Abstract
Context
Physical inactivity promotes insulin resistance and increases the risk of diabetes and cardiovascular disease. Recently introduced clustering based on simple clinical measures identified diabetes subgroups (clusters) with different risks of diabetes-related comorbidities and complications.
Objective
This study aims to determine differences in physical fitness and cardiovascular risk between diabetes subgroups and a glucose-tolerant control group (CON). We hypothesized that the severe insulin-resistant diabetes (SIRD) subgroup would be associated with lower physical fitness and increased cardiovascular risk.
Methods
The physical fitness and cardiovascular risk of 746 participants with recent-onset diabetes (diabetes duration of < 12 months, aged 18-69 years) and 74 CONs of the German Diabetes Study (GDS), a prospective longitudinal cohort study, were analyzed. Main outcome measures included physical fitness (VO2max from spiroerogometry), endothelial function (flow- and nitroglycerin-mediated dilation), and cardiovascular risk scores (Framingham Risk Scores for Coronary Heart Disease [FRS-CHD] and Atherosclerotic CardioVascular Disease [ASCVD] risk score).
Results
VO2max was lower in SIRD than in CON, severe autoimmune diabetes (SAID) (both P < .001), and mild age-related diabetes (MARD) (P < .01) subgroups, but not different compared to severe insulin-deficient diabetes (SIDD) (P = .98) and moderate obesity-related diabetes (MOD) subgroups (P = .07) after adjustment for age, sex, and body mass index. Endothelial function was similar among all groups, whereas SAID had lower FRS-CHD and ASCVD than SIRD, MOD, and MARD (all P < .001).
Conclusion
Despite comparable endothelial function across all groups, SIRD showed the lowest physical fitness. Of note, SAID had the lowest cardiovascular risk within the first year after diabetes diagnosis compared to the other diabetes subgroups.
Keywords: cardiovascular risk factors, diabetes subgroups, endothelial function, physical activity, physical fitness
Diabetes mellitus confers an about doubled risk of cardiovascular outcomes, as documented by a meta-analysis of 102 prospective studies (1). Chronic hyperglycemia contributed to higher cardiovascular risk by impairment of vascular function (2). However, other abnormalities such as adipose tissue dysfunction and insulin resistance with subsequent dyslipidemia and hyperinsulinemia may be at least as important not only for the development of type 2 diabetes, but also for worsening cardiovascular risk (3). Thus, it is of clinical relevance to assess the roles of the various diabetes-related risk factors for differences in premature morbidity and mortality related to cardiovascular complications in order to contribute to the road map of precision in medicine (4).
In this context, clustering of people with diabetes recently allowed us to identify subgroups (clusters) with distinct clinical features (5, 6). Out of the 5 subgroups, 1 subgroup (severe autoimmune diabetes [SAID]) overlaps with type 1 diabetes, while the others (severe insulin-deficient diabetes [SIDD], severe insulin-resistant diabetes [SIRD], mild obesity-related diabetes [MOD], and mild age-related diabetes [MARD]) reflect the classical type 2 diabetes (5, 6). Specifically, the SIRD subgroup is associated with a higher prevalence of diabetic nephropathy (5) and nonalcoholic fatty liver disease (6), both of which are associated with greater risk of cardiovascular events (3, 7). In line with these findings, endothelial dysfunction, characterized by impaired flow-mediated vasodilation due to decreased nitric oxide bioavailability (8), is a cardiovascular risk factor, which is more prevalent in people with insulin resistance, dyslipidemia, and type 2 diabetes (9). Moreover, 2 previous studies reported an at least nominally increased cardiovascular risk in individuals with SIRD (5, 10), while another analysis suggested that high glycated hemoglobin A1c (HbA1c) and low body mass index (BMI), typical for SIDD, may better explain the elevated risk of major adverse cardiovascular events and death (11). Currently, there is no explanation for the observed differences between the subgroups and the findings from different studies.
Lifestyle is the key modifiable cardiovascular risk factor, as even small improvements in physical fitness, reflecting cardiorespiratory fitness (CRF), can reduce the risk of type 2 diabetes (12) and regular moderate physical activity decreases cardiovascular risk (13). Exercise training enhances physical fitness, that is, maximal oxidative capacity or oxidative capacity at the anaerobic threshold, and insulin sensitivity in type 2 diabetes (14), but even habitual physical activity of lower intensity can improve insulin sensitivity (15). In general, individuals with overt type 2 diabetes exhibit lower physical activity levels (16) and lower tolerance to physical exhaustion than glucose-tolerant humans (17). Interestingly, physical fitness is not affected by hyperglycemia in people with type 1 diabetes (18). However, little is known about physical fitness and activity behavior, endothelial function, and cardiovascular risk in the novel diabetes subgroups. Because of the higher risk of sedentary lifestyle for the development of insulin resistance (19), we hypothesized that particularly SIRD is associated with (i) lower physical fitness, (ii) possibly due to reduced physical activity, (iii) lower endothelial dysfunction, and (iv) with increased cardiometabolic risk. To this end, we analyzed the data from the multicentric prospective German Diabetes Study (GDS), which follows people with diabetes from the first year after diagnosis and glucose-tolerant humans (20) and recently allowed us to validate insulin sensitivity and secretion of the diabetes cluster concept (6).
Materials and Methods
Study Population
This study included all consecutive participants with diabetes mellitus (n = 746) at the first visit (diabetes duration of < 12 months, aged 18-69 years) and a lean, glucose-tolerant control group (CON, n = 74) of the GDS (20), who had complete data sets with regard to age at diagnosis, BMI, glycemia, homoeostasis model estimates (homeostatic model assessment of insulin resistance [HOMA-IR] and homeostatic model assessment of β-cell activity [HOMA-B]), calculated from C-peptide values, glutamate acid decarboxylase antibodies (GADAs), and spiroergometry data. They underwent comprehensive phenotyping after giving written informed consent to the study, which was registered at clinicaltrials.gov (identifier No. NCT01055093), approved by the ethics boards of the Medical Faculty of Heinrich Heine University Düsseldorf (reference No. 4508) and of the associated centers, and performed according to the Declaration of Helsinki (20). Diagnosis of diabetes is based on criteria of the American Diabetes Association (21). CONs had no first-degree relatives with known diabetes and underwent a 75-g oral glucose tolerance test to exclude dysglycemia (21). Further, they had a BMI lower than 25. Exclusion criteria for all participants comprised diabetes of other causes; pregnancy; acute or severe chronic heart, hepatic, renal or psychiatric diseases; or immunosuppressive treatment. Comprehensive phenotyping was performed as described previously (20). Blood pressure was measured 3 times by a blood pressure monitor (Omron M400, Omron Healthcare GmbH) in supine position on the left arm. A 12-lead electrocardiogram and resting heart rate (HR) were recorded under resting conditions.
