Abstract
Cancer cachexia is a multifactorial, paraneoplastic syndrome that impacts roughly half of all cancer patients. It can negatively impact patient quality of life and prognosis by causing physical impairment, reducing chemotherapy tolerance, and precluding them as surgical candidates. While there is substantial research on cancer-induced skeletal muscle cachexia, there are comparatively fewer studies and therapies regarding cardiac cachexia in the setting of malignancy. A literature review was performed using the PubMed database to identify original articles pertaining to cancer-induced cardiac cachexia, including its mechanisms and potential therapeutic modalities. Seventy studies were identified by two independent reviewers based on inclusion and exclusion criteria. While there are multiple studies addressing the pathophysiology of cardiac-induced cancer cachexia, there are no studies evaluating therapeutic options in the clinical setting. Many treatment modalities including nutrition, heart failure medication, cancer drugs, exercise, and gene therapy have been explored in in vitro and mice models with varying degrees of success. While these may be beneficial in cancer patients, further prospective studies specifically focusing on the assessment and treatment of the cardiac component of cachexia are needed.
Keywords: cancer, cardiac cachexia, reactive oxygen species, TNFα
1. Introduction
Cachexia is a multifactorial and multi-organ syndrome characterized by sarcopenia, inflammation, and negative protein balance that cannot be reversed with conventional nutritional support [1,2,3,4,5]. Per international consensus, cachexia is diagnosed if the patient meets one of the following criteria: weight loss > 5% over six months, BMI < 20 and any weight loss > 2%, or appendicular skeletal muscle index indicative of sarcopenia and any weight loss > 2% [2]. Cachexia is noted in many chronic inflammatory conditions, including autoimmune disorders, chronic lung diseases, acquired immunodeficiency syndrome (AIDS), congestive heart failure (CHF), and cancer [3,5]. Cancer cachexia can have a detrimental impact on patient mortality and quality of life. Roughly 50% of cancer patients develop cachexia, with the highest incidence in the gastric and pancreatic cancer patient population [4,5]. In the clinical setting, patients present with lack of appetite, involuntary weight loss, and progressive physical impairment [4,6]. The resulting debilitation can impair the patient’s immune system and reduce tolerance to chemotherapy. Cachexia can also promote hepatic dysfunction, thus perpetuating the nutritional deficit and impacting cardiac and respiratory muscles leading to cardiopulmonary failure [7,8]. Patients that undergo surgical intervention are at increased risk for postoperative complications and have higher mortality rates [9,10]. Overall, cancer cachexia is a predictor of poor patient outcomes and is responsible for roughly 20% of cancer-associated deaths [4].
There have been many studies investigating the molecular pathogenesis of cancer-associated cachexia, particularly in skeletal muscle. The main driving force behind this syndrome is chronic systemic inflammation and tumor–host interaction. Tumor and host immune cells release pro-cachectic cytokines such as tumor necrosis factor (TNFα), interleukin 1 (IL-1), IL-6, and interferon gamma (IFNγ) [1,6,7,11]. TNFα drives skeletal muscle catabolism by inducing ubiquitin-mediated proteasome degradation (UPR) via the nuclear factor kappa-B (NF-κB) pathway [7,11,12]. TNFα also synergizes with IL-1 and IFNγ to impact appetite. They cross the blood–brain barrier and induce a series of neurohormonal alterations to promote anorexia and muscle wasting. They increase levels of available serotonin, reduce secretion of appetite-stimulating hormones such as neuropeptide Y and ghrelin, and trigger the hypothalamic–pituitary–adrenal axis, thus further promoting skeletal muscle and adipose tissue breakdown [7,8,11].
While there is heavy emphasis on skeletal muscle degradation, cancer cachexia can also have an impact on cardiac muscle and function. Independent of cardiotoxicity secondary to chemotherapy, cancer cachexia can cause cardiac muscle degradation, leading to heart failure [13,14]. Understanding the mechanisms behind cancer-induced cardiac cachexia can allow for improvements in patient management both in the setting of chemotherapy and surgical intervention. This article will review the current literature regarding cancer-induced cardiac cachexia, including mechanisms, diagnostic methods, and current therapeutic options.
2. Materials and Methods
A literature search was performed to identify original research pertaining to cardiac alterations secondary to cancer cachexia. An advanced search was performed on the PubMed Search Engine from 1960 through to 24 August 2021. The following search string was utilized: ((cardiac cachexia) AND (cancer)) OR ((cancer cachexia) AND (heart)) OR ((cardiac sarcopenia) AND (cancer)) OR ((cardiac cachexia) AND (methods)) OR ((cardiac sarcopenia) AND (methods)) OR ((cardiac muscle wasting) AND (cancer)) OR ((cardiac muscle wasting) AND (methods). Studies were limited to journal articles and those published in English. Studies were excluded if they were abstract only, not published in full, or were duplicate articles. Articles were considered eligible if the research addressed cardiac cachexia in the setting of cancer. Studies that pertained to cardiac toxicity secondary to chemotherapy or heart failure precipitating cachexia were excluded.
Two individuals (T.D. and V.V.) independently screened titles and abstracts based on the above search terms and inclusion/exclusion criteria. Articles were initially screened for relevance by title. The remaining articles were screened by abstract for eligibility. All articles that met inclusion criteria were independently reviewed by both reviewers in their entirety.
3. Results
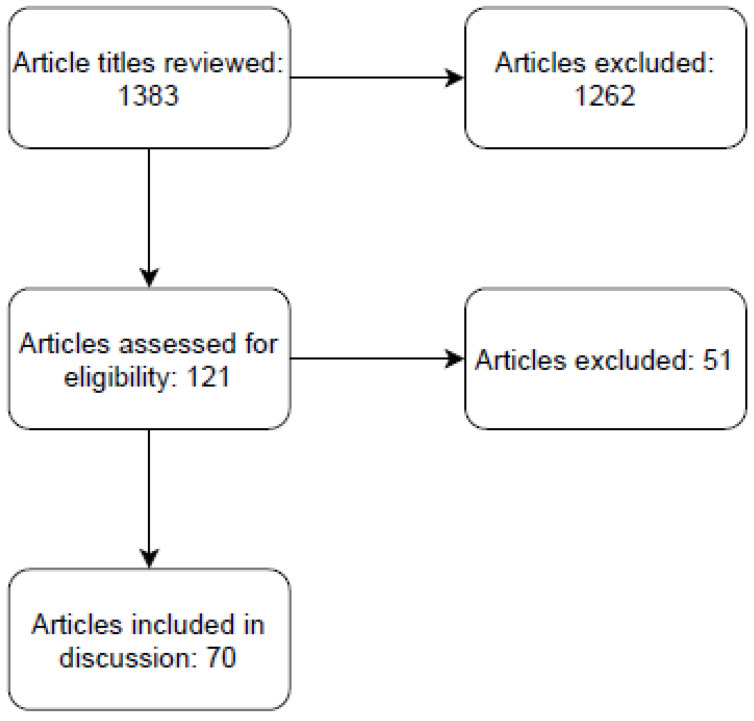
The resulting search yielded 1383 articles including duplicates (Figure 1). In total, 121 articles were selected based on title relevance. These articles were then screened based on either abstract or full text. Ultimately, 70 articles were identified that presented original research on cancer-induced cardiac cachexia.
Figure 1.
PRISMA diagram of literature search.
4. Discussion
4.1. Mechanisms
4.1.1. TNFα
Pro-inflammatory cytokines are known to play a critical role in the development of cancer cachexia. Markers such as TNFα, IL-6, and Ataxin-10 were observed to be elevated in cardiac tissue of colon-26 (C26) adenocarcinoma mice [15,16,17]. In early studies, TNFα was noted to have an impact on protein degradation rates in cardiac muscle after observing a decrease in protein loss in hepatoma mice receiving anti-TNF treatment [18]. Similarly, increased myocardial weight loss was observed in normal female rats treated with TNFα [19]. TNFα activates the NF-κB pathway, resulting in increased myocardial turnover via the UPR system and increased oxidative stress secondary to suppression of glutathione peroxidase production [20]. Furthermore, other moieties, such as high mobility group box protein 1 (HMGB1), which induce TNFα expression in cardiac myocytes were also increased in cancer cachexia models [21]. As in skeletal muscle, TNFα plays a pivotal role in cardiac cachexia via multiple pathways.
4.1.2. Autophagy
Increased autophagy is another mechanism involved in cancer cachexia. Autophagy involves degradation of intracellular proteins, organelles, and macromolecules secondary to cellular stress. In colorectal tumor-bearing mice, markers of increased lysosomal activation, including cathepsin L, beclin, and microtubule-associated protein light chain 3 (LC3), were elevated in the myocardium. Direct observation of autophagy was obtained via electron microscopy, demonstrating autophagic vacuoles containing mitochondria and cytoplasm [22]. Autophagy can be activated by multiple pathways, including alterations in AMP-activated protein kinase (AMPK) and PI3K/Akt/mTOR signaling.
