Abstract
Context
Fracture risk is underestimated in people with type 2 diabetes (T2D).
Objective
To investigate the longitudinal relationship of glycated hemoglobin (HbA1c) and common medications on fracture risk in people with T2D.
Methods
This retrospective population-based cohort study was conducted using de-identified claims and electronic health record data obtained from the OptumLabs Data Warehouse for the period January 1, 2007, to September 30, 2015. For each individual, the study was conducted within a 2-year HbA1c observation period and a 2-year fracture follow-up period. A cohort of 157 439 individuals with T2D [age ≥ 55 years with mean HbA1c value ≥ 6%] were selected from 4 018 250 US Medicare Advantage/Commercial enrollees with a T2D diagnosis. All fractures and fragility fractures were measured.
Results
With covariates adjusted, poor glycemic control in T2D individuals was associated with an 29% increase of all fracture risk, compared with T2D individuals who had adequate glycemic control (HR: 1.29; 95% CI, 1.22-1.36). Treatment with metformin (HR: 0.88; 95% CI, 0.85-0.92) and DPP4 inhibitors (HR: 0.93; 95% CI, 0.88-0.98) was associated with a reduced all fracture risk, while insulin (HR: 1.26; 95% CI, 1.21-1.32), thiazolidinediones (HR: 1.23; 95% CI, 1.18-1.29), and meglitinides (HR: 1.12; 95% CI, 1.00-1.26) were associated with an increased all fracture risk (All P value < 0.05). Bisphosphonates were associated similarly with increased fracture risk in the T2D and nondiabetic groups.
Conclusion
Longitudinal 2-year HbA1c is independently associated with elevated all fracture risk in T2D individuals during a 2-year follow-up period. Metformin and DPP4 inhibitors can be used for management of T2D fracture risk.
Keywords: HbA1c, fracture risk, T2D, metformin, bisphosphonates
Despite elevated bone mineral density (BMD), people with type 2 diabetes (T2D) are at greater risk for fracture which is underestimated by current standard of care tools (1-3). The underlying pathogenesis of T2D fractures is complex and involves factors beyond BMD. For example, lack of glycemic control is correlated with various diabetic comorbidities in people with T2D, one of which is poor bone quality (2). The altered glucose metabolism disrupts bone turnover (4, 5) and negatively impact bone material properties (6-9) which may lead to higher risk of fracture. Other T2D comorbidities, such as retinopathy, nephropathy, and hypertension, also have shown to elevate the fracture risk (10, 11). However, the duration and severity of T2D with its comorbidities are difficult to quantify. Glycated hemoglobin (HbA1c), as a reflection of glycemic control (12) and an independent indicator for T2D status, has been widely used to assess the risk of diabetic comorbidities (13) and mortality (14). The relationship between HbA1c and fracture risk is yet to be determined.
The measurement of HbA1c provides the average blood glucose level over the past 2 to 3 months (15). However, recent clinical studies have indicated that the deteriorated bone quality and elevated fracture risk may be related to high glycemic level over a prolonged period (3, 6, 16). Consequently, current methods evaluating the relationship between single time point measures of HbA1c (prior to fracture) with fracture incidences have not proven useful (17-20). In contrast, longitudinal HbA1c measurements over a moderate time period may provide a physiologically relevant clinical tool to assess and manage T2D fracture risk even in the absence of comorbidities information.
In addition to regulating blood glucose, treatments targeting T2D may reduce risk of fracture by better normalizing bone turnover (1, 21). For instance, metformin (a biguanide) is associated with a 19% reduction in fracture risk after adjusting for other covariates such as age, gender, and diabetes history (1). However, some antidiabetic medications may have direct adverse effects on bone tissue. For example, thiazolidinediones (TZDs) have been shown to increase fracture risk, as TZDs impair bone metabolism by decreasing osteoblast activities, causing a reduction in bone mass (22). Additionally, some antidiabetic medications, such as insulin and sulfonylureas, have been associated with an increased fracture risk due to an increased chance for a fall from temporary hypoglycemia (23-27). With other medications, such as meglitinides, dipeptidyl peptidase-4 (DPP4) inhibitors (28, 29), glucagon-like peptide-1 (GLP-1) receptor agonists (30), and α-blockers, there is currently either no evidence, or a contradictory evidence of their associations with alterations in fracture risk.
Anti-osteoporosis medications such as bisphosphonates can improve bone health through a reduction in bone resorption (31, 32). Previously, bisphosphonates have been shown to increase BMD in patients with diabetes (32). However, some studies showed that bisphosphonates do not favorably alter fracture risk in diabetes (31, 33) as bisphosphonates further delay the remodeling process (34, 35). In contrast, other medications, such as raloxifene (36), estrogen therapy (37), teriparatide, and denosumab (38), could normalize bone turnover among osteoporotic individuals. Therefore, more data regarding the impact of common treatments on fracture risk are needed for management and reduction of fracture risk in the T2D population.
To this end, the objectives of this study are to: (1) investigate the longitudinal relationship of HbA1c with bone fractures in a large cohort of patients with T2D, for improving the risk assessment of T2D-induced fractures; and (2) determine the associations of commonly used antidiabetic and anti-osteoporotic medications (with other risk factors adjusted) for managing fracture risk.
Methods
Data Collection
This study used de-identified administrative claims and electronic health record (EHR) data with linked laboratory results from the OptumLabs Data Warehouse. The database contains longitudinal health information on enrollees and patients, representing a diverse mixture of ages, ethnicities, and geographical regions across the United States (39). The claims data in OptumLabs Data Warehouse includes medical and pharmacy claims, laboratory results, and enrollment records for commercial and Medicare Advantage enrollees. The EHR–derived data include a subset of EHR data that have been normalized and standardized into a single database (39).
Study Population
This retrospective cohort study was conducted using de-identified data extracted for a period from January 1, 2007, to September 30, 2015. This study period was selected to ensure the EHR data were available for the period covered by the International Classification of Diseases, Ninth Revision (ICD-9) system. The index date for each individual was defined as the date of the first T2D diagnosis. The study cohort was further defined by using the following inclusion criteria: (1) age ≥ 55 years with known gender; (2) at least 3-year continuous enrollment of Medicare Advantage/Commercial coverage with available medication information; (3) At least 2 inpatient or outpatient visits with T2D diagnosis within 1 year from the index date; and (4) at least 1 HbA1c measurement within the 2-year observational period starting at 15 days before the first T2D diagnosis. For each individual with multiple HbA1c records, the HbA1c values were averaged. Since the American Diabetes Association suggests a 6.5% HbA1c threshold for diabetes (12), people with a mean HbA1c value less than 6% were not considered in the study. Patients with only 1 HbA1c measurement were not excluded, as doing so might potentially bias the population against the mild/moderate T2D cases, thereby limiting the applications of these findings to this clinically relevant cohort. Also, since the first HbA1c measurement was recorded a few days before or on the day of the first diagnosis, this requirement ensures that the HbA1c value provides a reasonable approximation of glycemic control over the longer 2-year longitudinal period as opposed to a single value measured in close proximity or at the time of fracture. Eventually, a total of 157 439 individuals, selected from 4 018 250 people with T2D, were included in the study cohort (Table 1).
Table 1.
Attrition table and cohort population
| N | |
|---|---|
| Individual with T2D diagnosis between 2007 and 2013 | 4 018 250 |
| Individual with at least 2 diagnosis service dates within 365 days | 3 027 830 |
| Individual meet age requirement and 3-year continuous enrollment | 557 627 |
| Individual with at least 1 HbA1c value within 730 days following first diagnosis | 187 185 |
| Individual with mean HbA1c value over 6% (inclusive) | 157 439 |
Abbreviations: HbA1c, glycated hemoglobin; T2D, type 2 diabetes.
