Summary
Background
The purpose of this study was to investigate prevalence of self-reported symptom burden during the acute phase of SARS-CoV-2 infection and associated factors including sex differences.
Methods
All Danish adolescents aged 15–18 years with laboratory confirmed SARS-CoV-2 infection between January 2020 and July 2021 were invited to participate. A survey covered the initial four weeks of SARS-CoV-2 infection and included questions regarding 17 symptoms associated with acute COVID-19, symptom burden and medical history. Statistical analyses included descriptive statistics and logistic regression.
Findings
A total of 24,315 adolescents with SARS-CoV-2 infection were invited and 6630 (27.3%) completed the questionnaire. The median age was 17.6 years, and 58.4% (n = 3873) were female. No symptoms were reported by 33.8% (n = 2241), mild perceived symptom burden by 57.2%(n = 3775), and severe symptom burden by 9.0 % (n = 594). Two thirds (n = 2999) of the symptomatic participants reported a symptom duration of 1–10 days. The most prevalent symptoms included headaches 39.2% (n = 2597), a reduced sense of smell 36.2% (n = 2398), cough 31.6% (n = 2093), sore throat 31.1% (n = 2063), and a reduced sense of taste 31.1% (n = 2062). Adolescents at the age of 18 years had higher odds of reporting ≥6 symptoms OR1.47 (95%CI, 1.23–1.76), p < 0.0001 and adolescents 18+ years old had higher odds of reporting a severe symptom burden OR1.98 (95%CI, 1.43–2.73) compared to the 15years old adolescents. A history of OCD/anxiety/depression was associated with reporting ≥6 symptoms OR 1.67 (95%CI, 1.34–2.09), p < 0.0001 and a history of allergy and OCD/anxiety/depression reporting severe symptom burden OR 1.64 (95%CI, 1.35–1.99), p < 0.0001 and OR 1.75 (95%CI, 1.28–2.36), p = 0.0004. Females reported more symptoms than males; median of three (IQR 0–6) vs. a median of two (IQR 0–4) symptoms, p < 0.0001.
Interpretation
Two in three experienced symptoms and the majority reported mild symptom burden. Headache, a reduced sense of smell and taste, cough and sore throat were most common. Female sex, asthma and previous Epstein-Barr virus were associated with more symptoms and higher symptom burden.
Funding
The study was funded by the AP Møller Foundation. The research was investigator initiated. The study funder played no role in the study.
Research in context.
Evidence before this study
We searched PubMed before designing this study in January 2021 to identify studies investigating symptoms of COVID in children and adolescents. The search terms used were “child” OR “children” OR “adolescent” AND “COVID-19” OR “SARS-CoV-2” AND “symptoms”.
Studies identified primarily concern symptoms in children or adolescent after admission or with in-patient hospital contacts during infection with SARS-CoV-2 and thereby represents the most severe cases. One national population study from England published in august 2021, the only one to our knowledge, reports self-reported symptoms within the first weeks after a positive SARS-CoV-2 test and found the most common symptom to be headache and fatigue. Illness duration were a median of 6 days, longest for the oldest children. Results were parent proxy reported.
Added value of this study
To our knowledge, this study is the first population based national study investigating self-reported symptoms with PCR confirmed SARS-CoV-2.
Implications of all the available evidence
Knowledge of symptoms of COVID in adolescents is important to guide clinical recognition and management of this condition, as well as a contribution to decisions about vaccine strategies. Also, findings on predictors for a severe symptom burden call for attention.
Alt-text: Unlabelled box
Introduction
Background/rationale
During the COVID-19 pandemic, children and adolescents have been considered less affected with a lower burden of symptoms and low rates of hospitalisation and mortality.1, 2, 3 However, recent studies indicate that symptoms may be more severe in children and adolescents than first anticipated, with one study showing that more than 50% of children experienced symptoms in the acute phase of infection with SARS-CoV-2.4 A meta-analysis has identified the most prevalent symptoms to be fever (47%) and cough (37%) and found that the majority of children and/or adolescents reported mild symptoms (79%) and that only 4% were critical.5,6
Another meta-analysis has indicated that shortness of breath and dry cough may be as prevalent in children as in adults.7
Factors associated with severe COVID-19 in children and adolescents leading to hospital admission have been investigated.8 Two studies found that comorbidities including obesity, asthma neurodevelopmental disorders, immunosuppression, malignancy, cardiac disease, diabetes, chronic lung disease, anxiety and depression were all associated with admission for COVID-19 in 8–19 year old children and adolescents.9,10 By contrast, a retrospective study from the United Kingdom found no differences with regard to comorbidities in children admitted to the ICU for mechanical ventilation.11
The severity of COVID-19 in the individual patient has been defined in various ways. The American Center for Disease Control defines special signs of warning and recommends attention towards symptoms including difficulty breathing, chest pain or pressure, confusion, inability to wake or stay awake and pale, grey or blue colored skin or lips.12 Other studies define severe COVID-19 as any SARS-CoV-2 infection requiring supplemental help to breathe and/or admission to a Pediatric Intensive Care Unit.8 Yet, little attention has been given to children and adolescents’ own perception of the disease and severity of symptoms, and non-hospitalized children and adolescents are underrepresented in the literature as are their own perceptions of the disease burden.
