Abstract
Context
Peripheral neuropathy (PN) is a frequent prediabetes and type 2 diabetes (T2D) complication. Multiple clinical studies reveal that obesity and dyslipidemia can also drive PN progression, independent of glycemia, suggesting a complex interplay of specific metabolite and/or lipid species may underlie PN.
Objective
This work aimed to identify the plasma metabolomics and lipidomics signature that underlies PN in an observational study of a sample of individuals with average class 3 obesity.
Methods
We performed plasma global metabolomics and targeted lipidomics on obese participants with (n = 44) and without PN (n = 44), matched for glycemic status, vs lean nonneuropathic controls (n = 43). We analyzed data by Wilcoxon, logistic regression, partial least squares–discriminant analysis, and group-lasso to identify differential metabolites and lipids by obesity and PN status. We also conducted subanalysis by prediabetes and T2D status.
Results
Lean vs obese comparisons, regardless of PN status, identified the most significant differences in gamma-glutamyl and branched-chain amino acid metabolism from metabolomics analysis and triacylglycerols from lipidomics. Stratification by PN status within obese individuals identified differences in polyamine, purine biosynthesis, and benzoate metabolism. Lipidomics found diacylglycerols as the most significant subpathway distinguishing obese individuals by PN status, with additional contributions from phosphatidylcholines, sphingomyelins, ceramides, and dihydroceramides. Stratifying the obese group by glycemic status did not affect discrimination by PN status.
Conclusion
Obesity may be as strong a PN driver as prediabetes or T2D in a sample of individuals with average class 3 obesity, at least by plasma metabolomics and lipidomics profile. Metabolic and complex lipid pathways can differentiate obese individuals with and without PN, independent of glycemic status.
Keywords: complex lipid, diacylglycerol, metabolomics, lipidomics, obesity, polyneuropathy
Peripheral neuropathy (PN) is a common prediabetes and the most common type 2 diabetes (T2D) complication (1). Multiple clinical studies have identified factors beyond glycemia that underlie PN onset and progression (2), specifically, components of metabolic syndrome (MetS) (3-5). Furthermore, we have shown in several population studies that MetS components are PN risk factors, independent of glycemic status (6-8). MetS encompasses a collection of conditions, which include obesity, dyslipidemia, insulin resistance, and hypertension, which frequently occur together. The criteria defining individuals with MetS are 3 out of 5 from the following: elevated waist circumference (WC; ≥ 102 cm men, ≥ 88 cm women), systolic (≥ 130 mm Hg) or diastolic blood pressure (≥ 85 mm Hg), triacylglycerols (TAGs, ie, triglycerides; ≥ 150 mg/dL), and fasting glucose (> 100 mg/dL) and lower high-density lipoprotein cholesterol (HDL-c; < 40 mg/dL men, < 50 mg/dL women) (9).
In our most recent clinical study, we identified WC, defined by National Cholesterol Education Program (NCEP) criteria, as the primary anthropometric PN driver, even in normoglycemic obese individuals (10, 11). This finding suggests that central obesity itself is a sufficient condition for PN development. This observation was replicated in our preclinical study of diet-induced obesity in mice, which also drives PN development independent of T2D status (12). In this integrated lipidomic-transcriptomic study, we found that high-fat diet dysregulated the nerve lipidome during PN progression. The sciatic nerve from obese prediabetic mice with PN exhibited a distinct lipid profile compared to lean mice without PN, which centered on differential TAG species and expression of diacylglycerol acyltransferase 2, the enzyme catalyzing the final and committed step in TAG synthesis. While the study suggested that lipidome dysregulation is a critical PN feature in obese murine models, whether these results translate to humans is unknown.
To overcome this gap (13), we undertook an observational study to characterize the metabolomic and lipidomic plasma profiles in a sample of individuals with average class 3 obesity with (n = 44; obese_PN) and without PN (n = 44; obese_No_PN), matched for glycemic status, vs lean controls without PN (n = 43). In this clinical cohort, metabolomic profiles between obese vs lean individuals correlated most strongly with gamma-glutamyl and branched-chain amino acid (BCAA) metabolism and within obese participants by PN status by alterations in polyamine, purine biosynthesis, and benzoate metabolism (a xenobiotics subpathway). Lipidomic profiles identified TAGs as strongly correlating with obesity compared to lean individuals, regardless of PN status. However, significant differences were present in diacylglycerols (DAGs) and in several complex lipid subpathways in obese individuals without PN compared to their obese counterparts with PN, supporting a role for dysregulation of specific lipid species in PN development. Stratifying the obese group by glycemic status did not affect discrimination by PN status. These results suggest obesity may be as strong a PN driver as prediabetes or T2D in individuals with class 3 obesity, at least by plasma metabolomics and lipidomics profile.
Materials and Methods
Study Participants and Diagnoses
Participants were recruited as part of two separate clinical trials from the University of Michigan Bariatric Surgery Clinic (1) and Investigational Weight Management Clinic (IWMC) (2), respectively. In parallel, lean controls were recruited for each trial. Controls did not have any MetS components, assessed by clinical testing, by the NCEP/Adult Treatment Panel III criteria. NCEP/Adult Treatment Panel III definitions for MetS included WC (> 102 cm men, > 88 cm women), systolic (> 130 mm Hg) or diastolic blood pressure (> 85 mm Hg), TAGs (> 150 mg/dL), HDL-c (< 40 mg/dL men, < 50 mg/dL women), and fasting glucose (> 100 mg/dL) (3). Baseline plasma samples were obtained from all participants. The present baseline observational study combines the demographic data from both studies and includes obese participants without PN (n = 44) and with PN (n = 44) and lean controls without MetS (n = 43) (Table 1).
Table 1.
Participant demographics at time of plasma collection for global metabolomics and lipidomics analysis
| Clinical parameter | Lean (n = 43) | Obese_PN (n = 44) | Obese_No_PN (n = 44) | P Obese_PN vs Lean | P Obese_No_PN vs Lean | P Obese_PN vs Obese_No_PN |
|---|---|---|---|---|---|---|
| Age, mean (SD), y | 43.93 ± 12.28 | 53.16 ± 8.68 | 52.75 ± 8.42 | < .001 | < .001 | .98 |
| Sex | ||||||
| Female | 35 (81.40%) | 24 (54.55%) | 24 (54.55%) | .011 | .011 | ≥ .99 |
| Male | 8 (18.60%) | 20 (45.45%) | 20 (45.45%) | .011 | .011 | ≥ .99 |
| BMI, mean (SD) | 22.89 ± 2.06 | 45.03 ± 6.84 | 43.03 ± 6.22 | < .001 | < .001 | .21 |
| Body weight, mean (SD), kg | 64.43 ± 10.01 | 137.32 ± 29.94 | 123.88 ± 21.91 | < .001 | < .001 | .015 |
| WC, mean (SD), cm | 80.64 ± 7.12 | 136.47 ± 16.92 | 124.49 ± 16.11 | < .001 | < .001 | < .001 |
| Blood pressure, mean (SD), mm Hg | ||||||
| Systolic | 108.40 ± 10.50 | 135.00 ± 15.39 | 130.50 ± 11.12 | .001 | < .001 | .22 |
| Diastolic | 66.30 ± 9.73 | 70.02 ± 11.17 | 71.36 ± 10.67 | .23 | .069 | .82 |
| Cholesterol, mean (SD), mmol/l | 181.81 ± 41.06 | 157.25 ± 41.60 | 162.27 ± 36.91 | .013 | .062 | .83 |
| TAGs, mean (SD), mmol/L | 72.42 ± 22.34 | 161.09 ± 113.90 | 151.25 ± 94.48 | < .001 | < .001 | .86 |
| HDL-c, mean (SD), mmol/l | 67.19 ± 16.23 | 43.34± 12.36 | 41.57 ± 9.99 | < .001 | < .001 | .80 |
| LDL-c, mean (SD), mmol/L | 102.77 ± 28.83 | 85.16 ± 34.91 | 99.26 ± 44.57 | .069 | .90 | .18 |
| FBG, mean (SD), mg/dL | 85.07 ± 6.46 | 128.61 ± 37.80 | 131.71 ± 66.70 | <0.001 | < .001 | .95 |
| Type 2 diabetes (yes/no) | 0/43 | 29/15 | 25/19 | < .001 | < .001 | .051 |
| Prediabetes (yes/no) | 0/43 | 7/37 | 13/31 | .012 | < .001 | .20 |
| Statin use (yes/no) | 0/43 | 22/22 | 21/23 | < .001 | < .001 | ≥ .99 |
| β-blocker use (yes/no) | 0/43 | 18/26 | 17/27 | < .001 | < .001 | ≥ .99 |
Age, BMI, body weight, WC, blood pressure (systolic, diastolic), cholesterol, TAGs, HDL-c, and LDL-c were analyzed by one-way analysis of variance with post hoc analysis with Tukey test; sex (female, male), diabetes status, prediabetes status, statin use, and β-blocker use were analyzed by Fisher test.
Abbreviations: BMI, body mass index; FBG, fasting blood glucose; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; obese_PN, obese with peripheral neuropathy; obese_No_PN, obese without peripheral neuropathy; TAGs, triacylglycerols; WC, waist circumference.
