Abstract
The pharmacokinetics of levofloxacin, administered in high doses and with extended dosing intervals, was studied in human immunodeficiency virus (HIV)-infected patients. Thirty patients received either 750 mg of the drug or a placebo once daily for 14 days, followed by 750 mg or 1,000 mg of the drug or a placebo three times weekly for an additional 14 days. Levofloxacin disposition was characterized by rapid oral absorption, with peak concentrations occurring approximately 1.5 h after dosing and elimination half-lives from 7.2 to 9.4 h. The overall incidence of any adverse effect was 70% (1,000 mg) to 95% (750 mg) for levofloxacin-treated patients and 71% for those taking the placebo. Levofloxacin pharmacokinetic parameters for HIV-infected patients were consistent with those observed in studies of healthy volunteers.
Patients infected with human immunodeficiency virus (HIV) are known to be highly susceptible to coinfection with mycobacterial species, especially Mycobacterium avium complex (MAC) and Mycobacterium tuberculosis (3, 7). For patients with HIV and disseminated MAC that is resistant to macrolides, clinicians often prescribe complex regimens that may include a quinolone, such as levofloxacin (1). In addition, for therapy of multidrug-resistant tuberculosis (MDR-TB), the use of levofloxacin may be considered. Levofloxacin has been shown to be active against tuberculosis in vitro with an MIC at which 90% of the isolates are inhibited of <1.0 μg/ml (9). Recent clinical data suggest that levofloxacin may be of some utility in patients with MDR-TB (10).
Levofloxacin is a fluorinated quinolone antibiotic with a broad spectrum of activity. It possesses a favorable pharmacokinetic profile, with wide distribution into tissues and macrophages, good oral absorption, and a long half-life (t1/2) which allows for once-daily dosing (2, 4). It is eliminated renally and thus does not cause interactions with the myriad of drugs used for HIV infection that are metabolized by the cytochrome P-450 system.
The pharmacokinetics of levofloxacin in healthy volunteers and in patients with asymptomatic HIV infection has been well described (4, 5). Its disposition in patients with advanced HIV infection has not been studied. Furthermore, the pharmacokinetics of levofloxacin when higher doses are administered over extended intervals has not been evaluated. Such doses, administered two or three times weekly, would facilitate directly observed therapy. The purpose of this study was to characterize the pharmacokinetics and safety of levofloxacin in HIV-infected patients when it is administered in higher doses at extended intervals.
This was a sequential, two-part, randomized, double-blind, placebo-controlled study of HIV-infected patients. Inclusion criteria included documented HIV infection (positive by enzyme-linked immunosorbent assay and Western blot test), an age of ≥18 years, and laboratory test results within defined protocol limits. Subjects were excluded if they had allergies to quinolones, had experienced vomiting or diarrhea within 48 h of screening, or had HIV wasting syndrome. Subjects were randomized to one of the three treatment groups listed below and were stratified by CD4 count (less than and greater than 250 cells/mm3). Patients received levofloxacin or the placebo once daily from days 1 to 14 and three times weekly from days 15 to 26. The doses were as follows: for group 1, 750 mg of levofloxacin throughout; for group 2, 750 mg of levofloxacin on days 1 to 14 and 1,000 mg of levofloxacin on days 15 to 26; and for group 3, placebo throughout.
The 750-mg dose consisted of one 500-mg tablet, two 125-mg tablets, and two placebo tablets. The 1-g dose consisted of one 500-mg tablet and four 125-mg tablets. Placebo and drug supplies appeared to be identical. Patients were instructed to fast for at least 6 h prior to the administration of the morning dose and to consume breakfast 2 h after each dose. In the event of nausea, subsequent doses were given with food (one patient).
