Abstract
Aging and aging-related diseases have emerged as increasingly severe health and social problems. Therefore, it is imperative to discover novel and effective therapeutics to delay the aging process and to manage aging-related diseases. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs), one of the classes of antihyperglycemic drugs, have been recommended to manage type 2 diabetes mellitus (T2DM). Moreover, GLP-1 RAs have been shown to protect against oxidative stress, cellular senescence and chronic inflammation, which are widely accepted as the major risk factors of aging. However, their significance in aging or aging-related diseases has not been elucidated. Herein, we explain the underlying mechanisms and protective roles of GLP-1 RAs in aging from a molecular, cellular and phenotypic perspective. We provide novel insights into the broad prospect of GLP-1 RAs in preventing and treating aging-related diseases. Additionally, we highlight the gaps for further studies in clinical applications of GLP-1 RAs in aging-related diseases. This review forms a basis for further studies on the relationship between aging-related diseases and GLP-1 RAs.
Keywords: glucagon-like peptide-1, GLP-1 receptor agonists, aging, aging-related diseases
1.Introduction
Aging due to molecular and cellular damage decreases the physical and mental capacity in a time-dependent manner. It is a significant risk factor for the higher incidences of mortality and morbidity associated with various chronic diseases [1]. The aging population is increasing worldwide and should be addressed. The number of people over 60 years is 1 billion based on the World Health Organization (WHO). Moreover, it is estimated that individuals over 60 years may possess 22% of the world’s population (about 2.1 billion) by 2050 [2]. The prevalence of aging-related diseases is becoming a growing concern due to the prolonged life expectancy and rapidly aging population. Aging-related diseases, such as type 2 diabetes mellitus (T2DM), renal function decline, cancers, etc., significantly affect the health and life quality of the elderly [1]. Aging-related diseases pose a great challenge to medical care due to the high incidence of senile diseases, poor treatment effects, and high coexistence rates of multiple diseases, thus bringing a substantial economic burden worldwide [3]. Therefore, new treatment strategies and drugs are needed for the management of aging-related diseases.
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs), including native GLP-1 derivatives (albiglutide, dulaglutide, liraglutide, and semaglutide) and exendin-4 derivatives (exenatide and lixisenatide), trigger the release of insulin from the pancreas, inhibiting the secretion of glucagon from pancreatic α cells. Besides, GLP-1 RAs are used for the clinical treatment of T2DM [4]. Table 1 summarizes the pharmacokinetic and toxicological features of GLP-1 RAs. Besides their strong hypoglycemic effects, GLP-1 RAs can significantly reduce the risk of hypoglycemia and lower lipid levels, maintain blood pressure, and enhance cardiovascular protection and renal protection [5-9]. Therefore, GLP-1 RAs can be used to reduce polypharmacy in older adults, especially those with multimorbidity [5, 10]. A recent study showed that GLP-1 RAs might protect against chronic inflammation, oxidative stress, cellular senescence, etc., all of which are the major risk factors of aging [11-14]. Furthermore, various clinical trials have demonstrated that GLP-1 RAs play important roles in delaying and treating aging-related diseases.
Table 1.
Pharmacokinetic and toxicological features of GLP-1 Ras.
| Agents | Liraglutide | Dulaglutide | Albiglutide | Semaglutide | Exenatide | Lixisenatide |
|---|---|---|---|---|---|---|
| Subcutaneous | Oral | Normal | Extended release | |||||
| T1/2 | 13h | 5d | 5d | 1w | 2.4h |/ | 3h |
| Tmax | 8-12h | 24-72h | 3-5d | 1-3d | 1h | 2.1h | 2w, 6-7w | 1-3.5h |
| Bioavailability | 55% | 65%, 47% | / | 89%, 0.4%-1% | / | / |
| Protein binding | >98% | / | / | >99% | / | / |
| Volume of distribution | 13L, 20-25L | 19.2L, 17.4 L | 11L | 12.5L, 8L | 28.3L | 100L |
| Metabolism | A similar manner to large proteins without a specific organ as a major route of elimination | May be degraded into its component amino acids by general protein catabolism pathways |
May be catabolized primarily in the vascular endothelium | Proteolytic cleavage of the peptide backbone and sequential beta-oxidation of the fatty acid sidechain |
May through glomerular filtration and proteolytic degradation. | May through glomerular filtration and proteolytic degradation |
| Excretion | urine 6%, feces 5% | / | / | urine 3%, feces/ | / | / |
| Effects of age on pharmacokinetics | None | None | None | None | None | None |
| Most common side effects | headache, nausea, diarrhea |
nausea, abdominal pain, diarrhea, vomiting, decreased appetite | upper respiratory tract infection, back pain, diarrhea, joint pain, nausea, inflammation of the sinuses, reactions at injection site, flu symptoms, cough |
nausea, vomiting, diarrhea, stomach pain, constipation |
nausea, feeling jittery, indigestion, vomiting, dizziness, constipation, diarrhea, headache, weakness a bump at the injection site, nausea |
nausea, vomiting, headache, diarrhea, feeling dizzy |
This article reviews the function of GLP-1 RAs in delaying aging and ameliorating aging-related diseases, thus providing a theoretical basis and scientific guidance for clinical application of GLP-1 RAs.