The aim of this observational study was to identify differential plasma metabolites and lipids by PN status in obesity, independent of glycemic status. Lean controls were normoglycemic, as determined by clinical testing, and did not meet any criteria for prediabetes or T2D. Lean controls had fasting blood glucose (FBG) of less than 100 mg/dL, 2-hour glucose of less than 140 mg/dL following a 75-g oral glucose tolerance test (OGTT) or a glycated hemoglobin (HbA1c) of less than 5.7% (39 mmol/mol). The obese participants with and without PN were matched for glycemic parameters. Prediabetes was defined based on an FBG (100-≤ 125 mg/dL) or a 2-hour glucose of 140 to 199 mg/dL following an OGTT or HbA1c of 5.7% (39 mmol/mol) to less than or equal to 6.4% (46 mmol/mol). T2D was defined as an FBG greater than 126 mg/dL or a 2-hour glucose greater than or equal to 200 mg/dL after an OGTT, according to the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (14). T2D was also determined based on a known diabetes diagnosis and/or medications or an HbA1c greater than or equal to 6.5% (48 mmol/mol). All participants without a known diagnosis of T2D had an OGTT, and HbA1c status was collected from available medical records if performed within 6 months of the study visit. Participants underwent PN diagnosis according to the Toronto consensus definition of probable PN, which requires 2 or more of the following: PN symptoms, abnormal sensory examination, and abnormal reflexes, as determined by 1 of 4 neuromuscular specialists (15). All participants gave their written informed consent for these studies, which were approved by the University of Michigan Institutional Review Board (HUM00092638 for the bariatric surgery clinic, HUM00039723 for the IWMC study).
Study Design
For each participant, we collected demographics (age, sex), anthropometric measures (body weight, height, body mass index [BMI], WC), vitals (systolic and diastolic blood pressure), and a fasting lipid profile (TAGs, total cholesterol, HDL-c, and low-density lipoprotein cholesterol [LDL-c]). For the primary study outcome, participants provided a plasma sample for metabolomics and lipidomics analysis before weight-loss intervention. Participants were asked to fast for 12 hours overnight and abstain from alcohol, smoking, medication use, and caffeine, and avoid vigorous exercise out of their normal routine. Participants with T2D were asked to monitor their blood sugar and adjust medication if needed. Blood was drawn from fasted participants using good clinical practice into EDTA tubes, which were temporarily kept at 4 °C for a maximum 2 hours’ duration. Tubes were then centrifuged (2000g, 10 min, 4 °C) and the plasma supernatants were saved in cryovials, which were directly transferred for storage at –80 °C.
Plasma Untargeted Metabolomics and Targeted Lipidomics Analysis
For metabolomics and lipidomics, plasma samples were shipped on dry ice to Metabolon, where they were stored at –80 °C. Untargeted metabolomics is a system-wide technique that agnostically and systematically detects and identifies metabolites from a biosample. Owing to the technical methodology, metabolomics generally captures polar, water-soluble metabolites, including more polar lipids. Thus, metabolomics analyses also contain certain lipid classes. Lipidomics is a technique that detects and identifies nonpolar as well as polar species, with an emphasis on lipid species. Targeted lipidomics specifically detects a predetermined lipid species panel.
Global untargeted metabolomics analysis was conducted by ultrahigh performance liquid chromatography–tandem mass spectroscopy (UPLC-MS/MS), using published Metabolon protocols (16, 17). Briefly, recovery and internal standards were added to plasma samples for evaluating extraction efficiency and instrument performance, respectively. Metabolites were extracted with methanol and analyzed by reverse-phase UPLC-MS/MS (positive and negative ion modes) and hydrophilic interaction chromatography UPLC-MS/MS. Metabolites were identified by automated ion peak comparison from each sample to a reference library of authenticated chemical standards with specific mass-to-charge ratios and retention times, followed by data curation. Each metabolite within a sample was quantified by its area under the curve and normalized to account for day-to-day variation by equating the metabolite median across all samples that day to 1 and normalizing the metabolite within each sample proportionately against the median.
The targeted Complex Lipid Panel was performed by differential mobility spectroscopy by Sciex SelexION at Metabolon. Differential mobility spectroscopy separates species beyond differences in mass-to-charge ratios and retention time, such as, additionally, by size and shape, facilitating lipid identification, even of highly similar species. Briefly, lipids were extracted from plasma in the presence of internal standards by butanol-methanol extraction (18), dried under nitrogen, and reconstituted in a dichloromethane:methanol solution containing ammonium acetate. Samples were analyzed via both positive and negative mode electrospray MS. Each lipid species concentration was quantified by a ratio of its sample signal intensity to an assigned internal standard, multiplied by the internal standard concentration in that sample. Each lipid class concentration was calculated by summing all lipids belonging to that class. Each fatty acid composition was calculated through the proportion of each class composed of individual fatty acids.
Metabolite Data Sets and Imputation Method
In sum, we detected 842 named metabolites from the metabolomics analysis and 983 named lipid species from the lipidomics analysis, both from Metabolon’s curated databases (19). We excluded from further analysis any metabolites not present in at least 80% of samples (ie, overall missingness > 20%), yielding 604 metabolites and 858 lipids (19). Missing values for metabolites retained in our analysis were imputed to the minimum observed value for each metabolite, per Metabolon protocols (16, 17).
Statistical Analysis
Descriptive analysis
Descriptive summaries of demographic and clinical characteristics were calculated for the following 4 groups: obese vs lean, obese with PN vs lean, obese without PN vs lean, and obese with vs without PN. Fisher exact tests and one-way analysis of variance with post hoc Tukey tests were used to determine the pairwise differences between groups (obese vs lean, obese with PN vs lean, obese without PN vs lean, and obese with vs without PN).
Identification of differential metabolites and lipids
Multiple approaches were employed to identify metabolites and lipids that statistically significantly differed between the groups (obese vs lean, obese with PN vs lean, obese without PN vs lean, and obese with vs without PN). Wilcoxon rank sum tests, referred to as unadjusted, were used to identify significant unadjusted differences in abundance for each metabolite and lipid between groups. Multivariable logistic regression models, referred to as adjusted, were created to determine the association between groups and each natural log-transformed and standardized metabolite and lipid, after adjusting for age and sex. Separate logistic regression models were created for each metabolite/lipid and for each comparison (obese vs lean, obese with PN vs lean, obese without PN vs lean, and obese with vs without PN). For both unadjusted (Wilcoxon rank sum tests) and adjusted (logistic regression) approaches, statistically significantly different metabolites and lipids were defined as those with a corresponding Benjamini-Hochberg–corrected P value of less than .05. The resulting adjusted and unadjusted P values were visualized using volcano plots and Manhattan plots, respectively.
Partial least squares-discriminant analysis (PLS-DA) was also performed on metabolites and lipids separately by using the R package mixOmics (20, 21). This dimensionality reduction tool identifies metabolite/lipid patterns that statistically significantly contributed to group separation as determined using a variable importance in projection (VIP) score greater than 1 as the cutoff.
We also performed group-lasso on log-transformed and standardized metabolites and lipids separately using the R package gglasso (22). Specifically, we used a 5-fold cross-validation to optimize the tuning parameter corresponding to a sparse model that was within 1 SE of the minimum cross-validation error. We then refit the group-lasso model to adjust for age and sex, and therefore generate the final model. Group-lasso results are represented by heatmaps with significant metabolites/lipids having βvalues greater than 0 or Manhattan plots with odds ratios (OR) greater than 1.
Prediabetes and type 2 diabetes status analysis
Despite matching, we reanalyzed the data stratified by glycemic status to determine whether there was any effect from plasma metabolomic and lipidomic profiles in prediabetes or T2D compared to the overall obesity profiles on PN separation. Specifically, we employed Wilcoxon rank sum tests and evaluated Benjamini-Hochberg–adjusted P values to identify differential metabolites/lipids between obese participants with and without T2D and with and without prediabetes. In addition, we used principal component analysis (PCA) to visualize groups by glycemic status in the entire cohort.
Metabolism and complex lipid pathway analysis
Pathway enrichment analysis was conducted by our in-house R package richR (https://github.com/hurlab/richR/). Superpathway and subpathway annotations were from Metabolon and were employed as background pathways. PLS-DA– and group-lasso–selected statistically significant metabolites were assessed for overrepresentation within each subpathway. A hypergeometric test was conducted for each candidate subpathway, which was considered statistically significant for P less than .05.
Two-way orthogonal partial least squares (O2PLS)
Two-way orthogonal partial least squares (O2PLS) was employed to integrate metabolomics and lipidomics to identify highly interassociated metabolites and lipids of biological significance (23), using an R package OmicsPLS (24). The network was built from 604 metabolites with established links to 858 lipids. Metabolomics and lipidomics data were scaled and transformed, according to published methods (25). The loading values for the joint covariance were extracted to identify highly correlated metabolites and lipids. The final metabolite-lipid correlation network was generated using the top 100 correlations between the 50 metabolites and 50 lipids with the highest loading values.
Spearman correlation analysis
Spearman rank correlation was calculated to determine correlation in metabolites and lipids in the obese participants with vs without PN. Heat maps were used to display the significant correlations (adjusted P < .05) for positive (strongest with the value of 1) and negative (strongest with the value of –1) correlations.
Statistical software
All statistical and prediction analyses were completed using the R statistical computing software version 4.0.2.
Results
Clinical Characteristics of the Obese Groups Compared to the Lean Group
In this observational study (see Table 1), age and sex differed in both obese groups vs lean, but did not differ between obese participants with vs without PN. All lean individuals were normoglycemic. Since obese participants were matched based on their glycemic status, the obese with vs without PN groups did not differ in the prevalence of prediabetes and T2D. Both obese groups had higher anthropometric measures (body weight, BMI, WC), systolic blood pressure (all P < .001), and TAGs (P < .001) and lower HDL-c (P < .001) vs lean controls. The mean BMIs of the obese group with PN and obese group without PN were 45.03 ± 6.84 and 43.03 ± 6.22, respectively, making these obese groups, on average, groups with class 3 obesity. There was a significantly lower total cholesterol level in obese participants with PN vs lean controls (P = .013) and a trending lower cholesterol level in obese participants without PN vs lean controls (P = .069). This, presumably, is because most obese individuals were on statins for hyperlipidemia (cholesterol level by statin use, “yes” vs “no,” P =9.4 × 10–6 by Wilcoxon in the obese vs lean comparison, P =.00051 by Wilcoxon in the obese with vs without PN comparison). Despite statin management, obese individuals had significantly higher plasma TAGs vs controls, which may be partly due to prevalent β-blocker use (26). While statins affect the lipidomic profile in obese participants, it does not affect PN development (27). WC was higher in obese participants with vs without PN, as anticipated (10, 11), as was body weight. Otherwise, importantly, obese groups with and without PN did not differ significantly in any other metabolic metric, including BMI, total cholesterol, TAGs, HDL-c, LDL-c, prediabetes status, and T2D status, underscoring our hypothesis that specific metabolite and/or lipid species may underlie PN rather than global dyslipidemia and/or glycemia alone.
