Abstract
Context
Hypophosphatemia, osteomalacia, and fractures are complications of certain intravenous iron formulations.
Objective
This study investigated risk factors for incident, severe, and persistent hypophosphatemia, and associated alterations in bone and mineral biomarkers following intravenous iron treatment.
Methods
We analyzed data from the PHOSPHARE-IDA randomized clinical trials, comprising 245 patients aged 18 years or older with iron deficiency anemia at 30 outpatient clinics in the United States who received intravenous ferric carboxymaltose (FCM) or ferric derisomaltose (FDI). Outcome measures included serum phosphate, intact fibroblast growth factor-23 (iFGF23), 1,25-dihydroxyvitamin D (1,25(OH)2D), ionized calcium, parathyroid hormone (PTH), and alkaline phosphatase.
Results
FCM was the only consistent risk factor for incident hypophosphatemia (< 2.0 mg/dL; odds ratio vs FDI: 38.37; 95% CI: 16.62, 88.56; P < 0.001). Only FCM-treated patients developed severe hypophosphatemia (< 1.0 mg/dL; 11.3%; 13/115) or persistent hypophosphatemia (< 2.0 mg/dL at study end; 40.0%; 46/115). More severe hypophosphatemia associated with significantly greater increases in iFGF23, PTH, and alkaline phosphatase, and more severe decreases in 1,25(OH)2D and ionized calcium (all P < 0.05). Patients with persistent vs resolved hypophosphatemia demonstrated significantly greater changes in iFGF23, PTH, 1,25(OH)2D, and N-terminal procollagen-1 peptide levels (all P < 0.01), but alkaline phosphatase increased similarly in both groups.
Conclusion
Treatment with FCM was the only consistent risk factor for hypophosphatemia. Patients who developed severe or persistent hypophosphatemia after FCM treatment manifested more severe derangements in bone and mineral metabolism. Changes in bone biomarkers continued beyond resolution of hypophosphatemia, suggesting ongoing effects on bone that may help explain the association of FCM with osteomalacia and fractures.
Keywords: hypophosphatemia, FGF23, intravenous iron, alkaline phosphatase, PTH, clinical trials
Ferric carboxymaltose (FCM; Injectafer in the United States, American Regent, Inc.; Ferinject in the European Union, Vifor Pharma; commercially available in the United States since 2013) and ferric derisomaltose (FDI; formerly known as iron isomaltoside; Monoferric in the United States; Monofer in the European Union, Pharmacosmos A/S; commercially available in the United States since 2020) are iron preparations that are available for intravenous use to rapidly correct iron deficiency (1-5). Both preparations increase hemoglobin comparably and with minimal risk of hypersensitivity reactions (3, 4, 6-10). FCM and FDI can decrease serum phosphate concentrations by increasing renal phosphate excretion, but the incidence of hypophosphatemia is significantly higher after FCM vs FDI treatment (11, 12). In addition, FCM but not FDI has been shown to cause severe hypophosphatemia (serum phosphate ≤ 1.0 mg/dL) and hypophosphatemia that can persist beyond 5 weeks after its initial administration (11-13).
Hypophosphatemia after FCM is mediated by increases in the phosphate-regulating hormone fibroblast growth factor-23 (FGF23) (14). High levels of biologically active, intact FGF23 (iFGF23) increase urinary phosphate excretion and suppress 1,25-dihydroxyvitamin D (1,25(OH)2D) concentrations by reducing the activation of 25-hydroxyvitamin D (25(OH)D) and accelerating the degradation of 1,25(OH)2D (15). The resultant reduction in serum calcium causes secondary hyperparathyroidism that maintains renal phosphate wasting via the phosphaturic effects of parathyroid hormone (PTH) (15). These biochemical changes were recently summarized as “6H-syndrome” (hypophosphatemia, high FGF23, high urinary phosphate excretion, hypocalcitriolemia, hypocalcemia, and secondary hyperparathyroidism) (16).
In the PHOSPHARE-IDA trials, which were a pair of identically designed, randomized clinical trials, 74.4% of patients treated with FCM developed hypophosphatemia (serum phosphate < 2.0 mg/dL) vs 8.0% of patients treated with FDI (12). Furthermore, 11.3% of patients treated with FCM developed severe hypophosphatemia (serum phosphate ≤ 1.0 mg/dL) vs none with FDI (12). The trials confirmed that FCM caused hypophosphatemia by inducing significant elevations of iFGF23 and PTH that were associated with significant changes in markers of bone metabolism (12). The aim of this analysis of the PHOSPHARE-IDA trials was to characterize the magnitude of derangements in bone and mineral metabolism among patients who developed hypophosphatemia of differing severity and duration. To support clinical decision making, we also investigated whether baseline characteristics could identify patients who were most likely to develop incident, severe, and/or persistent hypophosphatemia, and whether laboratory testing on days 7 and 14 could identify patients at highest risk of persistent hypophosphatemia.
Methods
Design and Outcomes
This is a secondary analysis of 2 identically designed, open-label randomized clinical trials, PHOSPHARE IDA-04 and PHOSPHARE IDA-05 (ClinicalTrials.gov, NCT03238911 and NCT03237065), for which the primary results were previously published (12). In brief, 245 patients aged 18 years or older with iron deficiency anemia (hemoglobin ≤ 11 g/dL and ferritin < 100 ng/mL), intolerance or unresponsiveness to oral iron, normal serum phosphate, and normal kidney function were recruited from 30 outpatient clinic sites in the United States between October 2017 and June 2018 (12). Patients were randomized (1:1) to either 2 doses of FCM (750 mg on day 0 and on day 7), or a single infusion of FDI (1000 mg on day 0), in accordance with their FDA-approved labeling (12, 17, 18). The primary outcome was incident hypophosphatemia, defined as serum phosphate < 2.0 mg/dL at any time from baseline to day 35 (12). In addition to hematological markers, serum phosphate, urinary phosphate excretion, ionized calcium, iFGF23, and C-terminal FGF23 (cFGF23), vitamin D metabolites [25(OH)D, 1,25(OH)2D, and 24,25-dihydroxyvitamin D (24,25(OH)2D)], bone biomarkers (N-terminal procollagen-1 peptide [P1NP], C-terminal telopeptide [CTx], alkaline phosphatase [total and bone-specific]), and PTH were measured on days 0, 1, 7, 8, 14, 21, and 35 (12). The trials were approved by a single institutional review board (Western Institutional Review Board, Puyallup, Washington; 98374-2115) and all patients provided written informed consent (12).
