmond, WA, USA) using a three-health state, partitioned survival model (PSM) (Figure 1). The clinical data for overall and progression-free survival (PFS) for brexu-cel were derived from the ZUMA-2 [16] study. In the absence of comparative randomized control trial (RCT) data, a historical control arm was constructed from a meta-analysis of studies that evaluated subsequent treatments in MCL patients who had been treated with a BTKi [21]. Survival estimates were extrapolated beyond the duration of the clinical studies using standard parametric fitted curves. A partitioned survival mixture cure modeling approach [22,23,24,25,26] was taken to characterize the potential to achieve long-term durable remissions with CAR T therapy in R/R MCL. Healthcare resource utilization and adverse event data were based on data from ZUMA-2 and validated through a series of structured interviews with Canadian clinical experts. The costs, reported in 2021 Canadian dollars, were derived from the publicly available Canadian cost databases and the published oncology literature. The future costs and future outcomes were discounted at 1.5% per year [27].
Figure 1.
Model structure.
The primary outcome was the incremental cost-utility ratio (ICUR). Sensitivity analyses were conducted to assess the robustness of the results. A cycle length of 1 month (30.4375 days) was applied. The model inputs and data sources are summarized in Table 1.
Table 1.
Summary of modeling approach.
| Elements | Description |
|---|---|
| Target population | ZUMA-2 trial population (R/R mantle cell lymphoma following treatment with a BTKi |
| Treatments | Brexucabtagene autoleucel vs. BSC |
| Model design | Partitioned survival mixture cure model for brexucabtagene autoleucel Partitioned survival model for BSC |
| Model inputs | Efficacy (PFS and OS), safety Utility values Treatment-related costs, disease-related costs, end-of-life costs |
| Outcomes of interest |
Costs by category LYs and QALYs Incremental costs, incremental LYs, incremental QALYs Incremental cost/LY and cost/QALY gained |
| Perspective | Canadian healthcare system perspective |
| Health states | Pre-progression survival Post-progression survival Death |
| Time horizon | Canadian healthcare system perspective |
| Discount | 1.5% per year for both costs and outcomes |
| Cycle length | 1 month |
| Year of cost and currency |
2021 Canadian dollar |
| Sensitivity analysis | One-way deterministic sensitivity analyses Probabilistic sensitivity analyses Scenario analyses |
| Programming software |
Microsoft Excel 365 |
Abbreviations: BSC, Best supportive care; BTKi, Bruton’s tyrosine kinase inhibitor; LYs, Life-years; OS, overall survival; PFS, progression-free survival; QALY, quality-adjusted life-year; R/R, relapsed or refractory.
2.1. Target Population
The target population for the economic analyses is defined as adult patients with relapsed or refractory mantle cell lymphoma (MCL) after two or more lines of systemic therapy, including a BTKi, in line with the intent-to-treat (ITT) patient population in the ZUMA-2 trial [16]. A total of 74 patients with confirmed R/R MCL were enrolled and underwent leukapheresis. Bridging therapy was administered at the investigator’s discretion, with the aim of ensuring the patient remained able to receive brexu-cel. Bridging therapy consisted of ibrutinib (560 mg daily) or acalabrutinib (100 mg twice daily) and/or dexamethasone (20–40 mg orally or IV daily for 1–4 days or an alternative corticosteroid) and was to be completed at least 5 days before the initiation of lymphodepletion chemotherapy. All patients received lymphodepletion chemotherapy, consisting of fludarabine (30 mg/m2 of body surface area [BSA] per day) and cyclophosphamide (500 mg/m2 of BSA per day), each given daily for 3 days (typically on Days −5 through −3 prior to receiving the CAR T cell infusion). Brexu-cel was successfully manufactured for 71 patients (96%) and administered to 68 patients (92%) and administered as a single intravenous infusion.
2.2. Comparators
As there is no recognized standard of care in R/R MCL, the primary comparator to brexu-cel is best supportive care (BSC), a blended comparator that includes multiple therapy options expressed as a single basket comparator with a single blended efficacy and safety profile, weighted by the proportion of patients expected to receive each therapy. In Canada, chemoimmunotherapy, such as bendamustine + rituximab, rituximab + cyclophosphamide + doxorubicin + vincristine + prednisone (R-CHOP), and R-DHAP, is most commonly used, with other therapies, including lenalidomide (+/− rituximab), bortezomib (+/− rituximab), and allogeneic transplant, being used less frequently.
2.3. Model Perspective
The model base case adopted a Canadian healthcare system perspective, which includes direct costs associated with the treatment and healthcare resource use of patients with R/R MCL.
2.4. Time Horizon, Discounting, and Cycle Length
Patients in the ZUMA-2 trial had a median age of 63.7 years, and the trial population included patients as young as 38 years old. In order to ensure that all the costs and clinical benefits related to the intervention and comparators were accounted for, the time horizon was set to 50 years. This approach should be considered appropriate, given that brexu-cel may be associated with sustained transformation of the natural history of the disease. Discount rates were set to 1.5% per year for both the costs and the benefits, in line with Canadian Agency for Drugs and Technologies in Health (CADTH) guidelines for the conduct of economic evaluations of health technologies [28].
2.5. Model Structure and Approach
2.5.1. Partitioned Survival Model
A partitioned survival model (PSM) with three health states (pre-progression, post-progression, and death) was selected as the model structure as it is widely used in oncology modeling and previous assessments of CAR T therapy (Figure 1) [29,30]. Costs and utility values were applied to each health state. To achieve a balance between the sensitivity and the complexity of the model along with consistency with previous analyses, a cycle length of 1 month (30.44 days) was implemented.
2.5.2. Partitioned Survival Mixture Cure Model
In previous CAR T clinical trials with longer-term follow-up available, a strong response dichotomy is consistently observed between CAR T treatments and their historic cohort comparators [31,32,33,34]. Mixture models allow the capture of this aspect of the data more clearly than in standard parametric models and thus better reflect the extrapolated clinical outcomes in a cost-effectiveness model. To capture the heterogeneity in population and outcomes in a partitioned survival framework, a partitioned survival mixture cure model (PS-MCM) approach was implemented which stratified the analysis according to a ‘functionally cured’ group and a ‘non-cured’ group [22]. While longer term trial evidence is required to substantiate this stratification, the `functionally cured’ patients are assumed to approach the age- and sex-adjusted mortality rates depicted in the Canadian life tables (2016–2018) [35,36]. The ‘non-cured’ patients are subject to cancer-specific hazards which are modelled and estimated using standard parametric functions.
2.6. Survival Estimates for Brexu-Cel
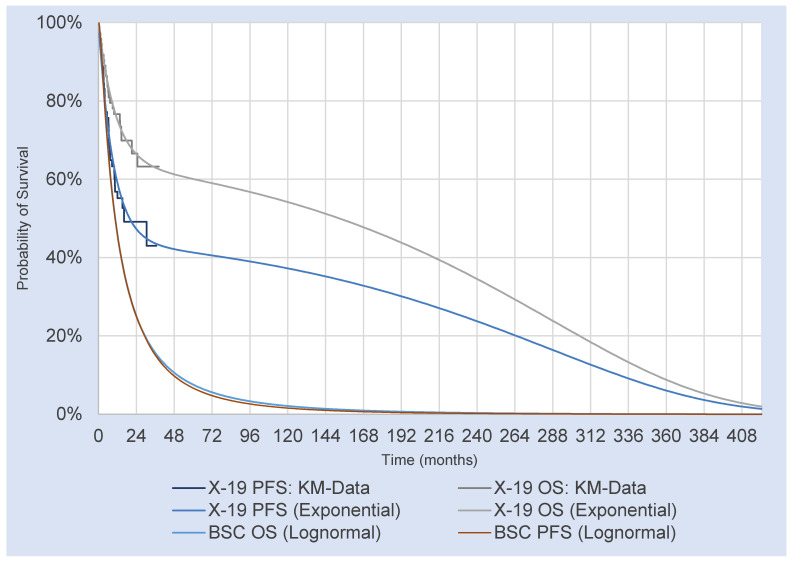
The OS and PFS Kaplan–Meier (KM) data used to inform the drug efficacy parameters were sourced from the ZUMA-2 primary efficacy analysis (data cut-off: July 24, 2019; median follow-up: 12.3 [range:7.0–32.3] months). Visual fit, statistical fit, and clinical plausibility were all considered when assessing the plausibility of different modeling and extrapolation approaches for OS and PFS, in accordance with the National Institute for Health and Care and Excellence (NICE) Decision Support Unit (DSU) 14 [37]. The initial parametric modeling of brexu-cel OS and PFS were performed by fitting the distributions recommended by CADTH for PSMs to the ZUMA-2 time-to-event data using maximum likelihood estimation [27,37]. The curves were selected based on the Akaike information criterion (AIC), Bayesian information criterion (BIC), face validity of the fit with the data and shape of the extrapolation, and clinical plausibility of the survival extrapolations. Overall survival for both treatment arms in the model was capped based on the general population mortality data taken from Canadian Life Tables 2016–2018 and adjusted for the patient characteristic profile of the ZUMA-2 ITT population [35].
2.7. Survival Estimates for Best Supportive Care
To model the efficacy parameters for the BSC comparator, a meta-analysis was conducted based on a systematic literature review to identify the studies that evaluated subsequent treatments in MCL patients who had been treated with BTKi [38]. The meta-analyses identified three studies for OS [9,10,13] and one study for PFS [10] that included relevant comparators, including various chemo-immunotherapies or systemic treatments, such as rituximab, bendamustine, and cytarabine (R-BAC). The parametric survival functions were fitted to reconstructed individual patient data (IPD) using the algorithm developed by Guyot et al., 2012 [39], from each relevant study. The parameters from the best fitting distribution were then pooled in a random effects meta-analysis model to provide an estimate of the absolute treatment effects in terms of OS and PFS.
2.8. Cost and Resource Use
All the costs presented and used in this analysis were adjusted for inflation to 2021 Canadian dollars [40]. A detailed breakdown of the costs is presented in Appendix A.
2.9. Drug Administration
The administration of BSC and lymphodepletion chemotherapies is assumed to include all the costs associated with the outpatient administration of chemotherapy, including the cost of physician services. Subsequent therapies were modelled using a basket approach: the costs of each comparator were weighted by the expected proportion of patients expected to receive each therapy, as per the approach taken in the cost-effectiveness model submitted to pCODR for the review of ibrutinib for MCL [5].
