Abstract
At present, most rheumatoid arthritis (RA) patients are at risk of osteoporosis (OP), which is increased by 1.5 times compared to non-RA individuals. Hence, we investigated overlapping targets related directly to the occurrence and development of RA and OP through public databases (DisGeNET, and OMIM) and literature. A total of 678 overlapping targets were considered as comorbid factors, and 604 out of 678 were correlated with one another. Interleukin 6 (IL-6), with the highest degree of value in terms of protein–protein interaction (PPI), was considered to be a core target against comorbidity. We identified 31 existing small molecules (< 1000 g/mol) as IL-6 inhibitors, and 19 ligands were selected by the 3 primary criteria (Lipinski’s rule, TPSA, and binding energy). We postulated that MD2-TLR4-IN-1 (PubChem ID: 138454798), as confirmed by the three criteria, was the key ligand to alleviate comorbidity between RA and OP. In conclusion, we described a promising active ligand (MD2-TLR4-IN-1), and a potential target (IL-6) against comorbidity of RA and OP, providing scientific evidence for a further clinical trial.
Keywords: rheumatoid arthritis, osteoporosis, comorbidity, interleukin 6, MD2-TLR4-IN-1
1. Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease that mainly causes severe pain and is associated with physical unfitness, diverse comorbidities, and diminished quality of life [1,2]. The main symptoms of RA are musculoskeletal pain, swelling, and stiffness of affected joints, linked deeply to synovial inflammation [3,4]. RA can present at all ages, and around 1% of this population suffers intractable pain, which entails enormous emotional stress and economic burden for the individual, and even for society [5]. Inflammation is the main driving factor that causes joint impairment, disorder, and unexpected comorbidity in RA patients, and anti-inflammation is the most significant therapeutic strategy [6]. At present, there are seven antibody drugs (biologics) for the treatment of RA: infliximab, adalimumab, etanercept, golimumab, tocilizumab, certolizumab, and abatacept [7]. In particular, disease-modifying anti-rheumatic drugs (DMARDs) are targeted against RA inflammation, limited to connective tissue damage [8]. Moreover, biological DMARDs are ineffective for improving bone density involved in the development of OP [9].
Osteoporosis (OP) is a severe health condition that weakens bones, making them fragile and more easily destroyed [10]. OP symptoms include back pain, loss of height, bone fractures, and change in posture [11]. Similarly, OP can also occur at all ages; mainly, primary osteoporosis develops ~10–15 years after menopause in women, and in elderly men between 75 and 80 years old [12]. In 2017, the International Osteoporosis Foundation announced that around 33% of women over 50 years old and 20% of men would experience OP in their lifespan [13]. Most recently, an emerging significant factor in OP was found to be inflammation that occurs upon bone turnover [14]. Thus, blockage of inflammation is a key clinical approach in OP patients [15]. Denosumab and odanacatib were used as biologics in the treatment of OP by enhancing bone mineral density [16,17]. Collectively, all biologics dampen the immune system, making it susceptible to common infections such as pneumonia, respiratory infections, urinary tract infections, and skin infections [18]. In addition, all antibody drugs are parenteral preparations for the patient, which have multiple risks, including hypersensitivity responses, risk of infection and emboli, and the absence of drug reversal [19,20,21]. Additionally, non-steroidal anti-inflammatory drugs (NSAIDs) are commonly used to relieve the pain related to RA and OP [22]. All NSAIDs are targeted to cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2)—the two cyclooxygenase (COX) isoforms in tissues, which have different expression levels [23]. Moreover, the inhibition of COX interferes with bone formation, angiogenesis, and soft tissue regeneration, which obstructs the bone healing process [24]. This implies that NSAIDs function merely as an analgesic for a short period of time.
Most commonly, OP has been considered to be a classical comorbidity in RA [25]. According to one report, in a cohort of 47,000 RA patients, the risk of osteoporotic destruction increased by 1.5 times compared with non-RA individuals [26]. Both RA and OP represent chronic inflammatory responses against cytokines such as interleukin 1 (IL-1), interleukin 6 (IL-6), and interleukin 17 (IL-17) [27]. This implies that inhibition of interleukin(s) might be a crucial strategy to alleviate the liaisons between RA and OP. The application of drug repositioning analysis on a data-driven approach is the most efficient methodology to obtain promising compounds [28].
Furthermore, drug repositioning is the procedure of obtaining new therapies for already-existing drugs [29]. This process has great synergistic effects, diminishing the cost of new drug development as well as securing its safety [30]. Previously, the output of drug repositioning was mainly due to fortuitous findings of unexpected therapeutic effects identified after testing with a given agent [31]. However, at present, the development of computational methodologies from holistic perspectives provides us with critical hints to reevaluate the additional efficacy of existing drugs [32].
Thus, the aim of this work was to discover the hierarchical target by which to manage both RA and OP via the computational approach method, thereby unveiling the most significant small molecule (<1000 g/mol) against the comorbidity of the two diseases.
2. Hypothesis
The identified overlapping targets between RA and OP were used to construct protein–protein interaction (PPI). We hypothesized that a target with the highest degree of value would be the most promising therapeutic point [33], while a ligand with the lowest binding energy would be the most significant compound against the comorbidity of RA and OP.
3. Methods
3.1. Retrieval of RA or OP Targets and Identification of Overlapping Targets
The targets linked to the occurrence and development of RA and OP were retrieved from DisGeNET (https://www.disgenet.org/) (accessed on 24 July 2021), OMIM (https://www.omim.org/) (accessed on 26 July 2021), and previous literature. InteractiVenn was utilized to identify the overlapping targets between RA and OP.
3.2. PPI Network Analysis
The overlapping targets analyzed by STRING (https://string-db.org/) (accessed on 29 July 2021) had their PPI constructed via an R package. One target with the highest degree of value was obtained via PPI analysis; we considered it to be the most significant target to manage the comorbidity of RA and OP.
3.3. Collection of Ligands
Based on the target, we prepared for its known ligands on a small molecule screen (<1000 g/mol), which can facilitate its biological activity or modify a target [34]. The ligands were retrieved from the website of the chemical supplier Selleckchem (https://www.selleckchem.com/) (accessed on 2 August 2021), which had input them into PubChem (https://pubchem.ncbi.nlm.nih.gov/) (accessed on 2 August 2021) for identification in the SMILES (simplified molecular-input line-entry system) format.