2.Aging-related changes from different perspectives
Aging is highly complex progress associated with many biological and pathological changes. This part elucidates the underlying alterations and mechanisms in aging from different perspectives (Fig. 1).
Figure 1.
The mechanisms of aging.
2.1 Aging-related molecular and cellular changes
Aging is associated with complex molecular and cellular changes (Fig. 2). Several biological hallmarks representing common aging characteristics, including epigenetic alteration, genomic instability, etc., have been systematically and comprehensively elucidated [1]. Genomic instability is caused by an imbalance between DNA damage and repair. Aging-related genomic damage is caused by both extrinsic and intrinsic factors [15]. Telomeres, repetitive DNA sequences, shorten every time cells divide. DNA damage signaling pathways and cellular senescence are triggered when telomeres are very short [16]. Epigenetic alterations, such as histone acetylation, chromatin remodeling, and DNA methylation, influence gene expression and genomic integrity and promote aging [17]. Non-coding RNAs mediate epigenetic modifications that may regulate aging and aging-related diseases [18]. The balance among protein synthesis, degradation and folding is key to maintaining protein homeostasis and thus ensuring longevity. Therefore, the loss of protein homeostasis leads to aging and diseases [19-21]. Moreover, insulin-like growth factor 1 (IGF-1), mammalian target of rapamycin (mTOR), adenosine 5’-monophosphate activated protein kinase (AMPK), and sirtuins-mediated metabolic signaling pathways are associated with the balance between nutrient anabolism and catabolism in cells, which maintain cellular homeostasis and play essential roles in aging [1, 22, 23]. Mitochondria are generally considered to be sources of cytotoxic reactive oxygen species (ROS). Many studies have suggested mitochondrial dysfunction is related to aging. Nevertheless, mitochondrial ROS are not always harmful and can even extend lifespan in mammals [24]. Therefore, the roles and mechanisms of mitochondrial dysfunction in aging should be further explored. Cellular senescence is characterized by secretory phenotype (SASP), macromolecular damage, altered metabolism, and irreversible cell-cycle withdrawal. It is involved in various biological processes and aging-related diseases [25]. Cellular senescence can be regarded as the positive compensatory response to injury. However, it may become detrimental and accelerate the aging process when the regenerative capacity of tissues is depleted [1]. Stem cell exhaustion causes a decline in tissue regeneration and significantly impacts the aging process. Moreover, the aging-related physiological decline can be protected by reducing stem cell senescence in the hypothalamus [26]. Furthermore, aging is often associated with changes in cell-to-cell communication, which are features of aging-related diseases, including Alzheimer’s disease (AD) and Parkinson’s disease (PD) [27]. Altogether, these biological hallmarks provide a better insight into the molecular and cellular changes in aging, building a framework for future studies.
Figure 2.
Molecular and cellular changes in aging.
2.2 Aging-related phenotypic changes
Molecular, phenotypic, and functional hierarchical domains establish the interlaced time and hierarchical relationship of aging. Aging is not a single cell death but a collection of phenotypes that respond to specific stimuli and follow particular dynamics. These specific aging processes are reflected in the physiological and pathological consequences of aging phenotypes. The elderly is predisposed to sarcopenia, osteoporosis, osteoarthritis, and fractures due to the progressive decline of muscle mass and strength, bone mineral density (BMD), and joint mobility. Endocrine imbalance and autonomic disorders cause dizziness, nausea, anxiety, and insomnia. Older people may also experience memory loss, mental retardation, cognitive impairment, and motor coordination deficits due to a gradual decline in the number and activity of neurons. Aging also increases arterial stiffness and decreases arterial elasticity and baroreceptor reflex sensitivity in the cardiovascular system, thus increasing blood pressure. Moreover, aging reduces the elasticity of the chest wall and lung tissue and pulmonary ventilation function in the respiratory system. Atrophy degeneration of the respiratory mucosa inhibits the removal of foreign bodies and bacterial defense, making individuals prone to respiratory infections. Aging is associated with poor digestion and absorption of nutrients, mainly due to tooth loss or loosening and atrophy of gastrointestinal mucosal. Many older adults suffer from constipation due to poor intestinal peristalsis. Renal aging is often accompanied by structural changes in the glomerulus, tubules, interstitium, and vasculature, and functional changes, including a decline in the estimated glomerular filtration rate (eGFR). These changes cause urinary disorders such as urinary frequency, urinary incontinence, or nocturia.
Herein, aging-related phenotypic changes, a multifaceted decline in histological structure and organismal function, and the corresponding susceptibility to aging-related diseases have been described.