Activation of phosphoinositide-3 kinase (PI3K) by insulin and insulin-like growth factor 1 (IGF-1) leads to downstream upregulation of Akt and mTOR, which subsequently enhances protein synthesis. In cancer patients, the utilization of glucose by tumor cells leads to reduced circulating insulin, thus reducing activation of this pathway. As confirmation, mTOR expression was shown to be reduced in preclinical models of colorectal cancer (CRC) and melanoma [20,23]. One of these studies, however, noted that while mTOR expression was suppressed, there was an increase in Akt phosphorylation. This pathway activation could be a compensatory mechanism by the myocardium to preserve cardiac mass [23].
AMPK is typically activated by decreased intracellular ATP/ADP ratio and serves to suppress energy demanding cellular activities. AMPK activation can induce upregulation of autophagy associated moieties and suppress mTOR formation, thus reducing protein synthesis. Manne et al. demonstrated the upregulation of AMPK and decrease in mTOR signaling in the cardiomyocytes of adenomatous polyposis coli mice (APC+). Interestingly, they also noted no evidence of increased protein ubiquitination or apoptosis in these hearts, thus placing greater weight on autophagy as a key molecular mechanism in cancer-induced cardiac cachexia [23].
4.1.3. Reactive Oxygen Species
Increased oxidative stress is the result of an imbalance between the production of reactive oxygen species (ROS) and their breakdown by antioxidant mechanisms, such as glutathione peroxidase. Elevated ROS can promote activation of proteolytic pathways such as ubiquitination and calcium-dependent proteolysis [24]. Hinch et al. demonstrated this imbalance in pro- and antioxidants in murine adenocarcinoma cell lines 13 (MAC13) mice by noting increased levels of xanthine oxidase and decreased expression of antioxidant superoxide dismutase in the myocardium [25]. This finding is corroborated by Lee et al., whose study showed increased ratios of oxidized to reduced molecules and decreased levels of ROS scavengers in Lewis lung carcinoma bearing mice. The resulting ROS also damages mitochondrial DNA, which can negatively impact the myocytes’ ability to process oxygen in the electron transport chain, thus accumulating more ROS [26].
4.1.4. Ubiquitination: Atrogin-1/MuRF1
Protein degradation in both skeletal and cardiac muscle requires ubiquitination of targeted proteins. These are typically done via E3 ligase enzymes. Atrogin-1 and MuRF1 (muscle ring finger 1) are two ligases that have been implicated in cardiac muscle proteolysis. These enzymes can be attenuated by many pathways, including NF-κB and Akt/mTOR. In their studies, both Tian et al. and Wysong et al. demonstrated increased expression of both ligases in the atrophic myocardium of C26 mice [27,28]. Matsuyama et al. also analyzed cytokine and Atrogin/MuRF expression levels in C26 mice. However, they also compared the impact of both intraperitoneal and subcutaneous implantation. Interestingly, the subcutaneous group had no reduction in cardiac weight and normal levels of both ligases in contrast to the intraperitoneal group. These findings demonstrate that tumor location plays a significant role in cancer cachexia phenotype and progression [29].
4.1.5. Cancer-Induced Cardiac Cachexia Pathways
There are many other mechanisms in the literature that have been implicated in cancer induced cardiac cachexia, including calcium-dependent proteolysis, decreased neural stimulation, activin activation, and metalloproteinases. Costelli et al. demonstrated the role of Ca2+-dependent proteolytic systems in cardiac atrophy. In this pathway, increased intracellular calcium leads to elevated levels of proteolytic enzymes, such as calpain. In their study, they evaluated cachexia in male Wistar rats inoculated with ascites hepatoma (AH-130) cells. Analysis of the cardiac muscle revealed elevated levels of calpain and decreased expression of Ca2+-ATPase (transport protein) and calpastatin (calpain inhibitor). However, the authors also note that calpains have been implicated in degradation of transcription factors such as NF-κB, thus suggesting that calpains may play a more complex role in attenuating myocardial damage [30,31]. Muhlfeld et al. noted decreased levels of nerve growth factor in stellate ganglion of myocardium derived from Lewis lung carcinoma bearing mice. This was associated with a decrease in vesicle number per axon and total length of axon in the left ventricle (LV). While the study noted the potential role of elevated IL-6 and TNFα, no causal relationship was established [16]. Activin molecules, which are TGF-B moieties, have been implicated in both cardiac and skeletal muscle wasting. These molecules bind to activin receptor type 2B (ACVR2B) and can induce degradation of skeletal muscle, cancellous bone, and cardiac function. Antagonism of ACVR2B has been shown to preserve cardiac function in CRC-bearing mice [32]. This pathway is further modulated by histone deacetylases, such as SIRT6, that block the expression of activin and ACVR2B [33]. Finally, one other pathway that has not been studied extensively is the alteration of the extracellular matrix (ECM). Devine et al. demonstrated that the cardiac and skeletal muscles in C26 bearing mice had increased protein levels of matrix metalloproteinases. These enzymes degrade the ECM, causing disruptions in cell-to-cell and cell-to-basement membrane interactions, thus promoting cardiac dysfunction. While this mechanism has been discussed in the setting of heart failure, this is the first study to address its role in cancer-induced cardiac dysfunction [34].
4.2. Models to Study Cardiac Cachexia
Establishing a reproducible animal model of cachexia is crucial to delineate the mechanisms leading to cardiac sarcopenia. Historically, the Lewis lung carcinoma, C26 colorectal adenocarcinoma, patient-derived xenograft, and genetically engineered mouse models have been used to investigate cachexia [35]. To standardize the well-studied C26 model for studying cancer cachexia, Bonetto et al. subcutaneously injected 1 × 106 C26 cells in each CD2F1 mouse. At the end of a 10–14-day period, significant losses in skeletal and cardiac muscle weights were observed [36]. In a subcutaneous Ehrlich ascites carcinoma (EAC) model in 129/SvJ mice, echocardiography performed at weekly intervals showed a decline in left ventricular (LV) thickness, ejection fraction (EF) and Fractional Shortening (FS) and an increase in LV internal diameter [37]. A pancreatic ductal adenocarcinoma (PDAC) cachexia model was developed by generating a tumor cell line from genetically modified mice with oncogenic KRAS G12D mutation and additional tumor suppressor P53 R172H mutation, and subsequently implanting the cells in wild-type C57BL/6 mice either subcutaneously, intraperitoneally or orthotopically. This study demonstrated PDAC induced cardiac cachexia in an autophagy-dependent manner. Additionally, mice with orthotopic and intraperitoneal implants demonstrated a reduction in cumulative food intake when compared to mice receiving subcutaneous implants [35].
Orthotopic tumor mouse models can also involve implantation of patient-derived xenografts into tissues matching the tumor histology, thereby preserving the tumor architecture and stromal environment. However, the requirement of immunosuppression and the potential loss in signaling pathways due to absence of species homology can potentially serve as limitations toward implementing this model extensively [35].
The lack of a standard approach toward developing a cachexia model poses a significant barrier in comparing existing data surveying this phenomenon. Bonetto et al. noted that the strain of the mouse, method and site of tumor implantation, tumor source and number of cells injected can influence outcomes pertaining to cachexia even within the C26 model [36]. In the EAC model—as in other animal models—cardiac dysfunction manifested as LV dilatation, but cancer cachexia generally presents as reduced ventricular volume in humans, even in the absence of chamber dilatation [37]. Therefore, further standardization and refinement of animal models are needed to achieve translationally viable outcomes.
4.3. Methods to Assess Cardiac Cachexia
Cardiac cachexia in cancer manifests with biochemical and functional impairments, which can be analyzed to monitor disease progression and efficacy of ongoing interventions. In preclinical studies, measurements of cardiac weight at necropsy—generally expressed as a ratio to total body weight—have been utilized to demonstrate cardiac sarcopenia. However, the presence of generalized edema in tumor bearing mice was shown to influence this ratio, reflecting a higher than expected value for normalized cardiac weights in a C26 model at day 14 after tumor implantation [15]. Therefore, metrics utilizing weight-loss relative to BMI as a parameter for cachexia must factor in variability stemming from study design prior to analysis and interpretation.