Exposure and Outcome Definition
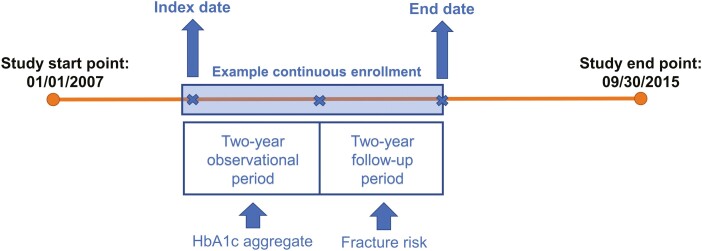
The study period is illustrated in Fig. 1. The mean HbA1c value for each individual in the cohort was binned into 2 types of predetermined categories: by every 1% change (6%-7%, 7%-8%, 8%-9%, 9%-10%, 10%-11%, 11%-12%, ≥ 12%), and by binary change (adequate glycemic control [6%-9%], poor glycemic control [≥ 9%]). The cutoff of 9% was selected based on previous studies (40-42). However, other classifications of adequate/poor glycemic control using 7% and 8% as cutoff were also examined. The standard deviation was calculated for people with more than 1 HbA1c record. If the individual had any HbA1c measurement during the follow-up period (third to fourth year), the values in follow-up period up to the first fracture incidence (if applicable) were also averaged and binned in the same manner.
Figure 1.
Study period. For individual patients, HbA1c measurements were collected from 15 days prior to their index date to 2 years after their index date. Patients with no HbA1c records during this period were not considered in the study cohort. Multiple HbA1c measurements in this window are averaged. The subsequent 2-year period is used to estimate risk of fracture stratified by the HbA1c value in observational period.
To investigate whether the duration of diabetes history may impact the association between fracture risk and longitudinal HbA1c, any diagnosis records of T2D for a period of 1 year prior to the study period (January 1, 2006 to December 31, 2006), were extracted and analyzed. If an individual had previous T2D diagnosis prior to the selected study period, the patient was considered to have longer history of T2D and was classified as with a case of prevalent T2D. Other individuals, who had the first T2D diagnosis during the study period, were classified as cases of incident T2D.
For a 2-year period from the index date, the records of antidiabetic and anti-osteoporotic medications were extracted. These included classes of biguanides (metformin), insulin, TZDs, sulfonylureas, meglitinides, DPP4 inhibitors, GLP-1 receptor agonists, α-blockers, bisphosphonates, selective estrogen receptor modulators (raloxifene), and estrogens. The generic drugs included for each class is listed in Table S1 (43). Other medications of interest, such as sodium-glucose cotransporter-2 inhibitors, teriparatide, and denosumab, were not included, as the drug classes were not approved by the Food and Drug Administration for use before the start of the study period (January 1, 2007). The diagnosis records of diabetic comorbidities were also extracted for the entire study period. The selected comorbidities, known to directly affect fracture risk, included osteoporosis, neuropathy, retinopathy, nephropathy, coronary artery disease, hypertension, stroke, and obesity (body mass index [BMI] ≥ 30) (10, 11). The use of glucocorticoids and a history of fragility fractures in the observational period were also considered as covariates. The total fracture incidences, as well as the total fragility fracture incidences, were obtained for a follow-up period of 2 years from the start of the third year to the end of fourth year after the index date. The fragility fracture incidences were defined based on the fracture site (with pathological fractures included) per ICD-9 codes (44) and these do not refer to the nature of the trauma. Individuals who did not sustain any fracture during the continuous enrollment period were censored. The ICD-9 codes used to identify the conditions listed above are summarized in Table S2 (43).
In order to further understand the relationship between bisphosphonates use and fracture risk, we identified a nondiabetic control cohort by randomly selecting 30% of commercial and Medicare Advantage enrollees for the same study period as the T2D group identified above. Individuals in the nondiabetic control group had no prior diagnosis of T2D, or prescription for insulin and/or thiazolidinediones. In this group, the use of bisphosphonates and fracture incidences were recorded and compared against the T2D cohort for identical periods.
Statistical Analysis
Within the 2-year follow-up period, the Kaplan-Meier estimation of fracture probability was computed as a univariate model using 2 different predetermined categories of HbA1c bins (by every 1% change or by adequate [6%-9%] vs poor glycemic control [≥ 9%]). Log-rank tests were performed on HbA1c groups to verify if the difference in fracture risk was significant. The Kaplan-Meier model was also applied to 3 additional cases: (1) the risk estimation of fragility fractures, based on mean HbA1c; (2) the risk estimation for all fractures based on mean HbA1c during the follow-up period but before an individual’s first fracture incidence (if applicable); and (3) the risk estimation of all fractures, stratified by higher or lower standard deviation of HbA1c from the cohort mean during the observational period.
Multivariate Cox proportional hazard models were employed to further investigate the instantaneous correlation between the fracture risk and the HbA1c groups, adjusted by various covariates. The hazard ratio “h(t)” with a 95% CI is calculated, indicating the instantaneous risk of suffering an event, i.e., fracture, at any given time “t”, corresponding to each variable. In these models, the following variables were selected for adjustment to normalize their confounding effects on fracture risk: age, gender (male coded as 1 and female coded as 0), comorbidities (yes or no), glucocorticoids use (yes or no), and previous fragility fractures during the observational period (yes or no). Furthermore, an additional covariate—prevalent T2D (yes or no)-was included to examine whether a longer diabetes history would affect the relationship between HbA1c with fracture risk.
The association of medication use was similarly evaluated by including longitudinal HbA1c categories as an additional covariate. For antidiabetic treatments, the association was also analyzed after the exclusion of people with osteoporosis diagnosis. To examine the possibility of confounding by indication, the association of bisphosphonates use with fracture risk was also evaluated in a nondiabetic control cohort defined above after adjustments of age, gender, comorbidities (excluding osteoporosis), glucocorticoids use, and previous fracture. Due to the high collinearity in the Cox proportional hazard model of nondiabetic control group, a penalizer of 0.3 was added in both T2D and nondiabetic models. The hazard ratios of bisphosphonates use were compared between the T2D and nondiabetic individuals.
To further understand the above relationships in different racial/ethnic groups, we compared the fracture risk among Asian, Black, Hispanic, and White populations. The comparisons were done with or without the adjustments of HbA1c categories and the confounding variables described above. Furthermore, Cox proportional hazard models were also applied to evaluate the association of HbA1c with fracture risk for the study population for each racial/ethnic group.
Data integration and organization were done using DbVisualizer software (DbVis Software AB, Stockholm, Sweden). All statistical analyses were conducted in Python. A P value less than 0.05 was considered significant.
Results
For the study period, we identified 4 018 250 people with T2D. Based on the inclusion criteria described above, a cohort of 157 439 individuals (50% male, 50% female) was identified for this study (Table 1). Table 2 shows the demographics of the cohort. The mean age was 66.0 years with a standard deviation of 7.3 years. Among the 8148 claims associated with a BMI value, 8.9% (N = 726) of individuals had BMI under 25; 17.2% (N = 1402) of individuals had BMI between 25 and 29; and the remaining 73.9% (N = 6020) individuals passed the BMI threshold of 30. Using the HbA1c binning described above, more than half of the individuals were in the 6% to 7% HbA1c group (54.8%, N = 86 211) and a quarter of the study individuals were in 7% to 8% range (25.5%, N = 40 204). The 4 bins classifying people with HbA1c ≥ 9% accounted to a total of 9.4% (N = 14 839). In the study cohort, 79.9% (N = 125 802) individuals were taking one or more classes of antidiabetic medication; and 10.4% (N = 16 416) were taking one or more classes of anti-osteoporosis medication. About 27.2% (N = 42 807) of the study population had T2D diagnosis prior to the study period. The numbers of patients with established comorbidities are also described in Table 2. For the first 2 years of the follow-up period, a total of 18 826 claims were extracted based on an individual’s first fracture date. Among these claims, vertebrae fracture was most prevalent (13.1%, N = 2458, pathological fractures included), followed by hip fracture (9.8%, N = 1854, pathological fractures included). The prevalence of common fracture sites is listed in Table 3. The most common fracture sites are similar to a previously report (45).
