Abstract
Context
Youth with type 1 diabetes (T1D) do not meet glycated hemoglobin A1c (HbA1c) targets.
Objective
This work aimed to assess HbA1c outcomes in children with new-onset T1D enrolled in the Teamwork, Targets, Technology and Tight Control (4T) Study.
Methods
HbA1c levels were compared between the 4T and historical cohorts. HbA1c differences between cohorts were estimated using locally estimated scatter plot smoothing (LOESS). The change from nadir HbA1c (month 4) to 12 months post diagnosis was estimated by cohort using a piecewise mixed-effects regression model accounting for age at diagnosis, sex, ethnicity, and insurance type. We recruited 135 youth with newly diagnosed T1D at Stanford Children’s Health. Starting July 2018, all youth within the first month of T1D diagnosis were offered continuous glucose monitoring (CGM) initiation and remote CGM data review was added in March 2019. The main outcomes measure was HbA1c.
Results
HbA1c at 6, 9, and 12 months post diagnosis was lower in the 4T cohort than in the historic cohort (–0.54% to –0.52%, and –0.58%, respectively). Within the 4T cohort, HbA1c at 6, 9, and 12 months post diagnosis was lower in those patients with remote monitoring than those without (–0.14%, –0.18% to –0.14%, respectively). Multivariable regression analysis showed that the 4T cohort experienced a significantly lower increase in HbA1c between months 4 and 12 (P < .001).
Conclusion
A technology-enabled, team-based approach to intensified new-onset education involving target setting, CGM initiation, and remote data review statistically significantly decreased HbA1c in youth with T1D 12 months post diagnosis.
Keywords: type 1 diabetes, pediatrics, continuous glucose monitoring, diabetes technology, glycemic control, new diagnosis
It has been almost 30 years since the Diabetes Control and Complications Trial (DCCT) demonstrated the benefits of intensive management of type 1 diabetes (T1D) (1, 2). However, these results have not been translated in clinical practice in the United States (3, 4). In 2018 the American Diabetes Association (ADA) Standards of Care set a glycated hemoglobin A1c (HbA1c) target of less than 7.5% and this target was subsequently lowered to less than 7% in 2020 (5). The International Society for Pediatric and Adolescent Diabetes (ISPAD) consensus also recommends an HbA1c target of less than 7% for most children and adolescents with T1D (6). Despite these recommendations, a majority of children and adolescents with T1D do not meet these targets (3, 7, 8). Among high-income countries, the United States has one of the highest average HbA1c values (7). Other regions have implemented quality improvement initiatives to improve outcomes (9-11), with the United States doing so only recently (12, 13).
Several factors have been shown to improve glycemic control in youth with T1D. The Hvidoere study and the SWEET diabetes registry show that consistent glucose target setting by the diabetes care team lowers HbA1c (14, 15). In the Type 1 Diabetes Exchange registry, increased glucose monitoring is associated with improved HbA1c (16). The development of continuous glucose monitoring (CGM) technology has allowed for more frequent glucose measurements (every 5-15 min) with less burden on the individual with diabetes. The ADA now recommends CGM use for all individuals with T1D on insulin (5). Recent data in youth with T1D has shown that CGM use lowered HbA1c (17), whereas loss of insurance coverage for CGM was associated with increased HbA1c (18). In addition, there is evidence to suggest that glycemic control early in the course of diabetes influences the subsequent HbA1c trajectory for an individual (19, 20).
We developed the 4T Program: Teamwork, Targets, Technology, and Tight Control, to intensify diabetes management in the first year of T1D diagnosis with the goal of improving long-term outcomes (21). We have previously shown that CGM initiation in the new-onset period resulted in continued CGM use and was valued by patients and families (22, 23). In the pilot phase of this intervention, children with new-onset diabetes were started on CGM in the first month of diagnosis and a subset had asynchronous data review by the diabetes care team. Here, we describe the 1-year glycemic outcomes of youth in the Pilot 4T cohort (including those who were part of the remote monitoring arm) compared to youth in our clinic’s historical cohort.
Materials and Methods
Participants
The protocol for the 4T Program has been previously described (22). Briefly, all youth with newly diagnosed T1D between July 2018 and June 2020 were offered the opportunity to start on CGM (Dexcom G6) in the first month of diabetes diagnosis following the clinic’s standard of care. Based on historical data, this was estimated to be between 70 and 100 youth. Those who chose to start on CGM had a follow up visit (either in person or virtual) with a certified diabetes care and education specialist (CDCES) to initiate CGM (Supplemental figure (24)). At this visit, they were provided a transmitter, 3 sensors, and a receiver by the 4T Study. The diabetes care team applied for ongoing insurance approval for CGM coverage. One week after initiating CGM, the youth and family met with a nurse practitioner via telemedicine for a follow-up visit for additional CGM education and continued with routine care. Institutional review board approval at Stanford was obtained to prospectively collect data.
Youth diagnosed in March 2019 or later were additionally offered the opportunity to participate in remote monitoring (clinical trial No. NCT03968055). Informed consent was obtained. Participants in this arm had their CGM data transferred to our electronic medical record (EMR) system using a previously described connection between Apple HealthKit and Epic MyChart (25). To facilitate CGM data transfer, individuals who did not have an iOS device were provided an iPod Touch for the duration of the study. Every week, CGM data were reviewed by a CDCES and dose adjustments, when necessary, were recommended using secure messaging within the electronic medical record. Initially, CDCESs manually reviewed data of every participant, but by January 2020, they relied on population health management tools to facilitate 4T Program scaling to a larger patient population (26). The health management tools were based on consensus guidelines for time in range (TIR) and hypoglycemia (27). The modified tool prioritized patients for CDCES review by identifying participants with TIR of less than 65%, percentage of time CGM worn less than 50% to increase scalability with constrained CDCES time but the flags for hypoglycemia were unchanged. The Stanford Institutional Review Board approved this protocol and consent (and assent for participants aged 7-18 y) was obtained for review of all participants.