In general, more boys are infected with SARS-CoV-2 than girls. In a meta-analysis including 5838 children and adolescents infected with SARS-CoV-2, 55% were males.13 Little is known about differences in sex with regard to the prevalence and severity of symptoms during the acute phase of infection. One study with 1754 SARS-CoV-2 positive 5–17 years old children showed that males were more likely to experience symptoms over a short period, <10 days (52.2% vs 47.8%), whereas females were more likely to experience long-term symptoms defined as a symptom duration ≥28 days (54.5 vs 45.5%).14 However, a thorough overview of the prevalence of acute symptoms and predicting factors for the experience of a severe symptom burden in adolescents is still lacking. Moreover, sex differences need to be further evaluated.
The overall aim of this study was to investigate the prevalence of self-reported acute symptoms, the self-reported symptom burden of COVID-19 in adolescents, and factors associated with having symptoms including comorbidities and sex differences.
Methods
Study design
The study was conducted as a national cross-sectional survey.
Setting
The population consisted of all adolescents aged 15–18 years who tested positive for SARS-CoV-2 (PCR laboratory confirmed) identified from the national COVID-19 database with complete national coverage in Denmark between January 1, 2020, and July 12, 2021. The survey was administered through e-Boks (a Nordic secure digital post-box that is used by public authorities to communicate with Danish citizens) to be answered in REDCap (a secure web application for online surveys)15 in the period July 20 to September 15, 2021 with one initial invitation and two reminders to non-responders.
Participants
Among 271,970 children aged 15–18 years living in Denmark during the study period, 24,315 (9.0%) had a positive test. All participants were under the age of 19 years at the time of having a positive SARS-CoV-2 test, but since every individual aged 18 years or younger in Denmark with a positive test since the beginning of the pandemic was included, some were 19 or 20 years old at the time of answering the questionnaire. No information on hospitalization was available for any of the participants.
No sample size calculation was performed as this is an explorative, descriptive study with all Danish SARS-CoV-2 positive adolescents forming the study population.
Variables
Acute symptoms were defined as symptoms experienced within the first four weeks after a positive PCR test for SARS-CoV-2.
Questionnaire survey data
The survey covered both the acute phase of SARS-CoV-2 infection and long-term symptoms following infection (long-term symptoms have been reported elsewhere).16 The part of the survey used in the present analyses comprised 39 items. A total of 17 common acute COVID symptoms were covered: fever, cold, sore throat, cough, stomach aches, rashes, touble breathing, reduced sense of smell, reduced sense of taste, chest pain, headaches, vomiting, diarrhoea, muscle pain, joint pain, pain, feeling unwell.13,17 Furthermore, participants answered one question regarding their self-perceived symptom burden to reflect the self-perceived severity in the acute phases. The self-reported burden of symptoms was categorised into three groups: no symptoms, mild symptom burden, and severe symptom burden.
The duration of symptoms was categorised into five groups: 1–3 days, 4–6 days, 7–10 days, 11–14 days, and more than 14 days.
Finally, participants answered and questions about previous comorbidities and height and weight to calculate body mass index (BMI).
The co-morbidities and potential risk factors included were chosen based on previously reported associations or suggestions from the literature, e.g. previous Epstein Barr virus infection was included as adolescents previously suffering from this infection might be more prone to suffer long term symptoms after viral infections.18
Pilot testing was done including representatives from the target population prior to sending out the survey. Small adjustments were made to layout and wording.
Bias
Several steps were taken to minimize the risk of bias. All adolescents with a positive test for SARS-CoV-2 were included to minimize selection bias. We used questionnaires distributed electronically and independently from the researchers. Finally, we sent two reminders to non-responders.
Approvals
This study was approved by the data protection agency (P-2021-195) and registered at clinicaltrials.gov (NCT04786353). Access to register data was granted by The Danish Health Data Authority (FSEID 00005625 and 00005757). Approval from the ethics committee is not needed for surveys in Denmark.
Data statement
In accordance with Danish data protection legislation data will not be made available.
Statistical methods
Categorical variables are presented as numbers and percentages. Continuous variables are presented as median and interquartile range (IQR).
Differences in prevalence between sexes were tested using two-sided χ2-tests for categorical variables. Continuous variables were tested for normality using the Kolmogorov-Smirnov test and the Wilcoxon Signed Rank test was used as data were not normally distributed.
Logistic regression models were used to calculate odds ratios (OR) and the corresponding 95% confidence intervals. Two outcomes of interest were chosen: reporting a number of symptoms in the upper quartile (yes/no) and reporting a severe acute symptom burden (yes/no). Each covariate was included separately in the models, but all models were adjusted for sex and age. Age was included as a categorical variable.
To correct for multiple testing Bonferroni correction was used. The significance level was set at p < 0.05 and we conducted 86 tests. Therefore, a p-value of <0.0006 was considered statistically significant.
WHO definitions for the classification of weight status in adolescents were used.19
As all questions had to be answered to submit the survey there were no issues with missing data.
All analyses are conducted using SAS 9.4 M5.