Spiroergometry
This gold-standard test to predict CRF measures physical fitness, which is described by the capacity of the circulatory and respiratory systems to deliver oxygenated blood to skeletal muscles during exercise (14). Measurements were performed by an exhaustive exercise test on a cycle ergometer (Ergometrics 900, Ergoline) (20), with continuous monitoring of HR, electrocardiogram, blood pressure, respiratory gas exchange ratio (RER), and peak power. During exercising, work rate was continually increased every 15 seconds and the incremental part of the test lasted 8 to 12 minutes. During the entire exercise test, the breath-by-breath system continuously records every single breath and parameters from gas measures are then given as averaged data over 8 breaths. VO2max refers to the average of the last 8 breaths of exercising. VO2max and VCO2max were recorded at maximal exertion, whereby the maximal respiratory minute volume (VEmax) indicates the volume of gas inhaled/exhaled per minute at maximal exertion. During incremental exercise, the aerobic-anaerobic transition indicates the first lactate increase. The first ventilatory threshold (VT1) is the respiratory response to these metabolic changes and is defined as an increase in ventilation (VE) disproportionally relative to VO2. As result of the decrease in pH and partial pressure of carbon dioxide, ventilation increases (respiratory compensation of the lactate increase), which can be measured during spiroergometry, that is, VE/VO2 exhibited a systematic increase without a concomitant increase in VE/VCO2 (22). Oxygen pulse describes the maximal amount of oxygen absorbed per maximal heartbeat during spiroergometry. All individuals performed cycling spiroergometry until exhaustion, as defined by RERmax, exceeding VT1, HR ,and ventilation. Spiroerometry was not been performed under fasted condition.
Baecke Questionnaire
The Baecke questionnaire (23) was used to assess habitual physical activity scores in the areas of work, sports, and leisure. The Baecke index represents the sum of individual scores. Scores range from 0 to 15, and high scores reflect high physical activity levels. The German translation was validated against 14-day exercise protocols (24) in adults aged 20 to 65.
Bioimpedance Analysis
Bioimpedance analysis (BIA) was used for the estimation of fat mass and fat-free mass and to calculate the deriving percentage fat mass (BioElectrical Impedance Analyzer System, RJL Systems) (20).
Endothelial Function Test
Flow-mediated dilation (FMD), nitroglycerin (glycerol trinitrate)-mediated dilation (NMD), flow velocity, preocclusion, and postocclusion diameter were measured as described previously using a B-mode ultrasonography Logiq S8 GE device (GE Healthcare) (20, 25). Endothelium-independent dilation was measured after sublingual application of 0.4 mg glycerol trinitrate. Flow velocity was measured at the brachial artery at rest, 15 seconds after deflation as well as 120 seconds after deflation. Furthermore, increase in brachial artery diameter was taken as surrogate for pulse-wave velocity (26) to assess arterial stiffness (27).
FMD was analyzed using the established edge-detection Brachial Analyzer software (Medical Imaging Applications). FMD was calculated as the quotient between the arterial baseline diameter and the diameter post–cuff deflation (25). Results were expressed in percentage [(diameter “stress” − diameter baseline)/diameter baseline], as possible advantages of allometric scaling have been challenged by others (eg, relationship between FMD and CVD is weaker (28); ability to examine the statistical relevance of individually values are limited) (29).
Cardiovascular Risk Scores
The Framingham Risk score for Coronary Heart Disease (FRS-CHD) is used to assess the 10-year risk of an individual to suffer under CHD. FRS-CHD was calculated based on age, sex, systolic blood pressure (SBP), total and high-density lipoprotein (HDL) cholesterol, smoking status, and treatment for hypertension (30). The Framingham Risk Score for Cardiovascular Disease (FRS-CVD) estimates the 10-year risk of an individual for a cardiovascular event in regard to age, sex, SBP, total and HDL cholesterol, smoking status, treatment for hypertension, and diabetes mellitus (31). The Atherosclerotic CardioVascular Disease (ASCVD) risk score estimates the 10-year risk for atherosclerotic CVD events based on age, sex, SBP, total and HDL cholesterol, smoking status, treatment for hypertension, diabetes mellitus, and race (32). The Systematic COronary Risk Evaluation (SCORE) for populations at high risk offers the estimation of total fatal cardiovascular risk. SCORE was calculated based on age, sex, SBP, total cholesterol, and smoking status (33). The triglycerides (TGs):HDL ratio is used as surrogate both of insulin resistance and CVD risk (34). We used several risk scores because of their different specificity and sensitivity for certain cardiovascular abnormalities, as they might differently affect the results in the novel subgroups.
Laboratory Analyses
Serum and plasma samples were analyzed in the biomedical laboratory as described previously (20).
Cluster Analyses and Statistics
The cluster analysis was based on the 6 variables age at diagnosis, BMI, HbA1c, HOMA2-B, HOMA2-IR, and GADAs. By using the nearest centroid approach, each participant is assigned to a predefined subgroup (cluster), that is, insulin-deficient individuals with positive GADA are allocated to the SAID subgroup, whereas those without GADAs to the SIDD subgroup. While all other subgroups show lower insulin sensitivity, SIRD is characterized by the most pronounced insulin resistance and MOD by the highest BMI. Individuals in the MARD subgroup are identified by their older age (5, 6). Recent studies also show different patterns of blood cell counts and biomarkers of inflammatory pathways between the subgroups (eg, highest biomarker levels and leukocyte counts in SIRD, but lowest biomarker levels in SIDD and lowest leukocyte counts in SAID) (35, 36). These findings underline possible pathophysiological differences between the subgroups. Data are presented as mean (SD) or median (interquartile range) for continuous variables and percentages for categorical variables (6). Skewed data were log-transformed before analysis. To account for multiple group comparisons, the Tukey-Kramer correction was applied. P values less than .05 were considered to indicate statistically significant differences. Associations between parameters of anthropometric and clinical characteristics and each cluster variable were evaluated using Pearson partial correlation adjusted for all remaining cluster variables. Statistical significance levels were adjusted for multiple comparisons by applying Bonferroni correction. Statistical analyses were performed with SAS (version 9.4; SAS Institute). Figures were drawn using GraphPad Prism (version 8.4.3; GraphPad Software).
Results
Clinical Characteristics of the Study Population
Table 1 shows anthropometric and clinical data of the participants with diabetes from the first year after diagnosis stratified according to the cluster analysis as well as of CONs. CONs comprised 38 (51%) women and 36 (49%) men, who were more likely to be of a younger age, and had a lower BMI (< 25) and no metabolic abnormalities. Members of SAID, representing 32% of the total cohort, were younger than members of SIRD, MOD, and MARD (all P < .001), but had similar serum low-density lipoprotein and TGs (both P ≥ .999) levels as CONs. Further, the SAID subgroup had lower BMI than SIRD, MOD, and MARD (all P < .001). The SIDD subgroup, representing 2% of the cohort, exhibited similar features as SAID and had poorer glycemic control than other subgroups. SIRD members, comprising 5% of the cohort, had higher waist-to-hip ratio (WHR) compared to individuals in all other subgroups and CONs (all P < .001). They also featured higher BMI and percentage body fat than SAID, SIDD, MARD, and CON members (all P < .001). The MOD subgroup, representing 25% of the cohort, showed similar BMI (P = .96) and percentage body fat (P = .87), but younger age than SIRD (P < .001). Members of MARD, comprising 27% of all participants, were generally older than those of the SAID, SIDD, and MOD and CON groups (all P < .001) and showed only minor metabolic abnormalities (see Table 1).
Table 1.