Study Outcomes
Our primary outcome was change in HbA1c from 4 months (nadir of HbA1c in our historical cohort) to 12 months post diagnosis. We calculated a sample size of 120 participants needed for 93% power to detect a reduction in mean HbA1c of 0.5% compared to historical controls. Our secondary outcome was the proportion of participants achieving the recommended HbA1c of less than 7.5% target (the ADA’s target at study initiation in 2018) (28). Exploratory outcomes assessed on the Pilot 4T cohort included glucose management indicator (GMI) and sensor glucose time in range (70-180 mg/dL, TIR) (29).
Point-of-care HbA1c was performed using a DCA Vantage Analyzer (Siemens). Owing to the COVID-19 pandemic, care beginning in March 2020 was transitioned primarily to telehealth. For clinical care, GMI was used as a substitute for point-of-care HbA1c. In November 2020 we incorporated home HbA1c measurements (University of Minnesota Advanced Research and Diagnostic Laboratory) (30, 31). A total of 80 home HbA1c values were received through June 2021, of which 51 measured HbA1c within 15 months of diabetes onset and 32 were considered in the 1-year analyses (representing 7% of 1-y HbA1c) measurements.
Cohort Descriptions
The Pilot 4T cohort consisted of all youth diagnosed between July 2018 and June 2020 who were started on CGM. This cohort was subdivided into those who did not receive remote monitoring (no remote monitoring—diagnosed July 2018 to February 2019) and those who did (remote monitoring—diagnosed March 2019 to June 2020).
The historical cohort has been described previously (20). Briefly, these were youth (n = 272) diagnosed with T1D between June 2014 and December 2016, who received standard new-onset education and received quarterly clinic visits (Fig. 1). During this time, technology was not consistently introduced in the first month of diabetes diagnosis. The median age at diagnosis was 9.7 years (range, 6.7-12.7 years), 50.4% were male, 42.6% were non-Hispanic White, and 72.4% had private insurance. The mean HbA1c at diagnosis was 10.7% ± 2.5%. In this cohort, 56.2% started on CGM but only 1.8% in the first 30 days of diabetes diagnosis. HbA1c measurements were performed in clinic using the same DCA Vantage Analyzer (Siemens).
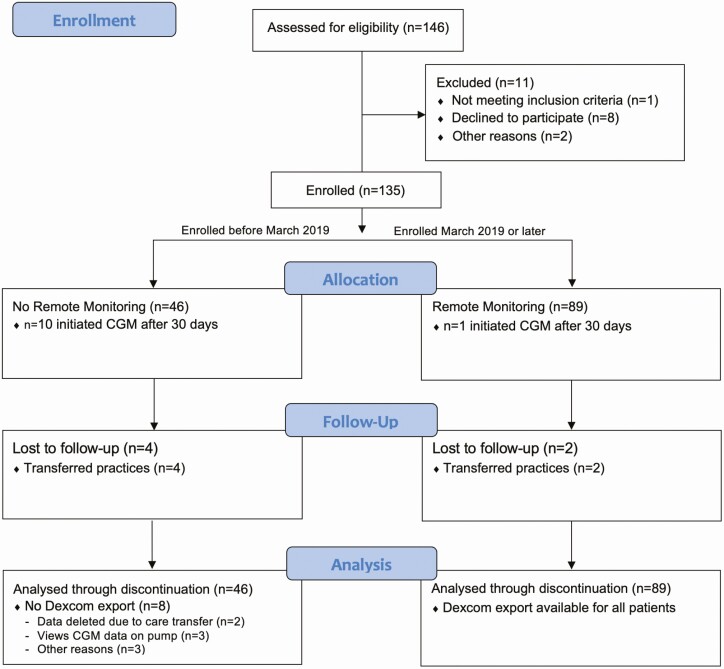
Figure 1.
CONSORT (Consolidated Standards of Reporting Trials) diagram of all participants.
Statistical Analysis
Participants were followed from their diagnosis date (baseline) to their discontinuation date or June 30, 2021. Baseline and follow-up characteristics of the historical and Pilot 4T cohorts were summarized as counts with percentages, quartiles, or means with SD.
For the Pilot 4T cohort, timing of study visits, HbA1c availability (point-of-care and home kit), and duration of follow-up were presented as a swimmer plot (Supplemental figure (32)). All participants who started in CGM in the first year were included in this analysis under the intention-to-treat principle. The GMI—an established estimator of HbA1c—was computed at 2-week intervals by applying the formula developed by Bergenstal et al (29) to CGM glucose readings, averaged across a lookback window of up to 90 days. Although other equations to estimate HbA1c are available (33-35), we chose GMI because it is the most widely used and the basis for the GMI calculation in the Dexcom Clarity report. CGM-based metrics (GMI, mean CGM glucose, and TIR) were visualized using locally estimated scatter plot smoothing (LOESS) over the first 15 months since diabetes onset. The level of smoothing in LOESS is determined by the span parameter, and we selected the value that minimized the mean squared error via 10-fold cross-validation. TIR was also visualized as box plots by glucose range and as stacked bar plots over time, with month 1 spanning the first 30 days after diagnosis and later time points spanning approximately 3-month intervals.
HbA1c trajectories of the historical and Pilot 4T cohorts were visualized using LOESS and compared. Differences in LOESS means between the Pilot 4T cohort and the historical cohort were calculated. A linear mixed-effects regression model that allows for 2 piecewise linear slopes of HbA1c levels to be estimated from diagnosis to 4 months post diagnosis (nadir in HbA1c) and from 4 months to 12 months post diagnosis was used to calculate cohort differences in 4- to 12-month slopes assessed via an interaction term. This model adjusted for characteristics at diagnosis (age, sex, Hispanic ethnicity, and public insurance), and within-subject correlation of HbA1c was accounted for using a subject-specific random effect. Regression analysis was performed on the full cohort, as well as on 2 subcohorts to gauge the sensitivity of findings to pump use and hybrid closed loop. The secondary outcome of achieving the ADA’s less than 7.5% target HbA1c was presented descriptively using bar plots over time. For all analyses, statistical significance was assessed at the .05 α level, and all analyses were conducted in the R statistical computing framework, version 4.0.
