Abstract
Background
Risk of type 2 diabetes mellitus (T2DM) in transgender and gender diverse (TGD) persons, especially those receiving gender-affirming hormone therapy (GAHT) is an area of clinical and research importance.
Methods
We used data from an electronic health record-based cohort study of persons 18 years and older enrolled in 3 integrated health care systems. The cohort included 2869 transfeminine members matched to 28 300 cisgender women and 28 258 cisgender men on age, race/ethnicity, calendar year, and site, and 2133 transmasculine members similarly matched to 20 997 cisgender women and 20 964 cisgender men. Cohort ascertainment spanned 9 years from 2006 through 2014 and follow-up extended through 2016. Data on T2DM incidence and prevalence were analyzed using Cox proportional hazards and logistic regression models, respectively. All analyses controlled for body mass index.
Results
Both prevalent and incident T2DM was more common in the transfeminine cohort relative to cisgender female referents with odds ratio and hazard ratio (95% CI) estimates of 1.3 (1.1-1.5) and 1.4 (1.1-1.8), respectively. No significant differences in prevalence or incidence of T2DM were observed across the remaining comparison groups, both overall and in TGD persons with evidence of GAHT receipt.
Conclusion
Although transfeminine people may be at higher risk for T2DM compared with cisgender females, the corresponding difference relative to cisgender males is not discernable. Moreover, there is little evidence that T2DM occurrence in either transfeminine or transmasculine persons is attributable to GAHT use.
Keywords: transgender, type 2 diabetes mellitus, cohort study
Transgender and gender diverse (TGD) people represent a sizeable proportion of the general population (1). TGD individuals are a diverse group and they share a common characteristic of having gender identity, expression, or perception that does not conform to the norms and stereotypes attributed to their gender assigned at birth (1, 2). Although many TGD people self-identify as men or women, there is also a large group of individuals who reject binary categories and present with a wide range of gender-diverse identities and behaviors (3). To capture the wide range of gender identities among TGD people, a more general term “transfeminine” (TF) is used to describe an individual who was assigned male gender at birth, and the term “transmasculine” (TM) is typically applied to an individual who was assigned female gender at birth. Some, but not all, TGD people seek gender-affirming care, which may include behavioral therapy to facilitate social transition, hormone therapy to achieve desired feminization or masculinization, as well as surgeries or other procedures to change appearance of the genitalia or secondary sex characteristics (4).
With the growing number of people who report identifying as TGD (5, 6), the likelihood that health care providers will encounter TGD patients in their practice is expected to increase. For all the previous reasons, better understanding of health issues facing TGD people is becoming increasingly important across a wide range of clinical disciplines. These health issues fall into 2 main categories: the general considerations that are not specific to TGD status such as the need to maintain a healthy lifestyle and adhere to recommended screening guidelines, and the considerations that are unique to the TGD population, such as the risks and benefits of gender-affirming hormone therapy (GAHT) (1, 7, 8).
An important priority of TGD health research is to better understand the metabolic changes induced by GAHT. In general, feminizing therapy for adult TF patients involves administration of estrogen and testosterone-lowering medications such as spironolactone, whereas masculinizing therapy for TM individuals usually includes testosterone (9, 10). A specific GAHT-related endpoint of interest in TGD persons is the occurrence of type 2 diabetes mellitus (T2DM) (11). There is evidence that GAHT may affect glucose metabolism and body weight, but how these changes influence the risk of T2DM is unclear (12). In addition, previous research suggests that testosterone may lower insulin resistance in cisgender men (13, 14); however, whether these findings are relevant to TM individuals is unknown.
With these knowledge gaps in mind, the objective of the present analysis was to examine the occurrence of T2DM in a cohort of TGD persons receiving GAHT. In addition, we sought to compare the measures of T2DM incidence and prevalence among TGD study participants with those observed in matched cisgender reference groups. In performing these analyses, we hypothesize that T2DM incidence among TGD people may be attributable to GAHT initiation. If so, we expect that the difference in T2DM incidence relative to cisgender controls of either gender will be most pronounced among TGD persons after they started receiving GAHT.
Methods
The present analysis used data from the Study of Transition Outcomes and Gender (STRONG), an electronic health record (EHR)-based cohort of TGD persons receiving care at 3 Kaiser Permanente (KP) health systems in Northern California, Southern California, and Georgia. The cohort ascertainment and data collection were coordinated by Emory University, and all activities were reviewed and approved by the institutional review boards of the 4 institutions with exemption of informed consent.
