Abstract
Context
Maturity onset diabetes of the young, type 8 (MODY8) is associated with mutations in the CEL gene, which encodes the digestive enzyme carboxyl ester lipase. Several diabetes cases and families have in recent years been attributed to mutations in CEL without any functional or clinical evidence provided.
Objective
To facilitate correct MODY8 diagnostics, we screened 2 cohorts of diabetes patients and delineated the phenotype.
Methods
Young, lean Swedish and Finnish patients with a diagnosis of type 2 diabetes (352 cases, 406 controls) were screened for mutations in the CEL gene. We also screened 58 Czech MODY cases who had tested negative for common MODY genes. For CEL mutation-positive subjects, family history was recorded, and clinical investigations and pancreatic imaging performed.
Results
Two cases (1 Swedish and 1 Czech) with germline mutation in CEL were identified. Clinical and radiological investigations of these 2 probands and their families revealed dominantly inherited insulin-dependent diabetes, pancreatic exocrine dysfunction, and atrophic pancreas with lipomatosis and cysts. Notably, hereditary pancreatitis was the predominant phenotype in 1 pedigree. Both families carried single-base pair deletions in the proximal part of the CEL variable number of tandem repeat (VNTR) region in exon 11. The mutations are predicted to lead to aberrant protein tails that make the CEL protein susceptible to aggregation.
Conclusion
The diagnosis of MODY8 requires a pancreatic exocrine phenotype and a deletion in the CEL VNTR in addition to dominantly inherited diabetes. CEL screening may be warranted also in families with hereditary pancreatitis of unknown genetic etiology.
Keywords: monogenic diabetes, MODY8, mutation screening, pancreatic exocrine function, chronic pancreatitis, pancreatic imaging
Maturity onset diabetes of the young (MODY) are inherited diabetes syndromes that account for 1% to 2% of all diabetes cases (1, 2). Usually, MODY manifests before the age of 25 years. The disease is therefore often misdiagnosed as type 1 diabetes (3). MODY genes are expressed in the pancreatic beta-cells, and the disease is generally considered to result from a primary beta-cell defect. An exception is, however, MODY8 or CEL-MODY (Online Mendelian Inheritance of Man Database, OMIM # 609812), which is caused by mutations in the gene encoding the digestive enzyme carboxyl ester lipase (CEL; EC 3.1.1.13) (4).
Pancreatic expression of CEL is restricted to the acinar cells, the islets of Langerhans being devoid of the protein (5). Nevertheless, the first identified MODY8 patients were investigated genetically due to the familial pattern of their diabetes, and it was the endocrine phenotype in combination with low fecal elastase values that eventually led to the discovery of the syndrome (4). In addition to endocrine and exocrine pancreatic dysfunction, imaging studies have revealed that MODY8 is characterized by pancreatic lipomatosis from childhood (6) and development of pancreatic cysts later in life (7).
The CEL gene maps to chromosome band 9q34.13, covers approximately 10 kilobase pairs of genomic sequence, and entails 11 exons (8). The last exon contains a guanine/cytosine-rich variable number of tandem repeats (VNTR) region, consisting of almost identical 33-base pair (bp) sequences. The VNTR is responsible for the extremely polymorphic nature of the gene (8), and repeat counts of between 3 and 23 have been observed across populations, with 16 being the predominating number (9-13). In addition, several structural variants of the CEL gene, including a hybrid allele associated with increased risk of chronic pancreatitis, have been observed (9, 14, 15).
After being secreted into the duodenum and activated by the presence of bile salts, the CEL protein participates in the digestion of cholesterols and lipid-soluble vitamins (16). CEL is also a component of mother’s milk, probably contributing to fat digestion in the intestine of newborns (17). The major portion of CEL consists of an N-terminal globular core encoded by exons 1-10, whereas the C-terminal part, encoded by the VNTR-containing exon 11, mainly consists of mucin-like amino acid repeats that are heavily O-glycosylated (5).
The causative mutations of the 2 first MODY8 families reported, both of Norwegian ancestry, were single-bp deletions in the first (c.1686delT) or fourth (c.1785delC) VNTR segment of CEL (4). They both result in a shift of the reading frame, leading to a premature stop codon in segment number 13 of the VNTR sequence. The CEL protein variants resulting from these mutations will hereafter be referred to as DEL1 and DEL4, respectively. Compared with the normal CEL protein, DEL1 and DEL4 contain a different and somewhat shorter tail region, consisting of aberrant 11-amino acid repeats with altered biochemical properties and reduced O-glycosylation potential (18).
After Ræder et al (4) described the 2 original MODY8 families, many reports have claimed to identify new CEL mutations that may cause diabetes (19-25). However, a MODY8-like phenotype has been convincingly demonstrated only for 1 recently identified Italian patient (26). Here, we have screened 2 European cohorts of selected diabetes patients and present 2 new families with dominantly inherited diabetes, pancreatic exocrine disease, and single-bp deletions in CEL exon 11. We compare the clinical findings with those of the previously reported families and provide recommendations for correct identification of MODY8 cases.
Methods
Patient Materials and Clinical Investigations
Two patient materials were used in the present study. One cohort was population-based and consisted of 352 diabetes cases and 406 controls from Southern Sweden and the Bothnia region of Finland (27). The cases had a diagnosis of type 2 diabetes and had been selected for low environmental risk as they were relatively lean and young. The controls were euglycemic individuals who were generally older and obese (27). The study was approved by the Ethical Committee of Lund University.
The other cohort originated from the Czech Republic and consisted of 58 probands who fulfilled clinical criteria for a MODY diagnosis (family history of diabetes in at least 2 generations, young onset of diabetes with negative pancreatic autoantibodies, and positive C-peptide several years after diagnosis). Sanger sequencing of the genes GCK, HNF1A, HNF4A, INS, and NEUROD1 had not identified any causal variant. The patients therefore constituted a “MODYX” cohort, that is, cases likely to have a monogenic form of diabetes but without any identified genetic cause. Six of the cases, including the proband described here, had previously undergone whole-exome sequencing without identifying any mutations in the CEL gene (28). The study was approved by the Ethics Committee of the University Hospital Motol and the Second Faculty of Medicine, Charles University, Prague.
The probands and tested family members gave their written informed consent for participation. Clinical investigations included detailed collection of family medical history, biochemical data checkup, fecal elastase test, pancreatic magnetic resonance imaging (MRI) with magnetic resonance cholangiopancreatography (MRCP), and a mixed-meal test (Czech family only).