Imaging techniques, including echocardiography and cardiac MRI, allow for assessment of changes in cardiac functional status in-vivo and in clinical studies. In a retrospective clinical analysis, a positive correlation was observed between BMI and LV and RV wall thickness (LVWT and RVWT) in GI cancer and LVWT in lung cancer [38]. In a C26 model implanted in CD2F1 female mice, changes in fractional shortening and posterior wall thickness (PWT) were noted in tumor bearing mice, but diastolic parameters were not affected [39]. In a comparative clinical study involving patients with colorectal cancer (CRC), CHF and controls, impairment in left ventricular ejection fraction (LVEF) was observed in CRC patients, although not to the degree observed in patients with CHF. Increased PWT was observed in CRC and CHF patients, but not in controls [40]. Alternatively, cardiac MRI might be more sensitive to changes in cardiac structure than can be detected via 2D echocardiography and is operator independent. While MRI studies in cardiac cachexia from cancer are lacking, analysis of CHF patients with and without cachexia showed down-trending cardiac weights in patients with cachexia versus an uptrend in patients without cachexia despite the CHF [41]. Further standardization of data from imaging studies can be attained by tracking indices combining data from individual parameters. For instance, the cachexia index (CXI) was formulated by incorporating skeletal muscle area and skeletal muscle index at the L3 level—as obtained from abdominal CT scan images [42]. However, due to the paucity of imaging data assessing cardiac cachexia in patient populations, further analysis is required prior to development of standardized indices for cancer associated cardiac cachexia.
Molecular and microscopic techniques offer a unique window into the subcellular changes associated with cachexia. In Tian et al., transverse electron microscopy (TEM) showed disruptions in myocardial ultrastructure [15]. In a C26 model, proteomic assessment of cardiac, soleus and gastrocnemius tissues showed dissolution of Z disc and M line proteins, along with downregulation of proteins involved in myocyte energetics and substrate metabolism [43]. Metabolomic data from a C26 model demonstrated a unique ‘footprint’ to cachexia-associated changes in skeletal muscle that is distinct from the changes seen purely from caloric restriction [44]. Finally, an elevated neutrophil-to-lymphocyte ratio (NLR)—as derived from a complete blood count with differentials—was associated with greater weight loss and cachexia in patients with advanced colon, lung and prostate cancers [45]. The baseline NLR status was also demonstrated to be a negative prognostic biomarker for patients with cachexia in a multicenter cohort study evaluating 2612 patients with cancer [46]. However, the significance of this ratio for tracking cancer associated cardiac cachexia is yet to be explored. Identifying structural and biochemical changes prior to overt clinical manifestation of cardiac cachexia is significant. In Xu et al., while diastolic dysfunction was not seen in echocardiography data, cardiomyocyte function assessed at a cellular level showed impaired relaxation kinetics. While cardiac weights were not reduced, the expression of MAFbx and Bnip3—known biomarkers for degradation in skeletal muscle—were upregulated [39]. Within echocardiography parameters, a decline in global longitudinal strain (GLS) can predate functional decline in EF, as changes in GLS were shown to correlate with a decline in LV mass in a clinical study involving patients with non-small cell lung cancer (NSCLC) [47]. Cramer et al. noted that heart rate variability (HRV), a parameter associated with intact autonomic regulation of the heart, was impaired in patients with CRC. While the CRC patients were not tachycardic, their heart rates were significantly elevated compared to patients with CHF and controls, and the authors speculated a potential utility of monitoring heart rate to assess progression toward clinically symptomatic cardiac cachexia [40]. Taken together, these findings underscore the importance of a multimodal approach in estimating cardiac cachexia in preclinical studies and patient populations.
4.4. Treatment
Potential therapies are wide-ranging and target various pathways known to play significant roles in cancer cachexia. Table 1 briefly summarizes the treatment modalities discussed below.
Table 1.
Treatments modalities for mitigating cancer-induced cardiac cachexia.
| Treatment | Mechanism | Benefit | Reference | |
|---|---|---|---|---|
| NF-κB Pathway Inhibitors | Compound A, NF-κB essential modulator, Luteolin | NF-κB inhibiting agents | Preserved cardiac mass and EF and reduction of inflammatory markers | [27,48] |
| Trabectedin, Lurbinectedin | Inhibits cytokine activation of NF-κB | Some survival benefit but no clear impact on NF-κB signaling in cardiac tissue | [49] | |
| Resveratrol | Inhibition of inflammatory pathways and improvement in myocardial calcium handling | Reduction in cardiac weight loss and preservation of anterior wall thickness | [50,51] | |
| ROS Inhibitors | SS-31 | Antioxidant that reduces ROS in mitochondria | Restored LV function, reduced proteolytic Calpain activity in heart | [52] |
| Ubiquinol | Antioxidant involved in ROS modulation | Increased muscle mass; however, did not improve LV diameter or protein degradation | [53] | |
| Pepstatin | Inhibition of lysosomal protease and oxidative stress | Reduced muscle degradation, but no clear impact on myocardial function | [54,55] | |
| Cardiovascular Drugs | Simvastatin | Decreases activity of matrix metalloproteinase-9 and reduces activity of various inflammatory markers | Decreased weight loss, improved LVEF, and increased SV | [56,57] |
| Bisoprolol and spironolactone | Beta receptor blockade and aldosterone inhibitor respectively | Preserved LV mass, body weight; improved LVEF; reduced cardiac fibrosis | [55] | |
| Losartan and Withaferin A | Angiotensin II inhibition | Preserved EF and SV; reduced fibrotic deposition | [58,59] | |
| Formoterol | B2 selective agonist | Non-significant increase in cardiac weight; significant increase in end-diastolic and systolic volumes | [60] | |
| Nutrition and Appetite | Rosiglitazone | Insulin sensitizer | Improved LVEF and cardiac output; decreased muscle wasting | [61] |
| Megestrol acetate | Appetite stimulant | Increased weight gain; improved LVEF | [49] | |
| Leucine | Decreased levels of chymotrypsin, myeloperoxidase, and caspase 3 and 7 | Improved myocardial function | [62] | |
| Lauric acid and Glucose | Reduced mitochondrial dysfunction and oxidative stress | Reduced myocardial atrophy and improved muscle maturity | [63] | |
| Total parenteral nutrition (TPN) | Intravenous nutrition supplementation | Increased cardiac mass | [64] | |
| Other Categories | EPO | Possible decrease in trypsin levels | Increase in cardiac weight, stroke volume, and physical activity | [65] |
| Oxypurinol | Xanthine oxidase inhibitor | Increased LVEF, total cardiac weight, and cardiac output | [66] | |
| Tandospirone | Antidepressant; serotonin receptor agonist | Preserved muscle mass, improvement in LV mass and EF; some survival benefit | [67] | |
| Testosterone | Unclear | Increase in SV and LVEF | [67] | |
| Exercise | Unclear; possible reduction in cardiac autophagy and NF-κB signaling | Impeded tumor growth, delayed onset of anorexia, improved EF | [68,69,70,71,72] | |
| Gene Therapy via Viral Vector | Upregulating SMAD7, known to inhibit overactivation of procachetic factors | Reduced skeletal and cardiac muscle atrophy | [73] | |
| Crytotanshinone | STAT3 inhibition | Decreased myocardial mass loss, body weight loss, and muscle wasting | [74] | |
| Minocycline | Matrix metalloproteinase inhibitor | Improved FS and EF | [75] |
4.4.1. Anti-Proteolytics
Dysfunctional regulation of proteolytic activity is a target of interest for pharmacologic intervention. Devine et al. found that minocycline, a matrix metalloproteinase (MMP) inhibitor, improved FS and EF in a murine model. Decreased collagen RNA expression confirmed attenuation of MMP mediated cardiac fibrosis [75]. Cathepsin D, a lysosomal protease, is another proteolytic enzyme elevated in tumor-bearing mice. While its inhibition in murine cardiac tissue was shown to be feasible with the aspartyl protease inhibitor Pepstatin in a study by Greenbaum and Sutherland, effects on cardiac function have yet to be described [54]. Nevertheless, in vitro studies in primary neonatal rat cardiomyocytes showed inhibition of oxidative stress and resultant apoptosis by Pepstatin A. [55]. Saitoh et al. led a study investigating applications of Erythropoietin (EPO), a hormone used for amelioration of cancer-induced anemia, in cancer cachexia. In their rat model of liver cancer cachexia, EPO-associated increases in cardiac weight, stroke volume (SV), FS, and physical activity were hypothesized to be attributed to observed decreased levels of Trypsin. High-dose EPO treated rats conferred a survival advantage [65]. While promising, further study is needed to take anti-proteolytic therapy to the clinical stage.
4.4.2. Statins
Applying statins’ anti-inflammatory effects to cancer cachexia has yielded dissimilar results. Muscaritoli et al. surprisingly found that simvastatin had negative effects on muscle wasting in Yoshida AH-13 ascites hepatoma rats and highlighted a 15% reduction in cardiac weight, prompting them to caution its administration in cachectic patients [56]. This was contrasted in a more recent study conducted by Palus et al. in AH-130 bearing rats. They found that simvastatin treatment reduced muscle and body weight loss, improved LVEF, and increased SV. Importantly, treatment reduced mortality. These contradictory findings necessitate additional study to clarify simvastatin’s effects [57].