Results
Participants
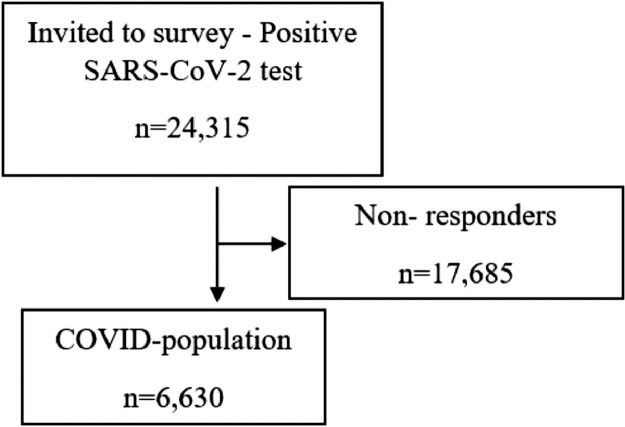
A total of 24,315 participants with a positive test for SARS-CoV-2 were invited. Of these 12,438 (51.2%) were male and 11,876 (48.8%) were female. In total, 6630 (27.3%) completed the survey (Figure 1). The median age of responders was 17.6 years (range 15–20) and of non-responders 17.4 years (range 15–20) (all were ≤18 years old at the time of positive SARS-CoV-2 test). Among responders, the proportion of females was 58.4% and males 41.6%. For non-responders, the figures were 45.3% and 54.7%. The participants’ major pre-existing co-morbidities included allergy, which was reported by 1248 (18.8%), asthma by 507 (7.7%) and eczema by 371 (5.6 %). Obsessive-compulsive disorder (OCD), anxiety or depression was reported by 365 (5.5%). Among participants, 121 (1.8%) were underweight and 866 (13.1%) were overweight (Table 1).
Figure 1.
Flow-chart.
Table 1.
Demographic and clinical profile.
| SARS-CoV-2 positive population (n = 6630) | |
|---|---|
| Age, median (range) (years) | 17.6 (15–20) |
| Sex, n (%) | |
| Female, n (%) | 3873 (58.4) |
| Male, n (%) | 2757 (41.6) |
| Body Mass Index (BMI)a | |
| Underweight, n (%) | 121 (1.8) |
| Normal weight, n (%) | 5312 (80.2) |
| Overweight, n (%) | 866 (13.1) |
| Obesity, n (%) | 325 (4.9) |
| Pre-existing co-morbidityb | |
| Asthma, n (%) | 507 (7.7) |
| Allergy, n (%) | 1248 (18.8) |
| Eczema, n (%) | 371 (5.6) |
| Tics, n (%) | 66 (1.0) |
| Lyme's disease, n (%) | <5 (0.03) |
| ADHD/ADS, n (%) | 176 (2.7) |
| Epstein-Barr Virus, n (%) | 166 (1.8) |
| Arthritis, n (%) | 26 (0.4) |
| ME/CFC, n (%) | 23 (0.4) |
| Autism, n (%) | 108 (1.6) |
| Urticaria, n (%) | <5 (0.1) |
| Dyspraxia, n (%) | <5 (0.02) |
| Hyper-mobility, n (%) | 124 (1.9) |
| HPV virus, n (%) | 5 (0.1) |
| OCD/anxiety/depression, n (%) | 365 (5.5) |
| Hyperthyroidism, n (%) | <5 (0.03) |
| Hypertonia, n (%) | <5 (0.1) |
| Other, n (%) | 177 (2.7) |
| Self-reported severity of COVID-19 | |
| No symptoms, n (%) | 2241 (33.8) |
| Mild symptom burden, n (%) | 3795 (57.2) |
| Severe symptom burden, n (%) | 594 (9.0) |
| Duration of acute COVID symptoms | |
| 1–3 days, n (%) | 794 (18.1) |
| 4–6 days, n (%) | 1236 (28.2) |
| 7–10 days, n (%) | 969 (22.1) |
| 11–14 days, n (%) | 512 (11.7) |
| More than 14 days, n (%) | 878 (20.0) |
SD= standard deviation, ADHD/ADS=Attention deficit hyperactivity disorder/Attention deficit syndrome, ME/CFC=Myalgic encephalomyelitis/Chronic fatigue syndrome, OCD=Obsessive-compulsive disorder.
6 participants had missing information on BMI.
Participants were asked about co-morbidity before COVID-19 infection.
The number of participants reporting a mild symptom burden decreased with time since positive SARS-CoV-2 PCR test, whereas the number of participants reporting a severe symptom burden increased with time since positive SARS-CoV-2 test (Table 2).
Table 2.
Self-reported severity of COVID-19 by time since positive test.
| Time since positive SARS CoV-2 test |
||||||
|---|---|---|---|---|---|---|
| ≤1 month | >1–3 months | 4–6 months | 7–9 months | 10–12 months | >12 months | |
| Self-reported severity of COVID-19, n (%) | ||||||
| No symptoms | 113 (30.9) | 388 (33.5) | 285 (33.3) | 1081 (34.2) | 283 (33.6) | 91 (37.6) |
| Mild symptoms | 233 (63.7) | 700 (60.5) | 497 (58.1) | 1788 (56.5) | 453 (53.7) | 124 (51.2) |
| Severe symptoms | 20 (5.5) | 70 (6.0) | 74 (8.6) | 296 (9.4) | 107 (12.7) | 27 (11.2) |
| Total population | 366 (5.5) | 1158 (17.5) | 856 (12.9) | 3165 (47.7) | 843 (12.7) | 242 (3.7) |
Outcome data
Acute symptoms
2241(33.8%) of participants reported no symptoms during the acute phase of SARS-CoV-2 infection, 3795 (57.2%) reported a mild symptom burden, and 594 (9.0%) reported a severe symptom burden. Two thirds of the symptomatic participants reported a symptom duration of 1–10 days. The acute COVID-19 symptoms lasted for more than two weeks for 878 (20.0%) of participants (Table 1).
