Abstract
Anautogenous female mosquitoes obtain the nutrients needed for egg development from vertebrate blood, and consequently they transmit numerous pathogens of devastating human diseases. Digestion of blood proteins into amino acids that are used for energy production, egg maturation and replenishment of maternal reserves is an essential part of the female mosquito reproductive cycle. However, the regulatory mechanisms underlying this process remain largely unknown. Here, we report that the transcription factor E93 is a critical factor promoting blood meal digestion in adult females of the major arboviral vector Aedes aegypti in response to the steroid hormone 20-hydroxyecdysone (20E). E93 was upregulated in the female mosquito midgut after a blood meal, and RNA interference (RNAi)-mediated knockdown of E93 inhibited midgut blood digestion. E93 RNAi silencing repressed late trypsin (LT), serine protease I (SPI), SPVI and SPVII, and activated early trypsin (ET) expression in the female mosquito midgut after a blood meal. Injection of 20E activated E93, LT, SPI, SPVI and SPVII, and repressed ET expression, whereas RNAi knockdown of the ecdysone receptor (EcR) repressed E93, LT, SPI, SPVI and SPVII, and activated ET expression in the midgut. Furthermore, E93 silencing resulted in a complete loss of 20E responsiveness of LT, SPVI and SPVII. Our findings reveal important mechanisms regulating blood meal digestion in disease-transmitting mosquitoes.
Keywords: E93, steroid hormone, 20-hydroxyecdysone, serine protease, blood meal digestion, Mosquito
Graphical Abstract

1. Introduction
Blood-feeding mosquitoes are effective vectors of many devastating human diseases, such as malaria, dengue fever, yellow fever, chikungunya and Zika virus, causing hundreds of thousands of human deaths annually, worldwide (Barrett and Higgs 2007; McNeil and Shetty 2017; Tsetsarkin, et al. 2016). The adult females require blood from a vertebrate host to provide the necessary nutrients for egg production, and multiple cycles of blood feeding and egg development thus promote pathogen transmission from host to host. The most effective approach to slowing the spread of mosquito-borne diseases is to control mosquito populations in areas of high pathogen transmission. However, increasing insecticide resistance of the mosquito and the collateral damage of insecticides to other organisms add to the urgency of exploring alternative strategies of vector control. After acquisition, the blood is quickly digested in the mosquito midgut into amino acids that are used by the fat body for biosynthesis of yolk protein precursors (YPPs), which are then packaged into developing oocytes for egg maturation (Briegel 2003; Raikhel and Dhadialla 1992). Therefore, inhibition of blood digestion in the mosquito midgut could reduce egg production and has been considered an effective strategy for population control (Isoe, et al. 2009b). A detailed understanding of blood-digestion regulation at the molecular level could provide valuable clues for designing novel approaches to control mosquito populations and, thus, mosquito-borne diseases.
Every reproductive cycle of a female mosquito is divided into two phases, posteclosion (PE) and post blood meal (PBM), which are governed by the sesquiterpenoid juvenile hormone (JH) and the steroid hormone 20-hydroxyecdysone (20E), respectively. In the Aedes aegypti female, the JH-dependent PE development, which lasts 3–5 days from adult eclosion to blood feeding, is critical for competence acquisition for blood feeding and subsequent egg maturation (Zhu, et al. 2003; Zou, et al. 2013). JH action is mediated by Methoprene-tolerant (Met), a member of the family of basic helix-loop-helix (bHLH)-Per-Arnt-Sim (PAS) transcription factors (Jindra, et al. 2013). The ingestion of blood by a female mosquito initiates a cascade of events in different tissues, including digestion of blood proteins into amino acids in the midgut, as well as massive synthesis and secretion of YPPs in the fat body and their subsequent accumulation by developing oocytes (Attardo, et al. 2005; Briegel 2003). The action of 20E is mediated by the ecdysone receptor (EcR), which requires Ultraspiracle (USP) to form a heterodimer complex. This complex directly activates several primary response genes, including E74, E75 and Broad, which in turn regulate many secondary response genes that control stage- and tissue-specific developmental responses to 20E (King-Jones and Thummel 2005; Nakagawa and Henrich 2009; Yamanaka, et al. 2013).
Vertebrate blood consists primarily of protein, with only small amounts of lipid and carbohydrate (Zhou, et al. 2004). Proteolytic enzymes secreted into the midgut lumen of the female mosquitoes after feeding are responsible for degrading blood proteins into oligopeptides and amino acids. The majority of secreted proteases are endoproteolytic serine proteases (SPs), aminopeptidases and carboxypeptidases. SPs function as the main enzymes in blood digestion, and Ae. aegypti express 12 SP-like genes in the midgut (Isoe, et al. 2009a). Transcription of early trypsin (ET) and JA15 occurs prior to blood feeding, whereas late trypsin (LT), SPI, SPVI and SPVII are expressed in the midgut after a blood meal. In contrast, SPII-V are constitutively expressed in the female midgut (Bian, et al. 2008; Brackney, et al. 2010; Noriega, et al. 1997). Previous studies have shown that JH controls expression of ET and JA15, and that insulin-like peptide 3 (ILP3) and the target of rapamycin pathway coordinately regulate LT and SPVI expression (Bian, et al. 2008; Gulia-Nuss, et al. 2011; Li, et al. 2011; Noriega, et al. 1997). However, the entire molecular complexity of regulatory mechanisms underlying blood digestion in female mosquitoes is not completely understood.
The helix-turn-helix transcription factor Eip93F (E93) was first characterized as a critical regulator of programmed cell death during Drosophila metamorphosis (Lee, et al. 2000; Yin and Thummel 2005). Later studies showed that E93 functions as the key determinant that promotes adult metamorphosis in both the hemimetabolous insect Blattella germanica and holometabolous insect Tribolium castaneum (Ureña, et al. 2014). Recent studies have indicated that E93 is also involved in female reproduction in the brown planthopper Nilaparvata lugens and T. castaneum (Eid, et al. 2020; Mao, et al. 2019). Our previous transcriptomic analyses revealed that the Ae. Aegypti E93 (AAEL004572) is strongly upregulated in female mosquitoes after a blood meal (Roy, et al. 2015), indicating its involvement in blood meal-activated reproductive events in female mosquitoes. In the present study, we have demonstrated that the transcription factor E93 is indispensable for blood meal digestion in Ae. aegypti adult females. E93 RNA interference (RNAi) disturbed midgut SP expression and reduced trypsin-like activity in the midguts. 20E activated E93, LT, SPI, SPVI and SPVII expression in the midgut. However, E93 silencing resulted in a complete loss of 20E responsiveness of LT, SPVI and SPVII. This study provides a better understanding of the molecular mechanisms underlying blood meal digestion in disease-transmitting mosquitoes and valuable clues for designing novel strategies to control mosquito populations and, thus, mosquito-borne diseases.