4.4.3. STAT3 Inhibition
STAT3 upregulation has various pro-cancer effects. Inhibition with Cryptotanshinone, a chemical with reported antiproliferative, anti-inflammatory, and anti-tumor properties, was explored by Chen et al. in CT26 tumor-bearing mice. While cellular analysis confirmed Cryptotanshinone induced STAT3 inhibition, treatment also decreased myocardial mass loss, body weight loss, muscle wasting, and epididymal fat [74]. Though understanding is limited, STAT3 inhibition is another pathway target in cancer cachexia.
4.4.4. Heart Failure Medication
Applying heart failure treatment to cardiac cachexia may alleviate shared symptoms.
To this end, Springer et al. explored bisoprolol (beta-blocker), imidapril (angiotensin converting enzyme inhibitor), and spironolactone (aldosterone inhibitor) in a rat hepatoma model. Treatment with spironolactone and bisoprolol preserved left ventricle mass, body weight, fat mass, and lean body mass in addition to improving LVEF and left ventricular FS with spironolactone showing greater effects. These agents also reduced caspase-3 and ubiquitin proteasome activity. Imidapril failed to produce similar outcomes. Specifically, in spironolactone-treated rats, cardiac fibrosis was reduced, which, when paired with observations of elevated aldosterone levels in human cancer patients, cements aldosterone’s significance in cancer cachexia [76]. These results parallel a related study of spironolactone’s application in an AH-130 hepatoma rat model, which found that treatment preserved LV diameter, increased LV mass, and downregulated neutrophil gelatinase-associated lipocalin, an aldosterone regulated gene upregulated in the heart failure and cachectic environment [77].
Angiotensin I and II inhibitors were also explored in cancer cachexia. Stevens et al. investigated losartan, an angiotensin II receptor blocker, in a C26 mouse model and found that losartan preserved EF, SV, and PWT while reducing left ventricular end-diastolic dimensions and normalizing calcium signaling dysfunction observed in tumor-bearing mice. Time to 90% muscle peak-shortening and 90% muscle re-lengthening were shorter in untreated tumor-bearing mice compared to treated mice. Interestingly, losartan impeded tumor cell proliferation [58]. Similar results were observed in an investigation of the steroidal lactone Withaferin A. Used for its anti-inflammatory characteristics and ability to impede tumor growth, Withaferin A administration to cachectic mice with ovarian cancer reduced tumor-associated increases in angiotensin II and subsequently reduced cardiomyocyte cross-sectional area loss, systolic and diastolic dysfunction, and fibrotic deposition [59]. These studies and the overlapping pathophysiology between cardiac cachexia and heart failure merit exploration of other heart failure agents.
4.4.5. Reactive Oxygen Species
Increased levels of ROS are key in cancer cachexia advancement. Smuder et al. recently explored ROS attenuation through restoration of mitochondrial function via the antioxidant peptide SS-31 in C26 mice. They reported that SS-31 decreased ROS production, restored left ventricular function, and diminished proteolytic Calpain activity in the heart when compared to saline-treated mice [52]. Other studies [78] emphasizing mitochondrial dysfunction in skeletal muscle atrophy supports further targeting of this pathway. Also involved in ROS modulation is Ubiquinol, an antioxidant deficient in various types of cancer. While its administration was associated with increased muscle mass in a C26 murine model, treatment failed to improve protein degradation, left ventricular diastolic diameter, PWT, and FS [53]. While in its early stages, development of therapeutics addressing ROS pathways underlying cancer cachexia represents a promising field of study.
4.4.6. NF-κB
NF-κB is a proinflammatory transcription factor whose activation by tumor derived-cytokines potentiates proteolysis. Shadfer et al. utilized the phytoalexin Resveratrol in a C26 mouse model to modulate NF-κB. In this study, they found that tumor-attributed decreases in cardiac weight to body weight ratios, anterior wall thickness (AWT), and PWT disappeared with Resveratrol treatment. Increases in NF-κB activity in the hearts of tumor-bearing mice were also ameliorated along with MuRF1 mRNA whose role in cardiac atrophy has been well documented [50,51]. Wysong et al. also targeted NF-κB through novel drugs Compound A and NF-κB essential modulator (NEMO) binding domain peptide in C26 mouse models. Separately, Compound A and NEMO binding domain peptide inhibited IkB kinase and subsequently NF-κB in the heart, preserving cardiac mass, EF, FS, cardiomyocyte area, and heart wall thickness [27]. Luteolin, a natural flavonoid, represents another NF-κB inhibiting agent, and its effects were discovered to include preservation of cardiac muscle mass, reduction of TNF-α and IL6 levels, and attenuation of increased MuRF1 levels in a Lewis lung cancer mouse model [48]. Distinctly, Aquila et al. used chemotherapy agents, trabectedin and lurbinectedin, to inhibit cytokine activation of NF-κB in C26 mice. While preliminary analysis of trabectedin and lurbinectedin demonstrated survival benefits, further study into lurbinectedin failed to impact NF-κB signaling in atrophying myotubes or exert cardiac protective effects [49]. Much like other disease processes, normalization of NF-κB activity is a key factor in addressing cachexia.
4.4.7. ActRIIB
Upregulation of the ACVR2B pathway is involved in numerous types of cancer and is associated with cancer cachexia. Zhou et al. found that ACVR2B antagonism reversed muscle wasting, protected against cardiac atrophy, and imparted a survival benefit. Cellular investigation connected the inhibition of ubiquitin-proteasome processes and ubiquitin ligases in muscle tissue with ACVR2B inhibition, thus further defining a mechanism for its effects [60]. The strength of these findings corroborates the ACVR2B pathway’s emergence as a therapeutic target in cancer cachexia.
4.4.8. Oxypurinol
Hyperuricemia is a risk factor for worse outcomes in cardiac cachexia. Uric acid regulation through inhibition of xanthine oxidase follows as a logical step to reduce hyperuricemia-related consequences. Oxypurinol (xanthine oxidase inhibitor) increased LVEF, FS, total cardiac weight, and cardiac output in a rat model of cancer cachexia [66]. Though promising, the mechanisms of these effects must be demonstrated in the pre-clinical setting before clinical application.
4.4.9. Antidepressants
Depression and anxiety are associated with cancer cachexia. Addressing these symptoms may not only provide direct relief but may also have secondary benefits. Elkina et al. explored the utilization of the antidepressant and anxiolytic drug tandospirone in a Yoshida hepatoma rat model. Treatment preserved muscle mass, locomotor activity, and food intake with accompanying improvement in left ventricular mass, SV, EF, and FS. Not to mention, a survival advantage was seen with treatment [67]. This study suggests antidepressants may have benefits outside their intended psychiatric impacts that require further investigation in future studies.
4.4.10. Weight and Muscle Gain
Promotion of muscle and weight gain have shown promise as therapeutic strategies. Rosiglitazone, an agent for type 2 diabetes mellitus, was found to not only improve LVEF, FS, and cardiac output in a cachectic rat model but also decrease muscle wasting and produce a survival benefit. Of note, previous observations of Rosiglitazone-induced cardiac hypertrophy were not replicated [61]. The application of other therapeutics used in diabetes such as Metformin have demonstrated potential utility in cancer-induced skeletal muscle cachexia mouse models but its significance in cancer-induced cardiac cachexia has never been explored [79,80]. Appetite-stimulating therapeutics like Megestrol acetate have also been investigated to promote weight gain. In cachectic tumor-bearing rats, improved LVEF, FS, and left ventricular end-systolic volume were associated with Megestrol acetate. Above all, mortality benefits were associated with treatment. Decreased Beclin-1 and LC3 indicated that Megestrol acetate suppressed autophagic pathways in cardiac tissue [81]. Formoterol, a potent B2 selective agonist known to generate skeletal muscle growth, was explored in a rat cachexia model. While treatment was associated with a non-significant increase in cardiac weight, preservation of left ventricular diameter and improved end-diastolic and end systolic volumes were all significantly associated with Formoterol [82]. Ojima et al. also studied the effects of promoting muscle growth in cachectic in vivo models by inhibiting negative regulation of skeletal muscle mass growth mediated by growth differentiation factor 8 (GDF-8). They found that by administering peptide 2 in tumor implanted C57BL/6 mice, they could inhibit GDF-8 signaling and promote myoblast differentiation. Although improvements in gross muscle mass with concomitant increase in grip strength were observed, cardiac muscle was excluded from these benefits [83]. These preclinical studies indicate that promoting muscle and weight gain hold potential for therapeutic applications in cancer cachexia. However, the limited data regarding these treatments necessitate further study.