Within the first four weeks of the SARS-CoV-2 infection, the median number of acute symptoms was two (IQR 0–5) for the total group of responders, four (IQR 2–6) for the group reporting mild symptom burden, and eight (IQR 5–11) for the group reporting a severe symptom burden, p < 0.0001. The most frequent symptoms among all SARS-CoV-2 positive adolescents were headache (39.2%), a reduced sense of smell (36.2%), cough (31.6%), sore throat (31.1%), and a reduced sense of taste (31.1%) (Table 3). For the group reporting a severe symptom burden, the most frequent symptoms were fever, reduced sense of smell, reduced sense of taste, cough, sore throat and feeling unwell. Symptoms were significantly more common in participants reporting a severe symptom burden compared to participants reporting a mild symptom burden (Table 3).
Table 3.
Differences in acute COVID-19 symptom prevalence between participants reporting mild symptom burden and severe symptom burden.
| SARS-CoV-2 positive population |
||||
|---|---|---|---|---|
| Total population (n = 6630) | Self-reported mild symptom burden (n = 3795) | Self-reported severe symptom burden (n = 594) | p-valuea | |
| Fever, n (%) | 1768 (26.7) | 1383 (36.4) | 385 (64.8) | <0.0001 |
| Cold, n (%) | 1939 (29.3) | 1594 (42.0) | 345 (58.1) | <0.0001 |
| Sore throat, n (%) | 2063 (31.1) | 1677 (44.2) | 386 (65.0) | <0.0001 |
| Cough, n (%) | 2093 (31.6) | 1711 (45.1) | 382 (64.3) | <0.0001 |
| Stomach aches, n (%) | 568 (8.6) | 390 (10.3) | 178 (30.0) | <0.0001 |
| Rashes, n (%) | 105 (1.6) | 72 (1.9) | 33 (5.6) | <0.0001 |
| Trouble breathing, n (%) | 1129 (17.0) | 787 (20.7) | 342 (57.6) | <0.0001 |
| Reduced sense of smell, n (%) | 2398 (36.2) | 2002 (52.8) | 396 (66.7) | <0.0001 |
| Reduced sense of taste, n (%) | 2062 (31.1) | 1689 (44.5) | 373 (62.8) | <0.0001 |
| Chest pain, n (%) | 393 (5.9) | 232 (6.1) | 161 (27.1) | <0.0001 |
| Headaches, n (%) | 2597 (39.2) | 2118 (55.8) | 479 (80.6) | <0.0001 |
| Vomiting, n (%) | 139 (2.1) | 76 (2.0) | 63 (10.6) | <0.0001 |
| Diarrhoea, n (%) | 281 (4.2) | 197 (5.2) | 84 (14.1) | <0.0001 |
| Muscle pain, n (%) | 1234 (18.6) | 903 (23.8) | 331 (55.7) | <0.0001 |
| Joint pain, n (%) | 748 (11.3) | 522 (13.8) | 226 (38.1) | <0.0001 |
| Pain, n (%) | 448 (6.8) | 250 (6.6) | 198 (33.3) | <0.0001 |
| Feeling unwell, n (%) | 1652 (24.9) | 1252 (33.0) | 400 (67.3) | <0.0001 |
| Number of symptoms (median, interquartile range) | 2 (0–5) | 4 (2–6) | 8 (5–11) | <0.0001 |
Differences in prevalence of symptom between participants reporting mild and severe symptom burden tested using two-sided χ2-tests.
Factors associated with acute COVID-19 symptoms
Increasing age was associated with ≥6 symptoms (upper quartile) with the oldest adolescents having the highest odds (17 years old OR 1.28 (1.06;1.55)); 18 years old (OR 1.47 (1.23;1.76)) and reporting a severe symptom burden (17 years old OR 1.47 (1.09;1.98) 18 years old OR 1.61 (1.21;2.14)). Females were more likely to report ≥6 symptoms, (OR 1.88 (1.67;2.12)), and a severe symptom burden (OR 1.41 (1.18;1.68)).
Comorbidities including asthma, allergy and eczema were all associated with reporting both ≥6 symptoms and a self-reported severe symptom burden (Table 3). Likewise, OCD/anxiety/depression were associated with having ≥6 symptoms, (OR 1.67 (1.34;2.09)), and having a severe symptom burden (OR 1.74 (1.28;2.36)). Finally, Epstein-Barr virus infection earlier in life was a predictor of both reporting ≥6 symptoms and a severe symptom burden, (OR 1.48 (1.00;2.19)), and (OR 2.26 (1.40;3.63)) (Table 4). When applying the conservative Bonferroni approach to correct for multiple testing only age 18 and 18+, sex, asthma and OCD/anxiety/depression remained significant.
Table 4.