Amplification of the CEL Exon 8-11 Region
Long-range polymerase chain reaction (PCR) of DNA extracted from EDTA blood samples was performed by modifying a previously published method (11). The primers L11F (5´-GTGCCTCACTCATTCTTCTATGGCAAC-3´) and VNTR-R (5´-TCCTGCAGCTTAGCCTTGGG-3´) were used to amplify the CEL exon 8-11 region. Each reaction mix (13 μL) consisted of 6.4 μL of 2×GC buffer-I (Takara Bio), 2.0 μL of dNTP mix (2.5mM of each dNTP), 2.5 μL betaine solution (5M), 0.063 μL LA Taq polymerase (5 U/μL; Takara Bio), 0.37 μL of each primer (20μM), 0.37 μL ddH2O and 1.0 μL (50 ng/μL) genomic DNA, and was run under the following conditions: a denaturation step at 94 °C for 1 minute, 14 cycles of 94 °C for 20 seconds and 60 °C for 10 minutes, then 20 cycles of 94 °C for 20 seconds and 62 °C for 10 minutes, followed by a final elongation step of 10 minutes at 72 °C. Size and integrity of the PCR products was verified by 1% agarose gel electrophoresis.
Sequencing of the CEL VNTR
After standard treatment of the CEL exon 8-11 PCR product with ExoSap-IT reagent (Thermo Fisher Scientific), Sanger sequencing of the VNTR region was performed with the primers EF (5´- CACACACTGGGAACCCT-3´) and VNTR-R (5´-TCCTGCAGCTTAGCCTTGGG-3´). The reaction mix (10 μL) consisted of 2.0 μL of the treated PCR product, 0.25 μL primer (20 μM), 2.0 μL betaine (5 M), 2.0 μL 5X Sequencing Buffer (Applied Biosystems), 2.75 μL ddH2O, and 1.0 μL BigDye Terminator v3.1 reagent (Applied Biosystems). Amplification consisted of an initial denaturation step at 96 °C for 10 minutes, followed by 25 cycles of denaturation at 96 °C for 10 seconds, annealing at 58 °C for 5 seconds and elongation at 60 °C for 4 minutes. The fluorescent-labeled sequencing products were purified using Sephadex G-50 Superfine (Sigma) and analyzed by capillary electrophoresis on a 3500xL Genetic Analyzer (Applied Biosystems). All sequences were evaluated manually with the help of the software SnapGene Version 4.1.9 (www.snapgene.com). CEL VNTR lengths were determined using a published method (11), using only the unlabeled forward primer Celex11F (5´-ACCGACCAGGAGGCCACCC-3´) and the fluorescently (NED) labeled reverse primer Celex11R-NED (5´-CCTGGGGTCCCACTCTTGT-3´).
Results
Screening of Diabetes Cohorts for CEL Mutations
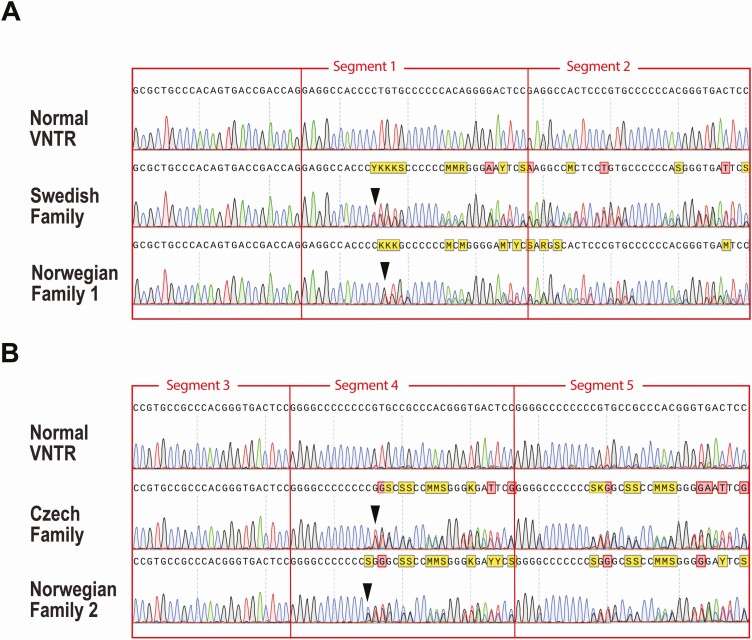
DNA sequencing was performed in 2 cohorts of diabetes patients, 1 from Sweden/Finland (27) and 1 from the Czech Republic (28). The Swedish/Finnish cohort consisted of 352 relatively lean and young patients with a diagnosis of type 2 diabetes and also included 406 controls (see Supplementary Table 1 in (29)). In total, 115 genes that either cause monogenic forms of diabetes or associate with type 2 diabetes were screened by targeted, high-throughput sequencing (27). In the Swedish material, 1 case had a heterozygous single-bp deletion in the first VNTR segment of the CEL gene (Fig. 1A). The mutation (c.1685delC; p.Pro562Leufs*133) was different at the DNA level from the previously described mutation in the Norwegian Family 1 of Ræder et al (4). However, the predicted change at the protein level was the same, that is, the aberrant CEL protein denoted DEL1. No CEL gene variants suspected to be pathogenic were detected in the Finnish cohort or among the controls.
Figure 1.
Molecular analysis of the CEL VNTR region in MODY8. A, Sanger sequencing of the VNTR showing DNA sequence of the Swedish family compared with corresponding sequence of the Norwegian Family 1 from Ræder et al (4) as well as normal VNTR sequence. Two different mutations affecting segment 1 of the VNTR are seen (arrowheads): c.1685delC in the Swedish and c.1686delT in the Norwegian family. B, DNA sequence of the Czech family compared with corresponding sequence of the Norwegian Family 2 from Ræder et al (4) as well as normal VNTR sequence. Two different mutations affecting segment 4 of the VNTR are seen (arrowheads): c.1786delG in the Czech and c.1785delC in the Norwegian family.
The Czech material consisted of 58 probands of families who had been screened for common MODY genes without finding any causal mutations. Clinical details about this MODYX cohort are given in Supplementary Table 1 in (29). The probands underwent targeted sequencing of the CEL exon 11 region (11). One subject had a heterozygous single-bp deletion in the fourth VNTR segment (Fig. 1B). The mutation (c.1786delG; p.Val596Cysfs*99) was different at the DNA level from the previously described mutation in the Norwegian Family 2 of Ræder et al (4). However, the predicted consequence at the protein level was the same, namely, the aberrant CEL protein denoted DEL4. No other CEL gene variants suspected to be pathogenic were detected among the Czech MODYX probands.
A Swedish MODY8 Family With Diabetes and Hereditary Pancreatitis
The Swedish case (proband II-2 of Family A in Fig. 2) was a male diagnosed with what was considered as type 2 diabetes at age 41. He had been treated with oral medication for 3 to 4 years after diagnosis, thereafter with insulin. He suffered from chronic diarrhea and was diagnosed with chronic pancreatitis at age 53 years. He had normal weight with body mass index (BMI) around 20. Due to pain and exocrine insufficiency, he was treated with strong opioid analgesics (ketobemidone) from age 55 years and pancreatic enzyme replacement from age 58. The clinicians had attributed his pancreatitis to alcohol abuse. He died from kidney failure at age 65 years.