Plasma Metabolomics Differs in Obese Groups vs Lean Group
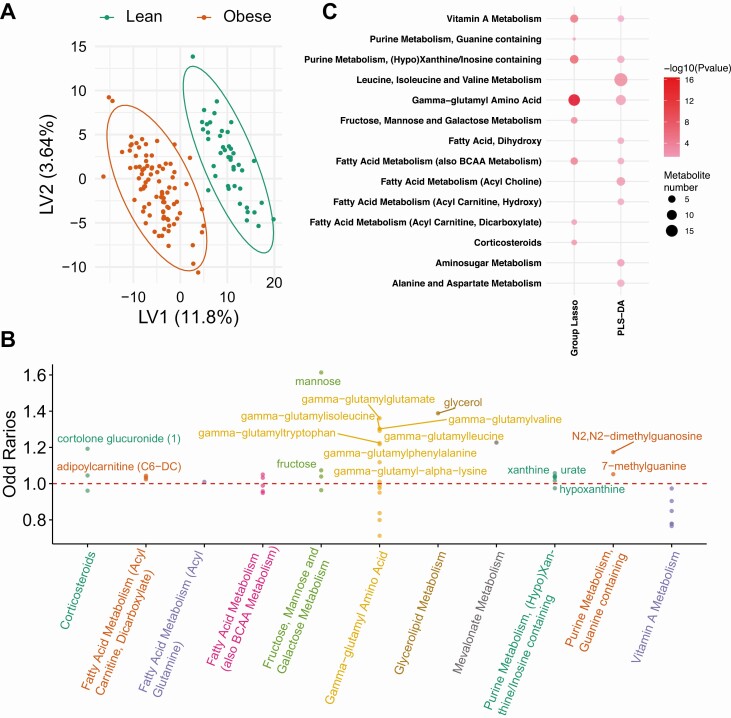
We first examined metabolite differences between the obese group as a whole (obese groups both with and without PN combined) vs the lean controls. Descriptive analyses are listed for Wilcoxon (19) and logistic regression (19). PLS-DA clearly separated obese from lean participants with 205 metabolites satisfying VIP greater than 1 (Fig. 1A) (19), whereas group-lasso identified 47 significant metabolites (Fig. 1B) (19). PLS-DA and group-lasso overlapped in several subpathways, including the most significant “gamma-glutamyl amino acid,” but also uniquely selected others, including several fatty acid metabolism subpathways (Fig. 1C).
Figure 1.
Metabolomics analysis in obese vs lean participants. A, Partial least squares–discriminant analysis (PLS-DA) fully separated obese (red, n = 88) from lean (green, n = 43) participants, and selected 205 significant metabolites that contributed to the separation with variable importance in projection (VIP) greater than 1. B, Group-lasso selected 11 subpathways containing 47 significant metabolites with an odds ratio (OR) greater than 1 (metabolite higher in obese) or OR less than 1 (metabolite lower in obese), adjusted for age and sex. C, Pathway analysis of PLS-DA– and group-lasso–selected metabolites. The circles represent selected enriched subpathways. Circle color indicates significance level from most (red) to least (lightest pink) significant. Circle size represents the number of selected metabolites belonging to the enriched subpathways. The subpathways also encompass several lipid pathways because the global metabolomics analysis detects some lipids.
Plasma Lipidomics Differs in Obese Groups vs Lean Group
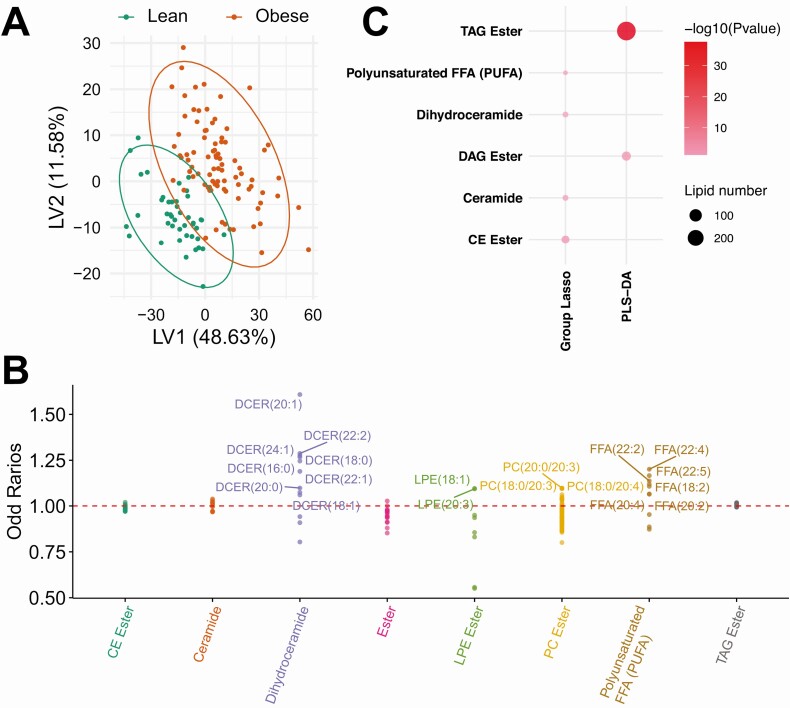
We observed more differences in lipid species than in general metabolites between the obese group as a whole (both groups) vs lean group; descriptive analyses are listed for Wilcoxon (19) and logistic regression (19). Interestingly, although PLS-DA fully resolved obese from lean participants by metabolites (Fig. 1A), the separation was less pronounced by lipids, although many more fulfilled VIP greater than 1 (Fig. 2A (19);). Moreover, group-lasso selected 668 statistically significant lipids (Fig. 2B) (19). Among lipid subpathways, PLS-DA and group-lasso were fully discordant (Fig. 2C); PLS-DA selected “TAG ester” (highest significance) and “DAG ester,” whereas group-lasso selected complex lipids, such as “dihydroceramide” and “ceramide.”
Figure 2.
Lipidomics analysis in obese vs lean participants. A, Partial least squares–discriminant analysis (PLS-DA) partly separated obese (red, n = 88) from lean (green, n = 43) participants, and selected 388 significant lipids that contributed to the separation with variable importance in projection (VIP) greater than 1. (B) Group-lasso selected 8 subpathways containing 668 significant lipids with an odds ratio (OR) greater than 1 (lipid higher in obese) or OR less than 1 (lipid lower in obese), adjusted for age and sex. PLS-DA and group-lasso both selected more lipids than metabolites (Fig. 1A and 1B) in obese vs lean comparisons. C, Pathway analysis of PLS-DA– and group-lasso–selected lipids. The circles represent selected enriched subpathways. Circle color indicates significance level from most (red) to least (lightest pink) significant. Circle size represents the number of selected lipids belonging to the enriched subpathways.
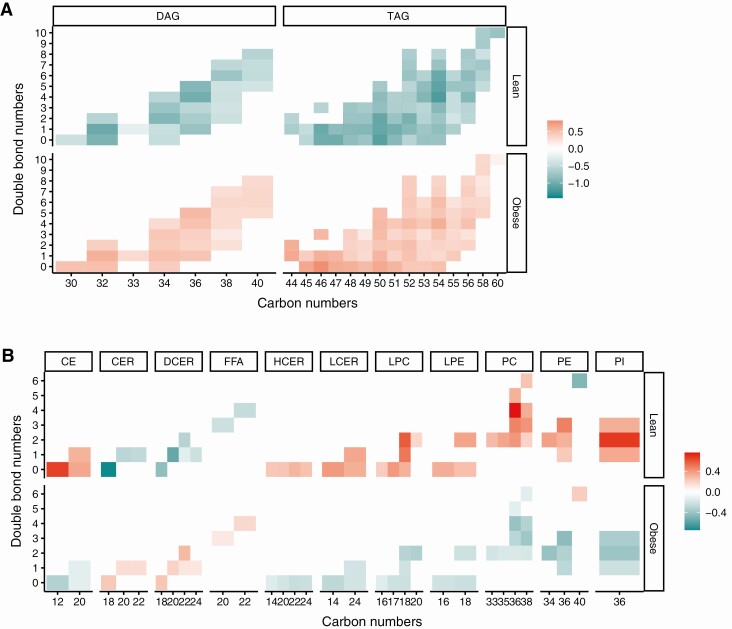
We next analyzed lipid species by chain length and saturation by generating heat maps of log2-transformed levels of lipids selected by PLS-DA (Fig. 3). TAGs and DAGs of all chain lengths and saturation were elevated in obese vs lean groups (see Fig. 3A). When we examined complex lipids, we found that the obese group was characterized by higher levels of free fatty acids, dihydroceramides, and ceramides of all chain lengths and saturation, and lower levels of cholesterol esters, hexosylceramides, lactosylceramides, lysophosphatidylcholines, lysophosphatidylethanolamines, phosphatidylcholines, phosphatidylethanolamines, and phosphatidylinositols (see Fig. 3B).
Figure 3.
Lipid abundance heat maps by chain lengths and saturation in obese vs lean participants. Heat maps of log2-transformed abundances of lipids selected by partial least squares–discriminant analysis (PLS-DA) for A, triacylglycerols (TAGs) and diacylglycerols (DAGs); and B, cholesterol esters (CE), ceramides (CER), dihydroceramides (DCER), free fatty acids (FFA), hexosylceramides (HCER), lactosylceramides (LCER), lysophosphatidylcholines (LPC), lysophosphatidylethanolamines (LPE), phosphatidylcholines (PC), phosphatidylethanolamines (PE), and phosphatidylinositols (PI).
