Abstract
Background
Congenital diaphragmatic hernia (CDH), is an uncommon but severe condition in which there is a developmental defect in the fetal diaphragm, resulting in liver and bowel migrating to the chest cavity and impairing lung development and function for the neonate. This condition can be diagnosed during pregnancy and as such, is potentially amenable to in‐utero prenatal intervention. Neonatal surgical repair is possible, but even with early surgical repair and improving neonatal management, neonatal morbidity and mortality is high. Prenatal interventions described to date have included maternal antenatal corticosteroid administration and fetal tracheal occlusion, with both methods aiming to improve lung growth and maturity. However surgical procedures have potential maternal complications, as the uterus and amniotic sac are breached in order to gain access to the fetus.
Objectives
To compare the effects of prenatal versus postnatal interventions for CDH on perinatal mortality and morbidity, longer‐term infant outcomes and maternal morbidity, and to compare the effects of different prenatal interventions with each other.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 August 2015) and reference lists of retrieved studies.
Selection criteria
All published (including those published in abstract form), unpublished, and ongoing randomised controlled trials comparing prenatal and postnatal interventions for fetuses with CDH. Quasi‐RCTs were eligible for inclusion but none were identified. Trials using a cross‐over design are not eligible for inclusion.
Data collection and analysis
Two review authors evaluated trials for inclusion and methodological quality without consideration of their results according to the stated eligibility criteria and extracted data independently. Data were checked for accuracy.
Main results
We identified 11 studies for potential inclusion. Of those, we included three studies involving 97 women. Two additional studies are ongoing.
Two trials examined in‐utero fetal tracheal occlusion with standard (postnatal) care in fetuses with severe diaphragmatic hernia. Whilst the trials utilised fetal interventions that were similar, there were important differences in how access was gained to the fetus and in the timing and mode of delivery. Therefore, we did not combine these trials in meta‐analysis and the results are examined in separate comparisons. One trial examined the effect of antenatal corticosteroids versus placebo. Overall, the methodological quality of the trials was variable and no data were available for a number of this review's secondary outcomes.
In‐utero fetal occlusion by maternal laparotomy versus standard postnatal management (one trial, 24 women)
For the primary infant outcome (perinatal mortality), there were no data suitable for inclusion in the analysis. There was no difference between groups in terms of long‐term infant survival (risk ratio (RR) 1.06, 95% confidence interval (CI) 0.66 to 1.69).
In‐utero fetal occlusion by minimally invasive fetoscopy versus standard postnatal management (one trial, 41 women)
The primary infant outcome (perinatal mortality) was not reported. Minimally invasive fetoscopy was associated with a small reduction in the mean gestational age at birth (mean difference (MD) ‐1.80 weeks, 95% CI ‐3.13 to ‐0.47), but there was no clear difference in the risk of preterm birth before 37 weeks (RR 1.75, 95% CI 0.78 to 3.92). Long‐term infant survival (three to six months) (RR 10.50, 95% CI 1.48 to 74.71) was increased with the intervention when compared with standard management, and there was a corresponding reduction in pulmonary hypertension (RR 0.58, 95% CI 0.36 to 0.93) associated with the intervention. There was no difference between groups in terms of preterm ruptured membranes (< 37 weeks) (RR 1.47, 95% CI 0.56 to 3.88) or maternal infectious morbidity (RR 3.14, 95% CI 0.14 to 72.92), and there were no maternal blood transfusions.
Antenatal corticosteroids versus placebo (one trial, 32 women)
We also included one trial (involving 32 women) examining the effect of antenatal corticosteroids versus placebo. There was no clear difference in the incidence of perinatal mortality (our primary infant outcome) between the group of women who received antenatal corticosteroids and the placebo control (RR 1.24, 95% CI 0.50 to 3.08). Data (mean only) were reported for two of our secondary outcomes (mechanical ventilation and days of hospital admission) but standard deviations (SDs) were not provided. For the purposes of this review and to permit further analysis we have estimated the SDs based on the reported P values reported in the trial report, although our estimation does assume that the SD is the same in both the intervention and control groups. There were no differences between the antenatal corticosteroid group and the placebo control in terms of days of mechanical ventilation (MD 18.00 days, 95% CI ‐14.77 to 50.77) or days of hospital admission (MD 17.00 days, 95% CI ‐13.93 to 47.93) .
Authors' conclusions
There is currently insufficient evidence to recommend in‐utero intervention for fetuses with CDH as a part of routine clinical practice. We identified three small studies, with only one study adequately reporting on the primary outcome of this review ‐ perinatal mortality, and there were few data pertaining to many of this review's secondary outcomes.
WIth regard to the administration of antenatal corticosteroids, there remains a gap in current research, and a large multicentre trial with adequate statistical power should be undertaken to answer this unresolved question. More studies are needed to further examine the effect of in‐utero fetal tracheal occlusion on important neonatal outcomes and long‐term infant survival and health. Long‐term follow‐up is of particular importance, and should include morbidity and mortality measures. Further studies should examine the benefits of an in‐utero intervention on subgroups with moderate and severe congenital diaphragmatic hernia. Indeed, there are three ongoing studies, being conducted by European, North and South American fetal medicine centres, which will contribute to this gap. Ongoing research and any implementation into clinical practice should include standardisation of the procedure, inclusion criteria and long‐term childhood follow‐up.
Plain language summary
Prenatal treatments for babies with congenital diaphragmatic hernia
A congenital diaphragmatic hernia (CDH) is a hole in the diaphragm, the muscle that helps with breathing and separates the chest and abdomen. This defect can allow the liver and bowel to move to the chest cavity and interfere with lung development, affecting lung and heart function in newborn babies. At birth, respiratory insufficiency and pulmonary hypertension contribute to poor outcomes. About one in every 3000 babies may be affected and the problem can be diagnosed during a routine mid‐pregnancy ultrasound at around 20 weeks. For babies born with a CDH, surgery in early life is necessary, but even with new surgical techniques there can be a poor outlook and many long‐term medical problems. Treatments are now possible in pregnancy. Interventions described to date include maternal antenatal corticosteroid administration and prenatal tracheal occlusion to improve lung growth and maturity by obstruction of the fetal trachea. This increases airway pressure by preventing secreted lung fluid from leaving the lungs resulting in growth and expansion of the lungs. There are potential side effects and complications for the mother with this procedure as the uterus and amniotic sac are entered in order to gain access to the unborn baby.
This review aimed to compare the new treatments used in pregnancy with standard current care, which is surgery to reduce the herniated abdominal contents and close the diaphragmatic defect in the first few days of life following stabilisation of the newborn in a neonatal intensive care unit.
We included three randomised controlled studies (involving 97 women). The quality of the studies was variable and a number of this review's important outcomes were not reported in the trials.
Two studies compared in‐utero fetal tracheal occlusion with standard postnatal repair, but differences between the two studies meant that we were unable to combine the data in our analyses. Neither study reported on perinatal deaths. In single studies, in‐utero fetal occlusion was associated with a slightly lower gestational age at birth but no clear difference in the risk of preterm birth before 37 weeks; the occurrence of pulmonary hypertension was reduced. there was no difference between groups in terms of preterm rupture of membranes < 37 weeks or maternal infectious morbidity and there were no maternal blood transfusions. Long‐term infant survival was improved with in‐utero tracheal occlusion in one study, but not in the other.