On days 1, 14, 15, and 26, patients were admitted to the hospital and serial blood samples were collected prior to dosing and then at 0.5, 1, 1.5, 2, 3, 4, 8, 12, 16, and 24 h after dosing. Samples were also drawn at 48 and 72 h after the final dose on day 26. Quantitative urine collections were performed between 0 and 2, 2 and 4, 4 and 8, 8 and 12, and 12 and 24 h on the study days listed above. Urine was also collected between 24 and 48 and 48 and 72 h following the last dose. Blood samples were collected just prior to and then 2 h after dosing on days 5, 13, and 24 to monitor adherence.
Patients were allowed to receive concomitant antiretroviral therapy during the study. Drugs were selected by each patient’s referring health care provider based on clinical judgment. Didanosine and levofloxacin doses were separated by at least 2 h.
The study was reviewed and approved by the Institutional Review Board of the National Institute of Allergy and Infectious Diseases.
Concentrations of levofloxacin in plasma were determined by high-pressure liquid chromatography by using a published assay (12). The assay was linear over the concentration range studied, and the lower limit of quantitation was 0.08 μg/ml. The inter- and intraday coefficient of variation was <10%.
Pharmacokinetic parameters were determined by standard noncompartmental analysis (8). Maximum concentration and time to maximum concentration (Tmax) were determined directly from concentration-time profiles. Area under the concentration-time curve (AUC) was calculated by the trapezoidal summation method. The elimination rate constant (kel) was determined by log-linear regression analysis of concentration-time data by using data points with a correlation coefficient of no less than 0.95. The t1/2 was calculated as 0.693/kel. The maximum concentration in urine was determined by inspection of urine concentration-versus-time profiles. The Ae was the cumulative amount of levofloxacin excreted in the urine. Renal clearance (CLR) was calculated by dividing the Ae over 24 h by the AUC from 0 to 24 h. Creatinine clearance (CLCR) at baseline was estimated by using the Cockcroft-Gault equation, with correction for gender (6).
The safety of levofloxacin was evaluated by clinical laboratory testing as well as subjective assessment by patient interview and record keeping.
Statistics.
The effects of prestudy CLCR, CD4 count (<250 or >250 cells/mm3), and dose on total body clearance on day 26 were studied by using general linear regression modeling of the data. The effects of these covariates were tested at the 5% level of significance with the SAS statistical program version 6.09. Similar regression models were also used to study the effects of CLCR, CD4 count, and dose on CLR on day 26. The adverse effects of different treatment regimens were analyzed by a Pearson’s chi-square test. A P value of <0.05 was considered statistically significant.
Subjects.
Thirty-one subjects were enrolled and randomized into the study. Results from one subject who did not receive study medication are not included in the analysis. Thirty subjects, including four subjects who discontinued the study prematurely and were replaced, received levofloxacin or the placebo. There were 28 males and 2 females, and the mean age was 35.5 ± 7.1 years. Eleven patients received 750 mg of levofloxacin throughout the study, twelve patients received 750 mg of levofloxacin daily (q.d.) followed by 1,000 mg of levofloxacin three times a week (t.i.w.), and seven patients received the placebo. Of those patients receiving levofloxacin, 11 had CD4 counts below 250 cells/mm3 (median, 123 cells/mm3; range, 4 to 249 cells/mm3) and 12 had CD4 counts above 250 cells/mm3 (median, 421 cells/mm3; range, 295 to 772 cells/mm3). Antiretroviral therapies were comparable between groups, although the majority of patients were receiving monotherapy or no therapy, since only four nucleoside analog reverse transcriptase inhibitors were available at the time of the study.
Safety.
Adverse effects experienced by treatment and placebo groups are shown in Table 1. Adverse events were noted with high frequency in both levofloxacin- and placebo-treated patients. The overall incidence of any adverse effect was 70% (for the group receiving 1,000 mg t.i.w.) to 95% (for the group receiving 750 mg q.d.) for levofloxacin-treated patients and 71% for those taking the placebo. No significant differences in the incidence of adverse effects in levofloxacin and placebo groups were noted. Most adverse effects were mild. Only two patients had to discontinue levofloxacin treatment, one because of fatigue, dizziness, and nausea and the other due to severe pruritus. Gastrointestinal side effects of nausea and diarrhea were the most common complaints, followed by headache, flatulence, and fatigue. Four subjects did not complete the study for non-medication-related reasons: two patients were excused for inability to obtain venous access, and two patients decided to withdraw. Levofloxacin concentrations were within the normal ranges on days 5, 13, and 24, suggesting that patients adhered to the prescribed regimen.