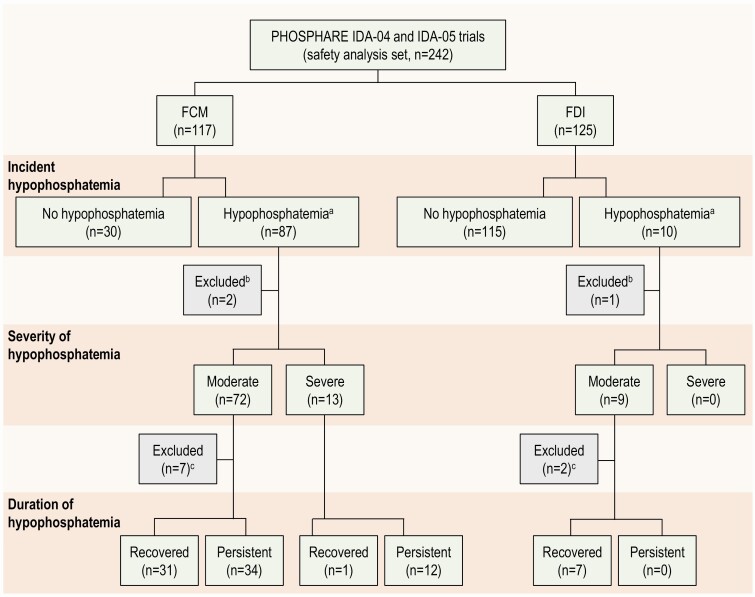
For the current study, we analyzed the pooled cohort of 242 patients who received at least 1 dose of trial drug (Fig. 1); the 3 randomized patients who were not treated were excluded. For analyses of incident hypophosphatemia, patients with no post-baseline phosphate measurements (n = 3) were imputed as hypophosphatemic (as in the trials’ primary analysis (12)). For analyses grouped by severity of hypophosphatemia, patients were categorized as: no hypophosphatemia (serum phosphate ≥ 2.0 mg/dL at all visits), moderate hypophosphatemia (serum phosphate > 1.0 to < 2.0 mg/dL at any post-baseline visit), or severe hypophosphatemia (serum phosphate ≤ 1.0 mg/dL at any post-baseline visit; Fig. 1). For analyses grouped by duration of hypophosphatemia, patients were considered to have persistent hypophosphatemia if they developed incident hypophosphatemia between days 1 and 14 and remained hypophosphatemic (serum phosphate < 2.0 mg/dL) on day 35; patients were considered to have recovered if they developed hypophosphatemia between day 1 and day 14, but their serum phosphate was ≥ 2.0 mg/dL on day 35 (Fig. 1). Patients with no post-baseline phosphate measurement were excluded from the analyses grouped by severity and duration of hypophosphatemia. Nine patients first developed hypophosphatemia on day 21 or day 35 (Fig. 1). These patients could not be classified as persistent or recovered based on our definitions and were excluded from the analyses of duration of hypophosphatemia.
Figure 1.
Patient flow according to study drug, hypophosphatemia severity, and hypophosphatemia duration. Abbreviations: FCM, ferric carboxymaltose; FDI, ferric derisomaltose. aFor patients with no post-baseline measurements, serum phosphate level was imputed as < 2.0 mg/dL (as in the primary clinical trial analysis). bPatients with no post-baseline serum phosphate measurements. cPatients who could not be categorized according to the definitions of “recovered” and “persistent”, due to low phosphate only on day 21 and/or day 35.
Statistical Analysis
We used SAS (version 9.4, Cary, NC) to perform all analyses. Baseline characteristics and demographics are presented as frequencies and percentages, or as means and standard deviations.
We investigated baseline risk factors for incident, severe, and persistent hypophosphatemia using univariate and multivariate logistic regression. Candidate risk factors included age, sex, race (White, African American, Asian, or other), ethnicity (Hispanic or Latino, or non-Hispanic or non-Latino), patient weight, cause of iron deficiency anemia (gynecological or other causes), hematological parameters (hemoglobin, ferritin, transferrin, and transferrin saturation), bone and mineral metabolism markers (serum phosphate, cFGF23, iFGF23, ionized calcium, PTH, 25(OH)D, 1,25(OH)2D, total alkaline phosphatase), and randomized drug assignment (FCM or FDI). First, we calculated univariate estimates for all candidate risk factors. We included variables with a P value < 0.1 on univariate analysis in intermediate multivariate models. From these models, we used a backward stepping approach in which we iteratively eliminated nonsignificant covariates until we reached final parsimonious models that included only those parameters that maintained statistical significance (P < 0.05).
Using the same modeling strategy, we analyzed risk factors that associated with the magnitude of change in serum phosphate from baseline to its nadir at any post-baseline visit in linear regression models. We also used a similar modeling strategy to investigate day 7 and day 14 characteristics as risk factors for persistent hypophosphatemia at day 35.
For the longitudinal analyses of bone and mineral markers stratified by severity of hypophosphatemia, we used a mixed model for repeated measures, including terms for parent trial (1 or 2), visit day (1, 7, 8, 14, 21, and 35), hypophosphatemia severity group (none, moderate, or severe), interactions between day and hypophosphatemia severity groups, and the baseline value of the parameter under investigation. We report least squares (LS) mean changes from baseline and 95% CI, and tested for statistical significance by assessing the P values corresponding to the day x hypophosphatemia severity group interaction terms. We performed similar analyses to compare longitudinal changes in bone and mineral markers according to hypophosphatemia duration, either persistent or recovered. The analyses stratified by hypophosphatemia severity and duration could only include patients in the FCM group because no patients in the FDI group developed severe or persistent hypophosphatemia (Fig. 1). To limit multiple testing, and given the focus of this report, tests for significance were restricted to the severe vs moderate hypophosphatemia groups, and the persistent vs recovered groups; for the sake of comparison and completeness, the results for the no hypophosphatemia group are also shown in the relevant figures.
