Abstract
Background: Canada has a publicly funded healthcare system with a complex drug funding process. After Health Canada approval to market a drug, the pan-Canadian Oncology Drug Review (pCODR) (now renamed the CADTH reimbursement review) makes a non-binding funding recommendation to the Canadian provinces (except Quebec), which each then decide whether the drug will be publicly funded. We identified the determinants of funding in this process. Methods: We analyzed drugs for advanced lung (n = 15), breast (n = 8), colorectal (CRC) (n = 7), melanoma (n = 10), and neuroendocrine (NET) (n = 3) cancers undergoing the funding decision process from 2011 to 2019. Determinants of funding assessed in the model included list price, cancer type, drug class, and pCODR recommendation. The primary outcome was the correlation between list price and time to funding (TTF: Health Canada approval to first provincial funding). Secondary outcomes included an exploratory analysis of predictors of drug funding. Results: We analyzed 43 drugs: targeted agents 72%, immunotherapy 20%, chemotherapy 7%. A total of 72% were funded in at least one province. Median TTF was 379 days (IQR 203–601). Median list price (28-day course) was CAD 8213 (IQR CAD 5391–9445). Higher list price was not correlated with TTF (correlation coefficient −0.20, p = 0.28). There was no association between list price and pCODR recommendation or the decision to fund in at least one province. A positive pCODR recommendation correlated with the provinces’ funding decisions (p < 0.001), where 89% of drugs with a positive recommendation were funded and 100% of drugs with a negative recommendation were not funded. Tumor type was predictive of TTF (p < 0.001): CRC drugs were the slowest at a median of 2541 days (IQR 702–4379), and NETs were the quickest at a median of 0 days (IQR 0–502). Cancer type predicted decision to fund in at least one province (p = 0.005), with funding for 100% of NET drugs at the high end and 29% of CRC drugs at the low end. Drug class was predictive of TTF (p = 0.01): 465 days (IQR 245–702) for targeted agents, 443 days (IQR 298–587) for chemotherapy, and 339 days (IQR 164–446) for immunotherapy. Conclusions: Determinants of drug funding included cancer type, drug class, and pCODR recommendation but not list price. Factors other than cost were more heavily weighted in the funding decisions of cancer drugs in Canadian provinces.
Keywords: drug funding, cancer drugs, drug access
1. Introduction
Cancer is the leading cause of death in Canada, with patients often diagnosed with advanced disease [1]. The development of new cancer drugs with novel mechanisms of action has contributed to improved survival rates in the setting of advanced malignancy [2]. However, the cancer drug regulatory and funding process in Canada is complex and may delay access. Once a drug demonstrates proof of efficacy (POE) in clinical studies (usually, but not exclusively, phase III clinical trials), the drug must receive Health Canada approval in order to be marketed in Canada [3,4]. With Health Canada approval, cancer drugs can be sold in the country, but their cost is not by default reimbursed by provincial and territorial payors. In order to receive public funding, cancer drugs must undergo a Health Technology Assessment (HTA) by the CADTH pan-Canadian Oncology Drug Review (pCODR) (since data collection for this manuscript it has been re-named the CADTH Reimbursement Review) [5]. The goal of the pCODR HTA is to make a legally non-binding, evidence-based funding recommendation to the provinces and territories of Canada (except Quebec, where the Institut National d’Excellence en Sante et en Services Sociaux (INESSS) undertakes a different process). The pCODR recommendation is based on clinical benefit, patient values, cost effectiveness, and feasibility of adoption into health systems. For each drug submitted for pCODR review, a recommendation is made to either fund the drug, not fund, or fund conditionally [6]. With a positive recommendation, a confidential price negotiation can occur with the pan-Canadian Pharmaceutical Alliance (pCPA). When a price is agreed upon, the HTA recommendation can then be considered by the provinces and territories individually to determine whether or not a given drug will be publicly reimbursed.
The complexity of this process may inadvertently restrict or delay access to new, life-prolonging medicines. It has been shown that the median time to public funding for cancer drugs in Canada is over 26 months from the date of proven drug efficacy. As a result, during the regulatory and funding process of 21 drugs used in advanced lung, breast, and colorectal cancer in Canada, over 39,000 potential life years were lost while awaiting public funding [3]. Similarly, in a study of 27 drugs used for advanced cancer, it was estimated that for each year eliminated from the regulatory and funding process, a median of 79,920 life years would be saved worldwide [7].
While it is known that the provincial and territorial payers base their funding decisions on the pCODR review, jurisdictional priorities, budget impacts, and program mandates [8], the factors most associated with funding decisions and timeliness are unknown [9,10,11]. To better understand the factors associated with positive funding decisions and timeliness, we investigated the determinants of the cancer drug funding process in Canada including the pCODR recommendation, list price, tumor type, and agent class.
2. Methods
We analyzed 43 drugs for advanced breast cancer, colorectal cancer (CRC), lung cancer melanoma, and neuroendocrine tumors (NETs) undergoing the funding decision process in Canada between 2011 and 2019.
The local research ethics board was consulted and determined that ethics approval was not required for this study because all data points collected and analyzed were publicly available.