Factors associated with reporting number of symptoms in the upper quartile and severe acute symptom burden.
| Reporting number of symptoms in the upper quartile (≥6) | p-value | Self-reported severe symptom burden | p-value | |
|---|---|---|---|---|
| OR (95%CI)a | OR (95%CI)a | |||
| Age | ||||
| 15 years | 1.0 (ref) | 1.00 (ref) | ||
| 16 years | 1.08 (0.88;1.31) | 0.4755 | 1.14 (0.83;1.57) | 0.4163 |
| 17 years | 1.28 (1.06;1.55) | 0.0098 | 1.47 (1.09;1.98) | 0.0110 |
| 18 years | 1.47 (1.23;1.76) | <0.0001 | 1.61 (1.21;2.14) | 0.0011 |
| +18 | 1.40 (1.13;1.74) | 0.0022 | 1.98 (1.43;2.73) | <0.0001 |
| Sex | ||||
| Female vs. male | 1.88 (1.67;2.12) | <0.0001 | 1.41 (1.18;1.68) | 0.0002 |
| Asthma | ||||
| Yes vs. No | 1.39 (1.14;1.70) | 0.0012 | 1.52 (1.15;2.00) | 0.0034 |
| Allergy | ||||
| Yes vs. no | 1.23 (1.07;1.41) | 0.0042 | 1.64 (1.35;1.99) | <0.0001 |
| Eczema | ||||
| Yes vs. no | 1.37 (1.09;1.72) | 0.0073 | 1.57 (1.15;2.15) | 0.0049 |
| OCD/anxiety/depression | ||||
| Yes vs. no | 1.67 (1.34;2.09) | <0.0001 | 1.74 (1.28;2.36) | 0.0004 |
| Epstein-Barr virus | ||||
| Yes vs. no | 1.48 (1.00;2.19) | 0.0500 | 2.26 (1.40;3.63) | 0.0008 |
Logistic regression model with reporting number of symptoms in the upper quartile (≥6) and reporting severe symptom burden as outcomes. Each covariate is included separately in the model but adjusted for sex and age.
Acute symptoms by sex
Overall, more females reported mild and severe symptom burden and more males reported no symptoms (no symptoms: females 29.9% vs. males 39.3%, mild symptom burden: females 60.0% vs. 53.4%, severe symptom burden: females 10.1% vs. males 7.3%, p < 0.0001). In addition, females reported a higher burden of acute symptoms with a median of three (IQR 0–6) symptoms compared to two (IQR 2–4) in males, p < 0.0001. Among those reporting severe symptom burden the largest difference between sexes was seen for trouble breathing, 42.1% in males compared to 65.6% in females, p < 0.0001 and headache, 66.3% for males compared to 88.0% for females, p < 0.0001 (Table 5).
Table 5.
Differences in self-reported mild and severe symptom burden divided by sex
| COVID population – mild and severe symptoms divided by sex |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total population (n = 6630) n (%) |
p-valuea | Self-reported mild symptom burden (n = 3795) n (%) |
p-valuea | Self-reported severe symptom burden (n = 594) n (%) |
p-valuea | ||||
| Male (n = 2757) | Female (n = 3873) | Male (n = 1471) | Female (n = 2324) | Male (n = 202) | Female (n = 392) | ||||
| Fever | 721 (26.2) | 1047 (27.0) | 0.4236 | 586 (39.8) | 797 (34.3) | 0.0005 | 135 (66.8) | 250 (63.8) | 0.4600 |
| Cold | 716 (26.0) | 1223 (31.6) | <0.0001 | 600 (40.8) | 994 (42.8) | 0.2280 | 116 (57.4) | 229 (58.4) | 0.8163 |
| Sore throat | 772 (28.0) | 1291 (33.3) | <0.0001 | 645 (43.9) | 1032 (44.4) | 0.7357 | 127 (62.8) | 259 (66.1) | 0.4386 |
| Cough | 796 (28.9) | 1297 (33.5) | <0.0001 | 661 (44.9) | 1050 (45.2) | 0.8824 | 135 (66.8) | 247 (63.0) | 0.3571 |
| Stomach aches | 168 (6.1) | 400 (10.3) | <0.0001 | 121 (8.2) | 269 (11.6) | 0.0009 | 47 (23.3) | 131 (33.4) | 0.0105 |
| Rashes | 24 (0.9) | 81 (2.1) | <0.0001 | 20 (1.4) | 52 (2.2) | 0.0534 | <5 (2.0) | 29 (7.4) | 0.0063 |
| Trouble breathing | 275 (10.0) | 854 (22.1) | <0.0001 | 190 (12.9) | 597 (25.7) | <0.0001 | 85 (42.1) | 257 (65.6) | <0.0001 |
| Reduced sense of smell | 828 (30.0) | 1570 (40.5) | <0.0001 | 708 (48.1) | 1294 (55.7) | <0.0001 | 120 (59.4) | 276 (70.4) | 0.0070 |
| Reduced sense of taste | 719 (26.1) | 1343 (34.7) | <0.0001 | 603 (41.0) | 1086 (46.7 | 0.0005 | 116 (57.4) | 257 (65.6) | 0.0520 |
| Chest pain | 105 (3.8) | 288 (7.4) | <0.0001 | 61 (4.2) | 171 (7.4) | <0.0001 | 44 (21.8) | 117 (29.9) | 0.0362 |
| Head aches | 808 (29.3) | 1789 (46.2) | <0.0001 | 674 (45.8) | 1444 (62.1) | <0.0001 | 134 (66.3) | 345 (88.0) | <0.0001 |
| Vomiting | 41 (1.5) | 98 (2.5) | 0.0035 | 27 (1.8) | 49 (2.1) | 0.5587 | 14 (6.9) | 49 (12.5 | 0.0368 |
| Diarrhoea | 82 (3.0) | 199 (5.1) | <0.0001 | 61 (4.2) | 136 (5.9) | 0.0211 | 21 (10.4) | 63 (16.1) | 0.0600 |
| Muscle pain | 378 (13.7) | 856 (22.1) | <0.0001 | 278 (18.9) | 625 (26.9) | <0.0001 | 100 (49.5) | 231 (58.9 | 0.0285 |
| Joint pain | 218 (7.9) | 530 (13.7) | <0.0001 | 151 (10.3) | 371 (16.0) | <0.0001 | 67 (33.2) | 159 (40.6) | 0.0787 |
| Pain | 130 (4.7) | 318 (8.2) | <0.0001 | 72 (4.9) | 178 (7.7 | 0.0008 | 58 (28.7) | 140 (35.7) | 0.0864 |
| Feeling unwell | 544 (19.7) | 1108 (28.6) | <0.0001 | 420 (28.6) | 832 (35.8) | <0.0001 | 124 (61.4) | 276 (70.4) | 0.0263 |
| Number of symptoms (median, interquartile range) | 2 (0–4) | 3 (0–6) | <0.0001 | 3 (2–6) | 4 (2–7) | <0.0001 | 7 (4–10) | 9 (6–11) | <0.0001 |
Differences in prevalence of symptoms between sexes tested using two-sided χ2-tests.