Plasma Metabolomics Differ in Obese Groups With and Without Peripheral Neuropathy vs Lean Controls
We next stratified the obese group by PN status and examined how obese participants with and without PN differed in plasma metabolomic profile compared to lean controls. Descriptive analyses for obese with PN vs lean and obese without PN vs lean were conducted by Wilcoxon and logistic regression (19). As expected in both instances, there was a good separation of obese with PN vs lean and obese without PN vs lean by PLS-DA (19); however, the 2 obese groups did not differ from lean participants in all the same metabolites (19). Group-lasso selected 45 and 46 statistically significant metabolites in obese with PN vs lean and obese without PN vs lean comparisons, respectively (19). In subpathways, obese with PN and obese without PN both differed from lean controls most significantly in “gamma-glutamyl amino acid,” as expected (19), and were discordant in only a few subpathways.
Plasma Lipidomics Differ in Obese Groups With and Without Peripheral Neuropathy vs Lean Controls
Descriptive analyses by Wilcoxon and logistic regression were conducted to identify lipid differences for obese with PN vs lean and obese without PN vs lean (19). When we compared obese groups by PN status to lean controls, we again did not see a complete group separation by lipids by PLS-DA but when compared to the number of metabolites, more lipids had VIP greater than 1 (19). Lipid species differing between obese with PN vs lean were highly shared with obese without PN vs lean. Group-lasso selected 670 and 165 statistically significant lipids in obese with PN vs lean and obese without PN vs lean comparisons, respectively (19). In obese with PN vs lean, “TAG ester” by PLS-DA was most statistically significant and encompassed the most lipid species; in obese without PN vs lean, “DAG ester” (group-lasso) was most statistically significant while “TAG ester” (PLS-DA) comprised the most lipids (19).
Plasma Metabolomic and Lipidomic Profiles Separate Obese Groups With and Without Peripheral Neuropathy
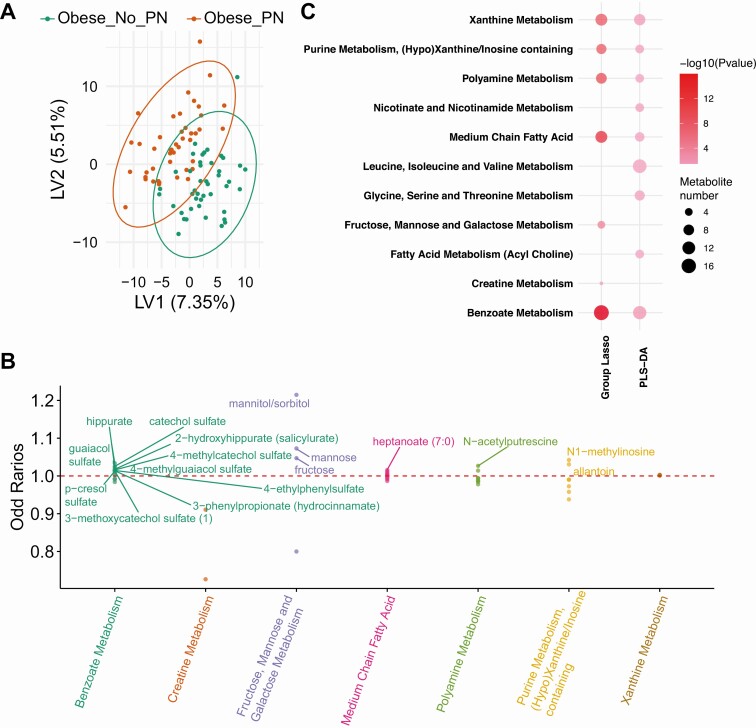
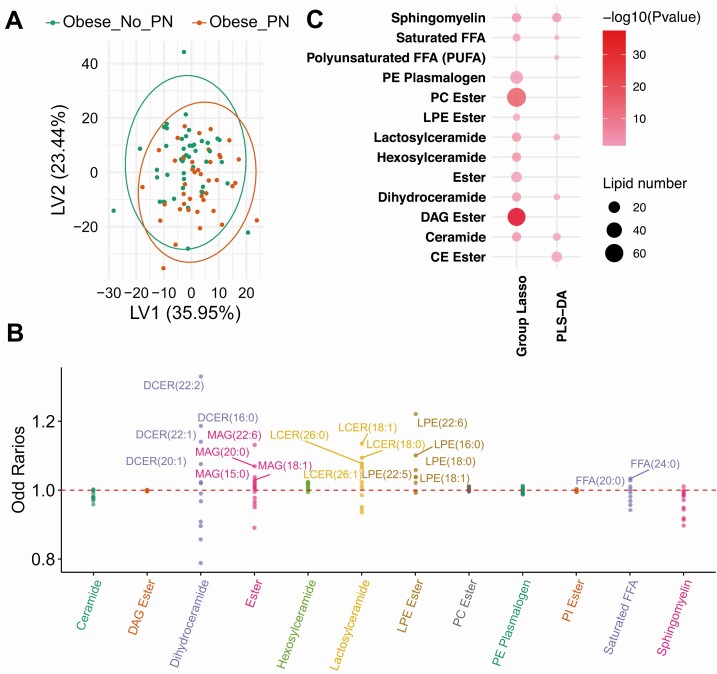
We next examined the metabolomic and lipidomic profiles of obese individuals with vs without PN (descriptive statistics in [19]). PLS-DA separated the 2 groups based on metabolites and lipids (Figs. 4A-5A) (19), but not to the same extent as obese vs lean individuals (Figs. 1A-2A), suggesting fewer differences in plasma metabolome and lipidome by PN status as opposed to obesity status. PLS-DA (VIP > 1) (19) and group-lasso (OR > 1 or OR < 1) selected 218 and 58 metabolites, respectively, and 256 and 249 lipids, respectively (Figs. 4B-5B) (19). By subpathway, “benzoate metabolism” contained the largest metabolite numbers and greatest statistical significance by PLS-DA and group-lasso overlap (Fig. 4C), but other subpathways were also mutually selected. The lipid subpathways, most significantly different by PN status, were primarily selected by group-lasso and comprised “DAG ester” and “PC (phosphatidylcholine) ester” (Fig. 5C). “Sphingomyelin” and “ceramide” subpathways differentiated PN status by both PLS-DA and group-lasso. Overall, complex lipid subpathways were most discriminating for obese participants with vs without PN.
Figure 4.
Metabolomics analysis in obese_PN vs obese_No_PN participants. A, Partial least squares–discriminant analysis (PLS-DA) partly separated obese_PN (red, n = 44) from obese_No_PN (green, n = 44) participants, and selected 218 significant metabolites that contributed to the separation with variable importance in projection (VIP) greater than 1. B, Group-lasso selected 7 subpathways containing 58 significant metabolites with an OR greater than 1 (metabolite higher in obese) or OR less than 1 (metabolite lower in obese), adjusted for age and sex. C, Pathway analysis of PLS-DA– and group-lasso–selected metabolites. The circles represent selected enriched subpathways. Circle color indicates significance level from most (red) to least (lightest pink) significant. Circle size represents the number of selected metabolites belonging to the enriched subpathways. The subpathways also encompass several lipid pathways because the global metabolomics analysis detects some lipids.
Figure 5.
Lipidomics analysis in obese_PN vs obese_No_PN participants. A, Partial least squares–discriminant analysis (PLS-DA) did not separate obese_PN (red, n = 44) from obese_No_PN (green, n = 44) participants, but selected 256 significant lipids that differed between groups with variable importance in projection (VIP) greater than 1. B, Group-lasso selected 12 subpathways containing 249 significant lipids with an odds ratio (OR) greater than 1 (lipid higher in obese) or OR less than 1 (lipid lower in obese), adjusted for age and sex. C, Pathway analysis of PLS-DA– and group-lasso–selected lipids. The circles represent selected enriched subpathways. Circle color indicates significance level from most (red) to least (lightest pink) significant. Circle size represents the number of selected lipids belonging to the enriched subpathways.
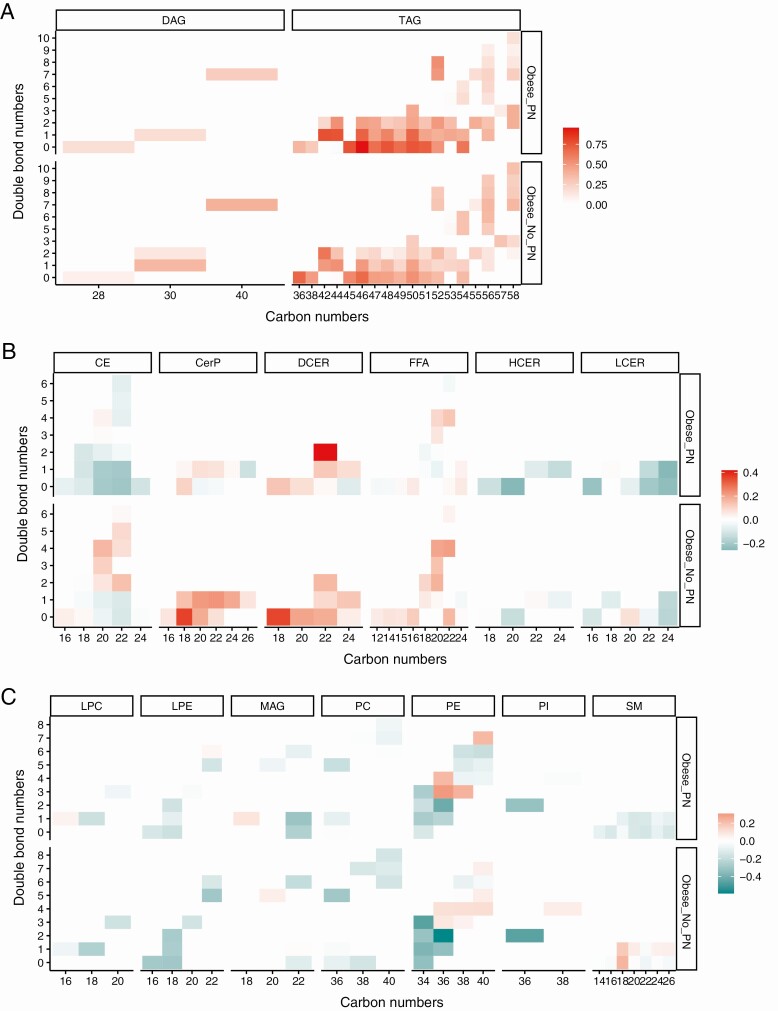
We also examined lipid species by chain length and saturation using heat maps of abundances of lipids selected by PLS-DA (Fig. 6). Saturated TAGs (no double bonds) and highly polyunsaturated and longer-chain TAGs tended to be higher in obese participants with PN vs without PN (see Fig. 6A). Among the complex lipids, obese participants with PN had lower ceramide and sphingomyelin levels across the whole spectrum of chain lengths and saturation vs obese participants without PN (see Fig. 6B). Trends in dihydroceramides differed across the 2 obese groups; dihydroceramides of lower carbon and double bond numbers were elevated in obese participants without PN, whereas dihydroceramides with more double bonds were elevated in obese participants with PN.