3. The roles of GLP-1 RAs in aging
GLP-1 RAs play crucial roles in aging. Activation of the GLP-1 receptor will enhance the DNA repair through the stimulation of apurinic/apyrimidinic endonuclease 1 (APE1) expression [28]. GLP-1 alleviates H2O2-induced senescence, modulates the antioxidant defense system, and attenuates cellular senescence and DNA damage caused by a series of oxidative stress [11, 29]. GLP-1 also has a neuroprotective effect, promoting DNA repair in neurodegenerative diseases [29, 30]. Additionally, liraglutide can ameliorate mitochondrial dysfunction via the cyclic AMP (cAMP)/PKA pathway [31]. Exenatide can correct mitochondrial energy crisis, improve mitochondrial morphology, and normalize mitochondrial dynamics [32]. Liraglutide also safeguards cardiomyocytes against mitochondrial dysfunction mainly caused by interleukin-1β. In H9c2 cardiomyoblasts, activating the GLP-1 receptor can significantly suppress methylglyoxal-induced mitochondrial dysfunctions [33, 34]. Moreover, activation of the GLP-1 receptor protects against apoptosis and inhibits ROS production and inflammatory reaction through acting Sirt1 [30, 35]. GLP-1 protects cell apoptosis via the PI3K/Akt/mTOR signaling pathway [36]. Liraglutide protects β cells apoptosis through the AMPK/mTOR signaling pathway [37]. Liraglutide also ameliorates inflammation through the mTORC1 signaling pathway [38]. Furthermore, hepatocyte steatosis can also be inhibited by GLP-1 through inducing the response of unfolded protein [39]. DPP4-GLP-1 axis modulates cellular senescence through AMPK/SIRT1/FOXO3a pathway [14]. Collectively, these findings demonstrate a range of anti-aging effects of GLP-1 RAs.
4. The roles of GLP-1 RAs in aging-related diseases
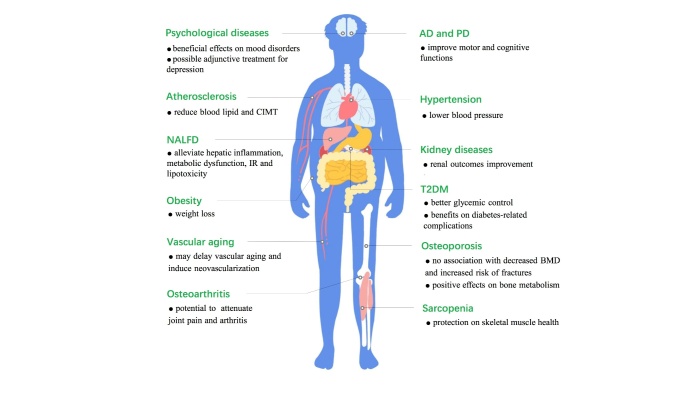
Mounting evidence has shown that GLP-1 RAs are involved in various aging-related diseases. These include metabolic, neurodegenerative, cardiovascular, kidney, degenerative musculoskeletal diseases (Fig. 3). This section discusses the protective roles of GLP-1 RAs in aging-related diseases in cultured cells and animal models (Table 2 and 3). It focuses on the roles and clinical evidence of GLP-1 RAs in aging-related diseases in middle-aged and aged patients (Table 4).
Figure 3.
The roles of GLP-1 RAs in aging-related diseases.
Table 2.
The roles of GLP-1 RAs in aging-related diseases in cultured cells.
| Agents | Diseases | Cell types | Dosing | Duration | Effects | Ref. |
|---|---|---|---|---|---|---|
| GLP-1 | Osteoporosis | Saos-2, TE-85, MG-63 cell lines | / | / | GLP-1 increases the viability levels of MG-63 and TE-85 osteoblastic cell lines. | [72] |
| GLP-1 | Osteoporosis | Saos-2, TE-85 cell lines | / | / | GLP-1 affects bone metabolism possibly through the ATP-induced c-Fos activation. | [73] |
| GLP-1 | Osteoporosis | ADSCs | 10, 100 nM | / | GLP-1 stimulates osteoblast differentiation in ADSCs via ERK signaling pathway, whereas it inhibits adipocyte differentiation. | [75] |
| exendin-4 | Alzheimer’s disease | SH-SY5Y cells, Primary neurons |
0, 50, 100, 200, 500 nM | 2h | Exendin-4 ameliorates the toxicity of Aβ and oxidative challenge in primary neuronal cultures and human SH-SY5Y cells in a concentration-dependent manner. | [89] |
| exenatide | Alzheimer’s disease | Brain ECs | 5nmol/kg/day | 4-5w | Exenatide strongly reverses aged mouse brain EC transcriptomic changes and BBB leakage. | [90] |
| GLP-1 exendin-4 |
Parkinson’s disease | SH-SY5Y cells | / | / | GLP-1 and exendin-4 stimulates cell proliferation and increased cell viability mainly via the PKA and PI3K signaling pathways. | [99] |
| GLP-1 exendin-4 |
Vascular aging | Human umbilical vein ECs | 10 nmol/L | 30min | GLP-1 prevents ROS-induced human umbilical vein endothelial cell senescence through the activation of PKA. | [11] |
| exendin-4 | Vascular aging | VSMCs | / | / | Inhibiting Rac1 activation via a cAMP/PKA-dependent pathway and activating Nrf2 contribute to the protective effects of exendin-4 against ANG II-induced senescence in VSMCs. | [104, 105] |