2.10. End-of-Life Costs
The cost of death was included for both treatment arms at an average cost of CAD 35,262 (2021 CAD dollars; inflated from CAD 24,015 in 2003 CAD dollars) based on a study of palliative services by patients in Ontario between 2002 and 2003 for adults who died with cancer [41].
2.11. Brexu-Cel Specific Treatment Costs
The costs associated with brexu-cel treatment included leukapheresis, lymphodepletion chemotherapy, bridging therapy, the acquisition cost of brexu-cel, and cell infusion and monitoring. For simplicity, all the administration costs associated with brexu-cel were assumed to be incurred in the first model cycle. Bridging therapy in ZUMA-2 was administered to patients at the discretion of the treating investigator: 36.8% of patients received bridging therapy, which included ibrutinib, acalabrutinib, or dexamethasone [42]. As acalabrutinib is not available for the treatment of MCL in Canada, it was assumed that ibrutinib and dexamethasone were the only therapies used for bridging. Aligned with the dosing observed in ZUMA-2, the model assumed that patients received 560 mg daily of ibrutinib via IV and 40 mg of oral dexamethasone daily for 4 days. The unit costs are presented in Appendix A. The administration costs were taken from the Ontario Ministry of Health and Long-Term Care (MoHLTC) Schedule of Benefits for Physician Services code G359 [43]. Lymphodepletion chemotherapy in ZUMA-2 included intravenous infusions of cyclophosphamide 500 mg/m2 and fludarabine 30 mg/m2 on the 5th, 4th, and 3rd days prior to the infusion of brexu-cel. The unit costs for cyclophosphamide and fludarabine were taken from a prior pCODR Economic Guidance Report for Ibrutinib for Mantle Cell Lymphoma [5] (Appendix A). The costs of the chemotherapy were derived after calculating the optimal combination of the different vial sizes, assuming an average body surface area (BSA) of the patients in ZUMA-2 [42]. Lymphodepletion chemotherapy was assumed to be conducted in an out-patient setting. The administration cost was taken from the Ontario Ministry of Health and Long-Term Care (MoHLTC) Schedule of Benefits for Physician Services code G359 [43]. Leukapheresis for CAR T cell manufacturing was included based on the unit costs described in a Canadian study examining the safety and cost-effectiveness of autologous stem cell transplantation in patients with multiple myeloma within a Canadian environment [44]. The cost of stem cell apheresis (not including the costs of filgrastim) was used as proxy for the cost of mononuclear cell leukapheresis. The infusion of brexu-cel and the subsequent monitoring were assumed to incur the cost of an elective hospitalization. The mean length of stay observed in the ZUMA-2 trial for patients treated with brexu-cel was 16.5 days [42]. To cost this in the model, the weighted average cost per day of an inpatient hospitalization for malignant lymphoma (Canadian Institute for Health Information Case Mix Group 615) [45] was multiplied by the mean length of stay reported in ZUMA-2. A proportion of patients from ZUMA-2 receiving brexu-cel also required monitoring within an intensive care unit (ICU) [42]. Due to a paucity of available data, this proportion was assumed to be 22% based on the use of vasopressors as a proxy for ICU admission. This proportion of patients incurred a cost per ICU day that was multiplied by the mean length of stay reported in ZUMA-2. The cost per ICU day was based on a study by Zheng et al., 2020, that reports the cost of patients with cancer that are admitted to the ICU [46].
2.12. BSC Specific Treatment Costs
As the BSC arm is applied as a blended comparator based on the use of a mixture of treatment regimens, the costs associated with the treatment have been weighted according to the number of patients on each treatment in the included studies. The treatments not currently publicly funded in Canada were excluded, and the treatment proportions were reweighted based on feedback from clinical experts. Table 2 presents the distribution of treatments based on proportions derived from the meta-analysis and validated by Canadian clinical experts. The treatment duration for the treatments included in the BSC arm follows the treatment regimen for each individual treatment. The proportion of patients receiving each treatment and dosage in BSC is sourced from the treatment protocols and Canadian clinical experts [47,48,49,50,51,52,53,54].
Table 2.
Proportion of patients on each of BSC treatments.
| Treatment | Proportion of Patients on Intervention in Base Case (%) |
|---|---|
| Rituximab | 68.2% |
| Bendamustine | 57.4% |
| Bortezomib | 5.5% |
| Anthracycline-based | 7.3% |
| Total | 138.5% |
Note: Sum is more than 100% as patients can be given these drugs in combination.
2.13. Drug Acquisition
The treatment dose for BSC in Canada was calculated based on body surface area (BSA). Wastage was considered in this model and dose intensity was assumed to be 100%. The costs and dosing are presented in Appendix A.
Health State Resource Use and Costs
Medical resource use is dependent on progression status and was therefore modelled according to health state. The subsequent therapies received in the post-progression health state were not explicitly modeled as there were no data available to enable this analysis. Healthcare resource use in each health state was estimated based on input from Canadian clinical experts. It was also assumed that patients who remain progression-free for at least 5 consecutive years are deemed to be in long-term remission. Consequently, these patients were assumed to utilize fewer medical resources (Appendix A). The unit costs of the healthcare resources were sourced from the Ontario Ministry of Health and Long-Term Care Schedule of Benefits [43,55] and the CIHI Patient Cost Estimator (PCE) [45], where applicable (Appendix A).
2.14. Adverse Events
Adverse events (AEs) were only applied to the brexu-cel treatment arm, and AE costs were applied as a one-off cost in the first model cycle (Appendix A). Consistent with a previously conducted study [30], the management of all AEs other than CRS was assumed to include the cost of one excess bed day; this is assumed to be captured in the reported mean length of stay of 16.5 days for ZUMA-2 patients. It was further assumed that the costs of AEs are covered in the length of stay for brexu-cel patients during cell infusion and monitoring and therefore costing each AE individually would result in double counting. The management costs for CRS were calculated using the method from the ZUMA-2 clinical study report [42].
2.15. Utility Values
Given the absence of published utility values for R/R MCL patients post-BTKi and the sparsity of EuroQol 5D (EQ5D) data collected in ZUMA-2, the health state utility values for both pre-progression and post-progression were sourced from the ibrutinib NICE R/R MCL submission [56] (Table 3). Based on similar demographics and the use of these values in other similar CADTH submissions, the UK estimates by Ara and Brazier (2010) [57] were used as a proxy for long-term survivors who were assumed to have a utility equal to the baseline general population utility of a 67-year-old.
Table 3.
Estimated utility values from literature used in model base case.
| Health States | Value | Standard Error | Reference |
|---|---|---|---|
| Pre-progression | 0.780 | 0.010 | NICE ibrutinib, 2016 [56] |
| Pre-progression for long-term survivors | 0.812 | 0.010 | Calculated from Ara and Brazier, 2010 [57] |
| Post-progression | 0.680 | 0.024 | NICE ibrutinib, 2016 [56] |
Abbreviation: NICE: National Institute for Health and Clinical Excellence.
3. Results
3.1. Deterministic Analysis
In the base case analysis over a lifetime horizon of 50 years, brexu-cel generated a total of 11.26 discounted life-years (LYs) compared to 1.70 LYs for BSC, leading to an incremental gain of 9.56 LYs (Table 4). In terms of QALYs, brexu-cel generated 8.34 QALYs compared to 1.31 QALYs for BSC, yielding an incremental gain of 7.03 QALYs (discounted). The total discounted costs were higher for brexu-cel (CAD 699,202) than for BSC (CAD 65,847), which led to an incremental cost difference of CAD 633,355 and an ICUR of CAD 88,503 per QALY gained.
Table 4.
Disaggregated deterministic results of brexucabtagene autoleucel vs. BSC.
| Brexucabtagene Autoleucel | Literature-Based Meta-Analysis | Incremental | |
|---|---|---|---|
| Median survival (years) | 12.71 | 0.88 | 11.83 |
| Total undiscounted years | 13.22 | 1.76 | 11.46 |
| Pre-progression | 9.30 | 1.68 | 7.63 |
| Post-progression | 3.92 | 0.09 | 3.83 |
| Total discounted years | 11.26 | 1.70 | 9.56 |
| Pre-progression | 7.95 | 1.63 | 6.33 |
| Post-progression | 3.31 | 0.08 | 3.23 |
| Total discounted QALYs | 8.34 | 1.31 | 7.03 |
| Pre-progression | 6.23 | 1.27 | 4.97 |
| Pre-Progression, pre-cure point | 1.95 | 1.11 | 0.85 |
| Pre-Progression, post-cure point | 4.28 | 0.16 | 4.12 |
| Post-progression | 2.14 | 0.05 | 2.09 |
| Adverse events | −0.04 | −0.01 | −0.03 |
| Total discounted costs | CAD 688,040 | CAD 66,108 | CAD 621,933 |
| Total treatment-related costs | CAD 589,375 | CAD 27,946 | CAD 561,429 |
| Total drug acquisition | CAD 533,523 | CAD 27,221 | CAD 506,302 |
| Total apheresis | CAD 1392 | CAD 0 | CAD 1392 |
| Total drug administration | CAD 211 | CAD 726 | CAD −515 |
| Total lymphodepletion chemotherapy | CAD 646 | CAD 0 | CAD 646 |
| Total bridging therapy | CAD 220 | CAD 0 | CAD 220 |
| Total hospitalization | CAD 53,383 | CAD 0 | CAD 3383 |
| Total disease management | CAD 57,739 | CAD 2490 | CAD 55,249 |
| Pre-progression | CAD 3939 | CAD 1225 | CAD 2714 |
| Post-progression | CAD 53,800 | CAD 1264 | CAD 52,535 |
| Other costs | CAD 40,926 | CAD 35,671 | CAD 5255 |
| End of life care | CAD 29,582 | CAD 34,589 | CAD −5007 |
| Adverse events | CAD 11,344 | CAD 1082 | CAD 10,262 |
| Cost/QALY | CAD 88,503 |
Abbreviations: BSC, best supportive care; QALYs: quality-adjusted life-years.