Figure 6.
Lipid abundance heat maps by chain lengths and saturation in obese_PN vs obese_No_PN participants. Heat maps of log2-transformed abundances of lipids selected by partial least squares–discriminant analysis (PLS-DA) for A, triacylglycerols (TAGs) and diacylglycerols (DAGs); B, cholesterol esters (CE), ceramides (CER), dihydroceramides (DCER), free fatty acids (FFA), hexosylceramides (HCER), lactosylceramides (LCER); and C, lysophosphatidylcholines (LPC), lysophosphatidylethanolamines (LPE), monoacylglycerols (MAG), phosphatidylcholines (PC), phosphatidylethanolamines (PE), phosphatidylinositols (PI), and sphingomyelins (SM).
Effect of Prediabetes and Type 2 Diabetes Status on Peripheral Neuropathy Status
There were no differences in prediabetes or T2D prevalence in the obese group with vs the obese group without PN because we matched participants for glycemic status (see Table 1). However, there were also no differences in basic lipid profiles (cholesterol, TAGs, HDL-c, LDL-c) between either obese group by PN status, although there were overall distinctions in total complex lipid profiles. Therefore, despite matching for prediabetes and T2D in the 2 obese groups, we reanalyzed the data stratified by glycemic status to evaluate if there was an influence from plasma metabolomic and lipidomic profile in the prediabetes and T2D setting compared to the overall obesity profiles on PN separation. First, we examined differential metabolite/lipid abundance by Wilcoxon within the obese groups, using a cutoff of adjusted P less than .05. By T2D status, 12 metabolites differed in obese participants with vs without T2D, of which 10 were shared with the 331 metabolites that differed in the obese vs lean comparison, and 2 were unique (annotated as “shared” and “unique” in [19]). Glucose was among the shared metabolites and was elevated in obese vs lean participants and in obese with vs without T2D, as anticipated. There were no differential metabolites by prediabetes status, and no differential lipids by either prediabetes or T2D status. Therefore, within the obese group, few metabolites differed in abundance by T2D status, but many differed from lean participants. When we examined by PN status, within either the obese groups with vs without PN, there were no differential metabolites or lipids by either prediabetes or T2D status. Overall, prediabetes and T2D status only marginally affected metabolite and lipid abundance by Wilcoxon.
Next, we examined group clustering by unsupervised PCA of the entire (obese and lean) cohort, stratifying obese participants, both with and without PN, by prediabetes and T2D status. We found that PCA of metabolites or lipids clustered obese participants with or without T2D together but still separated the obese participants (either with/without T2D) from lean participants (19). Moreover, there was no separation of participants with vs without T2D by PN status. Thus, in this highly obese sample of individuals (average class 3 obesity, average BMI 44.04) composed of normoglycemic, prediabetic, and diabetic participants, metabolic and lipidomic profiles by obesity status can separate participants from lean controls, independent of the glycemic state. As expected, we also determined that PCA of metabolites or lipids did not separate obese participants with or without prediabetes (19). As with the T2D analysis, there was no separation of participants with vs without prediabetes by PN status. Overall, at least by plasma metabolomic and lipidomic profiles, obesity and T2D differentiate PN status to similar extents in a sample of individuals with average class 3 obesity.
Plasma Metabolomic and Lipidomic Correlation Network
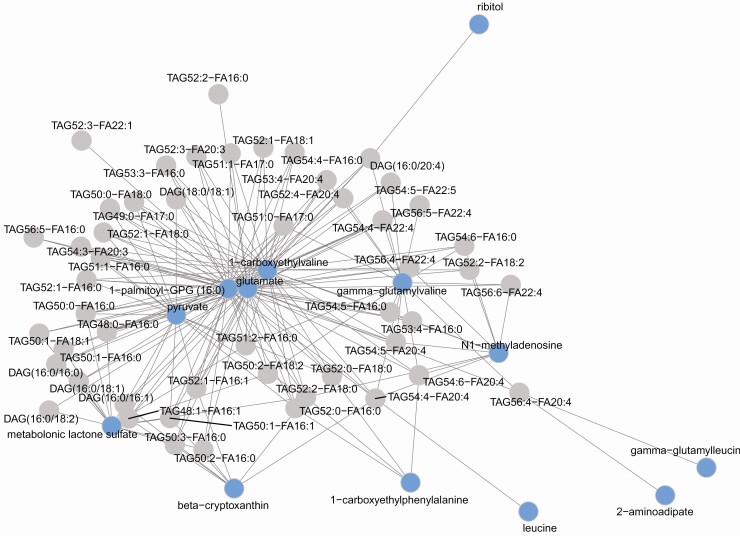
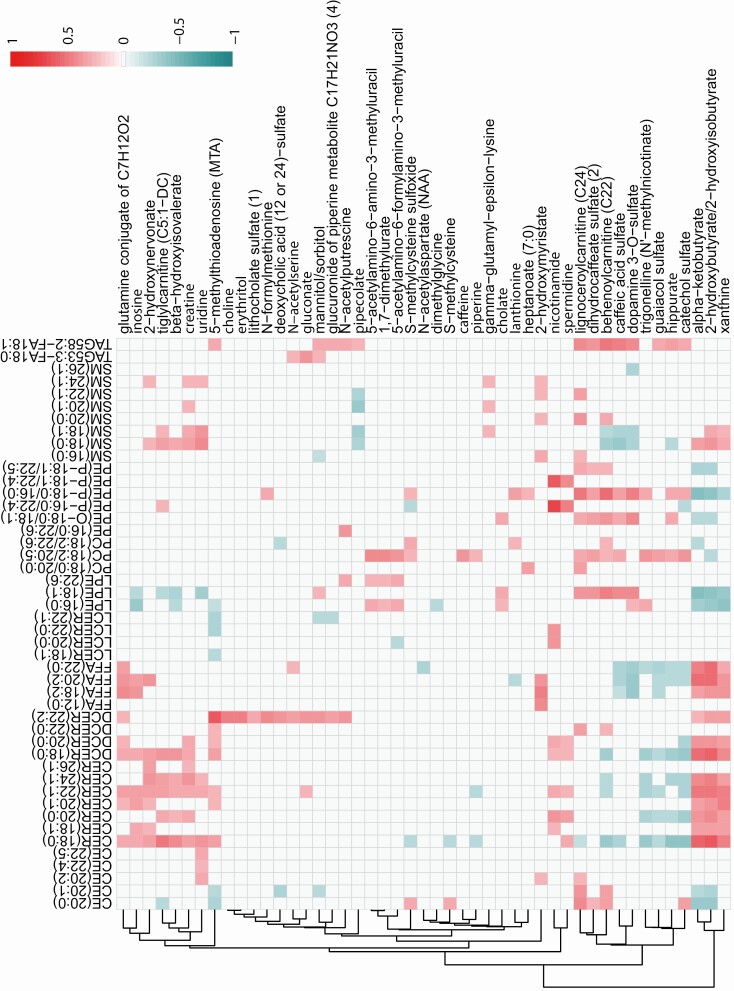
O2PLS integrated metabolites with lipids to construct a metabolite-lipid correlation across the obese vs lean comparison (Fig. 7) (19). The metabolite-lipid correlation network was constructed from correlations between the 50 metabolites and 50 lipids with the highest loading values. These 50 metabolites and 50 lipids generated 2500 statistically significant correlations (adjusted P < .05), of which the top 100 correlations are represented in the correlation network, which contains 13 metabolites and 50 lipids (Fig. 7). Candidates from the most statistically significant metabolite subpathways “gamma-glutamyl amino acid” (ie, gamma-glutamyl valine, gamma-glutamyl leucine) and “leucine, isoleucine and valine metabolism” (ie, leucine, 1-carboxyethylvaline) correlated to lipids from the most statistically significant subpathways lipid pathway “TAG ester.” Spearman correlation was performed between metabolites and lipids in the obese group with vs the obese group without PN comparisons (Fig. 8). A cluster of positive correlations occurred between ceramides, dihydroceramides, and sphingomyelins with 2-hydroxynervonate, β-hydroxyisovalerate, 2-hydroxymyristate, α-ketobutyrate, and 2-hydroxybutyrate/2-hydroxyisobutyrate, which negatively correlated with other complex lipids.
Figure 7.
Integrated metabolomics and lipidomics in obese vs lean groups. An integrative method, 2-way orthogonal partial least squares (O2PLS), was used to construct a metabolite-lipid correlation network using correlations between the 50 lipids and 50 metabolites with the highest loading values. These 50 metabolites and 50 lipids generated 2500 statistically significant correlations (adjusted P < .05), of which the top 100 correlations are represented in the correlation network, which contains 13 metabolites (blue circles) and 50 lipids (gray circles). Candidates from the most significant metabolite subpathways “gamma-glutamyl amino acid” (ie, gamma-glutamyl valine, gamma-glutamyl leucine) and “leucine, isoleucine and valine metabolism” (ie, leucine, 1-carboxyethylvaline) correlated to lipids from the most significant subpathways lipid pathway “TAG ester”.