The methods of cohort ascertainment were described in detail previously (15, 16). In brief, cohort selection involved a 3-step algorithm: an initial EHR search to identify cohort candidates (step 1), validation of TGD status (step 2), and determination of TM or TF status (step 3). Up to 10 male and 10 female cisgender KP members were matched to each member of the final validated TGD cohort on race/ethnicity (non-Hispanic white, non-Hispanic black, Asian/Pacific Islander, Hispanic, and other/unknown), year of birth (within a 5-year interval), study site, and calendar year of membership based on the index date to permit comparable follow-ups. Index date was defined as the first recorded evidence of TGD status. Each TGD participant was linked to both female and male cisgender referents. Both male and female cisgender reference groups were used because hormone serum concentrations among transgender persons can range from normal physiologic male to normal physiologic female levels, depending on the mode of delivery and dosage of hormone therapy, as well as individual characteristics (17). A 10:1 ratio was used to allow stratified and subgroup analyses while ensuring enough cisgender referents for each TGD cohort member. Each TGD cohort member was linked to matched referents via a unique cluster identification number to allow subanalyses. Follow-up time for TGD cohort members and their matched referents extended up to 11 years.
Only persons 18 years or older at the index date were included in the present analysis. Data collection methods and determination of GAHT have been described in detail previously (18). Receipt of GAHT was determined through EHR linkages to prescription data by using national drug codes. Information on other variables of interest, such as body mass index (BMI), was obtained from the clinical encounter records. The cohort ascertainment used EHR data from 2006 through 2014 and follow-up extended through the end of 2016.
In both the TGD and the reference cohorts, T2DM cases were ascertained using the Surveillance, Prevention, and Management of Diabetes Mellitus (SUPREME-DM) DataLink algorithm, adopted by a consortium of multiple integrated health care systems that include the 3 KP health plans participating in STRONG. The SUPREME-DM methods of T2DM identification have been described in detail previously (19). Briefly, the algorithm identifies enrolled members in the health systems with diabetes by searching inpatient and outpatient diagnosis codes, laboratory test results, and pharmacy records. For example, a person is characterized as having diabetes based on 2 or more hemoglobin A1c levels ≥ 6.5% or 2 or more fasting plasma glucose levels ≥ 126 mg/dL no more than 2 years apart. An outpatient visit diagnosis only qualified if it was from an ambulatory visit. Medications of interest, such as sulfonylurea, insulin, or biguanide, were only valid if either the laboratory or the diagnostic criteria were also met.
The analyses of T2DM occurrence among STRONG cohort members included 2 parts. In the first part, we focused on prevalent T2DM cases among TGD participants and matched controls at index date (baseline). The likelihood of having diabetes at index date was expressed as prevalence per 1000 subjects and compared across groups using logistic regression with results expressed as odds ratios with corresponding 95% confidence intervals (CI). The second part of the analysis focused on incident T2DM cases diagnosed among participants who were diabetes-free at the index date. The follow-up in these time-to-event analyses extended from the index date until the first occurrence of T2DM, disenrollment from the plan for more than 90 days, death, or the end of the study period (November 30, 2016). Date of T2DM diagnosis during follow-up was ascertained via the SUPREME-DM algorithm. Incident cases who either did not have follow-up (n = 3) or were diagnosed with diabetes after the follow-up time ended (n = 25) were excluded.
To assess the influence of GAHT on T2DM risk, a separate time-to-event analysis was conducted among participants who began GAHT at some time after the index date (GAHT initiation subcohort). The follow-up for the GAHT initiation subcohort started at the date of the first filled GAHT prescription. Matched referents were assigned the same date of start of follow-up.
Incidence of T2DM was calculated as the number of newly diagnosed cases per 1000 person-years and compared across groups by constructing cumulative incidence curves and by using multivariable Cox proportional hazards models. Results of Cox models were reported as hazard ratios (HR) and 95% CI, and the proportional hazard assumption was tested using “log-log” plots.
The logistic regression and Cox proportional hazards models controlled for BMI in addition to matched variables (age, race/ethnicity, calendar year, and site). Matching was accounted for by including the cluster identification number in the stratum statement of each model. Each analysis was conducted for all TGD participants and for a subset of TGD cohort members who had evidence of GAHT receipt. Type 1 diabetes (T1DM) cases were excluded from the analysis using available diagnostic codes. All data analyses were performed with SAS, version 9.4 (SAS Institute, Cary, NC).
Results
The descriptive characteristics of the study population are summarized in Table 1. Among 5002 TGD people included in this study, 2869 (57%) were TF and 2133 (43%) were TM. The TF cohort members were matched to 28 300 cisgender females (CFs) and 28 258 cisgender males (CMs), and the TM cohort members were matched to 20 997 CF and 20 964 CM referents. The TM cohort members were on average younger than their TF counterparts and more than one-half of participants (54% for TFs and 60% for TMs) were non-Hispanic Whites. About 18% and 14% of the TF and TM cohorts, respectively, were Hispanic and 7% to 9% in each group identified as Asian/Pacific Islander or non-Hispanic Black. The race/ethnicity of 12% in the TF cohort and 10% in the TM cohort was characterized as “other” or unknown. The proportions of participants with normal BMI at baseline were about the same in the TF and TM cohorts (36% vs 34%).
Table 1.