Results
Participant Demographics
During the study period, a total of 146 youth were diagnosed with T1D (see Fig. 1). Of these, 135 (93%) youth were enrolled, and 124 initiated CGM in the first month of diagnosis (Pilot 4T cohort) and 11 initiated CGM after 30 days of diagnosis. Among those who started CGM late, 2 participants initially declined CGM use, but later started on CGM. Six were initially excluded from study participation, one of them because they were traveling internationally and would not receive care in our practice. We initially could not offer CGM to 5 youth with public insurance since at that time Medi-CAL (California public insurance for children) did not cover CGM costs. However, we obtained philanthropic funding and were able to provide CGM devices to all participants on public insurance for 1 year. All 5 youth were eventually started on CGM within the first year of diagnosis, with median (interquartile range; IQR) time to start 117 days (IQR, 95-142 days). Forty-six participants were enrolled before the addition of remote CGM data review (no remote monitoring group), and 89 consented to participate in remote monitoring (see Fig. 1).
Among all 4T Pilot study participants, the median (IQR) age at diagnosis was 9.7 years (IQR, 6.8-12.7 years), 52.6% were male, 77.0% had with private insurance, 37.0% were non-Hispanic White, and 13.3% were non-English speaking (Table 1). The mean HbA1c at diagnosis was 12.2 ± 2.1%, and the median (IQR) time to CGM initiation was 7 days (IQR, 5-11 days). During this time period, 72 (53.3%) participants were started on insulin pumps with 32 (23.7%) started on insulin pumps with advanced hybrid closed loop. The median time to pump initiation was 212 days (range, 127-384 days) and for those on the advanced hybrid closed loop, the median time to pump initiation was 221 days (range, 126-402 days). In the historical cohort, 134 (49.3%) were on insulin pumps with a median time to initiation of 272 days (range, 157-453 days). During this time period, only the hybrid closed loop (MiniMed 670G system; Medtronic) was available and no patients were on the system.
Table 1.
Characteristics of historical and Pilot 4T cohorts
| Overall | Historical cohort | Pilot 4Ta cohort | Pilot 4T: no remote | Pilot 4T: remote | |
|---|---|---|---|---|---|
| (2014-2016) | (2018-2020) | Monitoring | Monitoring | ||
| No. of patients | 407 | 272 | 135 | 46 | 89 |
| Baseline characteristics | |||||
| Age at T1D diagnosis, median (Q1-Q3), y | 9.7 (6.7-12.7) | 9.7 (6.7, 12.7) | 9.7 (6.8-12.7) | 9.4 (5.6-11.6) | 9.7 (7.4-13.9) |
| Age group at diagnosis, n (%), y | |||||
| ≤ 6 | 90 (22.1) | 59 (21.7) | 31 (23.0) | 14 (30.4) | 17 (19.1) |
| 6-< 13 | 225 (55.3) | 154 (56.6) | 71 (52.6) | 25 (54.3) | 46 (51.7) |
| 13-< 20 | 92 (22.6) | 59 (21.7) | 33 (24.4) | 7 (15.2) | 26 (29.2) |
| Male, n (%) | 208 (51.1) | 137 (50.4) | 71 (52.6) | 25 (54.3) | 46 (51.7) |
| Race/Ethnicity, n (%) | |||||
| Non-Hispanic White | 166 (40.8) | 116 (42.6) | 50 (37.0) | 19 (41.3) | 31 (34.8) |
| Non-Hispanic Black | 5 (1.2) | 5 (1.8) | 0 (0) | 0 (0) | 0 (0) |
| Hispanic | 93 (22.9) | 68 (25.0) | 25 (18.5) | 8 (17.4) | 17 (19.1) |
| Asian or Pacific Islander | 46 (11.3) | 29 (10.7) | 17 (12.6) | 4 (8.7) | 13 (14.6) |
| American Indian or Alaska Native | 1 (0.2) | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) |
| Other | 11 (2.7) | 0 (0) | 11 (8.1) | 6 (13.0) | 5 (5.6) |
| Unknown | 85 (20.9) | 53 (19.5) | 32 (23.7) | 9 (19.6) | 23 (25.8) |
| HbA 1c (%) at T1D diagnosis, mean (SD) | 11.2 (2.5) | 10.7 (2.5) | 12.2 (2.1) | 11.9 (2.1) | 12.4 (2.2) |
| Insurance type, n (%) | |||||
| Private | 301 (74.0) | 197 (72.4) | 104 (77.0) | 37 (80.4) | 67 (75.3) |
| Public | 104 (25.6) | 73 (26.8) | 31 (23.0) | 9 (19.6) | 22 (24.7) |
| Unknown | 2 (0.5) | 2 (0.7) | 0 (0) | 0 (0) | 0 (0) |
| Primary language, n (%) | |||||
| English | 117 (28.7) | 0 (0) | 117 (86.7) | 39 (84.8) | 78 (87.6) |
| Non-English | 18 (4.4) | 0 (0) | 18 (13.3) | 7 (15.2) | 11 (12.4) |
| Unknown | 272 (66.7) | 272 (100) | 0 (0) | 0 (0) | 0 (0) |
| Follow-up characteristics | |||||
| CGM initiation, n (%) | 288 (70.8) | 153 (56.2) | 135 (100) | 46 (100) | 89 (100) |
| Early initiation (≤ 30 d) | 129 (31.7) | 5 (1.8) | 124 (91.9) | 36 (78.3) | 88 (98.9) |
| Late initiation (> 30 d) | 159 (39.1) | 148 (54.4) | 11 (8.1) | 10 (21.7) | 1 (1.1) |
| Days to CGM initiation, median (Q1-Q3) | 44 (8-248) | 221 (91-557) | 7 (5-11) | 7 (6-12) | 8 (5, 11) |
| Insulin pump use b , n (%) | 206 (50.6) | 134 (49.3) | 72 (53.3) | 27 (58.7) | 45 (50.6) |
| Open loop | 172 (42.3) | 134 (49.3) | 38 (28.1) | 16 (34.8) | 22 (24.7) |
| Predictive low glucose suspend | 18 (4.4) | 0 (0) | 18 (13.3) | 8 (17.4) | 10 (11.2) |
| Advanced hybrid closed loop | 32 (7.9) | 0 (0) | 32 (23.7) | 12 (26.1) | 20 (22.5) |
| None | 160 (39.3) | 97 (35.7) | 63 (46.7) | 19 (41.3) | 44 (49.4) |
| Unknown | 41 (10.1) | 41 (15.1) | 0 (0) | 0 (0) | 0 (0) |
| Days to pump initiation, median (Q1-Q3) | 241 (143-426) | 272 (157-453) | 212 (127-384) | 301 (144-497) | 182 (122-330) |
Abbreviations: 4T Study, Teamwork, Targets, Technology and Tight Control; CGM, continuous glucose monitoring; HbA1c, glycated hemoglobin A1c; n, number; Q, quartile; T1D, type 1 diabetes.