Figure 8.
Correlation analysis of metabolomics to lipidomics in obese_PN vs obese_No_PN groups. Spearman correlation was performed between metabolites (along the vertical y axis) and lipids (along the horizontal x axis) in the obese_PN vs obese_No_PN comparisons. Heat map shows significant correlations (adjusted P < .05) for positive (red, strongest with the value of 1) and negative (blue, strongest with the value of –1) correlations. A cluster of positive correlations occurs between CERs, DCERs, and SMs with 2-hydroxynervonate, β-hydroxyisovalerate, and 2-hydroxymyristate, which are negatively correlated with other lipid species. Additionally, CERs, DCERs, and SMs positively correlate with α-ketobutyrate and 2-hydroxybutyrate/2-hydroxyisobutyrate, which are negatively correlated with phospholipids (PCs, PEs), CEs, and LPEs. CE, cholesterol ester; CER, ceramide; DCER, dihydroceramide; FFA, free fatty acid; LCER, lactosylceramide; LPE, lysophosphatidylethanolamine; PC, phosphatidylcholine; PE, phosphatidylethanolamines; SM, sphingomyelin; TAG, triacylglycerol.
Discussion
In the present observational study, we performed plasma global metabolomics and lipidomics to identify species and pathways correlating with PN status in a sample of individuals with average class 3 obesity. Since our focus was obesity as a PN risk factor, we matched our obese groups with and without PN for glycemic status before our data analysis. However, we also conducted a post hoc subanalysis by prediabetes and T2D since dysglycemia remains a major risk factor for PN. The obese participants as a whole exhibited the anticipated characteristics vs the lean participants, for example, elevated body weight, BMI, WC, systolic blood pressure, and TAGs, as well as lower HDL-c. WC was higher in obese participants with vs without PN, as anticipated (10, 11), as was body weight. Otherwise, there were no additional demographic differences, allowing us to identify specific metabolic and lipidomic signatures underlying PN status in obese individuals.
As a first step, we examined the metabolites and lipid species that differed between the obese vs lean groups. We focused on subpathways because metabolites/lipids can exist within more than one network, which are further interconnected. The most prominent subpathway differentiating obese and lean individuals, selected by both PLS-DA and group-lasso, was “gamma-glutamyl amino acid,” which belongs to the gamma-glutamyl cycle responsible for glutathione antioxidant synthesis and degradation (28). Gamma-glutamyl transferase, a key gamma-glutamyl cycle enzyme, correlates positively with TAGs, BMI, and blood pressure and is linked to oxidative stress in obesity and MetS (29) and also potentially to T2D (30). Other emergent pathways when comparing the metabolome of obese vs lean participants were related to “leucine, isoleucine and valine metabolism” (or BCAA metabolism), as well as BCAA related to fatty acid metabolism. BCAA metabolism is centered around protein synthesis (through mechanistic target of rapamycin [mTOR]), glucose regulation, neurotransmission modulation, adiposity, and satiety, and is linked to obesity, where it correlates with insulin resistance and T2D-onset risk (31). Plasma metabolomics of participants of the Susceptibility to Particle Health Effects, miRNAs and Exosomes study (n = 1391) identified the highest association of BMI with aromatic amino acid and BCAA metabolism, after adjusting for age, sex, and smoking status (32). An integrative metabolomics and genome-wide association study in a population of Korean women (n = 77) concluded elevated BMI disrupted BCAA and aromatic amino acid catabolism, as well as β-oxidation, lipid metabolism, urea cycle, and purine/pyrimidine metabolism (33). Finally, our global metabolomics identified a number of fatty acid metabolism pathways that differed by obese vs lean status, as anticipated.
When we specifically examined lipids between the obese groups and the lean group, the largest and most significant pathway selected was “TAG ester,” followed by “DAG ester.” This agrees with the basic blood lipid profiles from our obese groups, which were higher in TAGs specifically containing long-chain fatty acids vs the lean group. Interestingly, group-lasso did not select the TAG subpathway, possibly because it contains the most candidates, at 518, and there may not have been sufficient significant TAGs for group-lasso to select the entire subpathway. On the other hand, group-lasso, which places greater emphasis on subpathway structure than PLS-DA, identified subpathways that were more centered on complex lipids, such as ceramides and dihydroceramides, as well as cholesteryl esters.
Our findings are aligned with several published studies, which have also investigated the correlation of plasma lipidomics signatures with obesity metrics, such as BMI, WC, and insulin resistance. Lipidomics analysis of plasma from participants enrolled in the Australian Diabetes, Obesity and Lifestyle Study (n = 10 339) and validated in the Busselton Health Study (n = 4207) found 577 lipids correlated with BMI, after adjusting for age and sex (34). Differential lipids dropped to 508, after additionally adjusting for total cholesterol, HDL-C, and triglycerides. TAGs and sphingolipids, especially shorter-chain ceramides and sphingomyelins, positively correlated with BMI, whereas gangliosides and hexosylceramides correlated negatively. In particular, Cer (18:1/18:0) and Cer (18:1/20:0) increased proportionately with BMI (both P < 10–10) (34); similarly, we had a statistically significant increase in Cer (18:1/18:0) and Cer(18:1/20:0) in obese participants with P values of 3.53 × 10–10 and 1.27 × 10–4, respectively, by Wilcoxon, along with several other statistically significant shorter-chained ceramides. These findings were mirrored in a cohort of 2302 ethnically Chinese Singaporeans, where ceramides associated positively with BMI, central obesity, measured by WC, and homeostatic model assessment of insulin resistance (HOMA-IR), whereas hexosylceramides associated negatively, after adjusting for age, sex, HDL-c, LDL-c, and triglycerides (35). Associations were also chain length and saturation dependent; as in other studies, shorter-chain ceramides, including Cer (18:1/18:0) and Cer (18:1/20:0), were elevated with BMI. In contrast, a smaller study (n = 28, BMI > 25; n = 23 lean) concluded a plasma lipidomic signature of higher BMI was characterized by elevated TAGs and lower plasmalogens, and lacking any association with sphingolipids (36). A plasma TAG signature also correlates with insulin resistance and T2D (37).
Our main interest focused on global metabolomic and complex lipidomic differences in obese participants by PN status. With regard to the metabolome, we identified 4 unique metabolomics signatures that are associated with PN in obese participants, “purine metabolism,” “polyamine metabolism,” “benzoate metabolism,” and “xanthine metabolism.” Purine metabolism is centered on the breakdown of adenosine monophosphate and guanosine monophosphate, which are involved in cellular energy metabolism and adenosine 5′-triphosphate synthesis. In alignment with these data, we previously identified altered nerve bioenergetics and decreased adenosine 5′-triphosphate levels (38-40) associated with PN in mouse models of prediabetes and T2D and in cultured primary sensory neurons treated with saturated fatty acids (41). Polyamines, such as spermidine, are part of “polyamine metabolism” and have proautophagy, immunomodulatory, and neuroprotective properties, and are present in high levels in the brain, where they sustain neuronal health (42). Polyamines have not been well studied in PN, although supplementation of polyamine biosynthesis precursors, L-arginine (43) and agmatine (44), are beneficial in treating neuropathic pain in diabetes rodent models. Two xenobiotics superpathways were identified in “benzoate metabolism,” possibly from benzoate additives in food, and “xanthine metabolism,” which contains several natural products and metabolized byproducts from coffee and tea, as well as pharmaceutical medications. Why metabolites from these pathways are associated with PN in only our obese group is unknown. One intriguing possibility lies in the idea that these food additives and byproducts alter the microbiome, which in turn is associated with PN (45), an area of active research by several groups (46, 47).
When examining distinct lipidomic signatures, there were more signaling and complex lipid classes associated with the obese group with PN. These results suggest that signaling dysregulation and complex, and possibly lipotoxic, lipids may in part underlie PN onset and progression. Among the largest and most significant signaling lipids were the DAGs, bioactive lipids that participate in several neurovascular mechanisms, such as blood flow and conduction velocity, through protein kinase C (PKC) activation (48). While earlier clinical trials of PKC inhibitors failed in the treatment of PN in diabetes cohorts (49), more recent efforts have focused on selective inhibition of different PKC isoforms for treating PN (50). Among the complex lipids, “ceramide,” “dihydroceramide,” “lactosylceramide,” and “sphingomyelin” are associated with obese participants with PN vs those obese participants without PN. This is of significant interest, and aligned with our prior studies, which found sphingolipid levels were altered in obese individuals with PN (51, 52).
Ceramides and dihydroceramides are a family of bioactive lipids that modulate apoptosis, senescence, and stress responses (53). As such, they are lipotoxic and accumulate in tissues or plasma during obesity and insulin resistance (54). Ceramide biosynthesis occurs through multiple pathways (53); however, under conditions of excessive dietary TAG intake, de novo synthesis may dominate (55), leading to an increase in dihydroceramides. Ceramides and dihydroceramides are linked to PN in patients with diabetes (56), and could be recurrent features in neuropathies, generally, for example, in hereditary neuropathies, such as in hereditary sensory and autonomic neuropathy type 1 (52). When comparing T2D participants with and without PN, we previously observed trends in ceramide acyl chain lengths (51), which influence their biological properties (57). The contributions of the various ceramide and dihydroceramide species length and saturation that contributed to PN status in this sample of individuals with average class 3 obesity are a future area of interest, along with a greater understanding of these lipotoxic lipids in prediabetes, T2D, and PN.
Sphingomyelins are important constituents of myelin-ensheathed nerves; however, they are less studied in obesity and diabetes, and associated PN (58). One study found higher sphingomyelins in cerebrospinal fluid from participants with acquired demyelinating peripheral neuropathies compared to axonal neuropathies (59). In neuropathic sural nerve, a loss of long-chain sphingomyelins occurs compared to healthy nerve and brain tissue (60). As with ceramides and dihydroceramides, the discovery of significant sphingomyelin species associated with PN opens a new avenue of research aimed at understanding PN pathogenesis.
