Abstract
Bactericidal/permeability-increasing protein (BPI) inhibited growth of cell wall-deficient Acholeplasma laidlawii and L forms of certain strains of Staphylococcus aureus and Streptococcus pyogenes. However, the same strains of S. aureus and S. pyogenes with intact cell walls were not susceptible to the growth-inhibitory effects of BPI.
Bactericidal/permeability-increasing protein (BPI), a 55-kDa basic protein found in azurophilic granules of polymorphonuclear leukocytes, has been reported to be cytotoxic for many gram-negative bacteria but not for gram-positive bacteria (1, 2). BPI binds to and permeabilizes the outer gram-negative bacterial lipopolysaccharide layer, initiating events that lead ultimately to cell death (9).
A recombinant N-terminal fragment (rBPI23) consisting of the first 199 amino acids of human BPI has been shown to retain the bactericidal activity of neutrophil-derived BPI (3, 10). We have produced several other recombinant BPI proteins, including full-length BPI (rBPI) (4, 5), a dimer of N-terminal recombinant BPI (rBPI42) (5), and rBPI21, an analog of rBPI23 consisting of the first 193 amino acids of BPI and lacking one of three native cysteines (at position 132, replaced with an alanine); unlike rBPI23, rBPI21 does not form a dimer (5).
In previous studies, BPI reportedly did not kill gram-positive bacteria (1) but was able to inhibit both oxygen consumption and energy-dependent amino acid transport by isolated membrane vesicles from both gram-positive and gram-negative bacteria (6). We hypothesized that the reported lack of sensitivity of intact gram-positive bacteria to BPI could be due to protection of the cytoplasmic membrane by the cell wall and that gram-positive bacteria growing without cell walls (i.e., as L forms [8]) might be susceptible to BPI. In this study, we examined the effects of BPI on two gram-positive bacteria growing with and without their cell walls and on Acholeplasma laidlawii, which naturally lacks a cell wall (7).
The rBPI21 and rBPI proteins used for these experiments were purified as previously described (4, 5). rBPI42 (dimer) was purified from a mixture of rBPI23 monomer and dimer by size exclusion chromatography. Proteins were serially diluted in 5 mM sodium citrate (pH 5.0)–150 mM NaCl prior to use.
Escherichia coli J5 (9) was grown as previously described (4, 5). Staphylococcus aureus bacterial form (ATCC 19636) was grown in heart infusion (HI) broth. S. aureus L form (ATCC 19640), derived from S. aureus ATCC 19636, was grown in HI broth containing 3.5% NaCl, 10 mM CaCl2, and 1,000 units of penicillin G per ml (to ensure maintenance of the L-form state). Streptococcus pyogenes bacterial form (ATCC 25663) was grown in brain heart infusion (BHI) broth. S. pyogenes L form (ATCC 27080), derived from S. pyogenes ATCC 25663, was maintained on BHI agar medium supplemented with 0.5% (wt/vol) yeast extract, 0.93% NaCl, 9.7% sucrose, 0.025% MgSO4, 1% horse serum, and 1,000 units of penicillin G per ml. For broth growth, small agar blocks containing S. pyogenes L-form colonies were transferred to the above medium supplemented with 10% horse serum and were incubated for ∼24 h at 37°C under 5% CO2. A. laidlawii (ATCC 23206) was grown in HI broth supplemented with 10% (vol/vol) yeast extract and 1% PPLO serum fraction (Difco, Detroit, Mich.).
For radial diffusion assays, cultures were grown in their respective broth media and added to molten 0.8% agarose medium at ∼3 × 105 cells/ml. The agarose media were the same as those used for broth growth except that the S. aureus L-form medium lacked CaCl2, the S. pyogenes L-form medium contained 1% horse serum, and the E. coli J5 medium consisted of HI broth. Five microliters of serially diluted rBPI21, rBPI42, or rBPI or of dilution buffer was added to 3-mm-diameter wells. The plates were incubated at 37°C for 24 h. Inhibition zone diameters were plotted versus BPI concentration. Each assay was performed at least three times for E. coli J5 and the gram-positive bacterial forms and L forms and twice for A. laidlawii.
