Abstract
OBJECTIVES
This study sought to evaluate the long-term differences in survival between multiple arterial grafts (MAG) and single arterial grafts (SAG) in patients who underwent coronary artery bypass grafting (CABG) in the SYNTAX study.
METHODS
The present analysis included the randomized and registry-treated CABG patients (n = 1509) from the SYNTAX Extended Survival study (SYNTAXES). Patients with only venous (n = 42) or synthetic grafts (n = 1) were excluded. The primary end point was all-cause death at the longest follow-up. Multivariable Cox regression was used to adjust for differences in baseline characteristics. Sensitivity analysis using propensity matching with inverse probability for treatment weights was performed.
RESULTS
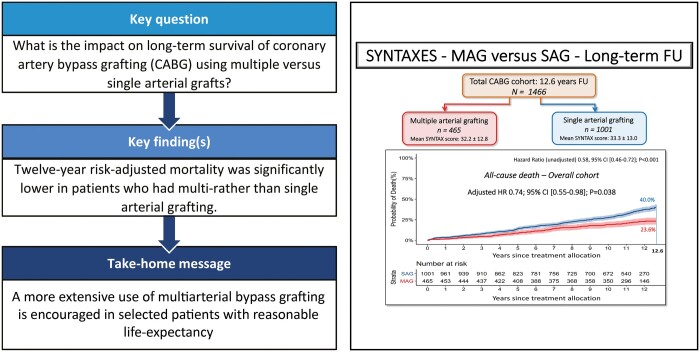
Of the 1466 included patients, 465 (31.7%) received MAG and 1001 (68.3%) SAG. Patients receiving MAG were younger and at lower risk. At the longest follow-up of 12.6 years, all-cause death occurred in 23.6% of MAG and 40.0% of SAG patients [adjusted hazard ratio (HR) 0.74, 95% confidence interval (CI) (0.55–0.98); P = 0.038], which was confirmed by sensitivity analysis. MAG in patients with the three-vessel disease was associated with significant lower unadjusted and adjusted all-cause death at 12.6 years [adjusted HR 0.65, 95% CI (0.44–0.97); P = 0.033]. In contrast, no significance was observed after risk adjustment in patients with the left main disease, with and without diabetes, or among SYNTAX score tertiles.
CONCLUSIONS
In the present post hoc analysis of all-comers patients from the SYNTAX trial, MAG resulted in markedly lower all-cause death at 12.6-year follow-up compared to a SAG strategy. Hence, this striking long-term survival benefit of MAG over SAG encourages more extensive use of multiple arterial grafting in selected patients with reasonable life expectancy.
Trial registration
SYNTAXES ClinicalTrials.gov reference: NCT03417050; SYNTAX ClinicalTrials.gov reference: NCT00114972.
Keywords: SYNTAX, Coronary artery disease, Revascularization, Coronary artery bypass grafting, Multiple arterial grafts, Survival
Whether coronary artery bypass grafting (CABG) should be performed with multiple arterial grafts (MAG) in patients requiring bypass surgery remains fiercely debated.
INTRODUCTION
Whether coronary artery bypass grafting (CABG) should be performed with multiple arterial grafts (MAG) in patients requiring bypass surgery remains fiercely debated. Observational studies report the benefit of using multiple arterial grafting [1]. Previous RCTs have failed to demonstrate a survival benefit of MAG with bilateral (BITA) over single internal thoracic artery (SITA) grafting because they were either underpowered [2] or, as in the case of the randomized Arterial Revascularization Trial (ART), were inconclusive due to discrepancies between treatment allocated and that received [3]. In the intention-to-treat analysis, approximately a quarter of patients in the SITA group received radial arteries as the second conduit and 16.4% of BITA patients crossed over to the SITA cohort, thereby preferentially benefitting the SITA group and depleting the BITA group of MAG patients. However, the as-treated analysis, which involved a comparison of outcomes in patients receiving MAG versus single arterial graft (SAG) irrespective of the randomization, demonstrated a significant survival benefit for the former group. The results of the ongoing Randomized comparison of the clinical Outcome of single versus Multiple Arterial grafts (ROMA) trial comparing MAG with SITA grafting, which was conceptualized to address the drawbacks mentioned above of the ART, are not expected at least until 2025 [4]. To provide credible evidence on this topic in the interim period, we decided to take advantage of the already available 10-year follow-up data of the SYNTAX trial (SYNTAX Extended Survival study) [5], which principally included patients amenable to undergo percutaneous coronary intervention (PCI) and CABG, and the SYNTAX CABG registry, which included patients not amenable for PCI [5]. The present sub-analysis of the SYNTAX Extended Survival study [5] only included patients undergoing CABG in the SYNTAX trial and registry. It aimed to evaluate the impact of MAG versus a SAG on long-term survival (>10 years) in patients with complex coronary artery disease (CAD).
METHODS
Study design
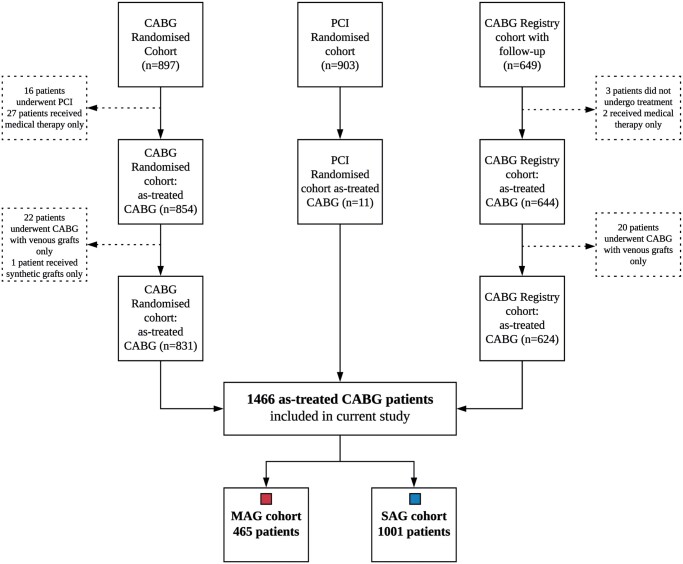
The design and outcomes of the SYNTAX (Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery) trial (NCT00114972) have been reported previously [6, 7]. In brief, the SYNTAX trial randomized patients with de novo three-vessel disease (3VD) and/or left main coronary artery disease (LMCAD) to undergo either PCI with paclitaxel-eluting stents or CABG. Patients ineligible for randomization were included in parallel nested registries for PCI-ineligible patients (CABG registry, n = 1077) and CABG-ineligible patients (PCI registry, n = 198). Out of the 1077 patients in the CABG registry, 649 were randomly allocated for a long-term follow-up, of whom 644 underwent CABG (as-treated). Of those, 20 patients received venous grafts only and were therefore excluded from the current study.