3.4. The Screening of Ligands
The screening methodology of the selected ligands was based on three criteria (Lipinski’s rule, TPSA, and binding energy), which were filtered using SwissADME (http://www.swissadme.ch/) (accessed on 4 August 2021). The three detailed selective conditions were as follows: (1) Lipinski’s rule violation (≤1) [35], (2) TPSA (<140 Å2) [36], and (3) binding energy (<−6.0 kcal/mol) [37].
3.5. The Preparation of Ligands and a Target for MDT
The identified ligands were converted from .sdf on PubChem into .pdb format via PyMOL; finally, the ligands were converted into .pdbqt format using AutoDock. Likewise, the PDB ID of the target was identified via RCSB PDB (https://www.rcsb.org/) (accessed on 7 August 2021), which was selected as .pdb format and converted to .pdbqt format via AutoDock (http://autodock.scripps.edu/ 15 December 2021). The existing positive ligands were docked with a target on AutoDock 4 by setting up 4 energy ranges and 8 levels of exhaustiveness as default to obtain 10 different poses of ligand molecules [38]. The grid box size was set to 40 Å × 40 Å × 40 Å. The 2D binding interactions were utilized on LigPlot+ v.2.2 (https://www.ebi.ac.uk/thornton-srv/software/LigPlus/) (accessed on 8 August 2021). After the molecular docking test (MDT), a key ligand accepted by three criteria with the lowest binding energy (highest affinity) was selected to visualize the ligand–target complex in PyMOL.
3.6. The Prediction of Toxicological Properties of the Key Ligand in Silico
Finally, we established the toxicological properties of the key ligand via the admetSAR web service tool (http://lmmd.ecust.edu.cn/admetsar1/predict/) (accessed on 10 August 2021) to develop new medication [39]. The workflow of this study is represented in Figure 1.
Figure 1.
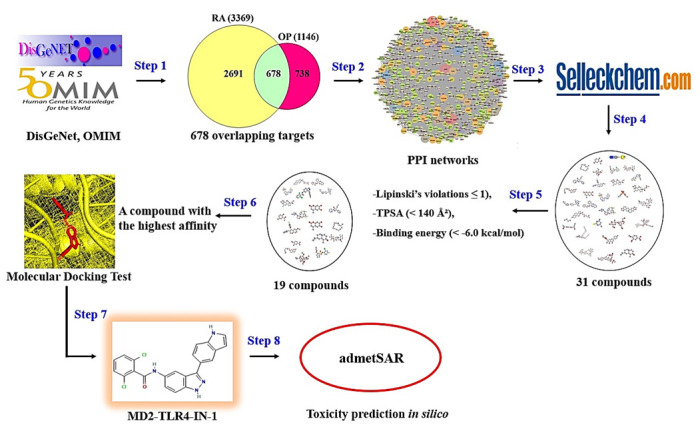
The workflow of this study.
4. Results
A total of 3369 targets associated with the occurrence and development of RA and a total of 1416 targets associated with OP were identified from DisGeNET (https://www.disgenet.org/ 15 December 2021), OMIM (https://www.omim.org/ 15 December 2021), and the literature (Supplementary Table S1). A total of 678 targets were overlapped between RA (3369 targets) and OP (1416 targets) (Figure 2) (Supplementary Table S2). Based on STRING analysis, 604 out of 678 targets were directly associated with comorbidity of RA and OP, suggesting 604 nodes and 16,705 edges (Figure 3); the 74 removed targets had no connectivity to the overlapping 678 targets. The nodes represented the total number of targets, while the edges stood for the number of relationships of each node. In PPI networks, IL-6 (432 degrees) had the greatest degree of value, and was considered a hierarchical target to manage the comorbidity of RA and OP (Table 1). The IL-6 (PDB ID: 4NI9) structure was revealed as two bound forms: apo-bound and receptor-bound [40,41]. The full length of IL-6 consisted of 212 amino acids linked to specific signal peptides with 29 amino acids, with a four-helix structure organized topologically [41,42]. In particular, 20 residues of the N-terminal did not form any secondary structure, and only the last 7 amino acids were identified as the crystal structure [43]. In contrast, the C–D loop of 10 residues (131–140 amino acids) was invisible in the crystal structure, and 17 (amino acids 44–60) out of 37 residues (amino acids 43–79) were unresolved in the crystal structure [43]. Then, IL-6 inhibitors of 31 small compounds (<1000 g/mol) to be retrieved from the Selleckchem website were screened by 3 criteria (Lipinski’s rule, TPSA, and binding energy) (Table 2); 19 out of 31 compounds were sorted by the three criteria. The MDT profiling of 31 known IL-6 inhibitors is given in Table 3.
Figure 2.
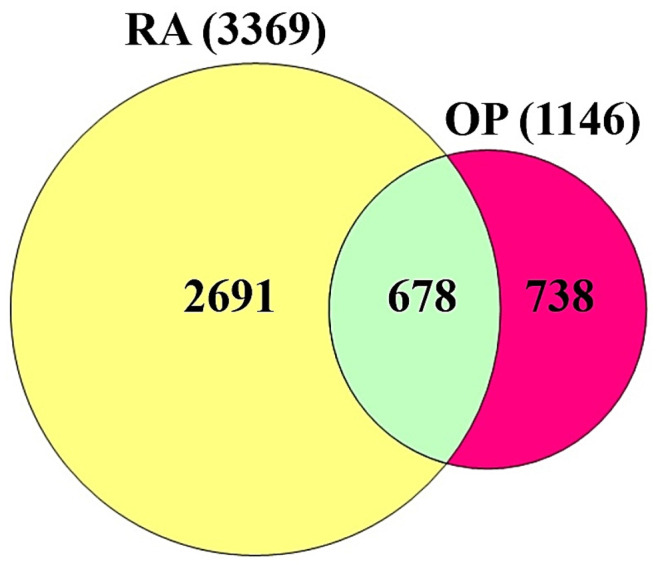
The overlapping targets between RA (3369 targets) and OP (1416 targets).
Figure 3.