2.1. Endpoints
The primary endpoint was the correlation between the publicly stated list price and time to funding (TTF) among the drugs that were funded in at least one province. TTF was defined as the time from Health Canada approval (Notice of Compliance (NOC)) to first decision to fund by a province or territory. List prices were standardized as the price for a 28-day course.
Secondary endpoints included an exploratory analysis of predictors of both the decision to fund as well as funding timeliness, including the pCODR recommendation, tumor type, agent class, and list price.
2.2. Eligibility Criteria
Drugs that were eligible for inclusion were all drugs used in the treatment of advanced breast cancer, CRC, lung cancer, melanoma, and NETs submitted to the pCODR HTA from 2011 to 2019. Data points for all included drugs were updated and current as of April 2019. Drugs still under provincial consideration were considered not funded for the purposes of the analysis. The time point of first public funding was set as the date on which the first province or territory agreed to publicly reimburse the drug. Drugs without NOC (approval by Health Canada) were excluded from the analysis due to missing essential data points (such as list price).
2.3. Calculations
When determining the drug cost per 28-day course, additional costs for loading doses in cycle 1 were excluded from the calculations. In the case of Ipilimumab, for the indication in the study (four courses only), the cost of the loading dose was included in the calculation. The cost of each drug was calculated separately with the exception of drugs submitted as doublets to pCODR, which were calculated as the cost for both drugs for a 28-day course.
When calculating median list price, all drugs in the dataset were included regardless of funding status.
To calculate the median TTF, the median time from Health Canada approval to first provincial or territorial decision to fund was determined. Only drugs that received funding in at least one province or territory were included in this calculation.
2.4. Statistics
Statistics were primarily descriptive due to the small sample size. The continuous variables were summarized using median (IQR) and compared using the Wilcoxon rank-sum test. Categorical variables were summarized using frequency (%) and compared using the chi-square test. Calculations of correlation coefficients were performed where applicable, and p < 0.05 was considered significant.
A logistic regression model was constructed for the outcome of decision to fund, using as predictors cost per 28-day course and agent class. The results are presented as odds ratios (95% CI). pCODR recommendation was not included in logistic regression as all drugs that received a “do not fund” recommendation were not funded, and the model required at least one instance of each possible outcome. The tumor type was not included in logistic regression due to the small sample size and five levels of the variable.
3. Results
We analyzed 43 cancer drugs that underwent the funding decision process between 2011 and 2019. These included drugs for breast cancer (n = 8), CRC (n = 7), lung cancer (n = 15), melanoma (n = 10), and NETs (n = 3). See Table 1 for the list of included drugs and descriptors. Of the included drugs, 3 were cytotoxic chemotherapies, 9 were immunotherapies, and 31 were targeted therapies. Among the 43 drugs observed, 35 received a favorable pCODR recommendation (to fund or fund conditionally), and 31 were funded in at least one province. The TTF ranged from 0 to 4379 days, with a median of 379 days. The list price ranged from CAD 1166 to CAD 34,305 for a 28-day course with a median list price of CAD 8213. Table 2 summarizes the study drug characteristics.
Table 1.
Drug descriptors.
| Cancer Type | Drug Name | Indication | Agent Class | pCODR Final Recommendation | List Price * per 28-Day Course | TTF (Days) |
|---|---|---|---|---|---|---|
| Breast | Eribulin mesylate | Third line | Chemo | Fund, Conditional | CAD 4569.33 | 587 |
| Breast | Everolimus | HR+, HER2/neu(−) | Targeted | Fund, Conditional | CAD 5208.00 | 302 |
| Breast | Fulvestrant | HER2/neu(−) | Targeted | Fund, Conditional | CAD 1165.79 | N/A |
| Breast | Lapatinib | HR+, HER2/neu+, in combination with letrozole | Targeted | Don’t fund | CAD 3948.00 | N/A |
| Breast | Palbociclib | HR+, HER2/neu(−), in combination with letrozole | Targeted | Fund, Conditional | CAD 6250.00 | 698 |
| Breast | Pertuzumab | HER2/neu+, in combination with trastuzumab and a taxane | Targeted | Fund, Conditional | CAD 8418.00 | 203 |
| Breast | Trastuzumab emtansine | Second line, HER2/neu+ | Targeted | Fund, Conditional | CAD 8426.88 | 202 |