Characteristics of the transgender and matched reference cohorts, n (%)
| Characteristics | TF cohort | Reference CF | Reference CM | TM cohort | Reference CF | Reference CM |
|---|---|---|---|---|---|---|
| (n = 2869) | (n = 28 300) | (n = 28 258) | (n = 2133) | (n = 20 997) | (n = 20 964) | |
| Membership site | ||||||
| KPNC | 1597 (55.7) | 15 727 (55.6) | 15 714 (55.6) | 1390 (65.2) | 13 647 (65.0) | 13 631 (65.0) |
| KPSC | 1194 (41.6) | 11 801 (41.7) | 11 775 (41.7) | 683 (32.0) | 6755 (32.2) | 6738 (32.1) |
| KPGA | 78 (2.7) | 772 (2.7) | 769 (2.7) | 60 (2.8) | 595 (2.8) | 595 (2.8) |
| Race/ethnicity | ||||||
| Non-Hispanic white | 1549 (54.0) | 15 240 (53.9) | 15 195 (53.8) | 1286 (60.3) | 12 600 (60.0) | 12 585 (60.0) |
| Hispanic | 526 (18.3) | 5202 (18.4) | 5206 (18.4) | 299 (14.0) | 2965 (14.1) | 2966 (14.2) |
| Asian/Pacific Islander | 268 (9.3) | 2654 (9.4) | 2652 (9.4) | 142 (6.7) | 1412 (6.7) | 1408 (6.7) |
| Non-Hispanic black | 195 (6.8) | 1919 (6.8) | 1920 (6.8) | 197 (9.2) | 1940 (9.2) | 1932 (9.2) |
| Other/unknown | 331 (11.5) | 3285 (11.6) | 3285 (11.6) | 209 (9.8) | 2080 (9.9) | 2073 (9.9) |
| Age at index date, y | ||||||
| 18-25 | 650 (22.7) | 6391 (22.6) | 6356 (22.5) | 749 (35.1) | 7350 (35.0) | 7342 (35.0) |
| 26-35 | 595 (20.7) | 5875 (20.8) | 5869 (20.8) | 703 (33.0) | 6918 (33.0) | 6902 (32.9) |
| 36-45 | 575 (20.0) | 5668 (20.0) | 5666 (20.1) | 343 (16.1) | 3382 (16.1) | 3384 (16.1) |
| 46-55 | 550 (19.2) | 5431 (19.2) | 5437 (19.2) | 218 (10.2) | 2154 (10.3) | 2149 (10.3) |
| 56-65 | 386 (13.5) | 3814 (13.5) | 3816 (13.5) | 90 (4.2) | 894 (4.3) | 893 (4.3) |
| >65 | 113 (3.9) | 1121 (4.0) | 1114 (3.9) | 30 (1.4) | 299 (1.4) | 294 (1.4) |
| Body mass index, kg/m 2 | ||||||
| Underweight (<18.5) | 79 (2.8) | 588 (2.1) | 254 (0.9) | 38 (1.8) | 539 (2.6) | 243 (1.2) |
| Normal weight (18.5-24.9) | 1028 (35.8) | 9908 (35.0) | 6507 (23.0) | 729 (34.2) | 8233 (39.2) | 5853 (27.9) |
| Overweight (25.0-29.9) | 822 (28.7) | 6900 (24.4) | 9343 (33.1) | 556 (26.1) | 4832 (23.0) | 6831 (32.6) |
| Obese (≥30.0) | 662 (23.1) | 7544 (26.7) | 7901 (28.0) | 658 (30.9) | 5409 (25.8) | 5428 (25.9) |
| Unknown | 278 (9.7) | 3360 (11.9) | 4253 (15.1) | 152 (7.1) | 1984 (9.5) | 2609 (12.5) |
Abbreviations: CF, cisgender female; CM, cisgender male; KPGA, Kaiser Permanente Georgia; KPNC, Kaiser Permanente Northern California; KPSC, Kaiser Permanente Southern California; TF, transfeminine; TM, transmasculine.
Overall, approximately 32% of TFs and 24% of TMs were on GAHT on or before the index date. Among transgender cohort members who initiated GAHT after the index date and who did not have a prior T2DM diagnosis, the mean follow-up time was 3.1 years for TFs and 2.8 years for TMs. The median (interquartile range) follow-up times were 2.5 (1.4, 4.1) years and 2.2 (1.1, 3.6) years for TF and TM, respectively.
Descriptive characteristics of TGD people with T2DM are summarized in Table 2. Among 287 TF persons with T2DM, approximately equal proportions were from KP Northern California and KP Southern California, whereas among the 131 TM persons with T2DM, nearly two-thirds were diagnosed at KP Northern California. Among TM patients, 21% were under the age of 36 years, compared with 13% among TF patients. With respect to timing of T2DM diagnosis, 175 (61%) of the 287 TF patients and 77 (59%) of the 131 TM patients had a diagnosis of T2DM at baseline (on or before the index date) and the rest developed diabetes at some time after the index date. Among the newly diagnosed cases, 94 TF patients and 44 TM patients were included in the time-to-event analyses because their date of diagnosis fell within the follow-up period.
Table 2.