In the third study, antenatal corticosteroids were compared with placebo and there was no difference in the number of perinatal deaths. Nor was there any difference in terms of the number of days that babies were given mechanical ventilation or the number of days babies spent in hospital.
We conclude that the current evidence is too limited by small numbers of pregnancies and the variable methodological quality of the trials to recommend intervention (treatment) in pregnancy for women and their unborn babies with CDH. Further high‐quality trials are needed in this area. WIth regard to the administration of antenatal corticosteroids, there remains a gap in current research, and a large, high‐quality trial should be undertaken to answer this unresolved question. More studies are needed to further examine the effect of in‐utero fetal tracheal occlusion on important neonatal outcomes and long‐term infant survival and health. Long‐term follow‐up is of particular importance, and should include morbidity and mortality measures. Further studies should examine the benefits of an in‐utero intervention in relation to the severity of the congenital diaphragmatic hernia (i.e. moderate and severe). Indeed, there are three ongoing studies, being conducted by European, North and South American fetal medicine centres, which will contribute to this gap. Ongoing research and any implementation into clinical practice should include standardisation of the procedure, inclusion criteria and long‐term childhood follow‐up.
Background
Description of the condition
Congenital diaphragmatic hernia (CDH) is a developmental defect that results in partial or complete absence of the diaphragm. The diaphragm is a large muscle that separates the chest from the abdominal cavity and is important in breathing. In Australia, the condition occurs in approximately one in 5000 births (0.2 per 1000) when live births and still births are included and is slightly more common (0.3 per 1000) when terminations of pregnancy are also included (AIHW 2004). The United States and Europe report similar rates, although data collection and reporting varies within and between countries and whether stillbirths and terminations of pregnancy are included (Done 2008). CDH is associated with other anatomical anomalies or chromosomal problems in 30% of babies and the presence of associated anomalies (anatomical and chromosomal) is frequently associated with poor outcomes for babies (Deprest 2004). The incompletely formed diaphragm is thought to result from an error during development when the diaphragm muscle joins itself to the chest wall. The right or left or both portions of the diaphragm may be absent resulting in a 'hernia' (Chiu 2008). The hernia in the diaphragm then allows abdominal contents (usually bowel) to move into the chest. This results in the fetal lungs being small and dysfunctional (hypoplastic) at birth. The defect is most commonly left‐sided (Deprest 2009a) and in some cases the liver may also be present in the chest instead of the abdomen. Prognosis is dependent upon the size of the lungs at birth. This may be assessed by evaluating the presence of the liver in the chest and also the lung‐‐head ratio (LHR). In cases of severe CDH (liver in the chest and low LHR), there is frequently high morbidity and mortality despite advances in neonatal care. Much of the disease in the neonatal population occurs because the lungs are small and do not function properly. Some authors believe that very abnormal/lethal lung function and size (hypoplasia) may be predicted during pregnancy by a fetal LHR less than one in the presence of liver in the chest (Laudy 2003). Increased understanding of the disease process and subsequent improved management has resulted in neonatal survival rates of up to 90% in some centres (Chiu 2006), although in most centres survival is in the order of 50% to 70% (Colvin 2005). Postnatal management requires access to a neonatal intensive care unit (NICU) for stabilisation and subsequent open repair. In some intensive care units, when technology is available "ECMO" (extracorporeal membrane oxygenation) is used. ECMO is a specialised way of providing respiratory support where blood is circulated through an artificial lung. This technology is not available in all centres. The overall aims of postnatal management are to support oxygenation and ventilation while minimising ventilator‐induced lung damage, maintain cardiovascular stability, and to minimise overall morbidity (Chiu 2008).
Description of the intervention
Surgical repair of CDH in the neonatal period was first described by Gross in 1946 (Gross 1946), and aims to reduce the herniated abdominal contents and close the diaphragmatic defect. The defect may be closed with or without the need for a patch. The underlying repair techniques have changed little over the last 20 years, although recent promising developments include the use of more advanced materials for patch repair, and the use of laparoscopic and thoracoscopic (key‐hole surgery) repair techniques. Currently, timing of repair tends to be individualised allowing for an initial stabilisation period after recognition that immediate repair can often be harmful in an unstable baby (Chiu 2008).
Advances in prenatal ultrasound now mean that most cases of CDH are diagnosed in the prenatal period. Prenatal surgical interventions/techniques have been developed with the aim of improving lung size and function (pulmonary hypoplasia) in utero and consequently improving lung function and neonatal outcome. Initial experimental work in animals has shown that intrauterine repair of diaphragmatic hernia with replacement of the bowel and liver into the abdomen can result in reversal of pulmonary hypoplasia and pulmonary hypertension, the main contributors to poor neonatal outcome.
Prenatal repair (Harrison 1997) was initially attempted via hysterotomy and fetal surgery; however, this technique seems to be associated with more maternal morbidity and is now less commonly practiced (Harrison 1997). Further animal studies showed that obstruction of the fetal trachea results in lung expansion and improved lung development and function (DiFiore 1994).
In 2004, a group of European colleagues (the FETO task group) reported the initial results of an intrauterine fetoscopic technique that uses a reversible balloon device to occlude the trachea in fetuses with severe CDH. This procedure is commonly called "FETO" (fetal endoscopic tracheal occlusion) (Deprest 2004). The average gestational age of performing the procedure is 26 weeks of pregnancy, and in initial reports, over 50% of women experienced post operative rupture of the membranes (Deprest 2004), which has significant implications for the health of both mother and baby. The procedure itself involves maternal general anaesthesia or combined spinal–epidural anaesthesia, fetal immobilisation with anaesthetic agents, and the placement of a cannula into the amniotic cavity. An endoscope/fetoscope is then placed through the cannula into the fetal mouth, airway and into the upper trachea, and the balloon inflated.
Balloon removal is undertaken via an intrauterine approach at 34 weeks (by fetal tracheoscopy, or by puncturing the balloon under ultrasound guidance), and if this is not possible, delivery is ideally by caesarean section and ex‐utero intrapartum treatment (EXIT). An EXIT procedure is performed as an adjunct to caesarean section and involves the fetal head and neck being removed from the uterus through a standard lower segment incision, and the airway then is able to be established whilst the baby remains connected to the placenta. The airway is usually secured via tracheoscopic retrieval of the balloon from its position in the trachea (Deprest 2004). In order to avoid spontaneous labour and to allow planning, an EXIT procedure is usually performed at a preterm gestation, and therefore a decision about timing and mode of delivery in this situation will need to balance risks associated with exposing the mother to surgical risks from a caesarean section, and fetal/neonatal risks associated with preterm delivery.
Initial case reports suggested the balloon occlusion technique was feasible and could possibly be associated with improved outcome (Deprest 2004). Since the establishment of prenatal tracheal occlusion as a treatment option, those working in the field have proposed a number of prognostic factors that may enable stratification of outcomes in cases of isolated CDH including the side of the lesion (right‐sided associated with poor outcomes), LHR and liver position (Deprest 2009a). These factors are also used to decide which cases might be suitable for prenatal therapy, which is often reserved for more severe cases.