4.4.11. Testosterone
Adjunct testosterone therapies have positive effects in cancer patients, but their impacts on cardiac function were only recently clarified. In 2019, Scott et al. published an analysis of a previous study assessing testosterone utilization in cachectic squamous cell carcinoma patients and evaluated testosterone’s effects on cardiac atrophy and dysfunction. Their investigation demonstrated testosterone-associated increases in SV and LVEF, as well as improvements in vascular parameters of arterial elastance and ventricular arterial coupling [84]. While promising, high powered randomized trials are necessary to elucidate testosterone’s mechanism of action and confirm its effects in cancer cachexia before clinical application.
4.4.12. Nutrition
Previous studies have suggested nutritional intervention as an adjunct therapy in cardiac cachexia. One rat model linked leucine’s anti-inflammatory, anti-oxidative stress, anti-proteolytic, and anti-apoptotic properties with attenuation of cancer cachexia-associated cardiac injury. Specifically, leucine decreased chymotrypsin, myeloperoxidase, tissue inhibitor of metalloproteinase, total plasminogen activator inhibitor 1, and caspases 3 and 7; all observed to be elevated in cachectic rats. Decreased levels of Tissue inhibitor of metalloproteinase 1 (TIMP-1), a marker of pathologic myocardial processes, further supported leucine administration [62]. A more recent study targeted oxidative stress mechanisms of myocardial damage stemming from mitochondrial dysfunction with co-administration of lauric acid and glucose. Nugaka et al. showed that this combination reduced mitochondria dysfunction in an in vitro cachexia model, leading to amelioration of oxidative stress and restoration of ATP. Their mice model also demonstrated that lauric acid and glucose improved levels of myocardial atrophy and muscle maturity [63]. Chance et al. separately studied the effects of dietary intervention in rats through maintenance with total parenteral nutrition and co-administration of acivicin (glutamine analog) and clenbuterol (beta-2 antagonist). They found that just sustaining sarcoma rats on TPN led to increased cardiac mass. Adding acivicin and clenbuterol enhanced the aforementioned effect while increasing skeletal muscle mass, inhibiting tumor growth, and promoting protein content [64]. Addition of over-the-counter nutritional supplements and medications may serve as valuable adjuncts to primary therapies. Some mouse model studies have suggested that non-steroidal anti-inflammatory drugs, glutamine, glycine, fish oils, carnitine and creatine may each hold value in the treatment of cancer-induced skeletal muscle cachexia [85,86,87,88,89,90]. However, these therapeutic effects have yet to be replicated or studied in the context of cancer-induced cardiac cachexia. Altogether, these positive results spanning various nutritional strategies merit further exploration.
4.4.13. Exercise
The benefits of exercise and conditioning in cancer patients are well established and may play a role in mitigating cardiac cachexia [68]. Parry and Hayward investigated the effects of exercise in a rat tumor model developed through inoculation of 13,762 MatBIII breast adenocarcinoma cells. They found that rats with running wheel training had reduced cardiac autophagic activity compared to sedentary tumor-bearing mice. Additionally, treatment impeded tumor growth and attenuated shifts in myosin heavy chain isoforms linked to reductions in left ventricular developed pressure [69]. A similar study in a C26 mouse model of cancer cachexia showed that resistance and aerobic exercise led to improved muscle mass, strength, and mitochondrial function [70]. Fernandes et al. alternatively approached exercise intervention by administering structured aerobic exercise training (AET) in a C26 mouse model. Treated mice had partially rescued cardiomyocyte cross-sectional diameter, improved EF, and a decrease in cardiac remodeling apparent in reduced necrosis, inflammation, and collagen deposition. Furthermore, AET attenuated the upregulation of BNIP3, a gene implicated in cardiac autophagy [71]. Another interventional AET study in a mammary tumorigenesis model advanced understanding of exercise’s mechanisms by identifying decreased cardiac TNF-related weak inducer of apoptosis (TWEAK) and NF-κB signaling, TRAF6, and atrogin-1 as causes for modulation of pathogenic cardiac remodeling [72]. Altogether, these preclinical studies have furthered the case for exercise in cardiac cachexia, and its ease of implementation supports inclusion into standard practice.
4.4.14. Gene Therapy
Gene therapy is a potential therapeutic avenue through targeting aberrant pathways. Winbanks et al. focused on the overactivation of the ActRIIB receptor, known to induce muscle wasting through binding of procachectic factors and downstream SMAD2/3 phosphorylation. SMAD2/3 proteins accumulate during muscle immobilization and are involved in skeletal muscle atrophy. By upregulating SMAD7, a SMAD2/3 negative regulator, with a recombinant viral vector, cachectic mice were protected from skeletal and cardiac muscle wasting via downregulation of ubiquitin ligases associated with SMAD2/3 linked atrophy [73]. Though still emerging, gene therapy has the potential to circumvent the shortcomings of other conventional approaches.
Research in the treatment of cardiac cachexia is still evolving but has yielded promising therapeutic targets. Continued collaboration between researchers and clinicians is needed to translate the results of current and future animal studies to the clinical setting.
5. Conclusions
Cancer cachexia is a debilitating syndrome that can impact a cancer patient’s quality of life, treatment tolerability, and mortality. The pro-inflammatory state that is characteristic of this condition can negatively impact the cardiac muscle architecture, thus promoting cardiac dysfunction. Despite the research discussed above, there are still many gaps in our understanding of cancer-induced cardiac cachexia, and increased knowledge of its molecular mechanisms can help identify more therapeutic targets. Furthermore, while there are many promising treatment modalities, including pharmacologic, nutritional, and genetic interventions, these are all in the preclinical phase. Further investigations need to be performed to determine the efficacy of these therapies and their long-term safety profiles in the patient population. Successful treatment of this disease process can potentially improve patient quality of life and improve survival.
Author Contributions
Conceptualization, J.G.T., V.V. and T.D.; methodology, V.V. and T.D.; validation, V.V. and T.D.; writing—original draft preparation, V.V., T.D. and C.L.; writing—review and editing, V.V., T.D., C.L., D.C.F., A.N.R., K.M.H. and J.G.T.; supervision, J.G.T.; project administration, J.G.T. All authors have read and agreed to the published version of the manuscript.
Funding
Authors and their research are supported by the National Human Genome Research Institute (T32 HG008958 to ANR, KMH), National Cancer Institute (T32 CA093423-13 to DCF, R01CA242003 to JGT, U54CA233444 to JGT, and U54CA233444-03S1 to ANR and JGT), and the Joseph and Ann Matella Fund for Pancreatic Cancer Research (JGT). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. ANR and KMH are also supported by the Collaborative Alliance for Pancreatic Education and Research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Blum D., Omlin A., Baracos V.E., Solheim T.S., Tan B.H., Stone P., Kaasa S., Fearon K., Strasser F. Cancer cachexia: A systematic literature review of items and domains associated with involuntary weight loss in cancer. Crit. Rev. Oncol. Hematol. 2011;80:114–144. doi: 10.1016/j.critrevonc.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Fearon K., Strasser F., Anker S.D., Bosaeus I., Bruera E., Fainsinger R.L., Jatoi A., Loprinzi C., MacDonald N., Mantovani G., et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 3.Herremans K.M., Riner A.N., Cameron M.E., Trevino J.G. The Microbiota and Cancer Cachexia. Int. J. Mol. Sci. 2019;20:6267. doi: 10.3390/ijms20246267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ni J., Zhang L. Cancer Cachexia: Definition, Staging, and Emerging Treatments. Cancer Manag. Res. 2020;12:5597–5605. doi: 10.2147/CMAR.S261585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadeghi M., Keshavarz-Fathi M., Baracos V., Arends J., Mahmoudi M., Rezaei N. Cancer cachexia: Diagnosis, assessment, and treatment. Crit. Rev. Oncol. Hematol. 