Abstract
Context
The clinical utility and implications of continuous glucose monitoring (CGM) in cystic fibrosis (CF) are unclear.
Objective
We examined the correlation between CGM measures and clinical outcomes in adults with CF, investigated the relationship between hemoglobin A1c (HbA1c) and CGM-derived average glucose (AG), and explored CGM measures that distinguish cystic fibrosis–related diabetes (CFRD) from normal and abnormal glucose tolerance.
Methods
This prospective observational study included 77 adults with CF who had CGM and HbA1c measured at 2 to 3 time points 3 months apart.
Results
Thirty-one of the 77 participants met American Diabetes Association–recommended diagnostic criteria for CFRD by oral glucose tolerance testing and/or HbA1c. In all participants, CGM measures of hyperglycemia and glycemic variability correlated with nutritional status and pulmonary function. HbA1c was correlated with AG (R2 = 0.71, P < 0.001), with no significant difference between this regression line and that previously established in type 1 and type 2 diabetes and healthy volunteers. Cutoffs of 17.5% time > 140 mg/dL and 3.4% time > 180 mg/dL had sensitivities of 87% and 90%, respectively, and specificities of 95%, for identifying CFRD. Area under the curve and percent of participants correctly classified with CFRD were higher for AG, SD, % time > 140, > 180, and > 250 mg/dL than for HbA1c.
Conclusion
CGM measures of hyperglycemia and glycemic variability are superior to HbA1c in distinguishing those with and without CFRD. CGM-derived AG is strongly correlated with HbA1c in adults with CF, with a similar relationship to other diabetes populations. Future studies are needed to investigate CGM as a diagnostic and screening tool for CFRD.
Keywords: cystic fibrosis–related diabetes, continuous glucose monitoring, HbA1c, average glucose
Cystic fibrosis–related diabetes (CFRD) is the most common nonpulmonary complication of cystic fibrosis (CF), affecting roughly 20% of adolescents and 35% to 50% of adults with CF (1). The diagnosis of CFRD is associated with decreased pulmonary function, lower body mass index (BMI), and earlier mortality; however, early diagnosis and treatment may improve these outcomes (1-9). Because CFRD onset is typically insidious, annual screening is recommended in all patients over the age of 10 years (10). The 2-hour oral glucose tolerance test (OGTT) is the recommended screening test because the diagnosis of diabetes based on this testing is correlated with important CF-specific outcomes, including nutritional status and pulmonary function (6, 7, 11). However, compliance with annual OGTT screening is low (1), likely related to the logistical burden and inconvenience of this testing in the setting of multiple competing medical priorities. For this reason, there is increasing interest in exploring alternative approaches to CFRD screening and diagnosis.
Hemoglobin A1c (HbA1c) has an important role in the diagnosis and management of type 1 and type 2 diabetes, but its utility in CFRD is less clear. The A1c Derived Average Glucose (ADAG) study established the strong correlation between HbA1c and average glucose (AG) derived from fingerstick glucose levels and continuous glucose monitoring (CGM) in adults with type 1 and type 2 diabetes and healthy volunteers via a simple linear regression equation (12). Historically, the relationship between AG and HbA1c was thought to differ in patients with CF, with the expectation that HbA1c underestimated true mean glycemia, potentially due to altered red blood cell kinetics (13-15). However, a recent study investigating CGM in youth with CF found that HbA1c correlated well with CGM-derived AG and that this relationship was similar to that in type 1 and type 2 diabetes populations (16). Further studies are needed to understand this relationship in older patients with CF over a broad range of glycemia.
CGM has been validated in patients with CF and has been shown to detect early glycemic variability otherwise missed on OGTT (4, 17-24). These early glucose abnormalities may be associated with worse pulmonary function, and glucose values > 200 mg/dL detected by CGM predicted future development of CFRD (4, 5, 18, 19, 25, 26). Although increasingly being used in clinical practice for the management of CFRD, few studies have investigated the role of CGM as a tool for the diagnosis of CFRD, and whether CGM measures can reliably distinguish CFRD from other categories of glucose tolerance is unknown. Long-term prospective data evaluating CGM as a screening tool to predict CF-specific outcomes unfortunately do not yet exist. However, if CGM is found to reliably predict the diagnosis of CFRD by OGTT or other standard diagnostic criteria, this represents an important step for the use of CGM as an alternative and less burdensome approach for diagnosing CFRD.
The goals of this prospective observational study were 3-fold: (1) to explore the correlation between CGM-derived measures of glycemic variability and important clinical outcomes in patients with CF, including pulmonary function and BMI; (2) to investigate the correlation between HbA1c and CGM-derived AG in adults with CF with and without CFRD, building on prior studies by adding longer duration of CGM data collected prospectively over multiple time points; and (3) to determine if CGM measures can distinguish those with and without CFRD, laying the groundwork for future studies investigating CGM as a diagnostic tool for CFRD.
Methods
Study Population
Primary analysis sample
Participants ages 18 to 70 years with an established diagnosis of CF were recruited from the Massachusetts General Hospital (MGH) CF Center and the Boston Children’s Hospital/Brigham and Women’s Hospital CF Center. Exclusion criteria included pregnancy, lung transplantation, known hemoglobinopathy, creatinine > 2.0 mg/dL, and a history of blood transfusion, CF exacerbation, or supraphysiologic systemic glucocorticoid treatment in the preceding 12 weeks. For participants with preexisting CFRD, the diagnosis of diabetes was confirmed by chart review based on the criteria established by both the American Diabetes Association and Cystic Fibrosis Foundation, including prior OGTT consistent with the diagnosis of CFRD and/or HbA1c ≥ 6.5% (10). None of the individuals with preexisting CFRD were diagnosed based on transient hyperglycemia occurring during a CF exacerbation or while on glucocorticoids. Individuals with CFRD managed their diabetes per usual care, and none of the participants utilized personal CGM devices during the study period. The study was approved by the Massachusetts General Brigham Institutional Review Board (IRB) with ceded review from the Boston Children’s Hospital IRB, and written informed consent was obtained from all participants.
Validation sample
As described in the Statistical Methods, CGM cutoffs calculated from the primary analysis sample were analyzed in a separate convenience population serving as a preliminary validation sample. This validation sample consisted of individuals ages 18 to 70 years with CF who were not enrolled in the primary study and either did not have CFRD or had early mild CFRD not yet treated with insulin. Clinical and CGM data in the validation sample were obtained from 2 separate IRB-approved protocols, a prospective study investigating CGM use in adults with CF and a retrospective chart review of patients seen at the CF Centers. The same exclusion criteria and definitions of CFRD were applied to both cohorts.