Anthropometric and clinical characteristics of the study population
| CONs (n = 74) | SAID (n = 261) | SIDD (n = 19) | SIRD (n = 42) | MOD (n = 204) | MARD (n = 220) | |
|---|---|---|---|---|---|---|
| Female, % | 51 | 47 | 11 | 19 | 49 | 26 |
| Age, y | 35.7 ± 12.6 | 36.7 ± 11.9 | 42.7 ± 12.2 | 55.4 ± 9.2 | 45.3 ± 9.9 | 56.7 ± 7.3 |
| BMI | 22.6 ± 1.6 | 24.7 ± 4.1 | 26.5 ± 2.8 | 34.0 ± 4.0 | 34.7 ± 6.0 | 27.3 ± 3.7 |
| Waist-to-hip ratio | 0.83 ± 0.07 | 0.86 ± 0.09 | 0.94 ± 0.06 | 1.01 ± 0.08 | 0.95 ± 0.07 | 0.94 ± 0.07 |
| Body fat, % | 22.6 ± 6.1 | 25.4 ± 8.3 | 26.7 ± 7.6 | 36.3 ± 5.8 | 37.5 ± 8.4 | 30.2 ± 6.5 |
| HbA1c, mmol/mol | 32 ± 3 | 48 ± 10 | 72 ± 14 | 44 ± 8 | 47 ± 9 | 45 ± 7 |
| HbA1c, % | 5.1 ± 0.3 | 6.5 ± 0.9 | 8.8 ± 1.3 | 6.2 ± 0.1 | 6.4 ± 0.1 | 6.3 ± 0.7 |
| FPG, mmol/L | 4.83 ± 0.83 | 7.34 ± 2.05 | 10.66 ± 3.55 | 6.60 ± 1.89 | 7.27 ± 1.67 | 6.83 ± 1.28 |
| LDL, mmol/L | 2.82 ± 0.98 | 2.87 ± 0.85 | 3.34 ± 0.75 | 3.24 ± 1.01 | 3.44 ± 0.98 | 3.29 ± 0.93 |
| HDL, mmol/L | 1.79 ± 0.44 | 1.63 ± 0.47 | 1.35 ± 0.28 | 1.01 ± 0.23 | 1.17 ± 0.28 | 1.32 ± 0.39 |
| TGs, mmol/L | 0.9 (0.6-1.2) | 0.9 (0.6-1.2) | 1.2 (0.8-2.3) | 2.4 (1.5-3.3) | 1.6 (1.1-2.3) | 1.3 (0.9-1.8) |
| TGs/HDL ratio | 1.1 (0.8-1.7) | 1.2 (0.8-2.1) | 1.9 (1.2-3.4) | 5.1 (2.9-10.3) | 3.3 (2.1-5.0) | 2.2 (1.5-3.7) |
| Oxygen pulse, mL/heartbeat | 14.3 ± 9.8 | 12.7 ± 3.9 | 11.8 ± 3.2 | 13.3 ± 3.3 | 13.1 ± 3.6 | 12.6 ± 3.6 |
| RERmax | 1.18 ± 0.09 | 1.18 ± 0.11 | 1.14 ± 0.09 | 1.12 ± 0.09 | 1.14 ± 0.11 | 1.16 ± 0.1 |
| Systolic BP, mm Hg | 122 ± 14 | 128 ± 16 | 137 ± 14 | 140 ± 16 | 138 ± 16 | 140 ± 19 |
| Diastolic BP, mm Hg | 74 ± 9 | 78 ± 10 | 81 ± 8 | 84 ± 12 | 85 ± 10 | 82 ± 10 |
| Heart rate, bpm | 63 ± 10 | 64 ± 10 | 70 ± 12 | 70 ± 11 | 69 ± 10 | 67 ± 10 |
Data are shown as mean ± SD for normally distributed parameters, median (first quartile to third quartile) for log-normally distributed parameters, or n (%).
Abbreviations: BMI, body mass index; BP, blood pressure; bpm, beats per minute; CONs, lean, glucose-tolerant controls; FPG, fasting plasma glucose; HbA1c, glycated hemoglobin A1c; HDL, high-density lipoprotein cholesterol; LDL, low-density lipoprotein cholesterol; MARD, moderate age-related diabetes; MOD, moderate obesity-related diabetes; RER, respiratory exchange ratio, SAID, severe autoimmune diabetes; SIDD, severe insulin-deficient diabetes; SIRD, severe insulin-resistant diabetes; TGs, triglycerides.
Physical Fitness and Physical Activity Behavior
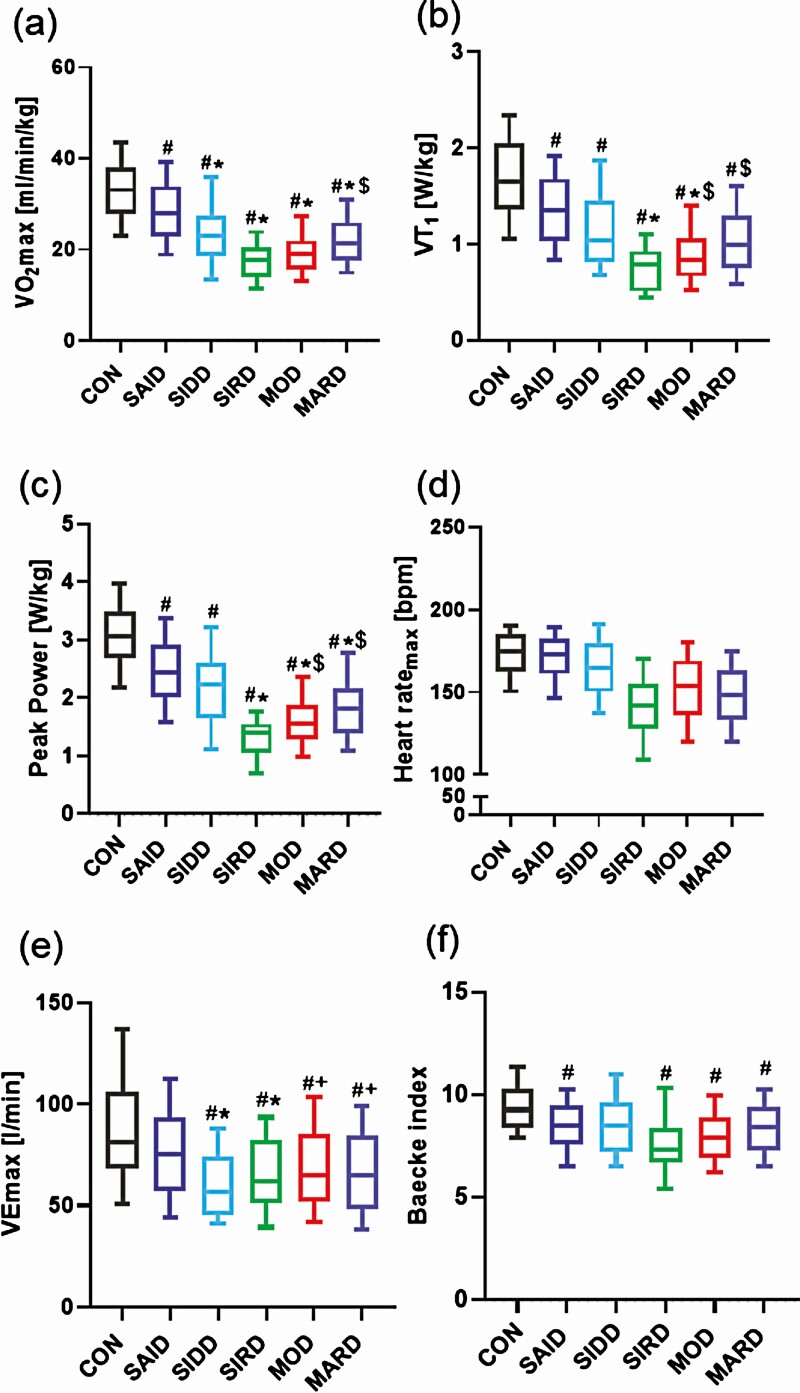
Physical fitness, as assessed by VO2max, was higher in SAID (28.5 ± 7.8 mL min–1 kg–1, P < .05) than in all other subgroups (Fig. 1A). On the other hand, VO2max was lower in SIRD than in SAID and MARD (both P < .01), but not different from SIDD and MOD (P = .98; P = .07) after adjustment for age, sex, and BMI. Further, power output at VT1 was lower in SIRD than in SAID (P < .001), MOD, and MARD (both P < .05) (Fig. 1B), but equal to SIDD (P = .88) after adjustment for age, sex, and BMI. Likewise, peak power output was lower in SIRD (1.33 ± 0.39 W/kg) than in SAID (2.50 ± 0.71 W/kg, P < .001), MOD (1.61 ± 0.53 W/kg, P < .001), and MARD (1.97 ± 0.65 W/kg, P < .01), but not different from SIDD (P = .38) after adjustment for age, sex, and BMI. Even after adjustment for the main confounders, peak power output was statistical higher in SAID compared to SIRD, MOD, and MARD (all P < .001) and tended to be higher than in SIDD (P = .05) (Fig. 1C). As expected, CONs exhibited higher physical fitness (VO2max: 33.0 ± 7.7 mL min–1 kg–1, all P < .001), power output at VT1 (1.70 ± 0.50 W/kg, all P < .001), and peak power (3.06 ± 0.64 W/kg, all P < .001) than individuals with diabetes independent of cluster classification after adjustments for age, sex, and BMI.