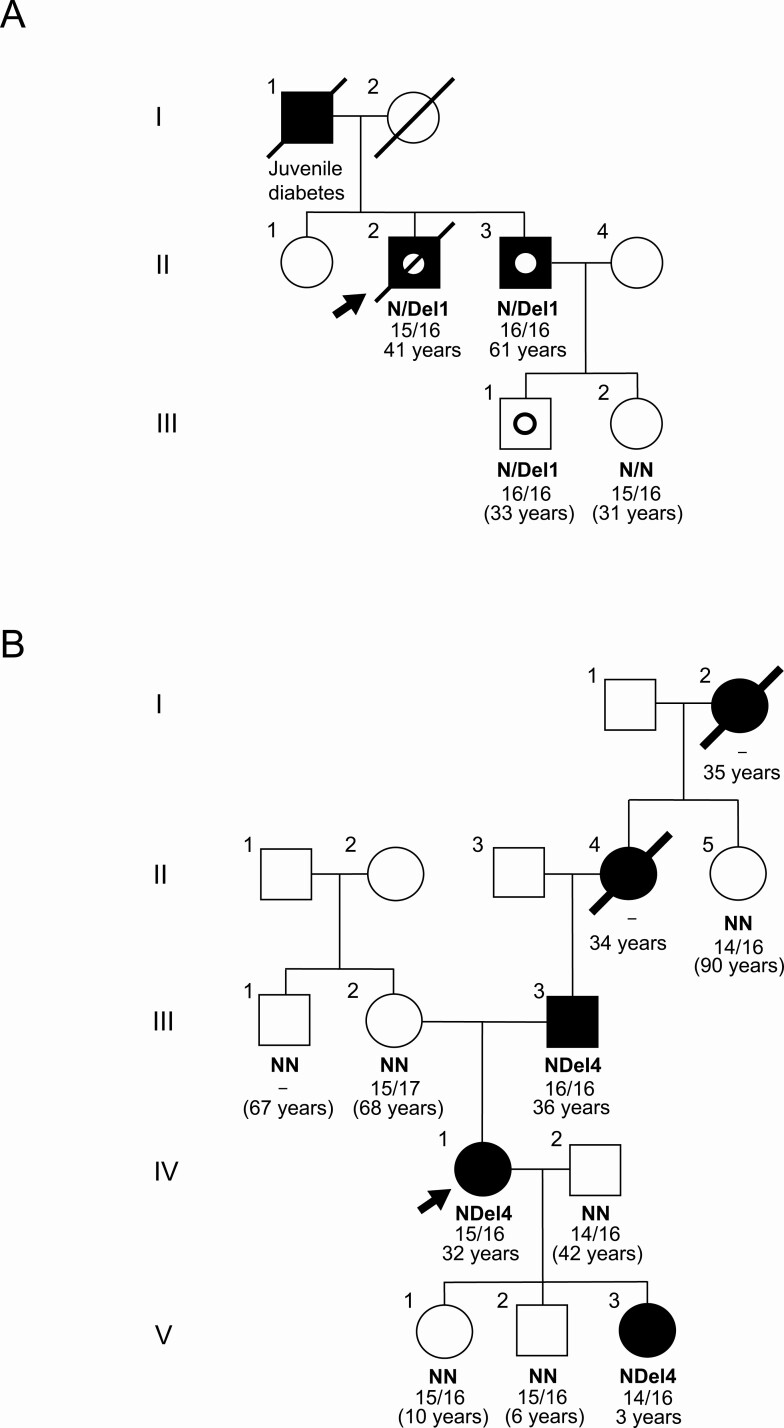
Figure 2.
Pedigrees of 2 new MODY8 families. Filled symbols represent subjects affected by diabetes. Squares symbolize male family members, circles are females. Arrows point to the probands and symbols with a slash are deceased family members. For family members with available DNA, N/N means 2 normal CEL alleles whereas N/DEL represents a heterozygous deletion mutation. The numbers below are the VNTR lengths of the subjects’ 2 CEL alleles. Age refers to diabetes onset or, when in parenthesis, age at evaluation for nondiabetic subjects. The pedigrees have been modified to protect the identity of the families. A, The Swedish MODY8 family carrying the mutation c.1685delC, a single-bp deletion in the first segment of the CEL VNTR. The subjects II-2, II-3, and III-1 with dotted symbols were diagnosed with chronic pancreatitis at ages 53, 54, and 28 years, respectively. B, The Czech MODY8 family carrying the mutation c.1786delG, a single-bp base deletion in the fourth segment of the CEL VNTR. Subject V-3 was diagnosed with type 1 diabetes in early childhood.
The father of the proband (I-1) died at age 72 years. He had diabetes with juvenile manifestation and was treated with insulin. There was no information about his exocrine pancreatic function. The proband’s mother (I-2) died in her nineties and did not have diabetes. Samples for genetic testing were not available from the parents.
The proband of Family A had 2 siblings of whom only a younger brother (II-3) was affected and available for testing (Fig. 2). Diabetes was diagnosed at age 61 years, and he was treated with insulin since age 63. Current HbA1c, fasting blood glucose, and BMI were 60 mmol/mol (7.6%), 7.0 mmol/L (126 mg/dL) and 21.9 kg/m2, respectively. His first attacks of abdominal pain occurred at age 13 years. He had acute pancreatitis at age 45 years and then again at age 50 years, when pancreatic atrophy with lipomatosis as well as steatosis of the liver was documented. He was diagnosed with chronic pancreatitis at age 54 years. Serum amylase was low and fecal elastase was very low (< 15 μg/g, normal value > 200 μg/g). He was treated with pancreatic enzyme replacement. At age 64 years, secretin-MRCP (30) demonstrated reduced secretion of pancreatic juice. At age 67 years, he underwent endoscopic papillectomy that demonstrated biliary low-grade dysplasia in biopsies. He also had multiple side-branch intraductal papillary mucinous neoplasms (IPMN) of the pancreas, with the largest lesion being 1.1 cm in diameter. Endoscopic retrograde cholangiopancreatography has been performed multiple times without any signs of malignancy. He also had a cholecystectomy at age 51 years due to gall stones and had a series of intestinal issues (adhesive ileus) that led to a removal of a portion of the small intestine. He has abstained from alcohol.
The affected brother II-3 had 2 children. The oldest (III-1, male) was diagnosed with chronic pancreatitis when he was 28 years old and has been on pancreatic enzyme replacement therapy since then. Due to episodes of acute abdominal pain after alcohol intake, he abstains from alcohol. He remains normoglycemic at age 33 years. The daughter (III-2) was healthy at the time of investigation (age 31 years).