Clinical Assessments
Participants in the primary analysis sample were evaluated at study visits at baseline and 3 months later. To address the potential confounding effect of hyperglycemia from illness or glucocorticoid use during the study period, those participants diagnosed with a CF exacerbation (defined by hospital admission and/or intravenous antibiotic use) and those requiring supraphysiologic systemic glucocorticoids had an additional “exacerbation visit” approximately 1 month after the exacerbation followed by the final study visit 3 months later.
Baseline clinical characteristics were obtained by questionnaire and included medical history, pancreatic insufficiency (defined as pancreatic enzyme replacement requirement), medications, hospitalizations, and pulmonary exacerbations over the past year. Race and ethnicity were self-reported. Height was measured on a wall-mounted stadiometer and weight on an electronic scale. Medical records were reviewed for CFTR genotype, confirmation of pulmonary exacerbations, and recent spirometry results closest to the baseline study visit (within 3 months), including percent predicted forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC).
Laboratory Procedures
At the baseline visit, participants in the primary analysis sample without preexisting CFRD underwent an OGTT after fasting for at least 8 hours. Glucola 75g was administered after HbA1c and fasting glucose levels were obtained, and repeat plasma glucose levels were drawn at 60 and 120 minutes. OGTT results were categorized into normal glucose tolerance (NGT, defined as fasting glucose < 100 mg/dL, 1-hour glucose < 200 mg/dL, and 2-hour glucose < 140 mg/dL), abnormal glucose tolerance (AGT, defined as fasting glucose 100-125 mg/dL, 1-hour glucose ≥ 200 mg/dL, and/or 2-hour glucose of 140-199 mg/dL), or CFRD (defined as fasting glucose ≥ 126 mg/dL and/or 2 hour glucose ≥ 200 mg/dL) based on American Diabetes Association and Cystic Fibrosis Foundation guidelines (10). Participants with preexisting CFRD underwent blood draw for HbA1c at baseline. HbA1c levels were repeated at the follow-up study visit and any exacerbation visits in all participants. HbA1c levels were measured via a high-performance liquid chromatography affinity assay (Trinity Biotech, Premier Hb9210 HbA1c analyzer, inter- and intra-assay CV < 2%) using an NGSP-certified instrument.
CGM Measures
Blinded CGM sensors (Freestyle Libre Pro, Abbott Laboratories, Illinois, mean absolute relative difference 12.3% (27)) were placed at each study visit. Participants wore each sensor for 14 days and then mailed the sensor to the MGH Diabetes Research Center for data capture. Participants who experienced sensor malfunction or loss with < 5 days of data collection were given the option of repeating the CGM wear. Participants who had a CF exacerbation requiring an interval exacerbation visit could have up to 3 14-day CGM sensor periods for inclusion in data analyses.
Primary CGM measures of interest included AG, % time 70 to 180 mg/dL, % time 70 to 140 mg/dL, hypoglycemia measures (% time < 70 mg/dL, % of time < 54 mg/dL), hyperglycemia measures (% time > 140 mg/dL, % time > 180 mg/dL, % of time > 250 mg/dL), and measures of glycemic variability (SD, coefficient of variation [CV], continuous overlying net glycemic action [CONGA] (28), and mean amplitude of glucose excursion [MAGE] (29)).
Statistical Analysis
Statistical analyses were performed using STATA (version 16, 2019; College Station, TX: StataCorp LLC). Bayesian modeling was conducted using the WinBUGS (The WinBUGS Project, http://www.mrc-bsu.cam.ac.uk/software/bugs/). Normality was assessed for all variables using the Shapiro-Wilk test. Baseline characteristics were compared between participants divided by glycemic category (NGT, AGT, and CFRD) using the Kruskal Wallis test, followed by pairwise comparisons with Tukey correction. Categorical variables were compared using chi- square tests. Mean HbA1c and CGM measures were calculated by combining all data from each visit and 2-week CGM collection period.
The association between CGM-derived glycemic measures and other variables including HbA1c, FEV1, and BMI were determined using Spearman correlation coefficients, followed by adjustment for age and gender using multivariable linear regression. The relationship between BMI and CGM measures were explored graphically via histograms, confirming that a linear relationship assumption was reasonable and that overweight habitus (BMI > 25 kg/m2) did not separately correlate with dysglycemia. To investigate for stability of glycemic measures over time, Wilcoxon signed rank test was used to assess if glycemic measures changed significantly between the baseline and final visit. Repeated measures analysis of variance (ANOVA) was used to analyze the subset of participants with additional exacerbation visits.
The relationship between mean HbA1c and AG was investigated using univariate linear regression, followed by multivariable regression adjusting for age, gender, race, and percent predicted FEV1. These confounders were chosen to reflect those analyzed in the ADAG study (age, race, and gender) (12), with the addition of FEV1 to determine if more compromised pulmonary function could alter the relationship between AG and HbA1c, potentially through postulated changes in red cell kinetics. In addition, we also applied Bayesian methods to estimate the relationships among mean HbA1c and CGM-derived average glucose using methods as previously described in the ADAG study (12). Determination of contrasts between the linear regression model and that of ADAG (12) and the recent study of CGM in youth with CFRD (16) was conducted via analysis of differences in the slope (β-coefficient) and intercept based on chi-squared statistics with 2 degrees of freedom.
The performance of HbA1c and CGM measures in distinguishing participants with CFRD from those without CFRD was evaluated using receiver-operating-characteristic (ROC) curves with calculation of area under the curve (AUC). The optimal cutoff maximizing sensitivity and specificity of each variable was estimated based on 1000 bootstrap samples. The approach to maximize both sensitivity and specificity was chosen because of the clinical importance of identifying CFRD for optimizing health and longevity (favoring high sensitivity), balanced with the significant treatment burden that a diagnosis places on a patient (favoring high specificity). The sensitivity, specificity, and percent correction classification were calculated for each glycemic measure.
Validation Sample
Cutoffs determined from ROC analyses were investigated in a preliminary validation sample independent from the participants enrolled in this study, focusing on patients without diagnosed CFRD and those with early mild CFRD not yet treated with insulin. CGM results in the validation sample were obtained using the Dexcom G6 and G6Pro (MARD 7%) and the Freestyle Libre Pro (MARD 12.3%). Those in the validation sample had 5 to 14 days of CGM data collected within 2 years of OGTT and/or had confirmed diagnosis of CFRD not treated with insulin at the time of CGM data collection. The sensitivity, specificity, positive predictive value (PPV) and negative predictive values (NPV) in this sample were calculated for each glycemic measure cutoff derived from the primary analysis sample.