2. Materials and Methods
2.1. Mosquito rearing
Ae. aegypti larvae were reared at 27 °C in water supplemented with complete larval food, as described previously (Roy, et al. 2007), and adults were reared at 27 °C and 80% humidity with unlimited access to water and 10% (wt/vol) sucrose solution. Four-day-old female mosquitoes were blood-fed on White Leghorn chickens. The use of vertebrate animals was approved by the University of California, Riverside, Institutional Animal Care and Use Committee.
2.2. dsRNA-mediated gene silencing
dsRNA was synthesized using the MEGAscript kit (Ambion) and purified using the MEGAclear Transcription Clean-Up Kit (Ambion), as previously described (Zou, et al. 2013). The bacterial luciferase gene was used to generate control iLuc dsRNA. Primers used for dsRNA synthesis are listed in Table S1. For E93, EcR and InR knockdown, 1 μg (0.5 μL of 2 μg/μL) of the desired dsRNA was injected into the thorax of cold-anesthetized female mosquitoes at 24 h PE using the Picospritzer II (General Valve). Mosquitoes were allowed to recover for 4 days before blood feeding. For Met knockdown, 1 μg (0.5 μL of 2 μg/μL) of dsMet was injected into the thorax of cold-anesthetized female mosquitoes at 6 h PE. The knockdown efficiency was examined using qPCR.
2.3. RNA isolation and qPCR
Total RNA was extracted from various tissues using TRIzol (Invitrogen) and treated with DNase I (Invitrogen) to remove genomic DNA, according to the manufacturer’s instructions. cDNAs were synthesized using ProtoScript II First Strand cDNA Synthesis Kit (New England BioLabs), and qPCR was performed using the SYBR Green Supermix (Bio-Rad), all according to the manufacturers’ instructions. All primers used are listed in Table S1. Each sample was measured in triplicate, and relative expression was calculated using the 2−ΔΔCt method and normalized to the housekeeping gene RPS7.
2.4. Analysis of blood digestion, ovary development, and fecundity
At 24 and 48 h PBM, female mosquitoes were dissected in 1 × PBS at room temperature. Midguts and ovaries were visualized under the Leica M165FC stereo microscope. Ovarian follicle size and egg length were measured under a Zeiss Axio Observer.A1 microscope. For oviposition, mated and blood fed females at 48 h PBM were kept individually in small cages lined with moist paper towel for three days. The number of eggs laid per female on the paper were then counted using a dissecting microscope. To determine egg hatching rate, the paper with eggs was stored under high humidity for three days and then submerged in 30 mL of distilled water under partial vacuum (25 lb per in2) for synchronous hatching (Barbosa and Peters 1969). The hatched larvae were recorded as a percentage of the total number of starting eggs using a dissecting microscope. Each treatment was repeated three times with 6–10 individuals in each replication.
2.5. Trypsin-like activity assay
Each midgut was dissected at different time points after a blood meal, transferred to 100 μL of 20 mM Tris (pH 8 with 20 mM CaCl2), homogenized on ice with a pellet pestle and then centrifuged at 14,000 × g for 2 min. The supernatants were frozen at ‒80 °C until use. 5-μL aliquots were added to 100 μL of 4 mM Nα-Benzoyl-DL-arginine p-nitroanilide hydrochloride (BAPNA) (Sigma) for 10 min. Absorbance was measured at 405 nm with SpectraMax 190 Absorbance plate reader. Each treatment was replicated five times and three biological replicates were repeated.
2.6. In vivo 20E injection
Female mosquitoes, with or without dsRNA treatment, at 96 h PE were used for 20E injection. A 2 × 10−2 M 20E stock solution was prepared by dissolving 20E (Sigma) in ethanol. A 10−4 M 20E working solution was prepared by dissolving 2 μL 20E stock solution in 400 μL 1 × PBS immediately before use. A control (solvent) working solution was prepared by dissolving 2 μL ethanol in 400 μL 1 × PBS. A 0.5-μL aliquot of the 20E or control working solution was injected into the thorax of cold-anesthetized mosquitoes using the Picospritzer II (General Valve). Midguts were dissected at 6 h after injection and used for qPCR analysis.
2.7. In vitro midgut culture
Midguts were dissected from female mosquitoes at 96 h PE and cultured in medium as previously described (Roy, et al. 2007). To examine the effect of 20E on SP expression, midguts were incubated in medium containing either solvent (ethanol) or 10 μM 20E for 6 h. To examine the effect of CHX on SP expression, a complete medium supplemented with a mixture of amino acids was used. The concentration of amino acids was diluted 10-fold compared with that described in the previous paper (Roy, et al. 2007). Midguts were incubated in the complete medium containing either solvent (ethanol), 20 μM CHX, 10 μM 20E, or 20 μM CHX and 10 μM 20E for 6 h. After these incubations, midguts were harvested, RNA was isolated by TRIzol, and SP expression was determined using qPCR.
2.8. In vivo insulin injection
A 2 mg/mL bovine insulin stock solution was prepared by dissolving 50 mg bovine insulin powder (Sigma) in 25 mL water acidified to pH 2 with 250 μL glacial acetic acid. A 0.5 mg/mL bovine insulin working solution was prepared by dissolving 125 μL bovine insulin stock solution in 375 μL 1 × PBS immediately before use. A control (solvent) working solution was prepared by dissolving 1.25 μL glacial acetic acid in 500 μL 1 × PBS. A 0.5-μL aliquot of the bovine insulin or control working solution was injected into the thorax of cold-anesthetized female mosquitoes at 96 h PE using the Picospritzer II (General Valve). Midguts were dissected at 6 h post-injection and used for qPCR analysis.
2.9. Methoprene topical application
To examine the effect of methoprene on the expression of PE SP before blood feeding, methoprene (1 ng/insect, Sigma) in acetone as solvent or acetone alone was applied topically to the abdomens of female mosquitoes within 1 h PE, and midguts were dissected at 9 h PE and used for qPCR analysis. To examine this same effect after a blood meal, methoprene (10 ng/insect) in acetone or acetone alone was applied topically to the abdomens of female mosquitoes within 1 h PBM, and midguts were dissected at 24 h PBM and used for qPCR analysis. Using this method, the efficiency of methoprene treatment was tested by verifying the induction of the established JH-activated gene Hairy.
2.10. Statistical Analysis
Data are presented as mean ± SEM of three independent biological replicates with three technical replicates, unless otherwise noted. All analyses were performed using SPSS (version 13) software. Differences were assessed using independent-samples t-tests at the following significance levels: *P < 0.05, **P < 0.01, and ***P < 0.001.
3. Results
3.1. E93 is induced in the midgut after a blood meal and indispensable for blood digestion and subsequent egg development in adult female mosquitoes
We first determined the expression levels of E93 among different tissues of female adults using quantitative PCR (qPCR). The results showed that the E93 transcript level was low in all tissues at 72 h PE, but significantly higher at 24 h PBM. By 48 h PBM, it decreased in the midgut, fat body and leftover tissue to the same level as that observed before blood feeding, but remained high in the ovary (Fig. 1A). Notably, at 24 h PBM, the expression was highest in the midgut, followed by the ovary and fat body (Fig. 1A). A more-detailed time-course expression analysis of E93 in the midgut showed that its transcript level was higher immediately after a blood meal, peaked at 36 h PBM, decreased rapidly at 48 h PBM and remained low thereafter (Fig. 1B).