The present analysis is a sub-study of the SYNTAX Extended Survival study (NCT03417050) and merged patients from both the randomized and registry cohorts who underwent CABG (831 patients from the randomized CABG cohort, 11 patients from the randomized PCI cohort, 624 patients from the CABG registry cohort) [5]. Patients who received only venous or synthetic grafts were excluded. Informed consent to obtain information on 10-year vital status was waived, and follow-up was performed in accordance with local law and regulations of each participating site and complied with the Declaration of Helsinki.
End points and definitions
The primary end point of the current study was all-cause death in patients who underwent CABG with MAG versus SAG (as-treated MAG versus SAG). Furthermore, the primary end point was examined in prespecified subgroups of patients (i) with 3VD, (ii) LMCAD, (iii) patients with medically treated diabetes and (iv) those without diabetes, and (v) according to SYNTAX score tertiles (low: 0–22, intermediate: 23–32, high: ≥33).
The MAG cohort consisted of patients who received 2 or more arterial grafts, irrespective of configuration or type of graft (internal thoracic arteries, radial artery, or gastroepiploic artery). The SAG cohort consisted of patients with only one SAG. Details regarding the definitions are reported in the Supplementary Materials.
Coronary artery bypass grafting techniques
Bypass surgery was performed with the aim to achieve complete revascularization of all vessels with a diameter of ≥1.5 mm or larger and with an angiographic diameter stenosis of ≥50% as quantified on coronary angiography and discussed during preoperative Heart Team meetings. The choice and configuration of bypass grafts, as well as the surgical technique utilized, were left at the discretion of the individual surgeon.
Statistical analyses
The present analysis was performed according to the as-treated principle, following standards of reporting and interpretation of subgroup analyses [8]. Discrete variables were expressed as percentages with frequencies and were compared by χ2 tests or Fisher’s exact test when the expected frequency in any cell was <5. Continuous variables were summarized as mean ± standard deviation and were compared by independent samples t-test if normally distributed, or the Wilcoxon rank-sum test if non-normally distributed. Patients with missing follow-up data were included in the analysis and censored at the time they were lost to follow-up or at 5 years if their recruiting hospital did not participate in the 10-year follow-up. Unadjusted cumulative all-cause death rates were estimated according to the Kaplan–Meier method and the difference between the use of MAG and SAG was evaluated with a log-rank test. Kaplan–Meier survival curves are truncated at a time-point in follow-up, when at least 10% of patients were still at risk, to avoid visual misinterpretation [9]. Exploratory analyses were performed for BITA versus SITA and total arterial revascularization versus without total arterial revascularization. Survival analyses for the use of MAG versus SAG were adjusted using multivariable Cox regression analysis that included the following combination of clinically and statistically relevant preoperative variables [10]: age (as a continuous variable; per 1-year increase), sex, body mass index ≥30 kg/m2, medically treated hypertension, medically treated hyperlipidaemia, history of myocardial infarction (MI), unstable angina, history of stroke and/or transient ischaemic attack, medically treated diabetes mellitus, peripheral vascular disease, carotid artery disease, creatinine >200 μmol/l, chronic obstructive pulmonary disease, left ventricular ejection fraction <50%, presence/absence of LMCAD and SYNTAX score (as a continuous variable). To further confirm results obtained with the multivariable Cox model, a sensitivity analysis was performed using propensity score-derived weighing. Detailed information regarding the statistical methods and relevant results is presented in the Supplementary Materials.
Statistical tests were reported as two-sided, and a P-value of <0.05 was considered statistically significant. Statistical analyses were performed with SPSS Statistics software, version 24 (IBM Corporation, Armonk, NY, USA) and R (The R Foundation for Statistical Computing, Vienna, Austria). The statistical analysis scripts may be requested from the corresponding author. Data will not be available for sharing on request.
RESULTS
Patient flow and characteristics
The as-treated CABG cohort consisted of 1466 patients, 465 in the MAG and 1001 in the SAG group (Fig. 1). Patients were enrolled in the SYNTAX trial and registry cohorts from March 2005 through April 2007. Vital status information was collected between 1 March 2017 and 17 June 2019 and was available in 94% of all included patients. The mean age of patients who received MAG was 62.3 vs 66.5 years in patients who received SAG (P < 0.001, Table 1). Patients receiving MAG were less likely to be female and had a lower cardiovascular risk profile. Approximately a quarter of patients had diabetes. The mean European System for Cardiac Operative Risk Evaluation (EuroSCORE) was 2.9 vs 4.4 (P < 0.001) and the mean SYNTAX score was 32.2 vs 33.3 (P = 0.14) among MAG versus SAG patients, respectively.
Figure 1:
Flow of the as-treated CABG patients through the SYNTAX trial (randomized and registry patients). CABG: coronary artery bypass grafting; MAG: multiple arterial grafting; PCI: percutaneous coronary intervention; SAG: single arterial grafting.
Table 1.