a Diagnosed through June 2020 and followed through June 30, 2021.
b Includes all patients in Pilot 4T (Pilot 4T: remote and Pilot 4T: no remote)
Compared to the historical cohort, the mean HbA1c at diagnosis was higher in the 4T cohort, but other baseline characteristics did not differ significantly. Comparing the no remote monitoring and remote monitoring cohorts, those who engaged in remote monitoring included a higher percentage of public insurance and minority participants.
Glycated Hemoglobin A1c Differences Between Cohorts
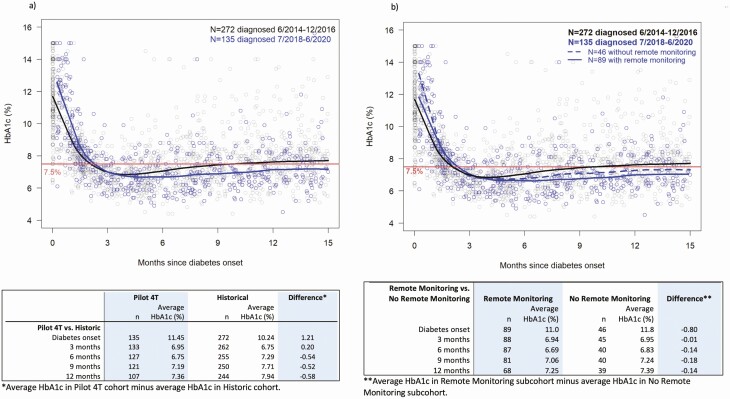
For both the historical cohort and the Pilot 4T cohort, HbA1c was highest at diagnosis and lowest at 4 months post diabetes diagnosis. Those in the historical cohort had an increase in their HbA1c after 4 months post diagnosis, and the HbA1c steadily increased over time (Fig. 2A). Those in the Pilot 4T cohort had a more gradual increase in HbA1c. LOESS-based differences in the mean HbA1c between the Pilot 4T cohort and the historical cohort were –0.54%, –0.52%, and –0.58% at months 6, 9, and 12 post diagnosis, respectively. Between months 4 and 12 post diabetes diagnosis, those in the historical cohort, on average, had an increase in the mean HbA1c of 1.47% (range, 1.36%-1.57%) compared to a statistically significantly lower increase of 1.32% (range, 1.20%-1.43%] in the Pilot 4T cohort after adjusting for age at diagnosis, sex, ethnicity, and insurance type (P < .001). The sensitivity analysis yielded a similar inference.
Figure 2.
Scatter plot of glycated hemoglobin A1c (HbA1c) levels over time, with locally estimated scatter plot smoothing (LOESS). Mean HbA1c is highest at diabetes diagnosis and reaches a nadir at approximately 4 months post diabetes diagnosis. A, Individuals in the Pilot 4T cohort had lower mean HbA1c at 12 months post diabetes diagnosis compared to those in the historical cohort. B, Those in the remote monitoring cohort had lower HbA1c at 12 months post diabetes diagnosis compared to those in the no remote monitoring cohort and those in the historical cohort.
When the participants in the Pilot 4T cohort were subdivided into remote monitoring and no remote monitoring, the HbA1c began to rise earlier and more quickly in the no remote monitoring group compared to those in the remote monitoring group (Fig. 2B). Both of these groups had a lower HbA1c at 12 months post diagnosis compared to the historical cohort. The mean HbA1c in these groups remained at less than 7.5% at 12 months post diagnosis while those in the historical cohort crossed this threshold at 9 months post diabetes diagnosis. LOESS-based differences in the mean HbA1c between the remote monitoring and no remote monitoring cohorts were –0.14%, –0.18%, and –0.14%, at months 6, 9, and 12 post diagnosis, respectively. Between months 4 and 12 post diabetes diagnosis, there was no statistically significant difference in the increase of HbA1c between those in the no remote monitoring group and those in the remote monitoring group (1.47% [range, 1.27%-1.67%], 1.54% [range, 1.33%-1.75%], respectively, (P = .220)].
Changes in Continuous Glucose Monitoring Metrics Over the First Year of Diagnosis
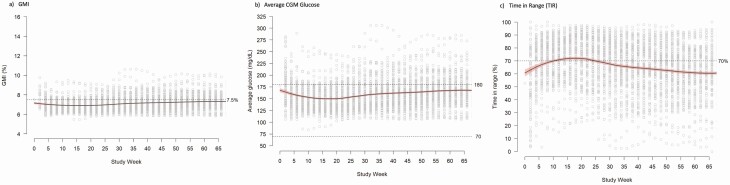
Owing to the COVID-19 pandemic, HbA1c was not available for all participants in the Pilot 4T cohort at recommended 3-month intervals. Therefore, to complement the HbA1c data, we calculated the GMI at 2-week intervals throughout the study period. CGM data was available for 127 (94.1%) participants. The median and interquartile range of the percentage of time with CGM data in this group was 90.8% (IQR, 60.9%-97.8%) after excluding the day of CGM initiation and study discontinuation, since the hour of CGM initiation and study end were not recorded. The GMI pattern was similar to the HbA1c pattern with the lowest GMI being between weeks 10-20 post diagnosis and a slow increase through the entire study period (Fig. 3A). Consistent with the HbA1c data, the mean GMI never crossed 7.5%. The pattern in mean CGM glucose calculated at 2-week intervals was also similar (Fig. 3B).
Figure 3.