Table 2.
Demographics table
| Overall population N = 157 439 | |||||
|---|---|---|---|---|---|
| Age | Mean | Std | |||
| 66.04 | 7.27 | ||||
| Age categories | N | % | Use of antidiabetic medications | N | % |
| 55-59 | 37 940 | 24.1% | All classes | 125 802 | 79.9% |
| 60-64 | 31 902 | 20.3% | Metformin | 95 342 | 60.6% |
| 65-69 | 35 062 | 22.3% | Insulin | 37 587 | 23.9% |
| 70-74 | 22 781 | 14.5% | TZD | 27 860 | 17.7% |
| 75-79 | 25 695 | 16.3% | Sulfonylureas | 59 542 | 37.8% |
| ≥ 80 | 4059 | 2.6% | DPP-4 inhibitors | 22 504 | 14.3% |
| Sex | N | % | GLP-1 receptor agonists | 6740 | 4.3% |
| Male | 78 530 | 49.9% | α-blockers | 882 | 0.6% |
| Female | 78 909 | 50.1% | Meglitinides | 2870 | 1.8% |
| Race | N | % | Use of other medications | N | % |
| Asian | 8195 | 5.2% | Bisphosphonates | 9122 | 5.8% |
| Black | 25 647 | 16.3% | Raloxifene | 1431 | 0.9% |
| White | 99 056 | 62.9% | Estrogens | 6797 | 4.3% |
| Hispanic | 14 888 | 9.5% | Glucocorticoids | 32 844 | 20.6% |
| Other/Unknown | 9653 | 6.1% | Comorbidities | N | % |
| HbA1c bins | N | % | Neuropathy | 40 062 | 25.4% |
| 6%-7% | 86 211 | 54.8% | Retinopathy | 30 006 | 19.1% |
| 7%-8% | 40 204 | 25.5% | Nephropathy | 40 371 | 25.6% |
| 8%-9% | 16 185 | 10.3% | Coronary artery disease | 47 443 | 30.1% |
| 9%-10% | 7525 | 4.8% | Stroke | 30 072 | 19.1% |
| 10%-11% | 3752 | 2.4% | Hypertension | 146 113 | 92.8% |
| 11%-12% | 1933 | 1.2% | Obesity | 35 442 | 22.5% |
| ≥ 12% | 1629 | 1.0% | Osteoporosis | 16 756 | 10.6% |
| T2D history | N | % | Previous fragility fractures (observational period) | N | % |
| Prevalent T2D | 42 807 | 27.2% | |||
| Incident T2D | 114 632 | 72.8% | Previous fractures | 4965 | 3.2% |
Please note the relatively low obesity percentage is due to limited availability of body mass index data through claims database.
Abbreviations: HbA1c, glycated hemoglobin; T2D, type 2 diabetes.
Table 3.
Common fracture sites for the 2-year follow-up period
| All claims since individual first fracture date = 18 826 | ||
|---|---|---|
| Common fracture sites | N | % |
| Vertebrae | 2458 | 13.1% |
| Femoral neck | 1854 | 9.8% |
| Rib | 1729 | 9.2% |
| Humerus | 1696 | 9.0% |
| Radius or ulna | 1584 | 8.4% |
| Ankle | 1565 | 8.3% |
| Tarsal or metatarsal | 1483 | 7.9% |
| Phalanges (foot) | 1027 | 5.5% |
| Tibia or fibula | 829 | 4.4% |
| Carpal or metacarpal | 697 | 3.7% |
| Phalanges (hand) | 673 | 3.6% |
| Facial bones | 604 | 3.2% |
| Femoral shaft | 521 | 2.8% |
| Pelvis | 507 | 2.7% |
For the 2-year follow-up period, common fracture sites out of the total 18 826 fracture claims on each individual’s first fracture date. Pathological fractures with specified locations are included. Fracture sites with less than 2% of claims, and unspecified fractures are not listed in this table.
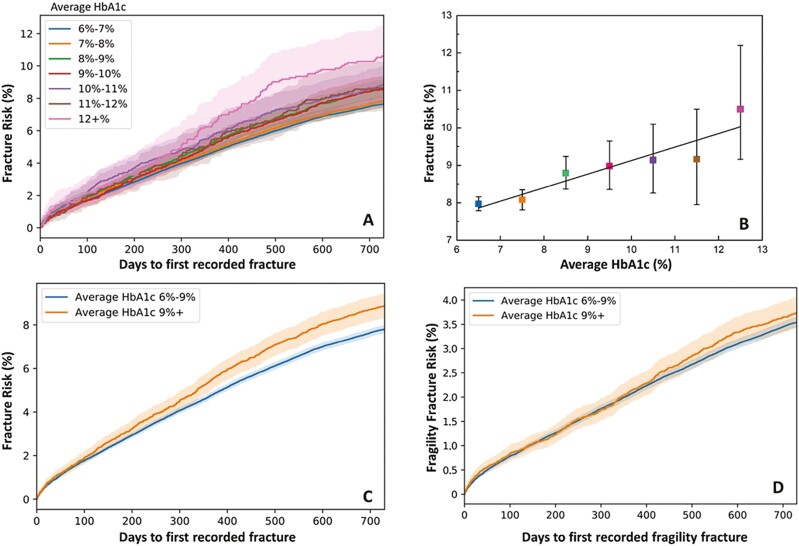
A univariate model was used to estimate the fracture probability in different longitudinal HbA1c cohorts. Figure 2A demonstrates a Kaplan-Meier survival estimation of 2-year cumulative risk of all fractures with 95% CI (stratified by longitudinal HbA1c bins per 1% change) after the 2-year observation period. Multi-group log-rank test indicated that the differences in fracture risk were statistically significant between longitudinal HbA1c groups from the observational period (P value < 0.001). The P values of the log-rank test between each individual groups are listed in Table S3 (43). At the end of the 2-year follow-up period, the cumulative fracture rate shows a significant increasing linear trend as HbA1c level increases (Fig. 2B, P = 0.003, R = 0.93). Figure 2C shows the estimation of fracture risk when HbA1c bins were classified as an adequate glycemic control group and a poor glycemic control group. Here, the group with poor glycemic control had significantly higher risk of fracture than the group with adequate glycemic control (P < 0.001). In contrast, the unadjusted longitudinal HbA1c did not have significant association with fragility fracture risk, as the fragility fracture risk was not statistically different between the adequate and poor glycemic control groups (P = 0.23, Fig. 2D).
Figure 2.
(A) Kaplan-Meier estimation of unadjusted fracture risk with 95% CI stratified by 1% difference of HbA1c groups for 730 days of additional follow-up period. (B) The “cross-section” of unadjusted fracture risk at the end of 2-year follow-up period shows a significant increasing linear correlation with the increase of longitudinal HbA1c percentage (P = 0.003, R = 0.93). (C) Kaplan-Meier estimation of unadjusted all fracture risk when longitudinal glycemic control is separated into 2 groups: adequate glycemic control (N = 142 600) and poor glycemic control (N = 14 839). (D) Kaplan-Meier estimation of unadjusted fragility fracture risk in poor and adequate glycemic control. The difference in fragility fracture risk between groups is not statistically significant (P = 0.23).