Results
Baseline demographic, hematological, and biochemical characteristics of the study population are summarized in Table 1. As detailed in the primary report, the incidence of hypophosphatemia was 8.0% (10/125) in FDI-treated patients, none of whom developed severe hypophosphatemia (12). In contrast, 74.4% (87/117) of FCM-treated patients developed hypophosphatemia, which was severe in 11.3% (13/115) of patients (12). In the current analysis, no FDI-treated patients developed persistent hypophosphatemia. Of all FCM-treated patients, 40.0% (46/115) had persistent hypophosphatemia on day 35, including 12 of the 13 who developed severe hypophosphatemia (Fig. 1).
Table 1.
Baseline demographic, hematologic, and biochemical characteristics
| Incident (n = 97) | Persistent (n = 46) | ||
|---|---|---|---|
| Demographics | All patients (n = 242) | Incident hypophosphatemia | Persistent hypophosphatemia |
| Age, years | 43.9 (11.6) | 44.2 (11.3) | 43.6 (11.3) |
| Sex | |||
| Female | 95.0% | 94.8% | 91.3% |
| Male | 5.0% | 5.2% | 8.7% |
| Race | |||
| White | 55.0% | 51.5% | 47.8% |
| Black or African American | 41.3% | 44.3% | 50.0% |
| Asian | 1.2% | 2.1% | 2.2% |
| Other | 2.5% | 2.1% | 0.0% |
| Ethnicity | |||
| Hispanic or Latino | 49.2% | 43.3% | 34.8% |
| Not Hispanic or Latino | 50.8% | 56.7% | 65.2% |
| Weight, kg | 83.1 (22.4) | 79.1 (19.2) | 80.8 (21.4) |
| IDA cause | |||
| Gynecological | 69.0% | 68.0% | 65.2% |
| Other | 31.0% | 32.0% | 34.8% |
| Phosphate and mineral metabolism | |||
| Phosphate, mg/dL | 3.3 (0.5) | 3.3 (0.5) | 3.2 (0.5) |
| Fractional phosphorus excretion, % | 10.3 (5.3) | 10.1 (4.6) | 10.4 (3.9) |
| cFGF23, RU/mL | 838 (1088) | 729 (725) | 889 (805) |
| iFGF23, pg/mL | 55 (38) | 50 (30) | 54 (37) |
| Ionized calcium, mg/dL | 5.1 (0.2) | 5.1 (0.2) | 5.1 (0.2) |
| PTH (intact), pg/mL | 55.5 (28.3) | 59.4 (32.4) | 61.7 (33.7) |
| 25(OH)D, ng/mL | 24.0 (9.1) | 24.7 (9.1) | 23.1 (9.0) |
| 1,25(OH)2D, pg/mL | 59.5 (18.5) | 62.5 (17.1) | 63.6 (15.9) |
| 24,25(OH)2D, ng/mL | 2.1 (1.3) | 2.2 (1.2) | 2.0 (1.3) |
| Alkaline phosphatase, IU/L | 72.7 (25.1) | 74.0 (27.4) | 76.2 (33.7) |
| Bone-specific alkaline phosphatase, μg/L | 12.2 (5.2) | 12.1 (5.6) | 12.5 (7.0) |
| P1NP, ng/mL | 59.3 (30.4) | 58.2 (29.6) | 60.7 (35.4) |
| CTx, ng/mL | 0.3 (0.2) | 0.3 (0.2) | 0.3 (0.2) |
| Hematology | |||
| Hemoglobin, g/dL | 9.6 (1.3) | 9.5 (1.3) | 9.3 (1.2) |
| Ferritin, ng/mL | 14.0 (30.5) | 13.3 (36.2) | 6.2 (6.2) |
| TSAT, % | 10.3 (17.6) | 8.1 (9.9) | 6.3 (7.9) |
| Transferrin, mg/dL | 323.9 (56.9) | 323.3 (55.6) | 333.1 (56.0) |
| Treatment | |||
| FCM | 48.3% | 89.7% | 100.0% |
| FDI | 51.7% | 10.3% | 0.0% |
Mean (SD) is presented for continuous parameters.
Incident hypophosphatemia: serum phosphate < 2.0 mg/dL at any time; persistent hypophosphatemia: serum phosphate < 2.0 mg/dL at any time on days 1–14 that was not resolved on day 35.
Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D; 24,25(OH)2D, 24,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; cFGF23, C-terminal fibroblast growth factor-23; CTx, carboxy-terminal collagen crosslinks; FCM, ferric carboxymaltose; FDI, ferric derisomaltose; IDA, iron deficiency anemia; iFGF23, intact fibroblast growth factor-23; P1NP, N-terminal propeptide of Type I collagen; PTH, parathyroid hormone; TSAT, transferrin saturation.
Baseline Risk Factors for Hypophosphatemia
Treatment with FCM was the strongest risk factor for incident hypophosphatemia (odds ratio [OR] vs FDI: 38.37; 95% CI: 16.62, 88.56; Table 2). Higher baseline PTH and lower body weight were also associated with increased risk of incident hypophosphatemia (Table 2). Since we defined incident hypophosphatemia using a fixed threshold that does not consider the baseline serum phosphate, we also analyzed baseline predictors of change in serum phosphate on a continuous scale after iron treatment. FCM vs FDI treatment was associated with the largest magnitude decrease in serum phosphate from baseline (−1.08 mg/dL; 95% CI: −1.22, −0.94; Table 2). Higher baseline serum phosphate and lower ferritin concentration were also associated with larger magnitude decreases in serum phosphate from baseline (Table 2). Unlike the analysis of incident hypophosphatemia, baseline PTH was not associated with the magnitude decrease in serum phosphate.