Baseline demographic and clinical characteristics of patients undergoing CABG in the SYNTAX trial
| Characteristics | CABG (n = 1466)a |
P-value | |
|---|---|---|---|
| MAG (N = 465) | SAG (N = 1001) | ||
| Age (years) | 62.3 ± 9.7 | 66.5 ± 9.2 | <0.001 |
| Female gender | 64 (13.8) | 224 (22.4) | <0.001 |
| BMI ≥30 (kg/m2) | 144 (31.0) | 310/1000 (31.0) | 0.99 |
| Medically treated diabetes | |||
| Oral medication or insulin | 103 (22.2) | 259 (25.9) | 0.12 |
| Insulin | 38 (8.3) | 102 (10.2) | 0.22 |
| History of nicotine abuse | 319/462 (69.0) | 657/994 (66.1) | 0.27 |
| History of chronic obstructive pulmonary disease | 34 (7.3) | 89 (8.9) | 0.31 |
| Carotid artery disease | 45 (9.7) | 101 (10.1) | 0.81 |
| Peripheral vascular disease | 49 (10.5) | 128 (12.8) | 0.22 |
| Creatinine >200 μmol/l | 6 (1.3) | 21 (2.1) | 0.29 |
| History of myocardial infarction | 128/457 (28.0) | 351/984 (35.7) | 0.004 |
| History of stroke or TIA | 39/464 (8.4) | 94/996 (9.4) | 0.52 |
| Medically treated hypertension (≥130/85 mmHg) | 344/459 (74.9) | 742/990 (74.9) | >0.99 |
| Medically treated hyperlipidaemia | 361/459 (78.6) | 751/985 (76.2) | 0.31 |
| Angina | |||
| Stable | 265 (57.0) | 610 (60.9) | 0.15 |
| Unstable | 119 (25.6) | 256 (25.6) | 0.99 |
| Impaired left ventricular ejection fraction (<50%)b | 74 (15.9) | 265/996 (26.6) | <0.001 |
| EuroSCORE I value | 2.9 ± 2.9 | 4.4 ± 4.9 | <0.001 |
| SYNTAX scorec | 32.2 ± 12.8 | 33.3 ± 13.0 | 0.14 |
| Number of lesionsc | 4.3 ± 1.7 | 4.4 ± 1.8 | 0.80 |
| Left main,d any | 181 (38.9) | 445 (44.5) | 0.046 |
| Three-vessel,d without left main involvement | 284 (61.1) | 556 (55.5) | 0.046 |
Values are shown as mean ± standard deviation or frequencies in percentages and (n) unless otherwise noted.
Data are reported according to the as-treated principle based on the randomized and registry as-treated CABG patients.
Impaired left ventricular ejection fraction was defined as <50%.
Core laboratory assessment.
Site reported.
BMI: body mass index; CABG; coronary artery bypass grafting; EuroSCORE: European System for Cardiac Operative Risk Evaluation; MAG: multiple arterial grafts; SAG: single arterial graft; TIA: transient ischaemic attack.
Both MAG and SAG patients received an average of 2.8 conduits and 3.4 distal anastomoses per patient (Table 2). BITA grafting was performed in 341 patients (73.3) who received MAG. In the SAG cohort, 995 patients (99.4%) received a single left internal thoracic artery, 2 patients a radial artery (0.2%) and 4 patients (0.4%) a single right internal thoracic artery, in addition to venous grafts. The rate of complete revascularization was similar among patients receiving MAG (68.0%) versus SAG (69.4%).
Table 2.
Surgical characteristics
| Characteristics | MAG (N = 465) | SAG (N = 1001) | P-value |
|---|---|---|---|
| Average number of conduits per patient | 2.8 ± 0.7 | 2.8 ± 0.8 | 0.74 |
| Average number of distal anastomoses per patient | 3.4 ± 0.9 | 3.4 ± 1.0 | 0.75 |
| Off-pump CABG | 101 (21.7) | 146 (14.5) | 0.003 |
| Grafts useda | |||
| LITA | 463 (99.6) | 995 (99.4) | 0.68 |
| LITA/RITA | 341 (73.3) | 0 (0) | <0.001 |
| Radial artery | 192 (41.3) | 2 (0.2) | <0.001 |
| Gastroepiploic artery | 1 (0.2) | 0 (0) | 0.14 |
| Venous | 245 (52.7) | 985 (98.4) | <0.001 |
| Arterial graft to LAD | 461 (99.8) | 977 (98.0) | 0.008 |
| Complete revascularization | 316 (68.0) | 695 (69.4) | 0.57 |
Values are shown as mean ± standard deviation or frequencies in percentages and (n) unless otherwise noted.
Four patients received a single RITA, in addition to venous graft(s) in the ‘single’ cohort.
CABG: coronary artery bypass grafting; LAD: left anterior descending artery; LITA: left internal thoracic artery; MAG: multiple arterial grafts; RITA: right internal thoracic artery; SAG: single arterial graft.
Clinical outcomes
At 12.6 years of follow-up, when at least 10% of patients were still at risk [9], all-cause death occurred in 23.6% of patients who received MAG versus 40.0% of those undergoing CABG with SAG [unadjusted hazard ratio (HR) 0.58, 95% confidence interval (CI) (0.46–0.72); P < 0.001, Fig. 2]. After correcting for preselected baseline variables, MAG continued to be associated with a significantly lower all-cause death rate as compared with those undergoing CABG with SAG [adjusted HR 0.74, 95% CI (0.55–0.98); P = 0.038, Table 3].