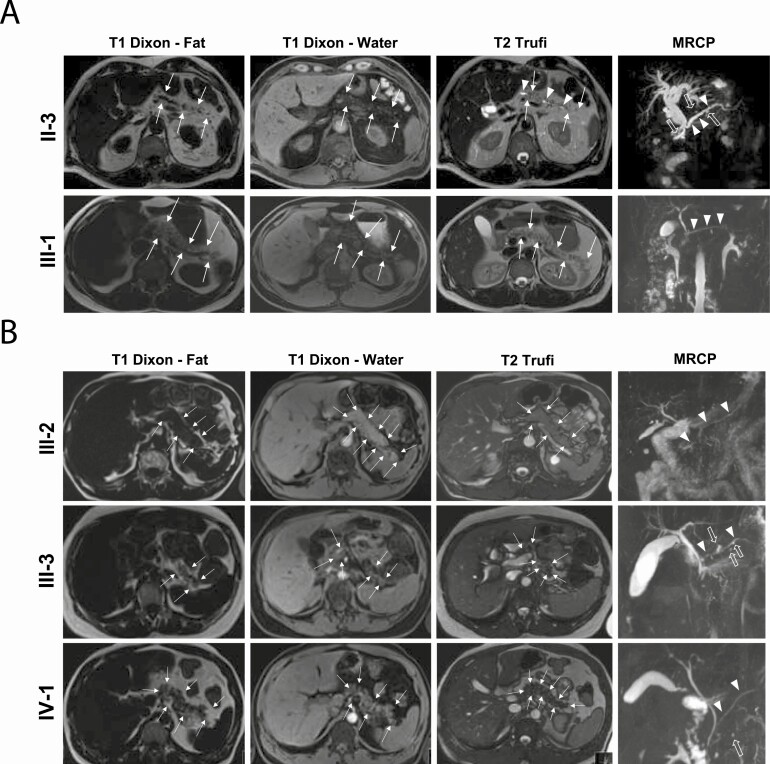
Pancreatic MRI with MRCP of the proband’s 2 affected relatives—the brother (II-3) and the nephew (III-1)—depicted altered pancreatic morphology with reduced organ size and lipomatosis in both patients (Fig. 3). In the brother, a dilated main pancreatic duct and 5 small pancreatic cysts were diagnosed, whereas these abnormalities were not observed in the nephew.
Figure 3.
Pancreatic imaging of MODY8 family members. A, The Swedish family. Magnetic resonance imaging (MRI) with magnetic resonance cholangiopancreatography (MRCP) was performed in the proband’s affected brother (II-3; upper panel) and nephew (III-1; lower panel). T1-weighted (Dixon series with fat and water images) and T2-weighted (Trufi) axial series at the level of the pancreatic body and tail depict altered pancreatic morphology with slightly reduced pancreatic size (arrows demarcate pancreatic boundaries) and increased pancreatic fat content (displayed by increased pancreatic signal intensity on the T1 fat image) in both subjects. T2-weighted MRI and MRCP indicate that the main pancreatic duct (arrowheads) is dilated in II-3 whereas it has normal caliber and contour in III-1. The affected brother II-3 also has 5 small pancreatic cysts (open arrows). B, The Czech family. The same imaging sequences as in A were performed in the healthy mother (III-2; upper panel), affected father (III-3; middle panel) and the proband (IV-1; lower panel). Altered pancreatic morphology with reduced pancreatic size and pancreatic lipomatosis is seen the 2 affected persons. The main pancreatic duct has normal caliber and contour in all subjects. Pancreatic small cysts are observed in the affected father III-3 (7 cysts) and in the proband IV-1 (1 cyst in the pancreatic head), but not in the healthy mother. Arrow symbols are same as in A.
The mutation c.1685delC in the CEL gene was confirmed by Sanger sequencing of a DNA sample from the proband II-2. Three other at-risk family members then underwent diagnostic sequencing of CEL exon 11. The affected subjects II-3 and III-1 tested positive whereas the healthy subject III-2 tested negative for the mutation.
A Czech MODY8 Family With Diabetes in 5 Generations
The Czech case (proband IV-1 of Family B in Fig. 2) was a female diagnosed with diabetes at the age of 32 years. From the time of diagnosis, she had been treated by daily insulin injections (dose at time of investigation 0.18 IU/kg/day). At age 42 years, her BMI was 28 kg/m2 and her diabetes well-regulated with HbA1c of 39 mmol/mol (5.7%). At the time of investigation, fasting values for blood glucose and C-peptide were 5.2 mmol/L (94 mg/dL) and 432 pmol/L, respectively, indicating some remaining insulin secretion from the beta-cells. The proband did not display signs of retinopathy or neuropathy, but hyperuricemia and hyperlipidemia were present. Fecal elastase was low (169 μg/g).
There were 4 other cases of diabetes in the family, spanning 5 generations (Fig. 2). The proband’s father (III-3) had been diagnosed with diabetes at age 36 years and had been treated by daily insulin injections since then (dose at time of investigation 0.45 IU/kg/day). His current age was 66 years, BMI was 24 kg/m2 and HbA1c was 71 mmol/mol (8.6%). At the time of investigation, fasting values for serum glucose and C-peptide were 9.6 mmol/L (173 mg/dL) and 181 pmol/L, respectively. Fecal elastase was very low (2 μg/g). He suffered from polyneuropathy and macroangiopathy as well as vitamin D deficiency with secondary hyperparathyreosis.
The youngest daughter of the proband (V-3) had been diagnosed with autoimmune diabetes (positive for anti-tyrosine phosphatase and anti-glutamic acid decarboxylase autoantibodies) without ketoacidosis at age 3 years. At the time of investigation, her HbA1c was 59 mmol/mol (7.5%) and C-peptide undetectable, and she used insulin at a dose of 0.86 IU/kg/day. Information about the status of the exocrine pancreas was not available. The paternal grandmother (II-4) of the proband had been diagnosed with diabetes at age 34 years and was treated with insulin. She died in her eighties. Similarly, the great-grandmother (I-2) had been diagnosed with diabetes at age 35 years and treated with insulin. She died in her nineties. Otherwise, no clinical information or pancreas specimens were available for the 2 latter individuals.