TABLE 1.
Number of subjects with reported adverse effects
| Adverse event or body system affected | No. of subjects from group:
|
||||
|---|---|---|---|---|---|
| 750 mg qda | 750 mg tiwb | 1,000 mg tiwb | Placeboc
|
||
| qd | tiw | ||||
| Any event | 22 | 9 | 7 | 5 | 5 |
| Gastrointestinal | 18 | 5 | 2 | 2 | 3 |
| Nausea | 12 | 1 | 2 | 2 | 1 |
| Diarrhea | 8 | 2 | 1 | 1 | 2 |
| Flatulence | 4 | 0 | 0 | 1 | 0 |
| General | 8 | 3 | 3 | 3 | 2 |
| Fatigue | 4 | 0 | 0 | 0 | 0 |
| Fever | 2 | 1 | 2 | 0 | 1 |
| Central nervous system | 8 | 0 | 2 | 3 | 0 |
| Headache | 7 | 0 | 0 | 3 | 0 |
| Skin | 6 | 2 | 4 | 2 | 2 |
| Pruritus | 2 | 0 | 2 | 1 | 0 |
| Rash | 2 | 1 | 2 | 2 | 1 |
Includes patients from groups 1 and 2; n = 23.
n = 10.
n = 7.
Pharmacokinetics.
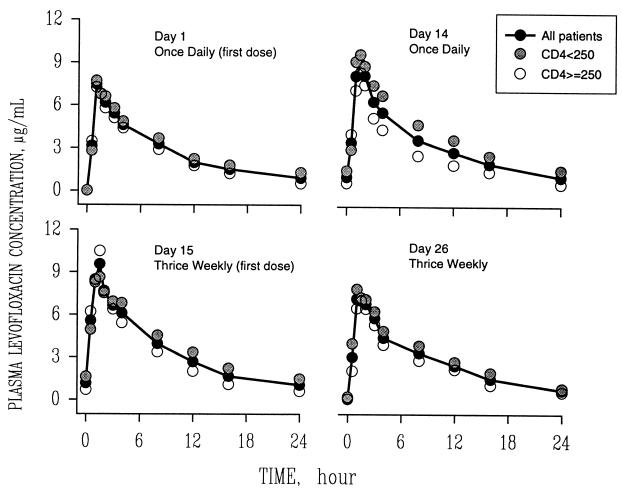
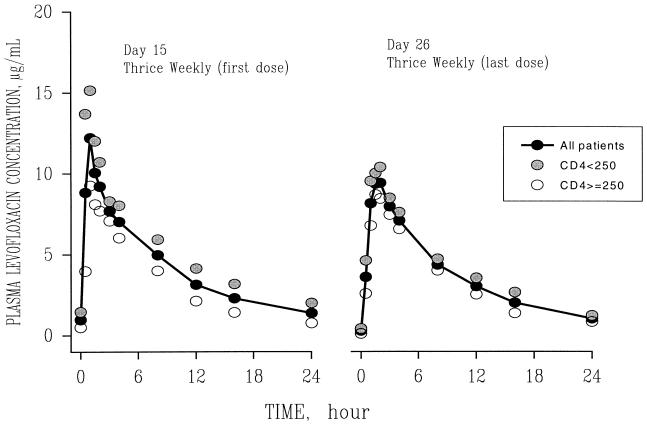
Mean concentration-time curves of levofloxacin for each dose group are shown in Fig. 1 and 2. Mean pharmacokinetic parameters are given in Table 2. Levofloxacin disposition was characterized by rapid oral absorption, with peak concentrations occurring approximately 1.5 h after dosing. Levofloxacin pharmacokinetic parameters remained linear and constant throughout study days 1, 14, 15, and 26. The mean interday coefficients of variation in Tmax, t1/2, and oral clearance (CL/F) were 28, 20, and 13%, respectively. Following multiple 750-mg q.d. doses, the mean ratio of the AUC on day 14 to that on day 1 was 1.29 (standard deviation, 0.33), indicating some accumulation upon multiple dosing.