3.2. Probabilistic Sensitivity Analysis
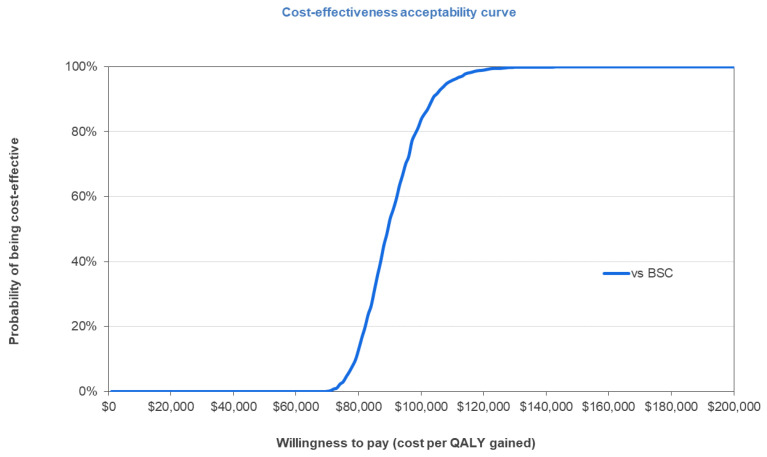
Using 1000 Monte Carlo simulations, over a lifetime horizon of 50 years, brexu-cel was associated with higher mean total QALYs (mean incremental QALYs of 7.00) with a higher mean total cost (mean incremental cost of CAD 621,571) when compared with BSC among patients with R/R MCL who had received prior treatment with a BTKi, resulting in an ICER of CAD 88,814 per QALY gained and corroborating the results of the deterministic base case analysis. Brexu-cel was found to be cost-effective in 82% of the simulations at the commonly cited willingness-to-pay (WTP) threshold of 100,000 CAD/QALY for oncology products in Canada (Figure 2) [58].
Figure 2.
Cost-utility acceptability curve of 1000 simulations. Abbreviations: BSC, best supportive care; QALY, quality-adjusted life year.
3.3. Univariate Sensitivity Analysis
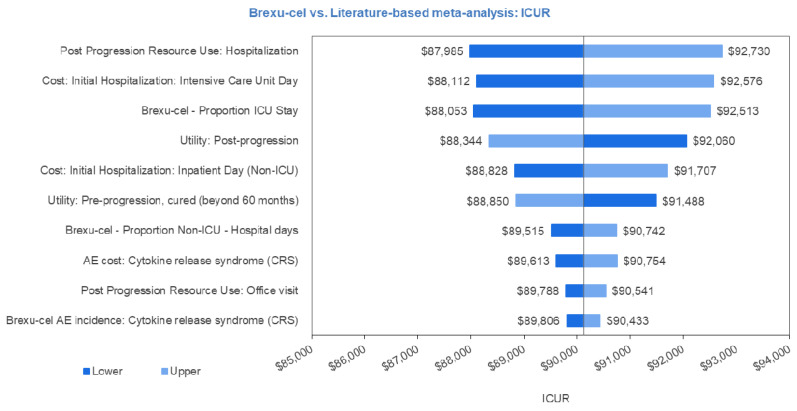
To characterize the uncertainty associated with individual input parameter values, a univariate sensitivity analysis was conducted in which each parameter was independently adjusted to its respective lower and upper ranges. The results of the 10 most influential parameters on the ICUR are plotted onto a tornado diagram presented in Figure 3. The most influential parameters were those around the cost and length of stay of hospitalizations and the utility values of pre- and post-progression.
Figure 3.
Univariate sensitivity analysis tornado plot. Abbreviations: AE, adverse event; ICU, intensive care unit.
3.4. Scenario Analysis
A special interest scenario analysis was also conducted in which the post-progression survival and post-progression costs associated with treatment with brexu-cel were set exactly equal to BSC. The results of this analysis yielded an ICER of CAD 115,396 per QALY gained.
4. Discussion
In this study, we show that brexu-cel is a potentially cost-effective use of medical resources compared with best supportive care in Canada. Our analysis was conducted in line with the approved Health Canada indication for brexu-cel, and we used clinical data from the ZUMA-2 trial to populate the model and included comparators that were reflective of current Canadian clinical practice. In order to examine the plausibility of comparing the available meta-analysis results with the outcomes observed in ZUMA-2, various adjustments were explored to support the comparability of these two sources of evidence.
The published evidence has suggested that patients with DLBCL who achieve event-free survival at 24 months after frontline therapy have a subsequent OS similar to that of the age- and sex-matched general population [36]; however, this was not deemed transferable to a relapsed/refractory MCL population a priori. We therefore felt that 5 years of progression-free survival was a more realistic assumption for when patients could potentially achieve OS similar to the age- and sex-matched general population, as suggested in previous studies [59]. This further supports the validity of our results and helps to diminish the uncertainty inherent in parametric survival extrapolations.
In Canada, both the Institut national d’excellence en santé et services sociaux (INESSS), the HTA body responsible for the province of Quebec, and CADTH have published recommendations for public funding of brexu-cel, conditional upon carrying out additional clinical follow-up and mitigation of the economic burden. INESSS suggested a base case ICUR of between 151,390 CAD/QALY and 338,510 CAD/QALY gained [60], and CADTH was unable to determine a base case ICUR21. In the UK, NICE reported a base case ICUR of 46,898 GBP/QALY gained [20]. A US study from 2021 evaluated the cost-effectiveness of brexu-cel compared with best supportive care [61]. Using a US payer perspective, the authors concluded that brexu-cel was cost-effective with a base case ICER estimated to be 31,985 USD/QALY gained. While there is no explicit willingness-to-pay threshold in Canada, 100,000 CAD/QALY gained is commonly cited for oncology products [58]. A lower (50,000 CAD/QALY) threshold, which is often used for non-oncology products in Canada, or a higher (150,000 USD/QALY) threshold, which is used by I.C.E.R. in the United States, could be applied to our analysis. However, the 100,000 CAD/QALY gained threshold may be the most appropriate for comparison with previous oncology cost-effectiveness research in Canada [58]. The assessment details described above demonstrate that cost-effectiveness results often differ across regions and HTA agencies, due largely to differences in healthcare systems and reimbursement submission requirements and can lead to potentially divergent recommendations. These differences highlight the need for jurisdiction-specific estimates of cost-effectiveness. In addition, not all HTA agencies publish the full details of their assessments, underscoring the need for peer-reviewed analyses to be available in the public domain.
A key limitation of the economic analysis is the lack of randomized control trial evidence comparing brexu-cel against BSC. While a direct comparison of results between randomized treatment arms may minimize bias and is ideal for establishing comparative efficacy, given the lack of a recognized standard of care in the post-BTKi setting and the inherent problem of clinical equipoise in comparing CAR T to commonly used regimens known to have poor outcomes in this MCL patient population, a phase III trial may not be methodologically or ethically feasible. In addition, while this is a limitation common to most, if not all clinical trial data, the clinical environment of ZUMA-2 was highly controlled, and therefore, the results of the trial may not be representative of patient experience in the real-world setting. However, recent RWE studies of CAR T therapies in DLBCL have suggested that adverse events, response rates, and efficacy are similar in the real-world setting [32,62]. The current analysis also lacks EQ5D data from ZUMA-2. Despite this shortcoming, a commonly accepted alternative approach was followed in which the utility values were derived from the published oncology literature in MCL [56].
Another limitation is that the potential use of further therapy after relapse following CAR T cell administration was not included in model, as it is not clear how frequently such intervention occurs. Due to a lack of data, subsequent therapies received in the post-progression health state were not explicitly modeled. However, the impact of this assumption is very likely to be minimal, given the lack of effective therapies available in the pre-progression health state and the fact that the therapies available for post-progression treatment are likely to be similar between the two treatment arms. The results from a single-centre case series study from the US suggest that a small subset of patients may receive a range of subsequent salvage therapies following brexu-cel, which can include chemo-immunotherapy with/without local radiation, venetoclax, acalabrutinib, copanlisib, and abemaciclib [63].
A further limitation concerns the immaturity of the ZUMA-2 trial data used in this analysis. The model estimated long-term survival based on survival estimates with a median follow-up of at least 12 months, and while neither median PFS nor median OS was met by this time, the results are broadly in line with previous trials in CAR T [64,65], which supports the present analyses. However, as a majority of the time horizon in the model was based on extrapolated data, the associated uncertainty and results should be interpreted with caution.
The recent regulatory approval of brexu-cel in the US, EU, and Canada highlights the promising impact of this novel therapy for R/R MCL patients. While clinical and economic uncertainty are inherent in economic modeling exercises, the results of our base case analysis suggest that brexu-cel may be a cost-effective use of medical resources compared with best supportive care in Canada. However, additional research is required to confirm our results, as residual uncertainty around clinical outcomes for patients post-brexu-cel, illustrated in the special interest sensitivity analysis, warrant further assessment with longer follow-up data. This will inform even more robust future cost-effectiveness analyses. Future studies could also examine the frequency with which relapsing patients receive available subsequent therapies to further build on these findings.
Appendix A
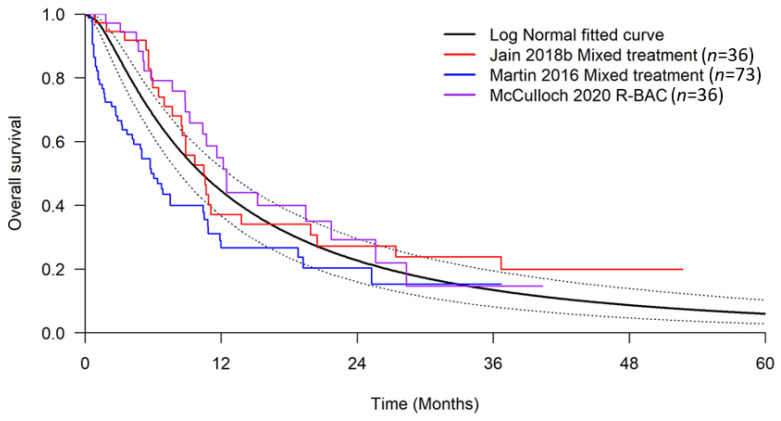
Figure A1.
Random effects meta-analysis (log-normal model) of overall survival Kaplan–Meier curves; studies with mixed treatments or R-BAC. Abbreviations: R-BAC = Rituximab-Bendamustine Cytarabine.
Figure A2.
Mixture cure parametric model extrapolations fitted to overall survival and progression-free survival data of brexucabtagene autoleucel (data from ZUMA-2 trial) and BSC (data from meta-analysis). Data derived from ZUMA-2 trial (Brexu-cel) and meta-analysis (BSC). Abbreviations: PFS: progression-free survival; OS: overall survival; ITT: intent-to-treat population; KM: Kaplan–Meier; PS-MCM: partitioned survival mixed cure model.
Table A1.