Fig. 1. E93 is upregulated and essential for blood digestion in the midgut of female mosquitoes after a blood meal.

(A) Relative mRNA levels of E93 in the midgut, fat body, ovary and leftover tissue of female mosquitoes at 72 h PE and at 24 and 48 h PBM. (B) Relative mRNA levels of E93 in the female mosquito midgut analyzed at 72 and 96 h PE and at 6, 24, 36, 48, 72 and 96 h PBM using qPCR. Fold changes are relative to the expression of E93 at 72 h PE, arbitrarily set to 1. Mean ± SEM from three independent experiments. (C) Schematic representation of the study design. (D) Effect of E93 knockdown on blood meal digestion in the midgut observed at 24 and 48 h PBM. Midguts were visualized under the Leica M165FC stereo microscope. Scale bars, 1 mm. Images are representative of three independent experiments, with a total of 40 females analyzed.
We then knocked down the expression of E93 using RNAi. Female mosquitoes at 24 h PE were injected with either dsE93 (iE93) or dsLuciferase (iLuc) as a control and were fed blood 4 days later (Fig. 1C). Compared with the iLuc control, E93 mRNA level was significantly lower in the midgut, fat body and ovary of iE93 female mosquitoes at 24 h PBM (Fig. S1A). Strikingly, the knockdown of E93 resulted in dramatic defects in blood digestion in the midgut of female mosquitoes. At 24 h PBM, all the midguts from the wild-type (WT) and iLuc female mosquitoes contained a compact, dark-brown bolus of digested blood, whereas 90% of the midguts from iE93 females were filled with bright-red blood, suggesting that the blood had not yet been digested. At 48 h PBM, the WT and iLuc mosquitoes have completed digestion of the ingested blood, as all of their midguts were empty of blood. In contrast, 87.5% of the midguts of iE93 mosquitoes were still filled with blood (Fig. 1D). Furthermore, E93 knockdown dramatically arrested the ovary development of female mosquitoes. The primary follicles of iE93 females were much smaller (171 and 287 μm on average at 24 and 48 h PBM, respectively) than those of the iLuc and WT mosquitoes (236–239 and 429–439 μm on average at 24 and 48 h PBM, respectively; Fig. S1B–E). In addition, the E93-knocked-down females displayed reduced fecundity, laying significantly fewer eggs (38 eggs per female on average) than the iLuc and WT controls (131–133 eggs per female on average; Fig. S1F). E93 knockdown also affected the egg shape and hatching rate. Eggs from iLuc and WT mosquitoes exhibited a normal elongated elliptical shape and a hatching rate of 94.5–95.2%, whereas those from iE93 mosquitoes were shorter along the major axis and only 9.1% of them hatched (Fig. S1G–I). Together, these results demonstrate that E93 is induced in the midgut after a blood meal and essential for blood digestion and subsequent egg development in the Ae. aegypti mosquito.
3.2. Knockdown of E93 disturbs midgut SP expression after a blood meal
In anautogenous mosquitoes, a prerequisite for oocyte maturation and egg production is the successful digestion of a blood meal (Attardo, et al. 2005; Isoe, et al. 2009a). Therefore, our subsequent investigations focused mainly on the function of E93 in blood digestion in the midgut of female mosquitoes. The principle midgut enzymes responsible for blood digestion are endoproteolytic SPs (Brackney, et al. 2010). The dramatic defects in blood digestion in E93-knocked-down mosquitoes imply that E93 may control the expression of midgut SPs. To test this hypothesis, we first analyzed the time-course expression of eleven SPs in the female mosquito midgut. This analysis allowed for separation of the examined SPs into three groups according to their expression patterns: Group 1, ET and JA15, upregulated PE and downregulated PBM; Group 2, LT, SPVI, SPVII and SPI, activated PBM (PBM SPs); and Group 3, CHYMO and SPII-V, activated PE and constitutively expressed thereafter (Fig. S2). Due to the extremely high degree of sequence similarity of SPII-V (Brackney, et al. 2010), a single primer pair targeting the common region of these four genes was used in qPCR analysis. However, the total transcript level of the four SPs was extremely low when compared with that of the other SPs (Fig. S2). Therefore, we focused our next experiments on only the seven highly expressed SPs: the three SPs (ET, JA15 and CHYMO) that are activated PE (PE SPs) and the four PBM SPs.
We examined the expression of these SPs in the midguts of E93-depleted females at 6, 24 and 36 h PBM. Of the three PE SPs, ET transcript level was significantly higher in the midguts of iE93 females than in iLuc control at all the three time points (Fig. 2A), suggesting that E93 is required to shut down the PE expression of ET after a blood meal. In contrast, the transcript levels of JA15 and CHYMO were dramatically lower in the midguts of iE93 females than the control at 36 h PBM (Fig. 2B and C). Strikingly, E93 knockdown markedly reduced the transcript levels of the four PBM SPs compared with the control (Fig. 2D–G), indicating that E93 is required to activate the expression of these SPs after a blood meal. Previous functional studies have shown that LT, SPVI and SPVII all contribute to blood meal digestion and subsequent egg development in Ae. aegypti mosquitoes. In particular, knockdown of SPVI caused a 77.6% decrease in late-phase, trypsin-like activity in the midgut extracts from female mosquitoes (Isoe, et al. 2009a). We then measured the amount of trypsin-like activity in the midguts of iE93 mosquitoes using the Nα-Benzoyl-DL-arginine p-nitroanilide hydrochloride (BAPNA) cleaving assay. Consistent with the decreased transcript levels of PBM SPs, the level of trypsin-like activity in the midgut extracts of iE93 females was shown to be 38.3, 68.6 and 59.7% lower than control at 6 h, 24 h and 36 h PBM, respectively (Fig. 2H). These results indicate that E93 is involved in controlling midgut SP expression after a blood meal.
Fig. 2. Knockdown of E93 disturbs midgut SP expression after a blood meal.

(A–G) Relative mRNA levels of SPs in the midgut of iLuc and iE93 female mosquitoes at 6, 24 and 36 h PBM. (H) Trypsin-like activity in the midgut of iLuc and iE93 female mosquitoes at 6, 24 and 36 h PBM. (A–H) Mean ± SEM from three independent experiments; *P < 0.05, **P < 0.01, ***P < 0.001 (independent-samples t-test).