How the intervention might work
The intervention of antenatal corticosteroids was developed from the observation that similarities existed between the lung in CDH and the lung in premature infants with insufficient surfactant leading to lung immaturity and hypoplasia (George 1987). Therefore, in the lung affected by CDH, corticosteroids are thought to reverse immaturity, an observation confirmed in animal studies (Lally 2006). However, observational and randomised studies in this area have commonly utilised multiple doses of corticosteroids, which may have implications for later growth and development of the infant.
Prenatal surgical interventions in particular have been developed with the aim of reducing pulmonary hypoplasia in utero and consequently improving lung function and neonatal outcome. Prenatal tracheal occlusion increases airway pressures by preventing secreted lung fluid from leaving the lungs, which is thought to result in proliferation and maturation of the airway spaces and associated blood vessels (Deprest 2009a). Potential side effects from both prenatal tracheal occlusion and open fetal surgery are significant, as is the morbidity and mortality of the untreated condition.
Maternal complications associated with the intervention can include those associated with the delivery of the intervention itself and then the subsequent delivery of the baby. Access to the fetus during pregnancy may occur via an entirely minimally invasive technique with a fetoscope (an endoscope designed for fetal interventions) being passed through the maternal abdomen and then into the uterus, or as in earlier studies, may take place via a large maternal abdominal incision (laparotomy) and a fetoscope then being passed into the uterus. Maternal laparotomy necessitates a general anaesthetic which may be associated with complications, and the laparotomy itself is a major procedure and associated with risks of pain, infection and bleeding. Entry into the uterine cavity with a fetoscope will in many cases be associated with later rupture of the membranes, a condition known to pose risks to the mother of infection in particular.
Why it is important to do this review
It is important to systematically review the topic of prenatal repair for CDH to allow a comparison of the benefits and risks of prenatal and postnatal interventions for this condition.
Objectives
To compare the effects of prenatal versus postnatal interventions for congential diaphragmatic hernia on perinatal mortality and morbidity, longer‐term infant outcomes and maternal morbidity. Also, to compare the effects of different types of prenatal repair on the same outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We considered for inclusion randomised and quasi‐randomised studies examining the effects of any prenatal intervention or repair compared with another intervention for congential diaphragmatic hernia. We did plan to include cluster‐randomised trials but not cross‐over design trials.
Types of participants
Women with a singleton pregnancy in which the fetus has been diagnosed with congenital diaphragmatic hernia, but excluding those with other congenital abnormalities or chromosomal abnormalities.
Types of interventions
Prenatal (in‐utero) repair or intervention for congenital diaphragmatic hernia compared with standard postnatal repair/intervention.
One type of prenatal repair/intervention compared with another type of prenatal repair/intervention.
Types of outcome measures
Primary outcomes
Infant outcomes
Perinatal mortality
Secondary outcomes
Infant outcomes
Gestational age at birth
Preterm birth before 37 weeks
Preterm birth before 34 weeks
Preterm birth before 32 weeks*
Days of mechanical ventilation
Days of hospital admission
Days of NICU admission
Survival to discharge
Longer‐term infant survival (as defined by trial authors)
Pulmonary hypertension (diagnosed by echo, definition according to trial authors)
Neonatal mortality
Stillbirth
Chronic lung disease (defined as the need for ventilatory support, chronic bronchodilators, diuretics, or oxygen supplementation)
Oxygen use at 30 days*
Infant neurodevelopmental outcomes (as defined by trial authors)
Maternal outcomes
Preterm ruptured membranes
Preterm ruptured membranes (before 32 weeks)*
Preterm labour before 37 weeks
Maternal infectious morbidity
Maternal blood transfusion
Emotional wellbeing and satisfaction with care
Maternal quality of life
Admission to intensive care
* Outcomes not prespecified in the protocol, see Differences between protocol and review.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 August 2015).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identify as a result of the search strategy. We resolved any disagreement through discussion or, if required, by consulting the third author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, by consulting the third author. We entered data into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We state whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we consider it likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference. We planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
We did not include cluster‐randomised trials or trials with a cross‐over design.
Dealing with missing data
For included studies, we noted levels of attrition. We explored the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we planned to carry out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we would have attempted to include all participants randomised to each group in the analyses, and analysed all participants in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial would have been the number randomised minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of the review, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We planned to use fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and we judged the trials’ populations and methods sufficiently similar. We planned that, if there was clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity was detected, we would use random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials is considered clinically meaningful. In future updates of the review, if we use random‐effects, we will treat the random‐effects summary as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials.
If we use random‐effects analyses, we will present the results as the average treatment effect with its 95% confidence interval, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
No studies were combined in meta‐analysis but in future updates, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if so, use random‐effects analysis to produce it.
We will carry out the following subgroup analyses:
severe congenital diaphragmatic hernia (CDH) versus non‐severe CDH; with severe CDH being defined as a LHR below 1.4.
gestational age at intervention (if comparing two in‐utero treatments).
We will restrict subgroup analysis to the primary outcomes.
We will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value
Sensitivity analysis
We planned to conduct sensitivity analysis by trial quality, excluding trials that used quasi‐random allocation methods.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies.
Results of the search
The search of the Pregnancy and Childbirth Group's Trials Register identified 15 reports, involving seven studies for potential inclusion. Of those, we included three studies (nine reports) with 65 women (Harrison 2003; Lally 2006; Ruano 2012), and excluded one study (Belfort 2011). There are a number of European and South and North American centres participating in ongoing studies (Deprest 2009b; Kohl 2006; Ruano 2013) (see:Figure 1).
1.
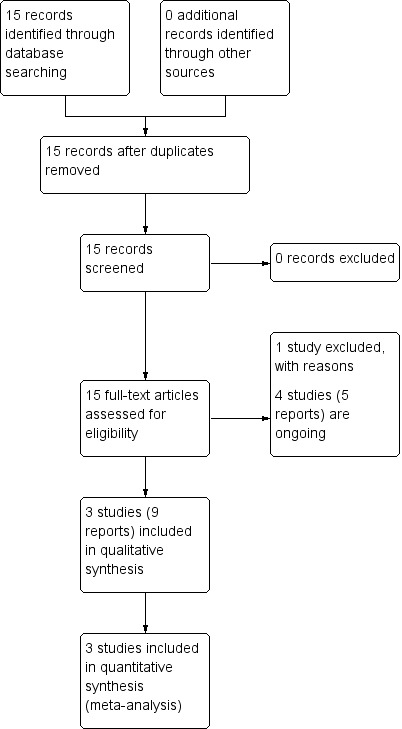
Study flow diagram.
Included studies
Participants and settings
Two trials involving 65 women (Harrison 2003; Ruano 2012) compared the effect of in‐utero fetal tracheal occlusion with standard (postnatal) care, all fetuses included were classified using lung‐head ratio (LHR) as having a severe diaphragmatic hernia. The first study was conducted in the United States (Harrison 2003), and the second study was conducted in Brazil (Ruano 2012). One study compared antenatal corticosteroids versus placebo for fetuses with congenital diaphragmatic hernia (Lally 2006) and randomised women from participating hospitals in the US, Italy, Germany and Australia.