| Breast | Ribociclib | First line, HR+, HER2/neu(−) | Targeted | Fund, Conditional | CAD 6249.99 | N/A |
| CRC | Aflibercept | Second+ line | Targeted | Don’t fund | CAD 2800.00 | N/A |
| CRC | Bevacizumab | First line, with capecitabine | Targeted | Fund, Conditional | CAD 4200.00 | 4379 |
| CRC | Cetuximab | First line, KRAS WT | Targeted | Don’t fund | CAD 6251.75 | N/A |
| CRC | Panitumumab | First line, KRAS WT, with contraindication to bevacizumab | Targeted | Fund, Conditional | CAD 5174.06 | 702 |
| CRC | Panitumumab | First line, KRAS WT, for left sided primary | Targeted | Do not fund | CAD 5391.29 | N/A |
| CRC | Regorafenib | Third+ line | Targeted | Do not fund | CAD 6237.00 | N/A |
| CRC | Trifluridine and tipiracil | Last line | Chemo | Do not fund | CAD 5631.00 | N/A |
| Lung | Afatinib | First line, EGFR+ | Targeted | Fund, Conditional | CAD 2240.00 | 291 |
| Lung | Alectinib | First line | Targeted | Fund, Conditional | CAD 9446.08 | 245 |
| Lung | Alectinib | Second line | Targeted | Fund, Conditional | CAD 9446.08 | 865 |
| Lung | Alectinib | NSCLC with CNS metastases | Targeted | Do not fund | CAD 9452.80 | N/A |
| Lung | Atezolizumab | Second line | I-O | Fund, Conditional | CAD 9034.67 | 311 |
| Lung | Ceritinib | ALK+ | Targeted | Fund, Conditional | CAD 9445.32 | 1210 |
| Lung | Crizotinib | First line, ALK+ | Targeted | Fund, Conditional | CAD 8213.34 | 1315 |
| Lung | Crizotinib | Second line, ALK+ | Targeted | Fund, Conditional | CAD 8213.34 | 524 |
| Lung | Dabrafenib and trametinib | Second line, BRAF+ | Targeted | Do not fund | CAD 15,669.70 | N/A |
| Lung | Nivolumab | Second line | I-O | Fund, Conditional | CAD 8213.31 | 369 |
| Lung | Osimertinib | First line, EGFR+ | Targeted | Fund, Conditional | CAD 8250.94 | N/A |
| Lung | Osimertinib | EGFR T790M mutation+ | Targeted | Fund, Conditional | CAD 8250.94 | 818 |
| Lung | Pembrolizumab | First line, PD-L1 ≥50% | I-O | Fund, Conditional | CAD 11,733.33 | 148 |
| Lung | Pembrolizumab | Second+ line, PD-L1+ | I-O | Fund, Conditional | CAD 8237.00 | 601 |
| Lung | Pemetrexed | Maintenance following first line pemetrexed and cisplatin | Chemo | Fund, Conditional | CAD 5834.00 | 298 |
| Melanoma | Cobimetinib | BRAF+ | Targeted | Fund, Conditional | CAD 7567.00 | 465 |
| Melanoma | Dabrafenib | BRAF+ | Targeted | Fund, Conditional | CAD 7093.00 | 381 |
| Melanoma | Dabrafenib and trametinib combo | BRAF+ | Targeted | Fund, Conditional | CAD 15,213.64 | 503 |
| Melanoma | Ipilimumab | First line, | I-O | Fund, Conditional | CAD 32,480.00 | 176 |
| Melanoma | Ipilimumab | Second line | I-O | Fund, Conditional | CAD 32,480.00 | 151 |
| Melanoma | Nivolumab | First+ line | I-O | Fund, Conditional | CAD 8213.31 | 523 |
| Melanoma | Nivolumab and ipilimumab combo | First+ line | I-O | Fund, Conditional | CAD 34,305.23 | N/A |
| Melanoma | Pembrolizumab | First+ line | I-O | Fund, Conditional | CAD 8213.34 | 367 |
| Melanoma | Trametinib | BRAF+ | Targeted | Fund, Conditional | CAD 8120.00 | 379 |
| Melanoma | Vemurafenib | BRAF+ | Targeted | Fund, Conditional | CAD 10,425.34 | 198 |
| NET | Everolimus | NETs of GI/lung origin | Targeted | Fund, Conditional | CAD 5602.38 | 502 |
| NET | Everolimus | NETs of pancreas origin | Targeted | Fund, Conditional | CAD 5208.00 | 0 |
| NET | Sunitinib | NETs of pancreas origin | Targeted | Fund, Conditional | CAD 5304.79 | 0 |
Abbreviations: ALK, anaplastic lymphoma kinase; BRAF, v-raf murine sarcoma viral oncogene homolog B1; CNS, central nervous system; CRC, colorectal cancer; EGFR, epidermal growth factor receptor; GI, gastrointestinal; HER2/neu, human epidermal growth factor receptor 2; HR, hormone receptor; I-O, immunotherapy; KRAS, Kirsten rat sarcoma; NET, neuroendocrine tumor; NSCLC, non-small-cell lung cancer; PD-L1, programmed death-ligand 1; TTF time to funding; WT, wildtype. * Available publicly from: Reimbursement Review Reports | CADTH: Available online: https://cadth.ca/reimbursement-review-reports (accessed on 8 January 2021).
Table 2.
Drug characteristics.
| Feature | Category | Result (n = 43) * |
|---|---|---|
| Tumor Type (N, %) | Breast | 8 (19%) |
| Colorectal | 7 (16%) | |
| Lung | 15 (35%) | |
| Melanoma | 3 (7%) | |
| Neuroendocrine | 10 (23%) | |
| Drug Class (N, %) | Chemotherapy | 3 (7%) |
| I-O | 9 (21%) | |
| Targeted | 31 (72%) | |
| Drugs with a positive pCODR recommendation (N, %) | 35 (81%) | |
| Drugs funded in at least one province (N, %) | 31 (72%) | |
| Time to funding (median, IQR) | 379 days (203–601) | |
| List price per 28-day course (median, IQR) | CAD 8213 (5391–9445) |
* Includes all drugs, irrespective of funding status, Abbreviations: I-O, immunotherapy.