3.3. EcR-mediated 20E signaling activates E93 and PBM SP expression in the midgut
20E is the major regulator of blood feeding-activated reproductive events in female mosquitoes. In Ae. aegypti, the 20E titer is low before blood feeding, increases after a blood meal, peaking at 18–24 h PBM, and decreases rapidly thereafter (Hagedorn, et al. 1975). Considering the positive correlations between the expression of E93 and the four PBM SPs and the titer of 20E in female mosquitoes after a blood meal, we investigated whether 20E regulates E93 and PBM SP expression in the female mosquito midgut. We first conducted an in vivo 20E injection assay. Female mosquitoes at 96 h PE (when endogenous 20E level is low) were injected with 0.5 μL 10−4 M 20E or solvent as a control. Midguts were dissected at 6 h after injection and used for qPCR analysis. The transcript levels of E93, SPI, LT, SPVI and SPVII were significantly higher in the midguts of 20E-treated mosquitoes than in the control (Fig. 3A and B). We then performed an in vitro midgut culture assay. After culture of 96 h PE midguts in medium containing 10−5 M 20E for 6 h, the transcript levels of E93, SPI, LT, SPVI and SPVII were significantly higher than the solvent treatment (Fig. 3C and D). These results demonstrate that 20E activates E93 and PBM SP expression in the female mosquito midgut.
Fig. 3. EcR-mediated 20E signaling activates E93 and PBM SP expression in the midgut.
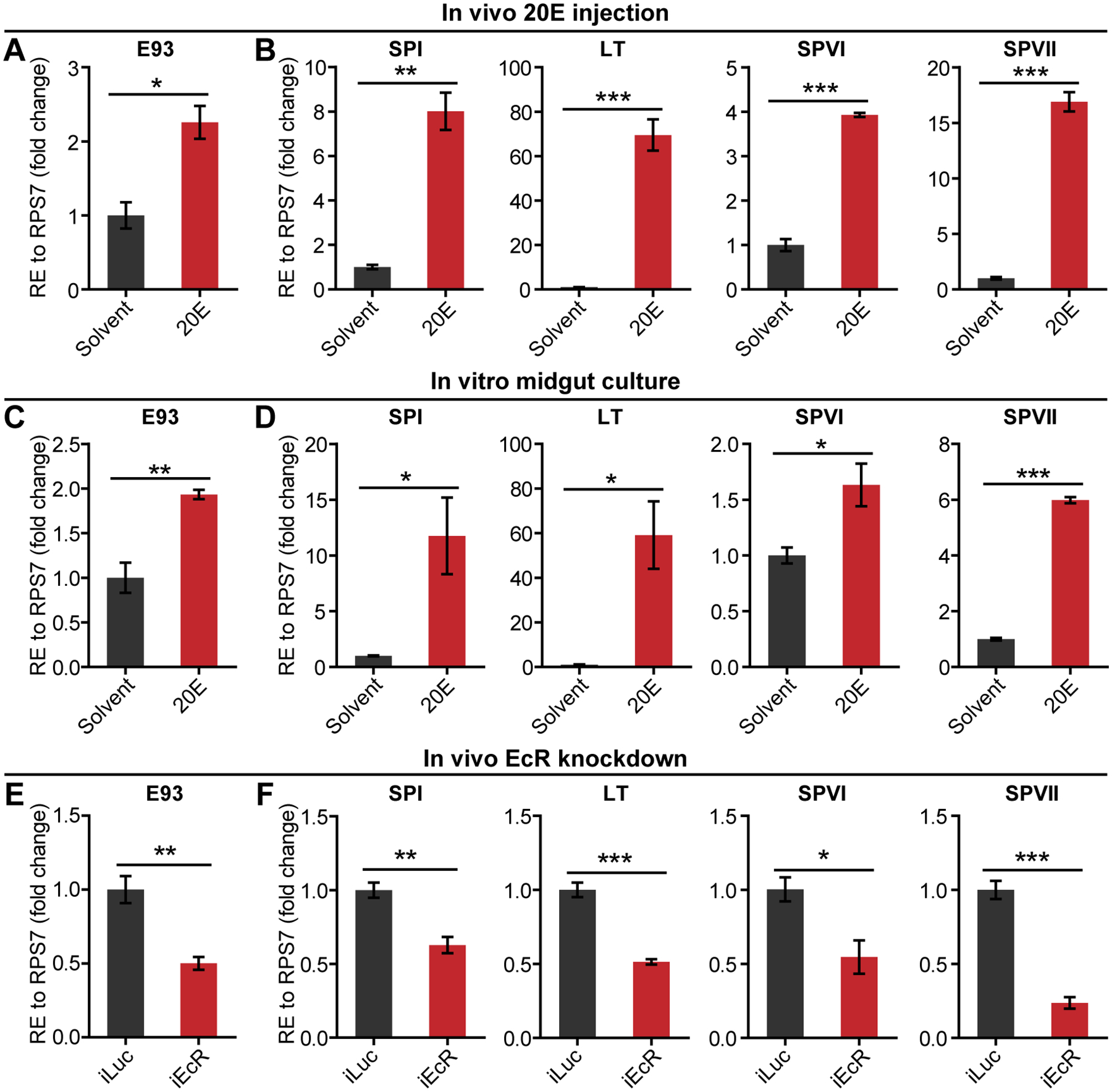
(A and B) Relative mRNA levels of E93 (A) and PBM SPs (B) in the midguts of 20E- or ethanol (solvent)-injected female mosquitoes at 96 h PE. (C and D) Relative mRNA levels of E93 (C) and PBM SPs (D) in the midguts dissected from female mosquitoes at 96 h PE and incubated in medium containing 20E (10 μM) or ethanol (solvent) for 6 h. (A–D) Fold changes are relative to expression in the solvent treatment, arbitrarily set to 1. (E and F) Relative mRNA levels of E93 (E) and PBM SPs (F) in the midguts of iLuc and iEcR female mosquitoes at 6 h (SPI) or 24 h (E93, LT, SPVI and SPVII) PBM. Fold changes are relative to expression in the iLuc control, arbitrarily set to 1. (A–F) Mean ± SEM from three independent experiments; *P < 0.05, **P < 0.01, ***P < 0.001 (independent-samples t-test).
To further verify the activation of these genes by 20E, we knocked down the expression of its receptor, EcR, using RNAi. Female mosquitoes at 24 h PE were injected with either dsEcR (iEcR) or dsLuc and were fed blood 4 days later. Compared with the iLuc control, EcR mRNA level was 69.1% lower in the midguts of iEcR females at 24 h PBM (Fig. S3A). qPCR analyses showed that the transcript levels of E93, SPI, LT, SPVI and SPVII were significantly lower in the midguts of iEcR mosquitoes than the control (Fig. 3E and F), confirming the activation of these genes by the EcR-mediated 20E signaling.
3.4. E93 is required for the 20E-dependent activation of PBM SP expression in the midgut
To examine whether an intermediate factor(s) is required for the 20E-dependent activation of PBM SPs, we utilized a classic approach using the protein synthesis inhibitor cycloheximide (CHX) (Li, et al. 2000). Midguts of female mosquitoes at 96 h PE were incubated in culture medium containing 10−5 M 20E in the presence or absence of 20 μM CHX for 6 h. CHX was ineffective at preventing the 20E activation of SPI, suggesting direct control of SPI by the 20E signaling pathway. In contrast, the addition of CHX to the culture medium inhibited the activation of LT, SPVI and SPVII, indicating the involvement of intermediate factors in the activation of these SPs by 20E (Fig. 4A).