Table 2.
Baseline predictors for development of incident, persistent and severe hypophosphatemia, and for magnitude of change in serum phosphate from baseline to nadir
| Hypophosphatemia | Change in serum phosphate | |||||||
|---|---|---|---|---|---|---|---|---|
| Incident (n = 238) | Persistent (n = 106)a | Severe (n = 114)a | From baseline to nadir (n = 225) | |||||
| Dependent variable | OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | p | β-coefficientof change (95% CI) | P |
| Weight, per 5 kg increase | 0.88 (0.80; 0.97) | 0.013 | − | − | − | − | − | − |
| Phosphate, per 0.5 mg/dL increase | − | − | 0.63 (0.40; 1.00) | 0.048 | − | − | −0.35 (−0.42; −0.28) | <0.001 |
| PTH, per 10 pg/mL increase | 1.23 (1.06; 1.42) | 0.006 | − | − | 0.74 (0.55; 1.00) | 0.047 | − | − |
| Ferritin, per 10 ng/mL increase | − | − | 0.59 (0.35; 0.99) | 0.046 | − | − | 0.03 (0.01; 0.05) | 0.015 |
| Treatment, FCM vs FDI | 38.37 (16.62; 88.56) | <0.001 | −a | −a | −a | −a | −1.08 (−1.22; −0.94) | <0.001 |
Results are from the final parsimonious multivariate models that originally considered the following candidate factors: age, sex, race (White, African American, Asian, or other), ethnicity (Hispanic or Latino, or non-Hispanic or non-Latino), patient weight, cause of iron deficiency anemia (gynecological or other causes), hematological parameters (hemoglobin, ferritin, transferrin, and transferrin saturation), bone and mineral metabolism markers (serum phosphate, cFGF23, iFGF23, ionized calcium, PTH, 25(OH)D, 1,25(OH)2D, total alkaline phosphatase), and randomized drug assignment (FCM or FDI). Incident hypophosphatemia: serum phosphate < 2.0 mg/dL at any time; persistent hypophosphatemia: serum phosphate < 2.0 mg/dL at any time on days 1–14 that was not resolved on day 35; severe hypophosphatemia: serum phosphate ≤ 1.0 mg/dL at any time. Only significant predictors from the final parsimonious models are shown.
Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; cFGF23, C-terminal fibroblast growth factor-23; FCM, ferric carboxymaltose; FDI, ferric derisomaltose; iFGF23, intact fibroblast growth factor-23; OR, odds ratio; PTH, parathyroid hormone.
aBaseline predictors of persistent hypophosphatemia and severe hypophosphatemia were assessed only in the FCM-treated patients as neither persistent nor severe hypophosphatemia occurred after FDI treatment.
In analyses of baseline risk factors for severe hypophosphatemia and persistent hypophosphatemia, only FCM-treated patients could be investigated because no FDI-treated patient developed severe or persistent hypophosphatemia (Fig. 1). Among FCM-treated patients, only lower baseline PTH was associated with the development of severe hypophosphatemia (Table 2). Lower baseline ferritin and lower baseline serum phosphate were the only independent baseline risk factors for persistent hypophosphatemia (Table 2).
Hypophosphatemia Severity
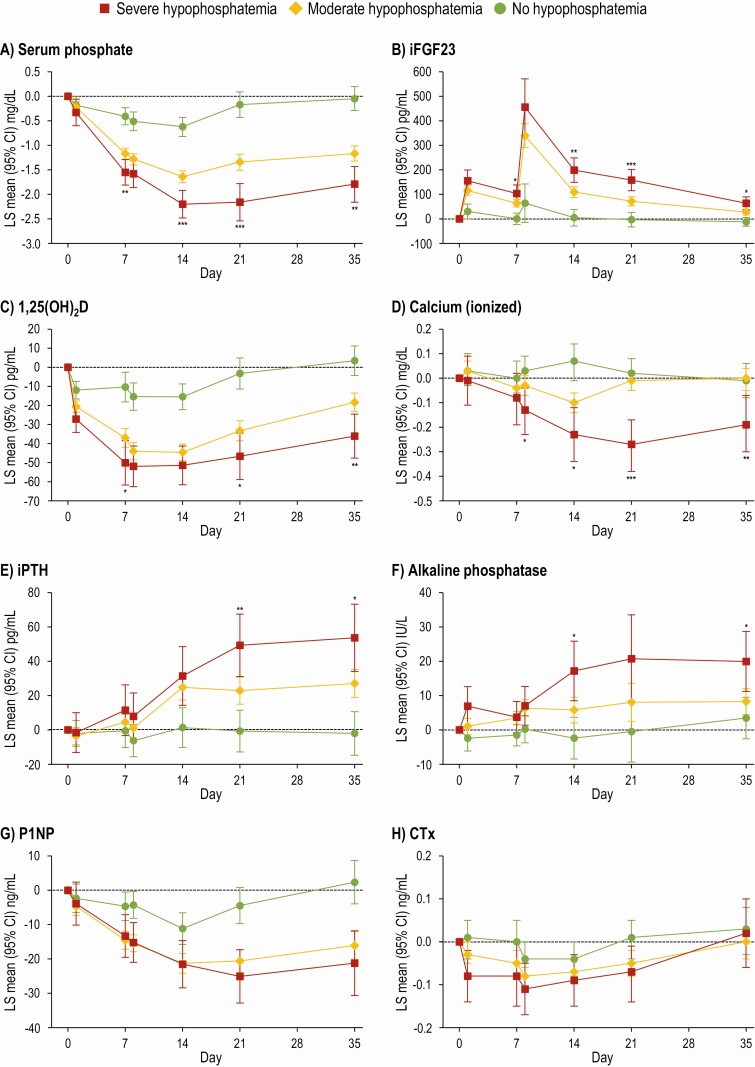
The primary manuscript reported results for the entire FCM and FDI groups (12), which may underestimate the magnitude of effects on bone and mineral parameters among patients who developed hypophosphatemia by mixing their data with data from those who did not develop hypophosphatemia. In longitudinal analyses according to the severity of hypophosphatemia (Fig. 2), more severe hypophosphatemia was associated with more severe derangements in iFGF23, ionized calcium, 1,25(OH)2D, PTH, alkaline phosphatase, and P1NP. Among patients with severe hypophosphatemia, the maximal LS mean increase in iFGF23 was 456 pg/mL (95% CI: 340, 571) on day 8 and the maximal LS mean reduction from baseline in serum phosphate was 2.20 mg/dL (95% CI: 1.92, 2.48) on day 14. PTH was significantly higher and 1,25(OH)2D was significantly lower in the severe vs the moderate hypophosphatemia group from day 21 onwards. Ionized calcium was significantly lower in the severe vs the moderate hypophosphatemia group from day 8 onwards. Alkaline phosphatase increased to significantly higher values in the severe vs the moderate hypophosphatemia group on day 14 and day 35.