Scatter plot of glucose management indicator (GMI), average glucose, and time in range (TIR) at 2-week intervals in the Pilot 4T cohort, with locally estimated scatter plot smoothing (LOESS). A, GMI is highest in the first month of diagnosis, lowest at approximately 4 months post diabetes diagnosis, and slowly increases until 12 months post diabetes diagnosis. B, Average glucose is highest at diagnosis, lowest at approximately 4 months post diabetes diagnosis, and slowly increased until 12 months post diabetes diagnosis. C, TIR is lowest in the first month of diagnosis, highest at approximately 4 months post diabetes diagnosis, and slowly decreases until 12 months post diabetes diagnosis.
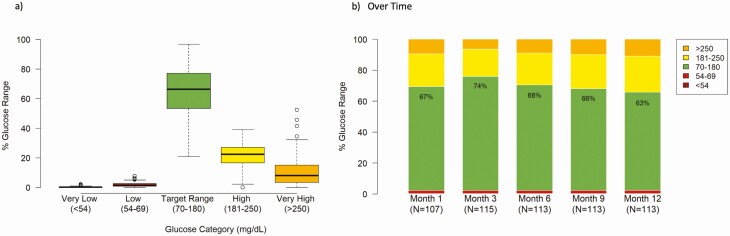
The TIR was highest in weeks 10 to 20 post diagnosis, and similarly to the GMI, decreased slowly through the study period (Fig. 3C). The mean TIR for the Pilot 4T cohort was 66% with a low incidence of time below range (54-69 mg/dL, 1.9%) and very low incidence of time below 54 mg/dL (0.4%) (Fig. 4A). By 12 months post diagnosis, the average TIR was 63% with a low incidence of hypoglycemia (54-69 mg/dL: 1.9%, < 54 mg/dL: 0.4%) (Fig. 4B).
Figure 4.
Glucose distribution in the Pilot 4T cohort. A, Over the first 12 months of diabetes diagnosis, time in range (TIR) was 66% in the 4T cohort with a low incidence of hypoglycemia. B, TIR is highest at month 3 of diagnosis with a low decrease in the first year. C, At 12 months post diagnosis TIR is 63% with a low incidence of hypoglycemia.
Participants Achieving Glycated Hemoglobin A1c Targets During the First Year of Diagnosis
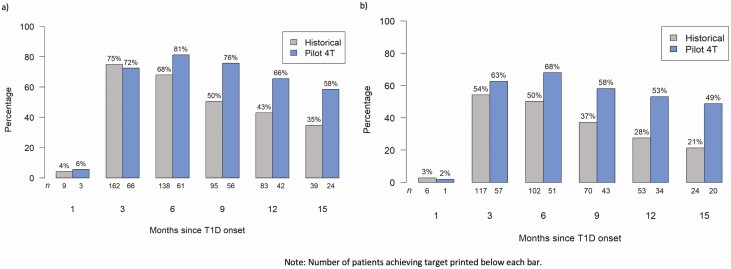
At 3 months post diagnosis, a higher percentage of those in the historical cohort met the ADA HbA1c target of less than 7.5%. However by 6 months post diagnosis, a higher percentage of those in the Pilot 4T cohort achieved an HbA1c below less than 7.5% (Fig. 5A) and this trend was sustained throughout the study period. At 12 months post diagnosis, 66% of those in the Pilot 4T cohort met HbA1c targets while only 43% of those in the historical pathway met HbA1c targets (P < .01). Although an HbA1c of less than 7% was not our target during this study, by 12 months post diagnosis, 53% of youth in the 4T program achieved an HbA1c of less than 7% while only 28% in the historical cohort achieved this target.
Figure 5.
Proportion achieving glycated hemoglobin A1c (HbA1c) targets over time. Proportion of participants achieving an HbA1c target of A, less than 7.5%, and B, less than 7% are shown in the Pilot 4T (blue) and historical (gray) cohorts.
Discussion
In this prospective single-center study, 135 youth enrolled in the Pilot 4T program consisting of a team-based approach for diabetes management with clear CGM glucose targets, and early CGM initiation. We observed that those in the Pilot 4T cohort obtained tighter glucose control than those in our historical comparison cohort, as measured by HbA1c, TIR, GMI, and the percentage of youth achieving ADA HbA1c targets. Between months 4 and 12, the mean HbA1c increase was statistically significantly lower in the 4T group compared to the historical cohort. We report that there may be a further improvement in HbA1c for youth in the Pilot 4T program who received remote monitoring when compared to those who did not. Youth started on CGM had a low incidence of hypoglycemia and no episodes of severe hypoglycemia (36). GMI increased slightly over the first year of diagnosis, consistent with HbA1c. We have previously shown that CGM use soon after diagnosis (ie, 7 d [5-11 d]) is welcomed by families (23). The effects of early CGM initiation are further enhanced by remote monitoring, which allows for more frequent dose adjustments.
A recent study from the SWEET diabetes registry showed that quality improvement initiatives combined with increased technology use was associated with improved HbA1c outcomes in youth with T1D (37). The 4T program has sought to operationalize the learning of the DCCT and international quality improvement programs that have lowered HbA1c in their pediatric T1D patients combined with advances in diabetes technology. Operationalizing frequent CGM data review and frequent dose adjustments was a team effort by the research team, clinical team, and families, and was supported by the development of population health management tools (26) that can help translate these learnings to other clinics. Frequent data review allowed for focused contact between families and the care team when dose adjustments were needed, including opportunities for diabetes education. In the 4T Study, at the population level, the mean difference LOESS plot HbA1c was improved in those with remote monitoring and frequent dose adjustments. However, when looking at difference in individual trajectories, the difference is not statistically significant. In our study, dose adjustments were provided by secure electronic health record–based messaging, and some participants may not engage with this modality of treatment. Further work would entail examining differences between those who interacted with the messaging and those who did not.
Implementing a program such as this requires systemic change for equitable access. A recent study from 2 registries showed that while gaps in diabetes technology access for those from lower socioeconomic status narrowed in the Germany, gaps in technology access increased in the United States (38, 39). These disparities in technology access can contribute to differences in diabetes outcomes. The 4T Study was specifically designed to provide CGM access to all newly diagnosed youth with T1D in our clinic, which was not possible initially, but then rectified once philanthropic funding was acquired (see Fig. 1).