FIG. 1.
Mean plasma concentration-versus-time profiles of levofloxacin in HIV-seropositive subjects following daily (days 1 to 14) and thrice-weekly (days 15 to 26) 750-mg oral doses.
FIG. 2.
Mean plasma concentration-versus-time profiles of levofloxacin in HIV-seropositive subjects following a dose change from 750 mg to 1 g on day 15 and after thrice-weekly 1-g doses of the drug were administered orally from days 15 to 26.
TABLE 2.
Levofloxacin pharmacokinetic parametersa
| Group,b dose, and day | Maximum concn in serum (mg/ml) | Tmax (h) | AUC (mg · h/ml)c | CL/F (ml/min) | t1/2 (h) | Maximum concn in urine (mg/ml) | Ae (% of dose)d | CLR (mg/ml) |
|---|---|---|---|---|---|---|---|---|
| A | ||||||||
| 750 mg | ||||||||
| 1 | 8.89 (2.64) | 1.5 (0.7) | 71.1 (17.3) | 151 (22) | 7.91 (1.50) | 563 (288) | 60.8 (22.2) | 115 (52) |
| 14 | 11.4 (2.4) | 1.4 (0.4) | 97.7 (28.6) | 139 (45) | 9.22 (2.06) | 718 (444) | 74.0 (29.0) | 103 (59) |
| 15 | 10.4 (3.3) | 1.9 (1.2) | 91.4 (28.9) | 153 (64) | 9.41 (1.94) | 825 (422) | 79.3 (27.3) | 120 (54) |
| 26 | 8.94 (1.29) | 1.6 (0.9) | 73.9 (14.0) | 142 (33) | 9.98 (4.32) | 856 (241) | 86.0 (32.4) | 139 (65) |
| 1 g | ||||||||
| 15 | 15.7 (4.5) | 1.2 (0.3) | 122 (28) | 133 (28) | 10.5 (1.9) | 1,156 (601) | 61.9 (9.7) | 89 (23) |
| 26 | 12.1 (1.8) | 1.4 (0.5) | 101 (28) | 151 (49) | 10.2 (4.2) | 826 (666) | 69.2 (8.4) | 103 (40) |
| B | ||||||||
| 750 mg | ||||||||
| 1 | 8.70 (2.37) | 1.4 (0.6) | 56.3 (8.0) | 208 (31) | 6.63 (1.17) | 393 (259) | 60.6 (17.2) | 135 (39) |
| 14 | 9.88 (2.38) | 1.5 (0.4) | 60.7 (8.5) | 210 (35) | 6.69 (0.68) | 684 (405) | 72.9 (23.4) | 150 (59) |
| 15 | 11.2 (3.5) | 1.3 (0.3) | 68.5 (8.7) | 185 (23) | 6.10 (0.88) | 725 (209) | 69.9 (22.6) | 124 (32) |
| 26 | 10.1 (1.4) | 1.4 (0.4) | 55.8 (8.6) | 207 (43) | 7.47 (1.86) | 517 (201) | 72.5 (24.1) | 162 (77) |
| 1 g | ||||||||
| 15 | 10.6 (1.9) | 1.4 (0.4) | 74.6 (19.9) | 224 (73) | 7.30 (2.36) | 902 (416) | 60.9 (35.5) | 144 (110) |
| 26 | 9.61 (1.55) | 2.2 (0.8) | 76.7 (17.2) | 194 (51) | 8.30 (1.08) | 839 (486) | 66.0 (11.9) | 135 (42) |
| All (A plus B) | ||||||||
| 750 mg | ||||||||
| 1 | 8.79 (2.45) | 1.4 (0.6) | 63.7 (15.2) | 184 (39) | 7.17 (1.43) | 475 (280) | 60.7 (19.4) | 125 (46) |
| 14 | 10.7 (2.4) | 1.4 (0.4) | 79.2 (28.0) | 175 (53) | 8.10 (2.03) | 701 (414) | 73.4 (25.1) | 130 (62) |
| 15 | 10.8 (3.2) | 1.6 (0.9) | 80.0 (23.4) | 169 (49) | 7.94 (2.28) | 775 (318) | 75.1 (24.3) | 122 (43) |
| 26 | 9.50 (1.41) | 1.5 (0.7) | 64.8 (14.5) | 178 (50) | 8.59 (3.24) | 686 (275) | 77.6 (26.1) | 152 (68) |
| 1 g | ||||||||
| 15 | 13.1 (4.2) | 1.3 (0.4) | 98.1 (33.7) | 174 (68) | 9.06 (2.57) | 1,029 (505) | 61.5 (22.8) | 113 (75) |