Figure 2.
LS mean change (95% CI) in biochemical parameters from baseline to day 35 in patients grouped by hypophosphatemia severity (FCM-treated patients only). *P < 0.05, **P < 0.01, ***P < 0.001 for comparisons of severe vs moderate hypophosphatemia; to limit multiple testing, comparisons vs no hypophosphatemia were omitted. Severe hypophosphatemia: serum phosphate ≤ 1.0 mg/dL at any time from baseline to day 35; moderate hypophosphatemia: serum phosphate > 1.0 to < 2.0 mg/dL at any time from baseline to day 35; no hypophosphatemia: serum phosphate ≥2.0 mg/dL at all times. Data are presented for FCM-treated patients only as no FDI-treated patients developed severe hypophosphatemia. Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D; CTx, C-terminal telopeptide; FCM, ferric carboxymaltose; iFGF23, intact fibroblast growth factor-23; iPTH, intact parathyroid hormone; P1NP, N-terminal procollagen-1 peptide.
Hypophosphatemia Duration
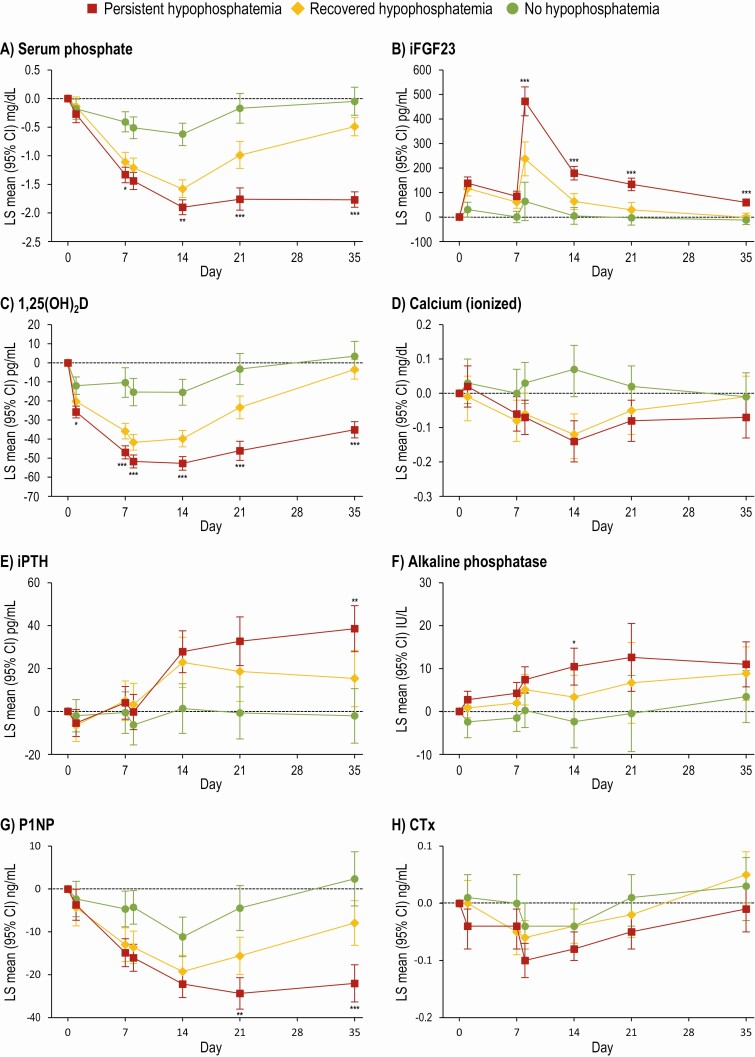
Comparisons of bone and mineral metabolism among FCM-treated patients who developed incident hypophosphatemia and either recovered (n = 32) or experienced persistent hypophosphatemia (n = 46) at day 35 are presented in Fig. 3; FCM-treated patients who did not develop hypophosphatemia are shown for comparison and completeness. Beginning on day 8 and throughout the remainder of follow-up, iFGF23 was significantly higher among those who remained persistently hypophosphatemic compared with those who recovered. In accordance with the known biological effects of FGF23, 1,25(OH)2D decreased significantly more by day 1 and remained significantly lower at all subsequent visits in the persistent hypophosphatemia vs the recovered group. Compared with the recovered group, PTH was significantly higher on day 35 and P1NP was significantly lower on day 21 and day 35 in the persistent hypophosphatemia group. Changes in CTx did not differ between groups. Although alkaline phosphatase increased from baseline in both the persistent and recovered hypophosphatemia groups, there was no difference between these groups at day 35.
Figure 3.
LS mean change (95% CI) in biochemical parameters from baseline to day 35 grouped by hypophosphatemia duration (FCM-treated patients only). *P < 0.05, **P < 0.01, ***P < 0.001 for comparisons of persistent vs recovered hypophosphatemia; to limit multiple testing, comparisons vs no hypophosphatemia were omitted. Persistent hypophosphatemia: serum phosphate < 2.0 mg/dL at any time on days 1–14 that was not resolved on day 35. Recovered hypophosphatemia: serum phosphate < 2.0 mg/dL at any time on days 1–14 that resolved by day 35; no hypophosphatemia: serum phosphate ≥ 2.0 mg/dL at all times. Data are presented for FCM-treated patients only as no FDI-treated patients developed persistent hypophosphatemia. Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D; CTx, C-terminal telopeptide; FCM, ferric carboxymaltose; iFGF23, intact fibroblast growth factor-23; iPTH, intact parathyroid hormone; P1NP, N-terminal procollagen-1 peptide.