These results should be interpreted in light of several limitations. The COVID-19 pandemic prevented measurement of HbA1c levels during shelter in place. However, we had CGM data with GMI available during this phase and eventually implemented a process for home kit HbA1c. A recent study has shown that home kit HbA1c measurements have similar accuracy to venous HbA1c and that the errors are unbiased, that is, that the average difference between home kit HbA1c and venous HbA1c is not statistically significantly different from zero (30). In our analysis, home kit HbA1c values had a positive bias (32). Furthermore, only 7% (n = 32) of the included readings were home kit HbA1c measurements. The other measurements were performed on the same DCA Vantage machines both in the historical and Pilot 4T cohorts. While this intervention was performed at a single academic medical center, the findings from this program can be implemented at other multidisciplinary diabetes clinics. In addition, at the time of study initiation, HbA1c targets in the United States were less than 7.5%. When looking at the lower target of less than 7%, which is now the ADA and ISPAD target, more youth in the 4T cohort achieved this target. We were fortunate to have philanthropic funding and policy changes that allowed us to provide CGM to our youth with T1D who had public insurance; however, this may not be possible everywhere. Ensuring equitable access to diabetes technology can increase access to programs with intensive glycemic management and we hypothesize help to close some gaps in HbA1c outcomes. In addition, we were also able to provide youth with devices to facilitate remote review of diabetes data, which may not be available everywhere. However, diabetes technology is moving to be more digitally connected, which will facilitate such interventions, and the COVID-19 pandemic has increased the use of telehealth (40).
For patients, one of the primary challenges has been consistent sharing of data without increasing patient burden. Newer diabetes technology has the ability to decrease this barrier by remote data-sharing features that require minimal intervention by users. However, disparities both in diabetes technology access and internet access can further exacerbate disparities in outcomes (39); therefore access to technology should be assessed at each visit (41). On the provider end, reviewing and analyzing large volumes of shared data is challenging with resource-constrained teams. The development of population health management tools can help improve the scalability of this intervention (26), and further work can help refine the cadence and flags for review as well as incorporate artificial intelligence–based decision-support tools (42). While we saw a consistent difference in HbA1c at months 6, 9, and 12 post diagnosis between those receiving remote monitoring and those who did not receive remote monitoring, it was not statistically significant perhaps because of the small sample size of these 2 populations, which was not intended to be powered to measure the difference. We had a higher percentage of children with public insurance in our remote monitoring group, likely because of the initiation of this study arm coinciding with philanthropic funding to support those with public insurance. Children with public insurance have historically had higher HbA1c (3, 8). In addition, a higher percentage of participants in the cohort without remote monitoring were on insulin pumps, including advanced hybrid closed loop systems, which could also have improved glycemic control.
Using a team-based approach to implement the 4T program, there was a statistically significant decrease in the rate of change of the slope of HbA1c after the HbA1c nadir at 4 months. Future work in the 4T program will assess the benefits of additional technology, such as automated insulin delivery systems, early in the course of diabetes to further improve glycemic outcomes. The use of automated insulin delivery systems in those with new-onset T1D may allow for further increases in TIR and reduced burden of care for youth. At the population level, a combination of CGM and automated insulin delivery may safely allow for the lowering of glucose targets in youth with diabetes. In addition, many children with T1D are not meeting the recommended physical activity guidelines (43), and in fact, are less active overall compared to youth without diabetes (44). This may be partially due to concerns about managing glucose during activity and fear of hypoglycemia (45). Children with T1D also have decreased physical activity, partially because of concerns about managing glucose during activity. In future work, incorporation of exercise education and physical activity tracking may help further improve outcomes. Improving glycemic control is only one aspect of diabetes care. T1D is associated with increased psychosocial comorbidities (46). Future work will assess the effect of the 4T program on quality of life measures. While this study occurred at a single site, the interventions could be translated to other centers and adapted for differences in resources.
In conclusion, teamwork, target setting, CGM use, and intensified early education were successful in improving glycemic outcomes in youth with T1D. Diabetes technology and related systems changes, when combined with a patient-centered approach, can help implement the findings of the DCCT. To facilitate programs for intensive glucose management, diabetes technology, such as CGM, needs to be accessible to all youth with T1D. Combining this patient-centered approach with population health management tools for high-volume data review and frequent dose adjustments by a diabetes care team can enable precision health approaches for all youth with T1D.
Acknowledgments
The authors would like to acknowledge all the youth who participated in the 4T Study. We would like to thank the other members of the 4T Study team members include: Nora Arrizon-Ruiz, Franziska Bishop, Barry Conrad, Ana Cortes, Rebecca Gardner, Carolyn Herrera, Julie Hooper, Brianna Leverenz, Jeannine Leverenz, Esli Osmanlliu, Erica Pang, Natalie Pageler, Piper Sagan, and Anjoli Martinez-Singh.
We thank the numerous SURF students who contributed to the development and deployment of the technology that facilitated remote data review: Oseas Oyardi, Angela Gu, Josh Grossman, Jacqualine Jil Valon, Anastasiya Vitko, Dianelys Perez Morales, Daniel Jun, Ryan Leonard, Michael Zikai Gao, Annie Chang, Prashant Yadav, Isha Thapa, and Johannes Opsahl Ferstad.
Financial Support: This work was supported in part by the National Institutes of Health P30DK116074 via the Stanford Diabetes Research Center and R18DK122422 to D.M.M. CGM supplies for the first month (transmitter, 3 sensors, and receiver per patient) were donated by Dexcom. Funding for iOS devices and some CGM supplies was provided by a grant through the Lucile Packard Children’s Hospital Auxiliaries Endowment.
Clinical Trial Information: Clinical trial registration number: NCT03968055 (registered May 30, 2019).
Author Contributions: D.M., P.P., M.D., and K.H. designed the interventions; V.D. and M.D. performed the data analyses; P.P and V.D. wrote the manuscript; and D.M., M.D., K.H., D.S., D.Z., A.A., and R.J. contributed to the discussion and reviewed/edited the manuscript.
Prior Presentation: Portions of these data were presented as 3 abstracts (69-OR, 913-P, 916-P) at the American Diabetes Association 81st Scientific Sessions (June 25-29, 2021) and as an oral presentation at the Advanced Technologies & Treatments for Diabetes conference (June 2-5, 2021).