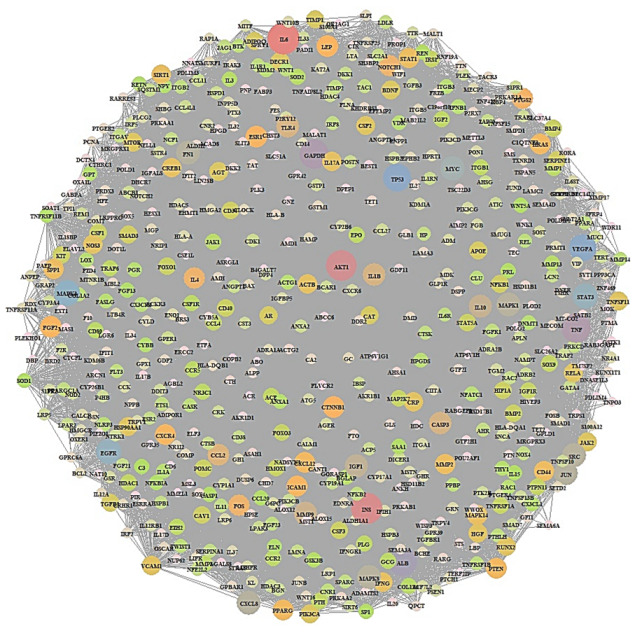
PPI network of 604 overlapping targets.
Table 1.
Degrees of value of the top 20 targets from PPI.
| No. | Target | Degrees of Value |
|---|---|---|
| 1 | IL-6 | 432 |
| 2 | INS | 317 |
| 3 | AKT1 | 312 |
| 4 | TNF | 289 |
| 5 | GAPDH | 288 |
| 6 | TP53 | 267 |
| 7 | VEGFA | 266 |
| 8 | MAPK3 | 242 |
| 9 | EGFR | 237 |
| 10 | STAT3 | 236 |
| 11 | CXCL8 | 216 |
| 12 | JUN | 216 |
| 13 | MAPK1 | 215 |
| 14 | SRC | 215 |
| 15 | MMP9 | 215 |
| 16 | IGF1 | 209 |
| 17 | IL-10 | 206 |
| 18 | CASP3 | 195 |
| 19 | IL-1B | 195 |
| 20 | TLR4 | 194 |
Table 2.
The physicochemical properties and classification of 31 compounds as IL-6 antagonists.
| Compounds | Lipinski Rules | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| MW | HBA | HBD | MLogP | Lipinski’s violations | Bioavailability Score | TPSA | Compound Classification | ||
| No. | <500 | <10 | ≤5 | ≤4.15 | ≤1 | >0.1 | <140 Å2 | ||
| 1 | Forsythoside B | 756.70 | 19 | 11 | −3.93 | 3 | 0.17 | 304.21 | Oligosaccharides |
| 2 | Pectolinarin | 622.57 | 15 | 7 | −3.03 | 3 | 0.17 | 227.20 | Flavonoid-7-O-glycosides |
| 3 | MD2-TLR4-IN-1 | 421.28 | 2 | 3 | 4.01 | 0 | 0.55 | 73.57 | Indazole |
| 4 | Aprepitant | 534.43 | 12 | 2 | 4.05 | 1 | 0.55 | 83.24 | Phenylmorpholines |
| 5 | Mulberroside A | 568.52 | 14 | 10 | −2.97 | 3 | 0.17 | 239.22 | Stilbene glycosides |
| 6 | Homoplantaginin | 462.40 | 11 | 6 | −1.89 | 2 | 0.17 | 179.28 | Flavonoid-7-O-glycosides |
| 7 | NE 52-QQ57 | 416.52 | 6 | 1 | 3.46 | 0 | 0.55 | 81.14 | Pyrazolo[1,5-a]pyrimidines |
| 8 | Madecassic acid | 504.70 | 6 | 5 | 3.33 | 1 | 0.55 | 118.22 | Triterpenoids |
| 9 | GSK583 | 398.45 | 5 | 2 | 3.36 | 0 | 0.55 | 96.12 | Aminoquinolines and derivatives |
| 10 | IQ 3 | 341.32 | 6 | 0 | 2 | 0 | 0.55 | 77.58 | Quinoxalines |
| 11 | Methylprednisolone | 374.47 | 5 | 3 | 1.52 | 0 | 0.55 | 94.83 | 21-Hydroxysteroids |
| 12 | Hydrocortisone hemisuccinate | 462.53 | 8 | 3 | 1.29 | 0 | 0.55 | 138.20 | Gluco/mineralocorticoids, progestogens, and derivatives |
| 13 | 20(S)-Ginsenoside Rh1 | 638.87 | 9 | 7 | 1.77 | 2 | 0.17 | 160.07 | Triterpene saponins |
| 14 | Stylopine | 323.34 | 5 | 0 | 2.56 | 0 | 0.55 | 323.34 | Protoberberine alkaloids and derivatives |
| 15 | Methylprednisolone Acetate | 416.51 | 6 | 2 | 1.86 | 0 | 0.55 | 100.90 | Gluco/mineralocorticoids, progestogens, and derivatives |
| 16 | Gardenoside | 404.37 | 11 | 6 | −2.62 | 2 | 0.11 | 175.37 | Iridoid O-glycosides |
| 17 | 4-Methylesculetin | 192.17 | 4 | 2 | 0.76 | 0 | 0.55 | 70.67 | 6,7-Dihydroxycoumarins |
| 18 | Auraptene | 298.38 | 3 | 0 | 3.51 | 0 | 0.55 | 39.44 | Terpene lactones |
| 19 | AX-024 HCl | 375.86 | 4 | 0 | 3.86 | 0 | 0.55 | 21.70 | Neoflavenes |
| 20 | APX-115 free base | 279.34 | 2 | 1 | 2.66 | 0 | 0.55 | 50.68 | Pyrazolylpyridines |
| 21 | Resatorvid | 361.82 | 5 | 1 | 2.44 | 0 | 0.55 | 80.85 | Sulfanilides |
| 22 | Myrislignan | 374.43 | 6 | 2 | 1.97 | 0 | 0.55 | 77.38 | Lignans, neolignans, and related compounds |
| 23 | Muscone | 238.41 | 1 | 0 | 3.92 | 0 | 0.55 | 17.07 | Cyclic ketones |
| 24 | 2′,5′-Dihydroxyacetophenone | 152.15 | 3 | 2 | 0.51 | 0 | 0.55 | 57.53 | Alkyl-phenylketones |
| 25 | α-Cyperone | 218.33 | 1 | 0 | 3.46 | 0 | 0.55 | 17.07 | Eudesmane, isoeudesmane, or cycloeudesmane sesquiterpenoids |
| 26 | Veratric acid | 182.17 | 4 | 1 | 1.06 | 0 | 0.85 | 55.76 | P-methoxybenzoic acids and derivatives |
| 27 | Triolein | 885.43 | 6 | 0 | 9.49 | 2 | 0.17 | 78.90 | Triacylglycerols |
| 28 | Methylthiouracil | 142.18 | 1 | 2 | −0.35 | 0 | 0.55 | 80.74 | Pyrimidones |
| 29 | Falcarindiol | 260.37 | 2 | 2 | 3.33 | 0 | 0.55 | 40.46 | Long-chain fatty alcohols |
| 30 | Diethyl phosphate | 154.10 | 4 | 1 | −0.43 | 0 | 0.85 | 65.57 | Dialkyl phosphates |
| 31 | Sodium thiocyanate | 81.07 | 1 | 0 | −1.01 | 0 | 0.55 | 23.79 | Metal thiocyanates |
Table 3.