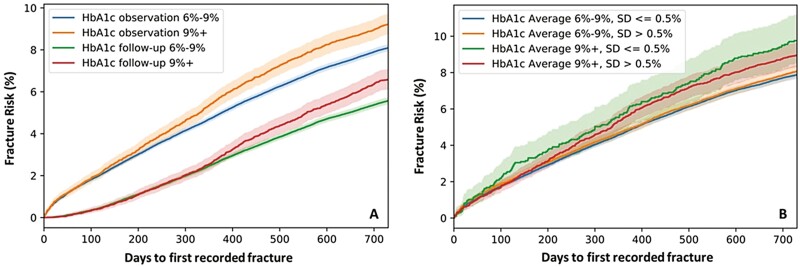
To show the value of utilizing 2-year HbA1c instead of HbA1c measurement near fracture incidences, the mean of HbA1c for both the observational period and follow-up period was used to estimate risk of all fractures. As shown in Fig. 3A, the fracture risk, assessed by mean HbA1c from the follow-up period, was severely underestimated in comparison to the assessment using 2-year longitudinal HbA1c from the observational period. Moreover, within the first year of the first fracture incidence during the follow-up period, the fracture risk cannot be stratified by glycemic control levels.
Figure 3.
(A) Unadjusted fracture risk estimation by HbA1c mean in observational period, and HbA1c mean in follow-up period (up to first fracture incidence if applicable). HbA1c measured in the same period as fracture incidences underestimates the risk of all fractures. N = 101 575 for mean HbA1c follow-up 6%-9%; N = 11 266 for mean HbA1c follow-up 9%+. (B) Unadjusted fracture risk estimation when stratified by high/low SD within each mean HbA1c level. The fracture risk is not statistically different when stratified by HbA1c SD. N = 74 418 for mean HbA1c 6%-9%, SD <= 0.5%; N = 30 748 for mean HbA1c 6%-9%, SD > 0.5%; N = 1968 for mean HbA1c 9%+, SD <= 0.5%; N = 7522 for mean HbA1c 9%+, SD > 0.5%.
Figure 3B shows the impact of variation in the HbA1c values during the 2-year observational period on the relationship between longitudinal glycemic control and fracture risk. Here, only individuals with more than one HbA1c measurements during the observational period were included in the analysis. Among this group, the mean SD for 2-year HbA1c values was 0.54%. Therefore, we used a SD of 0.5% as a cutoff for separating the high and low variation of HbA1c groups. For both poor and adequate glycemic control groups, the difference in fracture risk was not statistically significant when stratified by SD (Fig. 3B).
To exclude the effects of covariates on HbA1c, a multivariate Cox proportional hazard model was applied. Table 4A shows the hazard ratio of longitudinal HbA1c stratified by every 1% change and by adequate (6%-9%) vs poor (≥ 9%) glycemic control. The adjustment was done minimally (adjusted for age, gender, glucocorticoids use, and previous fracture), and with multiple covariates (adjusted for age, gender, glucocorticoids use, previous fracture, osteoporosis, neuropathy, retinopathy, nephropathy, coronary artery disease, hypertension, stroke, and obesity). The hazard ratio was 1.08 for each 1% increase in longitudinal HbA1c, (95% CI [1.07, 1.10]; P < 0.001). This indicates that for each 1% increase in 2-year HbA1c there was concomitant 8% increase in fracture risk for the following 2 years. When further adjusted for various comorbidities, the hazard ratio attenuated to 1.05 (95% CI [1.03, 1.06]; P < 0.001). Compared with the group with adequate glycemic control, the group with poor glycemic control had a 29% increase in fracture risk when adjusted minimally [hazard ratio: 1.29; 95% CI [1.22, 1.36]; P < 0.001], and a 19% increase in fracture risk when further adjusted for additional comorbidities [hazard ratio: 1.18; 95% CI [1.11, 1.25]; P < 0.001]. Adjusting the cutoff for poor glycemic control to > 7% and > 8% resulted in a change of the minimally adjusted hazard ratio to 1.13 (CI [1.09, 1.17]; P < 0.001) and 1.20 (CI [1.15, 1.25]; P < 0.001), respectively. Separating fractures as incident or prevalent (or by excluding prevalent T2D cohort) did not alter the hazard ratio determined using longitudinal HbA1c.
Table 4.
Hazard ratios estimated from Cox hazard proportional model
| Table 4a. Hazard ratio of longitudinal HbA1c in 2 binning methodsa | ||
|---|---|---|
| Longitudinal HbA1c | Minimally adjusted hazard ratio (95% CI) | Multivariate adjusted hazard ratio (95% CI) |
| Per 1% increase of longitudinal HbA1c | 1.08 (1.07, 1.10) | 1.05 (1.03, 1.06) |
| From adequate to poor glycemic control | 1.29 (1.22, 1.36) | 1.18 (1.11, 1.25) |
| Table 4b. Hazard ratios of medications use b | ||
| Medication | Hazard ratio (95% CI) | P value |
| Insulin | 1.26 (1.21, 1.32) | < 0.001 |
| TZDs | 1.23 (1.18, 1.29) | < 0.001 |
| Bisphosphonates | 1.15 (1.07, 1.22) | 0.004 |
| Meglitinides | 1.12 (1.00, 1.26) | 0.04 |
| DPP4 inhibitors | 0.93 (0.88, 0.98) | 0.005 |
| Metformin | 0.88 (0.85, 0.92) | 0.003 |
Abbreviations: DPP4, dipeptidyl peptidase-4; HbA1c, glycated hemoglobin; TZD, thiazolidinedione.
aMinimally adjusted: adjusted for age, gender, glucocorticoids use, and previous fracture; multivariate adjustment: adjusted for age, gender, glucocorticoids use, previous fracture, osteoporosis, neuropathy, retinopathy, nephropathy, coronary artery disease, hypertension, stroke, and obesity. All P values < 0.001.
bHazard ratios of medications use, adjusted for age, gender, longitudinal HbA1c, glucocorticoids use, previous fracture, osteoporosis, neuropathy, retinopathy, nephropathy, coronary artery disease, hypertension, stroke, and obesity. Only medications with statistically significant associations are presented.
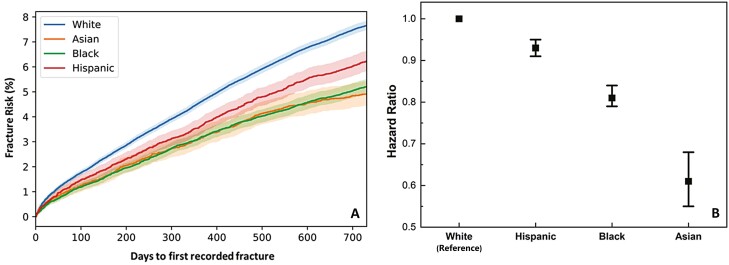
Figure 4A demonstrates the unadjusted fracture risk assessment in the White, Asian, Black, and Hispanic populations. The fracture risk was significantly higher in the White population than the other 3 racial groups. The Black population had higher risk of fracture than the Asian and Hispanic populations, while the risk was not significantly different between the Asian and Hispanic groups. With confounding variables adjusted, compared with the White population (hazard ratio set to 1), the fracture risk was lowered by 7% in the Hispanic population (hazard ratio: 0.93; CI [0.91, 0.95]), 19% in the Black population (hazard ratio: 0.81; CI [0.79, 0.84]), and 39% in the Asian population (hazard ratio: 0.61; CI [0.55, 0.68]). Interestingly, when comparing the effect of poor and adequate glycemic control on fracture risk in the 4 racial/ethnic groups separately, we discovered that the relationship remained unchanged in the White and Black populations but was no longer significant in Asian population with minimal adjustment, or in the Asian and Hispanic populations with multivariate adjustment (Table 5).
Figure 4.
(A) Unadjusted fracture risk estimation stratified by 4 racial groups (White population: N = 99 056; Asian population: N = 8195; Black population: N = 25 647, Hispanic population: N = 14 888; Other/nonspecified not considered). (B) Using White population as a reference point (hazard ratio set to 1), the hazard ratios for other racial groups with covariates adjustment. Hazard ratio for Hispanic population: 0.93 (CI 0.91-0.95); for Black population: 0.81 (CI 0.79-0.84); for Asian population: 0.61 (CI 0.55-0.68). All groups are significantly different from each other.
Table 5.