Glossary
Abbreviations
- 4T Study
Teamwork, Targets, Technology and Tight Control
- ADA
American Diabetes Association
- CDCES
certified diabetes care and education specialist
- CGM
continuous glucose monitoring
- DCCT
Diabetes Control and Complications Trial
- GMI
glucose management indicator
- HbA1c
glycated hemoglobin A1c
- IQR
interquartile range
- ISPAD
International Society for Pediatric and Adolescent Diabetes
- LOESS
locally estimated scatter plot smoothing
- T1D
type 1 diabetes
- TIR
time in range
Additional Information
Disclosures: D.P.Z. has received speakers honoraria from Medtronic Diabetes, Ascensia Diabetes, and Insulet Canada; and research support from the Helmsley Charitable Trust and ISPAD-JDRF Research Fellowship. D.M.M. has received research support from the National Institutes of Health, JDRF, NSF, and the Helmsley Charitable Trust; and his institution has received research support from Medtronic, Dexcom, Insulet, Bigfoot Biomedical, Tandem, and Roche. He has consulted for Abbott, the Helmsley Charitable Trust, Sanofi, Novo Nordisk, Eli Lilly, and Insulet, and is supported by grant number P30DK116074. K.H. has received research support from Dexcom, Inc, for investigator-initiated research and consultant fees from the Lilly Innovation Center, LifeScan Diabetes Institute, and MedIQ. The remaining authors have nothing to disclose.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 2. Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group. J Pediatr. 1994;125(2):177-188. [DOI] [PubMed] [Google Scholar]
- 3. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D Exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wood JR, Miller KM, Maahs DM, et al. ; T1D Exchange Clinic Network . Most youth with type 1 diabetes in the T1D Exchange Clinic Registry do not meet American Diabetes Association or International Society for Pediatric and Adolescent Diabetes clinical guidelines. Diabetes Care. 2013;36(7):2035-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. American Diabetes Association. 13. Children and adolescents: Standards of Medical Care in Diabetes—2021. Diabetes Care. 2021;44(Suppl 1):S180-S199. [DOI] [PubMed] [Google Scholar]
- 6. DiMeglio LA, Acerini CL, Codner E, et al. ISPAD clinical practice consensus guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes. 2018;19(Suppl 27):105-114. [DOI] [PubMed] [Google Scholar]
- 7. Charalampopoulos D, Hermann JM, Svensson J, et al. Exploring variation in glycemic control across and within eight high-income countries: a cross-sectional analysis of 64,666 children and adolescents with type 1 diabetes. Diabetes Care. 2018;41(6):1180-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller KM, Beck RW, Foster NC, Maahs DM. HbA1c levels in type 1 diabetes from early childhood to older adults: a deeper dive into the influence of technology and socio-economic status on HbA1c in the T1D Exchange Clinic Registry findings. Diabetes Technol Ther. 2020;22(9):645-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanberger L, Birkebaek N, Bjarnason R, et al. Childhood diabetes in the Nordic countries: a comparison of quality registries. J Diabetes Sci Technol. 2014;8(4):738-744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hofer SE, Raile K, Fröhlich-Reiterer E, et al. ; Austrian/German Diabetes Patienten Verlaufsdokumentation DPV Initiative; German Competence Network for Diabetes Mellitus . Tracking of metabolic control from childhood to young adulthood in type 1 diabetes. J Pediatr. 2014;165(5):956-961.e1. [DOI] [PubMed] [Google Scholar]
- 11. Samuelsson U, Åkesson K, Peterson A, Hanas R, Hanberger L. Continued improvement of metabolic control in Swedish pediatric diabetes care. Pediatr Diabetes. 2018;19(1):150-157. [DOI] [PubMed] [Google Scholar]
- 12. Alonso GT, Corathers S, Shah A, et al. Establishment of the T1D Exchange Quality Improvement Collaborative (T1DX-QI). Clin Diabetes. 2020;38(2):141-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Divan V, Greenfield M, Morley CP, Weinstock RS. Perceived burdens and benefits associated with continuous glucose monitor use in type 1 diabetes across the lifespan. J Diabetes Sci Technol. Published online December 24, 2020. doi:10.1177/1932296820978769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cameron FJ, de Beaufort C, Aanstoot HJ, et al. ; Hvidoere International Study Group . Lessons from the Hvidoere International Study Group on childhood diabetes: be dogmatic about outcome and flexible in approach. Pediatr Diabetes. 2013;14(7):473-480. [DOI] [PubMed] [Google Scholar]
- 15. Van Loocke M, Battelino T, Tittel SR, et al. ; SWEET study group . Lower HbA1c targets are associated with better metabolic control. Eur J Pediatr. 2021;180(5):1513-1520. [DOI] [PubMed] [Google Scholar]
- 16. Miller KM, Beck RW, Bergenstal RM, et al. ; T1D Exchange Clinic Network . Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D Exchange Clinic Registry participants. Diabetes Care. 2013;36(7):2009-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laffel LM, Kanapka LG, Beck RW, et al. ; CGM Intervention in Teens and Young Adults with T1D (CITY) Study Group; CDE10 . Effect of continuous glucose monitoring on glycemic control in adolescents and young adults with type 1 diabetes: a randomized clinical trial. JAMA. 2020;323(23):2388-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Addala A, Maahs DM, Scheinker D, Chertow S, Leverenz B, Prahalad P. Uninterrupted continuous glucose monitoring access is associated with a decrease in HbA1c in youth with type 1 diabetes and public insurance. Pediatr Diabetes. 2020;21(7):1301-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nirantharakumar K, Mohammed N, Toulis KA, Thomas GN, Narendran P. Clinically meaningful and lasting HbA1c improvement rarely occurs after 5 years of type 1 diabetes: an argument for early, targeted and aggressive intervention following diagnosis. Diabetologia. 2018;61(5):1064-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Prahalad P, Yang J, Scheinker D, Desai M, Hood K, Maahs DM. Hemoglobin A1c trajectory in pediatric patients with newly diagnosed type 1 diabetes. Diabetes Technol Ther. 2019;21(8):456-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prahalad P, Zaharieva DP, Addala A, et al. Improving clinical outcomes in newly diagnosed pediatric type 1 diabetes: teamwork, targets, technology, and tight control—the 4T Study. Front Endocrinol (Lausanne). 