| liraglutide | Atherosclerosis | Human THP-1 macrophages, bone marrow-derived macrophages |
250 nmol/l | 6h | Liraglutide decreases inflammatory response in MΦ0 THP-1 macrophages and bone marrow-derived macrophages | [117] |
| liraglutide | Atherosclerosis | VMSCs | 100 nM / 1 μM | 120h | Liraglutide may inhibit Ang II-induced VSMC proliferation by activating AMPK signaling and inducing cell cycle arrest, thus delaying the progression of atherosclerosis | [120] |
| exendin-4 | Hypertension | LLC-PK1 cell line | 1nM | 30min | Exendin-4 regulates Na+/H+ exchanger NHE3 in renal proximal tubule cells. | [134] |
| exendin-4 | Kidney diseases | HK-2 cells | 0, 0.1, 1, 10, 100?nM | 48h | Exendin-4 ameliorates high glucose-induced fibrosis by inhibiting the secretion of miR-192 from injured renal tubular epithelial cells. | [13] |
| exendin-4 | Kidney diseases | MCs | 0.1, 1, 10, 100nM | 12,24,48 h | Exendin-4 alleviates high glucose-induced rat MC dysfunction through the AMPK pathway. | [147] |
| liraglutide | Osteoarthritis | Chondrocytes | 100/500nM | 24h | Liraglutide protects chondrocytes against endoplasmic reticulum stress and apoptosis induced by IL-1β or TGs. | [166] |
| liraglutide | Osteoarthritis | Human primary chondrocytes | 50/100 nM | 24h | Liraglutide suppresses TNF-α-induced degradation of extracellular matrix in human chondrocytes. | [167] |
| liraglutide | Sarcopenia | C2C12 cells | 1μM | / | Liraglutide induces myogenesis in C2C12 myoblasts via a cAMP-dependent complex network of signaling events. | [168] |
| exendin-4 | Sarcopenia | C2C12 cells | 20 nM | 60min/6h | Exendin-4 suppresses the expression of MSTN and muscle atrophic factors such as atrogin-1 and MuRF-1 in Dex-treated C2C12 myotubes. | [169] |
ADSCs: adipose-derived stem cells; ANG: angiotensin; atrogin-1: F-box only protein 32; BBB: blood brain barrier; Dex: dexamethasone; ECs: endothelial cells; MCs: mesangial cells; MSTN: myostatin; MuRF-1: muscle RING-finger protein-1; TGs: triglycerides.
Table 4.
The roles of GLP-1 RAs in aging-related diseases in clinical trials.
| Agents | Type of study | Sample information | Dosing | Findings | Ref. |
|---|---|---|---|---|---|
| T2DM | |||||
| lixisenatide | phase I, single-centre, open-label study | 18 (≥65)/ 18 (18-45) |
20 µg a single |
Mean exposure and the terminal half-life was higher in elderly subjects; C(max), t(max) and adverse events were comparable in both groups | [50] |
| lixisenatide | pooled analysis | 2565(<65)/ 623 (≥65) |
20 µg, 12/24 m once daily |
Lixisenatide significantly reduced HbA1c vs placebo in all age groups | [50] |
| lixisenatide plus OADs | meta-analysis | 501 (≥65) |
20 µg, 24 w once daily |
Lixisenatide plus OADs improved glycemic control | [51] |
| lixisenatide plus basal insulin | post hoc analysis | 108 (≥70) |
20 µg, 24 w once daily |
Lixisenatide significantly reduced HbA1c, 2-hour PPG, average seven-point SMPG, and body weight | [52] |
| oral semaglutide | review | / | / | Age did not affect glycemic efficacy of oral semaglutide | [53] |
| GLP-1 RAs | retrospective analysis | 90094 (≥66) |
/ | GLP-1RAs had similar MACE risk, increased HHF risk, and decreased risk of DKA, LLA, and genital infections vs SGLT2i | [54] |
| GLP-1 RAs | systematic review and meta-analysis | 93502 (≥65) |
/ | GLP-1 RAs reduced MACE | [55] |
| GLP-1 RAs plus SGLT2i | observational, prospective, multicenter study | 113 (>65) |
/ | Combination therapy was tolerated well and reduced A1C level, body weight and SBP | [56] |
| Obesity | |||||
| semaglutide | STEP 2 trial | 1210 (55.3±10.6) |
2.4/1.0 mg, 14 w once weekly |
Semaglutide (2.4mg) was associated with significant reduced bodyweight and higher incidence of adverse events | [6] |
| liraglutide | double-blind study | 68 (58±8) |
0.6-1.8 mg, 14 w once daily |