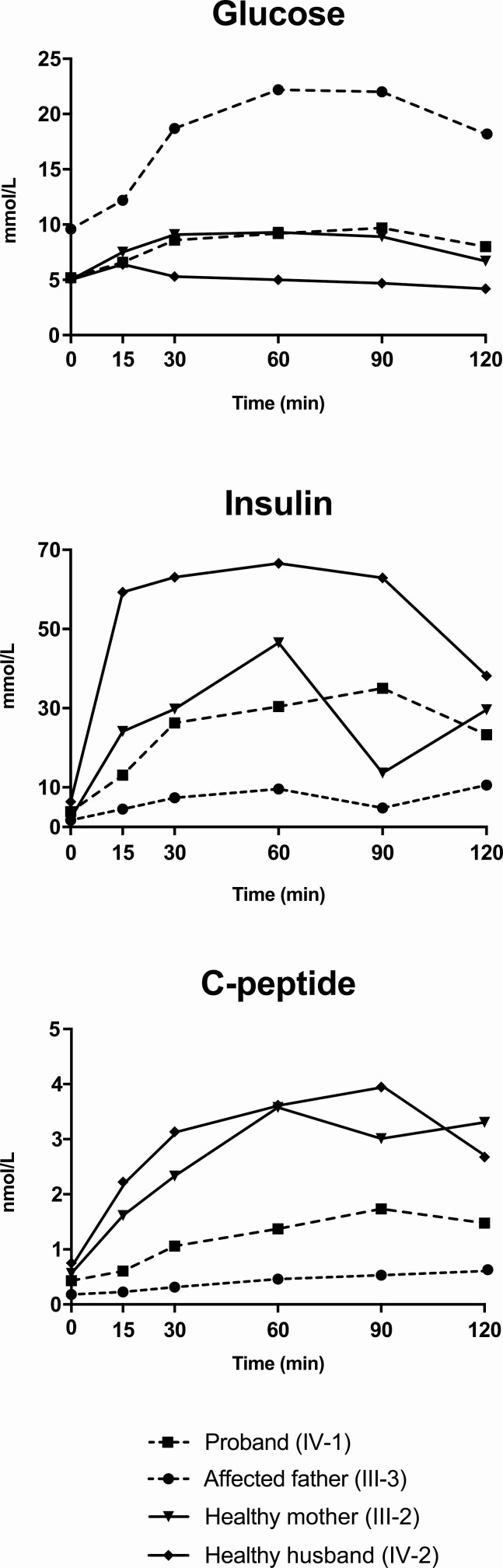
The proband, her affected father, as well as her healthy husband (IV-2) and healthy mother (III-2) underwent a mixed-meal test (31). After an overnight fast (last available insulin injection was long-acting insulin analog in the previous evening), a liquid meal (Ensure Plus; Abbott Laboratories) was administered at a dose of 4 mL/kg. Samples were obtained for glucose, insulin, and C-peptide measurements at time 0 (before meal administration) and after 30, 60, 90, and 120 minutes. The proband’s glucose profile appeared similar to that of her 2 healthy family members (Fig. 4). Her affected father had elevated fasting glucose and developed hyperglycemia after the meal, not reaching normal levels after 120 minutes. Moreover, the proband exhibited an insulin response similar to one of her healthy family members, whereas the response in the affected father was virtually absent. The proband had reduced and the father only residual C-peptide levels during the mixed-meal test (Fig. 4).
Figure 4.
Glucose homeostasis in the Czech MODY8 family. Glucose, insulin and C-peptide were measured in blood serum from 2 affected and 2 healthy family members at the indicated time points after an overnight fast and consumption a mixed meal. Subject numbers correspond to those of Family B in Fig. 2. The 2 affected members received insulin injections in the evening before undergoing the test.
At pancreatic MRI with MRCP, the proband (IV-1) and her affected father (III-3) exhibited pancreatic atrophy and increased pancreatic fat content (Family B in Fig. 3). The father also had 7 small pancreatic cysts (all with size < 5 mm) in the pancreatic body and tail, whereas the proband had a single 3-mm pancreatic cyst in the pancreatic head. Normal pancreas was observed in the healthy mother (III-2).
DNA samples were available from 8 of the proband’s family members (see Family B in Fig. 2). The CEL mutation c.1786delG was found in the 2 affected subjects at risk (III-3 and V-3), whereas it was not present in the 2 healthy family members at risk (V-1 and V-2) or in the 4 healthy individuals not at risk (II-5, III-1, III-2, and IV-2). Thus, co-segregation between the CEL mutation and diabetes was present. Notably, the diabetes of the proband’s daughter V-3 was regarded as type 1 due to the presence of islet autoantibodies and early age of diagnosis.
VNTR Length Determination
Finally, we determined the number of VNTR segments on the mutated allele in both the Swedish and Czech families. This was accomplished by measuring the size of amplified DNA fragments from the CEL exon 11 region for the available family members and evaluating the pattern of segregation within each pedigree (Fig. 2). In both families, the CEL deletion mutation had occurred on an allele that carried 16 VNTR repeats, which is the most common VNTR length of all populations tested so far (8).
Discussion
The Phenotype of MODY8
MODY8 is a rare MODY form where the primary genetic defect affects pancreatic exocrine tissue and not the islets. Still, diabetes was the most prominent phenotype of the 2 previously published MODY8 families, and after age 45 all mutation carriers had developed diabetes (4, 7). Their pancreatic exocrine dysfunction was not the presenting symptom, although some patients reported abdominal pain and diarrhea. By testing, however, fecal elastase levels were consistently low in all mutation carriers, and imaging demonstrated atrophic pancreas with lipomatosis and cysts (4, 6, 7).
The phenotype of the Czech family described here (Family B in Fig. 2) is similar to that of the 2 Norwegian families. The proband and 3 of her ancestors presented with insulin-dependent diabetes in their thirties. Moreover, for the proband and her father there were low fecal elastase levels and abnormal findings on pancreatic imaging. Interestingly, both in the Czech pedigree and in the Norwegian Family 1 (4), there was one case of confirmed type 1 diabetes occurring in a mutation-positive child. Identification of more MODY8 families and HLA risk allele genotyping will be needed to determine if this is an incidental finding or whether CEL VNTR mutations increase the risk for type 1 diabetes development.
In the Swedish family, 1 mutation carrier (subject II-3 of Family A in Fig. 2) had received a diagnosis of acute and subsequent chronic pancreatitis several years before developing diabetes. Subject III-1 was diagnosed with chronic pancreatitis at age 28 and is still normoglycemic. For the deceased mutation carrier (subject II-2), diabetes was diagnosed first; however, he died with severe chronic pancreatitis. Thus, the phenotype of the Swedish family is consistent with that of hereditary pancreatitis. In fact, also several mutation carriers in the 3 other MODY8 pedigrees fulfill criteria for chronic pancreatitis (32). We therefore emphasize that single-bp deletion mutations of the CEL VNTR should be recognized not only as triggers of MODY but also as a cause of hereditary pancreatitis.
Most CEL Mutations Do Not Cause MODY8
After being classified as a MODY gene, CEL has become part of gene panels used when screening for monogenic forms of diabetes. In recent years, several studies from different countries have published patients suggested to carry pathogenic variants of CEL. Yu et al, in the Joslin Medalist Study, listed several USA cases with rare CEL variants (19). For 1 case, who had the variant p.Lys496Gln, pancreatic autopsy material was available and showed overall normal morphology. In another American study, Todd et al studied youth with a clinical diagnosis of type 2 diabetes and reported 1 variant in the CEL gene classified as pathogenic/likely pathogenic (25). Moreover, when examining Iranian families with diabetes, Sarmadi et al (20) concluded that the CEL variant p.Ile488Thr causes MODY, even if present at an allele frequency of 0.01 in various Middle Eastern populations. Other single cases or families proposed to carry pathogenic CEL mutations outside the VNTR have been published from India (21), Siberia (22), China (23), and South Korea (24).