Characteristics of TGD cohort members with T2DM, n (%)
| Characteristics | TF cohort | TM cohort |
|---|---|---|
| (n = 287) | (n = 131) | |
| Membership site | ||
| KPNC | 142 (49.5) | 83 (63.4) |
| KPSC | 141 (49.1) | 44 (33.6) |
| KPGA | 4 (1.4) | 4 (3.1) |
| Race/ethnicity | ||
| Non-Hispanic white | 161 (56.1) | 82 (62.6) |
| Hispanic | 60 (20.9) | 18 (13.7) |
| Asian/Pacific Islander | 29 (10.1) | 10 (7.6) |
| Non-Hispanic black | 28 (9.8) | 11 (8.4) |
| Other/unknown | 9 (3.1) | 10 (7.6) |
| Age at index date, y | ||
| 18-25 | 11 (3.8) | 7 (5.3) |
| 26-35 | 26 (9.1) | 20 (15.3) |
| 36-45 | 53 (18.5) | 37 (28.2) |
| 46-55 | 77 (26.8) | 30 (22.9) |
| 56-65 | 84 (29.3) | 28 (21.4) |
| >65 | 36 (12.5) | 9 (6.9) |
| Body mass index, kg/m 2 | ||
| Under or normal weight (<25.0)a | 33 (11.5) | 7 (5.3) |
| Overweight (25.0-29.9) | 58 (20.2) | 29 (22.1) |
| Obese (≥30.0) | 154 (53.7) | 82 (62.6) |
| Unknown | 42 (14.6) | 13 (9.9) |
| Timing of T2DM diagnosis | ||
| Prevalent at baseline | 175 (61.0) | 77 (58.8) |
| Diagnosed at any time after index date | 112 (39.0) | 54 (41.2) |
| Diagnosed during enrollment and follow-upb | 94 (32.8) | 44 (33.6) |
Abbreviations: CF, cisgender female; CM, cisgender male; KPGA, Kaiser Permanente Georgia; KPNC, Kaiser Permanente Northern California; KPSC, Kaiser Permanente Southern California; T2DM, type 2 diabetes mellitus; TF, transfeminine; TM, transmasculine.
a Underweight and normal weight categories were collapsed because of small numbers of underweight (<5).
b A subset of patients who were included in the time-to-event analyses (excludes cases with no enrollment information and those with diagnosis after end of follow-up).
The baseline prevalence of T2DM is shown in Table 3. Of the 175 prevalent TF cases and 77 prevalent TM cases, 56 (32%) and 19 (34%) cases, respectively, were on GAHT, either initiated before or on the index date. The baseline prevalence of T2DM was 61 per 1000 participants (about 6%) both in the overall TF group and among TF participants on GAHT. The corresponding prevalence estimates for TMs (per 1000 people) were 36 overall and 37 for persons receiving GAHT (about 4%). Logistic regression analyses demonstrated significantly higher odds of having prevalent T2DM in the full TF cohort vs CF referents with adjusted odds ratio estimate of 1.3 (95% CI, 1.1-1.5). By contrast, the same analyses in the remaining groups, with and without GAHT receipt, demonstrated no significant departures from the null value (Table 3).
Table 3.
Logistic regression analyses comparing baseline prevalence of T2DM in TGD cohort members and matched reference groups
| Analysis typea | T2DM cases | T2DM prevalenceb | vs reference CF | vs reference CM |
|---|---|---|---|---|
| at baseline | (95% CI) | OR (95% CI) | OR (95% CI) | |
| All TF participants | 175 | 61 (52.5-70.4) | 1.3 (1.1-1.5) | 0.9 (0.8-1.1) |
| TF participants on GAHT | 56 | 61 (46.6-78.7) | 1.0 (0.7-1.3) | 0.7 (0.5-1.0) |
| All TM participants | 77 | 36 (28.6-44.9) | 1.2 (0.9-1.5) | 1.1 (0.8-1.4) |
| TM participants on GAHT | 19 | 37 (22.7-57.8) | 1.1 (0.7-1.9) | 1.0 (0.6-1.6) |
CF, cisgender females; CI, confidence interval; CM, cisgender males; GAHT, gender-affirming hormone therapy; OR, odds ratio; T2DM, type 2 diabetes mellitus; TF, transfeminine; TM, transmasculine.
a All models adjusted for body mass index.
b Per 1000 participants.