Relationship between longitudinal HbA1c (poor/adequate glycemic control) and fracture risk in 4 racial groups, minimally adjusted and multivariate adjusted
| From adequate to poor glycemic control | Minimally adjusted hazard ratio (95% CI; P value) | Multivariate adjusted hazard ratio (95% CI; P value) |
|---|---|---|
| Asian | 1.33 (0.95, 1.87; 0.09) | 1.20 (0.84, 1.70; 0.31) |
| Black | 1.25 (1.08, 1.44; 0.002) | 1.19 (1.02, 1.38; 0.02) |
| Hispanic | 1.21 (1.01, 1.45; 0.04) | 1.10 (0.92, 1.33; 0.30) |
| White | 1.32 (1.22, 1.42; < 0.001) | 1.21 (1.13, 1.31; < 0.001) |
Statistically significant hazard ratios are in bold.
The significant associations with medications use, adjusted for multiple variables listed above, are shown in Table 4B. The use of metformin and DDP4 inhibitors were associated with a 12% decrease (hazard ratio: 0.88; 95% CI [0.85, 0.92]; P = 0.003), and a 7% decrease (hazard ratio: 0.93; 95% CI [0.88, 0.98]; P = 0.005) in fracture risk, respectively. In contrast, meglitinides, TZDs, and insulin use were correlated with 12%, 20%, and 24% higher fracture risk, respectively (hazard ratio for meglitinides: 1.12; 95% CI [1.00, 1.26]; P = 0.04; hazard ratio for TZDs: 1.23; 95% CI [1.18, 1.29]; P < 0.001; hazard ratio for insulin: 1.26; 95% CI [1.21, 1.32]; P < 0.001). Interestingly, bisphosphonates use was also associated with a 15% increase in fracture risk within the T2D group (hazard ratio: 1.15; 95% CI [1.07, 1.22]; P < 0.001). However, the elevated fracture risk in the T2D group was not different than in the nondiabetic group, as the difference between hazard ratios was not statistically significant (hazard ratio in T2D group: 1.16; 95% CI [1.13, 1.20]; hazard ratio in nondiabetic control group: 1.13; 95% CI [1.11, 1.14], both P < 0.001). For each of above treatment with significant relationship with fracture risk, the user distribution of longitudinal HbA1c categories, and the occurrence rate of comorbidities, are illustrated in Figure S1 and Figure S2, respectively (43). Exclusion of the osteoporotic cohort from the analysis (instead of adjusting for osteoporosis condition) did not significantly alter the hazard ratios for the antidiabetic treatments (Table S4) (43). Other antidiabetic medications (sulfonylureas, GLP-1 receptor agonists, α-blockers) and other anti-osteoporotic medications (raloxifene and estrogens) did not show any significant impact on fracture risk (hazard ratios and P values are listed in Table S5 (43)).
Discussion
The increased fracture risk in people with T2D is not accurately evaluated by a BMD-based assessment, and the efficacy of common medications in rescuing type 2 diabetic fracture risk is not established. In this study, we report a significant independent correlation between longitudinal HbA1c, commonly used medications, and 2-year fracture risk in a large cohort with 157 439 T2D individuals.
The study has several strengths. First, it was conducted in a nationwide database containing more than 4 million commercial and Medicare Advantage enrollees with T2D, with a broad distribution of geographical regions and races. Second, all clinical fractures were evaluated, including typical fracture types attributed to T2D, such as hip and vertebrae fractures. Third, our study identified a simple, universal, and cost-effective blood-based measurement, HbA1c, as a potential predictor for fracture. Here, we demonstrate the superiority of using 2-year longitudinal HbA1c over HbA1c measured close to fracture incidences.
The association between the longitudinal HbA1c and the fracture risk is more evident and more physiologically relevant than fasting blood glucose (45) or single time point HbA1c measurements either at baseline or just prior to fracture (17-20). For example, one of the essential mechanisms for the association between long-term poor glycemic control and fracture could be nonenzymatic glycation (NEG). NEG is a systemic diffusion-based process where the accumulation of advanced glycation end-products (AGEs) is driven by blood glucose concentration and the time of exposure seen commonly with poor glycemic control in T2D (3, 16, 46). Accumulation of AGEs through NEG disrupts bone turnover (4, 5) and alters bone quality (6-9). Mechanistically, the 2-year longitudinal HbA1c may serve as a suitable indicator of the level of deterioration in bone tissue from elevated blood glucose over time, and may therefore associate with fracture risk, as was found in this study.
Here, over the 2-year period, we also included the individuals with only 1 HbA1c measurement (N = 42 783, 27.2%), since excluding this subgroup might bias the population against the mild/moderate T2D cases. We found that even by excluding this subgroup, the change to this association between HbA1c and fracture risk is minimal (hazard ratio for poor to adequate glycemic control, minimally adjusted: 1.28; 95% CI [1.16, 1.41], vs 1.29; 95% CI [1.22, 1.36] without exclusion). This result suggests that the association between HbA1c and fracture risk is valid as long as there is at least 1 HbA1c record extended across 2 years, as opposed to a HbA1c value measured near to fracture.
The univariate model distinguished the time-to-fracture probability in people with different values of longitudinal glycemic levels and provided a method to assess fracture risk solely based on a relatively short term (2-year) HbA1c level. In contrast, 2-year longitudinal HbA1c did not significantly stratify risk of fragility fractures (defined based on fracture sites only, not referring to the degree of trauma). Such an outcome can be partially explained due to the reduced size of cohort with fractures at specific sites associated with fragility (N = 13 309 by the end of the 2-year follow-up, compared with N = 58 510 for all fractures). More importantly, selecting only fragility fracture sites could increase the proportion of fractures caused by osteoporosis within the cohort, and, unlike T2D fractures, the occurrence of osteoporotic fractures may be dominated by factors other than glycemic control. Although previous studies have demonstrated that T2D does increase fragility fracture risk (47), here we show that the association with longitudinal HbA1c and fracture risk is significant when all fractures in the T2D population are considered.
We also find that, once the HbA1c mean is controlled, the variation and fluctuation of HbA1c within the 2-year period do not have a statistical impact on fracture risk. This outcome may be explained by noting that the impact of glycemic control on bone via a diffusion-based process, such as NEG, is dominated by average value over a period of time (48-50). Consequently, once the mean glycemic level is reached over a moderate period, the fluctuation of HbA1c may not have major effects on bone quality and bone turnover. Thus, our results suggest that people with high variance of HbA1c do not present higher risk of T2D fractures. Consistent with this observation above, a previous study also reports that intensive glycemic control treatment strategies leading to more dramatic decrease in HbA1c did not alter the risk of fall or fracture (51).
Furthermore, the multivariate model in our study demonstrates the independent fracture risk of differences in longitudinal HbA1c. We find that a 1% increase of longitudinal HbA1c is responsible for an 8% higher risk of fractures. After adjusting for multiple diabetic comorbidities, there is still a 5% increased fracture risk directly associated with a 1% elevation in longitudinal HbA1c. It is noteworthy that the seemingly low hazard ratio corresponds to a small difference in HbA1c (1%) within a relatively short 2-year follow-up period. If the patient remains within a poorly controlled glycemic level for 2 years, the risk of fracture is increased by 29%, in comparison with patients maintaining adequate glycemic control. A 29% increase in fracture risk in the United States alone will account for more than 1 million additional fractures in people with poor glycemic control within a period of 2 years (52). Poor glycemic control can also account for the higher risk of fracture related to diabetic comorbidities. For example, the risk of developing retinopathy and/or neuropathy is dependent on the longitudinal HbA1c level. Both conditions could lead to higher risk of fall and hence increased fracture risk (24), although others have shown that fracture risk in T2D remains unchanged after adjustment for higher fall incidences (53). For example, our study shows that 2-year longitudinal HbA1c significantly correlated to the occurrence rate of other diabetic comorbidities (Figure S3 (43), retinopathy R2 = 0.73, neuropathy R2 = 0.79, nephropathy R2 = 0.89, all P < 0.01) and can therefore partially account for the increased fracture risk caused by diabetic comorbidities.