We doubt that any of these reports have found new MODY8 cases. Rare missense variants in the CEL gene are not necessarily detrimental, even if predicted to be pathogenic by bioinformatic analyses. The CEL variants known to be pathogenic are frameshift mutations that lead to a new tail region of the enzyme. This abnormal tail contains many cysteine residues, which are likely to induce misfolding and aggregation of the CEL protein (18). Hence, the MODY8-causing mutations are considered to have a dominant-negative, gain-of-function effect, consistent with the autosomal dominant inheritance pattern of the disease. Mutations causing amino acid substitutions therefore need to be evaluated individually to verify that they functionally affect the protein and co-segregate with the disease. Moreover, mice carrying knockout alleles for the CEL gene exhibit no pancreatic phenotype, neither in the heterozygous nor in the homozygous state, and there is so far no evidence that loss-of-function mutations of CEL cause diabetes (33).
The only recent paper that we have come across that convincingly demonstrates a MODY8 case, is the report of Pellegrini et al (26). They describe a normal-weight patient with young onset nonimmune diabetes. He had experienced abdominal symptoms (cramps, bloating), and pancreatic lipomatosis and atrophy was demonstrated by MRI. There was a familial disposition of diabetes leading to suspicion of monogenic diabetes. Among 31 candidate genes sequenced, a single-bp deletion was found in the fifth VNTR segment of CEL, predicted to give rise to a CEL protein of the DEL5 type. Although no family tree was shown and no investigations were performed in relatives, we regard the Italian patient as a very likely MODY8 case.
Of importance, the known MODY8 mutations affect only the most proximal VNTR segments of the CEL gene. In population-based materials, rare single-bp deletions have been observed in VNTR segment 9, and there is so far no evidence that the distal deletions can cause MODY8 (34). We therefore recommend that single-bp deletions beyond CEL VNTR segment 5 should be interpreted with caution.
The CEL VNTR Is Susceptible to Frameshifts
The CEL VNTR contains a considerable number of segments, each containing many consecutive guanine and cytosine bases. Such sequences represent a challenge during DNA replication as insertion/deletion mutations tend to arise in homopolymeric stretches (35). Notably, the 2 MODY8-causing mutations found earlier (4), those presented here and the recently described Italian mutation (26) are all single-bp deletions within or adjacent to homopolymeric tracts. The fact that none of the mutations are identical shows that the families are unrelated and supports our presumption of a hypermutable VNTR of CEL.
This notion is further illustrated by the presence of single-bp insertions in the CEL VNTR region. Such insertions may be carried by up to 10% of the population, and VNTR segment number 9 seems to be a hotspot in this regard (8). The predicted effect of these insertions at the protein level is, however, very distinct from that of the deletions identified in the MODY8 families. The insertions cause a C-terminal tail that is truncated almost immediately after the affected position, and there is so far no convincing evidence linking these alterations to human disease.
CEL Protein Forms Encoded by VNTR Single-bp Deletions: Variation Over a Common Theme
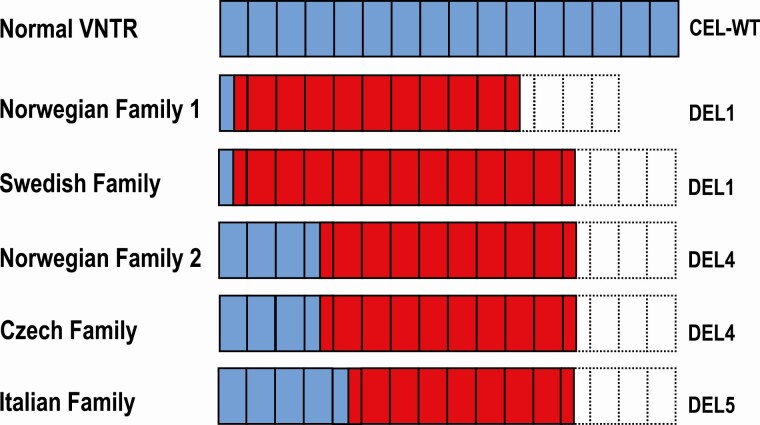
The Swedish mutation occurs on a background of 16 VNTR segments. As the reading frame for CEL alleles with single-bp deletions in proximal VNTR repeats will terminate in segment number 13, the Swedish mutation is predicted to result in 12 aberrant, still repetitive segments, present in a CEL protein of the DEL1 type (Fig. 5). In the Norwegian Family 1, also with DEL1, the mutation is present on an allele with 14 repeat segments (4), implying that 10 aberrant protein segments will be produced. The 2 DEL4 proteins and the Italian DEL5 protein are all encoded by VNTR alleles with 16 repeats, resulting in 9 and 8 aberrant protein segments, respectively (Fig. 5).
Figure 5.
Aberrant CEL protein tails predicted by single-bp deletion mutations within the CEL VNTR. Blue boxes represent normal protein segments, whereas red boxes depict aberrant segments due to shifts in the reading frame. The most common normal allele with 16 VNTR segments is indicated as reference. Stippled boxes represent VNTR segments not translated due to a premature stop codon encountered in the altered reading frame. In the Norwegian Family 1 with the DEL1 protein, the mutation is present on an allele with 14 VNTR segments implying that 10 aberrant protein segments will be produced. The Swedish mutation occurs on a background of 16 VNTR segments and results in 12 aberrant segments of the DEL1 protein. The two DEL4 proteins and the DEL5 protein all arise from mutations on VNTR alleles with 16 segments, resulting in 9 and 8 aberrant protein segments, respectively. The Italian case is from Pellegrini et al (26).
Thus, the mutation in the Swedish family is predicted to produce the longest aberrant CEL C-terminal sequence. Notably, the pancreatitis phenotype seems to be particularly prominent in this family compared with that of the other 3 pedigrees. As there is a correlation with the length of the aberrant CEL protein sequence and negative cellular impact (34), we speculate that the strong exocrine affection of the Swedish pedigree may be explained by having the highest number of aberrant repeats in the CEL protein tail. The exact disease mechanism of MODY8 is not known, but it is likely to involve protein misfolding/aggregation, endoplasmic reticulum stress and proteotoxicity (8, 34, 36-38). Adherence to the plasma membrane and endocytosis of the aggregated CEL protein by neighboring islet cells might also be part of the pathogenic process (39).