Fig. 4. E93 is required for 20E-dependent activation of PBM SP expression in the midgut.

(A) Relative mRNA levels of PBM SPs in the midguts dissected from female mosquitoes at 96 h PE and cultured for 6 h in medium under indicated conditions. Fold changes are relative to expression in the solvent treatment, arbitrarily set to 1. (B) Relative mRNA levels of PBM SPs in the midguts of iLuc and iE93 female mosquitoes after 20E or solvent injection at 96 h PE. Fold changes are relative to expression in the iLuc females after solvent treatment, arbitrarily set to 1. (A and B) Mean ± SEM from three independent experiments; n.s., not significant, *P < 0.05, **P < 0.01 (independent-samples t-test).
Our previous results indicate that 20E activates E93 (Fig. 3) and that E93 is required to activate the expression of PBM SPs after a blood meal (Fig. 2D–G). We thus asked whether E93 is required for the 20E-dependent activation of PBM SPs. To answer this question, we examined the effect of 20E on PBM SP expression in E93-knocked-down mosquitoes. Female mosquitoes were first injected with either dsE93 or dsLuc at 24 h PE, and then with 20E or solvent at 96 h PE. Midguts were dissected at 6 h after the second injection and used for qPCR analysis. The expression of E93 was successfully impaired in the midguts of iE93 females at 96 h PE compared with the control (Fig. S3B). Although the expression of the four PBM SPs was dramatically induced by 20E in the midguts of iLuc mosquitoes, no increase of LT, SPVI or SPVII transcripts was detected in iE93 mosquitoes after 20E treatment relative to the solvent control (Fig. 4B), indicating that E93 confers 20E responsiveness of these three SPs in the midgut. 20E treatment still significantly increased the transcript level of SPI in iE93 females compared with the solvent treatment; however, the overall transcript levels of SPI in iE93 mosquitoes were lower than in iLuc control (Fig. 4B), suggesting that E93 is required to allow full 20E responsiveness of SPI. Therefore, E93 is required for the 20E-dependent activation of PBM SP expression in the female mosquito midgut.
3.5. 20E acts independently of the insulin pathway to activate PBM SP expression
A previous study has shown that ILP3 stimulates LT and SPVI expression in the midgut of female Ae. aegypti (Gulia-Nuss, et al. 2011). It binds to the insulin receptor (InR) with high affinity and activates the insulin signaling pathway in Ae. aegypti (Brown, et al. 2008; Wen, et al. 2010). To investigate the role of this pathway in regulation of PBM SP expression, we first conducted an in vivo insulin injection assay. Female mosquitoes were injected at 96 h PE with bovine insulin, which has been used previously to activate the insulin pathway in Ae. aegypti (Brown, et al. 2008; Riehle and Brown 1999), or with solvent as a control. Midguts were dissected at 6 h after injection and used for qPCR analysis. With insulin treatment, the transcript levels of the four PBM SPs in midguts were significantly higher than in controls (Fig. 5A). Next, we knocked down the expression of InR using RNAi. Female mosquitoes were injected at 24 h PE with either dsInR (iInR) or dsLuc and were fed blood 4 days later. Compared with the control, the InR mRNA level was 69.4% lower in the midguts of iInR females at 24 h PBM (Fig. S3C). The transcript levels of LT, SPVI and SPVII were significantly lower than control in the midguts of iInR mosquitoes at 24 h PBM (Fig. 5B). These data indicate that the insulin pathway is also involved in activation of PBM SPs in female mosquitoes after a blood meal.
Fig. 5. 20E acts independently of the insulin pathway to activate PBM SP expression.

(A) Relative mRNA levels of PBM SPs in the midguts of bovine insulin- or solvent-injected female mosquitoes at 96 h PE. Fold changes are relative to expression in the solvent-treated animals, arbitrarily set to 1. (B) Relative mRNA levels of PBM SPs in the midguts of iLuc and iInR female mosquitoes at 24 h PBM. Fold changes are relative to expression in the iLuc control, arbitrarily set to 1. (C) Relative mRNA levels of PBM SPs in the midguts of iLuc and iInR female mosquitoes after 20E or solvent injection at 96 h PE. Fold changes are relative to expression in the iLuc females after solvent treatment, arbitrarily set to 1. (A–C) Mean ± SEM from three independent experiments; n.s., not significant, *P < 0.05, **P < 0.01 (independent-samples t-test).
It has been shown that ILP3 activates LT and SPVI expression in the female mosquito midgut in the absence of 20E (Gulia-Nuss, et al. 2011). Therefore, insulin can act independently of the 20E pathway to activate LT and SPVI expression. We then asked whether 20E acts independently of or through the insulin pathway to activate PBM SP expression. To answer this question, we examined the effect of 20E on PBM SP expression in InR-depleted mosquitoes. Female mosquitoes were first injected with either dsInR or dsLuc at 24 h PE, and then with 20E or solvent at 96 h PE. Midguts were dissected at 6 h after the second injection and used for qPCR analysis. The expression of InR was successfully impaired in the midguts of iInR females at 96 h PE relative to the control (Fig. S3C). Nevertheless, the transcript of the four PBM SPs in iInR females was induced by 20E to a similar level to that in the iLuc group after 20E treatment (Fig. 5C), suggesting that 20E acts independently of the insulin pathway to activate PBM SP expression.
3.6. Multifactorial regulation of PE SP expression
The development of the female mosquito before blood feeding is controlled mainly by JH. In Ae. aegypti, the JH III titer is low at eclosion, increases significantly by 24 h PE, peaking at around 72 h PE (Zhao, et al. 2016). Consistent with the activation of ET and JA15 expression by JH, as reported previously (Bian, et al. 2008; Noriega, et al. 1997), the transcript abundance of these two SPs increased gradually after eclosion (Fig. S2). To verify the role of JH in regulating PE SP expression, we topically applied methoprene, a JH III analogue, or solvent (acetone) onto the abdomens of female mosquitoes within 1 h PE. Midguts were dissected at 9 h PE and used for qPCR analysis. The transcript level of Hairy, an established downstream gene involved in the JH pathway (Saha, et al. 2016), was significantly higher after methoprene than solvent treatment (Fig. 6A), confirming activation of the JH pathway. The methoprene application resulted in dramatically higher transcript levels of ET, JA15 and CHYMO than in control (Fig. 6B). We further knocked down the expression of the JH receptor Met using RNAi. Female mosquitoes were injected at 6 h PE with either dsMet (iMet) or dsLuc, and their midguts were dissected at 96 h PE and used for qPCR analysis. Compared with the iLuc control, the transcript level of Met was 64.2% lower in the midguts of iMet females (Fig. 6C). The transcript levels of the three PE SPs were significantly lower in the midguts of iMet mosquitoes than in control (Fig. 6D), confirming that JH and its receptor Met activate the expression of PE SPs in the female mosquito midgut before blood feeding.