Interventions and comparisons
The two studies comparing fetal tracheal occlusion with standard care utilised fetal interventions that were similar, with important differences in how access was gained to the fetus and in the timing and mode of delivery. The earlier study (Harrison 2003) utilised maternal laparotomy at 24 to 25 weeks' gestation and then used fetoscopes to enter the uterine cavity and for delivery, all fetuses with a tracheal balloon were delivered via caesarean section and EXIT procedure (Harrison 2003). The second study used an entirely fetoscopic approach at a slightly later gestational age (26 to 30 weeks), and also required all fetuses with a tracheal balloon to be born via caesarean section and EXIT procedure (see background for description of EXIT procedure) (Ruano 2012). Both studies that compared fetal tracheal occlusion with standard postnatal care only included fetuses with severe congenital diaphragmatic hernia. The definition of severe CDH was different between the two studies, with Harrison 2003 allowing a LHR of up to 1.4, and Ruano 2012 using a LHR threshold of 1.0, however when examining baseline characteristics from both studies, it can be appreciated that the mean LHR was less than 1.0 in both studies.
Lally 2006 compared a corticosteroid (betamethasone) with a placebo control (although the composition of the placebo was not described). The intervention was two doses of betamethasone (12.5 mg) 24 hours apart (given at 34 weeks), followed by two weekly doses.
Outcomes
In all the included studies, reporting of prespecified outcomes of the systematic review was variable; one study (Lally 2006) reported on perinatal mortality (this review's primary outcome) but this was not reported in the other two studies (Harrison 2003; Ruano 2012). Few outcome data were available for this review's secondary outcomes. Harrison 2003 and Ruano 2012 both reported on long‐term infant survival (three to six months). In the Harrison 2003 report, only data for 90 day survival were reported using an intention‐to‐treat analysis, the remainder were reported using an actual treatment‐received analysis. Data were unable to be restored to an intention‐to‐treat form and therefore outcomes other than 90‐day survival were not able to be included in the meta‐analysis. Ruano 2012 also reported on gestational age at birth, preterm birth before 37 weeks, pulmonary hypertension, preterm ruptured membranes (< 37 weeks), maternal infectious morbidity,and maternal blood transfusion.
In terms of this review's secondary outcomes, Lally 2006 also reported on days of mechanical ventilation and days of hospital admission, but these data were provided as mean values only (i.e. no standard deviations were provided), so we calculated an estimate of the standard deviation from the P values detailed in the trial report.
Excluded studies
One study was excluded because it was a cohort study reporting general follow‐up from the FETO consortium (Belfort 2011).
Risk of bias in included studies
Overall, the methodological quality of the trials was variable (see Figure 2, Figure 3, Description of studies, and Characteristics of included studies).
2.
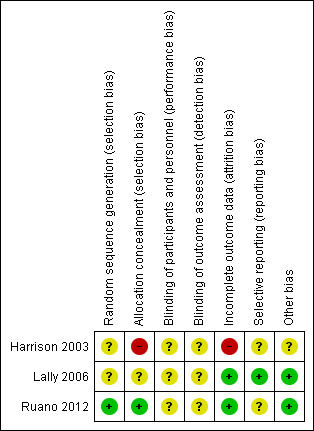
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.
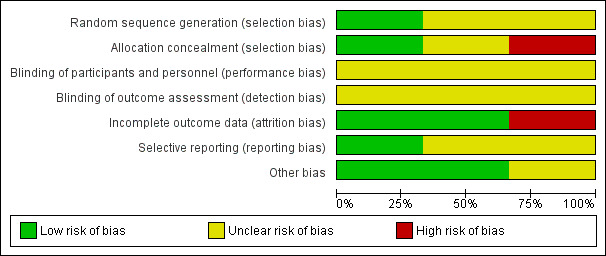
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
While all of the studies were stated to be randomised, the method of randomisation was adequately described in only one trial (Ruano 2012). In this study, allocation concealment was assessed as adequate (described by authors as involving a computer‐generated randomisation sequence). Selection bias for Ruano 2012 has therefore been assessed as low risk (Ruano 2012). Harrison 2003 did not specifically report random sequence generation (unclear risk), and allocation concealment has been assessed as high risk (two women allocated to fetal tracheal occlusion opted to have standard care) (Harrison 2003). Lally 2006 only described the method of randomisation as 'central randomisation centre' with no further information given ‐ this trial was assessed as unclear risk of bias for both sequence generation and allocation concealment.
Blinding
Blinding of participants and personnel was not reported/described in all three studies, and therefore all three studies have been assessed as at unclear risk of performance bias. Blinding of outcome assessor was not described or stated in Harrison 2003 and Ruano 2012, therefore both studies have been assessed as at unclear risk of detection bias. Lally's paper however report that investigators were blinded but does not mention other personnel, therefore this study has also bees assessed as at unclear risk of detection bias (Lally 2006).
Incomplete outcome data
The main report from the Harrison study (Harrison 2003) reported most outcomes by actual treatment received (as two women allocated to tracheal occlusion actually received standard care), not intention‐to‐treat and it was not possible from the information provided to restore participants to their randomised groups). The only outcome that was reported by intention‐to‐treat was 90‐day survival. For this reason, we have assessed Harrison as high risk for attrition bias. In the study by Lally (Lally 2006), one woman in each group withdrew without outcome data being available (total 2/34 or 6% of patients without outcome data). Lally has therefore been assessed as at low risk of attrition bias due to incomplete outcome reporting, as has Ruano (where the follow‐up to the stage of reporting primary outcomes is complete) (Ruano 2012). For the estimation of effects of interventions, all data were obtained from published manuscripts and no additional source was used.
Selective reporting
One study (Lally 2006) was assessed as low risk of reporting bias but the other two trials (Harrison 2003; Ruano 2012) were assessed as being at an unclear risk of reporting bias.
Other potential sources of bias
The main report from the Harrison study (Harrison 2003) reported some outcomes by actual treatment received, not intention‐to‐treat and it was not possible from the information provided to restore participants to their randomised groups.
There were no other identified potential sources of bias in the other two studies (Lally 2006; Ruano 2012).
Effects of interventions
Effect of in‐utero fetal tracheal occlusion
In‐utero fetal occlusion by maternal laparotomy versus standard postnatal management (one study, 24 women)
Primary outcomes
For the primary infant outcome of perinatal mortality, there were no data suitable for inclusion in the analysis.
Secondary outcomes
In‐utero fetal occlusion via maternal laparotomy was associated with a no difference in long‐term infant survival (risk ratio (RR) 1.06, 95% confidence interval (CI) 0.66 to 1.69; participants = 24) (long‐term infant survival was defined by this review as survival to age three to six months and reported by the authors of Harrison 2003 at 90 days). See Analysis 1.1.
1.1. Analysis.
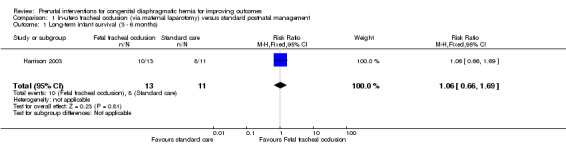
Comparison 1 In‐utero tracheal occlusion (via maternal laparotomy) versus standard postnatal management, Outcome 1 Long‐term infant survival (3 ‐ 6 months).
For this comparison, there were no other data that we could include in the meta‐analysis, due to the issues with reporting in Harrison 2003 that have already been described above.
In‐utero fetal occlusion by minimally invasive fetoscopy versus standard postnatal management (one study, 41 women)
Primary outcomes
For the primary infant outcome of perinatal mortality, there were no data for inclusion in the analysis.