For the broth microdilution assay, E. coli J5 and S. aureus L-form and bacterial-form cells were grown to log phase in their respective media and diluted to ∼3 × 105 cells/ml in the same media. Five microliters of rBPI21, rBPI42, and rBPI dilutions were added to 95 μl of cells in 96-well plates and were incubated for ∼18 h at 37°C. The results from duplicate samples are expressed in relation to the control (buffer only), for which the value was set at 100%.
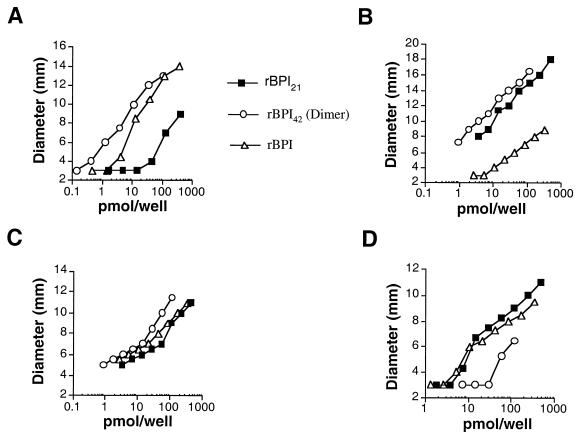
Radial diffusion assays (Fig. 1) demonstrated that the tested BPI proteins inhibited growth of A. laidlawii and the L forms of S. aureus and S. pyogenes at concentrations as low as 1 pmol/well (Fig. 1A, B, and C). By comparison, none of the tested BPI proteins, up to ∼470 pmol/well, inhibited growth of the tested S. aureus and S. pyogenes bacterial forms (not shown). As expected, the BPI proteins inhibited growth of E. coli J5 (Fig. 1D). The L forms, the mycoplasma, and E. coli J5 were similarly sensitive to the various BPI proteins. On a molar basis, rBPI42 was most potent against A. laidlawii and the L forms but least potent against E. coli J5. Conversely, rBPI21 was most potent against E. coli J5 but was least potent against A. laidlawii and the S. aureus L form. rBPI was more potent than rBPI21 against A. laidlawii and the S. aureus L form but was least potent against the S. pyogenes L form.
FIG. 1.
Growth inhibition of a mycoplasma and the L forms of gram-positive bacteria by BPI in a radial diffusion assay. The measured diameter includes the 3-mm hole to which the samples were added. (A) S. aureus L form. (B) S. pyogenes L form. (C) A. laidlawii. (D) E. coli J5.
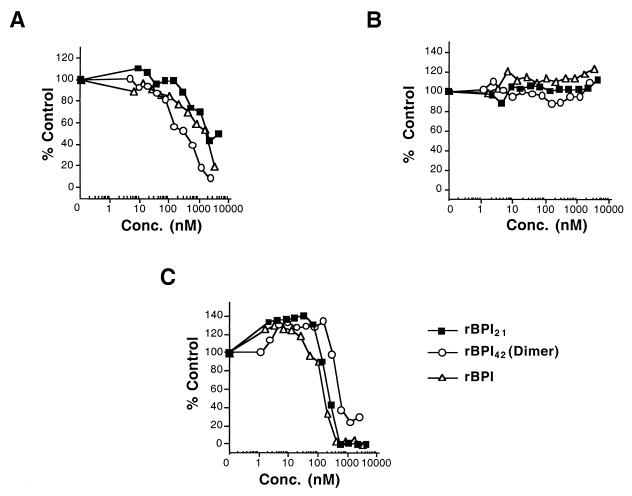
We next examined the effects of rBPI21, rBPI42, and rBPI on S. aureus and its L form in a broth microdilution assay. The tested BPI molecules inhibited growth of the S. aureus L form (Fig. 2A) but not the bacterial form (Fig. 2B). rBPI42 was the most potent while rBPI21 was the least potent protein against this L form. Against E. coli J5 (Fig. 2C), rBPI21 and rBPI were the most potent while rBPI42 was the least potent protein.
FIG. 2.
Growth inhibition of S. aureus and E. coli J5 in a broth microdilution assay. (A) S. aureus L form. (B) S. aureus bacterial form. (C) E. coli J5.