3.1. Determinants of Time to Funding (TTF)
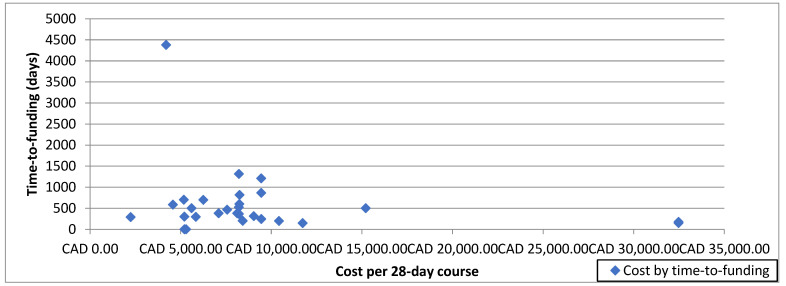
There was no correlation between list price and TTF (correlation coefficient −0.20, p = 0.28) (Figure 1). However, there were significant differences in TTF based on drug characteristics. Among the different tumor types, drugs for NETs had the shortest median TTF of 0 days (IQR 0–502), while drugs to treat CRC had the longest median TTF at 2541 days (IQR 702–4379) (p < 0.001). Among different classes of drugs, immunotherapies had the shortest median TTF at 339 days (IQR 164–446), while targeted therapies had the longest median TTF of 465 days (IQR 245–702) (p = 0.01). See Table 3 for the TTF based on drug characteristics.
Figure 1.
Scatter plot of time-to-funding by cost per 28-day course.
Table 3.
Time to funding based on drug characteristics.
| Drug Characteristic (n = 31) * | Result | p-Value |
|---|---|---|
| Cost | Correlation coefficient | |
| List price | −0.20 | 0.28 |
| Tumor Type | TTF (IQR) | |
| Breast | 302 days (203–587) | |
| Colorectal | 2541 days (702–4379) | |
| Lung | 447 days (295–842) | |
| Melanoma | 379 days (198–465) | |
| Neuroendocrine | 0 days (0–502) † | <0.001 |
| Agent Class | TTF (IQR) | |
| Chemo | 443 days (298–587) | |
| I-O | 339 days (164–446) | |
| Targeted | 465 days (245–702) | <0.01 |
* Includes only drugs that were funded in at least one province (n = 31), † Everolimus and sunitinib for pancreas NETs were funded in British Columbia before Health Canada approval (this is a highly unusual situation). Abbreviations: I-O, immunotherapy; TTF time to funding.
3.2. Determinants of Provincial/Territorial Funding Decisions
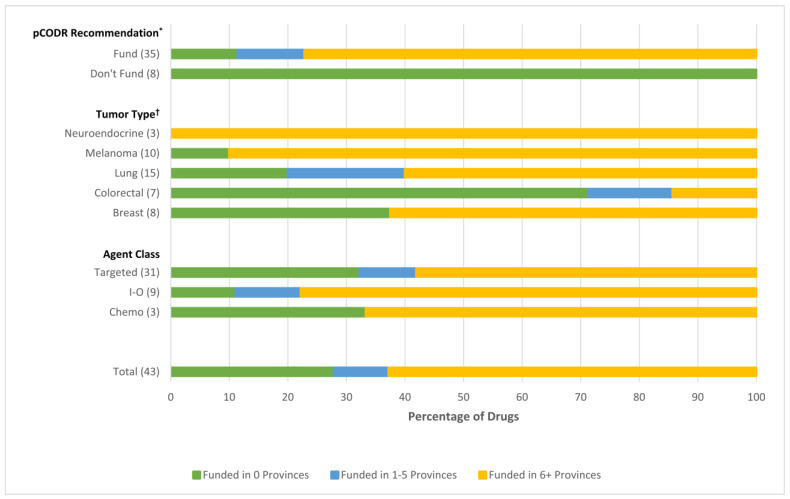
The pCODR recommendation was significantly associated with the provincial/territorial funding decisions (p < 0.001). Of the 35 drugs that received a positive pCODR recommendation, 31 (89%) were funded in at least one province by the time of data cutoff. Of those funded in at least 1 province (n = 31), 4 (13%) were funded in 1–5 provinces, and 27 (87%) were funded in 6 or more provinces. Of the eight drugs that received a negative pCODR recommendation, none have been funded in any province (0% uptake).
Tumor type predicted the decision to fund, with funding in at least one province for 100% of NET drugs, 90% of melanoma drugs, 80% of lung cancer drugs, 63% of breast cancer drugs, and 29% of CRC drugs (p = 0.005) (Figure 2).
Figure 2.
Determinants of drug funding * p < 0.0001, † p = 0.0347. Abbreviations: I-O, immunotherapy.
There was no statistically significant difference in drug funding by drug class, with funding in at least one province for 89% of immunotherapies, 68% of targeted therapies, and 67% of chemotherapies (p = 0.52). In comparison to immunotherapy drugs, the odds ratio (OR) for the decision to fund chemotherapy drugs was 0.11 (95% CI 0.002–6.48), and for targeted drugs was 0.12 (95% CI 0.005–3.09). Statistical significance could not be established due to the small sample size.