2018;127:91–104. doi: 10.1016/j.critrevonc.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 6.Sorensen J. Lung Cancer Cachexia: Can Molecular Understanding Guide Clinical Management? Integr. Cancer Ther. 2018;17:1000–1008. doi: 10.1177/1534735418781743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan C.R., Yaffee P.M., Jamil L.H., Lo S.K., Nissen N., Pandol S.J., Tuli R., Hendifar A.E. Pancreatic cancer cachexia: A review of mechanisms and therapeutics. Front. Physiol. 2014;5:88. doi: 10.3389/fphys.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peixoto da Silva S., Santos J.M.O., Costa E.S.M.P., Gil da Costa R.M., Medeiros R. Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia and asthenia. J. Cachexia Sarcopenia Muscle. 2020;11:619–635. doi: 10.1002/jcsm.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein P.D., Goldman J., Matta F., Yaekoub A.Y. Diabetes mellitus and risk of venous thromboembolism. Am. J. Med. Sci. 2009;337:259–264. doi: 10.1097/MAJ.0b013e31818bbb8b. [DOI] [PubMed] [Google Scholar]
- 10.Simonsen C., de Heer P., Bjerre E.D., Suetta C., Hojman P., Pedersen B.K., Svendsen L.B., Christensen J.F. Sarcopenia and Postoperative Complication Risk in Gastrointestinal Surgical Oncology: A Meta-analysis. Ann. Surg. 2018;268:58–69. doi: 10.1097/SLA.0000000000002679. [DOI] [PubMed] [Google Scholar]
- 11.Porporato P.E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis. 2016;5:e200. doi: 10.1038/oncsis.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skipworth R.J., Stewart G.D., Dejong C.H., Preston T., Fearon K.C. Pathophysiology of cancer cachexia: Much more than host-tumour interaction? Clin. Nutr. 2007;26:667–676. doi: 10.1016/j.clnu.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Kazemi-Bajestani S.M., Becher H., Fassbender K., Chu Q., Baracos V.E. Concurrent evolution of cancer cachexia and heart failure: Bilateral effects exist. J. Cachexia Sarcopenia Muscle. 2014;5:95–104. doi: 10.1007/s13539-014-0137-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murphy K.T. The pathogenesis and treatment of cardiac atrophy in cancer cachexia. Am. J. Physiol. Heart Circ. Physiol. 2016;310:H466–H477. doi: 10.1152/ajpheart.00720.2015. [DOI] [PubMed] [Google Scholar]
- 15.Tian M., Nishijima Y., Asp M.L., Stout M.B., Reiser P.J., Belury M.A. Cardiac alterations in cancer-induced cachexia in mice. Int. J. Oncol. 2010;37:347–353. doi: 10.3892/ijo_00000683. [DOI] [PubMed] [Google Scholar]
- 16.Muhlfeld C., Das S.K., Heinzel F.R., Schmidt A., Post H., Schauer S., Papadakis T., Kummer W., Hoefler G. Cancer induces cardiomyocyte remodeling and hypoinnervation in the left ventricle of the mouse heart. PLoS ONE. 2011;6:e20424. doi: 10.1371/journal.pone.0020424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schafer M., Oeing C.U., Rohm M., Baysal-Temel E., Lehmann L.H., Bauer R., Volz H.C., Boutros M., Sohn D., Sticht C., et al. Ataxin-10 is part of a cachexokine cocktail triggering cardiac metabolic dysfunction in cancer cachexia. Mol. Metab. 2016;5:67–78. doi: 10.1016/j.molmet.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costelli P., Carbo N., Tessitore L., Bagby G.J., Lopez-Soriano F.J., Argiles J.M., Baccino F.M. Tumor necrosis factor-alpha mediates changes in tissue protein turnover in a rat cancer cachexia model. J. Clin. Investig. 1993;92:2783–2789. doi: 10.1172/JCI116897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llovera M., Lopez-Soriano F.J., Argiles J.M. Effects of tumor necrosis factor-alpha on muscle-protein turnover in female Wistar rats. J. Natl. Cancer Inst. 1993;85:1334–1339. doi: 10.1093/jnci/85.16.1334. [DOI] [PubMed] [Google Scholar]
- 20.Pietzsch S., Ricke-Hoch M., Stapel B., Hilfiker-Kleiner D. Modulation of cardiac AKT and STAT3 signalling in preclinical cancer models and their impact on the heart. Biochim. Biophys. Acta Mol. Cell Res. 2020;1867:118519. doi: 10.1016/j.bbamcr.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 21.Miyagawa Y., Nukaga S., Mori T., Fujiwara-Tani R., Fujii K., Mori S., Goto K., Kishi S., Sasaki T., Nakashima C., et al. Evaluation of cancer-derived myocardial impairments using a mouse model. Oncotarget. 2020;11:3712–3722. doi: 10.18632/oncotarget.27759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cosper P.F., Leinwand L.A. Cancer causes cardiac atrophy and autophagy in a sexually dimorphic manner. Cancer Res. 2011;71:1710–1720. doi: 10.1158/0008-5472.CAN-10-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manne N.D., Lima M., Enos R.T., Wehner P., Carson J.A., Blough E. Altered cardiac muscle mTOR regulation during the progression of cancer cachexia in the ApcMin/+ mouse. Int. J. Oncol. 2013;42:2134–2140. doi: 10.3892/ijo.2013.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borges F.H., Marinello P.C., Cecchini A.L., Blegniski F.P., Guarnier F.A., Cecchini R. Oxidative and proteolytic profiles of the right and left heart in a model of cancer-induced cardiac cachexia. Pathophysiology. 2014;21:257–265. doi: 10.1016/j.pathophys.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Hinch E.C., Sullivan-Gunn M.J., Vaughan V.C., McGlynn M.A., Lewandowski P.A. Disruption of pro-oxidant and antioxidant systems with elevated expression of the ubiquitin proteosome system in the cachectic heart muscle of nude mice. J. Cachexia Sarcopenia Muscle. 2013;4:287–293. doi: 10.1007/s13539-013-0116-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee D.E., Brown J.L., Rosa-Caldwell M.E., Perry R.A., Brown L.A., Haynie W.S., Washington T.A., Wiggs M.P., Rajaram N., Greene N.P. Cancer-induced Cardiac Atrophy Adversely Affects Myocardial Redox State and Mitochondrial Oxidative Characteristics. JCSM Rapid Commun. 2021;4:3–15. doi: 10.1002/rco2.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wysong A., Couch M., Shadfar S., Li L., Rodriguez J.E., Asher S., Yin X., Gore M., Baldwin A., Patterson C., et al. NF-kappaB inhibition protects against tumor-induced cardiac atrophy in vivo. Am. J. Pathol. 2011;178:1059–1068. doi: 10.1016/j.ajpath.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian M., Asp M.L., Nishijima Y., Belury M.A. Evidence for cardiac atrophic remodeling in cancer-induced cachexia in mice. Int. J. Oncol. 2011;39:1321–1326. doi: 10.3892/ijo.2011.1150. [DOI] [PubMed] [Google Scholar]
- 29.Matsuyama T., Ishikawa T., Okayama T., Oka K., Adachi S., Mizushima K., Kimura R., Okajima M., Sakai H., Sakamoto N., et al. Tumor inoculation site affects the development of cancer cachexia and muscle wasting. Int. J. Cancer. 2015;137:2558–2565. doi: 10.1002/ijc.29620. [DOI] [PubMed] [Google Scholar]
- 30.Costelli P., De Tullio R., Baccino F.M., Melloni E. Activation of Ca2+-dependent proteolysis in skeletal muscle and heart in cancer cachexia. Br. J. Cancer. 2001;84:946–950. doi: 10.1054/bjoc.2001.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pin F., Minero V.G., Penna F., Muscaritoli M., De Tullio R., Baccino F.M., Costelli P. Interference with Ca2+-Dependent Proteolysis Does Not Alter the Course of Muscle Wasting in Experimental Cancer Cachexia. Front. Physiol. 2017;8:213. doi: 10.3389/fphys.2017.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huot J.R., Pin F., Narasimhan A., Novinger L.J., Keith A.S., Zimmers T.A., Willis M.S., Bonetto A. ACVR2B antagonism as a countermeasure to multi-organ perturbations in metastatic colorectal cancer cachexia. J. Cachexia Sarcopenia Muscle. 2020;11:1779–1798. doi: 10.1002/jcsm.12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Samant S.A., Kanwal A., Pillai V.B., Bao R., Gupta M.P. The histone deacetylase SIRT6 blocks myostatin expression and development of muscle atrophy. Sci. Rep. 2017;7:11877. doi: 10.1038/s41598-017-10838-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devine R.D., Bicer S., Reiser P.J., Velten M., Wold L.E. Metalloproteinase expression is altered in cardiac and skeletal muscle in cancer cachexia. Am. J. Physiol. Heart Circ. Physiol. 2015;309:H685–H691. doi: 10.1152/ajpheart.00106.