Figure 1.
Physical fitness and physical activity of the study population. Physical fitness was assessed by A to E, cycling Spiroergometry, and physical activity was assessed by F, Baecke index, in individuals with newly diagnosed severe autoimmune diabetes (SAID), severe insulin-deficient diabetes (SIDD), severe insulin-resistant diabetes (SIRD), moderate obesity-related diabetes (MOD), and moderate age-related diabetes (MARD) and glucose-tolerant controls (CONs). Box plot with median, first quartile to third quartile and whiskers for tenth to 90th percentile. #P less than .05 vs CON. *P less than .05 vs SAID. +P less than .05 vs SIDD. $P less than .05 vs SIRD. Statistical significance levels were adjusted for age, sex, and body mass index and Tukey-Kramer for 10 pairwise comparisons. VEmax, maximal respiratory minute volume; VT1, first ventilatory threshold.
Differences in maximal HR lost statistical significance after adjustment for age, sex, and BMI between subgroups and CONs (all P > .05) (Fig. 1D). Additionally, oxygen pulse was statistical higher in CONs than in SIDD, SIRD (both P < .01), and MARD (P < .05) and tended to be higher than in MOD (P = .05) but was equal to SAID (P = .4) after adjustment for age, sex, and BMI. There was no difference between subgroups with nonautoimmune diabetes (all P > .05) (see Table 1).
Finally, SAID presented with a higher VEmax (76 ± 27 L/min) compared to SIDD and SIRD (both P < .001), but not to MOD (P = .14), MARD (P = .08), and CON groups (P = .13), whereas SIDD had lower VEmax (60 ± 18 L/min) than SAID, MARD (both P < .001), and MOD (P < .01) after adjustment for age, sex, and BMI. There was no difference between SIDD and SIRD (P = .36). CONs showed a higher VEmax (92.6 ± 61.8 L/min) than SIDD, SIRD (both P < .001), MOD, and MARD (both P < .05) after adjustment for age, sex, and BMI (Fig. 1E).
Physical activity behavior, as assessed by the Baecke index, was similar between subgroups on adjustment for age, sex, and BMI, whereas CONs presented with higher habitual physical activity (9.4 ± 1.4) than SAID, SIRD, MOD (all P < .001), and MARD (all P < .01) (Fig. 1F).
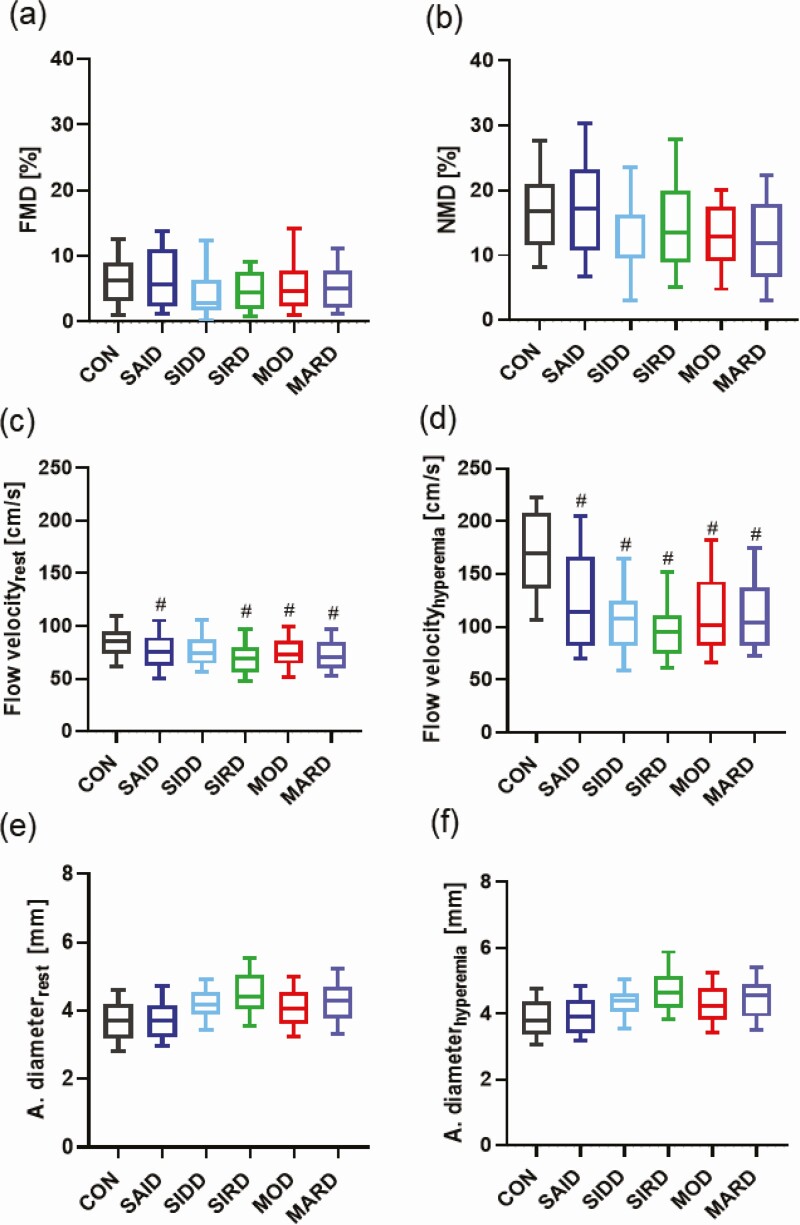
Endothelial Function
There were no differences between subgroups and CONs in FMD after adjustment for age, sex, and BMI (P < .05) (Fig. 2A). Moreover, NMD was similar between subgroups and CONs after previous mentioned adjustments (Fig. 2B). At rest, flow velocity was similar between subgroups after adjustment for age, sex, and BMI (P > .05). CONs showed a higher flow velocity than individuals in the SAID (P < .05), SIRD, MOD, and MARD groups (all P < .005), but no difference compared with SIDD after adjustments for age, sex, and BMI (P = .47) (Fig. 2C). In line with these results, the subgroups had equal flow velocity 15 seconds after deflation after adjustment for the main confounders (P > .05), while in CONs flow velocity was higher than in all the diabetes subgroups after adjustments for age, sex, and BMI (P < .001) (Fig. 2D). Preocclusion and postocclusion, brachial artery diameter was similar between subgroups and glucose-tolerant individuals after adjustments for age, sex, and BMI (P > .05) (Fig. 2E-2F). Also, there were no differences in NMD response of brachial artery diameter between all groups after adjustment for age, sex, and BMI (P > .05) (data not shown).