Liraglutide plus calorie restriction significantly reduced weight and improved IR, SBP, glucose, and TG | [70] |
| liraglutide | perspective case series | 9 (68.22±3.86) |
3.0 mg, 24 w once daily |
Liraglutide was associated with decrease in BMI, weight, fat mass and android fat | [71] |
| Osteoporosis | |||||
| exenatide | clinical trial | 69 (59 ± 8) | /, 44 w,/ | Exenatide had no adverse effects on BMD | [79] |
| GLP-1 RAs | cohort study | 79964 (Mean: 55) |
/ | GLP-1 RAs were not significantly associated with increased risk for fracture | [81] |
| GLP-1 RAs | meta-analysis | 4255 (56.4 ± 1.9) |
/ | GLP-1RA did not modify the risk of bone fracture | [82] |
| Alzheimer’s disease | |||||
| liraglutide | RCT | 18(Mean:63.1)/ 20(Mean:66.6)/ 6(63±3) |
0.6-1.8mg, 26 w once daily |
Liraglutide significantly raised blood-brain glucose transfer and prevented the decrease of CMRglc | [91] |
| dulaglutide | exploratory analysis of the REWIND trial | 9901 (≥50 y) |
1.5 mg, median:5.4 y once weekly |
Long-term use of dulaglutide may reduce cognitive impairment | [92] |
| liraglutide | RCT | 38 (50-80) |
0.6-1.8 mg, 26 w once daily |
Liraglutide was associated with numerical increase in CMRglc, but no influence on in Aβ levels and cognitive scores | [93] |
| exenatide | RCT | 21 (> 60) |
5-10µg, 12 w twice daily |
No differences in cortical thickness and volume, cognitive measures, or biomarkers between exenatide groups and placebo | [94] |
| liraglutide | RCT | 43 (45-70) |
0.6-1.8 mg, 12 w once daily |
No detectable cognitive differences were found between liraglutide groups | [95] |
| Parkinson’s disease | |||||
| exenatide | RCT | 20(61.4±6.0)/ 24 (59.4 ±8.4) |
5-10μg, 12 m twice daily |
Exenatide group showed a significant improvement in motor and cognitive functions, and these improvements persist for a long period after exenatide withdrawal | [100, 101] |
| exenatide | RCT | 31(61.6±8.2)/ 29 (57.8 ±8.0) |
2 mg, 48 w once weekly |
Exenatide group showed improvements in practically defined off-medication motor scores | [102] |
| Atherosclerosis | |||||
| liraglutide | clinical trial | 64 (63 ± 8) |
0.6-1.2 mg, 8 m once daily |
Liraglutide reduced TC, TG, LDL-C, and CIMT, whereas increased HDL-C | [7] |
| liraglutide | clinical trial | 29(61 ± 10)/ 29 (61 ± 8) |
0.6-1.2 mg, 8 m once daily |
Liraglutide significantly reduced CIMT in patients with T2DM and NAFLD, but not in T2DM patients without NAFLD | [121] |
| liraglutide | clinical trial | 121 (62 ± 9) |
0.6-1.2 mg, 18 m once daily |
Liraglutide significantly reduced waist circumference, BMI, fasting glycemia, HbA1c, TC, LDL-C, TG, and CIMT | [122] |
| Hypertension | |||||
| liraglutide | LEADER trial | 9340 (≥50) |
1.8mg, median:3.8 y once daily |
Liraglutide decreased SBP by 1.2mmHg | [8] |
| semaglutide | SUSTAIN-6 trial | 3297 (64.6±7.4) |
0.5/1.0 mg, 104 w once weekly |
The mean SBP in the semaglutide group was 1.3 mm Hg and 2.6 mm Hg lower in the group receiving 0.5 mg and 1.0 mg vs placebo, respectively | [125] |
| oral semaglutide | PIONEER 6 trial | 3183 (≥50) |
14mg, median:15.9 m once daily |
The mean SBP in the oral semaglutide group was 2.6 mmHg lower | [126] |
| exenatide and liraglutide | meta-analysis | 5860 (Mean: 55) |
/ | Exenatide and liraglutide reduced SBP and DBP by 1 to 5 mmHg vs some other hypoglycemic agents | [127] |
| GLP-1 RAs | systematic review and meta-analysis | 26654 (Average:55.88) |
/ | GLP-1RAs were associated with modest reduction on BP, but no significant association with hypertension | [128] |
| Kidney diseases | |||||
| liraglutide | LEADER trial | 9340 (≥50) |
1.8mg, median:3.8 y once daily |
Liraglutide was associated with significant reduction in new-onset severely increased albuminuria and lower rates of DKD events, but no association with increased risk of AKI | [8, 9] |
| exenatide plus insulin glargine | clinical trial | 92 (56.0± 8.4) |
5-10μg, 24 w twice daily |
Exenatide plus insulin glargine significantly reduced albuminuria in patients with T2DM and DKD | [149] |