Table 5 presents the evidence of the same relationship between poor/adequate glycemic control and fracture risk in different racial groups. With confounding factors fully adjusted, the hazard ratios between the Asian, Black, and White populations are indeed similar. The lack of statistical significance in both models for the Asian population could be attributed to the much smaller sample size (N = 8195). However, this relationship does seem to attenuate in Hispanic population, which warrants further investigation. As the T2D fracture risk in different racial groups is essentially different (Fig. 4), other factors, not included in the current model, such as bone structure and geometry, dietary habit, and socioeconomic status, should be explored as potential contributors to increased fracture risk in T2D.
In clinical practice, it is usually difficult to rigorously assess the patient’s diabetes history, as precise information on the onset of T2D is generally not available. Here, we separated our cohort as an incident T2D group and a prevalent T2D group whose T2D history is potentially longer. The prevalent T2D group had slightly higher unadjusted risk of fractures than the incident T2D group, possibly due to higher mean age. However, exclusion of prevalent T2D cohort or its inclusion as a covariate did not alter the relationship between longitudinal HbA1c and fracture risk in the multivariate model. Two previous studies show that bone material strength and fracture risk are impacted by the duration of diabetes (9, 54). However, these studies did not adjust for long-term glycemic levels and, when adjusted for long-term average HbA1c, the duration of diabetes was no longer found to be associated with bone material strength (3). Our results show that the 2-year HbA1c observational window is indeed sufficient to account for any impact of diabetes duration and to adequately evaluate the fracture risk. These findings emphasize the clinical importance of proper maintenance of glycemic control in reduction of fracture risk with T2D.
In terms of medications, 6 types of medications selected in our model significantly correlated with the alteration of fracture risk after adjusting for multiple covariates (Table 4B). The results from our observational study should, however, be interpreted with caution as this study is not a randomized controlled trial. Thus, the associations of medications with the fracture risk do not indicate a direct causal relationship between the medications and fracture risk.
We found that metformin was associated with a 12% lower fracture risk. This finding is consistent with recent reports showing reduced diabetic fracture risk with metformin use (1, 55). It is noteworthy that metformin not only lowers HbA1c level, but it also reverses the negative effects of AGEs on osteoblastic cells and normalizes the bone forming process (56-58). Similarly, in preclinical studies, GLP-1 receptor agonists have been shown to stimulate osteoblast differentiation (59) while suppressing osteoclast activities (60). Although a decrease of fracture risk with GLP-1 receptor agonists was reported in a meta-regression study (61), here we did not observe any significant alteration in fracture risk associated with GLP-1 receptor agonist use. We report that DPP4 inhibitors were associated with a 7% decrease in fracture risk, which agrees with a meta-analysis of random controlled trials (29). It has been suggested that DPP4 inhibitors can promote incretin levels which have a positive effect on BMD (29). Conversely, use of TZDs damages osteoblasts and subsequently leads to decrease in BMD (22). Thus, consistent with recent findings (55), we found that the use of TZDs was indeed correlated to a 23% increase in all fracture risk.
Insulin use was associated with a 26% increase in fracture risk. This finding agrees with a propensity-matched study (25) and a case-control study (62). In particular, peripheral hyperinsulinemia, induced by insulin use in T2D, was demonstrated to negatively influence osteoclastogenesis (25). The resulting impairment in bone turnover from hyperinsulinemia could therefore result in higher fracture risk. Insulin users may also have higher level of severity in diabetes/diabetic complications (25) that are not accounted for covariates considered here. Although the difference in longitudinal HbA1c was normalized when considering the effects of medications, insulin use can also lead to temporary hypoglycemia (subsequently recovered) and a consequent increase in the risk of fall related fractures (23, 24). Interestingly, sulfonylureas, which is also likely to induce temporary hypoglycemia and known to increase fracture risk (27), appeared to have no association with fracture risk in our model. There is currently no clinical evidence on fracture risk with 2 classes of antidiabetic medication use (meglitinides and α-blockers). Our study revealed that meglitinides are associated with a 12% increase in all fracture risk. Thus, careful evaluations of bone fractures in relation to meglitinides is suggested for future studies.
Anti-osteoporotic medications are primarily used to prevent fragility fractures in osteoporosis by normalizing bone turnover. While raloxifene and estrogens did not impact T2D associated fracture risk, we discovered a 15% increase in fracture risk with bisphosphonates use, comparable to a previous observational study (33). Because the increased fracture risk associated with bisphosphonates use in T2D was similar to that in nondiabetic individuals, such association is likely due to confounding by indication. However, it is worth noting that, as bisphosphonates prevent fractures by inhibiting osteoclast activity, the increased fracture risk in bisphosphonates users in both T2D and control may be linked to suppression of bone turnover and attenuated remodeling with long-time use of bisphosphonates (63). Among the bisphosphonates users in this study cohort, prior to the follow-up period, 56% had cumulated use of bisphosphonates for more than a year while 31% had bisphosphonates therapy for more than 2 years. Therefore, prescribing bisphosphonates to people with poor glycemic control should be considered cautiously, on a case-by-case basis.
Several limitations should be considered while interpreting the results of our study. First, as mentioned above, because BMD is not commonly measured for people with T2D and is not available in the claims database, it was not adjusted as a covariate in the model. However, since BMD-based evaluations underestimate fracture risk in people with T2D, our motivation was to evaluate a HbA1c-based measure, that is more accessible and can be measured easily through a simple blood test. Second, our study included data available from commercial and Medicare Advantage enrollees. Factors such as unhealthy lifestyles and socioeconomic disadvantages in people with poor glycemic control should also be considered to understand the increase in fracture risk associated with the increase in longitudinal HbA1c level. Third, as mentioned, our study was done retrospectively using de-identified data. Therefore, our results on the impact of medications provided only the initial evidence of the associations of medication with fracture risk when the longitudinal HbA1c for the same period is adjusted. In the current analysis, the duration of medication use, as well as the medication continuation in the follow-up period have not been utilized. However, our results regarding the impact of medication use on fracture risk in T2D, as noted above, are consistent with previous studies. Further investigations with large random controlled trials are warranted to fully understand the relationship between medication use, longitudinal HbA1c, and fracture risk.
This study investigated all types of incident fractures but, since ICD-9 fracture codes do not differentiate between high or low energy trauma, high trauma fractures were not identified and hence were not excluded. Additionally, the fragility fractures are defined here based on the fracture sites and do not refer to the nature of the trauma. Some high trauma fractures at selected fracture sites could therefore be misclassified as fragility fractures. In contrast to the above notion, a previous study shows that if the high trauma fractures are excluded from the analysis, the prevalence of fragility fractures can be underestimated in the osteoporosis population because individuals with osteoporosis have increased risk of both high and low trauma fractures (64). To determine the impact of this particular limitation, we conducted an additional analyses where fractures, not directly attributed to T2D including skull fractures, finger fractures, toe fractures, and fractures at unspecified sites were excluded from the analyses presented here. The relationship between longitudinal HbA1c and fracture risk remained the same, suggesting that the increased fracture risk in T2D found here is valid for multiple fracture sites associated with both high and low energy trauma.
In summary, this study presents evidence to indicate that longitudinal HbA1c measurement is a significant and effective tool for fracture risk assessment. We found that medication (metformin, insulin, TZDs, DPP4 inhibitors, meglitinides, and bisphosphonates) use in T2D population over a period of 2 years was associated with alteration in fracture risk for the following period. Our data provide important clinical input on management and reduction of fracture risk in people with T2D through monitoring and management of the longitudinal glycemic control, and the use of metformin and/or DPP4 inhibitors.