Fig. 6. The role of JH signaling in regulating PE SP expression.

(A and B) Relative mRNA levels of Hairy (A) and PE SPs (B) in the midguts of methoprene (Meth)- or acetone (solvent)-treated female mosquitoes at 9 h PE. Female mosquitoes were treated topically on the abdomen with Meth (1 ng/insect) or acetone within 1 h PE and dissected at 9 h PE for qPCR analysis. (C and D) Relative mRNA levels of Met (C) and PE SPs (D) in the midguts of iLuc and iMet female mosquitoes at 96 h PE. Fold changes are relative to expression in the iLuc control, arbitrarily set to 1. (E and F) Relative mRNA levels of Hairy (E) and PE SPs (F) in the midguts of Meth- or solvent-treated female mosquitoes at 24 h PBM. Female mosquitoes were treated topically on the abdomen with Meth (10 ng/insect) or acetone within 1 h PBM and dissected at 24 h PBM for qPCR analysis. (A–B and E–F) Fold changes are relative to expression in the solvent-treated animals, arbitrarily set to 1. (A–F) Mean ± SEM from three independent experiments; n.s., not significant, *P < 0.05, **P < 0.01, ***P < 0.001 (independent-samples t-test).
After a blood meal, the JH III titer falls rapidly during the first 6 h, and remains low until 48 h PBM, when it starts to rise again (Shapiro, et al. 1986; Zhao, et al. 2016). Considering the upregulation of PE SP by JH before blood feeding, the reduced transcript levels of PE SPs after a blood meal are likely due to the decreased titer of JH III. To test this hypothesis, we topically applied methoprene or solvent onto the abdomens of female mosquitoes within 1 h PBM. Midguts were dissected at 24 h PBM and used for qPCR analysis. The transcript level of Hairy in midguts was significantly more induced by methoprene than by the solvent control (Fig. 6E). Compared with solvent treatment, methoprene application resulted in significantly higher transcript levels of ET and JA15, but not of CHYMO in the midguts (Fig. 6F). This suggests that the decline of JH III titer contributes to downregulation of ET and JA15, but that other factors control the expression of CHYMO after a blood meal.
Remarkably, the transcript level of ET was dramatically higher in the midguts of iE93 females than in controls after a blood meal (Fig. 2A), suggesting repression of ET by E93. Given that E93 is activated by 20E, we tested whether 20E plays a role in repressing PE SP expression. In vivo injection of 20E (performed in a similar manner to that described in the previous section) resulted in a significantly lower transcript level of ET, but not of JA15 or CHYMO in the midguts of female mosquitoes at 96 h PE compared with solvent treatment (Fig. 7A). EcR RNAi silencing (performed similarly to that described in the previous section) further confirmed the repression of ET, but not of JA15 or CHYMO in the midguts of female mosquitoes at 24 h PBM by EcR (Fig. 7B). Therefore, the increased 20E titer contributes to the downregulation of ET, but not of JA15 or CHYMO after a blood meal.
Fig. 7. The role of 20E signaling in regulating PE SP expression.

(A) Relative mRNA levels of PE SPs in the midguts of 20E- or ethanol (solvent)-injected female mosquitoes at 96 h PE. Fold changes are relative to expression in the solvent-treated animals, arbitrarily set to 1. (B) Relative mRNA levels of PE SPs in the midguts of iLuc and iEcR female mosquitoes at 24 h PBM. Fold changes are relative to expression in the iLuc control, arbitrarily set to 1. (A and B) Mean ± SEM from three independent experiments; n.s., not significant, *P < 0.05 (independent-samples t-test).
4. Discussion
Anautogeny is a successful reproductive strategy utilized by many mosquito species. A detailed understanding of the mechanisms underlying anautogeny in mosquitoes is of great importance, because this reproductive strategy is the driving force behind the transmission of diseases to millions of people (Attardo, et al. 2005). The digestion of blood proteins into amino acids that are further used for energy production, egg maturation and replenishment of maternal reserves is an essential part of the reproductive cycle and understanding how this process is regulated could lead to safe, specific and effective ways to control mosquito populations and, thus, mosquito-borne diseases. It is recognized that the mosquito midgut SPs function as the principle enzymes in blood-meal protein digestion (Brackney, et al. 2010; Isoe, et al. 2009a). However, how they are controlled to coordinate their activity with the demands of mosquito reproduction remains largely unknown. In this study, we have identified transcription factor E93 as the critical factor that controls the expression of several midgut SPs. E93 is required not only to activate the expression of SPI, LT, SPVI and SPVII, but also to shut down ET expression after a blood meal. The present work thus represents a significant step toward understanding the molecular mechanisms underlying anautogeny in mosquitoes.
Studies have shown that E93 is a 20E primary response gene in insects such as D. melanogaster, Bombyx mori and Ae. aegypti (Baehrecke and Thummel 1995; Liu, et al. 2015; Wang, et al. 2021). Consistently, the E93 transcript is higher in 20E-treated midguts and lower in EcR-depleted midguts of female mosquitoes. So far, little has been known about how 20E signaling affects blood digestion in anautogenous mosquitoes. Here, we show that 20E activates the expression of four PBM SPs in the midgut of female Ae. aegypti mosquitoes. 20E indirectly activates LT, SPVI and SPVII, because the addition of CHX to the culture medium inhibited the activation. In contrast, the activation of SPI is likely to be directly mediated by EcR, as CHX failed to prevent 20E activation. In line with these results, the activation of LT, SPVI and SPVII by 20E was completely abolished in E93-knocked-down mosquitoes; however, 20E treatment still significantly increased the transcript level of SPI in E93-depleted females. Nevertheless, it is obvious that E93 is also required to allow full 20E responsiveness of SPI. Notably, the expression of SPI peaks at 6 h PBM and decreases dramatically at 24 h PBM, when the 20E level is high, suggesting that other factors repress its expression at this developmental stage.
It has been shown that ILP3 directly stimulates LT and SPVI expression in the midgut of female Ae. aegypti (Gulia-Nuss, et al. 2011). Here, we confirmed the role of the insulin pathway in activating PBM SP expression in female mosquitoes. We also showed that 20E acts independently of the insulin pathway to activate PBM SP expression, as the knockdown of InR had no influence on PBM SP activation by 20E. Previous research has shown that insulin signaling is essential for ecdysteroid production by the female mosquito ovary after a blood meal (Brown, et al. 2008; Gulia-Nuss, et al. 2011). Therefore, it is most likely that the insulin pathway acts both directly and indirectly through promoting ecdysteroid production by the ovary to activate PBM SP expression after a blood meal.