Binding energy of 31 known IL-6 inhibitors (<1000 g/mol).
| Grid Box | Hydrogen Bond Interactions | Hydrophobic Interactions | |||||
|---|---|---|---|---|---|---|---|
| Protein | Ligand | PubChem ID | Binding Energy (kcal/mol) | Center | Dimension | Amino Acid Residue | Amino Acid Residue |
| IL6 (PDB ID: 4NI9) | Forsythoside B | 23928102 | −11.4 | x = 11.213 | size_x = 40 | Asp34,Tyr31,Glu110 | Gly35,Gln111,Ala114 |
| y = 33.474 | size_y = 40 | ||||||
| z = 11.162 | size_z = 40 | ||||||
| Pectolinarin | 168849 | −10.4 | x = 11.213 | size_z = 41 | Asp34,Gln111 | Ala38 | |
| y = 33.474 | size_z = 42 | ||||||
| z = 11.162 | size_z = 43 | ||||||
| (*) MD2-TLR4-IN-1 | 138454798 | −9.9 | x = 11.213 | size_z = 44 | N/A | Glu110,Ala114 | |
| y = 33.474 | size_z = 45 | ||||||
| z = 11.162 | size_z = 46 | ||||||
| (*) Aprepitant | 135413536 | −9.6 | x = 11.213 | size_z = 47 | N/A | Tyr31,Asp34,Gly35 | |
| y = 33.474 | size_z = 48 | Gln111 | |||||
| z = 11.162 | size_z = 49 | ||||||
| Mulberroside A | 6443484 | −9.5 | x = 11.213 | size_z = 50 | Glu110,Ser37,Asp34 | Ala114,Gln111 | |
| y = 33.474 | size_z = 51 | Tyr31 | |||||
| z = 11.162 | size_z = 52 | ||||||
| Homoplantaginin | 5318083 | −9.5 | x = 11.213 | size_z = 53 | Asp34,Gln111 | Ala38 | |
| y = 33.474 | size_z = 54 | ||||||
| z = 11.162 | size_z = 55 | ||||||
| (*) NE 52-QQ57 | 68379135 | −9.4 | x = 11.213 | size_z = 56 | Ser37 | Asp34,Tyr31,Ala114 | |
| y = 33.474 | size_z = 57 | Gln111 | |||||
| z = 11.162 | size_z = 58 | ||||||
| (*) Madecassic acid | 73412 | −9.4 | x = 11.213 | size_z = 59 | Glu110 | Ala114,Tyr31 | |
| y = 33.474 | size_z = 60 | ||||||
| z = 11.162 | size_z = 61 | ||||||
| (*) GSK583 | 67469084 | −9.0 | x = 11.213 | size_z = 62 | N/A | Gln111,Ala38 | |
| y = 33.474 | size_z = 63 | ||||||
| z = 11.162 | size_z = 64 | ||||||
| (*) IQ 3 | 777728 | −9.0 | x = 11.213 | size_z = 65 | N/A | Tyr31,Glu110 | |
| y = 33.474 | size_z = 66 | ||||||
| z = 11.162 | size_z = 67 | ||||||
| (*) Methylprednisolone | 6741 | −9.0 | x = 11.213 | size_z = 68 | N/A | Tyr31,Glu110 | |
| y = 33.474 | size_z = 69 | ||||||
| z = 11.162 | size_z = 70 | ||||||
| (*) Hydrocortisone hemisuccinate | 16623 | −8.9 | x = 11.213 | size_z = 71 | N/A | Glu110,Ala114,Gln111 | |
| y = 33.474 | size_z = 72 | ||||||
| z = 11.162 | size_z = 73 | ||||||
| 20(S)-Ginsenoside Rh1 | 12855920 | −8.8 | x = 11.213 | size_z = 74 | Gln111 | Asp34,Tyr31 | |
| y = 33.474 | size_z = 75 | ||||||
| z = 11.162 | size_z = 76 | ||||||
| Stylopine | 6770 | −8.8 | x = 11.213 | size_z = 77 | N/A | Gln111,Ala114,Glu110 | |
| y = 33.474 | size_z = 78 | ||||||
| z = 11.162 | size_z = 79 | ||||||
| (*) Methylprednisolone Acetate | 5877 | −8.6 | x = 11.213 | size_z = 80 | N/A | Gln111,Tyr31,Ala114 | |
| y = 33.474 | size_z = 81 | Glu110 | |||||
| z = 11.162 | size_z = 82 | ||||||
| Gardenoside | 24721095 | −7.8 | x = 11.213 | size_z = 83 | Tyr31,Asp34,Gln111 | N/A | |
| y = 33.474 | size_z = 84 | ||||||
| z = 11.162 | size_z = 85 | ||||||
| (*) 4-Methylesculetin | 5319502 | −7.6 | x = 11.213 | size_z = 86 | Arg24,Arg16 | Pro18 | |
| y = 33.474 | size_z = 87 | ||||||
| z = 11.162 | size_z = 88 | ||||||
| (*) Auraptene | 1550607 | −7.6 | x = 11.213 | size_z = 89 | N/A | Asp34,Glu110,Ala114 | |
| y = 33.474 | size_z = 90 | Tyr31 | |||||
| z = 11.162 | size_z = 91 | ||||||
| (*) AX-024 HCl | 129909862 | −7.5 | x = 11.213 | size_z = 92 | N/A | Gln111,Tyr31,Ala114 | |
| y = 33.474 | size_z = 93 | ||||||
| z = 11.162 | size_z = 94 | ||||||
| (*) APX-115 free base | 51036475 | −7.2 | x = 11.213 | size_z = 95 | Tyr31 | Glu110,Gln111,Asp34 | |
| y = 33.474 | size_z = 96 | ||||||
| z = 11.162 | size_z = 97 | ||||||
| (*) Resatorvid | 11703255 | −7.1 | x = 11.213 | size_z = 98 | Tyr31,Gln111 | Glu110,Asp34 | |
| y = 33.474 | size_z = 99 | ||||||
| z = 11.162 | size_z = 100 | ||||||