| 26 | 10.9 (2.1) | 1.8 (0.8) | 88.9 (25.5) | 170 (51) | 9.37 (3.21) | 833 (550) | 67.6 (9.9) | 119 (42) |
Values are means (standard deviations). HIV-seropositive subjects received 750-mg q.d. oral doses of levofloxacin for 2 weeks, followed by 750-mg or 1-g t.i.w. oral doses of levofloxacin for 2 weeks.
Group A, subjects with a CD4 count of <250 cells/mm3; group B, subjects with a CD4 count of >250 cells/mm3.
AUC values were calculated from 0 to 24 h.
Data for days 1, 14, and 15 are based on 24-h urine recovery values, and data for day 26 are based on 72-h urine recovery values.
There appeared to be an increase in the terminal t1/2 of levofloxacin and a decreased oral clearance in patients with CD4 counts that were <250 cells/mm3 (Table 2). However, regression modeling demonstrated that CD4 count did not have a statistically significant effect on oral clearance (P = 0.117) or CLR (P = 0.631). Renal function, as estimated by prestudy CLCR values, was on average 23% lower in subjects with lower CD4 counts. CLCR ranged from 5 to 140 ml/min/1.73 m2 (mean, 83 ml/min/1.73 m2) in subjects with CD4 counts that were <250 cells/mm3 and ranged from 81 to 182 ml/min/1.73 m2 (mean, 108 ml/min/1.73 m2) in subjects with CD4 counts that were >250 cells/mm3. However, regression analysis did not show CLCR to significantly affect total body clearance (P = 0.06).
Since coinfection with HIV and TB or MAC is frequently encountered, we evaluated the pharmacokinetics of levofloxacin in HIV-infected patients receiving high doses and extended-interval doses that could be used to treat TB or disseminated MAC. Fluoroquinolones such as levofloxacin may be useful in drug-resistant mycobacterial infections in which there are a limited number of drugs available and a long duration of therapy is required (1, 7). This study provides preliminary pharmacokinetic data for high-dose, extended-interval dosing. The usefulness of this strategy in patients with MDR-TB remains to be determined in clinical trials.
Pharmacokinetic parameters of levofloxacin in this study were consistent with those found in previous studies of healthy patients (4). When levofloxacin was used thrice weekly at a dose of 1,000 mg, maximum concentrations were in excess of 12 μg/ml. In vitro studies have shown the MIC of levofloxacin to be <1.0 μg/ml for M. tuberculosis and 4 μg/ml for MAC (9, 11). Since levofloxacin likely demonstrates concentration-dependent killing of mycobacteria, there is rationale to evaluate doses higher than those used for common community-acquired infections (10). In addition, the use of extended-interval dosing supports the recent emphasis on directly observed therapy.
