Abstract
The in vitro antimalarial activities of 46 alkaloids and extracts from Strychnos species were evaluated. Two types of quasidimeric alkaloids exhibit high and selective activities against Plasmodium. Strychnopentamine and isostrychnopentamine were active against chloroquine-sensitive and -resistant strains (50% inhibitory concentration [IC50] ≈ 0.15 μM), while dihydrousambarensine exhibited a 30-fold higher activity against the chloroquine-resistant strain (IC50 = 0.03 μM) than it did against the chloroquine-sensitive strain.
Malaria is the major parasitic infection in many tropical and subtropical regions. An increase in the resistance of Plasmodium falciparum to conventional treatments is a worldwide problem, and few alternative drugs are under development, necessitating urgent efforts to identify new classes of antimalarial drugs. The clinical utility of quinine and quinidine, isolated from Cinchona tree bark, and the Chinese discovery of artemisinin from the herb Artemisia annua have stimulated much interest in plants as potential sources of new antimalarial drugs.
The in vitro antiplasmodial, antiamoebic, and cytotoxic activities of alkaloids isolated from Strychnos usambarensis have been previously reported (5, 12, 17). In this article, we report the antiplasmodial activities against two additional P. falciparum strains of 19 total extracts, 21 new Strychnos alkaloids, and 6 alkaloids which were previously tested against the K1 strain of P. falciparum (17).
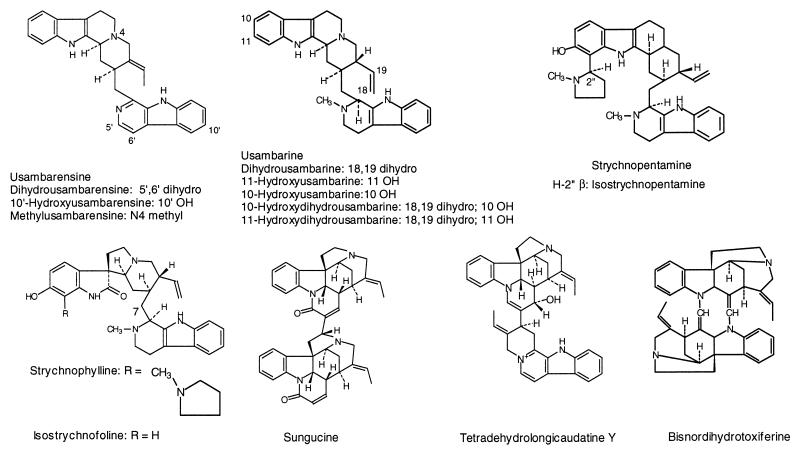
Dihydrousambarensine, usambarensine, Nb-methylusambarensine, 10′-hydroxyusambarensine, malindine, usambarine, dihydrousambarine, strychnopentamine, isostrychnopent-amine, isostrychnofoline, strychnophylline, 10-hydroxyusambarine, 11-hydroxyusambarine, 10-hydroxydihydrousambarine, 11-hydroxydihydrousambarine, and tetradehydrolongicaudatine Y were isolated from Strychnos usambarensis ‘Gilg’ root bark (1, 7, 10), leaves (2), or stem bark (9). The root bark and leaves of Strychnos icaja ‘Baill.’ were the sources of 14-hydroxyepoxynovacine, epoxynovacine, icajine, sungucine, and bisnordihydrotoxiferine (11) and of vomicine and novacine (4), respectively. Holstiine and diaboline, strychnochromine, and guianensine were obtained from Strychnos henningsii ‘Gilg’ root bark, Strychnos gossweileri ‘Exell.’ root bark, and Strychnos guianensis ‘Aubl.’ stem bark, respectively (3, 6, 14). The chemical structures of most alkaloids are shown in Fig. 1. The total plant extracts were prepared by maceration of powdered material (5 g) with ethanol (EtOH) or ethyl acetate (EtOAc) (4 × 50 ml). The extracts were assembled and concentrated to dryness under vacuum. Voucher specimens of the different plants used in this study were deposited in the herbarium of the Pharmaceutical Institute at Liège, Belgium. P. falciparum strains were continuously maintained in culture by the method of Trager and Jensen (16) and as described previously (10). Tests for antimalarial activity were adapted from methods described earlier (8, 10, 13). Chloroquine diphosphate (Sigma) and quinine base (Aldrich) were used as antimalarial references. Parasite growth was estimated by [3H]hypoxanthine incorporation. The results are expressed as percentage of growth inhibition. The sigmoid dose-response curve was used to derive 50% inhibitory concentration values (IC50s) as the means of two experiments. The human cancer cell lines KB and HeLa were cultured, and the IC50s were determined as previously described (5).