Figure 2:
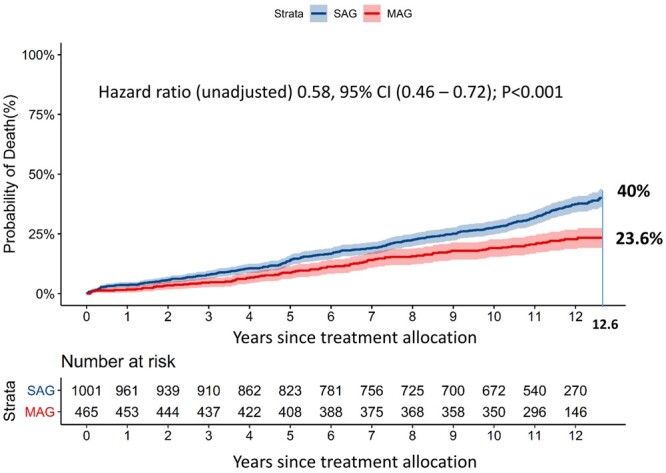
This graph presents the cumulative mortality for single versus multiple arterial grafting patients in our study. CI: confidence interval; MAG: multiple arterial grafts; SAG: single arterial graft. The axis label ‘years since treatment allocation’ indicates a follow-up period starting from the day of randomization or allocation to the percutaneous coronary intervention-ineligible coronary artery bypass grafting registry, which does not necessarily correlate to the operative day.
Table 3.
Multivariable Cox regression model: unadjusted and adjusted outcomes (as-treated)
| Cohort | MAG 12.6-year deaths (%) | SAG 12.6-year deaths (%) | Unadjusted HR (95% CI), P-value | P for interaction | Adjusted HR (95% CI), P-value |
|---|---|---|---|---|---|
| Overall | 23.6 | 40.0 | 0.58 (0.46–0.72), P < 0.001 | 0.74 (0.55–0.98), P = 0.038a | |
| Three-vessel disease | 22.5 | 38.5 | 0.56 (0.41–0.75), P < 0.001 | 0.73 | 0.65 (0.44–0.97), P = 0.033 |
| Left main disease | 24.9 | 42.3 | 0.60 (0.43–0.85), P = 0.004 | 0.85 (0.54–1.34), P = 0.49 | |
| Diabetes | 39.5 | 56.7 | 0.67 (0.46–0.97), P = 0.036 | 0.43 | 0.73 (0.43–1.24), P = 0.25 |
| No diabetes | 19.1 | 34.6 | 0.55 (0.42–0.73), P < 0.001 | 0.76 (0.54–1.09), P = 0.14 | |
| Coronary complexity | 0.86 | ||||
| SYNTAX score 0–22 | 17.5 | 35.3 | 0.47 (0.28–0.80), P = 0.005 | 0.83 (0.41–1.66), P = 0.60 | |
| SYNTAX score 23–32 | 28.4 | 41.7 | 0.70 (0.48–1.01), P = 0.060 | 0.74 (0.44–1.24), P = 0.25 | |
| SYNTAX score ≥33 | 23.6 | 41.0 | 0.58 (0.42–0.81), P = 0.001 | 0.71 (0.47–1.09), P = 0.11 |
Cox regression model on the primary outcome of 12.6 years all-cause death. At 12.6 years, at least 10% of patients were still at risk [9]. Data are reported according to the as-treated principle. Variables used in the full multivariable Cox regression analysis: age, sex, hypertension, hyperlipidaemia, stroke or TIA, diabetes mellitus, peripheral vascular disease, carotid artery disease, chronic obstructive pulmonary disease, creatinine >200 μmol/l, left ventricular ejection fraction <50% and SYNTAX score (as a continuous variable).
This result was confirmed with a weighted Cox proportional hazards model [HR 0.75, 95% CI (0.57–0.99); P = 0.039].
BMI: body mass index (kg/m2); CABG: coronary artery bypass grafting; CI: confidence interval; COPD: chronic obstructive pulmonary disease; HR: hazard ratio; LVEF: left ventricular ejection fraction; MAG: multiple arterial grafts; SAG: single arterial graft.
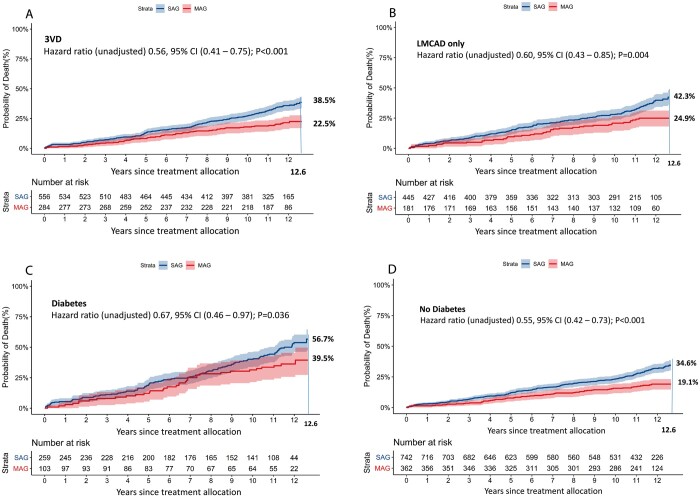
CABG with MAG was associated with lower unadjusted all-cause death rates in the subgroups of patients with 3VD and LMCAD [unadjusted HR 0.56, 95% CI (0.41–0.75); P < 0.001 and HR 0.60, 95% CI (0.43–0.85); P = 0.004, respectively; P for interaction = 0.73] (Fig. 3A and B and Table 3) that were prespecified in the original SYNTAX trial. After the multivariable adjustment, the survival benefit of MAG over SAG remained significant only in patients with 3VD [adjusted HR 0.65, 95% CI (0.44–0.97); P = 0.033, Table 3], while non-significant differences were observed in the subgroup of patients with LMCAD. Similarly, other subgroup comparisons based on the presence or absence of diabetes (Fig. 3C and D) and complexity of CAD defined by SYNTAX score tertiles (as reflected by low and high SYNTAX scores, Fig. 4A and C) also showed a non-significant trend towards lower mortality in favour of MAG. The inverse probability for treatment weights sensitivity analysis confirmed that MAG was associated with lower mortality [HR 0.75, 95% CI (0.57–0.99); P = 0.039; Supplementary Material, Figs S1–S3]. The E-value, representing the potential impact of unobserved variables, was 1.7 [11].