Figure 2.
Endothelial function of the study population. Parameters of endothelial function and flow velocity in severe autoimmune diabetes (SAID), severe insulin-deficient diabetes (SIDD), severe insulin-resistant diabetes (SIRD), moderate obesity-related diabetes (MOD), and moderate age-related diabetes (MARD) subgroups and glucose-tolerant controls (CONs) showing A, flow-mediated dilation (FMD); B, nitroglycerin-mediated dilation (NMD); C, flow velocity at rest; and D, 15 seconds after deflation (hyperemia); and E, brachial artery diameter at rest, and F, 15 seconds after deflation (hyperemia). Box plot with median, first quartile to third quartile and whiskers for tenth to 90th percentile. #P less than .05 vs CON. Statistical significance levels were adjusted for age, sex, and body mass index and Tukey-Kramer for 10 pairwise comparisons.
SBP was higher in the CON than in the MARD groups (P < .01) after adjustment for age, sex, and BMI, but there were no further differences between groups. Additionally, HR was higher in MARD than in CONs and SAID and lower in SIRD than in CONs (all P < .05) after adjustment for age, sex, and BMI. But again, there were no further differences between groups (see Table 1).
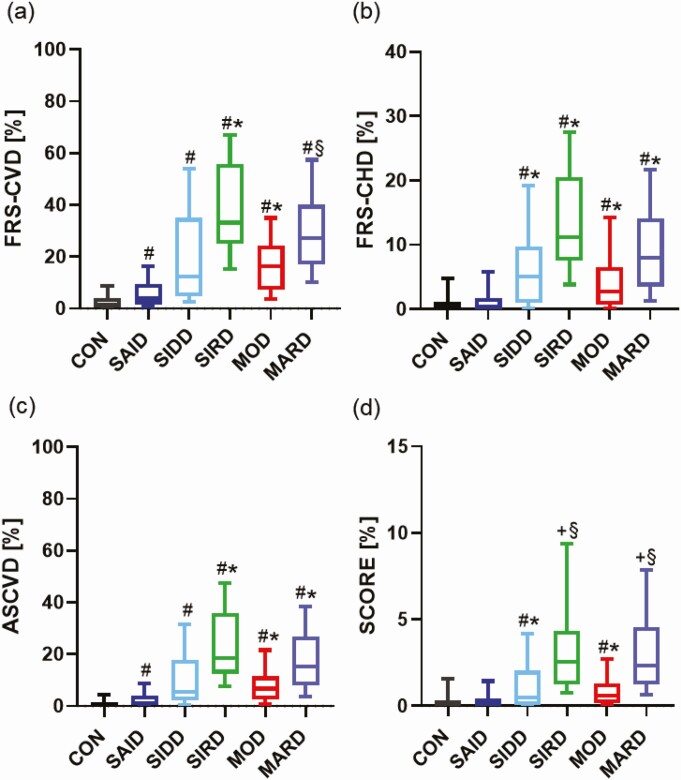
Cardiovascular Risk
Regarding the novel diabetes subgroups, all cardiovascular risk scores indicated the CVD risk to be highest for SIRD and lowest for SAID. According to the FRS, the SAID subgroup had the lowest 10-year risk for CHD (0.3% [0.02%-1.7%]) compared to all other subgroups (all P < .05) and a lower CVD (3.9% [1.6%-9.4%]) than SIRD (P < .05) and MOD (P < .001) even after adjustment for age, sex, and BMI. FRS-CVD presented a higher CVD risk for individuals with diabetes mellitus compared to CONs after adjustment for age, sex, and BMI (all P < .001). Along this line, FRS-CHD was lower in CONs than in the SIDD, SIRD, MOD, and MARD subgroups (all P < .001) after adjustments for the main confounders. However, there was no difference between the CON and SAID groups (P = .19) after the previous-mentioned adjustments (Fig. 3A-3B). Using the ASCVD score, SAID (1.04% [0.3%-3.9%]) had a lower CVD risk than SIRD (18.4% [12.4%-35.9%]), MOD (6.7% [2.5%-11.5%]), and MARD (15.1% [7.9%-26.7%]) (all P < .001) after adjustments for age, sex, and BMI. As for FRS-CVD, ASCVD showed a higher CVD risk for members of all diabetes subgroups compared to CONs after adjustments for age, sex, and BMI (all P < .001). (Fig. 3C). Finally, SCORE indicated a higher 10-year risk for major adverse cardiac events for participants in the SIRD (2.5% [1.3%-4.3%]) and MARD (2.3% [1.2%-4.6%]) subgroups compared to SIDD (P < .05; P < .001) and MOD (both P < .001) after adjustments for the main confounders. Furthermore, SCORE was similar between the CON and SAID (P = .06), SIRD (P = .96), and MARD groups (P = .98) after adjustments for age, sex, and BMI, but CONs had a lower SCORE than SIDD and MOD (both P < .001) (see Fig. 3A).
Figure 3.
Diabetes-related cardiovascular complications of study population. Calculation of the 10-year risk of heart disease in individuals with newly diagnosed severe autoimmune diabetes (SAID), severe insulin-deficient diabetes (SIDD), severe insulin-resistant diabetes (SIRD), moderate obesity-related diabetes (MOD), and moderate age-related diabetes (MARD) and glucose-tolerant control (CON). The 10-year risk was estimated by A, Framingham Risk Score–Cardiovascular Disease (FRS-CVD); B, Framingham Risk Score–Coronary Heart Disease (FRS-CHD); C, Atherosclerotic CardioVascular Disease (ASCVD) risk score; and D, Systematic COronary Risk Evaluation (SCORE). Box plot with median, first quartile to third quartile and whiskers for tenth to 90th percentile. #P less than .05 vs CON. *P less than .05 vs SAID. +P less than .05 vs SIDD. §P less than .05 vs MOD. Statistical significance levels were adjusted for age, sex, and body mass index and Tukey-Kramer for 10 pairwise comparisons.
The overall higher risk of SIRD is supported by the greater TGs:HDL ratio compared to SAID (P < .001), MOD (P < .05), and MARD (P < .01) after adjustment for age, sex, and BMI (all P < .05) (see Table 1) and by the strong association between HOMA-IR and HDL cholesterol (r = –0.24; P < .001) and SBP (r = 0.16; P = .001) in volunteers with nonautoimmune diabetes (SIDD, SIRD, MOD, and MARD subgroups) of the present study (data not shown).
Discussion
This study detected differences in CRF and cardiovascular risk among novel diabetes subgroups, already within the first year after diabetes diagnosis. Further, the analyses show a lower physical fitness and activity in individuals with diabetes compared to glucose-tolerant humans, supporting the concept of a tight relationship between cardiovascular complications and diabetes. In detail, members of the SIRD subgroup have the lowest physical fitness, despite comparable physical activity levels among all subgroups, and SAID individuals have the lowest scores for cardiovascular complications within the first year after diabetes diagnosis. However, these results were not reflected by parallel alterations of endothelial function, as both FMD and NMD were comparable among all subgroups.