FIG. 1.
Chemical structures of Strychnos alkaloids.
The in vitro antimalarial activities of extracts and alkaloids against the two strains are shown in Table 1. The different total extracts displayed a wide range of antiplasmodial activities. Higher activities (IC50 < 1 μg/ml) were obtained with root and leaf extracts from S. usambarensis and with root extracts from S. icaja. The EtOAc extract from S. icaja root was about five times more active than the EtOH extract. The stem bark extract of the liana form of S. usambarensis also had an IC50 of <1 μg/ml, while the tree form extract had an IC50 of about 25 μg/ml. This can be explained by the difference in alkaloid compositions between these two forms (15). Four Strychnos batches were found to exhibit moderate activities, with IC50s ranging between 10 and 30 μg/ml, namely, Strychnos variabilis root bark, Strychnos angolensis leaves, S. angolensis root, and Strychnos memecyloides leaf.
TABLE 1.
In vitro activities of crude extracts and alkaloids from some Strychnos species against two P. falciparum strainsa
| Compound N° | FCA 20 Ghana (chloroquine-sensitive strain)
|
W2 Indochina (chloroquine-resistant strain)
|
||||
|---|---|---|---|---|---|---|
| IC50 | IC90 | nb | IC50 | IC90 | nb | |
| Total extracts | ||||||
| S. usambarensis roots, EtOAc extract | (0.457 ± 0.005) | (2.345) | 2 | (0.039 ± 0.0029) | (0.597) | 3 |
| S. usambarensis leaves, EtOH extract | (0.287 ± 0.022) | (1.977) | 3 | (0.970 ± 0.141) | (4.853) | 2 |
| S. usambarensis bark liana variety, EtOAc extract | (0.766 ± 0.022) | (2.378) | 2 | (0.226 ± 0.039) | (2.897) | 2 |
| S. usambarensis bark tree variety, EtOH extract | (23.507) | (47.855) | 1 | (24.832) | (46.854) | 1 |
| S. scheffleri bark, EtOH extract | Inactive at (30) | 1 | NDc | |||
| S. mattogrossensis roots, EtOH extract | Inactive at (30) | 1 | ND | |||
| S. henningsii leaves, EtOH extract | (≅40) | (≅200) | 1 | ND | ||
| S. longicaudata roots, EtOH extract | Inactive at (50) | 1 | ND | |||
| S. variabilis bark, EtOH extract | (17.702) | (≅50) | 1 | ND | ||
| S. camptoneura roots, EtOH extract | (≅50) | 1 | ND | |||
| S. innocua roots, EtOH extract | Inactive at (40) | 1 | ND | |||
| S. angolensis leaves, EtOH extract | (29.935) | (≅80) | 1 | ND | ||
| S. angolensis roots, EtOH extract | (20.895) | (≅60) | 1 | ND | ||
| S. gossweileri roots, EtOH extract | (43.505) | (≅400) | 1 | ND | ||
| S. icaja leaves, EtOH extract | (40.421) | (94.159) | 1 | ND | ||
| S. icaja roots, EtOH extract | (1.433 ± 0.068) | (4.834) | 5 | (1.002 ± 0.054) | (4.628) | 2 |
| S. icaja roots, EtOAc extract | (0.286 ± 0.061) | (1.381) | 3 | (0.303 ± 0.022) | (1.998) | 2 |
| S. spinosa bark, EtOH extract | Inactive at (80) | 1 | ND | |||
| S. memecyloides leaves, EtOH extract | (13.489) | (48.75) | 1 | ND | ||