3.3. Association of Drug Characteristics and List Price
There were significant differences in list price (for a 28-day course) among drugs for the different tumor types. Melanoma drugs cost the most with a median list price of CAD 9319 (IQR CAD 8120–32,480) while breast cancer drugs had the lowest median list price of CAD 5729 (IQR CAD 4259–7334) (Table 4). Per CAD 500 decrease in list price, the OR for the decision to fund was 1.03 (95% CI 0.96–1.11), and per CAD 1000 decrease in list price, the OR for the decision to fund was 1.06 (95% CI 0.92–1.22). With regard to agent class, immunotherapy had the most expensive list price with a median of CAD 9305 (IQR CAD 8213–32,480), while chemotherapy had the lowest list price at a median of CAD 5631 (IQR CAD 4569–5834). There was no association between list price and the pCODR recommendation or the decision to fund in at least one province (Table 3).
Table 4.
Association of drug features and list price.
| Feature | Category | List Price (Median, IQR) | p-Value |
|---|---|---|---|
| Tumor Type | Breast | CAD 5279 (CAD 4259–7334) | |
| Colorectal | CAD 5391 (CAD 4200–6237) | ||
| Lung | CAD 8251 (CAD 8213–9446) | ||
| Melanoma | CAD 9319 (CAD 8210–32,480) | ||
| Neuroendocrine | CAD 5305 (CAD 5208–5602) | 0.0004 | |
| Agent Class | Chemo | CAD 5631 (CAD 4569–5834) | |
| I-O | CAD 9035 (CAD 8213–32,480) | ||
| Targeted | CAD 7093 (CAD 5208–8427) | 0.0088 | |
| pCODR Recommendation | Fund | CAD 8213 (CAD 5602–9445) | |
| Do not fund | CAD 5934 (CAD 4670–7852) | 0.27 | |
| Funding Decision | Funded in at least one province | CAD 8213 (CAD 5602–9445) | |
| Not funded | CAD 6244 (CAD 4670–8852) | 0.34 |
4. Discussion
The drug regulatory and funding process in Canada is complex and may result in delays in public access to life-prolonging anticancer therapies. While clinicians prescribe medications based on clinical judgement, risks, and benefits, the regulatory approval and funding decisions of cancer drugs in Canadian provinces are ultimately political processes. The specific factors correlated with positive provincial funding decisions and timeliness have been minimally investigated. We examined factors potentially influencing the drug regulatory and funding process including drug price, pCODR recommendation, tumor type, and agent class, to better understand the determinants of cancer drug funding decisions in Canada.
4.1. Impact of Drug Price
While our study showed that the cost of cancer drugs varies widely, it negated the intuitive assumption that the list price of anticancer therapies significantly influences regulatory and funding decisions or their timeliness. In fact, our study suggested no correlation between the list price and the speed with which cancer drugs are funded, and logistic regression did not reveal increased odds of funding due to decreased price. Similarly, there were no significant differences among list price between drugs that received positive or negative pCODR recommendations or between those that did or did not ultimately receive funding in at least one province. These findings may suggest that cost is not the most important driving factor in the drug funding process in Canada. It should be noted though that the publicly available list price does not represent the final price paid by the provinces. This is confidentially negotiated at pCPA prior to provincial listings.
While our study found no association between list price and funding, it is clear that the economic burden of cancer in Canada is a growing area of concern. One study found that between 2005 and 2012, the average annual economic burden of cancer care in Canada increased from CAD 2.9 to 7.5 billion [12]. A 2018 study that surveyed policymakers with cancer drug funding decision-making experience across nine Canadian provinces found that the most common reason cited for a drug to not receive funding despite a positive pCODR recommendation was budget constraints, that said, the majority (64%) of policymakers also cited that negative funding decisions may be made if the drug in question is not a priority for the local tumor group [13]. This may indicate that drug- and cancer-specific factors may play a larger role. Therefore, drugs addressing an unmet need may be considered at the provincial level differently to those where an existing drug in the same class is already available.
Assessing the role of drug cost in drug funding decisions and timeliness may be an over simplification, as cost in isolation makes no comment on the value of the drug in question. Drugs may be better analyzed by their cost effectiveness, which reports the cost of a drug adjusted by its clinical efficacy (for example: the cost per life year gained). A study of cancer care funding decisions in British Columbia up to 2008 [14] showed that there was a negative association between the incremental cost-effectiveness ratio (ICER) per life year gained and the likelihood of receiving a positive funding recommendation. Furthermore, the study found a significant difference between the mean ICER per life year gained among cancer drugs that received positive reimbursement recommendations (CAD 42,006) and those that received negative recommendations (CAD 156,967), indicating that drugs with better cost effectiveness were associated with positive funding recommendations. It would, therefore, seem that raw cost may not be as meaningful in decision-making as cost relative to drug efficacy. Focus groups have identified several factors that the Canadian public considers important when determining cost effectiveness of cancer drugs, including improvement in quality of life, duration of life, restoring patient independence, and improving patient mental health [15].