Acknowledgments
D.V. is the principal investigator of the study, and M.J.Z., R.S.B., B.S.G., and G.N.N. are co-investigators. All authors contributed to study design. B.W., Z.W., and A.A.P. contributed to data collection and organization. Data were analyzed by B.W., and interpreted by B.W., Z.W., D.V., M.J.Z., R.S.B., B.S.G., and G.N.N. Also, B.W. drafted the manuscript and revisions were suggested by all other authors. The final version was approved by all authors.
This work was supported by National Institute of Health (NIH) National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) R21AR071681. We would like to thank Tanya Natwick, Laura Becker, and Donna Spencer of OptumLabs for providing support in dataset construction. Authors gratefully acknowledge discussions and suggestions from Dr. David B. Burr (Indiana University), Dr. Thomas O. Carpenter (Yale University), Dr. Andrew F. Stewart (Icahn School of Medicine at Mount Sinai), Dr. Vijay K. Yechoor (University of Pittsburgh), and Dr. Grażyna Sroga (Rensselaer Polytechnic Institute).
Glossary
Abbreviations
- AGE
advanced glycation end-product
- BMD
bone mineral density
- BMI
body mass index
- DPP4
dipeptidyl peptidase-4
- EHR
electronic health record
- GLP-1
glucagon-like peptide-1
- HbA1c
glycated hemoglobin
- ICD-9
International Classification of Diseases, Ninth Revision
- NEG
nonenzymatic glycation
- T2D
type 2 diabetes
- TZD
thiazolidinedione
Financial Support
This work was funded by National Institute of Arthritis and Musculoskeletal and Skin Diseases. Award number: R21 AR071681; and Dr and Ms Sands and Sands Family for Orthopaedic Research. Grant Recipient: Deepak Vashishth.
Disclosures
The authors have no conflict of interest to disclosure.
Data Availability
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
- 1. Vestergaard P, Rejnmark L, Mosekilde L. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005;48(7):1292-1299. [DOI] [PubMed] [Google Scholar]
- 2. Compston J. Type 2 diabetes mellitus and bone. J Intern Med. 2018;283(2):140-153. [DOI] [PubMed] [Google Scholar]
- 3. Farr JN, Drake MT, Amin S, Melton LJ III, McCready LK, Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res. 2014;29(4):787-795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rubin MR, Patsch JM. Assessment of bone turnover and bone quality in type 2 diabetic bone disease: current concepts and future directions. Bone Res. 2016;4:16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanches CP, Vianna AGD, Barreto FC. The impact of type 2 diabetes on bone metabolism. Diabetol Metab Syndr. 2017;9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP. Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone. 2001;28(2):195-201. [DOI] [PubMed] [Google Scholar]
- 7. Poundarik AA, Wu PC, Evis Z, et al. . A direct role of collagen glycation in bone fracture. J Mech Behav Biomed Mater. 2015;52:120-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schwartz AV, Garnero P, Hillier TA, et al. ; Health, Aging, and Body Composition Study. . Pentosidine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab. 2009;94(7):2380-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Furst JR, Bandeira LC, Fan WW, et al. . Advanced glycation endproducts and bone material strength in type 2 diabetes. J Clin Endocrinol Metab. 2016;101(6):2502-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oei L, Rivadeneira F, Zillikens MC, Oei EH. Diabetes, diabetic complications, and fracture risk. Curr Osteoporos Rep. 2015;13(2):106-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moayeri A, Mohamadpour M, Mousavi SF, Shirzadpour E, Mohamadpour S, Amraei M. Fracture risk in patients with type 2 diabetes mellitus and possible risk factors: a systematic review and meta-analysis. Ther Clin Risk Manag. 2017;13:455-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35 Suppl 1:S64-S71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stratton IM, Adler AI, Neil HA, et al. . Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care. 2011;34(6):1329-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Diabetes Association. A1C and eAG. 2021. https://www.diabetes.org/diabetes/a1c-test-meaning/a1c-and-eag. Accessed July 28, 2021.
- 16. Sihota P, Yadav RN, Dhaliwal R, et al. . Investigation of mechanical, material, and compositional determinants of human trabecular bone quality in type 2 diabetes. J Clin Endocrinol Metab. 2021;106(5):e2271-e2289. [DOI] [PubMed] [Google Scholar]
- 17. Li CI, Liu CS, Lin WY, et al. . Glycated hemoglobin level and risk of hip fracture in older people with type 2 diabetes: a competing risk analysis of Taiwan diabetes cohort study. J Bone Miner Res. 2015;30(7):1338-1346. [DOI] [PubMed] [Google Scholar]
- 18. Lee RH, Sloane R, Pieper C, et al. . Glycemic control and insulin treatment alter fracture risk in older men with type 2 diabetes mellitus. J Bone Miner Res. 2019;34(11):2045-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Valentini A, Cianfarani MA, De Meo L, et al. . FRAX tool in type 2 diabetic subjects: the use of HbA1c in estimating fracture risk. Acta Diabetol. 2018;55(10):1043-1050. [DOI] [PubMed] [Google Scholar]
- 20. Puar TH, Khoo JJ, Cho LW, et al. . Association between glycemic control and hip fracture. J Am Geriatr Soc. 2012;60(8):1493-1497. [DOI] [PubMed] [Google Scholar]
- 21. Adil M, Khan RA, Kalam A, et al. . Effect of anti-diabetic drugs on bone metabolism: Evidence from preclinical and clinical studies. Pharmacol Rep. 2017;69(6):1328-1340. [DOI] [PubMed] [Google Scholar]
- 22. Kaku K, Hashiramoto M. Thiazolidinediones and bone fractures. J Diabetes Investig. 2011;2(5):354-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berlie HD, Garwood CL. Diabetes medications related to an increased risk of falls and fall-related morbidity in the elderly. Ann Pharmacother. 2010;44(4):712-717. [DOI] [PubMed] [Google Scholar]
- 24. Schwartz AV, Hillier TA, Sellmeyer DE, et al. . Older women with diabetes have a higher risk of falls: a prospective study. Diabetes Care. 2002;25(10):1749-1754. [DOI] [PubMed] [Google Scholar]
- 25. Losada-Grande E, Hawley S, Soldevila B, et al. . Insulin use and excess fracture risk in patients with type 2 diabetes: a propensity-matched cohort analysis. Sci Rep. 2017;7(1):3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rajpathak SN, Fu C, Brodovicz KG, Engel SS, Lapane K. Sulfonylurea use and risk of hip fractures among elderly men and women with type 2 diabetes. Drugs Aging. 2015;32(4):321-327. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Z, Cao Y, Tao Y, et al. . Sulfonylurea and fracture risk in patients with type 2 diabetes mellitus: a meta‐analysis. Diabetes Res Clin Pract. 2020;159:107990. [DOI] [PubMed] [Google Scholar]
- 28. Chen Q, Liu T, Zhou H, Peng H, Yan C. Risk of fractures associated with dipeptidyl peptidase-4 inhibitor treatment: a systematic review and meta-analysis of randomized controlled trials. Diabetes Ther. 2019;10(5):1879-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Monami M, Dicembrini I, Antenore A, Mannucci E. Dipeptidyl peptidase-4 inhibitors and bone fractures: a meta-analysis of randomized clinical trials. Diabetes Care. 2011;34(11):2474-2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Su B, Sheng H, Zhang M, et al. . Risk of bone fractures associated with glucagon-like peptide-1 receptor agonists’ treatment: a meta-analysis of randomized controlled trials. Endocrine. 2015;48(1):107-115. [DOI] [PubMed] [Google Scholar]
- 31. Anagnostis P, Paschou SA, Gkekas NN, et al. . Efficacy of anti-osteoporotic medications in patients with type 1 and 2 diabetes mellitus: a systematic review. Endocrine. 2018;60(3):373-383. [DOI] [PubMed] [Google Scholar]