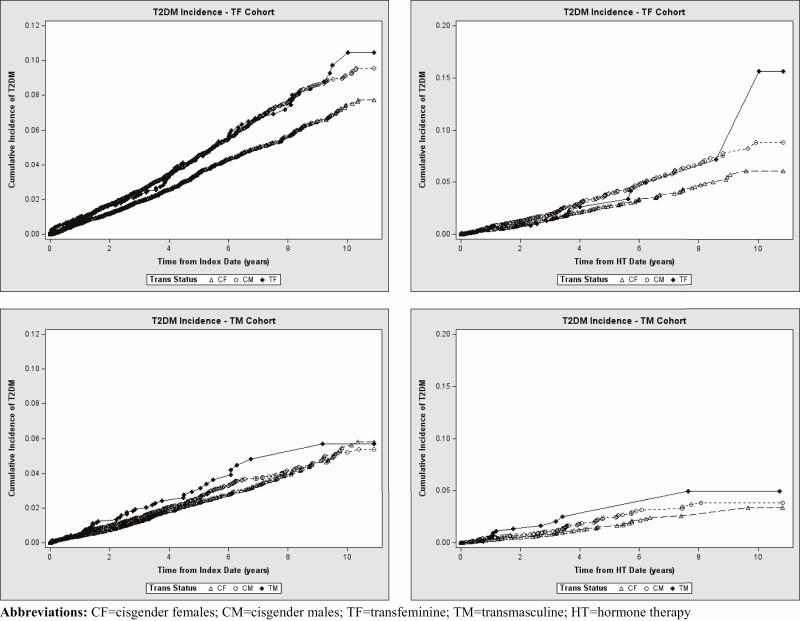
As shown in Table 4, there were 94 incident T2DM cases in the TF cohort and 44 incident T2DM cases in the TM cohort. Among 94 incident TF cases, 17 (18%) were diagnosed after initiation of GAHT. Similarly, 12 (27%) of the 44 incident TM cases developed T2DM following GAHT initiation. For the remaining 77 and 32 incident TF and TM cases, respectively, the participants were either not on GAHT during the study period or began receiving GAHT after T2DM diagnosis. In Table 4, the incidence of T2DM (per 1000 person-years) was 9.3 (95% CI, 7.6-11.3) among all TF participants, and 5.9 (95% CI, 3.6-9.4) among TF participants in the GAHT initiation subcohort. The corresponding incidence rate estimates (per 1000 person-years) for TM participants were 6.2 (95% CI, 4.6-8.4) overall and 5.5 (95% CI, 3.1, 9.7) for persons who initiated GAHT after the index date. The cumulative incidence curves (Fig. 1) showed only minor differences in T2DM risk between TGD study participants and the reference groups. An apparent separation of the curves between 8 and 10 years of follow up among TF persons receiving GAHT is likely attributable to sparse data with only 2 new cases diagnosed at the end of the observation period. Cox proportional hazard analyses showed that the overall TF cohort experienced moderately higher T2DM rate compared with CF referents (HR = 1.4; 95% CI, 1.1-1.8), but not when compared with the CM group (HR = 1.2; 95% CI, 0.9-1.5). The corresponding analyses for the TM cohort produced HR (95% CI) estimates of 1.3 (0.9-1.8) and 1.2 (0.9-1.7) relative to CF and CM reference cohorts, respectively. As also shown in Table 4, all HR values for the GAHT initiation subcohorts ranged from 1.0 to 1.4, and all 95% CIs included 1.0.
Table 4.
Cox proportional hazards analyses comparing incidence of T2DM in TGD cohort members and matched reference groups
| Analysis typea | Incident | T2DM incidenceb | vs. reference CF | vs. reference CM |
|---|---|---|---|---|
| T2DM cases | (95% CI) | HR (95% CI, P value) | HR (95% CI, P value) | |
| Full TF cohortc | 94 | 9.3 (7.6-11.3) | 1.4 (1.1-1.8) | 1.2 (0.9-1.5) |
| TF GAHT initiation subcohortd | 17 | 5.9 (3.6-9.4) | 1.4 (0.8-2.4) | 1.0 (0.6-1.6) |
| Full TM cohortc | 44 | 6.2 (4.6-8.4) | 1.3 (0.9-1.8) | 1.2 (0.9-1.7) |
| TM GAHT initiation subcohortd | 12 | 5.5 (3.1-9.7) | 1.4 (0.7-2.9) | 1.3 (0.7-2.6) |
CF, cisgender female; CM, cisgender male; GAHT, gender-affirming hormone therapy; HR, hazard ratio; T2DM, type 2 diabetes mellitus; TF, transfeminine; TM, transmasculine.
a All models stratified by cluster identification number and adjusted for body mass index.
b Rate per 1000 person-years.
c Follow-up starts at index date.
d Follow-up starts at first filled GAHT prescription.
Figure 1.
Cumulative incidence curves comparing incidence of T2DM in TGD participants and their matched referents. Top row shows results for the overall TF cohort followed from the index date (left panel) and for the TF cohort followed from HT initiation date (right panel). Bottom row shows results for the overall TM cohort followed from the index date (left panel) and for the TM cohort followed from HT initiation date (right panel). Abbreviations: CF, cisgender female; CM, cisgender male; HT, hormone therapy; T2DM, type 2 diabetes mellitus; TF, transfeminine; TGD, transgender and gender nonconforming; TM, transmasculine.
Discussion
This cohort study indicates that both incident and prevalent T2DM may be more common among TF persons compared with cisgender women, but not compared with cisgender men, which likely reflects the known gender disparity in T2DM risk in the general population (20). By contrast, the results for the TM cohort showed no significant differences compared with either reference group. There was little evidence that T2DM prevalence or incidence become more pronounced when the analysis is restricted to TGD people receiving GAHT.