Summary of health state resource use frequency, based on Canadian Clinical Expert feedback.
| Resource | Progression-Free | Progression-Free Post-5 Years | Progressed | ||||
|---|---|---|---|---|---|---|---|
| % of Patients |
Frequency per Cycle | % of Patients |
Frequency per Cycle | % of Patients |
Frequency per Cycle | ||
| Physician visits | Specialist visit | 100% | 0.33 | 100% | 0.17 | 100% | 1.00 |
| Laboratory tests | Complete Blood Count | 100% | 0.33 | 0% | 0.00 | 100% | 1.00 |
| Lactate Dehydrogenase | 100% | 0.33 | 0% | 0.00 | 100% | 1.00 | |
| Blood glucose | 100% | 0.33 | 0% | 0.00 | 100% | 1.00 | |
| Radiology | CT scan | 100% | 0.33 | 0% | 0.00 | 100% | 0.33 |
| X-ray | 100% | 0.17 | 0% | 0.00 | 100% | 0.33 | |
| Hospitalization | 0% | 0.00 | 0% | 0.00 | 100% | 0.08 | |
Abbreviations: CT, computed tomography.
Table A2.
Extended cost table, including unit cost per medical resource.
| Resource | Unit Cost | Reference |
|---|---|---|
| Specialist visit | CAD 157.00 | Ontario MoHLTC Schedule of Benefits—Physician Services, Haematology Consultation [43] |
| Complete blood count | CAD 3.98 | Ontario MoHLTC Schedule of Benefits—Laboratory Services, Hematology—CBC [55] |
| Lactate dehydrogenase | CAD 1.28 | Ontario MoHLTC Schedule of Benefits—Laboratory Services, Lactate Dehydrogenase [55] |
| Blood glucose | CAD 1.28 | Ontario MoHLTC Schedule of Benefits—Laboratory Services, C-reactive Protein [55] |
| CT scan (abdominal, thorax) | CAD 195.00 | Ontario MoHLTC Schedule of Benefits—Physician Services, CT abdomen and thorax, with and without IV contrast. [43] |
| X-ray | CAD 32.25 | Ontario MoHLTC Schedule of Benefits—Physician Services, Skeletal survey studies; assumed 3 views. [43] |
| Hospitalization | CAD 12,756.57 | CIHI Patient Cost Estimator [45] |
| IV administration | CAD 54.25 | Ontario Schedule of Benefits for Physician Services. [43] |
| Conditional chemotherapy administration | CAD 105.15 | Ontario Schedule of Benefits for Physician Services. [43] |
| Palliative care (one-off) | CAD 34,037 | Walker et al. 2011. [41] |
| Office visit | CAD 157.00 | Ontario Schedule of Benefits for Physician Services. [43] |
| Brexucabtagene autoleucel one-time treatment cost | CAD 533,523.10 | Kite list price |
| Brexucabtagene autoleucel administration | CAD 185.00 | Ontario Schedule of Benefits for Physician Services. [43] |
| Apheresis | CAD 1343.98 | Holbro et al. 2013. [44]. |
| Adverse event: cytokine release syndrome | CAD 18,366.96 | Cost of 6 days of tocilizumab treatment and 11 days hospitalized. Fifty-nine percent of patients were treated with tocilizumab. Cost per hospital day is weighted average of cost per inpatient day from CIHI patient cost estimator. [45] |
Abbreviations: CIHI, Canadian Institute for Health Information; CT, computed tomography; MoHLTC, Ministry of Health and Long-Term Care.
Table A3.
Unit drug costs.
| Resource | Unit Cost | Reference |
|---|---|---|
| Rituximab 100 mg | CAD 482.31 | Ontario Exceptional Access Program [47] |
| Bendamustine 25 mg | CAD 312.50 | pCODR Economic Guidance Report for Bendamustine [48] |
| Lenalidomide 25 mg | CAD 424.00 | Ontario Exceptional Access Program [47] |
| Lenalidomide 20 mg | CAD 403.00 | Ontario Exceptional Access Program [47] |
| Lenalidomide 15 mg | CAD 382.00 | Ontario Exceptional Access Program [47] |
| Lenalidomide 10 mg | CAD 361.00 | Ontario Exceptional Access Program [47] |
| Lenalidomide 5 mg | CAD 340.00 | Ontario Exceptional Access Program [47] |
| Lenalidomide 2.5 mg | CAD 329.00 | Ontario Exceptional Access Program [47] |
| Bortezomib 3.5 mg | CAD 1402.42 | pCODR Economic Guidance Report for Daratumumab [49] |
| Anthracycline 1 mg | CAD 5.05 | pCODR Economic Guidance Report for Pertuzumab-Trastuzumab [50] |
| Fludarabine 50 mg | CAD 255.00 | pCODR Economic Guidance Report for Ibrutinib [5] |
| Cyclophosphamide 1g | CAD 52.06 | pCODR Economic Guidance Report for Ibrutinib [5] |
| Ibrutinib 140 mg | CAD 97.60 | Ontario Exceptional Access Program [47] |
| Dexamethasone 4 mg | CAD 0.30 | Ontario Drug Benefit Formulary [66] |
| Tocilizumab 20 mg | CAD 182.80 | Ontario Exceptional Access Program [47] |
Abbreviations: MoHLTC, Ministry of Health and Long-Term Care; pCODR, pan-Canadian Oncology Drug Review.
Table A4.
Dosing of best supportive care therapies.
| Chemotherapy | Admin Route | mg/m2/day | Frequency | mg/Unit | Cost/Unit | Source |
|---|---|---|---|---|---|---|
| Rituximab | IV | 375 | Q4W for 6 cycles [54] | 100 | 482.31 | Ontario EAP [47] |
| Bendamustine | IV | 70 | Q4W 2 days for 6 cycles [54] | 25 | 312.50 | pCODR economic review of Bendamustine [48] |
| Lenalidomide | Oral | 25 | 21 days on, 7 off [53] | 25 | 424.00 | Ontario EAP [47] |
| Bortezomib | IV | 1.3 | Q3W 4 days, 9 cycles [51] | 3.5 | 1402.42 | pCODR economic review of Daratumumab [49] |
| Anthracycline | IV | 50 | Q3W [52] | 1 | 5.05 | pCODR economic review of Pertuzumab-Trastuzumab [50] |
Abbreviations: BSC, best supportive care; EAP, Exceptional Access Program; IV, intravenous; pCODR, pan-Canadian Oncology Drug Review; Q3W, every 3 weeks; Q4W, every 4 weeks.
Table A5.
Hospitalization of Patients.
| Item | Value | S.E. | Source |
|---|---|---|---|
| Proportion of brexu-cel patients who visit ICU | 22.7% | ZUMA-2 Clinical Study Report [38] | |
| % of BSC patients who visit ICU | 0 | 0 | ~ |
| Average duration in ICU | 21.2 days | 1.80 | ZUMA-2 Clinical Study report [38] |
| Total hospitalization cost | CAD 63,758.81 | 0 | ~ |
Abbreviation: BSC, best supportive care.
Table A6.
Adverse event rates from ZUMA-2.
| Incidence | ||
|---|---|---|
| Adverse Events | Brexucabtagene Autoleucel (%) (se) |
BSC (%) (se) |
| Cytokine release syndrome (CRS) Grade ≥2 | 62 (6) | 0 (0) |
| Pyrexia | 13 (4) | 0 (0) |
| Anemia | 50 (6) | 0 (0) |
| Platelet count decreased | 38 (6) | 0 (0) |
| Hypotension | 22 (5) | 0 (0) |
| Neutrophil count decreased | 50 (6) | 0 (0) |
| White blood cell count decreased | 40 (6) | 0 (0) |
| Hypoxia | 21 (5) | 0 (0) |
| Hypophosphatemia | 22 (5) | 0 (0) |
| Neutropenia | 34 (6) | 0 (0) |
| Hyponatremia | 10 (4) | 0 (0) |
| ALT increased | 9 (3) | 0 (0) |
| Encephalopathy | 19 (5) | 0 (0) |
| Hypokalemia | 7 (3) | 0 (0) |
| Hypocalcemia | 6 (3) | 0 (0) |
| Thrombocytopenia | 16 (4) | 0 (0) |
| AST increased | 10 (4) | 0 (0) |
| Confusional state | 12 (4) | 0 (0) |
| Hyperglycemia | 6 (3) | 0 (0) |
| Hypertension | 13 (4) | 0 (0) |
| Acute Kidney Injury | 7 (3) | 0 (0) |
| Leukopenia | 13 (4) | 0 (0) |
| Lymphocyte count decreased | 9 (3) | 0 (0) |
| Pneumonia | 9 (3) | 0 (0) |
| Respiratory Failure | 6 (3) | 0 (0) |
| Sepsis | 6 (3) | 0 (0) |
Abbreviations: ALT: alanine aminotransferase; AST aspartate aminotransferase; BSC, best supportive care; SE, standard error.
Table A7.
Disaggregated cost and outcomes of brexucabtagene autoleucel vs. BSC based on 1000 iterations.
| Brexucabtagene Autoleucel | BSC | Incremental | |
|---|---|---|---|
| Total discounted years | 11.21 | 1.72 | 9.49 |
| Pre-progression | 8.01 | 1.50 | 6.51 |
| Post-progression | 3.20 | 0.22 | 2.98 |
| Total discounted QALYs | 8.31 | 1.31 | 7.00 |
| Pre-progression | 6.28 | 1.17 | 5.11 |
| Pre-progression, pre-cure point | 1.99 | 1.03 | 0.95 |
| Pre-progression, post-cure point | 4.29 | 0.14 | 4.15 |
| Post-progression | 2.07 | 0.14 | 1.92 |
| Adverse events | −0.04 | −0.01 | −0.03 |
| Total discounted costs | CAD 689,636 | CAD 68,066 | CAD 621,571 |
| Total treatment-related costs | CAD 592,182 | CAD 27,701 | CAD 564,480 |
| Total drug acquisition | CAD 533,523 | CAD 26,989 | CAD 506,534 |
| Total apheresis | CAD 1374 | CAD 0 | CAD 1374 |
| Total drug administration | CAD 211 | CAD 713 | CAD −502 |
| Total lymphodepletion chemotherapy | CAD 646 | CAD 0 | CAD 646 |
| Total bridging therapy | CAD 222 | CAD 0 | CAD 222 |
| Total hospitalization | CAD 56,206 | CAD 0 | CAD 56,206 |
| Total disease management | CAD 56,500 | CAD 4692 | CAD 51,808 |
| Pre-progression | CAD 3996 | CAD 1145 | CAD 2851 |
| Post-progression | CAD 52,504 | CAD 3547 | CAD 48,957 |
| Other costs | CAD 40,954 | CAD 35,672 | CAD 5282 |
| End-of-life care | CAD 29,603 | CAD 34,591 | CAD −4988 |
| Adverse events | CAD 11,351 | CAD 1081 | CAD 10,270 |
| Cost/QALY | CAD 88,814 |
Abbreviations: BSC, best supportive care; LYs, life-years; QALYs, quality-adjusted life-years.