- 32. Keegan TH, Schwartz AV, Bauer DC, Sellmeyer DE, Kelsey JL; Fracture Intervention Trial. . Effect of alendronate on bone mineral density and biochemical markers of bone turnover in type 2 diabetic women: the fracture intervention trial. Diabetes Care. 2004;27(7):1547-1553. [DOI] [PubMed] [Google Scholar]
- 33. Vestergaard P, Rejnmark L, Mosekilde L. Are antiresorptive drugs effective against fractures in patients with diabetes? Calcif Tissue Int. 2011;88(3):209-214. [DOI] [PubMed] [Google Scholar]
- 34. Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos Int. 2009;20(6):887-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vashishth D, Bertholon C, Ginevts E, Chavassieux P, Boivin G, Delmas P. Increased non-enzymatic glycation of cancellous bone due to decrease in remodeling during alendronate therapy of osteoporotic women. J Bone Miner Res. 2008;23: S22-S22. [Google Scholar]
- 36. Maricic M, Adachi JD, Sarkar S, Wu W, Wong M, Harper KD. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis. Arch Intern Med. 2002;162(10):1140-1143. [DOI] [PubMed] [Google Scholar]
- 37. Gambacciani M, Levancini M. Hormone replacement therapy and the prevention of postmenopausal osteoporosis. Prz Menopauzalny. 2014;13(4):213-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Idolazzi L, Rossini M, Viapiana O, et al. . Teriparatide and denosumab combination therapy and skeletal metabolism. Osteoporos Int. 2016;27(11):3301-3307. [DOI] [PubMed] [Google Scholar]
- 39. OptumLabs. OptumLabs and OptumLabs Data Warehouse (OLDW) Descriptions and Citation. Eden Prairie, MN: OptumLabs; 2020. [Google Scholar]
- 40. Juarez DT, Sentell T, Tokumaru S, Goo R, Davis JW, Mau MM. Factors associated with poor glycemic control or wide glycemic variability among diabetes patients in Hawaii, 2006-2009. Prev Chronic Dis. 2012;9. doi: 10.5888/pcd9.120065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Feldman BS, Cohen-Stavi CJ, Leibowitz M, et al. . Defining the role of medication adherence in poor glycemic control among a general adult population with diabetes. PLoS One. 2014;9(9):e108145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang B, Glicksberg BS, Nadkarni GN, Vashishth D. Evaluation and management of COVID-19-related severity in people with type 2 diabetes. BMJ Open Diabetes Res Care. 2021;9(1):e002299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang B, Wang Z, Poundarik AA, et al. . Online supplemental material to: unmasking fracture risk in type 2 diabetes: the association of longitudinal glycemic hemoglobin level and medications. [Internet]. 2021. Doi: 10.6084/m9.figshare.16550061. https://figshare.com/articles/figure/Supplementary_Materials_docx/16550061. Accessed October 12, 2021. [DOI]
- 44. United States Bone and Joint Initiative. The Burden of Musculoskeletal Diseases in the United States: Osteoporosis ICD-9-CM and ICD-10-CM Codes. 2021. https://www.boneandjointburden.org/fourth-edition/iva8/icd-9-cm-and-icd-10-cm-codes. Accessed July 28, 2021.
- 45. Strotmeyer ES, Cauley JA, Schwartz AV, et al. . Nontraumatic fracture risk with diabetes mellitus and impaired fasting glucose in older white and black adults: the health, aging, and body composition study. Arch Intern Med. 2005;165(14):1612-1617. [DOI] [PubMed] [Google Scholar]
- 46. Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014;18(1):1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li G, Prior JC, Leslie WD, et al. ; CaMos Research Group. . Frailty and risk of fractures in patients with type 2 diabetes. Diabetes Care. 2019;42(4):507-513. [DOI] [PubMed] [Google Scholar]
- 48. Karim L, Tang SY, Sroga GE, Vashishth D. Differences in non-enzymatic glycation and collagen cross-links between human cortical and cancellous bone. Osteoporos Int. 2013;24(9):2441-2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dyer DG, Blackledge JA, Thorpe SR, Baynes JW. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J Biol Chem. 1991;266(18):11654-11660. [PubMed] [Google Scholar]
- 50. Grandhee SK, Monnier VM. Mechanism of formation of the Maillard protein cross-link pentosidine. Glucose, fructose, and ascorbate as pentosidine precursors. J Biol Chem. 1991;266(18):11649-11653. [PubMed] [Google Scholar]
- 51. Schwartz AV, Margolis KL, Sellmeyer DE, et al. . Intensive glycemic control is not associated with fractures or falls in the ACCORD randomized trial. Diabetes Care. 2012;35(7):1525-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ali SN, Dang-Tan T, Valentine WJ, Hansen BB. Evaluation of the clinical and economic burden of poor glycemic control associated with therapeutic inertia in patients with type 2 diabetes in the United States. Adv Ther. 2020;37(2):869-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Karim L, Bouxsein ML. Effect of type 2 diabetes-related non-enzymatic glycation on bone biomechanical properties. Bone. 2016;82:21-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Majumdar SR, Leslie WD, Lix LM, et al. . Longer duration of diabetes strongly impacts fracture risk assessment: the Manitoba BMD cohort. J Clin Endocrinol Metab. 2016;101(11):4489-4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Salari-Moghaddam A, Sadeghi O, Keshteli AH, Larijani B, Esmaillzadeh A. Metformin use and risk of fracture: a systematic review and meta-analysis of observational studies. Osteoporos Int. 2019;30(6):1167-1173. [DOI] [PubMed] [Google Scholar]
- 56. Schweizer A, Couturier A, Foley JE, Dejager S. Comparison between vildagliptin and metformin to sustain reductions in HbA1c over 1 year in drug-naïve patients with Type 2 diabetes. Diabet Med. 2007;24(9):955-961. [DOI] [PubMed] [Google Scholar]
- 57. Cortizo AM, Sedlinsky C, McCarthy AD, Blanco A, Schurman L. Osteogenic actions of the anti-diabetic drug metformin on osteoblasts in culture. Eur J Pharmacol. 2006;536(1-2):38-46. [DOI] [PubMed] [Google Scholar]
- 58. Schurman L, McCarthy AD, Sedlinsky C, et al. . Metformin reverts deleterious effects of advanced glycation end-products (AGEs) on osteoblastic cells. Exp Clin Endocrinol Diabetes. 2008;116(6):333-340. [DOI] [PubMed] [Google Scholar]
- 59. Nuche-Berenguer B, Moreno P, Portal-Nuñez S, Dapía S, Esbrit P, Villanueva-Peñacarrillo ML. Exendin-4 exerts osteogenic actions in insulin-resistant and type 2 diabetic states. Regul Pept. 2010;159(1-3):61-66. [DOI] [PubMed] [Google Scholar]
- 60. Yamada C, Yamada Y, Tsukiyama K, et al. . The murine glucagon-like peptide-1 receptor is essential for control of bone resorption. Endocrinology. 2008;149(2):574-579. [DOI] [PubMed] [Google Scholar]
- 61. Zhang YS, Weng WY, Xie BC, et al. . Glucagon-like peptide-1 receptor agonists and fracture risk: a network meta-analysis of randomized clinical trials. Osteoporos Int. 2018;29(12):2639-2644. [DOI] [PubMed] [Google Scholar]
- 62. Monami M, Cresci B, Colombini A, et al. . Bone fractures and hypoglycemic treatment in type 2 diabetic patients: a case-control study. Diabetes Care. 2008;31(2):199-203. [DOI] [PubMed] [Google Scholar]
- 63. Black DM, Greenspan SL, Ensrud KE, et al. ; PaTH Study Investigators. . The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349(13):1207-1215. [DOI] [PubMed] [Google Scholar]
- 64. Sanders KM, Pasco JA, Ugoni AM, et al. . The exclusion of high trauma fractures may underestimate the prevalence of bone fragility fractures in the community: the geelong osteoporosis study. J Bone Miner Res. 1998;13(8):1337-1342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of some or all data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.