Screening Strategy for Pathogenic CEL VNTR Mutations
The 2 original Norwegian kindreds were both discovered among diabetes patients with suspected MODY. The Czech proband described here was also identified in a MODY cohort. In contrast, the Swedish proband was identified in a population-based cohort of patients with a diagnosis of type 2 diabetes (27). However, this sample had been selected to consist of cases that were leaner and younger than the classical type 2 diabetes patient, explaining why a MODY8 proband could be present. We recommend genetic testing for MODY8 in patients fulfilling the MODY criteria, but only when the most common MODY genes (HNF1A, HNF1B, HNF4A, GCK) have been excluded and there is some evidence of pancreatic exocrine dysfunction. Conversely, it will be worthwhile to screen for MODY8 in materials of familial chronic pancreatitis when mutations in more common pancreatitis genes such as PRSS1, SPINK1, CTRC, and CFTR (40, 41) have been ruled out, especially when there are subjects with a MODY-like diabetic phenotype in the family.
On the molecular level, sequencing of CEL exon 11 has in many laboratories proved challenging due to the high guanine/cytosine content in combination with the considerable length variation of the VNTR (9-13) and copy number variants involving the CEL locus (9, 14, 15). We recommend the sequencing method described here, starting with PCR amplification of exons 8-11 by a high-fidelity DNA polymerase that in or hands has worked well for copying the CEL VNTR. Amplification of a long PCR product is necessary to avoid inappropriate copying of the adjacent CEL pseudogene (CELP), which otherwise may seriously disturb the analysis.
As demonstrated by the discovery of the Swedish MODY8 family, deletions that affect the most proximal segment of the CEL VNTR, may also be captured by high-throughput sequencing. This could represent an effective way of identifying new MODY8 families by mining existing data sets of diabetes or pancreatitis patients. Nevertheless, the complexity of the CEL gene is such that we generally recommend genotyping methods that specifically target this locus. In this respect, it is worth noting that the Czech family had undergone whole-exome sequencing without revealing the deletion mutation in the CEL VNTR segment number 4 (28).
In summary, we provide evidence that the only definitely pathogenic mutations of CEL reported so far are single-bp deletions in the proximal VNTR segments. For other rare CEL variants to be classified as disease-causing in the context of MODY, evidence of a pancreatic exocrine phenotype needs to be present in addition to diabetes. Further support for pathogenicity must be provided by proving co-segregation of mutation and disease in the affected family and/or by functional studies of the protein product showing effects on aggregation and endoplasmic reticulum stress.
Acknowledgments
We are thankful to all the patients and family members who participated in the investigation.
Financial Support: This research was funded by a postdoctoral fellowship to K. E. J. from the University of Bergen and by grants from Western Norway Regional Health Authority (Helse Vest #912057) and Research Council of Norway (FRIMEDBIO program #289534) to A.M. The study was also supported by grants to P.R.N. from Stiftelsen Kristian Gerhard Jebsen (Translational Medical Center), Novo Nordisk Foundation (Grant #54741) and the Norwegian Diabetes Association. The analysis of Czech samples was supported by the Ministry of Health of the Czech Republic grant number NV18-01-00078.
Glossary
Abbreviations
- BMI
body mass index
- bp
base pair
- CEL
carboxyl ester lipase
- HbA1c
glycosylated hemoglobin A1c
- MODY
maturity onset diabetes of the young
- MRCP
magnetic resonance cholangiopancreatography
- MRI
magnetic resonance imaging
- PCR
polymerase chain reaction
- VNTR
variable number of tandem repeats
Author Contributions
P.R.N. and A.M., with the help of S.J., conceived and directed the study. K.E.J. wrote the manuscript with supervision of A.M. and prepared all figures. P.D., E.T., S.P., L.G., and J-M.L. recruited the patients and provided or evaluated the clinical data. I.S.H. evaluated the radiological data. K.E.J., J.M., B.B.J., K.F., and S.J. performed genetic analyses. All authors contributed to the revision of the manuscript and approved the final version.
Additional Information
Disclosures: All authors declare that there are no conflicts of interest.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Molven A, Njølstad PR. Role of molecular genetics in transforming diagnosis of diabetes mellitus. Expert Rev Mol Diagn. 2011;11(3):313-320. [DOI] [PubMed] [Google Scholar]
- 2. De Franco E. From biology to genes and back again: gene discovery for monogenic forms of beta-cell dysfunction in diabetes. J Mol Biol. 2020;432(5):1535-1550. [DOI] [PubMed] [Google Scholar]
- 3. Johansson BB, Irgens HU, Molnes J, et al. Targeted next-generation sequencing reveals MODY in up to 6.5% of antibody-negative diabetes cases listed in the Norwegian Childhood Diabetes Registry. Diabetologia. 2017;60(4):625-635. [DOI] [PubMed] [Google Scholar]
- 4. Ræder H, Johansson S, Holm PI, et al. Mutations in the CEL VNTR cause a syndrome of diabetes and pancreatic exocrine dysfunction. Nat Genet. 2006;38(1):54-62. [DOI] [PubMed] [Google Scholar]
- 5. El Jellas K, Johansson BB, Fjeld K, et al. The mucinous domain of pancreatic carboxyl-ester lipase (CEL) contains core 1/core 2 O-glycans that can be modified by ABO blood group determinants. J Biol Chem. 2018;293(50):19476-19491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ræder H, Haldorsen IS, Ersland L, et al. Pancreatic lipomatosis is a structural marker in nondiabetic children with mutations in carboxyl-ester lipase. Diabetes. 2007;56(2):444-449. [DOI] [PubMed] [Google Scholar]
- 7. Ræder H, McAllister FE, Tjora E, et al. Carboxyl-ester lipase maturity-onset diabetes of the young is associated with development of pancreatic cysts and upregulated MAPK signaling in secretin-stimulated duodenal fluid. Diabetes. 2014;63(1):259-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johansson BB, Fjeld K, El Jellas K, et al. The role of the carboxyl ester lipase (CEL) gene in pancreatic disease. Pancreatology. 2018;18(1):12-19. [DOI] [PubMed] [Google Scholar]
- 9. Dalva M, El Jellas K, Steine SJ, et al. Copy number variants and VNTR length polymorphisms of the carboxyl-ester lipase (CEL) gene as risk factors in pancreatic cancer. Pancreatology. 2017;17(1):83-88. [DOI] [PubMed] [Google Scholar]
- 10. Bengtsson-Ellmark SH, Nilsson J, Orho-Melander M, Dahlenborg K, Groop L, Bjursell G. Association between a polymorphism in the carboxyl ester lipase gene and serum cholesterol profile. Eur J Hum Genet. 2004;12(8):627-632. [DOI] [PubMed] [Google Scholar]