Results
Baseline clinical characteristics of all enrolled participants (n = 77) are summarized in Table 1. Twenty-five participants had preexisting CFRD diagnosed based on prior OGTT (n = 11) or HbA1c ≥ 6.5% (n = 14), and 52 participants underwent an OGTT at the baseline visit to determine their glycemic category. Based on OGTT results, 6 were diagnosed with CFRD, 22 with AGT, and 24 with NGT (Table 1). Participants with CFRD were older and had lower baseline FEV1 than those with NGT and AGT. More hospitalizations in the preceding year were noted in the CFRD group compared to the NGT group, and those with NGT were more likely to have pancreatic sufficiency. Roughly half of all participants were treated with CFTR modulators at the time of enrollment with no differences between groups. Of the participants with known CFRD at enrollment, the age at CFRD diagnosis was 26 ± 13 years, CFRD duration was 13 ± 13 years, and 76% were using insulin. Four participants (1 with CFRD, 1 with AGT, and 2 with NGT) had no CGM data available due to sensor malfunction and/or loss to follow-up. Data from the remaining 73 participants were included in glycemic data analyses. Interval exacerbation visits were required in 12 participants, 4 without CFRD and 8 with CFRD. Due to participants lost to follow-up as well as research closures and safety concerns related to the COVID-19 pandemic, follow-up visits could not be performed for 15 participants. Average participant CGM sensor wear time across all study visits (including exacerbation visits) was 25.5 ± 9 days, and those with CFRD had slightly longer sensor wear than those with AGT (28 ± 8 vs 22 ± 7 days, P = 0.049).
Table 1.
Clinical characteristics and glycemic measures
| Total | NGT | AGT | CFRD | P value | |
|---|---|---|---|---|---|
| Baseline characteristics | n = 77 | n = 24 | n = 22 | n = 31 | |
| Age (years) | 33.1 ± 1.3 | 33.5 ± 2.9 | 28.6 ± 2.0 | 36.1 ± 1.9 | 0.035 |
| Female | 52 (67.5%) | 17 (70.8%) | 14 (63.6%) | 21 (67.7%) | 0.873 |
| Race | 0.542 | ||||
| White | 73 (95%) | 24 (100%) | 21 (95.5%) | 28 (90%) | |
| Black | 3 (4%) | 0 | 1 (4.5%) | 2 (6.5%) | |
| Asian | 1 (1%) | 0 | 0 | 1 (3%) | |
| Other | 0 | 0 | 0 | 0 | |
| Ethnicity | 0.873 | ||||
| Hispanic | 5 (6%) | 2 (8%) | 1 (4.5%) | 2 (6.5%) | |
| Non-Hispanic | 72 (94%) | 22 (92%) | 21 (95.5%) | 29 (93.5%) | |
| Genotype | |||||
| F508del homozygous | 30 (39%) | 8 (33%) | 9 (41%) | 13 (42%) | 0.583 |
| F508del heterozygous | 34 (44%) | 11 (46%) | 9 (41%) | 14 (45%) | 0.978 |
| Other | 13 (17%) | 5 (21%) | 4 (18%) | 4 (13%) | 0.725 |
| Pancreatic Insufficiency | 68 (88%) | 17 (71%) | 21 (96%) | 30 (97%) | 0.006 a,c |
| BMI, kg/m 2 | 23.2 ± 0.4 | 24.3 ± 0.7 | 23 ± 0.9 | 22 ± 0.5 | 0.086 |
| FEV1, % predicted | 74 ± 3 | 85 ± 4 | 78 ± 5 | 61 ± 4 | 0.002 a,b |
| FVC, % predicted | 86 ± 2 | 93 ± 4 | 91 ± 4 | 78 ± 4 | 0.028 a |
| Modulator use | 35 (46%) | 13 (54%) | 13 (59%) | 9 (29%) | 0.857 |
| Elexacaftor-tezacaftor-ivacaftor | 11 (14%) | 4 (17%) | 4 (18%) | 3 (10%) | |
| Tezacaftor-ivacaftor | 12 (16%) | 6 (25%) | 5 (23%) | 1 (3%) | |
| Lumacaftor-ivacaftor | 7 (9%) | 1 (4%) | 3 (14%) | 3 (10%) | |
| Ivacaftor | 5 (6%) | 2 (8%) | 1 (5%) | 2 (6%) | |
| CF exacerbations in past year | 1.8 ± 0.2 | 1.2 ± 0.2 | 1.6 ± 0.4 | 2.2 ± 0.3 | 0.259 |
| Hospitalizations in past year | 0.5 ± 0.1 | 0.3 ± 0.1 | 0.5 ± 0.1 | 0.7 ± 0.1 | 0.014 a |
| Mean glycemic outcomes throughout the study period | n = 73 | n = 22 | n = 21 | n = 30 | |
| HbA1c, % (mmol/mol) | 5.9 ± 0.1 (41 ± 1.4) | 5.2 ± 0.1 (33 ± 0.7) | 5.6 ± 0.1 (38 ± 0.9) | 6.6 ± 0.3 (49 ± 2.73) | <0.001 a,b |
| AG, mg/dL | 116 ± 5 | 91 ± 2 | 100 ± 3 | 145 ± 8 | <0.001 a,b |
| SD, mg/dL | 36.2 ± 2.6 | 21.2 ± 1.0 | 26.7 ± 1.3 | 53.9 ± 4.5 | <0.001 a,b |
| CV | 29.6 ± 1.0 | 23.4 ± 1.1 | 26.8 ± 1.0 | 36.1 ± 1.5 | <0.001 a,b |
| % time 70-180 mg/dL | 80.4 ± 2.1 | 85.2 ± 3.7 | 88.3 ± 1.7 | 71.5 ± 3.7 | <0.001 a,b |
| % time 70-140 mg/dL | 70.9 ± 2.5 | 82.1 ± 3.6 | 81.7 ± 1.5 | 55.1 ± 3.9 | <0.001 a,b |
| % time >140 mg/dL | 19.8 ± 2.7 | 3.6 ± 0.7 | 8.8 ± 1.4 | 39.5 ± 4.3 | <0.001 a,b |
| % time >180 mg/dL | 10.3 ± 2.1 | 0.5 ± 0.1 | 2.2 ± 0.6 | 23.1 ± 4.0 | <0.001 a,b |
| % time >250 mg/dL | 3.7 ± 1.1 | 0.01 ± 0.01 | 0.2 ± 0.1 | 8.9 ± 2.4 | <0.001 a,b |
| % time <70 mg/dL | 9.2 ± 1.4 | 14.1 ± 3.7 | 9.5 ± 1.9 | 5.5 ± 1.0 | 0.020 a |
| % time <54 mg/dL | 2.1 ± 0.5 | 3.4 ± 1.5 | 1.9 ± 0.7 | 1.3 ± 0.3 | 0.49 |
| CONGA, mg/dL | 34.3 ± 1.5 | 24.6 ± 1.7 | 31.7 ± 2.7 | 43.3 ± 2.0 | <0.001 a,b |
| MAGE, mg/dL | 89.0 ± 6.1 | 51.9 ± 3.1 | 67.7 ± 4.6 | 131.1 ± 10.1 | <0.001 a,b |
Data displayed as mean ±SE or n (%) unless otherwise indicated.