Discussion
Key results
In this study, we investigated acute COVID-19 symptoms in Danish SARS-CoV-2 infected adolescents aged 15–18 years. Within four weeks after a positive test, one in three participants had no symptoms, the majority had a mild symptom burden, and one in ten had a self-reported severe symptom burden. Two thirds of the symptomatic participants reported a symptom duration of 1–10 days. The most frequent symptoms reported by approximately one third were headache, reduced senses of smell and taste, cough, and sore throat. Increasing age and comorbidities such as asthma, allergy, eczema, mental health disorders such as anxiety and depression, and previous Epstein-Barr virus infection were all associated with a worse symptom burden or having six or more symptoms. Overall, females reported significantly more symptoms compared to males.
Strength and limitations
The study is strengthened by a large, nationwide study population and access to the Danish national registers that include all PCR test results from the total Danish population. Furthermore, the use of a national, secure digital post-box reaching out to the whole population also strengthens it.
The study also has limitations. Comorbidity is self-reported and not medical record confirmed diagnoses. For previous Epstein Barr virus infection most often it is those most severely affected by the infection who get diagnosed which strengthens the validity of the data. The number of symptoms suggested to be related to COVID-19 is very high and is increasing over time14,20 and the list may not be complete as no international agreement exists regarding this yet.
It might have been challenging for the participants to discriminate between symptoms e.g., “muscular pain” and “pain”. We did not investigate patterns of symptoms and the ability to predict adverse outcomes in the present paper. It could be very relevant for future research to examine possible symptom clusters in the acute phase of SARS-CoV-2 infection.
The number of participants was high, but the response rate was only 27.3%. The response rate was slightly lower than in other studies of other diseases affecting adolescents,21 yet much higher than in recent studies investigating COVID-19 symptoms (less than 13.0%).14,22, 23, 24 Responders and non-responders were comparable in age, but males were slightly underrepresented among responders. In general, adult non-responders are believed to have a riskier health behavior compared to responders25 which might have affected results, but whether this applies for a group of young persons with SARS-CoV-2 infection is unclear. Our study does not provide us with data regarding sociodemographic variables or ethnicity, nor does is describe whether participants were in contact with the health care system. We are aware that this might jeopardize the generalizability. Comparison of the symptom burden between SARS-CoV-2 positive adolescents and adolescent with no previous positive test has been reported elsewhere. Here, we found that comorbidities between the controls and SARS-CoV-2 infected participants were fairly balanced, but with a tendency towards less comorbidities in the SARS-CoV-2 infected group.16
No information on hospitalizations among participants was available. According to the Danish COVID-19 surveillance at Statens Serum Institut, 83 adolescents aged 15–18 years were hospitalized for SARS-CoV-2 infection in Denmark during the entire study period, 5 were admitted to the intensive care unit.26 From the data it is not known whether SARS-CoV-2 was the primary reason for the admissions, or it was a secondary finding. Furthermore, we do not know if the 83 adolescents were responders or non-responders to our survey. However, as the hospitalized adolescents represent a very small proportion of the total population, it is less likely that the lack of this information affects the overall findings.
In general, SARS-CoV-2 infections are more common in males. In a meta-analysis of 5829 children and adolescents with SARS-CoV-2 infection 55% were males13 which is slightly more than in our study where 51% were males.
The assessment of symptoms and symptom burden (no symptoms, mild symptoms, and severe symptoms) was self-reported and subjective by nature. No criteria for their assessment were given. Furthermore, up to 12+ months retrospective recall for those infected in the beginning of the pandemic may have caused a risk of recall bias. Nevertheless, this new disease, COVID-19 has received an enormous amount of attention and personal experiences with the illness may have caused increased awareness of it in the individual.
Interpretation
Prevalence and severity
It is notable that one in three SARS-CoV-2 infected adolescents had no symptoms which is in line with a previous study.2 Importantly, the majority had mild symptom burden, and only one in ten adolescents with a SARS-CoV-2 infection perceived their symptom burden as severe. This is not the same as a severe case of the disease with hospitalisation and oxygen required.8 However, it suggests that adolescents can be burdened in the acute phase.