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michaelis K.A., Zhu X., Burfeind K.G., Krasnow S.M., Levasseur P.R., Morgan T.K., Marks D.L. Establishment and characterization of a novel murine model of pancreatic cancer cachexia. J. Cachexia Sarcopenia Muscle. 2017;8:824–838. doi: 10.1002/jcsm.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonetto A., Rupert J.E., Barreto R., Zimmers T.A. The Colon-26 Carcinoma Tumor-bearing Mouse as a Model for the Study of Cancer Cachexia. J. Vis. Exp. 2016;117:e54893. doi: 10.3791/54893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mishra S., Tamta A.K., Sarikhani M., Desingu P.A., Kizkekra S.M., Pandit A.S., Kumar S., Khan D., Raghavan S.C., Sundaresan N.R. Subcutaneous Ehrlich Ascites Carcinoma mice model for studying cancer-induced cardiomyopathy. Sci. Rep. 2018;8:5599. doi: 10.1038/s41598-018-23669-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barkhudaryan A., Scherbakov N., Springer J., Doehner W. Cardiac muscle wasting in individuals with cancer cachexia. ESC Heart Fail. 2017;4:458–467. doi: 10.1002/ehf2.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu H., Crawford D., Hutchinson K.R., Youtz D.J., Lucchesi P.A., Velten M., McCarthy D.O., Wold L.E. Myocardial dysfunction in an animal model of cancer cachexia. Life Sci. 2011;88:406–410. doi: 10.1016/j.lfs.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cramer L., Hildebrandt B., Kung T., Wichmann K., Springer J., Doehner W., Sandek A., Valentova M., Stojakovic T., Scharnagl H., et al. Cardiovascular function and predictors of exercise capacity in patients with colorectal cancer. J. Am. Coll. Cardiol. 2014;64:1310–1319. doi: 10.1016/j.jacc.2014.07.948. [DOI] [PubMed] [Google Scholar]
- 41.Florea V.G., Moon J., Pennell D.J., Doehner W., Coats A.J., Anker S.D. Wasting of the left ventricle in patients with cardiac cachexia: A cardiovascular magnetic resonance study. Int. J. Cardiol. 2004;97:15–20. doi: 10.1016/j.ijcard.2003.05.050. [DOI] [PubMed] [Google Scholar]
- 42.Jafri S.H., Previgliano C., Khandelwal K., Shi R. Cachexia Index in Advanced Non-Small-Cell Lung Cancer Patients. Clin. Med. Insights Oncol. 2015;9:87–93. doi: 10.4137/CMO.S30891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shum A.M.Y., Poljak A., Bentley N.L., Turner N., Tan T.C., Polly P. Proteomic profiling of skeletal and cardiac muscle in cancer cachexia: Alterations in sarcomeric and mitochondrial protein expression. Oncotarget. 2018;9:22001–22022. doi: 10.18632/oncotarget.25146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Der-Torossian H., Gourin C.G., Couch M.E. Translational implications of novel findings in cancer cachexia: The use of metabolomics and the potential of cardiac malfunction. Curr. Opin. Supportive Palliat. Care. 2012;6:446–450. doi: 10.1097/SPC.0b013e328359b695. [DOI] [PubMed] [Google Scholar]
- 45.Barker T., Fulde G., Moulton B., Nadauld L.D., Rhodes T. An elevated neutrophil-to-lymphocyte ratio associates with weight loss and cachexia in cancer. Sci. Rep. 2020;10:7535. doi: 10.1038/s41598-020-64282-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q., Song M.M., Zhang X., Ding J.S., Ruan G.T., Zhang X.W., Liu T., Yang M., Ge Y.-Z., Tang M., et al. Association of systemic inflammation with survival in patients with cancer cachexia: Results from a multicentre cohort study. J. Cachexia Sarcopenia Muscle. 2021;12:1466–1476. doi: 10.1002/jcsm.12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kazemi-Bajestani S.M.R., Becher H., Butts C., Basappa N.S., Smylie M., Joy A.A., Sangha R., Gallivan A., Kavsak P., Chu Q., et al. Rapid atrophy of cardiac left ventricular mass in patients with non-small cell carcinoma of the lung. J. Cachexia Sarcopenia Muscle. 2019;10:1070–1082. doi: 10.1002/jcsm.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen T., Li B., Xu Y., Meng S., Wang Y., Jiang Y. Luteolin reduces cancerinduced skeletal and cardiac muscle atrophy in a Lewis lung cancer mouse model. Oncol. Rep. 2018;40:1129–1137. doi: 10.3892/or.2018.6453. [DOI] [PubMed] [Google Scholar]
- 49.Aquila G., Re Cecconi A.D., Forti M., Frapolli R., Bello E., Novelli D., Russo I., Licandro S.A., Staszewsky L., Martinelli G.B., et al. Trabectedin and Lurbinectedin Extend Survival of Mice Bearing C26 Colon Adenocarcinoma, without Affecting Tumor Growth or Cachexia. Cancers. 2020;12:2312. doi: 10.3390/cancers12082312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shadfar S., Couch M.E., McKinney K.A., Weinstein L.J., Yin X., Rodriguez J.E., Guttridge D.C., Willis M. Oral resveratrol therapy inhibits cancer-induced skeletal muscle and cardiac atrophy in vivo. Nutr. Cancer. 2011;63:749–762. doi: 10.1080/01635581.2011.563032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willis M.S., Rojas M., Li L., Selzman C.H., Tang R.H., Stansfield W.E., Rodriguez J.E., Glass D.J., Patterson C. Muscle ring finger 1 mediates cardiac atrophy in vivo. Am. J. Physiol. Heart Circ. Physiol. 2009;296:H997–H1006. doi: 10.1152/ajpheart.00660.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smuder A.J., Roberts B.M., Wiggs M.P., Kwon O.S., Yoo J.K., Christou D.D., Fuller D.D., Szeto H.H., Judge A.R. Pharmacological targeting of mitochondrial function and reactive oxygen species production prevents colon 26 cancer-induced cardiorespiratory muscle weakness. Oncotarget. 2020;11:3502–3514. doi: 10.18632/oncotarget.27748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clark Y.Y., Wold L.E., Szalacha L.A., McCarthy D.O. Ubiquinol reduces muscle wasting but not fatigue in tumor-bearing mice. Biol. Res. Nurs. 2015;17:321–329. doi: 10.1177/1099800414543822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Greenbaum L.M., Sutherland J.H. Host cathepsin D response to tumor in the normal and pepstatin-treated mouse. Cancer Res. 1983;43:2584–2587. [PubMed] [Google Scholar]
- 55.Roberg K. Relocalization of cathepsin D and cytochrome c early in apoptosis revealed by immunoelectron microscopy. Lab Investig. 2001;81:149–158. doi: 10.1038/labinvest.3780222. [DOI] [PubMed] [Google Scholar]
- 56.Muscaritoli M., Costelli P., Bossola M., Grieco G., Bonelli G., Bellantone R., Doglietto G.B., Fanelli F.R., Baccino F.M. Effects of simvastatin administration in an experimental model of cancer cachexia. Nutrition. 2003;19:936–939. doi: 10.1016/j.nut.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 57.Palus S., von Haehling S., Flach V.C., Tschirner A., Doehner W., Anker S.D., Springer J. Simvastatin reduces wasting and improves cardiac function as well as outcome in experimental cancer cachexia. Int. J. Cardiol. 2013;168:3412–3418. doi: 10.1016/j.ijcard.2013.04.150. [DOI] [PubMed] [Google Scholar]
- 58.Stevens S.C., Velten M., Youtz D.J., Clark Y., Jing R., Reiser P.J., Bicer S., Devine R.D., McCarthy D.O., Wold L.E., et al. Losartan treatment attenuates tumor-induced myocardial dysfunction. J. Mol. Cell. Cardiol. 2015;85:37–47. doi: 10.1016/j.yjmcc.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kelm N.Q., Straughn A.R., Kakar S.S. Withaferin A attenuates ovarian cancer-induced cardiac cachexia. PLoS ONE. 2020;15:e0236680. doi: 10.1371/journal.pone.0236680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou X., Wang J.L., Lu J., Song Y., Kwak K.S., Jiao Q., Rosenfeld R., Chen Q., Boone T., Simonet W.S., et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142:531–543. doi: 10.1016/j.cell.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 61.Trobec K., Palus S., Tschirner A., von Haehling S., Doehner W., Lainscak M., Anker S.D., Springer J. Rosiglitazone reduces body wasting and improves survival in a rat model of cancer cachexia. Nutrition. 2014;30:1069–1075. doi: 10.1016/j.nut.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 62.Toneto A.T., Ferreira Ramos L.A., Salomao E.M., Tomasin R., Aereas M.A., Gomes-Marcondes M.C. Nutritional leucine supplementation attenuates cardiac failure in tumour-bearing cachectic animals. J. Cachexia Sarcopenia Muscle. 2016;7:577–586. doi: 10.1002/jcsm.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nukaga S., Mori T., Miyagawa Y., Fujiwara-Tani R., Sasaki T., Fujii K., Mori S., Goto K., Kishi S., Nakashima C., et al. Combined administration of lauric acid and glucose improved cancer-derived cardiac atrophy in a mouse cachexia model. Cancer Sci. 2020;111:4605–4615. doi: 10.1111/cas.14656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chance W.T., Cao L., Zhang F.S., Fischer J.E. Clenbuterol plus acivicin decrease tumor growth and increase muscle mass in rats maintained on total parenteral nutrition. Am. J. Surg. 1991;161:51–56. doi: 10.1016/0002-9610(91)90360-P. [DOI] [PubMed] [Google Scholar]