Our results are somewhat inconsistent with those found in an earlier Dutch study, which noted that the prevalence of T2DM was markedly higher in both TM and TF participants compared with cisgender controls (21). The authors of the Dutch study compared 214 TF and 138 TM participants to age- and gender-matched CF and CM referents seen at a sex and gender health center at a large university hospital. All TGD participants in the Dutch study were on GAHT. The prevalence of T2DM (per 1000 persons) was 42.0 in the TF group, and 14.9 and 6.2 among CF and CM referents, respectively. Prevalence of T2DM in the TM group (also per 1000 persons) was 36.2, higher than the corresponding estimates of 21.4 among CF and 19.2 among CM referents.
It is important to point out, however, that the prevalence estimates among TGD participants in the Dutch study were similar or even lower than those observed at baseline in our cohort. By contrast, prevalence of T2DM was much lower among Dutch controls than among cisgender referents in our study, which resulted in the differences of observed prevalence ratios.
Although published data on the occurrence of overt T2DM in TGD populations are sparse, other studies have addressed a related research question by investigating the impact of GAHT on laboratory markers of insulin resistance and glucose metabolism. Two studies measured glucose utilization using euglycemic hyperinsulinemic clamp assays after GAHT initiation. The first study performed the assay among 13 TM and 18 TF individuals who had been receiving GAHT for 4 months and concluded that GAHT can induce insulin resistance in both groups, but only if insulin levels remained within physiologic range (22). The second study conducted a similar laboratory evaluation among 20 TF individuals and 17 TM individuals at 3 time points: baseline and 2 months and 12 months of follow-up (23). The authors reported that TF persons experienced increases in glucose utilization following GAHT initiation, whereas the same result for TM study participants demonstrated no discernable changes from the baseline.
Several research groups examined the association between GAHT and changes in glucose and insulin levels, as well Homeostatic Model Assessment for Insulin Resistance (HOMA-IR). One of the first published studies addressing this research question analyzed data on 47 TM individuals receiving testosterone therapy at a specialized clinic in Spain (24). Compared with baseline, there was a small but statistically significant decrease in HOMA-IR as well as in serum insulin at both 1-year and 2-year intervals following GAHT initiation. In another study conducted in Germany, the authors reported no significant differences in HOMA-IR or other markers of glucose metabolism, both within the TM group and in comparison, to women with polycystic ovarian syndrome (25). The lack of statistically significant association between masculinizing GAHT and changes in glucose levels or insulin resistance was also found in several more recent clinical studies that were conducted in Spain, Italy, and Belgium (26-30). By contrast, studies conducted among TF patients were more likely to report increases in insulin resistance following administration of feminizing GAHT (24, 28, 29, 30). For example, a study of 55 TF participants enrolled at a Ghent, Belgium, site of a multicenter European Network for the Investigation of Gender Incongruence study compared baseline laboratory measures with those observed after a year of GAHT that included antiandrogen cyproterone acetate and oral estradiol valerate (29). The analyses demonstrated a significant increase in HOMA-IR and insulin levels, but no changes in fasting glucose. A similarly designed study from Italy followed 79 TF patients for 2 years after starting feminizing therapy with transdermal estradiol gel in combination with oral cyproterone acetate (28). There were statistically significant increases in all 3 laboratory parameters: fasting glucose, serum insulin, and HOMA-IR.
Another study assessing various metabolic markers in 24 TF and 45 TM individuals enrolled in the previously mentioned European Network for the Investigation of Gender Incongruence cohort collected data at baseline and then 12 months after GAHT initiation (30). The authors of that study reported that fasting insulin levels and HOMA-IR increased among TF participants and decreased among the TM cohort members; however, no changes in fasting glucose were evident in either group.
These observations are generally consistent with the conclusions of a recent systematic review of the literature (12). Based on the summary of 26 studies, Spanos and colleagues observed that masculinizing GAHT may produce an increase in lean mass, decrease in fat mass, but no changes in insulin resistance. Conversely, the same review found evidence to suggest that TF individuals on GAHT experience opposite changes in lean mass and fat mass, and possibly worsening insulin resistance.
Taken together, the published literature indicates that measures of glucose metabolism and insulin resistance do not appear to be adversely influenced by testosterone in TM individuals but may be affected by estradiol therapy among TF individuals. These observations notwithstanding, the clinical significance of the reported associations is not clear, and it is possible that small GAHT-related changes in laboratory parameters do not translate into higher risk of overt T2DM.
Perhaps the most important methodological feature that distinguishes our study from previous research is the longitudinal design that allowed evaluating incidence rather than only prevalence of T2DM. This enabled establishing a temporal relation between the receipt of GAHT and a new diagnosis of T2DM. Other notable strengths of this study are the use of deidentified real-world EHR-based data, and a well-defined sampling frame that included all persons enrolled in 3 large community-based health plans. These study characteristics ensured inclusion of all eligible individuals without a need for subject opt-in and offered an opportunity to match each TGD participant to cisgender referents who shared the same demographic characteristics, resided in the same geographic areas, and were enrolled in the same health system.