| Alkaloids | ||||||
| S. usambarensis | ||||||
| Dihydrousambarensine | 0.857 ± 0.061 (0.371) | 2.486 | 5 | 0.032 ± 0.002 (0.013) | 4.676 | 4 |
| Usambarensined | 1.516 ± 0.031 (0.655) | 4.412 | 6 | 0.594 ± 0.052 (0.257) | 4.831 | 3 |
| 10′-Hydroxyusambarensine | 1.071 ± 0.031 (0.480) | 3.619 | 3 | 0.357 ± 0.035 (0.160) | 6.851 | 3 |
| Nb-Methylusambarensined | 5.045 (2.436) | 9.835 | 1 | 3.919 ± 0.083 (1.893) | ≅20 | 2 |
| Strychnopentamined | 0.117 ± 0.033 (0.064) | 0.443 | 4 | 0.145 ± 0.020 (0.079) | 2.982 | 4 |
| Isostrychnopentamine | 0.120 ± 0.042 (0.066) | 0.450 | 2 | 0.152 ± 0.009 (0.070) | 0.628 | 2 |
| Usambarined | 2.501 (1.125) | 9.207 | 1 | 2.358 ± 0.015 (1.061) | 7.294 | 2 |
| Dihydrousambarined | 1.247 (0.673) | 6.051 | 1 | 1.408 ± 0.013 (0.636) | 5.515 | 2 |
| 11-Hydroxyusambarine | 0.487 ± 0.015 (0.227) | 1.770 | 5 | 1.538 ± 0.109 (0.716) | 4.535 | 3 |
| 10-Hydroxyusambarine | 1.134 ± 0.012 (0.528) | 3.384 | 2 | 1.958 ± 0.115 (0.912) | 5.803 | 2 |
| Strychnophylline | 1.009 ± 0.035 (0.570) | 4.491 | 2 | 2.523 ± 0.134 (1.425) | 9.310 | 2 |
| Isostrychnofoline | 5.657 ± 0.126 (3.2923) | 25.959 | 2 | 2.598 ± 0.420 (1.519) | 15.745 | 2 |
| 10-Hydroxydihydrousambarine | 0.937 ± 0.044 (0.438) | 5.522 | 3 | 4.699 ± 0.373 (2.199) | 35.687 | 3 |
| 11-Hydroxydihydrousambarine | 2.864 ± 0.046 (1.340) | 7.023 | 2 | 5.449 ± 0.019 (2.550) | 15.772 | 2 |
| Malindine | 162.63 (49.43) | ≅800 | 1 | Inactive at 60 (20) | 1 | |
| Tetradehydrolongicaudatine Y | 1.236 (0.823) | 7.970 | 1 | 0.958 ± 0.129 (0.543) | 12.087 | 2 |
| Strychnos icaja | ||||||
| Vomicine | Inactive at 30 (12) | 1 | ND | |||
| Novacine | Inactive at 90 (40) | 1 | ND | |||
| 14-Hydroxyepoxynovacine | Inactive at 50 (20) | 1 | ND | |||
| Epoxynovacine | Inactive at 50 (20) | 1 | ND | |||
| Icajine | Inactive at 80 (30) | 198.16 | 2 | 95 ± 5 (34.7) | ≅28 | 2 |
| Sungucine | 2.292 ± 0.049 (1.435) | 11.182 | 2 | 1.659 ± 0.089 (1.051) | 12.327 | 2 |
| Bisnordihydrotoxiferine | 3.826 (2.112) | 22.30 | 1 | 4.480 ± 0.092 (2.470) | 13.123 | 2 |
| Strychnine | Inactive at 20 (7) | 1 | 23 (8.5) | ≅50 | 1 | |
| Other Strychnos species | ||||||
| Holstiine | ≅80 (30) | 1 | 34 (12.9) | ≅100 | 1 | |
| Diaboline | Inactive at 33 (12) | 1 | ND | |||
| Strychnochromine | 40.046 (11.93) | ≅200 | 1 | 16 (4.82) | ≅70 | 1 |
| Guianensine | 5.920 (3.694) | ≅20 | 1 | 7309 ± 486 (4.561) | 24.765 | 2 |
| Reference compounds | ||||||
| Chloroquine | 0.020 ± 0.002 (0.006) | 0.119 | 9 | 0.284 ± 0.017 (0.091) | 1.745 | 6 |
| Quinine | 0.269 ± 0.006 (0.087) | 1.913 | 3 | 0.413 ± 0.011 (0.134) | 1.718 | 2 |
Data are expressed as mean micromolar concentrations ± standard deviations. Values in parentheses are given in micrograms per milliliter.
n, number of independent experiments. All experiments were realized in duplicate.