2020;11:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Prahalad P, Addala A, Scheinker D, Hood KK, Maahs DM. CGM initiation soon after type 1 diabetes diagnosis results in sustained CGM use and wear time. Diabetes Care. 2020;43(1):e3-e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tanenbaum ML, Zaharieva DP, Addala A, et al. “I was ready for it at the beginning”: parent experiences with early introduction of continuous glucose monitoring following their child’s Type 1 diabetes diagnosis. Diabet Med. 2021;38(8):e14567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Prahalad P, Ding VY, Zaharieva DP, et al. Supplementary data for “Teamwork, Targets, Technology, and Tight Control in Newly Diagnosed Type 1 Diabetes: the Pilot 4T Study.” Posted October 2, 2021. https://figshare.com/s/7d62fe5be048b026336c [DOI] [PMC free article] [PubMed]
- 25. Kumar RB, Goren ND, Stark DE, Wall DP, Longhurst CA. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J Am Med Inform Assoc. 2016;23(3):532-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ferstad JO, Vallon JJ, Jun D, et al. Population-level management of type 1 diabetes via continuous glucose monitoring and algorithm-enabled patient prioritization: precision health meets population health. Pediatr Diabetes. 2021;22(7):982-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Battelino T, Danne T, Bergenstal RM, et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. American Diabetes Association . 12. Children and adolescents: Standards of Medical Care in Diabetes—2018. Diabetes Care. 2018;41(Suppl 1):S126-S136. [DOI] [PubMed] [Google Scholar]
- 29. Bergenstal RM, Beck RW, Close KL, et al. Glucose management indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care. 2018;41(11):2275-2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beck RW, Bocchino LE, Lum JW, et al. An evaluation of two capillary sample collection kits for laboratory measurement of HbA1c. Diabetes Technol Ther. 2021;23(8):537-545. [DOI] [PubMed] [Google Scholar]
- 31. Zaharieva D, Addala A, Prahalad P, et al. Good concordance between home kit A1c, glucose management indicator, and point-of-care A1c: potential solutions to glycemic assessment during the SARS CoV-2 pandemic. Diabetes. 2021;70(Supplement 1):913-P. [Google Scholar]
- 32. Prahalad P, Ding VY, Zaharieva DP, et al. Supplementary data for “Teamwork, Targets, Technology, and Tight Control in Newly Diagnosed Type 1 Diabetes: the Pilot 4T Study.” Posted October 2, 2021. https://figshare.com/s/c13377a590754ed2e58a [DOI] [PMC free article] [PubMed]
- 33. Fabris C, Heinemann L, Beck R, Cobelli C, Kovatchev B. Estimation of hemoglobin A1c from continuous glucose monitoring data in individuals with type 1 diabetes: is time in range all we need? Diabetes Technol Ther. 2020;22(7):501-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Grossman J, Ward A, Crandell JL, Prahalad P, Maahs DM, Scheinker D. Improved individual and population-level HbA1c estimation using CGM data and patient characteristics. J Diabetes Complications. 2021;35(8):107950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu Y, Grimsmann JM, Karges B, et al. Personal glycation factors and calculated hemoglobin A1c for diabetes management: real-world data from the Diabetes Prospective follow-up (DPV) Registry. Diabetes Technol Ther. 2021;23(6):452-459. [DOI] [PubMed] [Google Scholar]
- 36. Addala A, Zaharieva DP, Gu AJ, et al. Clinically serious hypoglycemia is rare and not associated with time-in-range in youth with new-onset type 1 diabetes. J Clin Endocrinol Metab. 2021;106(11):3239-3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gerhardsson P, Schwandt A, Witsch M, et al. The SWEET project 10-year benchmarking in 19 countries worldwide is associated with improved HbA1c and increased use of diabetes technology in youth with type 1 diabetes. Diabetes Technol Ther. 2021;23(7):491-499. [DOI] [PubMed] [Google Scholar]
- 38. Addala A, Auzanneau M, Miller K, et al. A decade of disparities in diabetes technology use and HbA1c in pediatric type 1 diabetes: a transatlantic comparison. Diabetes Care. 2021;44(1):133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lipman TH, Hawkes CP. Racial and socioeconomic disparities in pediatric type 1 diabetes: time for a paradigm shift in approach. Diabetes Care. 2021;44(1):14-16. [DOI] [PubMed] [Google Scholar]
- 40. Phillip M, Bergenstal RM, Close KL, et al. The digital/virtual diabetes clinic: the future is now—recommendations from an international panel on diabetes digital technologies introduction. Diabetes Technol Ther. 2021;23(2):146-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Klonoff DC, Shang T, Zhang JY, Cengiz E, Mehta C, Kerr D. Digital connectivity: the sixth vital sign. J Diabetes Sci Technol. Published online May 12, 2021. doi:10.1177/19322968211015241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nimri R, Battelino T, Laffel LM, et al. ; NextDREAM Consortium . Insulin dose optimization using an automated artificial intelligence-based decision support system in youths with type 1 diabetes. Nat Med. 2020;26(9):1380-1384. [DOI] [PubMed] [Google Scholar]
- 43. Lascar N, Kennedy A, Hancock B, et al. Attitudes and barriers to exercise in adults with type 1 diabetes (T1DM) and how best to address them: a qualitative study. PLoS One. 2014;9(9):e108019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sundberg F, Forsander G, Fasth A, Ekelund U. Children younger than 7 years with type 1 diabetes are less physically active than healthy controls. Acta Paediatr. 2012;101(11):1164-1169. [DOI] [PubMed] [Google Scholar]
- 45. Brazeau AS, Mircescu H, Desjardins K, et al. The Barriers to Physical Activity in Type 1 Diabetes (BAPAD-1) scale: predictive validity and reliability. Diabetes Metab. 2012;38(2):164-170. [DOI] [PubMed] [Google Scholar]
- 46. Johnson B, Eiser C, Young V, Brierley S, Heller S. Prevalence of depression among young people with Type 1 diabetes: a systematic review. Diabet Med. 2013;30(2):199-208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.