In previous studies, neutrophil-derived BPI did not kill gram-positive bacteria even at concentrations up to 1,000-fold higher than those typically effective against gram-negative bacteria (1). Under the assay conditions of this study, several recombinant human BPI forms were not cytotoxic for S. aureus and S. pyogenes, whereas they inhibited growth of the L forms of these gram-positive bacteria. They also inhibited growth of A. laidlawii. The L forms and Acholeplasma were approximately as sensitive to BPI as the gram-negative bacterium E. coli J5. Based on these results, we conclude that BPI must have similar direct cytotoxic effects on the cytoplasmic membranes of these organisms. These results indicate further that the cell walls of the tested gram-positive bacteria protect them from the BPI proteins used in this study.
Acknowledgments
We thank Marc Better, Steve Carroll, Pat Scannon, and Randolph Wall for critical reading of the manuscript.
This work was supported by XOMA (U.S.) LLC.
REFERENCES
- 1.Elsbach P, Weiss J. Oxygen-independent antimicrobial systems of phagocytes. In: Gallin J I, Goldstein I M, Snyderman R, editors. Inflammation: basic principles and clinical correlates. 2nd ed. New York, N.Y: Raven Press; 1992. pp. 603–636. [Google Scholar]
- 2.Elsbach P, Weiss J. The bactericidal/permeability-increasing protein (BPI), a potent element in host-defense against gram-negative bacteria and lipopolysaccharide. Immunobiology. 1993;187:417–429. doi: 10.1016/S0171-2985(11)80354-2. [DOI] [PubMed] [Google Scholar]
- 3.Gazzano-Santoro H, Parent J B, Grinna L, Horwitz A, Parsons T, Theofan G, Elsbach P, Weiss J, Conlon P J. High-affinity binding of the bactericidal/permeability-increasing protein and a recombinant amino-terminal fragment to the lipid A region of lipopolysaccharide. Infect Immun. 1992;60:4754–4761. doi: 10.1128/iai.60.11.4754-4761.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horwitz A H, Williams R E, Nowakowski G. Human lipopolysaccharide binding protein potentiates bactericidal activity of human bactericidal/permeability-increasing protein. Infect Immun. 1995;63:522–527. doi: 10.1128/iai.63.2.522-527.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horwitz A H, Leigh S D, Abrahamson S, Gazzano-Santoro H, Liu P-S, Williams R E, Carroll S F, Theofan G. Expression and characterization of cysteine-modified variants of an amino terminal fragment of bactericidal/permeability-increasing protein. Protein Expr Purif. 1996;8:28–40. doi: 10.1006/prep.1996.0071. [DOI] [PubMed] [Google Scholar]
- 6.In’t Veld G, Mannion B, Weiss J, Elsbach P. Effects of the bactericidal/permeability-increasing protein of polymorphonuclear leukocytes on isolated bacterial cytoplasmic membrane vesicles. Infect Immun. 1988;56:1203–1208. doi: 10.1128/iai.56.5.1203-1208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenney G. Mycoplasmas. In: Balows A, Hausler W Jr, Herrmann K L, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C: American Society for Microbiology; 1991. pp. 478–482. [Google Scholar]
- 8.Madoff S. Introduction to the bacterial L-forms. In: Madoff S, editor. The bacterial L-forms. New York, N.Y: Marcel Dekker, Inc.; 1986. pp. 1–20. [Google Scholar]
- 9.Mannion B A, Weiss J, Elsbach P. Separation of the sub-lethal and lethal effects of the bactericidal/permeability-increasing protein on Escherichia coli. J Clin Investig. 1990;85:853–860. doi: 10.1172/JCI114512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiss J, Elsbach P, Shu C, Castillo J, Grinna L, Horwitz A, Theofan G. Human bactericidal/permeability-increasing protein and a recombinant N-terminal fragment cause killing of serum-resistant gram-negative bacteria in whole blood and inhibit TNF release induced by the bacteria. J Clin Investig. 1992;90:1122–1130. doi: 10.1172/JCI115930. [DOI] [PMC free article] [PubMed] [Google Scholar]