The insulin-resistant SIRD subgroup showed not only lowest VO2max, but also peak power at VT1 and peak power output. These findings confirm the close association between VO2max and insulin sensitivity (37, 38) as well as between CRF and muscular strength with the risk of type 2 diabetes (12). Among others, VO2max reflects mitochondrial function and density, as demonstrated by its positive relationship with muscle adenosine 5′-triphosphate synthesis in humans (39). Impairment in mitochondrial function may result from inherited and acquired factors, for example, sedentary lifestyle, age, and body fat mass, and their interaction with whole-body insulin sensitivity, possibly via lipotoxicity (40). These results were underlined by higher physical fitness levels of glucose-tolerant individuals compared to people with diabetes as shown in the present and previous studies (41). Interestingly, the lower physical fitness in SIRD was largely independent of age, sex, and BMI. Surprisingly, differences in physical fitness between the diabetes subgroups could not be attributed to physical activity, as habitual physical activity behavior from the Baecke index was similar between all subgroups after adjusting for age, sex, and BMI. However, glucose-tolerant people exhibited greater physical activity than individuals with diabetes. This is in line with previous studies as physical activity levels seem to be lower in long-standing type 1 (42) and type 2 diabetes compared to glucose-tolerant patients (16). Taken together, these data suggest the presence of distinct abnormalities in mitochondrial plasticity (39, 43) and/or other mechanisms (44), not simply resulting from aging, adiposity, or physical inactivity.
Recent studies indicate that cardiovascular autonomic neuropathy is associated with newly diagnosed diabetes, albeit more prominently with type 2 diabetes than type 1 diabetes, and is linked to insulin resistance and hyperglycemia (45). The present study supports this finding in that oxygen pulse was reduced in all nonautoimmune diabetes subgroups, classically summarized as type 2 diabetes, but not in SAID when compared to the CON group after adjustment for age, sex, and BMI. Thus, these findings may help to explain the slight differences in maximal HR observed between subgroups before adjustment for age, sex, and BMI (data not shown) suggesting impaired compensatory HR elevation during spiroergometry, specifically in SIRD.
Nevertheless, some of the findings may be explained by body fat mass, as assessed roughly by BMI and WHR in the present study. For example, VEmax, that is, the product of tidal volume and breathing rate, was comparable between the most obese MOD and the leanest SAID subgroups, which is likely due to the compensatory higher breathing rates for lower tidal volumes in the obese individuals (46). Also, VEmax was greater in the lowest (SAID) than in the highest (SIRD) WHR subgroup, suggesting an additional role for visceral adiposity aside from contributing to insulin resistance. This is supported by the inverse correlation between WHR and respiratory function reported for 9674 men and 11 876 women (47). It is tempting to speculate that the increased ventilation rates could cause breathing discomfort and premature exercise termination in insulin-resistant individuals with (visceral) obesity and type 2 diabetes. However, impaired pulmonary function is strongly related to type 2 diabetes, due to chronic glycemic exposure (48), which would be supported by the present study.
Endothelial function, as assessed by FMD, was not different between the novel diabetes subgroups and glucose-tolerant individuals after adjustment for the main confounders. These results cannot be attributed to confounders for FMD such as differences in blood flow, artery diameter, and/or arterial stiffness, as intergroup differences in flow velocity stayed stable between rest and 15 seconds after deflation, and preocclusion and postocclusion diameter were similar between all groups. Rather, specific features of the baseline GDS population, such as recent-onset diabetes, good glucometabolic control, and absence of clinically relevant diabetes-related complications or comorbidities, may explain these results. Additionally, the data in the present study are supported by larger cohort studies, which also detected no association between diabetes and FMD (49) or lower FMD only in people with type 2 diabetes, but not in those with impaired glucose metabolism (8). Finally, the present results for FMD% are within the range from about 3.6 ± 2.3% to 6.4 ± 3.6% in people with type 2 diabetes reported by previous studies (50). Thus, the present study supports data challenging the concept of a close, direct relationship between endothelial dysfunction and diabetes mellitus. Previous results from Scandinavian cohorts suggested an higher CVD risk in SIRD and MARD subgroups, which disappeared after adjusting for age and sex (5). Further, a recent meta-analysis indicated an association between higher CRF and a lower risk of all-cause mortality, mostly linked to decreased CVD/CHD risks (13). Along this line, the data from the GDS cohort first identify the lowest CVD/CHD risk in the glucose-tolerant control group. Second, the present study reveals differences in cardiovascular risk between diabetes subgroups as SAID individuals have the lowest CVD/CHD risk—based on FRS-CHD and ASCVD scores—which mostly remained low on adjustment for age, sex, and BMI. On the other hand, the SIRD and MARD subgroups showed higher CVD risk using ASCVD and SCORE, which were only partly retained after the adjustments. These findings underscore the importance of overweight and BMI (51) and diabetes per se(52) as drivers of increased cardiovascular risk. Nevertheless, this study confirms the strong inverse association between HOMA-IR and FRS-CHD, FRS-CVD, and ASCVD and pointing to a higher risk for CVD/CHD in insulin-resistant individuals. Of note, the present analyses of the novel diabetes subgroups rely mainly on short duration of disease and do not take into account cluster instability (6). Also, SIRD features additional possibly detrimental risk factors, which may determine long-term CVD risk, including dyslipidemia, progressive nonalcoholic fatty liver disease (6, 53), low-grade inflammation (35), as well as impaired CRF, as shown in the present study. Finally, this group also had the greatest TGs:HDL ratio, another surrogate both of insulin resistance (34) and CVD risk (54).
An additional recent analysis of three intervention trials suggested the highest risk of major adverse cardiovascular events and cardiovascular death for members of their cluster A, corresponding to the SIDD subgroup (11). The present study only found higher FRS-CHD score and SCORE in SIDD when compared to SAID, but not in other subgroups. Longer diabetes duration (14-16 years), worse HbA1c (96-98 mmol/mol (10.9-11.1%)) and proportion of participants at high CVD risk (80-85%) likely explains the observed difference.
The strengths of the present study are the large sample size of well-characterized individuals with a known diabetes duration of less than 1 year and the use of gold-standard methods to assess whole-body insulin sensitivity, physical fitness, and endothelial function. One limitation is the nominally small number of individuals in the SIDD and SIRD subgroups, although proportionally representative of the distribution shown in previous study populations (5, 6, 10). Consequently, some associations may not reach statistical significance in the present analysis. Also, other mediating factors, such as specific dietary patterns (20), were not assessed in the context of the present analysis, but may affect the results. Moreover, the glucose-tolerant CON group illustrated the variables in lean, healthy people but did not allow for direct comparison with each subgroup of the study. Further, there are also other factors that can affect physical fitness, such as the circulatory system, and left and right ventricle function. Of note, differences in resting HR, SBP, and age between groups could affect the results of FMD (55), a method with already very high intrinsic variability. Furthermore, resting HR and SBP were recorded before but not continuously monitored during FMD measurements.
Taken together, the novel diabetes subgroups (clusters) exhibit distinct differences in cardiovascular risk already within the first year after diabetes diagnosis. Specifically, the SAID subgroup has lower risk scores for CVDs among diabetes subgroups, whereas the SIRD subgroup exhibits the lowest physical fitness, despite similar physical activity and endothelial function compared to other groups.
Acknowledgements
Parts of this study were accepted to be presented at national and international scientific meetings (DDG 2021, American Diabetes Association 2021 and ÖDG 2021).