The most common acute symptoms differed between the adolescents reporting mild and severe symptom burdens. In agreement with previous studies we found that the most common symptoms, each reported by more than 30%, were sore throat, cough, reduced senses of smell and taste, and headache.4,13,14,20 Furthermore 20% experienced acute symptoms lasting more than two weeks which could potentially extend the contamination period. More data are needed to investigate the duration of e.g., fever and feeling unwell.
Associating factors
Age was associated with more symptoms and a more severe symptom burden. This was a pattern discovered early in the pandemic in the adult population27 and more recently among adolescents.14
COVID-19 causes respiratory symptoms and adolescents with asthma and allergy experience more symptoms and a more severe symptom burden.9 Previous register based research found that asthma was associated with hospitalization among children up to 16 years, OR 1.09 (95%CI 0.98;1.21).9
Anxiety and depression were also associated with severe symptom burden. There are reports that anxiety and depression rates have increased during the pandemic,28 which might support the connection. Perception of SARS-CoV-2 as being dangerous due to the massive media exposure could possibly increase the focus on symptoms in adolescents with anxiety and depression, and low mental resilience could perhaps increase the perception of the symptom burden.
We found previous Epstein-Barr virus infection to be associated with high symptom burden and more symptoms. Several factors could explain that. One relevant point could potentially be that the virus shares an immunological response pattern with COVID-19. Further, Epstein-Barr virus infection may be reactivated, as seen in adults with SARS-CoV-2 infection, which could explain the more severe symptom burden.18 Infection with Epstein-Barr virus is often asymptomatic and adolescents in our study that indicated they had Epstein-Barr virus in the past probably had a symptomatic infection. As such, these adolescents may have an immune function that allows symptomatic viral infections.
All symptoms, except fever, were most prevalent among females. It is noticeable that the only symptom that is measured objectively is similar between the sexes. It could point to the sensitivity of body signals and body awareness as influencing factors. Sex differences in perceived health and quality of life increase with age above 12 years and follow the imbalance of hormonal status, the prevalence of stressful life events and coping preferences. Female adolescents are reported to be generally more worried, sensitive, and concerned with their well-being.29, 30, 31
Generalisability
The pandemic is handled differently between countries. Denmark is a small Western country with six million citizens and a free health care service. Schools were open from March 2020 to June 2021 with varying periods of home-schooling. The population was advised to reduce social contact. Accessibility to testing moved from being by medical referral at the beginning of the pandemic to easily accessible walk-in centres from May of 2020. Throughout the pandemic, testing was free of charge. Furthermore, comorbidity and other relevant health factors such as nutrition and body weight may have influenced outcomes.
The results from this study represent the period in which the previous variants, e.g. alpha and delta were dominant, and therefore results might not be generalizable to children and adolescents infected with the recent omicron variant.
Conclusion
One in three SARS-CoV-2 infected adolescents aged 15–18 years had no symptoms and the majority reported mild symptom burden. Two out of three reported symptoms within the first four weeks with headaches, reduced sense of smell, cough, sore throat, and reduced sense of taste being most prevalent. Increasing age and comorbidities such as asthma and previous Epstein-Barr virus infection were related to a higher symptom burden. Females reported more symptoms than males.
Trial registration
Clinicaltrials.gov (NCT04786353).
Data sharing statement
Data will not be made available for others.
Contributors
SKB conceptualized the study with input from all the co-authors. SKB, SDN, UN and HB are the co-senior authors. AVC conducted the statistical analysis. PP and SKB wrote the first draft. All the authors provided critical scholarly feedback on the manuscript. All the co-authors approved the final version of the manuscript. SKB is the guarantor. SKB and AVC have directly assessed and verified the underlying data. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding
The study was funded by the AP Møller Foundation (2021-00661). The research presented was investigator initiated. The study funder played no role in the study.
Declaration of interests
SDN declares a research grant from Novo Nordic Foundation, a travel grant from Gilead, and that she is on the Advisory board for Gilead, GSK, MSD. None of the other authors has anything to declare.
Acknowledgments
We are thankful to the participants who took the time to participate in the survey. The Danish Departments of Clinical Microbiology (KMA) and Statens Serum Institut carried out the laboratory analysis, registration, and release of the national SARS-CoV-2 surveillance data for the present study.