Figure 3:
(A–D) These graphs present the cumulative mortality in SAG and MAG patients in specific clinically important subgroups. (A) 3VD, (B) LMCAD, (C) preoperative diabetes mellitus and (D) no preoperative diabetes mellitus. 3VD: three-vessel disease; CI: confidence interval; LMCAD: left main coronary artery disease; MAG: multiple arterial grafts; SAG: single arterial graft. The axis label ‘years since treatment allocation’ indicates a follow-up period starting from the day of randomization or allocation to percutaneous coronary intervention-ineligible coronary artery bypass grafting registry, which does not necessarily correlate to the operative day.
Figure 4:
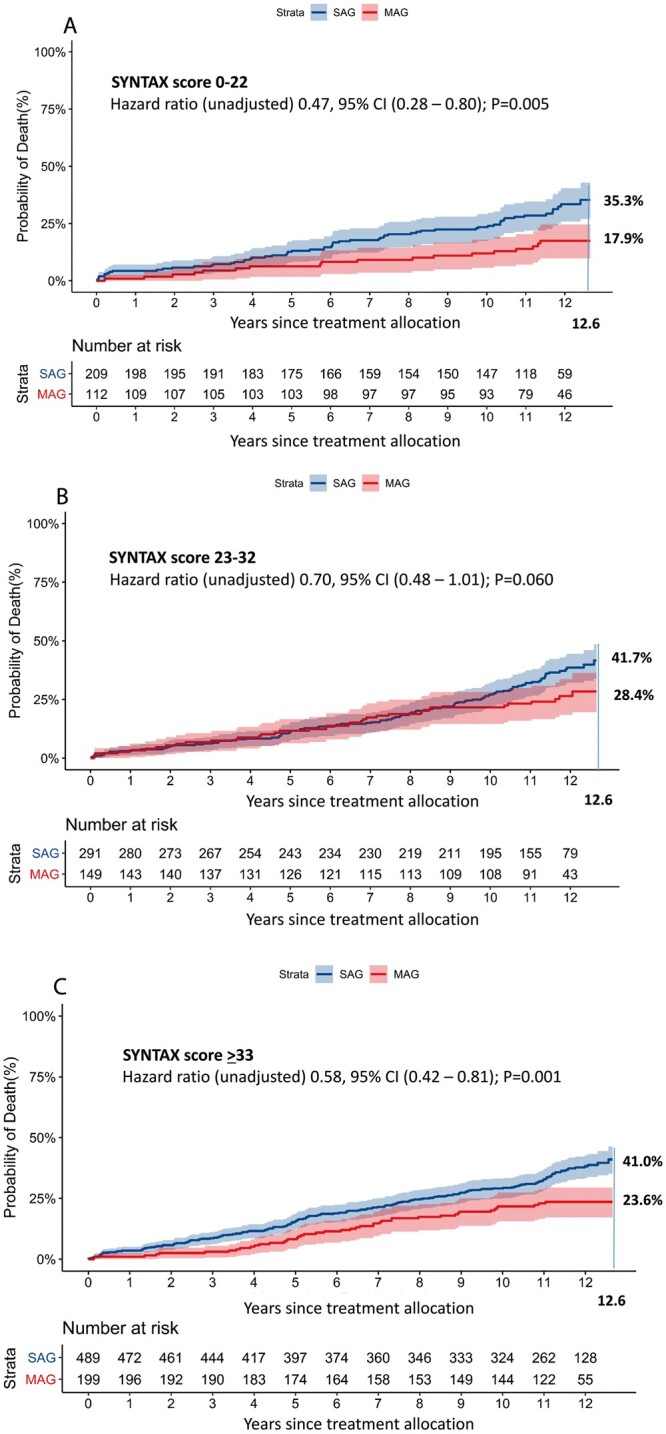
(A–C) This graph presents the cumulative mortality in the MAG and SAG groups stratified by their preoperative SYNTAX score. CI: confidence interval; MAG: multiple arterial grafts; SAG: single arterial graft. The axis label ‘years since treatment allocation’ indicates a follow-up period starting from the day of randomization or allocation to percutaneous coronary intervention-ineligible coronary artery bypass grafting registry, which does not necessarily correlate to the operative day.
The exploratory analyses are reported in the Supplementary Materials.
DISCUSSION
CABG using MAG, compared to using a SAG, was associated with lower all-cause death at 12.6 years follow-up in patients with de novo three-vessel and LMCAD included in the SYNTAX trial and registry, even after adjusting for differences in baseline characteristics. We further validated our results with inverse probability-weighted Cox regression analysis to address the main concern of selection bias in favour of MAG in observational studies. It is common knowledge that MAG is preferentially used by surgeons in younger patients with a low-risk profile. Similarly, the current analysis also determined that MAG in patients with 3VD was associated with significantly lower all-cause death compared to SAG at long-term follow-up. In contrast, other subgroups that were prespecified in the original SYNTAX trial such as LMCAD and those with and those without diabetes, demonstrated a non-significant trend towards better survival at the 12.6-year follow-up mark following the use of MAG.
Although from a pathophysiological standpoint it is reasonable to expect that arterial grafts improve graft-patency and clinical outcomes compared with venous grafts, proof remains limited to observational data [1, 12–16]. While the ART trial showed no difference in survival between BITA versus SITA revascularization, it was fraught with several drawbacks. The major one was that 40% of patients did not receive the therapy they were assigned to during randomization [3]. An excessively higher crossover of patients from BITA to SITA (14%) was associated with poorer clinical outcomes [17], whereas a high rate (22%) of use of radial artery grafts in the SITA group could have improved results in this group, because radial artery grafts have been shown to have superior angiographic patency compared to vein grafts (92% vs 80% at 5 years) [18]. However, multiple arterial grafting in the as-treated analyses demonstrated a significant survival benefit compared with a single arterial grafting strategy [19]. Some of the methodological limitations of the ART guided the design of the ROMA trial, which aims to determine the impact of using at least 2 arterial grafts to the left coronary system on 10-year survival in 4300 patients [4]; however, the first study results are likely to be published only after 2025. The present study, which is a post hoc analysis of the SYNTAXES study that provided the first randomized data meticulously collected with a high follow-up rate of 94% at 12.6 years, provides the best possible evidence in the interim period.