| (*) Myrislignan | 21636106 | −7.1 | x = 11.213 | size_z = 101 | Gln111 | Tyr31,Gly35,Asp34 | |
| y = 33.474 | size_z = 102 | ||||||
| z = 11.162 | size_z = 103 | ||||||
| (*) Muscone | 10947 | −6.7 | x = 11.213 | size_z = 104 | N/A | N/A | |
| y = 33.474 | size_z = 105 | ||||||
| z = 11.162 | size_z = 106 | ||||||
| (*) 2′,5′-Dihydroxyacetophenone | 10279 | −6.5 | x = 11.213 | size_z = 107 | N/A | Gln17,Pro18 | |
| y = 33.474 | size_z = 108 | ||||||
| z = 11.162 | size_z = 109 | ||||||
| (*) α-Cyperone | 6452086 | −6.3 | x = 11.213 | size_z = 110 | N/A | Gln111,Glu110 | |
| y = 33.474 | size_z = 111 | ||||||
| z = 11.162 | size_z = 112 | ||||||
| (*) Veratric acid | 7121 | −6.1 | x = 11.213 | size_z = 113 | Arg16 | Gln17,Pro18 | |
| y = 33.474 | size_z = 114 | ||||||
| z = 11.162 | size_z = 115 | ||||||
| Triolein | 5497163 | −5.5 | x = 11.213 | size_z = 116 | N/A | N/A | |
| y = 33.474 | size_z = 117 | ||||||
| z = 11.162 | size_z = 118 | ||||||
| Methylthiouracil | 667493 | −5.4 | x = 11.213 | size_z = 119 | N/A | Arg24 | |
| y = 33.474 | size_z = 120 | ||||||
| z = 11.162 | size_z = 121 | ||||||
| Falcarindiol | 5281148 | −5.2 | x = 11.213 | size_z = 122 | Glu110 | Ala114,Tyr31,Gln111 | |
| y = 33.474 | size_z = 123 | Glu110 | |||||
| z = 11.162 | size_z = 124 | ||||||
| Diethyl phosphate | 654 | −4.9 | x = 11.213 | size_z = 125 | N/A | Arg16,Gln17 | |
| y = 33.474 | size_z = 126 | ||||||
| z = 11.162 | size_z = 127 | ||||||
| Sodium thiocyanate | 516871 | −2.6 | x = 11.213 | size_z = 128 | N/A | Arg16,Gln17 | |
| y = 33.474 | size_z = 129 | ||||||
| z = 11.162 | size_z = 130 | ||||||
(*): The indication of 19 compounds accepted by the three criteria: (1) Lipinski’s rule violation (≤1), (2) TPSA (<140 Å2), and (3) binding energy (<−6.0 kcal/mol).
Particularly, forsythoside B (PubChem ID: 23928102) and pectolinarin (PubChem ID: 168849), with a higher affinity for IL-6 than MD2-TLR4-IN-1 (PubChem ID: 138454798), were not accepted by the Lipinski’s rule (Lipinski’s violations ≤ 1) and TPSA (<140 Å2) criteria.
A total of 19 out of 31 compounds were accepted by Lipinski’s rule (Lipinski’s violations ≤ 1), TPSA (<140 Å2), and binding energy (<−6.0 kcal/mol), among which MD2-TLR4-IN-1 (PubChem ID: 138454798) (Figure 4), with the highest binding energy (−9.9 kcal/mol), was selected as the most important ligand to dampen comorbidity of RA and OP. MDT studies suggest that aprepitant (PubChem ID: 135413536), NE 52-QQ57 (PubChem ID: 68379135), and madecassic acid (PubChem ID: 73412) could be potential ligands with positive effects in alleviating comorbidity. The MDT of MD2-TLR4-IN-1 (PubChem ID: 138454798) on IL-6 (PDB ID: 4NI9) is displayed in Figure 5. The MDT results showed that the complex of IL-6 (PDB ID: 4NI9) with MD2-TLR4-IN-1 (PubChem ID: 138454798) had two hydrophobic interactions (Glu110 and Ala114), and there was no hydrogen bonding; this implies that hydrophobic interactions exert a strong binding effect on the complex of IL-6 (PDB ID: 4NI9) with MD2-TLR4-IN-1 (PubChem ID: 138454798). Furthermore, MD2-TLR4-IN-1 (PubChem ID: 138454798) on IL-6 had 4–22 donors and 3–7 acceptors; however, aprepitant (PubChem ID: 135413536), with the second highest affinity for IL-6, had 7–23 donors and 1–13 acceptors, while NE 52-QQ57 (PubChem ID: 6837915), with the third highest affinity for IL-6, had 11–24 donors and 2–7 acceptors. In parallel, these results shed light on the significance of the number of acceptors involved in target–ligand interactions. Finally, we demonstrated the toxicity of MD2-TLR4-IN-1 (PubChem ID: 138454798) via the admetSAR web-based tool. Our results showed that MD2-TLR4-IN-1 (PubChem ID: 138454798) had no Ames toxicity, carcinogenic properties, acute oral toxicity, or rat acute toxicity properties (Table 4).
Figure 4.
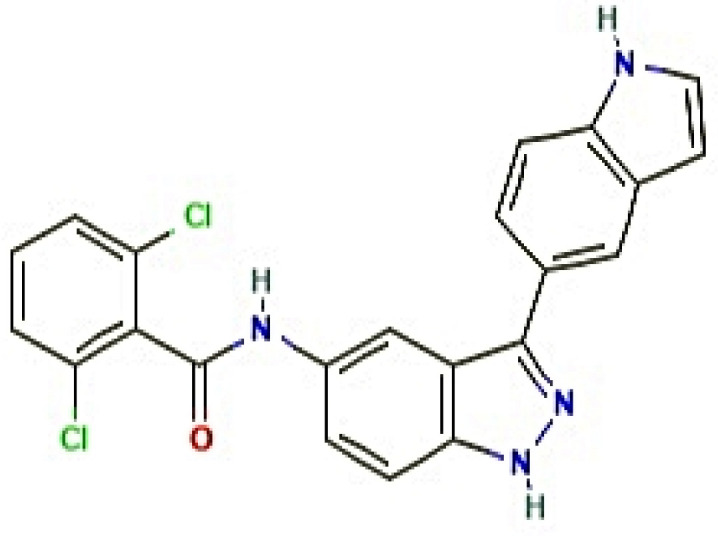
2D structure of MD2-TLR4-IN-1 (PubChem ID: 138454798).
Figure 5.