Postinfusion Risk Factors for Persistent Hypophosphatemia
In the United States, FCM is administered in 2 doses of 750 mg each, 1 week apart (17). Before administering the second dose, clinicians have the opportunity to assess the effects of the first dose on mineral metabolism, which can be used to determine whether to give the second dose. Therefore, we assessed risk factors for development of persistent hypophosphatemia according to parameters measured on day 7 before the second FCM dose was administered. Among the biochemical parameters, lower 1,25(OH)2D and lower serum phosphate concentrations on day 7 were independent predictors for development of persistent hypophosphatemia (Table 3).
Table 3.
Day 7 and day 14 predictors of persistent hypophosphatemia in FCM-treated patients
| Day 7 predictors (n = 101) | Day 14 predictors (n = 68) | |||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| Ethnicity: Hispanic or Latino vs not Hispanic or Latino | 0.31 (0.11; 0.85) | 0.023 | − | − |
| Phosphate, per 0.5 mg/dL increase | 0.51 (0.29; 0.88) | 0.017 | − | − |
| iFGF23, per 25 pg/mL increase | − | − | 1.35 (1.04; 1.74) | 0.022 |
| 1,25(OH)2D, per 10 pg/mL increase | 0.58 (0.38; 0.89) | 0.012 | 0.36 (0.14; 0.88) | 0.025 |
Results are from the final parsimonious multivariate models. Persistent hypophosphatemia: serum phosphate < 2.0 mg/dL at any time on days 1–14 that was not resolved on day 35; recovered hypophosphatemia: serum phosphate < 2.0 mg/dL at any time on days 1–14 that resolved by day 35. Only significant predictors are shown.
Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D; FCM, ferric carboxymaltose; iFGF23, intact fibroblast growth factor-23; OR, odds ratio.
It is also important for clinicians to be able to identify which patients with FCM-induced hypophosphatemia will require the closest follow-up for persistent hypophosphatemia. Since rates of hypophosphatemia were highest on day 14, we investigated risk factors for development of persistent hypophosphatemia according to parameters measured on day 14. Lower 1,25(OH)2D and higher iFGF23 concentrations on day 14 were independent predictors for development of persistent hypophosphatemia (Table 3).
Discussion
In this secondary analysis of the PHOSPHARE trials, we aimed to identify risk factors for the incidence, severity, and duration of hypophosphatemia following intravenous iron treatment and their associations with the magnitude of changes in markers of bone and mineral metabolism. FCM treatment was the most potent and the only consistent risk factor for development of incident hypophosphatemia and for the magnitude of change in serum phosphate from baseline to its post–iron infusion nadir. Differences in patient weight and baseline serum phosphate, ferritin, and PTH were among the few other risk factors that were identified for incident, severe, or persistent hypophosphatemia, but their effects were of modest magnitude and were inconsistent across the analyses (Table 2). These results are broadly concordant with those from the FIRM trial that compared FCM with ferumoxytol, and also found that FCM use was the strongest risk factor for development of incident and persistent hypophosphatemia (19). In aggregate, these results demonstrate that there are few characteristics beyond FCM use that can inform clinicians which patients are at higher risk of developing hypophosphatemia after receiving intravenous iron.
Longitudinal analyses demonstrated that serum phosphate decreased by 2.20 mg/dL from baseline among patients who were treated with FCM and developed severe hypophosphatemia. By comparison, a decrease of 1.46 mg/dL was reported in the overall FCM group (12). Thus, the current analysis demonstrates that the severity of hypophosphatemia in the most affected patients can be underestimated when considering mean changes in an overall population that also includes unaffected patients. The longitudinal analyses stratified by hypophosphatemia severity also exposed the true magnitude of the derangements in markers of mineral metabolism among FCM-treated patients who developed moderate or severe hypophosphatemia compared to the overall FCM group. In support of the underlying mechanism of FCM-induced hypophosphatemia, these analyses show that worsening severity of hypophosphatemia is associated with more marked elevation of iFGF23, lower 1,25 (OH)2D, and more pronounced hypocalcemia and secondary hyperparathyroidism.
More severe and more prolonged hypophosphatemia after FCM treatment were also associated with changes in bone biomarkers, including increased alkaline phosphatase and decreased P1NP, but little change in CTx. The divergence of the P1NP and CTx responses suggests uncoupling of normal bone remodeling in a pattern that is reminiscent of the effect of corticosteroids, which are known to cause bone loss and increase the risk of fracture (20). The elevated alkaline phosphatase is a typical finding in osteomalacia (21), and numerous case reports describe elevated alkaline phosphatase in the setting of osteomalacia and fractures after FCM treatment (22). However, in the absence of bone histology or sophisticated bone imaging, we can only speculate why the bone formation markers P1NP and alkaline phosphatase diverged. It is possible that the pattern of bone biomarkers differs when well-established osteomalacia comes to clinical attention after a longer and highly variable duration of bone disease than the current situation in which we studied patients soon after the onset of bone injury, the exact timing of which was known. Alternatively, the mechanism of bone disease due to FCM might differ from other causes of osteomalacia and produce a different pattern of bone biomarkers. While further research is needed to investigate the mechanisms of bone disease after FCM and the associated evolution of changes in bone biomarkers over time, it is noteworthy that elevated alkaline phosphatase and reduced P1NP persisted through the end of the study period in patients who developed FCM-induced hypophosphatemia regardless of its duration. This suggests that bone injury can occur after a single course of FCM and can continue even after serum phosphate returns to normal, as has been previously reported (23). As a corollary, resolution of hypophosphatemia after FCM treatment does not necessarily signify normalization of bone and mineral metabolism. Thus, the current data support recent updates to the product label for FCM in the United States, to include a warning on symptomatic hypophosphatemia, and in the European Union and in Brazil, to inform prescribers that FCM-induced hypophosphatemia can cause osteomalacia and fractures (17, 24, 25). They also support the need for long-term studies of bone health in patients with a history of FCM-induced hypophosphatemia of any duration.