When we integrated metabolites with lipids to build an O2PLS correlation network for all obese participants (both groups) vs the lean group, the most significant metabolite subpathways gamma-glutamyl amino acid (ie, gamma-glutamyl valine, gamma-glutamyl leucine) and BCAA (ie, leucine, 1-carboxyethylvaline) correlated to lipids from the most significant lipid subpathways, TAGs, suggesting that global dysregulation of both the metabolome and lipidome occurred in concert in all obese participants. When we performed Spearman correlation analyses between metabolites and lipids in the obese groups based on PN status, we found a cluster of positive correlations between ceramides, dihydroceramides, and sphingomyelins to 2-hydroxy fatty acids, namely 2-hydroxynervonate, β-hydroxyisovalerate, and 2-hydroxymyristate. 2-Hydroxy fatty acids condense with ceramides and sphingolipids to generate 2-hydroxy fatty acid–ceramides and 2-hydroxy fatty acid–sphingolipids, which are important for normal neural function (61). Additionally, ceramides, dihydroceramides, and sphingomyelins positively correlated with α-ketobutyrate and 2-hydroxybutyrate. α-Ketobutyrate is the oxidized form of α-hydroxybutyrate, which is linked to insulin resistance and impaired glucose regulation, particularly in nondiabetes populations, and could constitute a link to dyslipidemia (62).
Our study has limitations. First, although it involved a real-life cohort, which is a strength, most participants were on statins to manage their hyperlipidemia, which affected their basic plasma lipid profiles, for example, cholesterol. Thus, data analysis could place a greater emphasis on accounting for medication use, that is, statins, β-blockers, antidiabetic medications. However, since statin use does not affect PN status in T2D (27) and was evenly balanced between both obese groups with and without PN in the present study, we anticipate that the identified metabolites and lipids present in the obese PN participants represent an association with PN. In addition, though our primary focus was on the effects of obesity on PN, insulin resistance and hyperglycemia are frequently comorbid conditions with obesity in patients. However, our study was limited by a lack of sensitive measures of insulin resistance, like HOMA-IR. Sex and age differences were not evaluated in this study, which could affect the plasma lipidome (63-66). Sex and age differences could be relevant to this study, particularly regarding the sex and age imbalance in lean vs obese participants, though our logistic regression and group-lasso models accounted for these factors as covariates. Importantly, the study also faced a tissue issue, since plasma may not be representative of nerve tissue-specific metabolome and lipidome differences, which is not an accessible tissue in human participants. Additionally, the study was not longitudinal, rendering it difficult to make causal inferences. Furthermore, this study was in individuals with severe obesity, and whether these results generalize to other populations is unknown.
Finally, our sample size included 88 obese individuals and 43 lean controls, which likely limited our power to detect small differences in metabolites and lipids between groups with vs without PN. This was even more pronounced in our subanalysis by prediabetes (n = 15) and T2D (n = 43) status. Analysis of larger cohorts may find that metabolic and lipidomic profiles in T2D may outperform obesity profiles for PN separation. Although we statistically accounted for the number of comparisons, their sheer number, due to a large number of detected metabolites and lipids relative to the number of study participants could led to some false associations. Future studies are needed to confirm our results.
In conclusion, when compared to lean individuals, this observational study identified differences in the metabolome and lipidome of a sample of individuals with average class 3 obesity, especially gamma-glutamyl amino acid and BCAA metabolism and TAG lipid metabolism. When obese individuals were stratified by PN status, possible novel research avenues emerged in the metabolome with polyamine biosynthesis, as well as better established defects in bioenergetics through purine biosynthesis. With respect to the lipidome, bioactive and complex lipids distinguished the obese group with PN from the obese group without PN. It will be critical, moving forward, to identify trends in chain length and saturation level of specific bioactive lipid classes and particular species that contribute to PN. We anticipate lipid signaling and complex lipids may be exciting avenues of investigation for PN associated with metabolic dysfunction, that is, obesity, prediabetes, and T2D. Inhibitors of complex lipid signaling, such as ceramides (67, 68), are an active area of research as antiobesity, anti-T2D, and anti-insulin resistance therapies, highlighting the potential significance of this research direction in PN.
Acknowledgments
The authors would like to thank the study participants and Dr Evan Reynolds and Dr Lucy Hinder, University of Michigan, for help in data management. They also thank Emily Villegas-Umana for plasma collection.
Financial Support: This work was supported by the National Institutes of Health (grant Nos. R24DK082841 to E.L.F., 1R21NS102924 to E.L.F. and J.H., 1K99DK119366 to A.E.R., and 1R01DK115687 and K23NS079417 to B.C.C.); the Novo Nordisk Foundation Challenge Programme (grant No. NNF14OC0011633 to E.L.F. and B.C.C.); the NeuroNetwork for Emerging Therapies; and the A. Alfred Taubman Medical Research Institute.
Author Contributions: K.G. analyzed data and performed bioinformatics analysis; M.G.S. analyzed data and wrote and revised the manuscript for intellectual content; A.E.R. analyzed data, contributed to discussion, and revised the manuscript for intellectual content; F.M.A analyzed data and performed bioinformatics analysis; B.C.C. contributed to the study design and revised the manuscript for intellectual content; J.H. contributed to the study design, analyzed data, performed bioinformatics analysis, and revised the manuscript for intellectual content, and E.L.F. contributed to the study design, analyzed data, and wrote and revised the manuscript for intellectual content.
Glossary
Abbreviations
- BCAA
branched-chain amino acid
- BMI
body mass index
- DAG
diacylglycerol
- FBG
fasting blood glucose
- HbA1c
glycated hemoglobin A1c
- HDL-c
high-density lipoprotein cholesterol
- HOMA-IR
homeostatic model assessment of insulin resistance
- IWMC
Investigational Weight Management Clinic
- LDL-c
low-density lipoprotein cholesterol
- MetS
metabolic syndrome
- NCEP
National Cholesterol Education Program
- O2PLS
2-way orthogonal partial least squares
- OGTT
oral glucose tolerance test
- OR
odds ratio
- PCA
principal component analysis
- PKC
protein kinase C
- PLS-DA
partial least squares–discriminant analysis
- PN
peripheral neuropathy
- T2D
type 2 diabetes
- TAG
triacylglycerol
- UPLC-MS/MS
ultrahigh performance liquid chromatography–tandem mass spectroscopy
- VIP
variable importance in projection
- WC
waist circumference
Additional Information
Disclosures: B.C.C. consults for a PCORI grant, DynaMed, and performs medical legal consultations including consultations for the Vaccine Injury Compensation Program. The other authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Feldman EL, Callaghan BC, Pop-Busui R, et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):41. [DOI] [PubMed] [Google Scholar]
- 2. Callaghan BC, Little AA, Feldman EL, Hughes RAC. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev. 2012;6(6):CD007543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Christensen DH, Knudsen ST, Gylfadottir SS, et al. Metabolic factors, lifestyle habits, and possible polyneuropathy in early type 2 diabetes: a nationwide study of 5,249 patients in the Danish Centre for Strategic Research in Type 2 Diabetes (DD2) cohort. Diabetes Care. 2020;43(6):1266-1275. [DOI] [PubMed] [Google Scholar]
- 4. Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A; KORA Study Group . Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care. 2008;31(3):464-469. [DOI] [PubMed] [Google Scholar]
- 5. Smith AG, Singleton JR. Obesity and hyperlipidemia are risk factors for early diabetic neuropathy. J Diabetes Complications. 2013;27(5):436-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Callaghan BC, Xia R, Banerjee M, et al. ; Health ABC Study . Metabolic syndrome components are associated with symptomatic polyneuropathy independent of glycemic status. Diabetes Care. 2016;39(5):801-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Callaghan BC, Xia R, Reynolds E, et al. Association between metabolic syndrome components and polyneuropathy in an obese population. JAMA Neurol. 2016;73(12):1468-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Callaghan BC, Gao L, Li Y, et al. Diabetes and obesity are the main metabolic drivers of peripheral neuropathy. Ann Clin Transl Neurol. 2018;5(4):397-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735-2752. [DOI] [PubMed] [Google Scholar]
- 10. Callaghan BC, Reynolds EL, Banerjee M, et al. The prevalence and determinants of cognitive deficits and traditional diabetic complications in the severely obese. Diabetes Care. 2020;43(3):683-690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Callaghan BC, Reynolds E, Banerjee M, Chant E, Villegas-Umana E, Feldman EL. Central obesity is associated with neuropathy in the severely obese. Mayo Clin Proc. 2020;95(7):1342-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. O’Brien PD, Guo K, Eid SA, et al. Integrated lipidomic and transcriptomic analyses identify altered nerve triglycerides in mouse models of prediabetes and type 2 diabetes. Dis Model Mech. 2020;13(2):dmm042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Savelieff MG, Callaghan BC, Feldman EL. The emerging role of dyslipidemia in diabetic microvascular complications. Curr Opin Endocrinol Diabetes Obes. 2020;27(2):115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Genuth S, Alberti KG, Bennett P, et al. ; Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160-3167. [DOI] [PubMed] [Google Scholar]
- 15. Tesfaye S, Boulton AJ, Dyck PJ, et al. ; Toronto Diabetic Neuropathy Expert Group . Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dehaven CD, Evans AM, Dai H, Lawton KA. Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J Cheminform. 2010;2(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Evans A, Bridgewater B, Mitchell M, et al. High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics. 2014;4(2):1000132. [Google Scholar]