We would like to acknowledge the support given to this project by the GDS Group, which consists of M. Roden (speaker), H. Al-Hasani, B. F. Belgardt, V. Burkart, A. E. Buyken, G. Geerling, V. Schrauwen-Hinderling, C. Herder, A. Icks, K. Jandeleit-Dahm, S. Kahl, J. Kotzka, O. Kuss, E. Lammert, S. Trenkamp, W. Rathmann, J. Szendroedi, D. Ziegler, and their colleagues who are responsible for the design and conduct of the GDS.
Financial Support: The GDS was initiated and financed by the German Diabetes Center (DDZ), which is funded by the German Federal Ministry of Health (Berlin, Germany) and the Ministry of Culture and Science of the state of North Rhine-Westphalia (Düsseldorf, Germany) and by the German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD e.V.). M.R. is further supported by grants from the European Funds for Regional Development (No. EFRE-0400191), EUREKA Eurostars-2 (No. E!-113230-DIA-PEP), the German Research Foundation (DFG; Nos. CRC/SFB 1116/2 B12 and RTG/GRK 2576), and the Schmutzler Stiftung. M.K. and N.G. are also supported by grants from the German Research Foundation (CRC1116-B12 to M.K. and CRC1116-B09 to N.G.). The funding sources were not involved in the design of the study; the collection, analysis, or interpretation of data; writing the report; and did not impose any restrictions regarding the publication of the report.
Author Contributions: N.S. wrote the first draft of the manuscript and researched data. O.P.Z. researched data. K.S. performed the statistical analyses. O.P.Z., D.P., V.B., J.S., N.G., M.K., M.R. initiated the study, contributed to the discussion, and reviewed/edited the manuscript. All authors critically reviewed the manuscript. M.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript. Authors affirm that each person listed as authors participated in the study in a substantive manner, in accordance with ICMJE authorship guidelines, and is prepared to take public responsibility for it. All authors consent to the investigation of any improprieties that may be alleged regarding the study. Each author further releases and holds harmless the Endocrine Society from any claim or liability that may arise therefrom.
Clinical Trial Information: Clinicaltrials.gov registration number NCT01055093 (registered January 25, 2010).
Glossary
Abbreviations
- ASCVD
Atherosclerotic CardioVascular Disease
- BIA
bioimpedance analysis
- BMI
body mass index
- CON
lean, glucose-tolerant control
- CRF
cardiorespiratory fitness
- FMD
flow-mediated dilation
- FRS-CHD
Framingham Risk Score for Coronary Heart Disease
- FRS-CVD
Framingham Risk Score for Cardiovascular Disease
- GADA
glutamate acid decarboxylase antibodies
- GDS
German Diabetes Study
- HbA1c
glycated hemoglobin A1c
- HDL
high-density lipoprotein cholesterol
- HOMA-IR
homeostatic model assessment of insulin resistance
- HR
heart rate
- LDL
low-density lipoprotein cholesterol
- MARD
moderate age-related diabetes
- MOD
moderate obesity-related diabetes
- NMD
nitroglycerin (glycerol trinitrate)-mediated dilation
- RER
respiratory exchange ratio
- SAID
severe autoimmune diabetes
- SBP
systolic blood pressure
- SCORE
Systematic COronary Risk Evaluation
- SIDD
severe insulin-deficient diabetes
- SIRD
severe insulin-resistant diabetes
- TGs
triglycerides
- VCO2max
maximal carbon dixoide production
- VE
ventilation
- VEmax
maximal respiratory minute volume
- VO2max
maximal oxygen consumption
- VT1
first ventilatory threshold
- WHR
waist-to-hip ratio.
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided. The data are subject to national data protection laws. Therefore, data cannot be made freely available in a public repository. However, data can be requested through an individual project agreement with the Steering Committee of the GDS (speaker: Michael Roden, michael.roden@ddz.de).
References
- 1. Sarwar N, Gao P, Seshasai SR, et al. . Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Makimattila S, Virkamaki A, Groop PH, et al. . Chronic hyperglycemia impairs endothelial function and insulin sensitivity via different mechanisms in insulin-dependent diabetes mellitus. Circulation. 1996;94(6):1276-1282. [DOI] [PubMed] [Google Scholar]
- 3. Roden M, Shulman GI. The integrative biology of type 2 diabetes. Nature. 2019;576(7785):51-60. [DOI] [PubMed] [Google Scholar]
- 4. Chung WK, Erion K, Florez JC, et al. . Precision medicine in diabetes: a consensus report from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63(9):1671-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahlqvist E, Storm P, Käräjämäki A, et al. . Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6(5):361-369. [DOI] [PubMed] [Google Scholar]
- 6. Zaharia OP, Strassburger K, Strom A, et al. ; German Diabetes Study Group . Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: a 5-year follow-up study. Lancet Diabetes Endocrinol. 2019;7(9):684-694. [DOI] [PubMed] [Google Scholar]
- 7. Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589-600. [DOI] [PubMed] [Google Scholar]
- 8. Henry RM, Ferreira I, Kostense PJ, et al. . Type 2 diabetes is associated with impaired endothelium-dependent, flow-mediated dilation, but impaired glucose metabolism is not; the Hoorn Study. Atherosclerosis. 2004;174(1):49-56. [DOI] [PubMed] [Google Scholar]
- 9. Flammer AJ, Anderson T, Celermajer DS, et al. . The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126(6):753-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tanabe H, Saito H, Kudo A, et al. . Factors associated with risk of diabetic complications in novel cluster-based diabetes subgroups: a Japanese retrospective cohort study. J Clin Med. 2020;9(7):2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kahkoska AR, Geybels MS, Klein KR, et al. . Validation of distinct type 2 diabetes clusters and their association with diabetes complications in the DEVOTE, LEADER and SUSTAIN-6 cardiovascular outcomes trials. Diabetes Obes Metab. 2020;22(9):1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tarp J, Støle AP, Blond K, Grøntved A. Cardiorespiratory fitness, muscular strength and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetologia. 2019;62(7):1129-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kodama S, Saito K, Tanaka S, et al. . Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024-2035. [DOI] [PubMed] [Google Scholar]
- 14. Ross R, Blair SN, Arena R, et al. . Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 2016;134(24):e653-e699. [DOI] [PubMed] [Google Scholar]
- 15. Herzig KH, Ahola R, Leppäluoto J, Jokelainen J, Jämsä T, Keinänen-Kiukaanniemi S. Light physical activity determined by a motion sensor decreases insulin resistance, improves lipid homeostasis and reduces visceral fat in high-risk subjects: PreDiabEx study RCT. Int J Obes (Lond). 2014;38(8):1089-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fagour C, Gonzalez C, Pezzino S, et al. . Low physical activity in patients with type 2 diabetes: the role of obesity. Diabetes Metab. 2013;39(1):85-87. [DOI] [PubMed] [Google Scholar]
- 17. Sayer AA, Dennison EM, Syddall HE, Gilbody HJ, Phillips DI, Cooper C. Type 2 diabetes, muscle strength, and impaired physical function: the tip of the iceberg? Diabetes Care. 2005;28(10):2541-2542. [DOI] [PubMed] [Google Scholar]
- 18. Stettler C, Jenni S, Allemann S, et al. . Exercise capacity in subjects with type 1 diabetes mellitus in eu- and hyperglycaemia. Diabetes Metab Res Rev. 2006;22(4):300-306. [DOI] [PubMed] [Google Scholar]