Previous studies have shown that ET and JA15 are induced by JH, leading to the assumption that the natural fall of JH is responsible for the downregulation of these genes after a blood meal (Bian, et al. 2008; Li, et al. 2011; Noriega, et al. 1997). Importantly, our results show that this idea is not entirely correct as ET repression also depends on E93 activation. E93 or EcR silencing resulted in dramatically higher ET transcript levels in the midguts of female mosquitoes after a blood meal. This upregulation of ET is unlikely due to increased JH pathway activity, because the expression level of JA15 was not induced by these treatments. In addition, a 38-fold induction of ET transcript level was also observed in the midguts of methoprene-treated female mosquitoes at 24 h PBM. In this regard, our data suggest that the strong downregulation of ET expression after a blood meal is due to the strong upregulation of E93 expression by 20E and to the critical decline of JH. The reinduction of JA15 expression in the blood-fed female midgut by methoprene indicates that the decline of the JH titer may be the major factor that leads to the downregulation of JA15 after a blood meal. JH activates CHYMO expression before blood feeding. However, the manipulation of JH and 20E pathways had no influence on CHYMO expression in the blood-fed mosquito midgut, suggesting the involvement of other factors in the regulation of this gene after a blood meal.
The Ae. aegypti E93 contains two conserved DNA binding domains of the Pipsqueak (Psq) family (Fig. S4) (Siegmund and Lehmann 2002). The Psq DNA binding domains of the honeybee Mblk-1 have been shown to bind DNA (Park, et al. 2002). Moreover, the B. mori E93 binds to GAGA-containing motifs present in the Atg1 promoter via the two Psq domains to induce gene expression (Liu, et al. 2015). A recent study reported that E93 functions as an Atg8-positive regulator that interacts with the Atg8 promoter region in Ae. aegypti (Wang, et al. 2021). Therefore, E93 may activate the expression of PBM SPs by directly binding to the upstream regulatory regions of these genes. In addition, The Ae. aegypti E93 contains three nuclear receptor interaction motifs (LXXLL). The LXXLL motif is a signature sequence that facilitates the interaction of co-activators with nuclear receptors (Heery, et al. 1997). The mammalian ortholog of E93, ligand-dependent co-repressor (LCoR), was found to interact with a range of ligand-bound nuclear receptors (Song, et al. 2012; White, et al. 2004). In B. mori, E93 binds to the EcR/USP complex via a physical association with USP (Liu, et al. 2015). Thus, another possible mechanism underlying the implication of E93 in 20E-dependent activation of PBM SPs is that E93 interacts with nuclear receptors of the 20E hierarchy, synergistically activating these genes. The E93 protein also contains a co-repressor C-terminal binding protein (CtBP) interaction motif (PXDLSXK/H) (Mannervik, et al. 1999; Mou, et al. 2012). LCoR represses transcription by recruiting CtBP via the tandem N-terminal PXDLS motifs (Fernandes, et al. 2003; Palijan, et al. 2009). Drosophila CtBP mediates transcriptional repression by Knirps, Krüppel and Snail in the embryo via binding to these repressors through such motifs (Nibu, et al. 1998a; Nibu, et al. 1998b). The mosquito CtBP may be involved in the repression of ET expression by E93. Finally, the Drosophila E93 has been found to control temporal identity by directly regulating chromatin accessibility across the genome. It not only increases the accessibility of late-acting enhancers, but also decreases the accessibility of early-acting enhancers in the Drosophila wing (Uyehara, et al. 2017). We thus speculate that E93 may control midgut SP expression through regulation of chromatin accessibility of these genes in a hormone-dependent manner in mosquitoes. Future study of which mechanism is used by E93 for controlling midgut SP expression will greatly improve our understanding of mosquito reproductive biology.
Supplementary Material
Highlights.
E93 was highly upregulated in the female Aedes aegypti midgut after a blood meal.
E93 RNAi silencing inhibited midgut blood digestion and reduced fecundity.
E93 silencing disturbed midgut expression of serine protease (SP) genes.
20E treatment activated E93, LT, SPI, SPVI and SPVII, and repressed ET expression.
E93 silencing resulted in the loss of 20E responsiveness of LT, SPVI and SPVII.
Acknowledgments
We would like to thank Vladimir A. Kokoza for assistance in assessing mosquito egg hatching rate. This work was supported by the National Institutes of Health Grant R01 AI036959 (to A.S.R.) and R01 AI113729 (to A.S.R.).
References
- Attardo Geoffrey M, Hansen Immo A, and Raikhel Alexander S 2005. Nutritional regulation of vitellogenesis in mosquitoes: implications for anautogeny. Insect Biochem Mol Biol 35:661–675. [DOI] [PubMed] [Google Scholar]
- Baehrecke Eric H, and Thummel Carl S 1995. The Drosophila E93 gene from the 93F early puff displays stage- and tissue-specific regulation by 20-hydroxyecdysone. Dev Biol 171:85–97. [DOI] [PubMed] [Google Scholar]
- Barbosa P, and Peters TM 1969. A comparative study of egg hatching techniques for Aedes aegypti (L.). Mosq News 29:548–551. [Google Scholar]
- Barrett Alan DT, and Higgs Stephen 2007. Yellow fever: a disease that has yet to be conquered. Annu Rev Entomol 52:209–229. [DOI] [PubMed] [Google Scholar]
- Bian Guo Wu, Raikhel Alexander S, and Zhu Jin Song 2008. Characterization of a juvenile hormone-regulated chymotrypsin-like serine protease gene in Aedes aegypti mosquito. Insect Biochem Mol Biol 38:190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackney Doug E, et al. 2010. Expression profiling and comparative analyses of seven midgut serine proteases from the yellow fever mosquito, Aedes aegypti. J Insect Physiol 56:736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briegel Hans 2003. Physiological bases of mosquito ecology. J Vector Ecol 28:1–11. [PubMed] [Google Scholar]
- Brown Mark R, et al. 2008. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A 105:5716–5721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid Duaa Musleh, Chereddy Shankar CRR, and Palli Subba Reddy 2020. The effect of E93 knockdown on female reproduction in the red flour beetle, Tribolium castaneum. Arch Insect Biochem Physiol 104(4):e21688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes Isabelle, et al. 2003. Ligand-dependent nuclear receptor corepressor LCoR functions by histone deacetylase-dependent and-independent mechanisms. Mol Cell 11:139–150. [DOI] [PubMed] [Google Scholar]
- Gulia-Nuss Monika, et al. 2011. Insulin-like peptides and the target of rapamycin pathway coordinately regulate blood digestion and egg maturation in the mosquito Aedes aegypti. PLoS One 6:e20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagedorn HH, et al. 1975. The ovary as a source of alpha-ecdysone in an adult mosquito. Proc Natl Acad Sci U S A 72:3255–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heery David M, et al. 1997. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature 387:733–736. [DOI] [PubMed] [Google Scholar]