- 18. Löfgren L, Ståhlman M, Forsberg GB, Saarinen S, Nilsson R, Hansson GI. The BUME method: a novel automated chloroform-free 96-well total lipid extraction method for blood plasma. J Lipid Res. 2012;53(8):1690-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo K, Savelieff MG, Rumora AE, et al. Supplementary data for “Plasma metabolomics and lipidomics different iate obese individuals by peripheral neuropathy status. Figshare. Posted September 9, 2021. https://figshare.com/articles/figure/Plasma_metabolomics_and_lipidomics_differentiate_obese_individuals_by_peripheral_neuropathy_status/16559154
- 20. Rohart F, Gautier B, Singh A, Lê Cao KA. mixOmics: an R package for ‘omics feature selection and multiple data integration. PLoS Comput Biol. 2017;13(11):e1005752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Galindo-Prieto B, Eriksson L, Trygg J. Variable influence on projection (VIP) for orthogonal projections to latent structures (OPLS). J Chemom. 2014;28(8):623-632. [Google Scholar]
- 22. Yang Y, Zou H. A fast unified algorithm for solving group-lasso penalize learning problems. Stat Comput. 2015;25(6): 1129-1141. [Google Scholar]
- 23. Trygg J, Wold S. O2-PLS, a two-block (X-Y) latent variable regression (LVR) method with an integral OSC filter. J Chemomet. 2003;17(1):53-64. [Google Scholar]
- 24. Bouhaddani SE, Houwing-Duistermaat J, Salo P, Perola M, Jongbloed G, Uh HW. Evaluation of O2PLS in omics data integration. BMC Bioinformatics. 2016;17(Suppl 2):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bylesjö M, Eriksson D, Kusano M, Moritz T, Trygg J. Data integration in plant biology: the O2PLS method for combined modeling of transcript and metabolite data. Plant J. 2007;52(6):1181-1191. [DOI] [PubMed] [Google Scholar]
- 26. Bell DS. Advantages of a third-generation beta-blocker in patients with diabetes mellitus. Am J Cardiol. 2004;93(9A):49B-52B. [DOI] [PubMed] [Google Scholar]
- 27. Kristensen FP, Christensen DH, Callaghan BC, et al. Statin therapy and risk of polyneuropathy in type 2 diabetes: a Danish cohort study. Diabetes Care. 2020;43(12):2945-2952. [DOI] [PubMed] [Google Scholar]
- 28. Ristoff E, Larsson A. Inborn errors in the metabolism of glutathione. Orphanet J Rare Dis. 2007;2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lee DS, Evans JC, Robins SJ, et al. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2007;27(1):127-133. [DOI] [PubMed] [Google Scholar]
- 30. Gasecka A, Siwik D, Gajewska M, et al. Early biomarkers of neurodegenerative and neurovascular disorders in diabetes. J Clin Med. 2020;9(9):2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Holeček M. Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements. Nutr Metab (Lond). 2018;15:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frigerio G, Favero C, Savino D, et al. Plasma metabolomic profiling in 1391 subjects with overweight and obesity from the SPHERE study. Metabolites. 2021;11(4):194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim MJ, Kim JH, Kim MS, Yang HJ, Lee M, Kwon DY. Metabolomics associated with genome-wide association study related to the basal metabolic rate in overweight/obese Korean women. J Med Food. 2019;22(5):499-507. [DOI] [PubMed] [Google Scholar]
- 34. Beyene HB, Olshansky G, T Smith AA, et al. High-coverage plasma lipidomics reveals novel sex-specific lipidomic fingerprints of age and BMI: evidence from two large population cohort studies. PLoS Biol. 2020;18(9):e3000870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chew WS, Torta F, Ji S, et al. Large-scale lipidomics identifies associations between plasma sphingolipids and T2DM incidence. JCI Insight. 2019;5(13):e126925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tonks KT, Coster AC, Christopher MJ, et al. Skeletal muscle and plasma lipidomic signatures of insulin resistance and overweight/obesity in humans. Obesity (Silver Spring). 2016;24(4):908-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rhee EP, Cheng S, Larson MG, et al. Lipid profiling identifies a triacylglycerol signature of insulin resistance and improves diabetes prediction in humans. J Clin Invest. 2011;121(4):1402-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vincent AM, Edwards JL, McLean LL, et al. Mitochondrial biogenesis and fission in axons in cell culture and animal models of diabetic neuropathy. Acta Neuropathol. 2010;120(4):477-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hinder LM, Vivekanandan-Giri A, McLean LL, Pennathur S, Feldman EL. Decreased glycolytic and tricarboxylic acid cycle intermediates coincide with peripheral nervous system oxidative stress in a murine model of type 2 diabetes. J Endocrinol. 2013;216(1):1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sas KM, Kayampilly P, Byun J, et al. Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight. 2016;1(15):e86976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rumora AE, Lentz SI, Hinder LM, et al. Dyslipidemia impairs mitochondrial trafficking and function in sensory neurons. FASEB J. 2018;32(1):195-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ghosh I, Sankhe R, Mudgal J, Arora D, Nampoothiri M. Spermidine, an autophagy inducer, as a therapeutic strategy in neurological disorders. Neuropeptides. 2020;83:102083. [DOI] [PubMed] [Google Scholar]
- 43. Rondón LJ, Farges MC, Davin N, et al. L-Arginine supplementation prevents allodynia and hyperalgesia in painful diabetic neuropathic rats by normalizing plasma nitric oxide concentration and increasing plasma agmatine concentration. Eur J Nutr. 2018;57(7):2353-2363. [DOI] [PubMed] [Google Scholar]
- 44. Karadag HC, Ulugol A, Tamer M, Ipci Y, Dokmeci I. Systemic agmatine attenuates tactile allodynia in two experimental neuropathic pain models in rats. Neurosci Lett. 2003;339(1):88-90. [DOI] [PubMed] [Google Scholar]
- 45. Lin B, Wang Y, Zhang P, Yuan Y, Zhang Y, Chen G. Gut microbiota regulates neuropathic pain: potential mechanisms and therapeutic strategy. J Headache Pain. 2020;21(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tanase DM, Gosav EM, Neculae E, et al. Role of gut microbiota on onset and progression of microvascular complications of type 2 diabetes (T2DM). Nutrients. 2020;12(12):3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vendrik KEW, Ooijevaar RE, de Jong PRC, et al. Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol. 2020;10:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106(8):1319-1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bansal D, Badhan Y, Gudala K, Schifano F. Ruboxistaurin for the treatment of diabetic peripheral neuropathy: a systematic review of randomized clinical trials. Diabetes Metab J. 2013;37(5):375-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. He Y, Wang ZJ. Nociceptor beta II, delta, and epsilon isoforms of PKC differentially mediate paclitaxel-induced spontaneous and evoked pain. J Neurosci. 2015;35(11):4614-4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rumora AE, Guo K, Alakwaa FM, et al. Plasma lipid metabolites associate with diabetic polyneuropathy in a cohort with type 2 diabetes. Ann Clin Transl Neurol. 2021;8(6):1292-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fridman V, Zarini S, Sillau S, et al. Altered plasma serine and 1-deoxydihydroceramide profiles are associated with diabetic neuropathy in type 2 diabetes and obesity. J Diabetes Complications. 2021;35(4):107852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20(6):1010-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Aburasayn H, Al Batran R, Ussher JR. Targeting ceramide metabolism in obesity. Am J Physiol Endocrinol Metab. 2016;311(2):E423-E435. [DOI] [PubMed] [Google Scholar]
- 55. Bandet CL, Tan-Chen S, Bourron O, Le Stunff H, Hajduch E. Sphingolipid metabolism: new insight into ceramide-induced lipotoxicity in muscle cells. Int J Mol Sci. 2019;20(3):479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mandal N, Grambergs R, Mondal K, Basu SK, Tahia F, Dagogo-Jack S. Role of ceramides in the pathogenesis of diabetes mellitus and its complications. J Diabetes Complications. 2021;35(2):107734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Turpin-Nolan SM, Brüning JC. The role of ceramides in metabolic disorders: when size and localization matters. Nat Rev Endocrinol. 2020;16(4):224-233. [DOI] [PubMed] [Google Scholar]
- 58. Iqbal J, Walsh MT, Hammad SM, Hussain MM. Sphingolipids and lipoproteins in health and metabolic disorders. Trends Endocrinol Metab. 2017;28(7):506-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Capodivento G, Visigalli D, Garnero M, et al. Sphingomyelin as a myelin biomarker in CSF of acquired demyelinating neuropathies. Sci Rep. 2017;7(1):7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Heipertz R, Pilz H, Seidel D, Klauke W, Goebel HH. Fatty acid composition of myelin lipids (cerebrosides, sulphatides and sphingomyelin) from normal human sural nerve, and changes in peripheral neuropathy. Neuropathol Appl Neurobiol. 1978;4(3):197-207. [DOI] [PubMed] [Google Scholar]
- 61. Hama H. Fatty acid 2-hydroxylation in mammalian sphingolipid biology. Biochim Biophys Acta. 2010;1801(4):405-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gall WE, Beebe K, Lawton KA, et al. ; RISC Study Group . Alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS One. 2010;5(5):e10883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Torretta E, Barbacini P, Al-Daghri NM, Gelfi C. Sphingolipids in obesity and correlated co-morbidities: the contribution of gender, age and environment. Int J Mol Sci. 2019;20(23):5901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Santosa S, Jensen MD. The sexual dimorphism of lipid kinetics in humans. Front Endocrinol (Lausanne). 2015;6:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maekawa K, Okemoto K, Ishikawa M, Tanaka R, Kumagai Y, Saito Y. Plasma lipidomics of healthy Japanese adults reveals gender- and age-related differences. J Pharm Sci. 2017;106(9):2914-2918. [DOI] [PubMed] [Google Scholar]
- 66. Ishikawa M, Maekawa K, Saito K, et al. Plasma and serum lipidomics of healthy white adults shows characteristic profiles by subjects’ gender and age. PLoS One. 2014;9(3):e91806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Stith JL, Velazquez FN, Obeid LM. Advances in determining signaling mechanisms of ceramide and role in disease. J Lipid Res. 2019;60(5):913-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Raichur S, Brunner B, Bielohuby M, et al. The role of C16:0 ceramide in the development of obesity and type 2 diabetes: CerS6 inhibition as a novel therapeutic approach. Mol Metab. 2019;21:36-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.