References
- 1.Bhopal S.S., Bagaria J., Olabi B., Bhopal R. Children and young people remain at low risk of COVID-19 mortality. Lancet Child Adolesc Heath. 2021;5(5):e12–e13. doi: 10.1016/S2352-4642(21)00066-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li B., Zhang S., Zhang R., Chen X., Wang Y., Zhu C. Epidemiological and clinical characteristics of COVID-19 in children: a systematic review and meta-analysis. Front Pediatr. 2020;8 doi: 10.3389/fped.2020.591132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludvigsson J.F., Engerström L., Nordenhäll C., Larsson E. Open schools, covid-19, and child and teacher morbidity in Sweden. N Engl J Med. 2021;384(7):669–671. doi: 10.1056/nejmc2026670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dawood F.S., Porucznik C.A., Veguilla V., et al. Incidence rates, household infection risk, and clinical characteristics of SARS-CoV-2 infection among children and adults in Utah and New York City, New York. JAMA Pediatr. 2021 doi: 10.1001/jamapediatrics.2021.4217. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A., Rinaldi E., Zusi C., Beatrice G., Saccomani M.D., Dalbeni A. Coronavirus disease 2019 (COVID-19) in children and/or adolescents: a meta-analysis. Pediatr Res. 2021;89(4):733–737. doi: 10.1038/s41390-020-1015-2. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A., Rinaldi E., Zusi C., Beatrice G., Saccomani M.D., Dalbeni A. COVID-19 outbreak in children and/or adolescents. Pediatr Res. 2021 doi: 10.1038/s41390-021-01787-x. Published online. [DOI] [PubMed] [Google Scholar]
- 7.Chai S., Li Y., Li X., Tan J., Abdelrahim M.E.A., Xu X. Effect of age of COVID-19 inpatient on the severity of the disease: a meta-analysis. Int J Clin Pract. 2021;75(10) doi: 10.1111/ijcp.14640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsankov B.K., Allaire J.M., Irvine M.A., et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246–256. doi: 10.1016/j.ijid.2020.11.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kompaniyets L., Agathis N.T., Nelson J.M., et al. Underlying medical conditions associated with severe COVID-19 illness among children. JAMA Netw Open. 2021 doi: 10.1001/jamanetworkopen.2021.11182. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zachariah P., Johnson C.L., Halabi K.C., et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children's hospital in New York City, New York. JAMA Pediatr. 2020;174(10) doi: 10.1001/jamapediatrics.2020.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Issitt R.W., Booth J., Bryant W.A., et al. Children with COVID-19 at a specialist centre: initial experience and outcome. Lancet Child Adolesc Heal. 2020 doi: 10.1016/S2352-4642(20)30204-2. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Control C.D. When to seek emergency medical attention. Published 2021. Accessed December 3, 2021. https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/steps-when-sick.html
- 13.Cui X., Zhao Z., Zhang T., et al. A systematic review and meta-analysis of children with coronavirus disease 2019 (COVID-19) J Med Virol. 2021;93(2):1057–1069. doi: 10.1002/jmv.26398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molteni E., Sudre C.H., Canas L.S., et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health. 2021;5(10):708–718. doi: 10.1016/s2352-4642(21)00198-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berg S.K., Nielsen S.D., Nygaard U., et al. Long COVID symptoms in SARS-CoV-2-positive adolescents and matched controls (LongCOVIDKidsDK): a national, cross-sectional study. Lancet child Adolesc Health. 2022;4642(22):4–9. doi: 10.1016/S2352-4642(22)00004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.https://www.longcovidkids.org/. Published 2021. https://www.longcovidkids.org/
- 18.Simonnet A., Engelmann I., Moreau A.S., et al. High incidence of Epstein–Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infect Dis Now. 2021;51(3):296–299. doi: 10.1016/j.idnow.2021.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Onis M., Onyango A.W., Borghi E., Siyam A., Nishida C., Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu X., Zhang L., Du H., et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382(17):1663–1665. doi: 10.1056/nejmc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richards J., Wiese C., Katon W., et al. Surveying adolescents enrolled in a regional health care delivery organization: mail and phone follow-up - what works at what cost? J Am Board Fam Med. 2010;23(4):534–541. doi: 10.3122/jabfm.2010.04.100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephenson T., Shafran R., De Stavola B., et al. Long COVID and the mental and physical health of children and young people: national matched cohort study protocol (the CLoCk study) BMJ Open. 2021;11(8):1–5. doi: 10.1136/bmjopen-2021-052838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stephenson T., Shafran R., De Stavola B., et al. Long COVID - the physical and mental health of children and non-hsopitalised young people 3 months after SARS-CoV-2 infection; a national matched cohort study (The CLoCk) Study. doi:10.21203/rs.3.rs-798316/v1
- 24.Zimmermann P., Pittet L.F., Curtis N. How common is long COVID in children and adolescents? Pediatr Infect Dis J. 2021 doi: 10.1097/inf.0000000000003328. Publish Ah. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christensen A.I., Ekholm O., Gray L., Glümer C., Juel K. What is wrong with non-respondents? Alcohol-, drug- and smoking-related mortality and morbidity in a 12-year follow-up study of respondents and non-respondents in the Danish health and morbidity survey. Addiction. 2015;110(9):1507–1514. doi: 10.1111/add.12939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schønning K., Dessau R.B., Jensen T.G., et al. Electronic reporting of diagnostic laboratory test results from all healthcare sectors is a cornerstone of national preparedness and control of COVID-19 in Denmark. APMIS. 2021;129(7):438–451. doi: 10.1111/apm.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallo Marin B., Aghagoli G., Lavine K., et al. Predictors of COVID-19 severity: a literature review. Rev Med Virol. 2021 doi: 10.1002/rmv.2146. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi L., Lu Z.A., Que J.Y., et al. Prevalence of and risk factors associated with mental health symptoms among the general population in China during the coronavirus disease 2019 pandemic. JAMA Netw Open. 2020;3(7) doi: 10.1001/jamanetworkopen.2020.14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michel G., Bisegger C., Fuhr D.C., Abel T. Age and gender differences in health-related quality of life of children and adolescents in Europe: a multilevel analysis. Qual Life Res. 2009;18(9):1147–1157. doi: 10.1007/s11136-009-9538-3. [DOI] [PubMed] [Google Scholar]
- 30.Steinberg L., Morris A.S. Adolescent development. Annu Rev Psychol. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- 31.Patton G.C., Viner R. Pubertal transitions in health. Lancet. 2007;369(9567):1130–1139. doi: 10.1016/S0140-6736(07)60366-3. (London, England) [DOI] [PubMed] [Google Scholar]