- 11. Torsvik J, Johansson S, Johansen A, et al. Mutations in the VNTR of the carboxyl-ester lipase gene (CEL) are a rare cause of monogenic diabetes. Hum Genet. 2010;127(1):55-64. [DOI] [PubMed] [Google Scholar]
- 12. Miyasaka K, Ohta M, Takano S, et al. Carboxylester lipase gene polymorphism as a risk of alcohol-induced pancreatitis. Pancreas. 2005;30(4):e87-e91. [DOI] [PubMed] [Google Scholar]
- 13. Fjeld K, Beer S, Johnstone M, et al. Length of variable numbers of tandem repeats in the Carboxyl Ester Lipase (CEL) gene may confer susceptibility to alcoholic liver cirrhosis but not alcoholic chronic pancreatitis. PLoS One. 2016;11(11):e0165567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fjeld K, Weiss FU, Lasher D, et al. A recombined allele of the lipase gene CEL and its pseudogene CELP confers susceptibility to chronic pancreatitis. Nat Genet. 2015;47(5): 518-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fjeld K, Masson E, Lin JH, et al. Characterization of CEL-DUP2: Complete duplication of the carboxyl ester lipase gene is unlikely to influence risk of chronic pancreatitis. Pancreatology. 2020;20(3):377-384. [DOI] [PubMed] [Google Scholar]
- 16. Hui DY, Howles PN. Carboxyl ester lipase: structure-function relationship and physiological role in lipoprotein metabolism and atherosclerosis. J Lipid Res. 2002;43(12):2017-2030. [DOI] [PubMed] [Google Scholar]
- 17. Bläckberg L, Lombardo D, Hernell O, Guy O, Olivecrona T. Bile salt-stimulated lipase in human milk and carboxyl ester hydrolase in pancreatic juice: are they identical enzymes? FEBS Lett. 1981;136(2):284-288. [DOI] [PubMed] [Google Scholar]
- 18. Johansson BB, Torsvik J, Bjørkhaug L, et al. Diabetes and pancreatic exocrine dysfunction due to mutations in the carboxyl ester lipase gene-maturity onset diabetes of the young (CEL-MODY): a protein misfolding disease. J Biol Chem. 2011;286(40):34593-34605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu MG, Keenan HA, Shah HS, et al. Residual β cell function and monogenic variants in long-duration type 1 diabetes patients. J Clin Invest. 2019;129(8):3252-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sarmadi A, Mohammadi A, Tabatabaei F, Nouri Z, Chaleshtori MH, Tabatabaiefar MA.Molecular genetic study in a cohort of Iranian families suspected to maturity-onset diabetes of the young, reveals a recurrent mutation and a high-risk variant in the CEL gene. Adv Biomed Res. 2020;9:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mohan V, Radha V, Nguyen TT, et al. Comprehensive genomic analysis identifies pathogenic variants in maturity-onset diabetes of the young (MODY) patients in South India. BMC Med Genet. 2018;19(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ovsyannikova AK, Rymar OD, Shakhtshneider EV, Voropaeva EN, Ivanoshchuk DE, Voevoda MI. MODY in Siberia – molecular genetics and clinical characteristics. Diabetes Mellitus. 2017;20:5–12. [Google Scholar]
- 23. Wang Y, Zhang J, Zhao Y, et al. COL4A3 gene variants and diabetic kidney disease in MODY. Clin J Am Soc Nephrol. 2018;13(8):1162-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cheon CK, Lee YJ, Yoo S, et al. Delineation of the genetic and clinical spectrum, including candidate genes, of monogenic diabetes: a multicenter study in South Korea. J Pediatr Endocrinol Metab. 2020;33(12):1539-1550. [DOI] [PubMed] [Google Scholar]
- 25. Todd JN, Kleinberger JW, Zhang H, et al. Monogenic diabetes in youth with presumed type 2 diabetes: results from the Progress in Diabetes Genetics in Youth (ProDiGY) Collaboration. Diabetes Care. 2021; dc210491. doi: 10.2337/dc21-0491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pellegrini S, Pipitone GB, Cospito A, et al. Generation of β Cells from iPSC of a MODY8 patient with a novel mutation in the Carboxyl Ester Lipase (CEL) gene. J Clin Endocrinol Metab. 2021;106(5):e2322-e2333. [DOI] [PubMed] [Google Scholar]
- 27. Flannick J, Thorleifsson G, Beer NL, et al. ; Go-T2D Consortium; T2D-GENES Consortium . Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nat Genet. 2014;46(4):357-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dusatkova P, Fang M, Pruhova S, et al. Lessons from whole-exome sequencing in MODYX families. Diabetes Res Clin Pract. 2014;104(3):e72-e74. [DOI] [PubMed] [Google Scholar]
- 29. El Jellas K, Dusatkova P, Haldorsen IS, et al. Online supplemental material to: Two new mutations in the CEL gene causing diabetes and hereditary pancreatitis: how to correctly identify MODY8 cases. Figshare. Dataset. Posted November 2, 2021. Available at: 10.6084/m9.figshare.16917574.v1 [DOI]
- 30. Matos C, Metens T, Devière J, et al. Pancreatic duct: morphologic and functional evaluation with dynamic MR pancreatography after secretin stimulation. Radiology. 1997;203(2):435-441. [DOI] [PubMed] [Google Scholar]
- 31. Greenbaum CJ, Mandrup-Poulsen T, McGee PF, et al. ; Type 1 Diabetes Trial Net Research Group; European C-Peptide Trial Study Group . Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care. 2008;31(10):1966-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Löhr JM, Dominguez-Munoz E, Rosendahl J, et al. ; HaPanEU/UEG Working Group . United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterol J. 2017;5(2):153-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vesterhus M, Ræder H, Kurpad AJ, et al. Pancreatic function in carboxyl-ester lipase knockout mice. Pancreatology. 2010;10(4):467–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gravdal A, Xiao X, Cnop M, et al. The position of single-base deletions in the VNTR sequence of the carboxyl ester lipase (CEL) gene determines proteotoxicity. J Biol Chem. 2021;296:100661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Garcia-Diaz M, Kunkel TA. Mechanism of a genetic glissando: structural biology of indel mutations. Trends Biochem Sci. 2006;31(4):206-214. [DOI] [PubMed] [Google Scholar]
- 36. Xiao X, Jones G, Sevilla WA, et al. A Carboxyl Ester Lipase (CEL) mutant causes chronic pancreatitis by forming intracellular aggregates that activate apoptosis. J Biol Chem. 2016;291(44):23224-23236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dalva M, Lavik IK, El Jellas K, et al. Pathogenic Carboxyl Ester Lipase (CEL) variants interact with the normal CEL protein in pancreatic cells. Cells. 2020;9(1):244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sahin-Tóth M. Genetic risk in chronic pancreatitis: the misfolding-dependent pathway. Curr Opin Gastroenterol. 2017;33(5):390-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Torsvik J, Johansson BB, Dalva M, et al. Endocytosis of secreted carboxyl ester lipase in a syndrome of diabetes and pancreatic exocrine dysfunction. J Biol Chem. 2014;289(42):29097-29111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14(2):141-145. [DOI] [PubMed] [Google Scholar]
- 41. Zator Z, Whitcomb DC. Insights into the genetic risk factors for the development of pancreatic disease. Therap Adv Gastroenterol. 2017;10(3):323-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.