| dulaglutide | AWARD-7 trial | 577 (64.7±8.8)/(64.7±8.6)/(64.3±8.4) |
0.75/1.5mg, 52 w once weekly |
Dulaglutide was associated with less eGFR decline vs insulin glargine | [151] |
| semaglutide | post-hoc analysis of the SUSTAIN 1-7 trials | 8416 (Mean: 53.7-64.6) |
0.5/1.0 mg, 30-104 w once weekly |
Semaglutide was associated with initial reductions in eGFR and marked reductions in UACR, but no association with increased risk of kidney adverse events | [152] |
| dulaglutide | exploratory analysis of the REWIND trial | 9901 (≥50) |
1.5 mg, median: 5.4 y once weekly |
Long-term use of dulaglutide reduced composite renal outcomes in people with T2DM | [153] |
| GLP-1RAs | systematic review and meta-analysis | 56004 (Mean: 60-66) |
/ | GLP-1RAs conferred a reduction in a broad composite kidney outcome | [154] |
| GLP-1RAs | Scandinavian cohort study | 38731 (59±10) |
/ | GLP-1 RAs reduced risk of serious renal events vs DPP4i | [155] |
| GLP-1RAs | systematic review and meta-analysis | 77242 (Mean: 60-65) |
/ | GLP-1 RAs had a less marked effect on preventing hospitalization for progression of kidney disease vs SGLT2i | [157] |
| Sarcopenia | |||||
| liraglutide | perspective case series | 9 (68.22±3.86) |
3.0 mg, 12/24 m once daily |
Liraglutide was associated with an improvement in SMI | [71] |
| NAFLD | |||||
| semaglutide | RCT | 320 (Mean: 55) |
0.1/0.2/0.4 mg, 72 w once daily |
Semaglutide resulted in a significantly higher percentage of patients with NASH resolution vs placebo | [172] |
AKI: acute kidney injury; DBP: diastolic blood pressure; DKD: diabetic kidney disease; BMD: Becker muscular dystrophy; BMI: body mass index; DPP4i: dipeptidyl peptidase 4 inhibitors; CIMT: constraint-induced movement therapy; CMRglc; cerebral metabolic rate for glucose; DKA: diabetic Ketoacidosis; eGFR: epidermal growth factor receptor; GLP-1 RAs: GLP-1 receptor agonists; HbA1c: glycated hemoglobin/hemoglobin A1c; HDL-C: high-density lipoprotein cholesterol; HHF: hospitalization for heart failure; IR: insulin resistance; LDL-C: low-density lipoprotein cholesterol; LLA: lower-limb amputations; MACE: major adverse cardiovascular events; NAFLD: non-alcoholic fatty liver disease; NASH: non-alcoholic steatohepatitis; OADs: oral antidiabetic drugs; PPG: prandial plasma glucose; RCT: randomized controlled trial; SBP: systolic blood pressure; SGLT2i: sodium-glucose cotransporter 2 inhibitors; SMPG: self-measured plasma glucose; T2DM: type 2 diabetes mellitus; TC: total cholesterol fasting blood glucose; TG: triglycerides; UACR: urinary albumin-creatinine ratio
4.1 The roles of GLP-1 RAs in aging-related metabolic diseases
Aging is associated with many aging-related metabolic diseases, which cause disability and death. Compelling evidence has substantiated on the roles of GLP-1 RAs in aging-related metabolic diseases, such as T2DM, obesity, and osteoporosis.
4.1.1 GLP-1 RAs and T2DM
Aging impairs β-cell function and reduces insulin sensitivity and secretion, thus predisposing T2DM development in the elderly [40, 41]. About 135.6 million people aged 65 to 99 years have diabetes globally, based on the latest data from the 9th edition of the Diabetes Atlas by the International Diabetes Federation (IDF). Moreover, the number is expected to increase to 195.2 million by 2030 and 276.2 million by 2045 [42]. GLP-1 levels can also be reduced by aging in a fasting and glucose-stimulated state [43]. However, GLP-1 can reverse the age-associated impairment of insulin sensitivity and glucose tolerance [44, 45]. GLP-1 stimulates pancreatic cell proliferation and differentiation, thus improving pancreatic β-cell function in elderly rodents [46, 47]. Exendin-4 enhances the secretion of insulin from adult human pancreatic β cells [48]. Moreover, GLP-1 therapy improves pulsatile insulin secretion in elderly diabetic patients [49].
Table 3.
The roles of GLP-1 RAs in aging-related diseases in animal models.