Secondary outcomes
In‐utero fetal occlusion by minimally invasive fetoscopy was associated with a small reduction in the mean gestational age at birth, with a mean difference (MD) of ‐1.80 weeks (95% CI ‐3.13 to ‐0.47; participants = 41 (Analysis 2.1)). There was no clear difference between groups with relation to preterm birth before 37 weeks (RR 1.75, 95% CI 0.78 to 3.92; participants = 41 (Analysis 2.2)). Long‐term infant survival (three to six months) was increased with the intervention when compared with standard management (RR 10.50, 95% CI 1.48 to 74.71; participants = 41 (Analysis 2.3)), and there was a corresponding reduction in pulmonary hypertension (RR 0.58, 95% CI 0.36 to 0.93; participants = 41 (Analysis 2.4)) associated with the intervention.
2.1. Analysis.
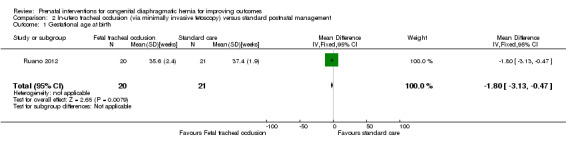
Comparison 2 In‐utero tracheal occlusion (via minimally invasive fetoscopy) versus standard postnatal management, Outcome 1 Gestational age at birth.
2.2. Analysis.
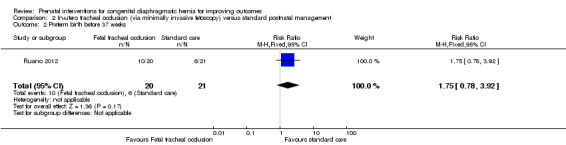
Comparison 2 In‐utero tracheal occlusion (via minimally invasive fetoscopy) versus standard postnatal management, Outcome 2 Preterm birth before 37 weeks.
2.3. Analysis.
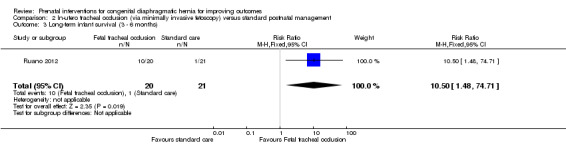
Comparison 2 In‐utero tracheal occlusion (via minimally invasive fetoscopy) versus standard postnatal management, Outcome 3 Long‐term infant survival (3 ‐ 6 months).
2.4. Analysis.
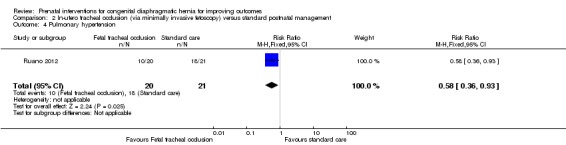
Comparison 2 In‐utero tracheal occlusion (via minimally invasive fetoscopy) versus standard postnatal management, Outcome 4 Pulmonary hypertension.
There was no clear difference in the risk of preterm ruptured membranes (less than 37 weeks) (RR 1.47, 95% CI 0.56 to 3.88; participants = 41 (Analysis 2.5)), maternal infectious morbidity (RR 3.14, 95% CI 0.14 to 72.92; participants = 41 (Analysis 2.6)).
2.5. Analysis.
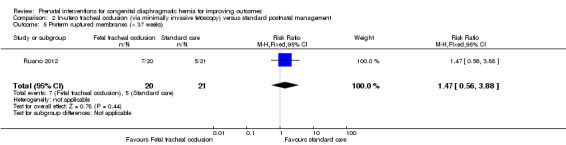
Comparison 2 In‐utero tracheal occlusion (via minimally invasive fetoscopy) versus standard postnatal management, Outcome 5 Preterm ruptured membranes (< 37 weeks).
2.6. Analysis.
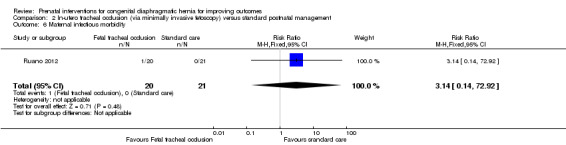
Comparison 2 In‐utero tracheal occlusion (via minimally invasive fetoscopy) versus standard postnatal management, Outcome 6 Maternal infectious morbidity.
There was no incidence of maternal blood transfusion in either the treatment or standard care groups (Analysis 2.7).
2.7. Analysis.
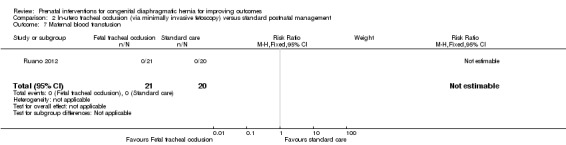
Comparison 2 In‐utero tracheal occlusion (via minimally invasive fetoscopy) versus standard postnatal management, Outcome 7 Maternal blood transfusion.
Effect of antenatal corticosteroids
Antenatal corticosteroids versus placebo (one study, 32 women)
Primary outcomes
For the primary infant outcome of perinatal mortality, there was no clear difference between the antenatal corticosteroids group and the placebo control (RR 1.24, 95% CI 0.50 to 3.08; participants = 32) (see Analysis 3.1).
3.1. Analysis.
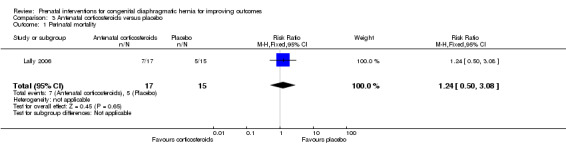
Comparison 3 Antenatal corticosteroids versus placebo, Outcome 1 Perinatal mortality.
Secondary outcomes
Data (mean values) were reported for two of our secondary outcomes (mechanical ventilation and days of hospital admission) but standard deviations were not provided. For the purposes of this review and to permit further analysis, we have estimated the standard deviations based on the P values detailed in the trial report, although our estimation does assume that the standard deviation is the same in both the intervention and control groups. There were no differences between the antenatal corticosteroid group and the placebo control in terms of days of mechanical ventilation (MD 18.00 days, 95% CI ‐14.77 to 50.77 (Analysis 3.2)), or days of hospital admission (MD 17.00 days, 95% CI ‐13.93 to 47.93 (Analysis 3.3))
3.2. Analysis.

Comparison 3 Antenatal corticosteroids versus placebo, Outcome 2 Days of mechanical ventilation.
3.3. Analysis.
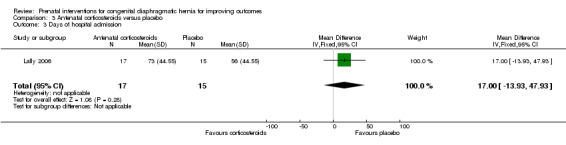
Comparison 3 Antenatal corticosteroids versus placebo, Outcome 3 Days of hospital admission.
For all of our other secondary outcomes, no data were reported by the study authors.
Discussion
Summary of main results
We included three studies involving 97 women (a further two studies are ongoing). Two trials examined in‐utero fetal tracheal occlusion with standard (postnatal) care in fetuses with severe diaphragmatic hernia, and one trial examined the effect of antenatal corticosteroids versus placebo. Overall, the methodological quality of the trials was variable and no data were available for a number of this review's secondary outcomes.