Subgroup analysis revealed that MAG, compared with SAG, remained associated with significantly lower all-cause death in patients with 3VD, even after adjusting for differences in characteristics between groups, while no difference in long-term survival between multiple versus single arterial grafting was observed in patients with left main disease [20].
The majority of patients with diabetes have diffuse and complex CAD. The present study found that 57% of patients with diabetes who received SAG have died during 12.6-year follow-up vs 40% of patients who received MAG. Although this survival difference was associated with a numerically decreased risk of all-cause death in favour of MAG, after adjusting for differences in baseline characteristics this difference remains statistically non-significant. The propensity-matched analysis by Yamaguchi et al. [21] reported decreased 12-year all-cause death rates with MAG versus SAG in patients with (35.1% vs 41.2%, P = 0.041) and without diabetes (28.6% vs 36.2%, P = 0.014). Besides, an increasing number of arterial grafts have been shown to have an incremental survival benefit in both patients with and without diabetes [22].
The major strength of the original SYNTAX trial was that all patients were discussed in a multidisciplinary heart team, consisting of a cardiac surgeon, a clinical cardiologist and an interventional cardiologist. Prior to receiving either PCI or CABG, all significantly stenosed coronary vessels were assessed and those suitable for revascularization determined. After myocardial revascularization, completeness of revascularization was verified based on the number of vessels revascularized compared to those deemed suitable for revascularization prior to intervention. Nonetheless, the rate of complete revascularization in both treatment groups in our study was lower than observed in previous studies [23–25]. These differences most certainly reflect the variation in definitions of completeness of revascularization used across clinical trials, yet could also be partly explained by the greater anatomical complexity of CAD in patients included in the SYNTAX trial and in the nested CABG registry (SYNTAX score 37.8 ± 13.3). Severely calcified and diffusely diseased coronary arteries and small-sized (<2 mm) vessels distal to the lesion were the most common rationale for incomplete revascularization in the CABG cohort of the SYNTAX trial [26]. Inability to graft such vessels is usually not associated with an increased risk of adverse events, which was clearly evident after 3-year follow-up of CABG patients in the SYNTAX trial who did not undergo complete revascularization. Additional prospective studies with longer follow-up (≥10 years) are warranted to determine the influence of complete revascularization on clinical outcomes beyond 3 years.
Strengths and limitations
The results of our study are hypothesis generating. Numerous differences in baseline characteristics existed between patients who received multiple versus singe arterial grafting. We have therefore performed multivariable adjustment, as well as propensity matching (Supplemental Materials), and also accounted for hospital-level differences in the use of multi-arterial grafting. However, in spite of this, no statistical approach, except randomization, can adjust for all residual cofounding factors that may account for the observed difference in mortality between groups. Factors such as incomplete revascularization and use of guideline-directed medical therapy during follow-up that were not adjusted for could also have influenced survival in both cohorts [6, 27]. We studied all-cause mortality as our primary end point and cannot present data regarding cause-specific mortality because the secondary end points such as spontaneous MI, stroke and repeat revascularization and cause of death were not collected during the extended follow-up of the SYNTAX trial.
CONCLUSION
In this post hoc analysis of 1466 SYNTAX trial participants undergoing CABG, all-cause mortality was substantially lower with multiple arterial grafting. This improved survival benefit of multi-arterial grafting at CABG strongly encourages more extensive use of multiple arterial grafting in selected patients with reasonable life expectancy.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Funding
The SYNTAX Extended Survival study was supported by the German Foundation of Heart Research (Frankfurt am Main, Germany). During a 0- to 5-year follow-up, the SYNTAX trial was funded by Boston Scientific Corporation (Marlborough, MA, USA). Both sponsors had no role in the study design, data collection, data analyses and interpretation of the study data, nor were they involved in the decision to publish the final manuscript. The principal investigators and authors had complete scientific freedom.
Conflict of interest: A. Pieter Kappetein reports to work as employee of Medtronic, outside the submitted work. Patrick W. Serruys reports personal consultancy fees from Abbott Laboratories, Biosensors, Cardialysis, Medtronic, Micell, Sino Medical Sciences Technology, Philips/Volcano, Xeltis and Heartflow. Michael J. Mack reports non-financial support from Edwards Lifesciences, non-financial support from Medtronic and non-financial support from Abbott, outside the submitted work. Niels J. Verberkmoes reports personal fees from Medtronic and personal fees from Atricure, outside the submitted work. Stuart J. Head reports to work as chief medical officer at GATT Technologies, outside the submitted work. All other authors declared no conflict of interest.