Abbreviations: AG, average glucose; AGT, abnormal glucose tolerance; BMI, body mass index (kg/m2); CFRD, cystic fibrosis–related diabetes; CONGA, continuous overall net glycemic action; CV, coefficient of variation; FEV1, forced expiratory volume; FVC, forced vital capacity; HbA1c, hemoglobin A1c; MAGE, mean amplitude of glycemic excursions; N, number; NGT, normal glucose tolerance.
a P < 0.05 comparing NGT vs CFRD;
b P < 0.05 comparing AGT vs CFRD;
c P < 0.05 comparing NGT vs AGT
Table 1 displays the mean HbA1c levels and CGM measures obtained throughout the study period divided across the 3 glycemic categories. HbA1c, CGM-derived AG, and CGM measures of hyperglycemia and glycemic variability were significantly greater in the CFRD group compared with both the NGT and AGT cohorts. Percent time < 70 mg/dL was significantly lower in individuals with CFRD compared to both NGT and AGT, while % time < 54 mg/dL did not significantly vary between glycemic categories. There were no differences in any glycemic measures between the AGT and NGT groups.
Table 2 presents the correlation coefficients between HbA1c, CGM variables, and key clinical outcomes in CF, namely BMI and FEV1. HbA1c was strongly correlated with CGM measures of hyperglycemia (% time > 140 mg/dL, > 180 mg/dL, and > 250 mg/dL) and glycemic variability (AG, CV, MAGE, and CONGA). BMI was negatively correlated with CGM measures of AG, hyperglycemia, and glycemic variability, but no relationship was noted between BMI and HbA1c. There was a significant negative correlation between FEV1 and most CGM measures as well as HbA1c, with AG and % time > 140 mg/dL having the strongest association with FEV1. Adjustment for age and gender did not affect the significance of these findings (data not shown).
Table 2.
Correlation between glycemic and clinical measures
| HbA1c | BMI | FEV1 | |
|---|---|---|---|
| HbA1c | --- | -0.20 | -0.46*** |
| AG | 0.78*** | -0.25* | -0.49* |
| SD | 0.80*** | -0.30* | -0.44*** |
| CV | 0.70*** | -0.36* | -0.35* |
| % time >140 mg/dL | 0.82*** | -0.27* | -0.47*** |
| % time >180 mg/dL | 0.82*** | -0.29* | -0.46*** |
| % time >250 mg/dL | 0.76*** | -0.28* | -0.40** |
| % time <70 mg/dL | -0.40** | 0.10 | 0.30* |
| % time <54 mg/dL | -0.25* | 0.09 | 0.15 |
| CONGA | 0.67*** | -0.36* | -0.45*** |
| MAGE | 0.79*** | -0.31* | -0.41** |
Data displayed are Spearman’s rho correlation coefficients.
Abbreviations: AG, average glucose; BMI, body mass index, kg/m2; CONGA, continuous overall net glycemic action; CV, coefficient of variation; FEV1, percent predicted forced expiratory volume in 1 second; HbA1c, hemoglobin A1c; MAGE, mean amplitude of glycemic excursions.
* P value ≤ 0.05; ** P value ≤ 0.001; *** P value ≤ 0.0001.
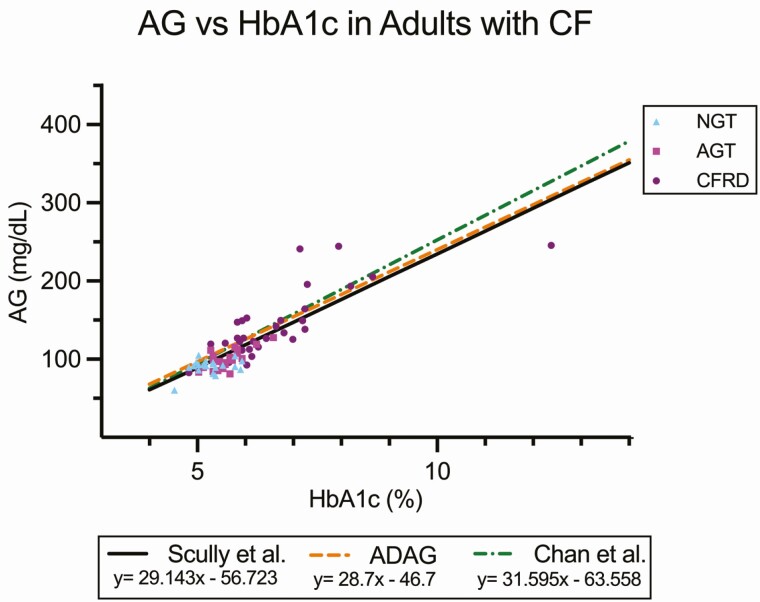
In addition, there was a strong correlation between mean HbA1c and AG (Fig. 1), defined by the regression equation eAG = 29.143(HbA1c)-56.723 (R2 = 0.71, P < 0.0001). This relationship remained significant after adjustment for age, gender, race, and percent predicted FEV1 (R2 = 0.77, P < 0.001, data not shown). The regression equation was separately determined using the Bayesian methods and was very similar to the linear regression, eAG = 30.62(HbA1c)-56.41, with 95% CI for the slope (29.10, 32.13) and intercept (−64.54, −48.26). In the Bayesian model, the correlation has a SD described by the equation SD = a*HbA1c^(b/2), where a = 0.005946 (95% CI = 0.000553, 0.054123) and b = 7.418 (95% CI = 4.19, 10.89).
Figure 1.
Regression line and scatterplot of HbA1c vs CGM-derived AG divided into OGTT glycemic category (NGT, AGT, CFRD). Regression lines are included from the ADAG study (adults with type 1 and type 2 diabetes and healthy volunteers) (12) and from the study published by Chan et al (youth with CF) (16). Scully et al represents the regression line from the present analysis.
Fig. 1 also displays the linear regression analyses of HbA1c and AG from 2 other notable published studies, showing no significant differences in the slopes or intercepts of the regression line calculated in our study compared to adults with type 1 and type 2 diabetes and healthy volunteers from the ADAG study (12) (difference in slope P = 0.84 and in intercept P = 0.45) and youth with CF published by Chan et al (16) (difference in slope P = 0.27 and intercept P = 0.61).