Although there was a high incidence of adverse effects with levofloxacin, it is important to note that members of this group of HIV-infected patients were taking various other medications for treatment of the underlying disease. Many of the generalized adverse effects, such as nausea, diarrhea, and headache, were likely attributable to concomitant medications used for HIV infection. This is demonstrated by the high incidence of adverse effects in the placebo groups.
Levofloxacin is likely to be used in HIV-infected patients for treatment of both common bacterial infections and drug-resistant mycobacterial infections. Its in vitro activity combined with good oral absorption, penetration into macrophages, and lack of drug interactions makes it a useful agent in this population. Additional studies are warranted to evaluate the role of levofloxacin in HIV-infected patients with concomitant mycobacterial disease.
REFERENCES
- 1.Alangaden G J, Lerner S A. The clinical use of fluoroquinolones for the treatment of mycobacterial diseases. Clin Infect Dis. 1997;25:1213–1221. doi: 10.1086/516116. [DOI] [PubMed] [Google Scholar]
- 2.Andrews J M, Honeybourne D, Jevons G, Brenwald N P, Cunningham B, Wise R. Concentrations of levofloxacin (HR 355) in the respiratory tract following a single oral dose in patients undergoing fibre-optic bronchoscopy. J Antimicrob Chemother. 1997;40:573–577. doi: 10.1093/jac/40.4.573. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Tuberculosis morbidity—United States, 1997. Morbid Mortal Weekly Rep. 1998;47:253–257. [PubMed] [Google Scholar]
- 4.Chien S-C, Rogge M C, Gisclon L G, Curtin C, Wong F, Natarajan J, Williams R R, Fowler C L, Cheung W K, Chow A T. Pharmacokinetic profile of levofloxacin following once-daily 500-milligram oral and intravenous doses. Antimicrob Agents Chemother. 1997;41:2256–2260. doi: 10.1128/aac.41.10.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chien S C, Chow A T, Rogge M C, Williams R R, Hendrix C W. Pharmacokinetics and safety of oral levofloxacin in human immunodeficiency virus-infected individuals receiving concomitant zidovudine. Antimicrob Agents Chemother. 1997;41:1765–1769. doi: 10.1128/aac.41.8.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cockcroft D W, Gault M H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 7.Cohn D L. Prevention strategies for Mycobacterium avium-intracellular complex (MAC) infection. A review of recent studies in patients with AIDS. Drugs. 1997;54(Suppl. 2):8–15. doi: 10.2165/00003495-199700542-00004. [DOI] [PubMed] [Google Scholar]
- 8.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1982. [Google Scholar]
- 9.Mor N, Vanderkolk J, Heifets L. Inhibitory and bactericidal activities of levofloxacin against Mycobacterium tuberculosis in vitro and in human macrophages. Antimicrob Agents Chemother. 1994;38:1161–1164. doi: 10.1128/aac.38.5.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peloquin C A, Iseman M D, Huitt G A, Berning S E. Levofloxacin for drug-resistant Mycobacterium tuberculosis. Ann Pharmacother. 1998;32:268–269. doi: 10.1345/aph.17167. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 11.Rastogi N, Goh K S, Bryskier A, Devallois A. Spectrum of activity of levofloxacin against nontuberculous mycobacteria and its activity against the Mycobacterium avium complex in combination with ethambutol, rifampin, roxithromycin, amikacin, and clofazimine. Antimicrob Agents Chemother. 1996;40:2483–2487. doi: 10.1128/aac.40.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wog F A, Juzwin S J, Flor S C. Rapid stereospecific high-performance liquid chromatographic determination of levofloxacin in human plasma and urine. J Pharm Biomed Anal. 1997;15:765–771. doi: 10.1016/s0731-7085(96)01890-0. [DOI] [PubMed] [Google Scholar]