Author contributions
Daniel J.F.M. Thuijs: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Visualization; Writing—original draft; Writing—review & editing. Piroze Davierwala: Funding acquisition; Investigation; Visualization; Writing—original draft; Writing—review & editing. Milan Milojevic: Conceptualization; Data curation; Investigation; Methodology; Writing—original draft; Writing—review & editing. Salil V. Deo: Conceptualization; Formal analysis; Methodology; Writing—original draft; Writing—review & editing. Thilo Noack: Funding acquisition; Writing—original draft; Writing—review & editing. A. Pieter Kappetein: Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Resources; Supervision; Writing—original draft; Writing—review & editing. Patrick W. Serruys: Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing—original draft; Writing—review & editing. Friedrich-Wilhelm Mohr: Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Supervision; Writing—review & editing. Marie-Claude Morice: Conceptualization; Investigation; Methodology; Supervision; Writing—review & editing. Michael J. Mack: Conceptualization; Investigation; Methodology; Supervision; Writing—review & editing. L. Elisabeth G.E. Ståhle: Conceptualization; Investigation; Methodology; Writing—review& editing. Niels J. Verberkmoes: Conceptualization; Investigation; Methodology; Writing—original draft; Writing—review & editing. David R. Holmes Jr: Conceptualization; Investigation; Writing—review & editing. Stuart J. Head: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Resources; Supervision; Writing—original draft; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Carlos A. Mestres and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
Supplementary Material
ABBREVIATIONS
- 3VD
Three-vessel disease
- ART
Arterial Revascularization Trial
- BITA
Bilateral internal thoracic artery
- CABG
Coronary artery bypass grafting
- CAD
Coronary artery disease
- CI
Confidence interval
- EuroSCORE
European System for Cardiac Operative Risk Evaluation
- HR
Hazard ratio
- LMCAD
Left main coronary artery disease
- MAG
Multiple arterial grafts
- MI
Myocardial infarction
- PCI
Percutaneous coronary intervention
- SAG
Single arterial graft
- SITA
Single internal thoracic artery
Contributor Information
Daniel J F M Thuijs, Department of Cardiothoracic Surgery, Erasmus University Medical Centre, Rotterdam, Netherlands.
Piroze Davierwala, University Department of Cardiac Surgery, Heart Centre Leipzig, Leipzig, Germany; Division of Cardiovascular Surgery, Peter Munk Cardiac Centre, Toronto General Hospital, 15 University Health Network, Toronto, Ontario, Canada; Department of Surgery, University of Toronto, Toronto, Canada.
Milan Milojevic, Department of Cardiothoracic Surgery, Erasmus University Medical Centre, Rotterdam, Netherlands; Department of Cardiac Surgery and Cardiovascular Research, Dedinje Cardiovascular Institute, Belgrade, Serbia.
Salil V Deo, Department of Cardiovascular Surgery, Louis Stokes Cleveland VA Medical Center, Cleveland, OH, USA.
Thilo Noack, University Department of Cardiac Surgery, Heart Centre Leipzig, Leipzig, Germany.
A Pieter Kappetein, Department of Cardiothoracic Surgery, Erasmus University Medical Centre, Rotterdam, Netherlands.
Patrick W Serruys, Department of Cardiology, National University of Ireland, Galway, Ireland.
Friedrich-Wilhelm Mohr, University Department of Cardiac Surgery, Heart Centre Leipzig, Leipzig, Germany.
Marie-Claude Morice, Department of Cardiology, Cardiovascular Institute Paris-Sud (ICPS), Hopital privé Jacques Cartier, Ramsay, Générale de Santé Massy, France.
Michael J Mack, Department of Cardiothoracic Surgery, Baylor University Medical Center, Dallas, TX, USA.
L Elisabeth G E Ståhle, Department of Thoracic and Cardiovascular Surgery, University Hospital, Uppsala, Sweden.
Niels J Verberkmoes, Department of Cardiothoracic Surgery, Catharina Hospital, Eindhoven, Netherlands.
David R Holmes, Jr, Department of Cardiovascular Diseases and Internal Medicine, Mayo Clinic, Rochester, MN, USA.
Stuart J Head, Department of Cardiothoracic Surgery, Erasmus University Medical Centre, Rotterdam, Netherlands.
Presented at the 33rd Annual Meeting of the European Association for Cardio-Thoracic Surgery, Lisbon, Portugal, 3–5 October 2019.
REFERENCES
- 1. Buttar SN, Yan TD, Taggart DP, Tian DH. Long-term and short-term outcomes of using bilateral internal mammary artery grafting versus left internal mammary artery grafting: a meta-analysis. Heart 2017;103:1419–26. [DOI] [PubMed] [Google Scholar]
- 2. Nasso G, Coppola R, Bonifazi R, Piancone F, Bozzetti G, Speziale G. Arterial revascularization in primary coronary artery bypass grafting: direct comparison of 4 strategies–results of the Stand-in-Y Mammary Study. J Thorac Cardiovasc Surg 2009;137:1093–100. [DOI] [PubMed] [Google Scholar]
- 3. Taggart DP, Benedetto U, Gerry S, Altman DG, Gray AM, Lees B et al. ; Arterial Revascularization Trial Investigators. Bilateral versus single internal-thoracic-artery grafts at 10 years. N Engl J Med 2019;380:437–46. [DOI] [PubMed] [Google Scholar]
- 4. Gaudino M, Alexander JH, Bakaeen FG, Ballman K, Barili F, Calafiore AM et al. Randomized comparison of the clinical outcome of single versus multiple arterial grafts: the ROMA trial-rationale and study protocol. Eur J Cardiothorac Surg 2017;52:1031–40. [DOI] [PubMed] [Google Scholar]
- 5. Thuijs D, Kappetein AP, Serruys PW, Mohr FW, Morice MC, Mack MJ et al. ; SYNTAX Extended Survival Investigators. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with three-vessel or left main coronary artery disease: 10-year follow-up of the multicentre randomised controlled SYNTAX trial. Lancet 2019;394:1325–34. [DOI] [PubMed] [Google Scholar]