Table A8.
Parameter values varied in deterministic sensitivity analyses.
| Parameter | Original Value | Lower Limit | Upper Limit |
|---|---|---|---|
| Patient Characteristics | |||
| Bodyweight | 81.8000 | 77.9723 | 85.6277 |
| BSA | 1.9780 | 1.9251 | 2.0309 |
| Resource Use | |||
| Pre-Progression Resource Use: Full blood count | 0.3333 | 0.2157 | 0.4761 |
| Pre-Progression Resource Use: X-ray | 0.1667 | 0.1079 | 0.2381 |
| Pre-Progression Resource Use: Blood glucose | 0.3333 | 0.2157 | 0.4761 |
| Pre-Progression Resource Use: Lactate dehydrogenase | 0.3333 | 0.2157 | 0.4761 |
| Pre-Progression Resource Use: CT Scan | 0.1667 | 0.1079 | 0.2381 |
| Pre-Progression Resource Use: Office visit | 0.1667 | 0.1079 | 0.2381 |
| Pre-Progression Cured: Resource Use: Office visit | 0.1667 | 0.1079 | 0.2381 |
| Post-Progression Resource Use: Full blood count | 1.0000 | 0.6471 | 1.4284 |
| Post-Progression Resource Use: X-ray | 0.3333 | 0.2157 | 0.4761 |
| Post-Progression Resource Use: Blood glucose | 1.0000 | 0.6471 | 1.4284 |
| Post-Progression Resource Use: Lactate dehydrogenase | 1.0000 | 0.6471 | 1.4284 |
| Post-Progression Resource Use: Office visit | 1.0000 | 0.6471 | 1.4284 |
| Post-Progression Resource Use: CT Scan | 0.3333 | 0.2157 | 0.4761 |
| Post-Progression Resource Use: Hospitalization | 0.0833 | 0.0539 | 0.1190 |
| End-of-life Resource Use: Palliative care (one-off) | 1.0000 | 0.6471 | 1.4284 |
| Brexucabtagene autoleucel—Proportion ICU Stay | 0.2200 | 0.1402 | 0.3119 |
| Brexucabtagene autoleucel—Proportion Non-ICU—Hospital days | 16.5000 | −4.5696 | 37.5696 |
| Bridging Therapy Proportion | 0.3676 | 0.3133 | 0.4237 |
| Cost: Initial Hospitalization: Intensive Care Unit Day | CAD 8,343.7300 | CAD 5399.6221 | 1 CAD 1918.2165 |
| Cost: Initial Hospitalization: Inpatient Day (Non-ICU) | CAD 1580.5300 | CAD 1022.8357 | CAD 2257.6352 |
| Cost: Stem cell transplant | CAD 166,855.5300 | CAD 10,7980.1014 | CAD 23,8337.0904 |
| Cost: Office visit | CAD 174.6400 | CAD 113.0178 | CAD 249.4565 |
| Cost: Palliative care (one-off) | CAD 35,262.4800 | CAD 22,820.0178 | CAD 50,369.0641 |
| Cost: Full blood count | CAD 4.1200 | CAD 2.6662 | CAD 5.8850 |
| Cost: X-ray | CAD 23.1500 | CAD 14.9815 | CAD 33.0676 |
| Cost: Blood glucose | CAD 1.3300 | CAD 0.8607 | CAD 1.8998 |
| Cost: Lactate dehydrogenase | CAD 1.3300 | CAD 0.8607 | CAD 1.8998 |
| Cost: Inpatient stay | CAD 1580.5300 | CAD 1022.8357 | CAD 2257.6352 |
| Cost: CT Scan | CAD 195.0000 | CAD 126.1937 | CAD 278.5388 |
| Cost: Hospitalization | CAD 13,215.8400 | CAD 8552.5948 | CAD 18,877.5574 |
| Utility | |||
| Utility: Pre-progression (up to 60 months) | 0.7800 | 0.7601 | 0.7993 |
| Utility: Pre-progression, cured (beyond 60 months) | 0.7852 | 0.7653 | 0.8045 |
| Utility: Post-progression | 0.6800 | 0.6321 | 0.7261 |
| Adverse Events | |||
| Brexucabtagene autoleucel AE incidence: Hypotension | 0.2206 | 0.1305 | 0.3265 |
| Brexucabtagene autoleucel AE incidence: Neutrophil count decreased | 0.5294 | 0.4103 | 0.6468 |
| Brexucabtagene autoleucel AE incidence: White blood cell count decreased | 0.4118 | 0.2977 | 0.5308 |
| Brexucabtagene autoleucel AE incidence: Hypoxia | 0.2059 | 0.1186 | 0.3097 |
| Brexucabtagene autoleucel AE incidence: Hypophosphataemia | 0.2206 | 0.1305 | 0.3265 |
| Brexucabtagene autoleucel AE incidence: Neutropenia | 0.3382 | 0.2308 | 0.4548 |
| Brexucabtagene autoleucel AE incidence: Hyponatraemia | 0.1029 | 0.0427 | 0.1855 |
| Brexucabtagene autoleucel AE incidence: Alanine aminotransferase increased | 0.0882 | 0.0333 | 0.1663 |
| Brexucabtagene autoleucel AE incidence: Encephalopathy | 0.1765 | 0.0956 | 0.2756 |
| Brexucabtagene autoleucel AE incidence: Hypokalaemia | 0.1471 | 0.0735 | 0.2405 |
| Brexucabtagene autoleucel AE incidence: Hypocalcaemia | 0.0882 | 0.0333 | 0.1663 |
| Brexucabtagene autoleucel AE incidence: Thrombocytopenia | 0.1618 | 0.0844 | 0.2582 |
| Brexucabtagene autoleucel AE incidence: Aspartate aminotransferase increased | 0.1029 | 0.0427 | 0.1855 |
| Brexucabtagene autoleucel AE incidence: Confusional state | 0.1176 | 0.0526 | 0.2042 |
| Brexucabtagene autoleucel AE incidence: Hypertension | 0.1324 | 0.0629 | 0.2225 |
| Brexucabtagene autoleucel AE incidence: Acute Kidney Injury | 0.0735 | 0.0244 | 0.1465 |
| Brexucabtagene autoleucel AE incidence: Leukopenia | 0.1471 | 0.0735 | 0.2405 |
| Brexucabtagene autoleucel AE incidence: Lymphocyte count decreased | 0.0882 | 0.0333 | 0.1663 |
| Brexucabtagene autoleucel AE incidence: Pneumonia | 0.1324 | 0.0629 | 0.2225 |
| Brexucabtagene autoleucel AE incidence: Respiratory Failure | 0.0588 | 0.0163 | 0.1259 |
| Brexucabtagene autoleucel AE incidence: Sepsis | 0.0588 | 0.0163 | 0.1259 |
| Disutility Cytokine release syndrome | 0.7800 | 0.4087 | 0.9850 |
| Disutility Pyrexia | 0.1100 | 0.0707 | 0.1566 |
| Disutility Anaemia | 0.1200 | 0.0771 | 0.1708 |
| Disutility Platelet Count decreased | 0.1100 | 0.0707 | 0.1566 |
| Disutility Hypotension | 0.1500 | 0.0961 | 0.2133 |
| Disutility Neutrophil count decreased | 0.1500 | 0.0961 | 0.2133 |
| Disutility White blood cell count decreased | 0.1500 | 0.0961 | 0.2133 |
| Disutility Hypoxia | 0.1100 | 0.0707 | 0.1566 |
| Disutility Hypophosphataemia | 0.1500 | 0.0961 | 0.2133 |
| Disutility Neutropenia | 0.0900 | 0.0579 | 0.1282 |
| Disutility Hyponatraemia | 0.1500 | 0.0961 | 0.2133 |
| Disutility Alanine aminotransferase increased | 0.1500 | 0.0961 | 0.2133 |
| Disutility Encephalopathy | 0.1500 | 0.0961 | 0.2133 |
| Disutility Hypokalaemia | 0.1500 | 0.0961 | 0.2133 |
| Disutility Hypocalcaemia | 0.1500 | 0.0961 | 0.2133 |
| Disutility Thrombocytopenia | 0.1100 | 0.0707 | 0.1566 |
| Disutility Aspartate aminotransferase increased | 0.1500 | 0.0961 | 0.2133 |
| Disutility Confusional state | 0.1500 | 0.0961 | 0.2133 |
| Disutility Hypertension | 0.1500 | 0.0961 | 0.2133 |
| Disutility Acute Kidney Injury | 0.1500 | 0.0961 | 0.2133 |
| Disutility Leukopenia | 0.1500 | 0.0961 | 0.2133 |
| Disutility Lymphocyte count decreased | 0.1500 | 0.0961 | 0.2133 |
| Disutility Pneumonia | 0.1500 | 0.0961 | 0.2133 |
| Disutility Respiratory Failure | 0.1500 | 0.0961 | 0.2133 |
| Disutility Sepsis | 0.1500 | 0.0961 | 0.2133 |
| Duration Cytokine release syndrome | 4.0000 | 2.5886 | 5.7136 |
| Duration Pyrexia | 2.0000 | 1.2943 | 2.8568 |
| Duration Anaemia | 14.0000 | 9.0601 | 19.9977 |
| Duration Platelet Count decreased | 50.0000 | 32.3574 | 71.4202 |
| Duration Hypotension | 5.0000 | 3.2357 | 7.1420 |
| Duration Neutrophil count decreased | 17.0000 | 11.0015 | 24.2829 |
| Duration White blood cell count decreased | 40.0000 | 25.8859 | 57.1362 |
| Duration Hypoxia | 2.0000 | 1.2943 | 2.8568 |
| Duration Hypophosphataemia | 5.0000 | 3.2357 | 7.1420 |
| Duration Neutropenia | 47.0000 | 30.4159 | 67.1350 |
| Duration Hyponatraemia | 7.0000 | 4.5300 | 9.9988 |
| Duration Alanine aminotransferase increased | 7.0000 | 4.5300 | 9.9988 |
| Duration Encephalopathy | 9.0000 | 5.8243 | 12.8556 |
| Duration Hypokalaemia | 7.0000 | 4.5300 | 9.9988 |
| Duration Hypocalcaemia | 7.0000 | 4.5300 | 9.9988 |
| Duration Thrombocytopenia | 63.0000 | 40.7703 | 89.9894 |
| Duration Aspartate aminotransferase increased | 7.0000 | 4.5300 | 9.9988 |
| Duration Confusional state | 7.0000 | 4.5300 | 9.9988 |
| Duration Hypertension | 5.0000 | 3.2357 | 7.1420 |
| Duration Acute Kidney Injury | 7.0000 | 4.5300 | 9.9988 |
| Duration Leukopenia | 21.0000 | 13.5901 | 29.9965 |
| Duration Lymphocyte count decreased | 64.0000 | 41.4174 | 91.4178 |
| Duration Pneumonia | 7.0000 | 4.5300 | 9.9988 |
| Duration Respiratory Failure | 7.0000 | 4.5300 | 9.9988 |
| Duration Sepsis | 7.0000 | 4.5300 | 9.9988 |
| AE cost: Cytokine release syndrome | CAD 18,366.9647 | CAD 11,886.1311 | CAD 26,235.4440 |
Abbreviations: BSA = body surface area; AE = adverse events; ICU intensive care unit.