4.2. Impact of pCODR Recommendation
CADTH/pCODR is responsible for advising the provinces of Canada on cancer drug funding decisions. Clearly, the pCODR recommendation influences provincial funding decisions, particularly when negative, as no drugs in our study with a negative pCODR recommendation were funded in any province. A negative HTA recommendation generally indicates that price negotiations with pCPA will not occur, thereby inhibiting provinces from pursuing funding. pCODR recommendations are based on an assessment of clinical benefit, economics, and patient values [6]. However, there are no publicly stated, transparent weighting schemes or objective thresholds for these criteria [9]. The influences contributing to the pCODR drug funding recommendations are vitally important. Analyses have suggested that a high quality of clinical evidence and a maximum incremental cost effectiveness ratio (ICER) of approximately CAD 140,000 per quality adjusted life year (QALY) are factors related to positive pCODR recommendations [16]. Interestingly, recently, CADTH/pCODR recommended the funding of adjuvant osimertinib in NSCLC if the cost effectiveness can be improved to CAD 50,000 per QALY, at this time necessitating a price reduction of 82% from the current list price [17]. The implications of CADTH making recommendations on pricing, which is also addressed by both the PMPRB and pCPA, is uncertain but, at the time of writing, is adding some degree of confusion to understanding the process.
Given the importance and necessity of the pCODR recommendation prior to provincial funding decisions, the rapidity of the pCODR assessment is critical to timely drug access. While the pCODR does have publicly stated timeline targets to produce their recommendations, these targets are met only 50% of the time [18]. It is clear that pCODR recommendations play a significant role in drug funding decisions in Canada. Thus, timeliness of the pCODR process is necessary for prompt funding decisions and access to potentially life-prolonging cancer drugs. That being said, the HTA process does not represent the longest time delay in approval of new drugs. This can come from earlier in the process, with a delay in applying for Health Canada approval or submission to the HTA process after positive clinical trials, or later in the process, with delays in negotiating the final price to be paid by provinces. A previous review suggested the median time from HTA completion to first public funding is 6.9 months but may be as long as over 2 years [3].
4.3. Impact of Tumor Type
We found that there were significant differences in the decision to fund as well as TTF among drugs used to treat different tumor types. Drugs that treat NETs were frequently funded and had the quickest TTF. However, it should be noted that there was a very small sample size of NET drugs in our study, and two of the three drugs used to treat NETs received funding in British Columbia before Health Canada approval was obtained, which is a rare circumstance and, therefore, may not accurately reflect the average TTF among drugs in this category. After NETs, breast cancer drugs were funded the quickest. On the other end of the spectrum, drugs used to treat CRC were infrequently funded and, when funded, took the longest. In fact, bevacizumab for colorectal cancer had the longest TTF, largely due to a long interval between Health Canada approval and submission to the HTA [3]. The differences in the decisions to fund and TTF by tumor type suggest that the type of cancer may influence funding decisions and urgency. The specific reasons for variable funding and timeliness by tumor type are beyond the scope of this analysis; however, we may hypothesize that various factors may be contributing such as disease prevalence, mortality, other treatments available, and clinical benefit of the drugs. Comparing the analyzed breast cancer and colorectal cancer drugs in our study, a greater proportion of the breast cancer drugs were used in the first line, and the range of PFS and OS gains were objectively more impressive (data not shown), which may account for some differences.
Additionally, it is possible that variable drug funding decisions and timelines by tumor type are related to politics. As cancer drug funding is a highly politicized issue, studies have investigated the role of media in drug funding decisions. One study found that increased media attention toward a drug corresponded with a quicker TTF, for example, for the drug trastuzumab used in breast cancer [19]. Factors cited as potential reasons for the unusually rapid funding of trastuzumab included media coverage and survivorship [19]. Breast cancer is a highly publicized disease [20,21,22,23,24]. We may hypothesize that the strong media presence around breast cancer may contribute to political pressure to fund breast cancer drugs more quickly than therapeutics for other tumor types that are less prevalent in modern media. In fact, political pressure, in the form of advertisements or from pharmaceutical companies, has been cited as a top reason for funding drugs that received a negative pCODR recommendation [13], although this would seem to occur rarely, and there are no examples of this among the drugs included in our study. Therefore, it is possible that these same political pressures influence the timeliness of funding decisions in Canada. While pressures originating in the media may potentially influence drug funding decisions and timeliness, there is no evidence that drug funding decisions are influenced by upcoming political elections. Studies have failed to show a significant difference in drug funding decisions in the 60-day period preceding provincial elections [25]. As such, political factors influencing drug funding decisions may be complex and require further elucidation, and this was beyond the scope of our analysis.
4.4. Impact of Drug Class
We found statistically significant differences in TTF among drugs from different classes. Immunotherapy had the quickest TTF, while targeted therapies and chemotherapies took longer to receive funding approval. Immunotherapy is a relatively new and expanding area of cancer treatment, while chemotherapies have been used in the treatment of cancer since the early 20th century [26,27]. Therefore, there may be more pressure to approve and fund immunotherapies to expand this emerging treatment option. Relative efficacies and clinical benefits may play a role as well. In a systematic review, it was found that patients treated with immunotherapy were less likely to experience or die of treatment-related adverse events compared to those treated with chemotherapy [28]. Similarly, while there was no statistically significant difference in the decision to fund by agent class, numerically, a greater proportion of immunotherapy drugs were funded. The odds of funding chemotherapy drugs and targeted drugs were low in relation to those for immunotherapy drugs in logistic regression, although the sample size was too small to establish statistical significance. This may suggest a predisposition to fund novel agents such as immunotherapy.