To investigate the stability of HbA1c and CGM measures over time, we compared differences in these outcomes between baseline and final visits (Supplemental Table 1) (30). HbA1c, AG, and % time > 140 mg/dL significantly varied between the 2 study visits, with no significant difference noted in the remaining CGM measures. In the 12 participants who had an exacerbation visit, only % time > 140 mg/dL showed significant variation between their exacerbation visit and the other study visits (Supplemental Table 2) (30).
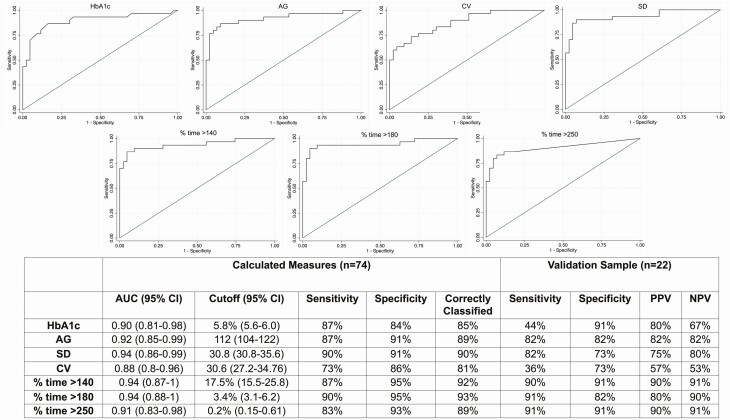
In this study, the diagnosis of CFRD in all participants was made based on either OGTT performed at baseline or documented prior OGTT or HbA1c diagnostic of CFRD, allowing us to accurately classify participants with and without CFRD according to current gold standard approaches and construct ROC curves for all glycemic variables of interest. Based on these data, the optimal cutoff value distinguishing those with and without CFRD was calculated for each variable. Focusing on data that can be easily obtained from standard CGM reports, Fig. 2 shows the ROC curves and corresponding AUC for the glycemic variables of greatest interest, along with the cutoff values for each variable and the corresponding sensitivity, specificity, and percent correctly classified. AUC and percent of participants correctly classified as having CFRD were higher for AG, SD, % time > 140 mg/dL, > 180 mg/dL, and > 250 mg/dL than for HbA1c. Of particular interest, % time > 180 mg/dL showed the best performance in terms of AUC, sensitivity, specificity, and number correctly classified. ROC analyses and cutoff data for CONGA and MAGE were similar to SD and CV (Supplemental Figure 1) (30).
Figure 2.
ROC analyses and calculated cutoffs for the prediction of CFRD. All P values for AUC < 0.0001.
To test these glycemic cutoffs in an independent sample of patients with CF either without CFRD or with early CFRD not yet treated with insulin, we conducted a preliminary validation analysis in 22 participants not included in the original cohort (non-CFRD n = 11, CFRD n = 11, Fig. 2). This sample was 59% female with mean age 27 ± 9.8 years (range, 13-55 years) and FEV1 83 ± 21%. Ninety-five percent of this sample had pancreatic insufficiency, and all had at least one copy of the F508del mutation. Of the 11 individuals with preexisting CFRD, 4 were diagnosed with CFRD based on HbA1c levels ≥ 6.5%, and the remaining 7 individuals had prior OGTT results consistent with a diagnosis of CFRD. CGM devices used by these individuals included the Freestyle Libre Pro (n = 10), Dexcom G6 (n = 10), and Dexcom G6Pro (n = 2). The HbA1c cutoff of 5.8% had a sensitivity of 44% and a specificity of 91% for identifying patients with CFRD. In contrast, cutoff for % time spent > 180 mg/dL had an 91% sensitivity and ≥ 80% specificity, PPV, and NPV, and cutoffs for % time > 140 mg/dL and > 250 mg/dL had sensitivities, specificities, PPV, and NPV ≥ 90%.
Discussion
CGM-derived measures of hyperglycemia and glycemic variability are significantly correlated with important clinical outcomes in CF, including BMI and pulmonary function, and can reliably distinguish between participants with and without CFRD across a broad range of glucose tolerance more consistently than HbA1c. Although historically HbA1c was thought to be spuriously low in people with CF, we found that AG correlates strongly with HbA1c and that this relationship is similar to patients with type 1 and type 2 diabetes. However, HbA1c does not capture the glycemic variability that occurs in mild CFRD and therefore may not perform as well as CGM as a screening test for CFRD and in identifying the clinical effects of dysglycemia. These findings lay the groundwork for the use of CGM as a tool in the diagnosis of CFRD and support the need for additional studies validating this as a screening strategy in this patient population.
CF-related dysglycemia exists across a spectrum of β-cell dysfunction and insulin resistance, and early CFRD is typically characterized by brief postprandial hyperglycemia without fasting hyperglycemia that gradually progresses to more substantial insulin deficiency and glycemic deterioration over time. Measures of mean glucose like HbA1c may not reflect this brief postprandial hyperglycemia occurring early in the course of CFRD, therefore missing an opportunity to intervene in a timely manner, as illustrated by studies showing the poor sensitivity of HbA1c in diagnosing CFRD when compared with OGTT (31, 32). In addition, although microvascular complications are less common in patients with CFRD, these tend to occur at a lower HbA1c than in other diabetes populations (33), suggesting that alternative markers of glycemia and glycemic variability may be important in gauging the effects of diabetes in people with CF.
Emerging evidence suggests that CGM provides important information about glycemic abnormalities that have direct implications for the health of patients with CF. Several studies have reported significant correlation between CGM measures and clinical outcomes in children with CF, including FEV1, pulmonary inflammation, and infection rates, and that higher and more variable glucose levels on CGM correlate with lung function decline (4, 21, 34). We found that CGM measures of hyperglycemia and glycemic variability significantly correlated with important CF-specific outcomes, including FEV1 and BMI, and that these correlations were stronger than those of HbA1c. These findings suggest that the information obtained by CGM is clinically meaningful and perhaps more relevant for clinical care than HbA1c in CF.