| Agents | Models | Diseases | Dosing | Duration | Effects | Ref. |
|---|---|---|---|---|---|---|
| GLP-1 | Wistar rats | T2DM | 1.5 pmol/kg/min, s.c. |
48 h | GLP-1 treatment can increase pancreatic insulin, GLUT2, and glucokinase mRNA in the old rats. | [44] |
| GLP-1 | Wistar rats | T2DM | 0.05, 0.1, 0.2, 0.4, 0.5 nmol/kg, IVGTT |
-5, 0, 2, 4, 7, 10, 15, 20, 30 min | GLP-1 restores the acute insulin response to glucose and increases the clearance of glucose in the old animals. | [45] |
| GLP-1 | Normal male mice | T2DM | 25 nmol/kg/day, i.p. |
12 d | GLP-1 counters aspects of the age-related impairment of pancreatic β-cell function and insulin sensitivity. | [46] |
| GLP-1 | Wistar rats | T2DM | 1.5 pmol/kg/min, s.c. |
2 or 5 d | GLP-1 causes an up-regulation of PDX-1 expression in islets and total pancreas, induces pancreatic cell proliferation, and β-cell neogenesis. | [47] |
| exendin-4 | NSG mice | T2DM | 24 nmol/kg/day, s.c. |
/ | Exendin-4 stimulates insulin secretion by both juvenile and adult human β cells. | [48] |
| GLP-1 | Adult male rats | Obesity | / | / | Intracerebroventricular (ICV) GLP-1 powerfully inhibits feeding in fasted rats. | [61] |
| exenatide | C57BL/6J mice | Obesity | 24 nmol/kg, i.p. |
8 w | Exenatide promotes brown remodelling of WAT in a SIRT1-dependent manner. | [66] |
| GLP-1 liraglutide exenatide |
CD-1 mice, SD rats, cynomolgus monkeys |
OP | / | / | GLP-1 RAs stimulate calcitonin release, up-regulation of calcitonin gene expression, and subsequently C-cell hyperplasia in rodents. | [78] |
| liraglutide | SAMP8 mice | AD | 100 or 500 g/kg/day, s.c. |
4 m | Liraglutide delays the progressive decline in memory function associated with hippocampal neuronal loss. | [86] |
| liraglutide | APP/PS1 mice | AD | 25 nmol/kg | 8 w | Liraglutide reduces inflammation response, β-amyloid plaque count, dense-core plaque numbers, soluble amyloid oligomers levels, prevents memory impairments, synapse loss and deterioration of synaptic plasticity, and increases young neurons numbers. | [87] |
| liraglutide | APP/PS1 mice | AD | 25 nmol/kg, i.p. | 2 m | Liraglutide can reverse some of the key pathological hallmarks of AD and prevents the progression of it. | [88] |
| exendin-4 | 3xTg-AD mice | AD | 3.5 pM/kg/min, s.c. | 16w | Exendin-4 reduces brain levels of tau, Aβ protein precursor and Aβ in STZ 3xTg-AD mice. | [89] |
| liraglutide lixisenatide |
C57/BL6 mice | PD | 2.5, 25, 250 nmol/kg, i.p./ lixisenatide (25 nmol/kg, i.p.) |
5min,30min,3h/ 3week |
Liraglutide and lixisenatide can cross the BBB and enhance neurogenesis. | [97] |
| NLY01 | C57BL6, hA53T α-synuclein transgenic, TLR-2 KO mice |
PD | 3 mg/kg s.c., twice weekly |
/ | NLY01 exerts neuroprotective effects via the direct prevention of microglial mediated conversion of astrocytes to an A1 neurotoxic phenotype. | [98] |
| exenatide | ApoE-/-mice | Vascular aging | 5 µg/kg s.c., twice daily |
12w | Exenatide ameliorated vascular aging induced by high-fat diet. | [106] |
| exenatide | C57BL/6J mice | Vascular aging | 5 μg/kg/day, s.c. | 14d | Exenatide prevents vascular senescence. | [108] |
| liraglutide | ApoE-/-mice | Atherosclerosis | 300 µg/kg, s.c. twice daily |
4w | Liraglutide inhibits progression of atherosclerotic plaque formation and enhances plaque stability. | [113] |
| liraglutide | ApoE-/- mice | Atherosclerosis | 0.4 mg/kg/day, s.c. | 9w | Liraglutide ameliorates atherogenesis through reducing serum AGEs levels and RAGE. | [114] |
| exendin-4 | C57BL/6J mice | Atherosclerosis | 300 pmol/kg/day 24 nmol/kg/day, s.c. |
28d | Exendin-4 reduces monocyte/macrophage accumulation in the arterial wall by inhibiting the inflammatory response in macrophages. | [116] |
| liraglutide | ApoE-/- mice | Atherosclerosis | 300 μg/kg/day, s.c. | 6 or 4 w | Liraglutide regulates immune cell phenotypes in early and preestablished atherosclerosis. | [117, 118] |
| lixisenatide liraglutide |
Apoe -/-Irs2 +/- mice | Atherosclerosis | lixisenatide (10 μg/kg/day, s.c) liraglutide (400 μg/kg/day, s.c) |
1 m | Lixisenatide decreases atheroma plaque size and instability by reprogramming macrophages towards an M2 phenotype. | [119] |
| liraglutide | ApoE-/-mice | Atherosclerosis | 400 μg/day, s.c. | 4 w | Liraglutide suppresses atherosclerotic lesions and increases AMPK phosphorylation in the aortic wall. | [120] |
| GLP-1 | SD rats | Hypertension | 30 pmol/kg/min, IVGTT | 120 or 30min | GLP-1 acutely recruits microvasculature and increases basal glucose uptake in muscle via a NO-dependent mechanism | [129] |
| liraglutide | C57BL/6 mice | Hypertension | 27or30 μg/kg, i.p. twice daily |
/ | Liraglutide promotes vasorelaxation by inducing the secretion of ANP. | [130] |