- 19. Hamburg NM, McMackin CJ, Huang AL, et al. . Physical inactivity rapidly induces insulin resistance and microvascular dysfunction in healthy volunteers. Arterioscler Thromb Vasc Biol. 2007;27(12):2650-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Szendroedi J, Saxena A, Weber KS, et al. ; GDS Group . Cohort profile: the German Diabetes Study (GDS). Cardiovasc Diabetol. 2016;15:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. American Diabetes Association. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes–2020. Diabetes Care. 2020;43(Suppl 1):S14-S31. [DOI] [PubMed] [Google Scholar]
- 22. Wasserman K, Whipp BJ, Koyl SN, Beaver WL. Anaerobic threshold and respiratory gas exchange during exercise. J Appl Physiol. 1973;35(2):236-243. [DOI] [PubMed] [Google Scholar]
- 23. Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936-942. [DOI] [PubMed] [Google Scholar]
- 24. Wagner P, Singer R. Ein Fragebogen zur Erfassung der habituellen körperlichen Aktivität verschiedener Bevölkerungsgruppen. Sportwissenschaft. 2003;33:383-397. [Google Scholar]
- 25. Corretti MC, Anderson TJ, Benjamin EJ, et al. ; International Brachial Artery Reactivity Task Force . Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257-265. [DOI] [PubMed] [Google Scholar]
- 26. Naka KK, Tweddel AC, Doshi SN, Goodfellow J, Henderson AH. Flow-mediated changes in pulse wave velocity: a new clinical measure of endothelial function. Eur Heart J. 2006;27(3):302-309. [DOI] [PubMed] [Google Scholar]
- 27. Reference Values for Arterial Stiffness Collaboration. Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values.’ Eur Heart J. 2010;31(19):2338-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Al Mheid I, Quyyumi AA. Allometric scaling of endothelium-dependent vasodilation: brachial artery flow-mediated dilation coming of age? Vasc Med. 2013;18(6):368-371. [DOI] [PubMed] [Google Scholar]
- 29. McLay KM, Nederveen JP, Koval JJ, Paterson DH, Murias JM. Allometric scaling of flow-mediated dilation: is it always helpful? Clin Physiol Funct Imaging. 2018;38(4):663-669. [DOI] [PubMed] [Google Scholar]
- 30. Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837-1847. [DOI] [PubMed] [Google Scholar]
- 31. D’Agostino RB Sr, Vasan RS, Pencina MJ, et al. . General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743-753. [DOI] [PubMed] [Google Scholar]
- 32. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25Suppl 2):S49-S73. [DOI] [PubMed] [Google Scholar]
- 33. Conroy RM, Pyörälä K, Fitzgerald AP, et al. ; SCORE project group . Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987-1003. [DOI] [PubMed] [Google Scholar]
- 34. Brehm A, Pfeiler G, Pacini G, Vierhapper H, Roden M. Relationship between serum lipoprotein ratios and insulin resistance in obesity. Clin Chem. 2004;50(12):2316-2322. [DOI] [PubMed] [Google Scholar]
- 35. Herder C, Maalmi H, Strassburger K, et al. GDS Group . Differences in biomarkers of inflammation between novel subgroups of recent-onset diabetes. Diabetes. 2021;70(5):1198-1208. [DOI] [PubMed] [Google Scholar]
- 36. Ratter-Rieck JM, Maalmi H, Trenkamp S, et al. ; GDS Group . Leukocyte counts and T-cell frequencies differ between novel subgroups of diabetes and are associated with metabolic parameters and biomarkers of inflammation. Diabetes. 2021;70(11):2652-2662. [DOI] [PubMed] [Google Scholar]
- 37. Perseghin G, Price TB, Petersen KF, et al. . Increased glucose transport-phosphorylation and muscle glycogen synthesis after exercise training in insulin-resistant subjects. N Engl J Med. 1996;335(18):1357-1362. [DOI] [PubMed] [Google Scholar]
- 38. Apostolopoulou M, Strassburger K, Herder C, et al. ; GDS group . Metabolic flexibility and oxidative capacity independently associate with insulin sensitivity in individuals with newly diagnosed type 2 diabetes. Diabetologia. 2016;59(10):2203-2207. [DOI] [PubMed] [Google Scholar]
- 39. Kacerovsky-Bielesz G, Chmelik M, Ling C, et al. . Short-term exercise training does not stimulate skeletal muscle ATP synthesis in relatives of humans with type 2 diabetes. Diabetes. 2009;58(6):1333-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Szendroedi J, Phielix E, Roden M. The role of mitochondria in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2011;8(2):92-103. [DOI] [PubMed] [Google Scholar]
- 41. Nesti L, Pugliese NR, Sciuto P, Natali A. Type 2 diabetes and reduced exercise tolerance: a review of the literature through an integrated physiology approach. Cardiovasc Diabetol. 2020;19(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Valerio G, Spagnuolo MI, Lombardi F, Spadaro R, Siano M, Franzese A. Physical activity and sports participation in children and adolescents with type 1 diabetes mellitus. Nutr Metab Cardiovasc Dis. 2007;17(5):376-382. [DOI] [PubMed] [Google Scholar]
- 43. Phielix E, Meex R, Moonen-Kornips E, Hesselink MK, Schrauwen P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals. Diabetologia. 2010;53(8):1714-1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Peter I, Papandonatos GD, Belalcazar LM, et al. ; Look AHEAD Research Group . Genetic modifiers of cardiorespiratory fitness response to lifestyle intervention. Med Sci Sports Exerc. 2014;46(2):302-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kück JL, Bönhof GJ, Strom A, et al. ; GDS group . Impairment in baroreflex sensitivity in recent-onset type 2 diabetes without progression over 5 years. Diabetes. 2020;69(5):1011-1019. [DOI] [PubMed] [Google Scholar]
- 46. Sampson MG, Grassino AE. Load compensation in obese patients during quiet tidal breathing. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(4):1269-1276. [DOI] [PubMed] [Google Scholar]
- 47. Canoy D, Luben R, Welch A, et al. . Abdominal obesity and respiratory function in men and women in the EPIC-Norfolk Study, United Kingdom. Am J Epidemiol. 2004;159(12):1140-1149. [DOI] [PubMed] [Google Scholar]
- 48. Davis WA, Knuiman M, Kendall P, Grange V, Davis TM; Fremantle Diabetes Study . Glycemic exposure is associated with reduced pulmonary function in type 2 diabetes: the Fremantle Diabetes Study. Diabetes Care. 2004;27(3):752-757. [DOI] [PubMed] [Google Scholar]
- 49. Empen K, Lorbeer R, Völzke H, et al. . Do patients with type 1 and type 2 diabetes really have an impaired endothelial function? A population-based propensity score matching analysis. Cardiovasc Diabetol. 2013;12:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee JH, Lee R, Hwang MH, Hamilton MT, Park Y. The effects of exercise on vascular endothelial function in type 2 diabetes: a systematic review and meta-analysis. Diabetol Metab Syndr. 2018;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Global BMI Mortality Collaboration. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388(10046):776-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383(9922):1068-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zaharia OP, Strassburger K, Knebel B, et al. ; GDS Group . Role of patatin-like phospholipase domain-containing 3 gene for hepatic lipid content and insulin resistance in diabetes. Diabetes Care. 2020;43(9):2161-2168. [DOI] [PubMed] [Google Scholar]
- 54. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595-1607. [DOI] [PubMed] [Google Scholar]
- 55. West SG, Wagner P, Schoemer SL, et al. . Biological correlates of day-to-day variation in flow-mediated dilation in individuals with type 2 diabetes: a study of test-retest reliability. Diabetologia. 2004;47(9):1625-1631. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided. The data are subject to national data protection laws. Therefore, data cannot be made freely available in a public repository. However, data can be requested through an individual project agreement with the Steering Committee of the GDS (speaker: Michael Roden, michael.roden@ddz.de).