Several studies have investigated the relationship between AG and HbA1c in patients with CF using fingerstick glucose results, but with contradictory results (14, 35). In a landmark paper in youth with CF, Chan et al found that HbA1c was significantly correlated with CGM-derived AG and that this correlation was similar to that observed in the ADAG study and the Diabetes Control and Complications Trial (16). Because this study analyzed data from predominately pediatric patients without CFRD at a single timepoint, our study further builds on these results by including a large sample of older adults over a broader distribution of glycemia, using repeated measures of HbA1c and a longer duration of CGM data collected at 2 to 3 time points prospectively over a 3-month period. Together, these studies suggest that HbA1c reflects AG levels in this patient population similar to other diabetes populations. However, it is important to note that relatively few participants in our study had HbA1c levels above 7%, limiting the conclusions that can be drawn particularly at the higher HbA1c ranges. In addition, the R2 from the linear regression was lower than that noted in the ADAG study, suggesting that there may be more variability in the relationship between AG and HbA1c in CF than other diabetes populations.
In our study, all participants without the prior confirmed diagnosis of CFRD underwent an OGTT in order to categorize their glycemic status, providing the opportunity to investigate whether CGM can distinguish different categories of dysglycemia in CF. We found that participants with CFRD had significantly higher AG, longer time in hyperglycemic ranges, and greater glycemic variability than participants with NGT and AGT. However, CGM measures were not significantly different between those with NGT and AGT, indicating that CGM may not be the best approach for distinguishing milder degrees of glucose intolerance diagnosed by OGTT in patients with CF.
Given that glycemic control can often fluctuate over time in individuals with CF related to their underlying illness, we assessed changes in HbA1c and CGM measures between baseline and study completion. Unlike HbA1c, CGM measures such as SD, CV, % time > 180 mg/dL, and % time > 250 mg/dL did not vary significantly when measured 3 months apart, suggesting that these CGM findings are stable over this time, supporting similar findings observed in other studies (22). Interestingly, % time >140 mg/dL varied significantly between baseline and follow-up visits and in the subset of patients with exacerbation visits, suggesting that fluctuations in this marker of less severe hyperglycemia may occur more often than other CGM measures over time, making it less useful as a diagnostic criterion.
Determining a set of CGM cutoff values for identifying CFRD has long been a topic of interest (4, 16, 25); however, at present there are no standardized criteria for the use of CGM to diagnose diabetes in any patient population. Recently, reference sensor glucose ranges were investigated in 153 healthy individuals > 6 years of age without diabetes, reporting AG of 99 mg/dL, time > 140 mg/dL of 2.1%, and time > 180 mg/dL of 0.0% in this sample (36). In a cohort of 23 children at high risk for developing type 1 diabetes based on the presence of elevated autoantibodies, a CGM cutoff of 18% time > 140 mg/dL had 75% sensitivity and 100% specificity for predicting progression to diabetes, and a cutoff of 2% time > 180 mg/dL had 63% sensitivity and 87% specificity (37). Similarly, we calculated cutoff values for several key CGM measures distinguishing those with and without CFRD, most notably 3.4% time > 180 mg/dL and 17.5% time > 140 mg/dL. The corresponding ROC analyses were quite striking, with AUC values > 0.9 and with sensitivity and specificity results at >90% for these and other CGM outcomes, correctly classifying over 90% of enrolled participants. These results are promising and lay the foundation for the development of meaningful CGM criteria for the diagnosis of CFRD, which is greatly needed in this patient population.
This study was intentionally enriched with participants with CFRD to ensure that a broad range of glycemia was represented. Although a strength of the study, this could also impact analyses establishing cutoffs for distinguishing CFRD vs no CFRD, as these may be more obvious when applied to those with established CFRD and may not have the same sensitivity and specificity in those with milder disease in whom the diagnosis is not clear. In addition, the majority of the participants in the CFRD group were treated with insulin, which affected their CGM results and represents a different clinical situation than those undergoing screening. For this reason, we conducted a small validation analysis from a convenience population of 22 participants not enrolled in this study, half of whom did not have diabetes and the other half with mild CFRD not yet treated with insulin. Focusing on the measures easily obtainable from a standard CGM report, the analyses for time in hyperglycemic ranges (> 140 mg/dL, > 180 mg/dL, and > 250 mg/dL) all resulted in sensitivities and NPV > 90%, and specificities and PPV > 80%. All key CGM variables apart from CV outperformed HbA1c in the proportion of patients correctly classified with CFRD. Similar to our analysis, Burgess et al proposed an HbA1c cutoff of 5.8% for the diagnosis of CFRD, reporting a 93% sensitivity vs OGTT; however, a subsequent retrospective analysis of 207 patients using the same cutoff found only a 68.2% sensitivity and 60.5% specificity (15, 38), consistent with the results in our validation sample. Additional studies are needed to further assess the utility of these CGM cutoff measures as a screening approach and to test this screening strategy in a much larger, more robust validation sample.
At present, the OGTT is the recommended screening tests for CFRD because of its known prediction of important pulmonary and nutritional outcomes in patients with CF (6, 10). Ultimately, large long-term prospective studies will be needed to investigate if CGM will similarly predict clinical decline in CF. In the meantime, identifying CGM measures that correlate with the diagnosis of CFRD establishes an important first step in this process, particularly given the notable benefits of using CGM in this setting. For example, obtaining CGM data by placing a sensor at a clinic visit is easy and convenient, offering the potential to substantially improve CFRD screening rates. In addition, CGM provides a comprehensive assessment of glycemia occurring during a patient’s typical home environment, allowing for the identification of glycemic patterns to guide management decisions and, if needed, individualize insulin therapy in an efficient manner.
This study has several limitations that warrant consideration. First, the study was conducted at 2 centers located in one geographic location and had limited racial and ethnic representation. Participants provided glycemic data at 2 distinct time points, with some participants only having 1 complete study visit. Continuous measurement of CGM data throughout the 3-month period may have provided more accurate glycemic data. In addition, we used OGTT to determine the diagnosis of CFRD in this study; however, variability in OGTT results has been noted in patients with CF (1, 39, 40). The OGTT is the currently accepted gold standard diagnostic test for CFRD and therefore this limitation cannot be avoided; however, this underscores the need for long-term studies investigating the performance of OGTT vs CGM in the screening for CFRD. While the range of dysglycemia in our cohort was greater than prior published studies, relatively few individuals had elevated HbA1c values > 9% and approximately half had HbA1c levels < 6.5%, therefore limiting the conclusions that can be drawn in assessing the relationship between HbA1c and AG particularly in higher A1c ranges. The participants enrolled in this study were relatively healthy with mild impairment in lung function. Some groups have suggested that patients with more advanced lung disease may have more severely altered red blood cell kinetics, which could potentially impact the AG-HbA1c relationship (13, 14, 41). However, adjusting for FEV1 did not change the results of the linear regression, suggesting that pulmonary function did not significantly affect the HbA1c-AG relationship. This study was not designed to assess the utility of CGM as a screening strategy for CFRD; in this case, it would have been preferable to include only those individuals newly diagnosed with CFRD but not yet on treatment in our ROC analyses. Instead, our results focus on CGM as a diagnostic tool for distinguishing those with and without CFRD and support the need for future prospective studies aimed at determining its value as a screening method. In addition, different types of CGM devices were used in our validation cohort, which reflects real-world application of CGM but may potentially decrease the precision of the CGM data. Lastly, our validation sample assessing CGM cutoffs was small and included those with most recent OGTT data from a 2-year time period. It is possible that glycemic status may have changed in some individuals in this 2-year period, not captured by our data. Although this validation cohort offers a preliminary assessment of the proposed CGM cutoff values, a larger prospective study is needed to investigate this as a screening strategy in individuals without CFRD.