Table A9.
Parameter values varied in probabilistic sensitivity analyses.
| Parameter | Value | SE | Distribution |
|---|---|---|---|
| Patient Characteristics | |||
| Bodyweight | 81.8 kg | 1.95296 | Normal |
| BSA | 1.978 m2 | 0.026997 | Normal |
| Resource Use | |||
| Pre-Progression Resource Use: Full blood count | 0.33 | 0.066667 | Gamma |
| Pre-Progression Resource Use: X-ray | 0.17 | 0.033333 | Gamma |
| Pre-Progression Resource Use: Blood glucose | 0.33 | 0.066667 | Gamma |
| Pre-Progression Resource Use: Lactate dehydrogenase | 0.33 | 0.066667 | Gamma |
| Pre-Progression Resource Use: CT Scan | 0.17 | 0.033333 | Gamma |
| Pre-Progression Resource Use: Office visit | 0.17 | 0.033333 | Gamma |
| Post-Progression Resource Use: Full blood count | 1.00 | 0.2 | Gamma |
| Post-Progression Resource Use: X-ray | 0.33 | 0.066667 | Gamma |
| Post-Progression Resource Use: Blood glucose | 1.00 | 0.2 | Gamma |
| Post-Progression Resource Use: Lactate dehydrogenase | 1.00 | 0.2 | Gamma |
| Post-Progression Resource Use: Office visit | 1.00 | 0.2 | Gamma |
| Post-Progression Resource Use: CT Scan | 0.33 | 0.066667 | Gamma |
| Post-Progression Resource Use: Hospitalization | 0.08 | 0.016667 | Gamma |
| End-of-life Palliative care (one-off) | 1 | 0.20 | Gamma |
| Apheresis: One-Time cost | CAD 1392.37 | CAD 278.47 | Gamma |
| Tecartus Proportion ICU Stay | 0.22 | 0.04 | Beta |
| Hospital days, proportion ICU | 18 | 22.5 | Normal |
| Hospital days, proportion non-ICU | 16.50 | 10.75 | Normal |
| Proportion requiring bridging therapy | 0.37 | 0.03 | Beta |
| Cost: Initial Hospitalization: Intensive Care Unit Day | CAD 8343.73 | CAD 1668.75 | Gamma |
| Cost: Initial Hospitalization: Inpatient Day (Non-ICU) | CAD 1580.53 | CAD 316.11 | Gamma |
| Cost: Stem cell transplant | CAD 166,855.53 | CAD 33,371.11 | Gamma |
| Cost: Office visit | CAD 174.64 | CAD 34.93 | Gamma |
| Cost: Palliative care (one-off) | CAD 35,262.48 | CAD 7052.50 | Gamma |
| Cost: Full blood count | CAD 4.12 | CAD 0.82 | Gamma |
| Cost: X-ray | CAD 23.15 | CAD 4.63 | Gamma |
| Cost: Blood glucose | CAD 1.33 | CAD 0.27 | Gamma |
| Cost: Lactate dehydrogenase | CAD 1.33 | CAD 0.27 | Gamma |
| Cost: Inpatient stay | CAD 1580.53 | CAD 316.11 | Gamma |
| Cost: CT Scan | CAD 195.00 | CAD 39.00 | Gamma |
| Cost: Hospitalization | CAD 13,215.84 | CAD 2643.17 | Gamma |
| Utility | |||
| Utility: Pre-progression (up to 60 months) | 0.78 | 0.01 | Beta |
| Utility: Pre-progression, cured (beyond 60 months) | 0.7851841 | 0.01 | Beta |
| Utility: Post-progression | 0.68 | 0.024 | Beta |
| Adverse Events | |||
| Brexucabtagene autoleucel AE incidence: Cytokine release syndrome (CRS) | 62% | 0.059 | Beta |
| Brexucabtagene autoleucel AE incidence: Pyrexia | 15% | 0.043 | Beta |
| Brexucabtagene autoleucel AE incidence: Anaemia | 51% | 0.061 | Beta |
| Brexucabtagene autoleucel AE incidence: Platelet Count decreased | 38% | 0.059 | Beta |
| Brexucabtagene autoleucel AE incidence: Hypotension | 22% | 0.050 | Beta |
| Brexucabtagene autoleucel AE incidence: Neutrophil count decreased | 53% | 0.061 | Beta |
| Brexucabtagene autoleucel AE incidence: White blood cell count decreased | 41% | 0.060 | Beta |
| Brexucabtagene autoleucel AE incidence: Hypoxia | 21% | 0.049 | Beta |
| Brexucabtagene autoleucel AE incidence: Hypophosphataemia | 22% | 0.050 | Beta |
| Brexucabtagene autoleucel AE incidence: Neutropenia | 34% | 0.057 | Beta |
| Brexucabtagene autoleucel AE incidence: Hyponatraemia | 10% | 0.037 | Beta |
| Brexucabtagene autoleucel AE incidence: Alanine aminotransferase increased | 9% | 0.034 | Beta |
| Brexucabtagene autoleucel AE incidence: Encephalopathy | 18% | 0.046 | Beta |
| Brexucabtagene autoleucel AE incidence: Hypokalaemia | 15% | 0.043 | Beta |
| Brexucabtagene autoleucel AE incidence: Hypocalcaemia | 9% | 0.034 | Beta |
| Brexucabtagene autoleucel AE incidence: Thrombocytopenia | 16% | 0.045 | Beta |
| Brexucabtagene autoleucel AE incidence: Aspartate aminotransferase increased | 10% | 0.037 | Beta |
| Brexucabtagene autoleucel AE incidence: Confusional state | 12% | 0.039 | Beta |
| Brexucabtagene autoleucel AE incidence: Hypertension | 13% | 0.041 | Beta |
| Brexucabtagene autoleucel AE incidence: Acute Kidney Injury | 7% | 0.032 | Beta |
| Brexucabtagene autoleucel AE incidence: Leukopenia | 15% | 0.043 | Beta |
| Brexucabtagene autoleucel AE incidence: Lymphocyte count decreased | 9% | 0.034 | Beta |
| Brexucabtagene autoleucel AE incidence: Pneumonia | 13% | 0.041 | Beta |
| Brexucabtagene autoleucel AE incidence: Respiratory Failure | 6% | 0.029 | Beta |
| Brexucabtagene autoleucel AE incidence: Sepsis | 6% | 0.029 | Beta |
| Disutility Cytokine release syndrome | 0.78 | 0.156 | Beta |
| Disutility Pyrexia | 0.11 | 0.022 | Beta |
| Disutility Anaemia | 0.12 | 0.024 | Beta |
| Disutility Platelet Count decreased | 0.11 | 0.022 | Beta |
| Disutility Hypotension | 0.15 | 0.03 | Beta |
| Disutility Neutrophil count decreased | 0.15 | 0.03 | Beta |
| Disutility White blood cell count decreased | 0.15 | 0.03 | Beta |
| Disutility Hypoxia | 0.11 | 0.022 | Beta |
| Disutility Hypophosphataemia | 0.15 | 0.03 | Beta |
| Disutility Neutropenia | 0.09 | 0.018 | Beta |
| Disutility Hyponatraemia | 0.15 | 0.03 | Beta |
| Disutility Alanine aminotransferase increased | 0.15 | 0.03 | Beta |
| Disutility Encephalopathy | 0.15 | 0.03 | Beta |
| Disutility Hypokalaemia | 0.15 | 0.03 | Beta |
| Disutility Hypocalcaemia | 0.15 | 0.03 | Beta |
| Disutility Thrombocytopenia | 0.11 | 0.022 | Beta |
| Disutility Aspartate aminotransferase increased | 0.15 | 0.03 | Beta |
| Disutility Confusional state | 0.15 | 0.03 | Beta |
| Disutility Hypertension | 0.15 | 0.03 | Beta |
| Disutility Acute Kidney Injury | 0.15 | 0.03 | Beta |
| Disutility Leukopenia | 0.15 | 0.03 | Beta |
| Disutility Lymphocyte count decreased | 0.15 | 0.03 | Beta |
| Disutility Pneumonia | 0.15 | 0.03 | Beta |
| Disutility Respiratory Failure | 0.15 | 0.03 | Beta |
| Disutility Sepsis | 0.15 | 0.03 | Beta |
| Duration Cytokine release syndrome | 4 | 0.8 | Gamma |
| Duration Pyrexia | 2 | 0.4 | Gamma |
| Duration Anaemia | 14 | 2.8 | Gamma |
| Duration Platelet Count decreased | 50 | 10 | Gamma |
| Duration Hypotension | 5 | 1 | Gamma |
| Duration Neutrophil count decreased | 17 | 3.4 | Gamma |
| Duration White blood cell count decreased | 40 | 8 | Gamma |
| Duration Hypoxia | 2 | 0.4 | Gamma |
| Duration Hypophosphataemia | 5 | 1 | Gamma |
| Duration Neutropenia | 47 | 9.4 | Gamma |
| Duration Hyponatraemia | 7 | 1.4 | Gamma |
| Duration Alanine aminotransferase increased | 7 | 1.4 | Gamma |
| Duration Encephalopathy | 9 | 1.8 | Gamma |
| Duration Hypokalaemia | 7 | 1.4 | Gamma |
| Duration Hypocalcaemia | 7 | 1.4 | Gamma |
| Duration Thrombocytopenia | 63 | 12.6 | Gamma |
| Duration Aspartate aminotransferase increased | 7 | 1.4 | Gamma |
| Duration Confusional state | 7 | 1.4 | Gamma |
| Duration Hypertension | 5 | 1 | Gamma |
| Duration Acute Kidney Injury | 7 | 1.4 | Gamma |
| Duration Leukopenia | 21 | 4.2 | Gamma |
| Duration Lymphocyte count decreased | 64 | 12.8 | Gamma |
| Duration Pneumonia | 7 | 1.4 | Gamma |
| Duration Respiratory Failure | 7 | 1.4 | Gamma |
| Duration Sepsis | 7 | 1.4 | Gamma |
| AE cost: Cytokine release syndrome | CAD 18,366.96 | CAD 3673.39 | Gamma |
Abbreviations: BSA = body surface area; AE = adverse events; ICU intensive care unit.