ND, not determined.
These six alkaloids were previously tested against P. falciparum with essentially the same IC50 as that for the W2 strain (17).
A large number of alkaloids from S. usambarensis were highly active against the chloroquine-sensitive FCA 20 strain. Dihydrousambarensine (IC50 = 0.857 μM), 11-hydroxyusambarine (0.487 μM), strychnopentamine (0.117 μM), and isostrychnopentamine (0.120 μM) were found to be the most active alkaloids, with IC50s of <1 μM. Eight other alkaloids (usambarensine, 10′-hydroxyusambarensine, usambarine, dihydrousambarine, 10- and 11-hydroxyusambarines, strychnophylline, and tetradehydrolongicaudatine Y) possess IC50s between 1 and 2 μM. The activities against the W2 strain were of the same order as those observed with the FCA strain. However, two compounds (usambarensine and 10′-hydroxyusambarensine) were more than twice as active against the resistant clone than the susceptible clone, and the IC50 for one compound (dihydrousambarensine) was 30-fold lower for the chloroquine-resistant strain (32 nM). Its 90% inhibitory concentration (IC90), however, remained higher, with a value of 4.6 μM. The IC50 of strychnopentamine for both strains was the same (0.15 μM), and its isomer, isostrychnopentamine, exhibited the same activity but with a lower IC90 for strain W2 (0.6 μM). On the other hand, some alkaloids, like strychnophylline and the hydroxyusambarines, were notably less active against the W2 strain.
Among the known alkaloids of S. icaja roots, only sungucine and bisnordihydrotoxiferine were slightly active, with an IC50 of 2 to 4 μM (≈1 and 2 μg/ml). However, these activities could not explain the high IC50 (0.3 μg/ml) of the EtOAc extract. Further investigations to find the compounds responsible for this activity are actually in progress.
The most active compounds were tested for cytotoxicity against the human cell lines KB and HeLa. All of these compounds exhibited a 6- to 400-fold higher activity against Plasmodium than against human cells, thus indicating some selectivity (Table 2). The most selective compounds were strychnopentamine and tetradehydrolongicaudatine Y, with, respectively, 70 to 100 and >40 to 50 times higher activities against the two Plasmodium strains. Dihydrousambarensine showed a good selectivity for the W2 strain only (400 times higher activity).
TABLE 2.
Cytotoxic activity toward human cancer cell lines (KB and HeLa) and antiprotozoal selectivity indexa of some Strychnos alkaloids
| Compound | IC50 (μM) | Selectivity indexa
|
|
|---|---|---|---|
| FCA | W2 | ||
| Dihydrousambarensine (KB) | 12 | 14 | 375 |
| Usambarensine (KB) | 9.7 | 6 | 16 |
| 10′-Hydroxyusambarensine (HeLa) | 20 | 19 | 56 |
| Strychnopentamine (KB) | 11.3 | 96 | 78 |
| Tetradehydrolongicaudatine Y (HeLa) | >50b | >40 | >52 |
Selectivity index is defined as the ratio of cytotoxicity (IC50) to antiplasmodial activity (IC50).
This compound has no effect at 50 μM.
All active compounds were tertiary dimers. There is no clear relationship which allows us to associate some structural features (stereochemistry or substitutions, etc.) with an increase in activity. Nevertheless, the results of the present study confirm the antimalarial activities of Strychnos bisindole alkaloids and more particularly of two alkaloid types. The usambarensine skeleton is linked with an important rise in the activity on chloroquine-resistant strains, more particularly for the 5′,6′-dihydro derivative. Strychnopentamine and its isomer demonstrate high and selective activities against the two strains. It would therefore be interesting to test closely related compounds that possess these kinds of structures.
Acknowledgments
This research was supported by the Belgian National Fund for Scientific Research (grant 3451997 and a fellowship to M.F.).
We thank J. Boniver (Anatomie et Cytologie Pathologiques, University of Liege) for liquid scintillation measurements, M. C. DePauw (Cytologie et Histologie, University of Liege) for cytotoxicity testing, M. Wéry (Tropical Medicine Institute, Antwerp, Belgium), and J. Le Bras (Hôpital Bichat-Claude Bernard, Laboratoire de Parasitologie, Paris, France) for providing the P. falciparum strains.