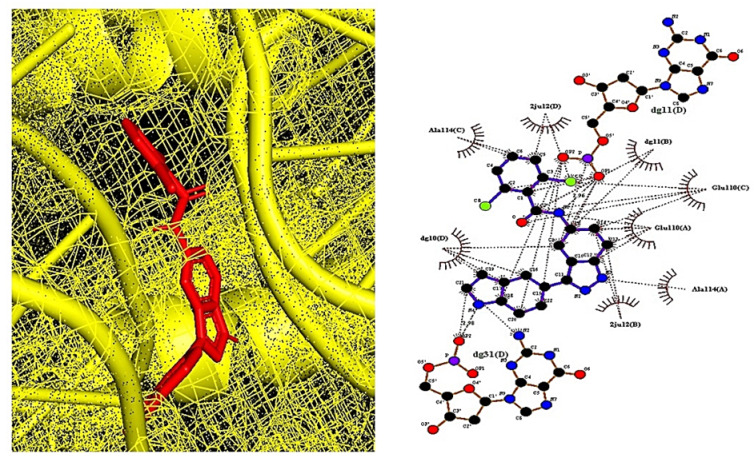
The molecular docking between IL-6 (PDB ID: 4NI9) and MD2-TLR4-IN-1 (PubChem ID: 138454798).
Table 4.
A prediction of toxicological propensity of MD2-TLR4-IN-1 (PubChem ID: 138454798).
| Parameters | Compound |
|---|---|
| MD2-TLR4-IN-1 | |
| Ames toxicity | NAT |
| Carcinogens | NC |
| Acute oral toxicity | Ⅲ |
| Rat acute toxicity | 2.2347 |
NAT: Non-Ames toxic; NC: non-carcinogenic; Category II: 50 mg/kg > Lethal Dose 50% (LD50) < 500 mg/kg; Category III: 500 mg/kg > Lethal Dose 50% (LD50) < 5000 mg/kg; Category IV: Lethal Dose 50% (LD50) > 5000 mg/kg; Rat acute toxicity: the treatment of 2.2347 mol/kg in rats shows LD50 toxicity.
5. Discussion
A total of 678 targets were involved in the occurrence and development of the liaison of comorbidity between RA and OP. IL-6, with the highest degree of value in PPI, was considered to be a core target to alleviate the level of pathological severity.
A report demonstrated that IL-6 inhibition prevents the progression of joint destruction in RA patients and interferes with bone resorption by blocking osteoclast formation [44]. IL-6 plays important roles in inflammatory processing—a continuation of autoimmunity via B-cell and T-helper 17 (Th17) differentiation [45]. Tocilizumab (TCZ), as a representative antagonist of IL-6, has been used to treat RA; however, its serious adverse side effect is that infectious disease related to C-reactive protein (CRP) cannot be recognized during TCZ treatment [46]; this implies that stealth infections without any specific signals can wreak havoc on patients’ condition.
Another report revealed that the expression level of IL-6 in OA patients is elevated noticeably, and that there is a considerable correlation between CRP and bone mineral density (BMD) [47]. Moreover, IL-6 expression levels were increased in the synovial fluid of RA patients, and a significant stimulator of bone resorption in OA patients [48]. Particularly, in an in vivo test, IL-6 aggravated the severity of osteoporosis, due to fewer osteoclasts and increased bone destruction [49]; this suggests that IL-6 plays a pivotal role in alleviating osteoporotic inflammation reactions.
Collectively, IL-6 inhibitors may be promising ligands to overcome comorbidity of RA and OP. Among the known IL-6 inhibitor ligands, MD2-TLR4-IN-1 (PubChem ID: 138454798), which is a derivative of indazole with a heterocyclic aromatic organic compound, was accepted by all three criteria (Lipinski’s rule, TPSA, and binding energy). The derivatives consist of a benzene ring and a pyrazole ring, which exert diverse biological activities, including antitumor, antibacterial, antifungal, antiarrhythmic, anti-HIV, and anti-inflammation activities [50,51]. A report demonstrated that derivatives of indazoles have potent anti-inflammatory efficacy, including anti-RA and anti-OP [52,53]. Thus, we suggest that MD2-TLR4-IN-1 (PubChem ID: 138454798) might be a potent ligand to alleviate the comorbidity of RA and OP.
6. Conclusions
To sum things up, the most significant targets—the key ligands for alleviation of the comorbidity of RA and OP—were IL-6 (PDB ID: 4NI9) and MD2-TLR4-IN-1 (PubChem ID: 138454798), respectively. This study gives us a hint at the value of imidazole derivatives to develop new medications against RA and OP.
Acknowledgments
This study has been worked with the support of a research grant of Kangwon National University in 2022.
Abbreviations
| BMD | Bone mineral density |
| COX | Cyclooxygenase |
| COX-1 | Cyclooxygenase-1 |
| COX-2 | Cyclooxygenase-2 |
| CRP | C-reactive protein |
| DMARDs | Disease-modifying anti-rheumatic drugs |
| IL-1 | Interleukin 1 |
| IL-6 | Interleukin 6 |
| IL-17 | Interleukin 17 |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| OP | Osteoporosis |
| PPI | Protein–protein interaction |
| RA | Rheumatoid arthritis |
| TCZ | Tocilizumab |
| TPSA | Topological polar surface area |
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cimb44030069/s1: Table S1: The 3377 rheumatoid arthritis (RA)-related targets, and 1426 osteoporosis (OP)-related targets; Table S2: The 678 overlapping targets.
Author Contributions
Conceptualization, methodology, formal analysis, investigation, visualization, data curation, and writing—original draft preparation, K.-K.O.; software, investigation, and data curation, K.-K.O. and M.A.; validation, writing—review and editing, M.A.; supervision, project administration, D.-H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
International Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Materials).