Author Contributions
Conceptualization, G.B., C.L. and M.D.S.; methodology, G.B. and D.C.; formal analysis, G.B. and D.C.; writing—original draft preparation, G.B.; writing—review and editing, C.L., D.C. and M.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
C.L. and M.S. received no external funding for this research. D.C. received funding from Gilead Sciences for analytical support. Publications charges for this manuscript were supported by a grant from Gilead Sciences.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Appendix A.
Conflicts of Interest
Graeme Ball is an employee of Gilead Sciences Canada. Matthew Seftel is an employee of Canadian Blood Services. Seftel has served on scientific advisory boards for Kite/Gilead and Novartis. Lemieux has served on scientific advisory boards for Kite/Gilead, BMS, and Novartis. Seftel’s and Lemieux’s employers played no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Klener P. Advances in Molecular Biology and Targeted Therapy of Mantle Cell Lymphoma. Int. J. Mol. Sci. 2019;20:4417. doi: 10.3390/ijms20184417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inamdar A.A., Goy A., Ayoub N.M., Attia C., Oton L., Taruvai V., Costales M., Lin Y.-T., Pecora A., Suh K.S. Mantle cell lymphoma in the era of precision medicine-diagnosis, biomarkers and therapeutic agents. Oncotarget. 2016;7:48692–48731. doi: 10.18632/oncotarget.8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Hamadani M., Habermann T.M., Cerhan J.R., Macon W.R., Maurer M.J., Go R.S. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: A longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am. J. Hematol. 2015;90:790–795. doi: 10.1002/ajh.24086. [DOI] [PubMed] [Google Scholar]
- 4.Ye X., Mahmud S., Skrabek P., Lix L., Johnston J.B. Long-term time trends in incidence, survival and mortality of lymphomas by subtype among adults in Manitoba, Canada: A population-based study using cancer registry data. Br. Med. J. Open. 2017;7:e015106. doi: 10.1136/bmjopen-2016-015106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan-Canadian Oncology Drug Review . Pan-Canadian Oncology Drug Review. Final Economic Guidance Report-Ibrutinib for Mantle Cell Lymphoma. Pan-Canadian Oncology Drug Review; Toronto, ON, Canada: 2016. [Google Scholar]
- 6.Smith A., Crouch S., Lax S., Li J., Painter D., Howell D., Patmore R., Jack A., Roman E. Lymphoma incidence, survival and prevalence 2004–2014: Sub-type analyses from the UK’s Haematological Malignancy Research Network. Br. J. Cancer. 2015;112:1575–1584. doi: 10.1038/bjc.2015.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain P., Wang M. Mantle cell lymphoma: 2019 update on the diagnosis, pathogenesis, prognostication, and management. Am. J. Hematol. 2019;94:710–725. doi: 10.1002/ajh.25487. [DOI] [PubMed] [Google Scholar]
- 8.Issa D.E., van de Schans S.A., Chamuleau M.E., Karim-Kos H.E., Wondergem M., Huijgens P.C., Coebergh J.W.W., Zweegman S., Visser O. Trends in incidence, treatment and survival of aggressive B-cell lymphoma in The Netherlands 1989–2010. Haematologica. 2015;100:525–533. doi: 10.3324/haematol.2014.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin P., Maddocks K., Leonard J.P., Ruan J., Goy A., Wagner-Johnston N., Rule S., Advani R., Iberri D., Phillips T., et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood. 2016;127:1559–1563. doi: 10.1182/blood-2015-10-673145. [DOI] [PubMed] [Google Scholar]
- 10.McCulloch R., Visco C., Eyre T.A., Frewin R., Phillips N., Tucker D.L., Quaglia F.M., McMillan A., Lambert J., Crosbie N., et al. Efficacy of R-BAC in relapsed, refractory mantle cell lymphoma post BTK inhibitor therapy. Br. J. Haematol. 2020;189:684–688. doi: 10.1111/bjh.16416. [DOI] [PubMed] [Google Scholar]
- 11.Cheah C.Y., Chihara D., Romaguera J.E., Fowler N.H., Seymour J.F., Hagemeister F.B., Champlin R.E., Wang M.L. Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Ann. Oncol. 2015;26:1175–1179. doi: 10.1093/annonc/mdv111. [DOI] [PubMed] [Google Scholar]
- 12.Epperla N., Hamadani M., Cashen A.F., Ahn K.W., Oak E., Kanate A.S., Calzada O., Cohen J.B., Farmer L., Ghosh N., et al. Predictive factors and outcomes for ibrutinib therapy in relapsed/refractory mantle cell lymphoma-a “real world” study. Hematol. Oncol. 2017;35:528–535. doi: 10.1002/hon.2380. [DOI] [PubMed] [Google Scholar]
- 13.Jain P., Kanagal-Shamanna R., Zhang S., Ahmed M., Ghorab A., Zhang L., Ok C.Y., Li S., Hagemeister F., Zeng D., et al. Long-Term Outcomes and Mutation Profiling of Patients with Mantle Cell Lymphoma (MCL) Who Discontinued Ibrutinib. Br. J. Haematol. 2018;183:578–587. doi: 10.1111/bjh.15567. [DOI] [PubMed] [Google Scholar]
- 14.Wang M., Schuster S.J., Phillips T., Lossos I.S., Goy A., Rule S., Hamadani M., Ghosh N., Reeder C.B., Barnett E., et al. Observational Study of Lenalidomide in Patients with Mantle Cell Lymphoma Who Relapsed/Progressed After or Were Refractory/Intolerant to Ibrutinib (MCL-004) J. Hematol. Oncol. 2017;10:171. doi: 10.1186/s13045-017-0537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dreyling M., Campo E., Hermine O., Jerkeman M., Le Gouill S., Rule S., Shpilberg O., Walewski J., Ladetto M., ESMO Guidelines Committee Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017;28:iv62–iv71. doi: 10.1093/annonc/mdx223. [DOI] [PubMed] [Google Scholar]
- 16.Wang M., Munoz J., Goy A., Locke F.L., Jacobsen C.A., Hill B.T., Timmerman J.M., Holmes H., Jaglowski S., Flinn I.W., et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020;382:1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.U.S. Food & Drug Administration Supplement Approval. [(accessed on 23 July 2021)];2021 Available online: https://www.fda.gov/media/146253/download.
- 18.Health Canada Notice of Compliance (NOC) Online Query. 2021. [(accessed on 16 August 2021)]. Available online: https://health-products.canada.ca/noc-ac/info.do?lang=en&no=26027.
- 19.Canadian Agency for Drugs and Technologies in Health Brexucabtagene Autoleucel. 2020. [(accessed on 22 April 2021)]. Available online: https://www.cadth.ca/brexucabtagene-autoleucel.
- 20.Final Appraisal Document Autologous Anti-CD19-Transduced CD3+ Cells for Treating Relapsed or Refractory Mantle Cell Lymphoma. 2021. [(accessed on 23 August 2021)]. Available online: https://www.nice.org.uk/guidance/ta677/documents/final-appraisal-determination-document.
- 21.Canadian Agency for Drugs and Technologies in Health (CADTH) Reimbursement Recommendation: Brexucabtagene Autoleucel (Tecartus) Canadian Agency for Drugs and Technologies in Health; Toronto, ON, Canada: 2021. [Google Scholar]
- 22.Lambert P.C. Modeling of the cure fraction in survival studies. Stata J. 2007;7:351–375. doi: 10.1177/1536867X0700700304. [DOI] [Google Scholar]
- 23.Roth J.A., Sullivan S.D., Lin V.W., Bansal A., Purdum A.G., Navale L., Cheng P., Ramsey S.D. Cost-effectiveness of axicabtagene ciloleucel for adult patients with relapsed or refractory large B-cell lymphoma in the United States. J. Med. Econ. 2018;21:1238–1245. doi: 10.1080/13696998.2018.1529674. [DOI] [PubMed] [Google Scholar]
- 24.Lin J.K., Muffly L.S., Spinner M.A., Barnes J.I., Owens D.K., Goldhaber-Fiebert J.D. Cost Effectiveness of Chimeric Antigen Receptor T-Cell Therapy in Multiply Relapsed or Refractory Adult Large B-Cell Lymphoma. J. Clin. Oncol. 2019;37:2105–2119. doi: 10.1200/JCO.18.02079. [DOI] [PubMed] [Google Scholar]
- 25.Liu R., Oluwole O.O., Diakite I., Botteman M.F., Snider J.T., Locke F.L. Cost effectiveness of axicabtagene ciloleucel versus tisagenlecleucel for adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy in the United States. J. Med. Econ. 2021;24:458–468. doi: 10.1080/13696998.2021.1901721. [DOI] [PubMed] [Google Scholar]
- 26.Ball G., Kuruvilla J., Boodoo C., Jain M.D. PCN108 Cost-Effectiveness of Axicabtagene Ciloleucel (AXI-CEL) and Tisagenlecleucel (TISA-CEL) in Adult Patients with Relapsed or Refractory (R/R) Large B-Cell Lymphoma (LBCL) in Canada. Value Health. 2021;24:S39. doi: 10.1016/j.jval.2021.04.200. [DOI] [Google Scholar]
- 27.CADTH . Guidelines for the Economic Evaluation of Health Technologies: Canada. 4th ed. CADTH; Ottawa, ON, Canada: 2018. [Google Scholar]
- 28.Guidelines for the Economic Evaluation of Health Technologies: Canada. 2015. [(accessed on 12 August 2021)]. Available online: https://www.cadth.ca/about-cadth/how-we-do-it/methods-and-guidelines/guidelines-for-the-economic-evaluation-of-health-technologies-canada.
- 29.NICE DSU Technical Support Document 19: Partitioned Survival Analysis for Decision Modelling in Health Care: A Critical Review. 2017. [(accessed on 2 January 2018)]. Available online: http://scharr.dept.shef.ac.uk/nicedsu/wp-content/uploads/sites/7/2017/06/Partitioned-Survival-Analysis-final-report.pdf.