Conclusions
CGM-derived measures of hyperglycemia and glycemic variability correlated more strongly than HbA1c with key clinical outcomes, including BMI and FEV1, and distinguished participants with and without CFRD. There is a clear correlation between CGM-derived AG and HbA1c levels in patients with CF, and this relationship is not significantly different than other diabetes populations. However, HbA1c does not capture the postprandial hyperglycemia and glycemic variability that characterize early CFRD and therefore does not perform well as a screening tool in this patient population. We identified cutoff values for CGM measures that may aid in the diagnosis of CFRD, laying the foundation for CGM to be used as a diagnostic tool for CFRD. Further large-scale, prospective studies are needed to explore how CGM measures can predict pulmonary and nutritional decline and microvascular complications in patients with CF and to investigate the use of CGM as a screening strategy in this patient population.
Acknowledgments
The authors thank the patients and the clinical and research teams of the Massachusetts General Hospital (MGH) and the Boston Children’s Hospital CF Centers as well as the MGH Diabetes Research Center personnel.
Financial Support: This study was funded by a Cystic Fibrosis Foundation Clinical Research Award (PUTMAN16A0) and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Heath (NIH) T32 (5T32DK007699).
Author Contributions: K.J.S. researched and analyzed data and wrote the manuscript. K.M., M.R., P.M., and M.L. researched data and reviewed and edited the manuscript. J.S.S., G.S.S., A.U., I.N., L.S. contributed to the discussion and reviewed and edited the manuscript. H.Z. contributed to the data analyses. M.S.P. and D.J.W. conceived and designed the study and reviewed and edited the manuscript. M.S.P is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation: Parts of this study were presented in abstract form at the North American Cystic Fibrosis Conference, Phoenix AZ, 21-23 October 2020.
Glossary
Abbreviations
- ADAG
A1c Derived Average Glucose
- AG
average glucose
- AGT
abnormal glucose tolerance
- AUC
area under the curve
- BMI
body mass index
- CF
cystic fibrosis
- CFRD
cystic fibrosis–related diabetes
- CGM
continuous glucose monitoring
- CONGA
continuous overall net glycemic action
- CV
coefficient of variation
- FEV1
percent predicted forced expiratory volume in 1 second
- FVC
forced vital capacity
- HbA1c
glycosylated hemoglobin A1c
- IRB
institutional review board
- MAGE
mean amplitude of glucose excursion
- NGT
normal glucose tolerance
- NPV
negative predictive value
- OGTT
oral glucose tolerance test
- PPV
positive predictive value
- ROC
receiver operating characteristic
Additional Information
Disclosures: G.S.S. and A.U. have served on advisory boards for Vertex Pharmaceuticals (unrelated to this work). G.S.S, A.U., and L.S. have received research funding from Vertex Pharmaceuticals (unrelated to this work). D.J.W. serves on data monitoring committees for Novo Nordisk (unrelated to this work). M.S.P. received research funding from Vertex Pharmaceuticals in the form of an Investigator Initiated Studies Grant (unrelated to this work). The other authors have nothing to disclose.
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Cystic Fibrosis Foundation. 2019 patient registry annual data report. 2019. Accessed March 10, 2021. https://www.cff.org/Research/Researcher-Resources/Patient-Registry/2019-Patient-Registry-Annual-Data-Report.pdf
- 2. Moran A, Pekow P, Grover P, et al. Insulin therapy to improve BMI in cystic fibrosis-related diabetes without fasting hyperglycemia results of the cystic fibrosis related diabetes therapy trial the cystic fibrosis related diabetes therapy study group*. 2009;32(10):1783-1788. [DOI] [PMC free article] [PubMed]
- 3. Norris AW, Ode KL, Merjaneh L, et al. Survival in a bad neighborhood: pancreatic islets in cystic fibrosis. J Endocrinol. 2019;241(1):R35-R50. doi: 10.1530/JOE-18-0468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prentice BJ, Ooi CY, Strachan RE, et al. Early glucose abnormalities are associated with pulmonary inflammation in young children with cystic fibrosis. J Cyst Fibros. 2019;18(6):869-873. doi: 10.1016/j.jcf.2019.03.010 [DOI] [PubMed] [Google Scholar]
- 5. Yi Y, Norris AW, Wang K, et al. Abnormal glucose tolerance in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 2016;194(8):974-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ode KL, Frohnert B, Laguna T, et al. Oral glucose tolerance testing in children with cystic fibrosis. Pediatr Diabetes. 2010;11(7):487-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Milla CE, Warwick WJ, Moran A. Trends in pulmonary function in patients with cystic fibrosis correlate with the degree of glucose intolerance at baseline. Am J Respir Crit Care Med. 2000;162(3 Pt 1):891-895. [DOI] [PubMed] [Google Scholar]
- 8. Mozzillo E, Franzese A, Valerio G, et al. One-year glargine treatment can improve the course of lung disease in children and adolescents with cystic fibrosis and early glucose derangements. Pediatr Diabetes. 2009;10(3):162-167. [DOI] [PubMed] [Google Scholar]
- 9. Lanng S, Thorsteinsson B, Nerup J, Koch C. Diabetes mellitus in cystic fibrosis: effect of insulin therapy on lung function and infections. Acta Paediatr. 1994;83(8):849-853. [DOI] [PubMed] [Google Scholar]
- 10. Moran A, Brunzell C, Cohen RC, et al. ; CFRD Guidelines Committee . Clinical care guidelines for cystic fibrosis-related diabetes: a position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33(12):2697-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care. 2009;32(9):1626-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ; A1c-Derived Average Glucose Study Group . Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31(8):1473-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hardin DS, Grilley K, Baron B, Hale KA. Accelerated red blood cell turnover can invalidate the use of hemoglobin a1c as a diagnostic test for cystic fibrosis related diabetes. Pediatr Res. 1999;45(4, Part 2 of 2):90A. [Google Scholar]