Conflicts of Interest
There are no conflicts of interest to be declared.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Llorente I., García-Castañeda N., Valero C., González-Álvaro I., Castañeda S. Osteoporosis in Rheumatoid Arthritis: Dangerous Liaisons. Front. Med. 2020:802. doi: 10.3389/fmed.2020.601618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McInnes I.B., Schett G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet. 2017;389:2328–2337. doi: 10.1016/S0140-6736(17)31472-1. [DOI] [PubMed] [Google Scholar]
- 3.Bullock J., Rizvi S.A.A., Saleh A.M., Ahmed S.S., Do D.P., Ansari R.A., Ahmed J. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med. Princ. Pract. 2019;27:501. doi: 10.1159/000493390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sweeney E.S., Firestein G.S. Rheumatoid arthritis: Regulation of synovial inflammation. Int. J. Biochem. Cell Biol. 2004;36:372–378. doi: 10.1016/S1357-2725(03)00259-0. [DOI] [PubMed] [Google Scholar]
- 5.Jiang Y., Zhong M., Long F., Yang R., Zhang Y., Liu T. Network Pharmacology-Based Prediction of Active Ingredients and Mechanisms of Lamiophlomis rotata (Benth.) Kudo Against Rheumatoid Arthritis. Front. Pharmacol. 2019:1435. doi: 10.3389/fphar.2019.01435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smolen J.S., Aletaha D., Koeller M., Weisman M.H., Emery P. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370:1861–1874. doi: 10.1016/S0140-6736(07)60784-3. [DOI] [PubMed] [Google Scholar]
- 7.Stevenson M., Archer R., Tosh J., Simpson E., Everson-Hock E., Stevens J., Hernandez M., Paisley S., Dickinson K., Scott D., et al. Adalimumab, etanercept, infliximab, certolizumab pegol, golimumab, tocilizumab and abatacept for the treatment of rheumatoid arthritis not previously treated with disease-modifying antirheumatic drugs and after the failure of conventional disease-modifying antirheumatic drugs only: Systematic review and economic evaluation. Health Technol. Assess. 2016;20:1–610. doi: 10.3310/HTA20350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betts K.A., Griffith J., Ganguli A., Li N., Douglas K., Wu E.Q. Economic Burden and Treatment Patterns of Cycling between Conventional Synthetic Disease-modifying Antirheumatic Drugs among Biologic-treated Patients with Rheumatoid Arthritis. Clinical Therapeutics. 2016;38:1205–1216. doi: 10.1016/j.clinthera.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka Y. Managing Osteoporosis and Joint Damage in Patients with Rheumatoid Arthritis: An Overview. J. Clin. Med. 2021;10:1241. doi: 10.3390/jcm10061241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osterhoff G., Morgan E.F., Shefelbine S.J., Karim L., McNamara L.M., Augat P. Bone mechanical properties and changes with osteoporosis. Injury. 2016;47:S11. doi: 10.1016/S0020-1383(16)47003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexandru D., So W. Evaluation and Management of Vertebral Compression Fractures. Perm. J. 2012;16:46. doi: 10.7812/TPP/12-037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji M.-X., Yu Q. Primary osteoporosis in postmenopausal women. Chronic Dis. Transl. Med. 2015;1:9. doi: 10.1016/J.CDTM.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sözen T., Özışık L., Başaran N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017;4:46. doi: 10.5152/eurjrheum.2016.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginaldi L., Di Benedetto M.C., Martinis M. De Osteoporosis, inflammation and ageing. Immun. Ageing I A. 2005;2:14. doi: 10.1186/1742-4933-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy R., Cooper M.S. Bone loss in inflammatory disorders. J. Endocrinol. 2009;201:309–320. doi: 10.1677/JOE-08-0568. [DOI] [PubMed] [Google Scholar]
- 16.Tella S.H., Gallagher J.C. Biological agents in management of osteoporosis. Eur. J. Clin. Pharmacol. 2014;70:1291–1301. doi: 10.1007/s00228-014-1735-5. [DOI] [PubMed] [Google Scholar]
- 17.Lewiecki E.M. New targets for intervention in the treatment of postmenopausal osteoporosis. Nat. Rev. Rheumatol. 2011;7:631–638. doi: 10.1038/nrrheum.2011.130. [DOI] [PubMed] [Google Scholar]
- 18.Ginosyan K., Jndoyan Z., Ghazinyan I., Vardanyan V. Benefits and disadvantages of biologic agents in chronic inflammatory arthritis. Int. J. Clin. Rheumatol. 2020;15:6–9. doi: 10.37532/1758-4272.2020.15(1). [DOI] [Google Scholar]
- 19.Thong B.Y.-H., Tan T.-C. Epidemiology and risk factors for drug allergy. Br. J. Clin. Pharmacol. 2011;71:684–700. doi: 10.1111/j.1365-2125.2010.03774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker S.E., Davey P., Davey P.G. Pharmacoeconomics of intravenous drug administration. Pharm. 1992;1:103–115. doi: 10.2165/00019053-199201020-00007. [DOI] [PubMed] [Google Scholar]
- 21.Gomes E., Demoly P. Epidemiology of hypersensitivity drug reactions. Curr. Opin. Allergy Clin. Immunol. 2005;5:309–316. doi: 10.1097/01.all.0000173785.81024.33. [DOI] [PubMed] [Google Scholar]
- 22.Crofford L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 2013;15:S2. doi: 10.1186/ar4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brooks P., Emery P., Evans J.F., Fenner H., Hawkey C.J., Patrono C., Smolen J., Breedveld F., Day R., Dougados M., et al. Interpreting the clinical significance of the differential inhibition of cyclooxygenase-1 and cyclooxygenase-2. Rheumatology. 1999;38:779–788. doi: 10.1093/rheumatology/38.8.779. [DOI] [PubMed] [Google Scholar]
- 24.Lisowska B., Kosson D., Domaracka K. Positives and negatives of nonsteroidal anti-inflammatory drugs in bone healing: The effects of these drugs on bone repair. Drug Des. Dev. Ther. 2018;12:1809. doi: 10.2147/DDDT.S164565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaubitz M. Osteoporose–häufige Komorbidität bei Rheumapatienten. Z. Für Rheumatol. 2019;78:249–254. doi: 10.1007/s00393-019-0622-y. [DOI] [PubMed] [Google Scholar]
- 26.Kim S.Y., Schneeweiss S., Liu J., Daniel G.W., Chang C.-L., Garneau K., Solomon D.H. Risk of osteoporotic fracture in a large population-based cohort of patients with rheumatoid arthritis. Arthritis Res. Ther. 2010;12:R154. doi: 10.1186/ar3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castañeda S., Garcés-Puentes M.V., Bernad Pineda M. Pathophysiology of osteoporosis in chronic inflammatory joint diseases. Rev. De Osteoporos. Y Metab. Miner. 2021;13:32–38. doi: 10.4321/S1889-836X2021000100006. [DOI] [Google Scholar]