- Isoe Jun, et al. 2009a. Molecular genetic analysis of midgut serine proteases in Aedes aegypti mosquitoes. Insect Biochem Mol Biol 39:903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoe Jun, Zamora Jorge, and Miesfeld Roger L 2009b. Molecular analysis of the Aedes aegypti carboxypeptidase gene family. Insect Biochem Mol Biol 39:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindra Marek, Palli Subba R, and Riddiford Lynn M 2013. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol 58:181–204. [DOI] [PubMed] [Google Scholar]
- King-Jones Kirst, and Thummel Carl S 2005. Nuclear receptors-a perspective from Drosophila. Nat Rev Genet 6:311–323. [DOI] [PubMed] [Google Scholar]
- Lee Cheng Yu, et al. 2000. E93 directs steroid-triggered programmed cell death in Drosophila. Mol Cell 6:433–443. [DOI] [PubMed] [Google Scholar]
- Li Chao, et al. 2000. Conserved molecular mechanism for the stage specificity of the mosquito vitellogenic response to ecdysone. Dev Biol 224:96–110. [DOI] [PubMed] [Google Scholar]
- Li Meng, Mead Edward A, and Zhu Jin Song 2011. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc Natl Acad Sci U S A 108:638–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Xi, et al. 2015. 20-hydroxyecdysone (20E) primary response gene E93 modulates 20E signaling to promote Bombyx larval-pupal metamorphosis. J Biol Chem 290:27370–27383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik Mattias, et al. 1999. Transcriptional coregulators in development. Science 284:606–609. [DOI] [PubMed] [Google Scholar]
- Mao Yiwen, et al. 2019. The direct interaction between E93 and Kr-h1 mediated their antagonistic effect on ovary development of the brown planthopper. Int J Mol Sci 20(10):2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil Candice J, and Shetty Avinash K 2017. Zika virus: A serious global health threat. J Trop Pediatr 63:242–248. [DOI] [PubMed] [Google Scholar]
- Mou Xiao Chun, et al. 2012. Control of target gene specificity during metamorphosis by the steroid response gene E93. Proc Natl Acad Sci U S A 109:2949–2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Yoshiaki, and Henrich Vincent C 2009. Arthropod nuclear receptors and their role in molting. FEBS J 276:6128–6157. [DOI] [PubMed] [Google Scholar]
- Nibu Yutaka, et al. 1998a. dCtBP mediates transcriptional repression by Knirps, Krüppel and Snail in the Drosophila embryo. EMBO J 17:7009–7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibu Yutaka, Zhang Hai Lan, and Levine Michael 1998b. Interaction of short-range repressors with Drosophila CtBP in the embryo. Science 280:101–104. [DOI] [PubMed] [Google Scholar]
- Noriega FG, Shah DK, and Wells MA 1997. Juvenile hormone controls early trypsin gene transcription in the midgut of Aedes aegypti. Insect Mol Biol 6:63–66. [DOI] [PubMed] [Google Scholar]
- Palijan Ana, et al. 2009. Ligand-dependent corepressor LCoR is an attenuator of progesterone-regulated gene expression. J Biol Chem 284:30275–30287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Jung Min, et al. 2002. DNA-binding properties of Mblk-1, a putative transcription factor from the honeybee. Biochem Biophys Res Commun 291:23–28. [DOI] [PubMed] [Google Scholar]
- Raikhel Alexander S, and Dhadialla TS 1992. Accumulation of yolk proteins in insect oocytes. Annu Rev Entomol 37:217–251. [DOI] [PubMed] [Google Scholar]
- Riehle Michael A, and Brown Mark R 1999. Insulin stimulates ecdysteroid production through a conserved signaling cascade in the mosquito Aedes aegypti. Insect Biochem Mol Biol 29:855–860. [DOI] [PubMed] [Google Scholar]
- Roy Saurabh G, Hansen Immo A, and Raikhel Alexander S 2007. Effect of insulin and 20-hydroxyecdysone in the fat body of the yellow fever mosquito, Aedes aegypti. Insect Biochem Mol Biol 37:1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy Sourav, et al. 2015. Regulation of gene expression patterns in mosquito reproduction. PLoS Genet 11:e1005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha Tusar T, et al. 2016. Hairy and Groucho mediate the action of juvenile hormone receptor Methoprene-tolerant in gene repression. Proc Natl Acad Sci U S A 113:E735–E743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AB, et al. 1986. Juvenile hormone and juvenile hormone esterase in adult females of the mosquito Aedes aegypti. J Insect Physiol 32:867–877. [Google Scholar]
- Siegmund Thomas, and Lehmann Michael 2002. The Drosophila Pipsqueak protein defines a new family of helix-turn-helix DNA-binding proteins. Dev Genes Evol 212:152–157. [DOI] [PubMed] [Google Scholar]
- Song Yi Yun, et al. 2012. Ligand-dependent corepressor acts as a novel corepressor of thyroid hormone receptor and represses hepatic lipogenesis in mice. J Hepatol 56:248–254. [DOI] [PubMed] [Google Scholar]
- Tsetsarkin Konstantin A, Chen Rubing, and Weaver Scott C 2016. Interspecies transmission and chikungunya virus emergence. Curr Opin Virol 16:143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ureña Enric, et al. 2014. Transcription factor E93 specifies adult metamorphosis in hemimetabolous and holometabolous insects. Proc Natl Acad Sci U S A 111:7024–7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyehara Christopher M, et al. 2017. Hormone-dependent control of developmental timing through regulation of chromatin accessibility. Genes Dev 31(9):862–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Xueli, et al. 2021. The ecdysone-induced protein 93 is a key factor regulating gonadotrophic cycles in the adult female mosquito Aedes aegypti. Proc Natl Acad Sci U S A 118(8):e2021910118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Zhi Mou, et al. 2010. Two insulin-like peptide family members from the mosquito Aedes aegypti exhibit differential biological and receptor binding activities. Mol Cell Endocrinol 328:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White John H, et al. 2004. Corepressor recruitment by agonist-bound nuclear receptors. Vitam Horm 68:123–143. [DOI] [PubMed] [Google Scholar]
- Yamanaka Naoki, Rewitz Kim F, and O’Connor Michael B 2013. Ecdysone control of developmental transitions: lessons from Drosophila research. Annu Rev Entomol 58:497–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Viravuth P, and Thummel Carl S 2005. Mechanisms of steroid-triggered programmed cell death in Drosophila. Semin Cell Dev Biol 16:237–243. [DOI] [PubMed] [Google Scholar]
- Zhao Bo, et al. 2016. Determination of juvenile hormone titers by means of LC-MS/MS/MS and a juvenile hormone-responsive Gal4/UAS system in Aedes aegypti mosquitoes. Insect Biochem Mol Biol 77:69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Guo Li, et al. 2004. Metabolic fate of [14C]-labeled meal protein amino acids in Aedes aegypti mosquitoes. J Insect Physiol 50:337–349. [DOI] [PubMed] [Google Scholar]
- Zhu Jin Song, Chen Li, and Raikhel Alexander S 2003. Posttranscriptional control of the competence factor βFTZ-F1 by juvenile hormone in the mosquito Aedes aegypti. Proc Natl Acad Sci U S A 100:13338–13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Zhen, et al. 2013. Juvenile hormone and its receptor, methoprene-tolerant, control the dynamics of mosquito gene expression. Proc Natl Acad Sci U S A 110:E2173–E2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