4.5. Limitations
There are imitations with respect to this study. First, while we analyzed correlations among various factors and subsequent provincial funding decisions and timeliness, the study was not designed to assess causation, and therefore, definitive conclusions on the specific determinants of cancer drug funding cannot be drawn. While a multivariate analysis was conducted for the odds of funding drugs by agent class and list price, all four variables could not be included in the analysis due to inherent limitations in the statistical model. It was also not possible to conduct multivariate analysis for the TTF endpoint due to the small sample size. Second, we used list price to compare cost among the different drugs analyzed; however, provinces receive discounted drug prices following confidential negotiations with pCPA. Thus, the list price may not be an accurate reflection of the actual cost of the drugs. Third, in our calculations of TTF, we measured time to first provincial funding, which may not accurately reflect time delays across the remainder of the provinces. This method was selected to provide the most conservative time delay estimate, recognizing that the remainder of the provinces may take longer to fund or not fund at all. Lastly, our paper did not consider the impact of less tangible or measurable factors, such as the impact of “thought-leaders” for individual tumor types.
Finally, since these data were collected, the pCODR program has been amalgamated into the broader CADTH Reimbursement Review process.
5. Conclusions
In our analysis of 43 cancer drugs undergoing the funding decision process in Canada, we found that tumor type, drug class, and pCODR recommendation were determinants of the decision to fund and TTF by Canadian provinces. Meanwhile, drug price did not appear to play a significant role in funding decisions. There are many factors that may contribute to these findings, including drug clinical efficacy, cost effectiveness, and political pressure, but definitive conclusions are beyond the scope of this analysis. Given the relative importance of the pCODR recommendation in comparison to the list price, we may focus our efforts on expediting the submission to the HTA, the HTA process itself, and the funding approval process post HTA completion. Future studies should further investigate the role that these factors play in cancer drug funding decisions.
Author Contributions
Conceptualization, J.G., D.J.S. and P.W.-P.; data curation, J.G. and J.J.W.S.; formal analysis, J.G. and R.M.; methodology, J.G., D.J.S., R.M. and P.W.-P.; supervision, P.W.-P.; writing—original draft, A.J.; writing—review and editing, J.G., D.J.S. and P.W.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study because all data points collected were publicly available.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data on drug details, approvals, and list prices: Reimbursement Review Reports; CADTH: Available online: https://cadth.ca/reimbursement-review-reports (accessed on 18 December 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Committee CCSA . Canadian Cancer Statistics 2019. Canadian Cancer Society; Toronto, ON, Canada: 2019. [Google Scholar]
- 2.Miller K.D., Siegel R.L., Lin C.C., Mariotto A.B., Kramer J.L., Rowland J.H., Stein K.D., Alteri R., Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 3.Gotfrit J., Shin J.J., Mallick R., Stewart D.J., Wheatley-Price P. Potential Life-Years Lost: The Impact of the Cancer Drug Regulatory and Funding Process in Canada. Oncolgist. 2020;25:e130–e137. doi: 10.1634/theoncologist.2019-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.How Drugs are Reviewed in Canada: Government of Canada. 2001. [(accessed on 8 January 2021)]. Available online: https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/fact-sheets/drugs-reviewed-canada.html.
- 5.The pCODR Expert Review Committeee (pERC): CADTH. 2015. [(accessed on 8 January 2021)]. Available online: https://www.cadth.ca/collaboration-and-outreach/advisory-bodies/pcodr-expert-review-committee-perc.
- 6.Procedures for the CADTH pan-Canadian Oncology Drug Review: CADTH. 2020. [(accessed on 8 January 2021)]. Available online: https://www.cadth.ca/sites/default/files/pcodr/pCODR%27s%20Drug%20Review%20Process/pcodr-procedures.pdf.
- 7.Stewart D.J., Stewart A.A., Wheatley-Price P., Batist G., Kantarjian H.M., Schiller J., Clemons M., Bradford J.-P., Gillespie L., Kurzrock R. The importance of greater speed in drug development for advanced malignancies. Cancer Med. 2018;7:1824–1836. doi: 10.1002/cam4.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CADTH Reimbursement Reviews: CADTH. 2020. [(accessed on 6 May 2021)]. Available online: https://cadth.ca/cadth-reimbursement-reviews.