The updated regulatory labeling for FCM also includes recommendations to monitor serum phosphate levels (17, 24). However, the optimal timing for when to monitor phosphate and whether additional biomarkers might also be relevant is not known. Given the FDA-approved administration schedule for FCM is 2 doses of 750 mg, 1 week apart (17), clinicians have a window to assess whether or not to administer the second dose. The results presented in this study could help guide the risk assessment of FCM treatment. We identified lower serum phosphate and 1,25(OH)2D on day 7 as independent predictors of persistent hypophosphatemia at day 35. Since the availability of testing for 1,25(OH)2D is inconsistent and the turnaround time can be long, we propose that all patients who receive FCM should have their serum phosphate tested at baseline and on day 7 to determine whether or not to administer the second dose of FCM. In clinical terms, each 0.5 mg/dL reduction in serum phosphate from baseline to day 7 approximately doubled the likelihood that a patient would remain hypophosphatemic to at least day 35 if they received the second dose (Table 3). Among patients who receive the second FCM dose, measuring their 1,25(OH)2D and iFGF23 levels 1 week later might also help clinicians identify which patients require the closest follow-up for persistent hypophosphatemia. Alternatively, using an iron formulation with low hypophosphatemia risk may help avoid downstream consequences of hypophosphatemia altogether.
Strengths of this study include the randomized design and the detailed biochemical characterization of patients at multiple time points. Limitations include the post hoc nature of this analysis, the use of different iron doses between the 2 treatment arms, and the preponderance of female participants that could limit generalizability to men. The relatively short duration of the trials precluded a determination of how long beyond day 35 hypophosphatemia and associated changes in bone and mineral metabolism persisted. While prior studies reported that hypophosphatemia persisted after a single course of FCM for 3 to 9 months in some patients (13, 26-28), there are limited data on the long-term effects of FCM-induced hypophosphatemia on bone. Another limitation of the short duration of follow-up is that we could not assess effects on clinical outcomes, such as fractures, that have been reported in multiple case reports of patients after treatment with FCM (29-31). Additional studies with long-term clinical surveillance are needed to assess the skeletal safety of FCM.
In conclusion, hypophosphatemia can occur after both FDI and FCM, but the incidence was higher after FCM, and severe and persistent hypophosphatemia was observed only after FCM treatment. Compared with moderate and recovered hypophosphatemia, severe and persistent hypophosphatemia were associated with greater and more prolonged elevations of iFGF23, greater reductions in 1,25(OH)2D, and more severe secondary hyperparathyroidism. These changes were associated with changes in biomarkers suggesting impaired bone metabolism that highlight the importance of preventing hypophosphatemia because its clinical consequences could extend beyond the duration of hypophosphatemia.
Acknowledgments
The study was funded by Pharmacosmos A/S, Holbæk, Denmark. The authors thank Jenny Muiry, PhD, of “Cambridge – a Prime Global Agency” for providing editorial support and Jens-Kristian Slott Jensen of “Slott Stat” for providing statistical support, which was funded by Pharmacosmos A/S.
Glossary
Abbreviations
- 1,25(OH)2D
1,25-dihydroxyvitamin D (active vitamin D)
- 24,25(OH)2D)
24,25-dihydroxyvitamin D
- 25(OH)D
25-hydroxyvitamin D
- cFGF23
C-terminal fibroblast growth factor 23
- CTx
C-terminal telopeptide
- FCM
ferric carboxymaltose
- FDI
ferric derisomaltose
- FGF23
fibroblast growth factor 23
- iFGF23
intact fibroblast growth factor 23
- LS
least squares
- OR
odds ratio
- P1NP
procollagen type 1 N-terminal propeptide
- PTH
parathyroid hormone
Disclosures
Dr. Schaefer reported receiving personal fees from Pharmacosmos A/S, and Vifor Pharma, and grants and personal fees from AbbVie, and Gilead outside the submitted work. Dr. Zoller reported receiving grants, personal fees, and nonfinancial support from AbbVie, Gilead, Pharmacosmos A/S, and Vifor Pharma; personal fees from Merck; personal fees and nonfinancial support from Bayer; grants from Merck Sharp & Dohme; and honoraria for lecturing from Bristol-Myers Squibb, Medice, Merz, and Novartis outside the submitted work. Dr. Wolf reported receiving personal fees from Akebia, Ardelyx, AstraZeneca, Bayer, Jnana, Pharmacosmos A/S, Unicycive, and Walden Biosciences outside the submitted work.
Data Availability
Research data are not shared.