REFERENCES
- 1.Angenot L, Bisset N G. Isolement et structure de nouveaux alcaloïdes extraits du Strychnos usambarensis GILG du Rwanda. J Pharm Belg. 1971;26:585–588. [Google Scholar]
- 2.Angenot L, Coune C, Tits M. Nouveaux alcaloïdes des feuilles du Strychnos usambarensis. J Pharm Belg. 1978;33:11–23. [PubMed] [Google Scholar]
- 3.Angenot L, Tits M. Isolement d’un nouvel alcaloïde et d’un triterpénoïde à partir de Strychnos henningsii du Zaïre. Planta Med. 1981;41:240–243. doi: 10.1055/s-2007-971709. [DOI] [PubMed] [Google Scholar]
- 4.Bisset N G, Das B, Parello J. Alkaloids from the leaves of Strychnos icaja Baill. Tetrahedron. 1973;29:4137–4148. [Google Scholar]
- 5.Bonjean K, De Pauw M C, Quetin-Leclercq J, Angenot L, Bassleer R. In vitro cytotoxic activity of two potential anticancer drugs isolated from Strychnos: strychnopentamine and usambarensine. Anticancer Res. 1996;16:1129–1138. [PubMed] [Google Scholar]
- 6.Bosly J. Etude des alcaloïdes du Strychnos Holstii. J Pharm Belg. 1951;6:150. [PubMed] [Google Scholar]
- 7.Caprasse M, Tavernier D, Anteunis M, Angenot L. Isolement de Nb-méthylantirhine, malindine et isomalindine à partir du Strychnos usambarensis. Planta Med. 1984;44:27–30. doi: 10.1055/s-2007-969613. [DOI] [PubMed] [Google Scholar]
- 8.Desjardins R, Canfield C, Haynes J, Chulay J. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979;16:710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frédérich M, Quetin-Leclercq J, GadiBiala R, Brandt V, Penelle J, Tits M, Angenot L. 3′,4′,5′,6′ Tetradehydrolongicaudatine Y, an anhydronium base from Strychnos usambarensis. Phytochemistry. 1998;48:1263–1266. [Google Scholar]
- 10.Frédérich M, Tits M, Hayette M P, Brandt V, Penelle J, De Mol P, Llabrés G, Angenot L. 10′-Hydroxyusambarensine, a new bisindole alkaloid from the roots of Strychnos usambarensis. J Nat Prod. 1999;62:619–621. doi: 10.1021/np980375m. [DOI] [PubMed] [Google Scholar]
- 11.Kambu K, Coune C, Angenot L. Nouveaux alcaloïdes des racines du Strychnos icaja. Planta Med. 1979;37:161–164. [Google Scholar]
- 12.Leclercq J, DePauw M C, Bassleer R, Angenot L. Screening of cytotoxic activities of Strychnos alkaloids. J Ethnopharmacol. 1986;15:305–316. doi: 10.1016/0378-8741(86)90169-8. [DOI] [PubMed] [Google Scholar]
- 13.Mirovsky P, Gay F, Bustos D, Mazier D, Gentilini M. Cloning of a fresh isolate of Plasmodium falciparum and drug sensitivity of the clones. Trans R Soc Trop Med Hyg. 1990;84:511–515. doi: 10.1016/0035-9203(90)90018-a. [DOI] [PubMed] [Google Scholar]
- 14.Quetin-Leclercq J, Llabrès G, Warin R, Belem-Pinheiro M, Mavar-Manga H, Angenot L. Guianensine, a zwitterionic alkaloid from Strychnos guianensis. Phytochemistry. 1995;40:1557–1559. [Google Scholar]
- 15.Quetin-Leclerq J, Tits M, Angenot L, Bisset N G. Alkaloids of Strychnos usambarensis stem bark. Planta Med. 1991;57:501–502. doi: 10.1055/s-2006-960185. [DOI] [PubMed] [Google Scholar]
- 16.Trager W, Jensen J B. Human malaria parasites in continuous cultures. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 17.Wright C W, Allen D, Cai Y, Chen Z, Phillipson J D, Kirby G, Warhurst D, Tits M, Angenot L. Selective antiprotozoal activity of some Strychnos alkaloids. Phytother Res. 1994;8:149–152. [Google Scholar]