When we compared in‐utero fetal occlusion by maternal laparotomy or via minimally invasive fetoscopy versus standard postnatal management, there were no data available for this review's primary outcome ‐ perinatal mortality. For the maternal laparotomy intervention, there was no difference in long‐term survival. For the intervention utilising minimally invasive fetoscopy, the intervention was associated with a small reduction in the mean gestational age at birth, although a difference in gestational age of less than two weeks is unlikely to have a large clinically significant impact, in a population already subject to intensive intervention in the newborn period. With few data from one small study (n = 41), there is insufficient evidence to assess the effect of the intervention in relation to preterm birth before 37 weeks, with confidence intervals for this outcome being consistent with either an increase or a decrease in the incidence of preterm birth. Long‐term infant survival (three to six months) was increased with the intervention when compared with standard management.
For infants/fetuses with congenital diaphragmatic hernia (CDH), the administration of antenatal corticosteroids (compared with a placebo control) does not improve perinatal mortality. There was no difference between groups in terms of the number of days of mechanical ventilation or hospital admission.
Whilst we aimed to assess adverse effects with the interventions, the availability of such data, particularly for maternal side effects and complications in the included studies was limited.
Overall completeness and applicability of evidence
The inability of this review to combine results in a single meta‐analysis leads to some limitations.The two included fetal intervention studies are substantially different, both in their provided intervention and the location/population where the studies took place. As such, the overall study population might be very different to the current patient population, hence applicability is limited even at the level of the individual studies included.
Fetal surgical techniques are generally complex with a long and steep "learning curve". This may partly be due to the small numbers of procedures performed at an institutional and even national level. For many relatively rare and complex conditions, including CDH, methods of diagnosis, perinatal management and treatments may vary across treating centres at any one time and across individual institutions over time. For example, some treatment centres will include the use of ECMO (extracorporeal membrane oxygenation), which is not universally available. The lack of ECMO use (due to it not being available in the institution) in the Ruano 2012 study may explain the low survival in their control group.
Interpretation and application of current literature and research findings is hampered by varying definitions of severity of CDH. For example, some authors have used liver location (above or below diaphragm), lung‐head ratio (LHR), and expected versus observed LHR. Standardised research and clinical definitions would improve research quality, enable comparison between studies and improve generalisability to clinical practice.
Quality of the evidence
Although Harrison 2003 reported longer‐term infant outcomes in other papers, the reporting of outcomes by actual treatment received increases the risk of bias and limits the ability to interpret the results.
Potential biases in the review process
At study level, the quality of the review overall is reflected in the risk of bias of included studies, and as such there is a residual risk of review bias due to the mixed quality of included studies. Whilst we identified a number of important outcomes, our ability to report on these is limited by their inclusion in the included studies.
At the review level, whilst we have attempted to minimise reporting bias and incomplete identification of studies, it is possible that we have failed to identify studies or data that would have been able to be included in the analysis, for example, if it had been possible to obtain data from Harrison 2003, this would have improved this aspect.
Agreements and disagreements with other studies or reviews
A recent review by Cundy et al, (Cundy 2013) aimed to "critically appraise controlled clinical trials investigating the role of FETO (fetal endoscopic tracheal occlusion) in moderate and severe isolated CDH" and discuss in a local context if FETO is "justified" in the Australasian region. The Cundy 2013 review identified the same randomised trials as we have in our review, but also included non‐randomised studies. The Cundy 2013 review takes a more narrative approach, which allows more individual aspects of the technique to be discussed, however their review team also identify similar issues raised in our review, including differences in procedural techniques.
Authors' conclusions
Implications for practice.
In‐utero fetal tracheal occlusion should only be conducted as part of an ongoing randomised trial. Current evidence relating to the use of in‐utero fetal tracheal occlusion or the administration of antenatal corticosteroids is limited by the small numbers of pregnancies that have been included in randomised trials to date. There is insufficient evidence to suggest that in‐utero fetal tracheal occlusion be implemented in clinical practice, outside a randomised controlled trial setting. FETO, however remains an potentially important treatment for this significant condition. As such, clinical practice can support ongoing research and practitioners could engage with and encourage the currently recruiting studies.
Implications for research.
More studies are needed to further examine the effect of in‐utero fetal tracheal occlusion on important neonatal outcomes and long‐term infant survival and health. Long‐term follow‐up is of particular importance, and should include morbidity and mortality measures. Further studies should examine the benefits of an in‐utero intervention on subgroups with moderate and severe congenital diaphragmatic hernia. Indeed, there are three ongoing studies being conducted by European, North and South American fetal medicine centres, which will contribute to this gap. Ongoing research and any implementation into clinical practice should include standardisation of the procedure, inclusion criteria and long‐term childhood follow‐up.
WIth regard to the administration of antenatal corticosteroids, there remains a gap in current research, and a large multicentre trial with adequate statistical power should be undertaken to answer this unresolved question.
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. In‐utero tracheal occlusion (via maternal laparotomy) versus standard postnatal management.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Long‐term infant survival (3 ‐ 6 months) | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.66, 1.69] |
Comparison 2. In‐utero tracheal occlusion (via minimally invasive fetoscopy) versus standard postnatal management.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gestational age at birth | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐1.80 [‐3.13, ‐0.47] |
| 2 Preterm birth before 37 weeks | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.78, 3.92] |
| 3 Long‐term infant survival (3 ‐ 6 months) | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.5 [1.48, 74.71] |
| 4 Pulmonary hypertension | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.36, 0.93] |
| 5 Preterm ruptured membranes (< 37 weeks) | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.56, 3.88] |
| 6 Maternal infectious morbidity | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.14 [0.14, 72.92] |
| 7 Maternal blood transfusion | 1 | 41 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Antenatal corticosteroids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perinatal mortality | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.50, 3.08] |
| 2 Days of mechanical ventilation | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 18.0 [‐14.77, 50.77] |
| 3 Days of hospital admission | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 17.0 [‐13.93, 47.93] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Harrison 2003.
| Methods | RCT. | |
| Participants | Women with left‐sided CDH at 22‐28 weeks' gestation, with LHR < 1.4, with normal fetal echo and karyotype. | |
| Interventions | Laparotomy and fetoscopic insertion of tracheal balloon, delivery by EXIT procedure at > 36 weeks in tertiary centre. Vaginal delivery planned unless CS required for obstetric indications. Standard care group was treated expectantly, with a planned return to the treating centre at 36 weeks of gestation, Antenatal steroids were administered if there was preterm labour or if the lung profile indicated immaturity. If spontaneous labour did not occur, labour was induced. Delivery was vaginal unless CS was indicated. | |
| Outcomes | Primary = survival to 90 days, need for ECMO, duration of ventilatory support, oxygen therapy, GI morbidity, neurological morbidity, survival to discharge, duration of hospital stay, maternal physical and psychological morbidity. | |
| Notes | Most outcomes for the primary report are reported as treatment actually received, although the Keller report of infant pulmonary function reported intention‐to‐treat analyses. Conducted in the United States. This research was funded by a grant (R01 HL62433) from the National Institute of Child Health and Human Development, National Institutes of Health (to Dr. Albanese), by the Nicholson Fund, and by Glaser Pediatric Research Network. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | High risk | 2 women randomly assigned to fetal tracheal occlusion opted to have standard care. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Some analysis performed/reported by actual treatment received, not intention‐to‐treat. |
| Selective reporting (reporting bias) | Unclear risk | Unable to make judgement. |
| Other bias | Unclear risk | Trial was terminated early based on data monitoring board recommendation that unlikely to detect difference with planned enrolment. |
Lally 2006.