- 28.Pushpakom S., Iorio F., Eyers P.A., Escott K.J., Hopper S., Wells A., Doig A., Guilliams T., Latimer J., McNamee C., et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2018;18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 29.Somolinos F.J., León C., Guerrero-Aspizua S. Drug repurposing using biological networks. Processes. 2021;9:1057. doi: 10.3390/pr9061057. [DOI] [Google Scholar]
- 30.Rudrapal M., Khairnar S.J., Jadhav A.G. Drug Repurposing-Hypothesis, Molecular Aspects and Therapeutic Applications. IntechOpen; London, UK: 2020. Drug Repurposing (DR): An Emerging Approach in Drug Discovery. [DOI] [Google Scholar]
- 31.Gil C., Martinez A. Is drug repurposing really the future of drug discovery or is new innovation truly the way forward? Expert Opin. Drug Discov. 2021;16:829–831. doi: 10.1080/17460441.2021.1912733. [DOI] [PubMed] [Google Scholar]
- 32.Hodos R.A., Kidd B.A., Shameer K., Readhead B.P., Dudley J.T. Computational Approaches to Drug Repurposing and Pharmacology. Wiley Interdiscip. Reviews. Syst. Biol. Med. 2016;8:186. doi: 10.1002/wsbm.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu G., Wang W., Wang X., Xu M., Zhang L., Ding L., Guo R., Shi Y. Network pharmacology-based strategy to investigate pharmacological mechanisms of Zuojinwan for treatment of gastritis. BMC Complementary Altern. Med. 2018;18:1–12. doi: 10.1186/s12906-018-2356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yip K.W., Liu F.-F. Small Molecule Screens. Encycl. Cancer. 2011:3451–3455. doi: 10.1007/978-3-642-16483-5_5376. [DOI] [Google Scholar]
- 35.Zhang M.-Q., Wilkinson B. Drug discovery beyond the “rule-of-five”. Curr. Opin. Biotechnol. 2007;18:478–488. doi: 10.1016/j.copbio.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Matsson P., Kihlberg J. How Big Is Too Big for Cell Permeability? J. Med. Chem. 2017;60:1662–1664. doi: 10.1021/acs.jmedchem.7b00237. [DOI] [PubMed] [Google Scholar]
- 37.Shityakov S., Förster C. In silico predictive model to determine vector-mediated transport properties for the blood–brain barrier choline transporter. Adv. Appl. Bioinform. Chem. AABC. 2014;7:23. doi: 10.2147/AABC.S63749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khanal P., Patil B.M., Chand J., Naaz Y. Anthraquinone Derivatives as an Immune Booster and their Therapeutic Option Against COVID-19. Nat. Prod. Bioprospecting. 2020;10:325. doi: 10.1007/s13659-020-00260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang H., Lou C., Sun L., Li J., Cai Y., Wang Z., Li W., Liu G., Tang Y. admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics. 2019;35:1067–1069. doi: 10.1093/bioinformatics/bty707. [DOI] [PubMed] [Google Scholar]
- 40.Boulanger M.J., Chow D.-c., Brevnova E.E., Garcia K.C. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science (New York N.Y.) 2003;300:2101–2104. doi: 10.1126/science.1083901. [DOI] [PubMed] [Google Scholar]
- 41.Somers W., Stahl M., Seehra J.S. 1.9 A crystal structure of interleukin 6: Implications for a novel mode of receptor dimerization and signaling. EMBO J. 1997;16:989–997. doi: 10.1093/emboj/16.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokota T., Arai N., De Vries J., Spits H., Banchereau J., Zlotnik A., Rennick D., Howard M., Takebe Y., Miyatake S., et al. Molecular Biology of Interleukin 4 and Interleukin 5 Genes and Biology of their Products that Stimulate B Cells, T Cells and Hemopoietic Cells. Immunol. Rev. 1988;102:137–187. doi: 10.1111/j.1600-065X.1988.tb00744.x. [DOI] [PubMed] [Google Scholar]
- 43.Gelinas A.D., Davies D.R., Edwards T.E., Rohloff J.C., Carter J.D., Zhang C., Gupta S., Ishikawa Y., Hirota M., Nakaishi Y., et al. Crystal Structure of Interleukin-6 in Complex with a Modified Nucleic Acid Ligand. J. Biol. Chem. 2014;289:8720. doi: 10.1074/jbc.M113.532697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hashizume M., Mihara M. The roles of interleukin-6 in the pathogenesis of rheumatoid arthritis. Arthritis. 2011;2011:765624. doi: 10.1155/2011/765624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 46.Ogata A., Kato Y., Higa S., Yoshizaki K. IL-6 inhibitor for the treatment of rheumatoid arthritis: A comprehensive review. Mod. Rheumatol. 2019;29:258–267. doi: 10.1080/14397595.2018.1546357. [DOI] [PubMed] [Google Scholar]
- 47.Li X., Zhou Z., Zhang Y., Yang H. IL-6 Contributes to the Defective Osteogenesis of Bone Marrow Stromal Cells from the Vertebral Body of the Glucocorticoid-Induced Osteoporotic Mouse. PLoS ONE. 2016;11:154677. doi: 10.1371/JOURNAL.PONE.0154677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roodman G.D. Perspectives: Interleukin-6: An osteotropic factor? J. Bone Miner. Res. 1992;7:475–478. doi: 10.1002/jbmr.5650070502. [DOI] [PubMed] [Google Scholar]
- 49.Ohshima S., Saeki Y., Mima T., Sasai M., Nishioka K., Nomura S., Kopf M., Katada Y., Tanaka T., Suemura M., et al. Interleukin 6 plays a key role in the development of antigen-induced arthritis. Proc. Natl. Acad. Sci. USA. 1998;95:8222–8226. doi: 10.1073/pnas.95.14.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Denya I., Malan S.F., Joubert J. Indazole derivatives and their therapeutic applications: A patent review (2013–2017) Expert Opin. Ther. Pat. 2018;28:441–453. doi: 10.1080/13543776.2018.1472240. [DOI] [PubMed] [Google Scholar]
- 51.Zhang S.-G., Liang C.-G., Zhang W.-H. Recent Advances in Indazole-Containing Derivatives: Synthesis and Biological Perspectives. Molecules. 2018;23:2783. doi: 10.3390/molecules23112783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheekavolu C., Muniappan M. In vivo and In vitro Anti-Inflammatory Activity of Indazole and Its Derivatives. J. Clin. Diagn. Res. JCDR. 2016;10:FF01. doi: 10.7860/JCDR/2016/19338.8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cerecetto H., Gerpe A., González M., Arán V.J., De Ocáriz C.O. Pharmacological properties of indazole derivatives: Recent developments. Mini Rev. Med. Chem. 2005;5:869–878. doi: 10.2174/138955705774329564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its Supplementary Materials).