- 9.Skedgel C., Younis T. The Politicization of Oncology Drug Funding Reviews in Canada. Curr. Oncol. 2016;23:139–143. doi: 10.3747/co.23.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younis T., Skedgel C. Timeliness of the Oncology Drug Review Process for Public Funding in Canada. Curr. Oncol. 2017;24:279–281. doi: 10.3747/co.24.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Driedger S.M., Cooper E., Annable G., Brouwers M. “There is always a better way”: Managing uncertainty in decision making about new cancer drugs in Canada. Int. J. Health Plann. Manag. 2018;33:e485–e499. doi: 10.1002/hpm.2492. [DOI] [PubMed] [Google Scholar]
- 12.de Oliveira C., Weir S., Rangrej J., Krahn M.D., Mittmann N., Hoch J.S., Chan K.K.W., Peacock S. The economic burden of cancer care in Canada: A population-based cost study. CMAJ Open. 2018;6:E1–E10. doi: 10.9778/cmajo.20170144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Srikanthan A., Penner N., Chan K., Sabharwal M., Grill A. Understanding the Reasons for Provincial Discordance in Cancer drug Funding—A Survey of Policymakers. Curr. Oncol. 2018;25:257–261. doi: 10.3747/co.25.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ismail Z., Peacock S.J., Kovacic L., Hoch J.S. Cost-effectiveness impacts cancer care funding decisions in british columbia, canada, evidence from 1998 to 2008. Int. J. Technol. Assess. Heal. Care. 2017;33:481–486. doi: 10.1017/S0266462317000642. [DOI] [PubMed] [Google Scholar]
- 15.Bentley C., Peacock S., Abelson J., Burgess M.M., Demers-Payette O., Longstaff H., Tripp L., Lavis J.N., Wilson M.G. Addressing the affordability of cancer drugs: Using deliberative public engagement to inform health policy. Heal. Res. Policy Syst. 2019;17:1–10. doi: 10.1186/s12961-019-0411-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skedgel C., Wranik D., Hu M. The Relative Importance of Clinical, Economic, Patient Values and Feasibility Criteria in Cancer Drug Reimbursement in Canada: A Revealed Preferences Analysis of Recommendations of the Pan-Canadian Oncology Drug Review 2011–2017. Pharmaco Economics. 2018;36:467–475. doi: 10.1007/s40273-018-0610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.CADTH CADTH Reimbursement Recommendation (Draft): Osimertinib (Tagrisso) 2021. [(accessed on 15 November 2021)]. Available online: https://cadth.ca/sites/default/files/DRR/2021/PC0246%20Tagrisso%20-%20Draft%20CADTH%20Recommendation%20For%20posting%20September%202%2C%202021.pdf.
- 18.Srikanthan A., Mai H., Penner N., Amir E., Laupacis A., Sabharwal M., Chan K.K.W. Impact of the Pan-Canadian Oncology Drug Review on Provincial Concordance with Respect to Cancer Drug Funding Decisions and Time to Funding. Curr. Oncol. 2017;24:295–301. doi: 10.3747/co.24.3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Booth C.M., Dranitsaris G., Gainford M.C., Berry S., Fralick M., Fralick J., Sue J., Clemons M. External influences and priority-setting for anti-cancer agents: A case study of media coverage in adjuvant trastuzumab for breast cancer. BMC Cancer. 2007;7:110–118. doi: 10.1186/1471-2407-7-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.About the Origins of Pink Ribbon International: Pink Ribbon International. [(accessed on 11 November 2021)]. Available online: http://pinkribbon.org/about/
- 21.AbiGhannam N., Chilek L.A., Koh H. Three Pink Decades: Breast Cancer Coverage in Magazine Advertisements. Heal. Commun. 2017;33:462–468. doi: 10.1080/10410236.2016.1278496. [DOI] [PubMed] [Google Scholar]
- 22.Our Corporate Partners: National Breast Cancer Foundation. [(accessed on 11 November 2021)]. Available online: https://www.nationalbreastcancer.org/nbcf-corporate-partners.
- 23.Our Partners: Breast Cancer Research Foundation. [(accessed on 11 November 2021)]. Available online: https://www.bcrf.org/partners/
- 24.Tan A.S.L. A Study of the Frequency and Social Determinants of Exposure to Cancer-Related Direct-to-Consumer Advertising Among Breast, Prostate, and Colorectal Cancer Patients. Heal. Commun. 2015;30:1102–1111. doi: 10.1080/10410236.2014.921752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srikanthan A., Gill S., Chan K. Provincial Elections and Timing of Cancer Drug Funding. Curr. Oncol. 2016;23:154–163. doi: 10.3747/co.23.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergman P.J. Cancer Immunotherapies. Vet. Clin. N. Am. Small Anim. Pract. 2019;49:881–902. doi: 10.1016/j.cvsm.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 27.DeVita V.T., Jr., Chu E. A History of Cancer Chemotherapy. Cancer Res. 2008;68:8643–8653. doi: 10.1158/0008-5472.CAN-07-6611. [DOI] [PubMed] [Google Scholar]
- 28.Magee D.E., Hird A.E., Klaassen Z., Sridhar S.S., Nam R.K., Wallis C.J.D., Kulkarni G.S. Adverse event profile for immunotherapy agents compared with chemotherapy in solid organ tumors: A systematic review and meta-analysis of randomized clinical trials. Ann. Oncol. 2020;31:50–60. doi: 10.1016/j.annonc.2019.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data on drug details, approvals, and list prices: Reimbursement Review Reports; CADTH: Available online: https://cadth.ca/reimbursement-review-reports (accessed on 18 December 2021).