| Methods | RCT. | |
| Participants | All women with fetus shown to have CDH prior to 34 weeks. | |
| Interventions | Intervention: Betamethasone (12.5 mg) 2 doses 24 hours apart (given at 34 weeks) followed by 2 weekly doses. Control: placebo (the composition of the placebo was not stated in the trial report). |
|
| Outcomes | Mortality, ventilator days, need for oxygen at 30 days, length of hospital stay, birth data, Apgar scores, treatments received. | |
| Notes | Women were randomised women from participating hospitals in the US, Italy, Germany and Australia. This trial was partly funded by NIH grants K24RR17050 and M01RR002558. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "central randomisation centre." |
| Allocation concealment (selection bias) | Unclear risk | Not stated other than "central randomisation centre". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Investigators were blinded", no other mention of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Investigators were blinded", no other mention of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One woman in each group withdrew with outcome data not available, analysis by intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | No apparent selective reporting. |
| Other bias | Low risk | Stopped early as interim analysis suggested unlikely that sufficient patients would be enrolled to determine an outcome. No apparent baseline differences. |
Ruano 2012.
| Methods | Individual RCT. | |
| Participants | Women at 22‐26 weeks with severe fetal CDH, no other anomalies and normal karyotype. LHR < 1.0, 1/3 of liver in abdomen. | |
| Interventions | FETO at 26‐30 weeks, with subsequent EXIT procedure at 38 weeks via hysterotomy. Control group all delivered by CS, also same neonatal protocol. | |
| Outcomes | Primary = survival at 6 months, maternal outcomes, severe pulmonary hypertension, length of time to repair. | |
| Notes | Trial conducted in Brazil. This study was sponsored by University of Sao Paulo General Hospital and in collaboration with the Ministry of Health, Brazil. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated 1:1 ratio. |
| Allocation concealment (selection bias) | Low risk | Computer‐generated. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | Unable to make judgement. |
| Other bias | Low risk | Reached sample size. |
CDH: congenital diaphragmatic hernia CS: caesarean section ECMO: extracorporeal membrane oxygenation EXIT: ex‐utero intrapartum treatment FETO: percutaneous ultrasound guided fetal endoscopic tracheal occlusion GI: gastrointestinal LHR: lung‐head ratio RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Belfort 2011 | This is a cohort study not an RCT, reporting ongoing follow‐up from the FETO group. |
FETO: percutaneous ultrasound guided fetal endoscopic tracheal occlusion RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Deprest 2009b.
| Trial name or title | Randomised trial of extracorporeal membrane oxygenation (FETO) versus expectant management during pregnancy in fetuses with left‐sided and isolated congenital diaphragmatic hernia and moderate/severe pulmonary hypoplasia. |
| Methods | Randomised trial. |
| Participants | Gestation no more than 31 weeks and 5 days at randomisation. |
| Interventions | FETO procedure between 20 and 32 weeks, or standard care. |
| Outcomes | |
| Starting date | |
| Contact information | http://www.totaltrial.eu |
| Notes | Ongoing study, 2 arms: 1 for moderate and 1 for severe hypoplasia. |
Kohl 2006.
| Trial name or title | Randomised clinical trial in order to assess the effect of fetoscopic tracheal balloon occlusion on the postnatal disease course in neonates with left‐sided congenital diaphragmatic hernia. |
| Methods | Randomised trial. |
| Participants | Left‐sided diaphragmatic hernia, fetal liver herniation into the chest; gestational age‐related lung volume between 20% to 25% of normal, as determined by magnetic resonance imaging between 30 + 0 ‐ 34 + 0 weeks + days of gestation. |
| Interventions | Fetoscopic tracheal balloon occlusion. |
| Outcomes | Primary outcome is need for postnatal ECMO therapy. |
| Starting date | January 2009. |
| Contact information | University Hospital, Bonn. |
| Notes |
Ruano 2013.
| Trial name or title | "Early" versus "standard" fetal endoscopic tracheal occlusion for severe congenital diaphragmatic hernia ‐ a randomised controlled trial |
| Methods | Allocation: randomised parallel assignment. |
| Participants | Severe congenital diaphragmatic hernia. |
| Interventions | FETO (Fetal Endoscopic Tracheal Occlusion) between 22‐24 weeks. |
| Outcomes | Safety/efficacy study ‐ neonatal and infant survival rate. |
| Starting date | January 2014. |
| Contact information | Rodrigo Ruano, MD PhD; rodrigoruano@usp.br |
| Notes | NCT01731509 |
ECMO: extracorporeal membrane oxygenation FETO: percutaneous ultrasound guided fetal endoscopic tracheal occlusion
Differences between protocol and review
We have added the following secondary outcomes that were not prespecified on our published protocol (Grivell 2011) but thought to add useful information to the review.
Infant outcomes
Preterm birth before 32 weeks
Oxygen use at 30 days
Maternal outcomes
Preterm ruptured membranes (before 32 weeks)
We have also updated some sections of our methods in line with Cochrane Pregnancy and Childbirth's standard methods text.
We have also edited the review's objectives to allow for the comparison of different types of prenatal repair on the same outcomes but such a comparison was not possible in this version of the review due to insufficient data.
Contributions of authors
Rosalie Grivell (RG) is guarantor for this review. RG registered the title and wrote the review with advice on content and outcomes from Chad Andersen and Jodie Dodd. All authors commented on drafts and approved the published review.
Declarations of interest
None known.
New
References
References to studies included in this review
Harrison 2003 {published data only}
- Albanese C, Farrell J. Congenital diaphragmatic hernia ‐ a randomized clinical trial. Frontiers in Fetal Health 2000;2(6):1‐4. [Google Scholar]
- Cortes RA, Keller RL, Townsend T, Harrison MR, Farmer DL, Lee H, et al. Survival of severe congenital diaphragmatic hernia has morbid consequences. Journal of Pediatric Surgery 2005;40(1):36‐45. [DOI] [PubMed] [Google Scholar]
- Harrison MR, Keller RL, Hawgood SB, Kitterman JA, Sandberg PL, Farmer DL, et al. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. New England Journal of Medicine 2003;349:1916‐24. [DOI] [PubMed] [Google Scholar]
- Keller RL, Hawgood S, Neuhaus JM, Farmer DL, Lee H, Albanese CT, et al. Infant pulmonary function in a randomized trial of fetal tracheal occlusion for severe congenital diaphragmatic hernia. Pediatric Research 2004;56(5):818‐25. [DOI] [PubMed] [Google Scholar]
Lally 2006 {published data only}
- Lally KP, Bagolan P, Hosie S, Lally PA, Stewart M, Cotten CM, et al. Corticosteroids for fetuses with congenital diaphragmatic hernia: can we show benefit?. Journal of Pediatric Surgery 2006;41(4):668‐74. [DOI] [PubMed] [Google Scholar]
Ruano 2012 {published data only}
- Ruano R. Tracheal occlusion guided by percutaneous fetoscopy in fetuses with severe isolated congenital diaphragmatic hernia. http://clinicaltrials.gov/ct2/show/record/NCT01302977 (accessed 16 December 2011) 2011.