The EHR-based design of this study is also associated with limitations. Unlike clinic-based cohorts, the data in STRONG were not collected at specified intervals and the number and frequency of clinical encounters differed across participants. Another limitation is that the algorithm did not explicitly distinguish between T1DM and T2DM. Although we excluded cases with T1DM-specific diagnostic codes, some misclassification was still possible. The analysis controlled for BMI at index date; however, a lack of repeated BMI measures precluded a more advanced adjustment for BMI as a time-varying covariate. It is also possible that a longer follow-up and an expanded case definition (to include both prediabetes and overt T2DM) are needed to detect clinically meaningful GAHT-induced changes in glucose metabolism.
An additional limitation of the present analysis is the lack of data on other T2DM risk factors, including family history, socioeconomic status, and, especially pertinent to TGD people, adverse childhood experiences and gender minority stress. Studies have shown that sexual and gender minority stress is related to a wide range of adverse clinical outcomes, including higher incidence of asthma and cardiovascular disease (31, 32). There is also evidence to suggest a link between stressful experiences and both elevated T2DM risk and poor glycemia control (33). Taking into consideration the apparent absence of the association between GAHT receipt and T2DM incidence in our study, additional research should focus on the hypothesized effect of gender minority stress and its interaction with lifestyle-related metabolic risk factors among TGD people.
Conclusion
In summary, there is little evidence that T2DM occurrence in either TF or TM persons is attributable to GAHT use. Whereas these findings provide reassurance, a more definitive conclusion regarding no effect of GAHT on T2DM risk may require larger, more detailed studies with longer follow-up. In the meantime, clinicians should continue monitoring cardiometabolic status of the TGD individuals.
Acknowledgments
Funding: Funding sources for this work included contract AD-12-11-4532 from the Patient Centered Outcome Research Institute and grant R21HD076387 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Glossary
Abbreviations
- BMI
body mass index
- CF
cisgender female
- CM
cisgender male
- EHR
electronic health record
- GAHT
gender-affirming hormone therapy
- HOMA-IR
Homeostatic Model Assessment for Insulin Resistance
- HR
hazard ratio
- KP
Kaiser Permanente
- STRONG
Study of Transition Outcomes and Gender
- SUPREME-DM
Surveillance, Prevention, and Management of Diabetes Mellitus
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
- TF
transfeminine
- TGD
transgender and gender diverse
- TM
transmasculine
Additional Information
Disclosures: None of the authors report any conflicts of interest.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Safer JD, Tangpricha V. Care of the transgender patient. Ann Intern Med. 2019;171(10):775-776. [DOI] [PubMed] [Google Scholar]
- 2. Winter S, Diamond M, Green J, et al. Transgender people: health at the margins of society. Lancet. 2016;388(10042):390-400. [DOI] [PubMed] [Google Scholar]
- 3. Rafferty J. Committee on psychosocial aspects of child and family health; committee on adolescence; section on lesbian, gay, bisexual, and transgender health and wellness. ensuring comprehensive care and support for transgender and gender-diverse children and adolescents. Pediatrics. 2018;142(4):e20182162. [DOI] [PubMed] [Google Scholar]
- 4. Puckett JA, Cleary P, Rossman K, Newcomb ME, Mustanski B. Barriers to gender-affirming care for transgender and gender nonconforming individuals. Sex Res Social Policy. 2018;15(1):48-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang Q, Goodman M, Adams N, et al. Epidemiological considerations in transgender health: a systematic review with focus on higher quality data. Int J Transgend Health. 2020;21(2):125-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goodman M, Adams N, Corneil T, Kreukels B, Motmans J, Coleman E. Size and distribution of transgender and gender nonconforming populations: a narrative review. Endocrinol Metab Clin North Am. 2019;48(2):303-321. [DOI] [PubMed] [Google Scholar]
- 7. Abeln B, Love R. Considerations for the care of transgender individuals. Nurs Clin North Am. 2019;54(4):551-559. [DOI] [PubMed] [Google Scholar]
- 8. Whitlock BL, Duda ES, Elson MJ, et al. Primary care in transgender persons. Endocrinol Metab Clin North Am. 2019;48(2):377-390. [DOI] [PubMed] [Google Scholar]
- 9. Ahmad S, Leinung M. The response of the menstrual cycle to initiation of hormonal therapy in transgender men. Transgend Health. 2017;2(1):176-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Angus L, Leemaqz S, Ooi O, et al. Cyproterone acetate or spironolactone in lowering testosterone concentrations for transgender individuals receiving oestradiol therapy. Endocr Connect. 2019;8(7):935-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Streed CG Jr, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M. Cardiovascular disease among transgender adults receiving hormone therapy: a narrative review. Ann Intern Med. 2017;167(4):256-267. [DOI] [PubMed] [Google Scholar]