References
- 1. Camaschella C. Iron-deficiency anemia. N Engl J Med. 2015;372(19):1832-1843. [DOI] [PubMed] [Google Scholar]
- 2. Pasricha SR, Tye-Din J, Muckenthaler MU, Swinkels DW. Iron deficiency. Lancet. 2021;397(10270):233-248. [DOI] [PubMed] [Google Scholar]
- 3. Onken JE, Bregman DB, Harrington RA, et al. A multicenter, randomized, active-controlled study to investigate the efficacy and safety of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Transfusion. 2014;54(2):306-315. [DOI] [PubMed] [Google Scholar]
- 4. Stein J, Walper A, Klemm W, Farrag K, Aksan A, Dignass A. Safety and efficacy of intravenous iron isomaltoside for correction of anaemia in patients with inflammatory bowel disease in everyday clinical practice. Scand J Gastroenterol. 2018;53(9):1059-1065. [DOI] [PubMed] [Google Scholar]
- 5. Blumenstein I, Shanbhag S, Langguth P, Kalra PA, Zoller H, Lim W. Newer formulations of intravenous iron: a review of their chemistry and key safety aspects - hypersensitivity, hypophosphatemia, and cardiovascular safety. Expert Opin Drug Saf. 2021;20(7):757-769. [DOI] [PubMed] [Google Scholar]
- 6. Bhandari S, Kalra PA, Berkowitz M, Belo D, Thomsen LL, Wolf M. Safety and efficacy of iron isomaltoside 1000/ferric derisomaltose versus iron sucrose in patients with chronic kidney disease: the FERWON-NEPHRO randomized, open-label, comparative trial. Nephrol Dial Transplant. 2021;36(1):111-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pollock RF, Muduma G. A systematic literature review and indirect comparison of iron isomaltoside and ferric carboxymaltose in iron deficiency anemia after failure or intolerance of oral iron treatment. Expert Rev Hematol. 2019;12(2):129-136. [DOI] [PubMed] [Google Scholar]
- 8. Auerbach M, Henry D, Derman RJ, Achebe MM, Thomsen LL, Glaspy J. A prospective, multi-center, randomized comparison of iron isomaltoside 1000 versus iron sucrose in patients with iron deficiency anemia; the FERWON-IDA trial. Am J Hematol. 2019;94(9):1007-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Derman R, Roman E, Modiano MR, Achebe MM, Thomsen LL, Auerbach M. A randomized trial of iron isomaltoside versus iron sucrose in patients with iron deficiency anemia. Am J Hematol. 2017;92(3):286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Onken JE, Bregman DB, Harrington RA, et al. Ferric carboxymaltose in patients with iron-deficiency anemia and impaired renal function: the REPAIR-IDA trial. Nephrol Dial Transplant. 2014;29(4):833-842. [DOI] [PubMed] [Google Scholar]
- 11. Schaefer B, Tobiasch M, Viveiros A, et al. Hypophosphataemia after treatment of iron deficiency with intravenous ferric carboxymaltose or iron isomaltoside-a systematic review and meta-analysis. Br J Clin Pharmacol. 2020;87(5):2256-2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wolf M, Rubin J, Achebe M, et al. Effects of iron isomaltoside vs ferric carboxymaltose on hypophosphatemia in iron-deficiency anemia: two randomized clinical trials. JAMA. 2020;323(5):432-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Detlie TE, Lindstrøm JC, Jahnsen ME, et al. Incidence of hypophosphatemia in patients with inflammatory bowel disease treated with ferric carboxymaltose or iron isomaltoside. Aliment Pharmacol Ther. 2019;50(4):397-406. [DOI] [PubMed] [Google Scholar]
- 14. Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;28(8):1793-1803. [DOI] [PubMed] [Google Scholar]
- 15. Edmonston D, Wolf M. FGF23 at the crossroads of phosphate, iron economy and erythropoiesis. Nat Rev Nephrol. 2020;16(1):7-19. [DOI] [PubMed] [Google Scholar]
- 16. Schaefer B, Meindl E, Wagner S, Tilg H, Zoller H. Intravenous iron supplementation therapy. Mol Aspects Med. 2020;75:100862. [DOI] [PubMed] [Google Scholar]
- 17.Injectafer® (ferric carboxymaltose). Prescribing Information. Vifor International, Inc.; 2020. [Google Scholar]
- 18.Monoferric® (ferric derisomaltose). Prescribing Information. Pharmacosmos A/S; 2020. [Google Scholar]
- 19. Wolf M, Chertow GM, Macdougall IC, Kaper R, Krop J, Strauss W. Randomized trial of intravenous iron-induced hypophosphatemia. JCI Insight. 2018;3(23):e124486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chotiyarnwong P, McCloskey EV. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat Rev Endocrinol. 2020;16(8):437-447. [DOI] [PubMed] [Google Scholar]
- 21. Peach H, Compston JE, Vedi S, Horton LW. Value of plasma calcium, phosphate, and alkaline phosphatase measurements in the diagnosis of histological osteomalacia. J Clin Pathol. 1982;35(6):625-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schaefer B, Tobiasch M, Wagner S, et al. Hypophosphatemia after intravenous iron therapy: comprehensive review of clinical findings and recommendations for management. Bone. 2021;154:116202. [DOI] [PubMed] [Google Scholar]
- 23. Klein K, Asaad S, Econs M, Rubin JE. Severe FGF23-based hypophosphataemic osteomalacia due to ferric carboxymaltose administration. BMJ Case Rep. 2018;2018:bcr2017222851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferinject® (ferric carboxymaltose). Summary of Product Characteristics. Vifor France; 2020. [Google Scholar]
- 25.Ferinject® (ferric carboxymaltose). Prescribing Information (Brazil). Vifor (International) Inc.; 2020. [Google Scholar]
- 26. Frazier R, Hodakowski A, Cai X, et al. Effects of ferric carboxymaltose on markers of mineral and bone metabolism: A single-center prospective observational study of women with iron deficiency. Bone. 2020;141:115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schaefer B, Würtinger P, Finkenstedt A, et al. Choice of high-dose intravenous iron preparation determines hypophosphatemia risk. PLoS One. 2016;11(12):e0167146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rosano G, Schiefke I, Göhring U-M, Fabien V, Bonassi S, Stein J. A pooled analysis of serum phosphate measurements and potential hypophosphataemia events in 45 interventional trials with ferric carboxymaltose. J Clin Med. 2020;9(11):3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zoller H, Schaefer B, Glodny B. Iron-induced hypophosphatemia: an emerging complication. Curr Opin Nephrol Hypertens. 2017;26(4):266-275. [DOI] [PubMed] [Google Scholar]
- 30. Sangrós Sahún MJ, Goñi Gironés E, Camarero Salazar A, Estébanez Estébanez C, Lozano Martínez ME. Symptomatic hypophosphataemic osteomalacia secondary to the treatment with iron carboxymaltose detected in bone scintigraphy. Rev Esp Med Nucl Imagen Mol. 2016;35(6): 391-393. [DOI] [PubMed] [Google Scholar]
- 31. Schaefer B, Glodny B, Zoller H. Blood and bone loser. Gastroenterology. 2017;152(6):e5-e6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Research data are not shared.