- Ruano R, Duarte S, Silva M, Tannuri U, Zugaib M. Fetal endoscopic tracheal occlusion in severe congenital diaphragmatic hernia using 1.0 mm fetoscope ‐ preliminary results of a randomized study. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S325. [Google Scholar]
- Ruano R, Yoshisaki CT, Silva MM, Ceccon ME, Grasi MS, Tannuri U, et al. A randomized controlled trial of fetal endoscopic tracheal occlusion versus postnatal management of severe isolated congenital diaphragmatic hernia. Ultrasound in Obstetrics & Gynecology 2012;39(1):20‐7. [DOI] [PubMed] [Google Scholar]
- Ruano S, Duarte SA, Silva MM, Tannuri, Zugaib M. Fetal endoscopic tracheal occlusion in severe congenital diaphragmatic hernia using 1.0 mm fetoscope ‐ a randomised study. Ultrasound in Obstetrics & Gynecology 2009;34:132. [Google Scholar]
References to studies excluded from this review
Belfort 2011 {published data only}
- Belfort M. The effectiveness of fetal endotracheal occlusion (feto) in the management of severe congenital diaphragmatic hernia. http://clinicaltrials.gov/ct2/show/NCT00881660 (accessed 1 December 2011) 2011.
References to ongoing studies
Deprest 2009b {published data only}
- Dekoninck P, Gratacos E, Mieghem T, Richter J, Lewi P, Ancel AM, et al. Results of fetal endoscopic tracheal occlusion for congenital diaphragmatic hernia and the set up of the randomized controlled TOTAL trial. Early Human Development 2011;87(9):619‐24. [DOI] [PubMed] [Google Scholar]
- Deprest J. Randomized control trial of fetoscopic endoluminal tracheal occlusion with a balloon versus expectant management during pregnancy in fetuses with left sided congenital diaphragmatic hernia and moderate pulmonary hypoplasia (TOTAL). http://clinicaltrials.gov/ct2/show/NCT00763737 (accessed 4 January 2009).
- Deprest J, Nicolaides K, Tibboel D, Ville Y, Reiss I, Gratacos E. Total randomized controlled trial for moderate pulmonary hypoplasia due to congenital diaphragmatic hernia (CDH). Ultrasound in Obstetrics & Gynecology 2009;34(Suppl 1):91. [Google Scholar]
Kohl 2006 {published data only}
- Kohl T. Randomized clinical trial in order to assess the effect of fetoscopic tracheal balloon occlusion on the postnatal disease course in neonates with left congenital diaphragmatic hernia. http://clinicaltrials.gov/ct2/show/record/NCT00373438 (accessed 19 December 2011).
Ruano 2013 {published data only}
- Ruano R. "Early" versus "standard" fetal endoscopic tracheal occlusion for severe congenital diaphragmatic hernia ‐ a randomized controlled trial. http://clinicaltrials.gov/show/NCT01731509 (accessed 24 October 2013).
Additional references
AIHW 2004
- AIHW National Perinatal Statistics Unit. Australia's Babies: Their Health and Wellbeing. Bulletin no. 21. AIHW cat. no. AUS 54. Camberra: AIHW NPSU, 2004. [Google Scholar]
Chiu 2006
- Chiu PP, Sauer C, Mihailovic A, Adatia I, Bohn D, Coates AL, et al. The price of success in the management of congenital diaphragmatic hernia: is improved survival accompanied by an increase in long‐term morbidity?. Journal of Pediatric Surgery 2006;41(5):888‐92. [DOI] [PubMed] [Google Scholar]
Chiu 2008
- Chiu P, Hedrick HL. Postnatal management and long‐term outcome for survivors with congenital diaphragmatic hernia. Prenatal Diagnosis 2008;28(7):592‐603. [DOI] [PubMed] [Google Scholar]
Colvin 2005
- Colvin J, Bower C, Dickinson J, Sokol J. Outcomes of congenital diaphragmatic hernia: a population‐based study in Western Australia. Pediatrics 2005;116(3):356‐63. [DOI] [PubMed] [Google Scholar]
Cundy 2013
- Cundy TP, Gardener GJ, Andersen CC, Kirby CP, McBride CA, Teague WJ. Fetoscopic endoluminal tracheal occlusion (FETO) for congenital diaphragmatic hernia in Australia and New Zealand: are we willing, able, both or neither?. Journal of Paediatrics and Child Health 2013;50(3):226‐33. [DOI] [PubMed] [Google Scholar]
Deprest 2004
- Deprest J, Gratacos E, Nicolaides KH, FETO Task Group. Fetoscopic tracheal occlusion (FETO) for severe congenital diaphragmatic hernia: evolution of a technique and preliminary results. Ultrasound in Obstetrics and Gynecology 2004;24(2):121‐6. [DOI] [PubMed] [Google Scholar]
Deprest 2009a
- Deprest JA, Gratacos E, Nicolaides K, Done E, Mieghem T, Gucciardo L, et al. Changing perspectives on the perinatal management of isolated congenital diaphragmatic hernia in Europe. Clinics in Perinatology 2009;36:329‐47. [DOI] [PubMed] [Google Scholar]
DiFiore 1994
- DiFiore JW, Fauza DO, Slavin R, Peters CA, Fackler JC, Wilson JM. Experimental fetal tracheal ligation reverses the structural and physiological effects of pulmonary hypoplasia in congenital diaphragmatic hernia. Journal of Pediatric Surgery 1994;29(2):248‐56. [DOI] [PubMed] [Google Scholar]
Done 2008
- Done E, Gucciardo L, Mieghem T, Jani J, Cannie M, Schoubroeck D, et al. Prenatal diagnosis, prediction of outcome and in utero therapy of isolated congenital diaphragmatic hernia. Prenatal Diagnosis 2008;28(7):581‐91. [DOI] [PubMed] [Google Scholar]
George 1987
- George DK, Cooney TP, Chiu BK, Thurlbeck WM. Hypoplasia and immaturity of the terminal lung unit (acinus) in congenital diaphragmatic hernia. American Review of Respiratory Disease 1987;136(4):947‐50. [DOI] [PubMed] [Google Scholar]
Gross 1946
- Gross RE. Congenital hernia of the diaphragm. American Journal of Diseases of Children 1946;71:579‐92. [DOI] [PubMed] [Google Scholar]
Harrison 1997
- Harrison MR, Adzick NS, Bullard KM, Farrell JA, Howell LS, Rosen MA, et al. Correction of congenital diaphragmatic hernia in utero. VII. A prospective trial. Journal of Pediatric Surgery 1997;32(11):1637‐42. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Laudy 2003
- Laudy JA, Gucht M, Dooren MF, Wladimiroff JW, Tibboel D. Congenital diaphragmatic hernia: an evaluation of the prognostic value of the lung‐to‐head ratio and other prenatal parameters. Prenatal Diagnosis 2003;23(8):634‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
References to other published versions of this review
Grivell 2011
- Grivell RM, Andersen C, Dodd JM. Prenatal interventions for congenital diaphragmatic hernia for improving outcomes. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD008925] [DOI] [PMC free article] [PubMed] [Google Scholar]