- 6. Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961–72. [DOI] [PubMed] [Google Scholar]
- 7. Mohr FW, Morice M-C, Kappetein AP, Feldman TE, Ståhle E, Colombo A et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013;381:629–38. [DOI] [PubMed] [Google Scholar]
- 8. Milojevic M, Nikolic A, Juni P, Head SJ. A statistical primer on subgroup analyses. Interact CardioVasc Thorac Surg 2020;30:839–45. [DOI] [PubMed] [Google Scholar]
- 9. Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet 2002;359:1686–9. [DOI] [PubMed] [Google Scholar]
- 10. Hickey GL, Dunning J, Seifert B, Sodeck G, Carr MJ, Burger HU et al. ; EJCTS and ICVTS Editorial Committees. Statistical and data reporting guidelines for the European Journal of Cardio-Thoracic Surgery and the Interactive CardioVascular and Thoracic Surgery. Eur J Cardiothorac Surg 2015;48:180–93. [DOI] [PubMed] [Google Scholar]
- 11. VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: introducing the E-Value. Ann Intern Med 2017;167:268–74. [DOI] [PubMed] [Google Scholar]
- 12. Lytle BW, Blackstone EH, Loop FD, Houghtaling PL, Arnold JH, Akhrass R et al. Two internal thoracic artery grafts are better than one. J Thorac Cardiovasc Surg 1999;117:855–72. [DOI] [PubMed] [Google Scholar]
- 13. Gaudino M, Rahouma M, Abouarab A, Tam DY, Di Franco A, Leonard J et al. Meta-analysis comparing outcomes of drug eluting stents versus single and multiarterial coronary artery bypass grafting. Am J Cardiol 2018;122:2018–25. [DOI] [PubMed] [Google Scholar]
- 14. Rocha RV, Tam DY, Karkhanis R, Nedadur R, Fang J, Tu JV et al. Multiple arterial grafting is associated with better outcomes for coronary artery bypass grafting patients. Circulation 2018;138:2081–90. [DOI] [PubMed] [Google Scholar]
- 15. Schwann TA, Hashim SW, Badour S, Obeid M, Engoren M, Tranbaugh RF et al. Equipoise between radial artery and right internal thoracic artery as the second arterial conduit in left internal thoracic artery-based coronary artery bypass graft surgery: a multi-institutional study. Eur J Cardiothorac Surg 2016;49:188–95. [DOI] [PubMed] [Google Scholar]
- 16. Goldman S, Zadina K, Moritz T, Ovitt T, Sethi G, Copeland JG et al. Long-term patency of saphenous vein and left internal mammary artery grafts after coronary artery bypass surgery: results from a Department of Veterans Affairs Cooperative Study. J Am Coll Cardiol 2004;44:2149–56. [DOI] [PubMed] [Google Scholar]
- 17. Benedetto U, Amrani M, Raja SG; Harefield Cardiac Outcomes Research Group. Guidance for the use of bilateral internal thoracic arteries according to survival benefit across age groups. J Thorac Cardiovasc Surg 2014;148:2706–11. [DOI] [PubMed] [Google Scholar]
- 18. Gaudino M, Benedetto U, Fremes S, Biondi-Zoccai G, Sedrakyan A, Puskas JD et al. Radial-artery or saphenous-vein grafts in coronary-artery bypass surgery. N Engl J Med 2018;378:2069–77. [DOI] [PubMed] [Google Scholar]
- 19. Taggart DP. Implications of the 10-year outcomes of the Arterial Revascularization Trial (ART) for multiple arterial grafts during coronary artery bypass graft. Eur J Cardiothorac Surg 2019;56:427–8. [DOI] [PubMed] [Google Scholar]
- 20. Thuijs D, Head SJ, Stone GW, Puskas JD, Taggart DP, Serruys PW et al. Outcomes following surgical revascularization with single versus bilateral internal thoracic arterial grafts in patients with left main coronary artery disease undergoing coronary artery bypass grafting: insights from the EXCEL trial. Eur J Cardiothorac Surg 2019;55:501–10. [DOI] [PubMed] [Google Scholar]
- 21. Yamaguchi A, Kimura N, Itoh S, Adachi K, Yuri K, Okamura H et al. Efficacy of multiple arterial coronary bypass grafting in patients with diabetes mellitus. Eur J Cardiothorac Surg 2016;50:520–7. [DOI] [PubMed] [Google Scholar]
- 22. Schwann TA, El Hage Sleiman AKM, Yammine MB, Tranbaugh RF, Engoren M, Bonnell MR et al. Incremental value of increasing number of arterial grafts: the effect of diabetes mellitus. Ann Thorac Surg 2018;105:1737–44. [DOI] [PubMed] [Google Scholar]
- 23. Briguori C, Condorelli G, Airoldi F, Focaccio A, D'Andrea D, Cannavale M et al. Comparison of coronary drug-eluting stents versus coronary artery bypass grafting in patients with diabetes mellitus. Am J Cardiol 2007;99:779–84. [DOI] [PubMed] [Google Scholar]
- 24. Buszman PE, Kiesz SR, Bochenek A, Peszek-Przybyla E, Szkrobka I, Debinski M et al. Acute and late outcomes of unprotected left main stenting in comparison with surgical revascularization. J Am Coll Cardiol 2008;51:538–45. [DOI] [PubMed] [Google Scholar]
- 25. Palmerini T, Barlocco F, Santarelli A, Bacchi-Reggiani L, Savini C, Baldini E et al. A comparison between coronary artery bypass grafting surgery and drug eluting stent for the treatment of unprotected left main coronary artery disease in elderly patients (aged > or =75 years). Eur Heart J 2007;28:2714–19. [DOI] [PubMed] [Google Scholar]
- 26. Head SJ, Mack MJ, Holmes DR Jr, Mohr FW, Morice MC, Serruys PW et al. Incidence, predictors and outcomes of incomplete revascularization after percutaneous coronary intervention and coronary artery bypass grafting: a subgroup analysis of 3-year SYNTAX data. Eur J Cardiothorac Surg 2012;41:535–41. [DOI] [PubMed] [Google Scholar]
- 27. Iqbal J, Zhang YJ, Holmes DR, Morice MC, Mack MJ, Kappetein AP et al. Optimal medical therapy improves clinical outcomes in patients undergoing revascularization with percutaneous coronary intervention or coronary artery bypass grafting: insights from the Synergy Between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery (SYNTAX) trial at the 5-year follow-up. Circulation 2015;131:1269–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.