- 30.Hettle R., Corbett M., Hinde S., Hodgson R., Jones-Diette J., Woolacott N., Palmer S. The assessment and appraisal of regenerative medicines and cell therapy products: An exploration of methods for review, economic evaluation and appraisal. Health Technol. Assess. 2017;21:1–204. doi: 10.3310/hta21070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuster S.J., Bishop M.R., Tam C.S., Waller E.K., Borchmann P., McGuirk J.P., Jäger U., Jaglowski S., Andreadis C., Westin J.R., et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 32.Nastoupil L.J., Jain M.D., Feng L., Spiegel J.Y., Ghobadi A., Lin Y., Dahiya S., Lunning M., Lekakis L., Reagan P., et al. Standard-of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results from the US Lymphoma CAR T Consortium. J. Clin. Oncol. 2020;38:3119–3128. doi: 10.1200/JCO.19.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasquini M.C., Hu Z.H., Curran K., Laetsch T., Locke F., Rouce R., Pulsipher M.A., Phillips C.L., Keating A., Frigault M.J., et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 2020;4:5414–5424. doi: 10.1182/bloodadvances.2020003092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Reagan P.M., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., et al. A Comparison of Two-Year Outcomes in ZUMA-1 (Axicabtagene Ciloleucel) and SCHOLAR-1 in Patients with Refractory Large B Cell Lymphoma. Blood. 2019;134:4095. doi: 10.1182/blood-2019-125792. [DOI] [Google Scholar]
- 35.Statistics Canada . Life Tables, Canada, Provinces and Territories 1980/1982 to 2016/2018. Statistics Canada; Ottawa, ON, Canada: 2020. [Google Scholar]
- 36.Maurer M.J., Ghesquières H., Jais J.P., Witzig T.E., Haioun C., Thompson C.A., Delarue R., Micallef I.N., Peyrade F., Macon W.R., et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J. Clin. Oncol. 2014;32:1066–1073. doi: 10.1200/JCO.2013.51.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.NICE DSU Technical Support Document 14: Survival Analysis for Economic Evaluations alongside Clinical Trials-Extrapolation with Patient-Level Data. 2011. [(accessed on 19 December 2017)]. Available online: http://scharr.dept.shef.ac.uk/nicedsu/wp-content/uploads/sites/7/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf.
- 38.Precision HEOR KP . Meta-Analysis and Indirect Comparison of Interventions for Relapsed or Refractory Mantle Cell Lymphoma Previously Treated with Bruton Tyrosine Kinase Inhibitors. Precision HEOR; Bethesda, MD, USA: 2020. [Google Scholar]
- 39.Guyot P., Ades A.E., Ouwens M.J., Welton N.J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bank of Canada @bankofcanada. 2020. [(accessed on 10 December 2021)]. Available online: https://www.bankofcanada.ca/
- 41.Walker H., Anderson M., Farahati F., Howell D., Librach S.L., Husain A., Sussman J., Viola R., Sutradhar R., Barbera L. Resource use and costs of end-of-Life/palliative care: Ontario adult cancer patients dying during 2002 and 2003. J. Palliat. Care. 2011;27:79–88. doi: 10.1177/082585971102700203. [DOI] [PubMed] [Google Scholar]
- 42.Kite Pharma Inc. Clinical Study Report, Primary Analysis: A Phase 2 Multicenter Study Evaluating the Efficacy of KTE-C19 in Subjects with Relapsed/Refractory Mantle Cell Lymphoma (ZUMA-2) Kite Pharma Inc.; Los Angeles, CA, USA: 2019. [Google Scholar]
- 43.Ministry of Health and Long Term Care . Schedule of Benefits: Physician Services under the Health Insurance Act. Ministry of Health and Long Term Care; Toronto, ON, Canada: 2020. [Google Scholar]
- 44.Holbro A., Ahmad I., Cohen S., Roy J., Lachance S., Chagnon M., LeBlanc R., Bernard L., Busque L., Roy D.C., et al. Safety and Cost-Effectiveness of Outpatient Autologous Stem Cell Transplantation in Patients with Multiple Myeloma. Biol. Blood Marrow Transplant. 2013;19:547–551. doi: 10.1016/j.bbmt.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Canadian Institute for Health Information . Patient Cost Estimator. Canadian Institute for Health Information; Ottawa, ON, Canada: 2020. [Google Scholar]
- 46.Zheng B., Reardon P.M., Fernando S.M., Webber C., Thavorn K., Thompson L.H., Tanuseputro P., Munshi L., Kyeremanteng K. Costs and Outcomes of Patients Admitted to the Intensive Care Unit with Cancer. J. Intensive Care Med. 2020;36:203–210. doi: 10.1177/0885066619899653. [DOI] [PubMed] [Google Scholar]
- 47.Ministry of Health and Long Term Care . Exceptional Access Program. Ministry of Health and Long Term Care; Toronto, ON, Canada: 2019. [Google Scholar]
- 48.Pan-Canadian Oncology Drug Review . Pan-Canadian Oncology Drug Review. Final Economic Guidance Report-Bendamustine. Pan-Canadian Oncology Drug Review; Toronto, ON, Canada: 2013. [Google Scholar]
- 49.Final Economic Guidance Report Daratumumab (Darzalex) + VMP for Multiple Myeloma. [(accessed on 21 May 2020)]. Available online: https://www.cadth.ca/sites/default/files/pcodr/pcodr_daratumumab_darzalex_mm_fn_rec.pdf.
- 50.Pan-Canadian Oncology Drug Review . Pan-Canadian Oncology Drug Review. Final Economic Guidance Report Pertuzumab-Trastuzumab for Early Breast Cancer. Pan-Canadian Oncology Drug Review; Toronto, ON, Canada: 2018. [Google Scholar]
- 51.Hambley B., Caimi P.F., William B.M. Bortezomib for the treatment of mantle cell lymphoma: An update. Ther. Adv. Hematol. 2016;7:196–208. doi: 10.1177/2040620716648566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anthracyclines. [(accessed on 14 September 2021)];2020 Available online: https://www.ncbi.nlm.nih.gov/books/NBK538187/
- 53.Desai M., Newberry K., Ou Z., Wang M., Zhang L. Lenalidomide in relapsed or refractory mantle cell lymphoma: Overview and perspective. Ther. Adv. Hematol. 2014;5:91–101. doi: 10.1177/2040620714532124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Visco C., Zambello R., Paolini R., Finotto S., Zanotti R., Zaja F., Nadali G., Trentin L., Rodella E., Lissandrini L., et al. Rituximab, Bendamustine and Cytarabine (R-BAC) Is a Very Active Regimen in Patients with Mantle Cell Lymphoma Not Eligible for Intensive Chemotherapy or Autologous Transplant. Blood. 2011;118:2677. doi: 10.1182/blood.V118.21.2677.2677. [DOI] [Google Scholar]
- 55.Ministry of Health and Long Term Care . Schedule of Benefits for Laboratory Services. Ministry of Health and Long Term Care; Toronto, ON, Canada: 2020. [Google Scholar]
- 56.Ibrutinib for Treating Relapsed or Refractory Mantle Cell Lymphoma. Technology Appraisal Guidance [TA502]-Committee Papers. 2016. [(accessed on 15 August 2019)]. Available online: https://www.nice.org.uk/guidance/ta502/documents/committee-papers.
- 57.Ara R., Brazier J.E. Populating an economic model with health state utility values: Moving toward better practice. Value Health. 2010;13:509–518. doi: 10.1111/j.1524-4733.2010.00700.x. [DOI] [PubMed] [Google Scholar]
- 58.Cameron D., Ubels J., Norström F. On what basis are medical cost-effectiveness thresholds set? Clashing opinions and an absence of data: A systematic review. Glob. Health Action. 2018;11:1447828. doi: 10.1080/16549716.2018.1447828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Assouline S., Li S., Gisselbrecht C., Fogarty P., Hay A., van den Neste E., Shepherd L.E., Schmitz N., Baetz T., Keating A., et al. The conditional survival analysis of relapsed DLBCL after autologous transplant: A subgroup analysis of LY.12 and CORAL. Blood Adv. 2020;4:2011–2017. doi: 10.1182/bloodadvances.2020001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.(INESSS) Indeesess Avis au Ministre-TECARTUS pour le Traitement du Lymphome à Cellules du Manteau. 2021. [(accessed on 27 November 2021)]. Available online: https://www.inesss.qc.ca/fileadmin/doc/INESSS/Inscription_medicaments/Avis_au_ministre/Juillet_2021/Tecartus__06.pdf.
- 61.Simons C.L., Malone D., Wang M., Maglinte G.A., Inocencio T., Wade S.W., Bennison C., Shah B. Cost-effectiveness for KTE-X19 CAR T therapy for adult patients with relapsed/refractory mantle cell lymphoma in the United States. J. Med. Econ. 2021;24:421–431. doi: 10.1080/13696998.2021.1894158. [DOI] [PubMed] [Google Scholar]
- 62.Tang K., Nastoupil L.J. Real-World Experiences of CAR T-Cell Therapy for Large B-Cell Lymphoma: How Similar Are They to the Prospective Studies. J. Immunother. Precis. Oncol. 2021;4:150–159. doi: 10.36401/JIPO-21-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jain P., Nastoupil L., Westin J., Lee H.J., Navsaria L., Steiner R.E., Ahmed S., Moghrabi O., Oriabure O., Chen W., et al. Outcomes and management of patients with mantle cell lymphoma after progression on brexucabtagene autoleucel therapy. Br. J. Haematol. 2021;192:e38–e42. doi: 10.1111/bjh.17197. [DOI] [PubMed] [Google Scholar]
- 64.Locke F.L., Ghobadi A., Jacobson C.A., Miklos D.B., Lekakis L.J., Oluwole O.O., Lin Y., Braunschweig I., Hill B.T., Timmerman J.M., et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20:31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bansal A., Sullivan S.D., Lin V.W., Purdum A.G., Navale L., Cheng P., Ramsey S.D. Estimating Long-Term Survival for Patients with Relapsed or Refractory Large B-Cell Lymphoma Treated with Chimeric Antigen Receptor Therapy: A Comparison of Standard and Mixture Cure Models. Med. Decis. Mak. 2019;39:294–298. doi: 10.1177/0272989X18820535. [DOI] [PubMed] [Google Scholar]
- 66.Ontario Drug Benefit Formulary/Comparative Drug Index 2021. [(accessed on 27 November 2021)]; Available online: https://www.formulary.health.gov.on.ca/formulary/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article and Appendix A.