- 12. Spanos C, Bretherton I, Zajac JD, Cheung AS. Effects of gender-affirming hormone therapy on insulin resistance and body composition in transgender individuals: a systematic review. World J Diabetes. 2020;11(3):66-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jones TH. Effects of testosterone on type 2 diabetes and components of the metabolic syndrome. J Diabetes. 2010;2(3):146-156. [DOI] [PubMed] [Google Scholar]
- 14. Simon D, Charles MA, Lahlou N, et al. Androgen therapy improves insulin sensitivity and decreases leptin level in healthy adult men with low plasma total testosterone: a 3-month randomized placebo-controlled trial. Diabetes Care. 2001;24(12):2149-2151. [DOI] [PubMed] [Google Scholar]
- 15. Roblin D, Barzilay J, Tolsma D, et al. A novel method for estimating transgender status using electronic medical records. Ann Epidemiol. 2016;26(3):198-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quinn VP, Nash R, Hunkeler E, et al. Cohort profile: Study of Transition, Outcomes and Gender (STRONG) to assess health status of transgender people. BMJ Open. 2017;7(12):e018121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869-3903. Erratum in: J Clin Endocrinol Metab. 2018;103(2):699. Erratum in: J Clin Endocrinol Metab. 2018;103(7):2758-2759. [DOI] [PubMed] [Google Scholar]
- 18. Getahun D, Nash R, Flanders WD, et al. Cross-sex hormones and acute cardiovascular events in transgender persons: a cohort study. Ann Intern Med. 2018;169(4):205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nichols GA, Desai J, Elston Lafata J, et al. ; SUPREME-DM Study Group . Construction of a multisite DataLink using electronic health records for the identification, surveillance, prevention, and management of diabetes mellitus: the SUPREME-DM project. Prev Chronic Dis. 2012;9:E110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kautzky-Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37(3):278-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wierckx K, Elaut E, Declercq E, et al. Prevalence of cardiovascular disease and cancer during cross-sex hormone therapy in a large cohort of trans persons: a case-control study. Eur J Endocrinol. 2013;169(4):471-478. [DOI] [PubMed] [Google Scholar]
- 22. Polderman KH, Gooren LJ, Asscheman H, Bakker A, Heine RJ. Induction of insulin resistance by androgens and estrogens. J Clin Endocrinol Metab. 1994;79(1):265-271. [DOI] [PubMed] [Google Scholar]
- 23. Elbers JM, Giltay EJ, Teerlink T, et al. Effects of sex steroids on components of the insulin resistance syndrome in transsexual subjects. Clin Endocrinol (Oxf). 2003;58(5):562-571. [DOI] [PubMed] [Google Scholar]
- 24. Yahyaoui R, Esteva I, Haro-Mora JJ, et al. Effect of long-term administration of cross-sex hormone therapy on serum and urinary uric acid in transsexual persons. J Clin Endocrinol Metab. 2008;93(6):2230-2233. [DOI] [PubMed] [Google Scholar]
- 25. Cupisti S, Giltay EJ, Gooren LJ, et al. The impact of testosterone administration to female-to-male transsexuals on insulin resistance and lipid parameters compared with women with polycystic ovary syndrome. Fertil Steril. 2010;94(7):2647-2653. [DOI] [PubMed] [Google Scholar]
- 26. Gava G, Mancini I, Cerpolini S, Baldassarre M, Seracchioli R, Meriggiola MC. Testosterone undecanoate and testosterone enanthate injections are both effective and safe in transmen over 5 years of administration. Clin Endocrinol (Oxf). 2018;89(6):878-886. [DOI] [PubMed] [Google Scholar]
- 27. Aranda G, Mora M, Hanzu FA, Vera J, Ortega E, Halperin I. Effects of sex steroids on cardiovascular risk profile in transgender men under gender affirming hormone therapy. Endocrinol Diabetes Nutr (Engl Ed). 2019;66(6):385-392. [DOI] [PubMed] [Google Scholar]
- 28. Colizzi M, Costa R, Scaramuzzi F, et al. Concomitant psychiatric problems and hormonal treatment induced metabolic syndrome in gender dysphoria individuals: a 2 year follow-up study. J Psychosom Res. 2015;78(4):399-406. [DOI] [PubMed] [Google Scholar]
- 29. Shadid S, Abosi-Appeadu K, De Maertelaere AS, et al. Effects of gender-affirming hormone therapy on insulin sensitivity and incretin responses in transgender people. Diabetes Care. 2020;43(2):411-417. [DOI] [PubMed] [Google Scholar]
- 30. Auer MK, Ebert T, Pietzner M, et al. Effects of sex hormone treatment on the metabolic syndrome in transgender individuals: focus on metabolic cytokines. J Clin Endocrinol Metab. 2018;103(2):790-802. [DOI] [PubMed] [Google Scholar]
- 31. Flentje A, Heck NC, Brennan JM, Meyer IH. The relationship between minority stress and biological outcomes: a systematic review. J Behav Med. 2020;43(5):673-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mereish EH, Goldstein CM. Minority stress and cardiovascular disease risk among sexual minorities: mediating effects of sense of mastery. Int J Behav Med. 2020;27(6):726-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lloyd C, Smith J, Weigner K. Stress and diabetes: a review of the links. Diabetes Spectr. 2005;18(2):121-127. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.