- 65.Saitoh M., Hatanaka M., Konishi M., Ishida J., Palus S., Ebner N., Döhner W., Von Haehling S., Anker S.D., Springer J. Erythropoietin improves cardiac wasting and outcomes in a rat model of liver cancer cachexia. Int. J. Cardiol. 2016;218:312–317. doi: 10.1016/j.ijcard.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 66.Springer J., Tschirner A., Hartman K., von Haehling S., Anker S.D., Doehner W. The xanthine oxidase inhibitor oxypurinol reduces cancer cachexia-induced cardiomyopathy. Int. J. Cardiol. 2013;168:3527–3531. doi: 10.1016/j.ijcard.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 67.Elkina Y., Palus S., Tschirner A., Hartmann K., von Haehling S., Doehner W., Mayer U., Coats A.J., Beadle J., Anker S.D., et al. Tandospirone reduces wasting and improves cardiac function in experimental cancer cachexia. Int. J. Cardiol. 2013;170:160–166. doi: 10.1016/j.ijcard.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 68.Alves C.R., da Cunha T.F., da Paixao N.A., Brum P.C. Aerobic exercise training as therapy for cardiac and cancer cachexia. Life Sci. 2015;125:9–14. doi: 10.1016/j.lfs.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 69.Parry T.L., Hayward R. Exercise Protects against Cancer-induced Cardiac Cachexia. Med. Sci. Sports Exerc. 2018;50:1169–1176. doi: 10.1249/MSS.0000000000001544. [DOI] [PubMed] [Google Scholar]
- 70.Ranjbar K., Ballaro R., Bover Q., Pin F., Beltra M., Penna F., Costelli P. Combined Exercise Training Positively Affects Muscle Wasting in Tumor-Bearing Mice. Med. Sci. Sports Exerc. 2019;51:1387–1395. doi: 10.1249/MSS.0000000000001916. [DOI] [PubMed] [Google Scholar]
- 71.Fernandes L.G., Tobias G.C., Paixao A.O., Dourado P.M., Voltarelli V.A., Brum P.C. Exercise training delays cardiac remodeling in a mouse model of cancer cachexia. Life Sci. 2020;260:118392. doi: 10.1016/j.lfs.2020.118392. [DOI] [PubMed] [Google Scholar]
- 72.Padrao A.I., Moreira-Goncalves D., Oliveira P.A., Teixeira C., Faustino-Rocha A.I., Helguero L., Vitorino R., Santos L.L., Amado F., Duarte J.A., et al. Endurance training prevents TWEAK but not myostatin-mediated cardiac remodelling in cancer cachexia. Arch. Biochem. Biophys. 2015;567:13–21. doi: 10.1016/j.abb.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 73.Winbanks C.E., Murphy K.T., Bernardo B.C., Qian H., Liu Y., Sepulveda P.V., Beyer C., Hagg A., Thomson R.E., Chen J.L., et al. Smad7 gene delivery prevents muscle wasting associated with cancer cachexia in mice. Sci. Transl. Med. 2016;8:348ra98. doi: 10.1126/scitranslmed.aac4976. [DOI] [PubMed] [Google Scholar]
- 74.Chen L., Yang Q., Zhang H., Wan L., Xin B., Cao Y., Zhang J., Guo C. Cryptotanshinone prevents muscle wasting in CT26-induced cancer cachexia through inhibiting STAT3 signaling pathway. J. Ethnopharmacol. 2020;260:113066. doi: 10.1016/j.jep.2020.113066. [DOI] [PubMed] [Google Scholar]
- 75.Devine R.D., Eichenseer C.M., Wold L.E. Minocycline attenuates cardiac dysfunction in tumor-burdened mice. J. Mol. Cell. Cardiol. 2016;100:35–42. doi: 10.1016/j.yjmcc.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Springer J., Tschirner A., Haghikia A., von Haehling S., Lal H., Grzesiak A., Kaschina E., Palus S., Pötsch M., von Websky K., et al. Prevention of liver cancer cachexia-induced cardiac wasting and heart failure. Eur. Heart J. 2014;35:932–941. doi: 10.1093/eurheartj/eht302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Musolino V., Palus S., Latouche C., Gliozzi M., Bosco F., Scarano F., Nucera S., Carresi C., Scicchitano M., von Haehling S., et al. Cardiac expression of neutrophil gelatinase-associated lipocalin in a model of cancer cachexia-induced cardiomyopathy. ESC Heart Fail. 2019;6:89–97. doi: 10.1002/ehf2.12372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.VanderVeen B.N., Hardee J.P., Fix D.K., Carson J.A. Skeletal muscle function during the progression of cancer cachexia in the male Apc(Min/+) mouse. J. Appl. Physiol. 2018;124:684–695. doi: 10.1152/japplphysiol.00897.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oliveira A.G., Gomes-Marcondes M.C. Metformin treatment modulates the tumour-induced wasting effects in muscle protein metabolism minimising the cachexia in tumour-bearing rats. BMC Cancer. 2016;16:418. doi: 10.1186/s12885-016-2424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bora V., Patel B.M. Investigation into the role of anti-diabetic agents in cachexia associated with metastatic cancer. Life Sci. 2021;274:119329. doi: 10.1016/j.lfs.2021.119329. [DOI] [PubMed] [Google Scholar]
- 81.Musolino V., Palus S., Tschirner A., Drescher C., Gliozzi M., Carresi C., Vitale C., Muscoli C., Doehner W., Von Haehling S., et al. Megestrol acetate improves cardiac function in a model of cancer cachexia-induced cardiomyopathy by autophagic modulation. J. Cachexia Sarcopenia Muscle. 2016;7:555–566. doi: 10.1002/jcsm.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Toledo M., Springer J., Busquets S., Tschirner A., Lopez-Soriano F.J., Anker S.D., López-Soriano F.J., Anker S.D., Argilés J.M. Formoterol in the treatment of experimental cancer cachexia: Effects on heart function. J. Cachexia Sarcopenia Muscle. 2014;5:315–320. doi: 10.1007/s13539-014-0153-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ojima C., Noguchi Y., Miyamoto T., Saito Y., Orihashi H., Yoshimatsu Y., Watabe T., Takayama K., Hayashi Y., Itoh F. Peptide-2 from mouse myostatin precursor protein alleviates muscle wasting in cancer-associated cachexia. Cancer Sci. 2020;111:2954–2964. doi: 10.1111/cas.14520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Scott J.M., Dillon E.L., Kinsky M., Chamberlain A., McCammon S., Jupiter D., Willis M., Hatch S., Richardson G., Danesi C., et al. Effects of adjunct testosterone on cardiac morphology and function in advanced cancers: An ancillary analysis of a randomized controlled trial. BMC Cancer. 2019;19:778. doi: 10.1186/s12885-019-6006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Solheim T.S., Fearon K.C., Blum D., Kaasa S. Non-steroidal anti-inflammatory treatment in cancer cachexia: A systematic literature review. Acta Oncol. 2013;52:6–17. doi: 10.3109/0284186X.2012.724536. [DOI] [PubMed] [Google Scholar]
- 86.Martins H.A., Sehaber C.C., Hermes-Uliana C., Mariani F.A., Guarnier F.A., Vicentini G.E., Bossolani G.D.P., Jussani L.A., Lima M.M., Bazotte R.B., et al. Supplementation with L-glutamine prevents tumor growth and cancer-induced cachexia as well as restores cell proliferation of intestinal mucosa of Walker-256 tumor-bearing rats. Amino Acids. 2016;48:2773–2784. doi: 10.1007/s00726-016-2313-1. [DOI] [PubMed] [Google Scholar]
- 87.Ham D.J., Murphy K.T., Chee A., Lynch G.S., Koopman R. Glycine administration attenuates skeletal muscle wasting in a mouse model of cancer cachexia. Clin. Nutr. 2014;33:448–458. doi: 10.1016/j.clnu.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 88.Togni V., Ota C.C., Folador A., Junior O.T., Aikawa J., Yamazaki R.K., Freitas F.A., Longo R., Martins E.F., Calder P.C., et al. Cancer cachexia and tumor growth reduction in Walker 256 tumor-bearing rats supplemented with N-3 polyunsaturated fatty acids for one generation. Nutr. Cancer. 2003;46:52–58. doi: 10.1207/S15327914NC4601_07. [DOI] [PubMed] [Google Scholar]
- 89.Liu S., Wu H.J., Zhang Z.Q., Chen Q., Liu B., Wu J.P., Zhu L. L-carnitine ameliorates cancer cachexia in mice by regulating the expression and activity of carnitine palmityl transferase. Cancer Biol. Ther. 2011;12:125–130. doi: 10.4161/cbt.12.2.15717. [DOI] [PubMed] [Google Scholar]
- 90.Cella P.S., Marinello P.C., Borges F.H., Ribeiro D.F., Chimin P., Testa M.T.J., Guirro P.B., Duarte J.A., Cecchini R., Guarnier F.A., et al. Creatine supplementation in Walker-256 tumor-bearing rats prevents skeletal muscle atrophy by attenuating systemic inflammation and protein degradation signaling. Eur. J. Nutr. 2020;59:661–669. doi: 10.1007/s00394-019-01933-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.