- 14. Godbout A, Hammana I, Potvin S, et al. No relationship between mean plasma glucose and glycated haemoglobin in patients with cystic fibrosis-related diabetes. Diabetes Metab. 2008;34(6 Pt 1):568-573. [DOI] [PubMed] [Google Scholar]
- 15. Boudreau V, Coriati A, Desjardins K, Rabasa-Lhoret R. Glycated hemoglobin cannot yet be proposed as a screening tool for cystic fibrosis related diabetes. J Cyst Fibros. 2016. doi: 10.1016/j.jcf.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 16. Chan CL, Hope E, Thurston J, et al. Hemoglobin A1c accurately predicts continuous glucose monitoring–derived average glucose in youth and young adults with cystic fibrosis. Diabetes Care. 2018;41(7):1406-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dobson L, Sheldon CD, Hattersley AT. Conventional measures underestimate glycaemia in cystic fibrosis patients. Diabet Med. 2004;21(7):691-696. [DOI] [PubMed] [Google Scholar]
- 18. Leclercq A, Gauthier B, Rosner V, et al. Early assessment of glucose abnormalities during continuous glucose monitoring associated with lung function impairment in cystic fibrosis patients. J Cyst Fibros. 2014;13(4):478-484. [DOI] [PubMed] [Google Scholar]
- 19. Brodsky J, Dougherty S, Makani R, Rubenstein RC, Kelly A. Elevation of 1-hour plasma glucose during oral glucose tolerance testing is associated with worse pulmonary function in cystic fibrosis. Diabetes Care. 2011;34(2):292-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brugha R, Wright M, Nolan S, Bridges N, Carr SB. Quantifying fluctuation in glucose levels to identify early changes in glucose homeostasis in cystic fibrosis. J Cyst Fibros. 2018;17(6):791-797. [DOI] [PubMed] [Google Scholar]
- 21. Prentice BJ, Chelliah A, Ooi CY, et al. Peak OGTT glucose is associated with lower lung function in young children with cystic fibrosis. J Cyst Fibros. 2020;19(2):305-309. [DOI] [PubMed] [Google Scholar]
- 22. O’Riordan SM, Hindmarsh P, Hill NR, et al. Validation of continuous glucose monitoring in children and adolescents with cystic fibrosis: a prospective cohort study. Diabetes Care. 2009;32(6):1020-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frost F, Dyce P, Nazareth D, Malone V, Walshaw MJ. Continuous glucose monitoring guided insulin therapy is associated with improved clinical outcomes in cystic fibrosis-related diabetes. J Cyst Fibros. 2018. doi: 10.1016/j.jcf.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 24. Jefferies C, Solomon M, Perlman K, Sweezey N, Daneman D. Continuous glucose monitoring in adolescents with cystic fibrosis. J Pediatr. 2005;147(3):396-398. [DOI] [PubMed] [Google Scholar]
- 25. Chan CL, Ode KL, Granados A, Moheet A, Moran A, Hameed S. Continuous glucose monitoring in cystic fibrosis - A practical guide. J Cyst Fibros. 2019;18 Suppl 2:S25-S31. [DOI] [PubMed] [Google Scholar]
- 26. Taylor-Cousar JL, Janssen JS, Wilson A, et al. Glucose >200 mg/dL during continuous glucose monitoring identifies adult patients at risk for development of cystic fibrosis related diabetes. J Diabetes Res. 2016;2016:1527932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Clinical Results and Outcomes. FreeStyle Libre Pro System | FreeStyle libre providers. Accessed March 30, 2021. https://provider.myfreestyle.com/freestyle-libre-pro-clinical-evidence.html
- 28. McDonnell CM, Donath SM, Vidmar SI, Werther GA, Cameron FJ. A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol Ther. 2005;7(2):253-263. [DOI] [PubMed] [Google Scholar]
- 29. Service FJ, Molnar GD, Rosevear JW, Ackerman E, Gatewood LC, Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19(9):644-655. [DOI] [PubMed] [Google Scholar]
- 30. Putman MS, Scully KJ. Continuous glucose monitoring in cystic fibrosis: glycemic relationships, clinical correlations, and implications for CFRD diagnosis. dryad, dataset. Posted online December 6,2021. doi: 10.5061/dryad.k3j9kd57s. [DOI]
- 31. Lanng S, Hansen A, Thorsteinsson B, Nerup J, Koch C. Glucose tolerance in patients with cystic fibrosis: five year prospective study. BMJ. 1995;311(7006):655-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holl RW, Buck C, Babka C, Wolf A, Thon A. HbA(1c) is not recommended as a screening test for diabetes in cystic fibrosis. Diabetes Care. 2000;23(1):126. [DOI] [PubMed] [Google Scholar]
- 33. Schwarzenberg SJ, Thomas W, Olsen TW, et al. Microvascular complications in cystic fibrosis-related diabetes. Diabetes Care. 2007;30(5):1056-1061. [DOI] [PubMed] [Google Scholar]
- 34. Chan CL, Vigers T, Pyle L, Zeitler PS, Sagel SD, Nadeau KJ. Continuous glucose monitoring abnormalities in cystic fibrosis youth correlate with pulmonary function decline. J Cyst Fibros. 2018;17(6):783-790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Brennan AL, Gyi KM, Wood DM, Hodson ME, Geddes DM, Baker EH. Relationship between glycosylated haemoglobin and mean plasma glucose concentration in cystic fibrosis. J Cyst Fibros. 2006;5(1):27-31. [DOI] [PubMed] [Google Scholar]
- 36. Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: a multicenter prospective study. J Clin Endocrinol Metab. 2019;104(10):4356-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steck AK, Dong F, Taki I, et al. Continuous glucose monitoring predicts progression to diabetes in autoantibody positive children. J Clin Endocrinol Metab. 2019;104(8):3337-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burgess JC, Bridges N, Banya W, et al. HbA1c as a screening tool for cystic fibrosis related diabetes. J Cyst Fibros. 2016;15(2):251-257. [DOI] [PubMed] [Google Scholar]
- 39. Balion CM, Raina PS, Gerstein HC, et al. Reproducibility of impaired glucose tolerance (IGT) and impaired fasting glucose (IFG) classification: a systematic review. Clin Chem Lab Med. 2007;45(9):1180-1185. [DOI] [PubMed] [Google Scholar]
- 40. Scheuing N, Holl RW, Dockter G, et al. High variability in oral glucose tolerance among 1128 patients with cystic fibrosis: a multicenter screening study. PLoS One. 2014;9(11):e112578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wagener JS, McNeill GC, Taussig LM, Corrigan JJ, Lemen R. Ferrokinetic and hematologic studies in cystic fibrosis patients. Am J Pediatr Hematol Oncol. 1983;5(2):153-160. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.