| GLP-1 | Wistar rats | Hypertension | 1 μg/kg·min, i.p. | 60min | GLP-1 can exert direct effects on relaxing rat conduit arteries, independently of NO and the endothelium. | [133] |
| liraglutide | SHR, WKY rats | Hypertension | 0.9?µg/3?µl/day, i.v. | 15d | Liraglutide attenuates the progression of hypertension in SHR through activating brainstem DBH neurons and suppressing sympathetic nerve activity. | [136] |
| liraglutide | STZ diabetic rats | Kidney diseases | 0.3 mg/kg/12 h, s.c. | 4w | Liraglutide against oxidative stress and diabetic nephropathy via a PKA-mediated inhibition of renal NAD(P)H oxidase. | [12] |
| liraglutide | Wistar rats | OA | 50?μg/kg/day, s.c. | 28d | Liraglutide ameliorates inflammation through the activation of the PKA/CREB pathway in OA rats. | [165] |
| liraglutide | SD rats | Sarcopenia | 200µg/kg, s.c. twice daily | / | Liraglutide ameliorates skeletal muscle atrophy in rodents. | [168] |
| exendin-4 dulaglutide |
C57BL/6, DBA/2J-mdx mice | Sarcopenia | exendin-4 (100 ng/day, s.c.) dulaglutide (1 mg/kg/week, s.c) |
exendin-4(8w) dulaglutide(3w) |
GLP-1 RAs ameliorate muscle wasting by suppressing MSTN and muscle atrophic factors and enhancing myogenic factors. | [169] |
AGEs: advanced glycation end products; DBH: dopamine beta-hydroxylase; MSTN: myostatin; OA: osteoarthritis; OP: osteoporosis; RAGE: receptor for advanced glycation end products; SD: Sprague-Dawley; SHR: spontaneously hypertensive rats; STZ: streptozotocin; WAT: white adipose tissue; WKY: Wister Kyoto rats
Lixisenatide is one of the most suitable GLP-1 RAs used for T2DM pathophysiology therapy in the elderly. A pooled analysis conducted on data from six GetGoal trials suggested that lixisenatide can effectively treat elderly patients with T2DM [50]. Moreover, lixisenatide, as an add-on to oral antidiabetics (OADs), can significantly improve glycemic control in T2DM patients aged ≥65 years [51]. Another post hoc analysis showed that lixisenatide combined with basal insulin could effectively treat T2DM in patients aged ≥70 years. Notably, the effectiveness and safety of lixisenatide are virtually not affected in moderate renal insufficiency patients [52]. Furthermore, oral semaglutide was recently approved for treating T2DM in elderly patients with or without comorbidities [53]. GLP-1 RAs have a lower risk of diabetic ketoacidosis, lower-limb amputations, and genital infections and similar major adverse cardiovascular events (MACE) risk among older adults compared with sodium-dependent glucose transporters 2 (SGLT2) inhibitors [54, 55]. Moreover, GLP-1 RAs combined with SGLT2 inhibitors positively impact systolic blood pressure (SBP), A1C level, and body weight in elderly T2DM patients [56].
4.1.2 GLP-1 RAs and obesity
Aging is associated with gains in fat mass and the loss of muscle mass, which leads to obesity among older individuals [57]. Several factors regulate these aging-related changes in body compositions, including physical inactivity, resting metabolic rate reductions, hormone levels decline, and blunted lipolysis [57-60]. However, the precise mechanisms underlying the changes in body composition associated with aging are unknown. GLP-1 RAs regulate body fat accumulation and weight loss via three direct or indirect ways. First, GLP-1 RAs can regulate satiety and suppress appetite through the central nervous system. Intracerebroventricular GLP-1 treatment strongly inhibits food intake in fasted rats [61]. A recent crossover, randomized, placebo-controlled trial showed that liraglutide could affect the neural reaction to food cues in middle-aged T2DM patients [62]. Notably, GLP-1 receptors were firstly proved to exist in human brains [62]. Second, GLP-1 RAs influence gastrointestinal function by inhibiting gastric motility and delaying gastric emptying [63-65]. Third, GLP-1 RAs directly act on adipose tissue, promote brown remodeling of white adipose tissue and fat mobilization, accelerating fat burning and thus achieving sustained weight loss [66].
Liraglutide was approved by the U.S. Food and Drug Administration (FDA) (2014) for the treatment of obesity and chronic weight management in obese adolescents aged 12-17 [67, 68]. Studies have also assessed the safety and effectiveness of semaglutide in obese patients with or without T2DM for an enhanced therapeutic approach in the future [69]. However, there are few studies related to a weight loss of GLP-1 RAs focusing on older adults. A STEP 2 study showed that semaglutide significantly decreases the weight of T2DM patients with overweight or obesity (55.3±10.6 years) [6]. A clinical trial with 68 older adults with obesity and prediabetes found that liraglutide can significantly enhance weight loss. Moreover, liraglutide-mediated weight loss substantially improves insulin resistance, glucose tolerance, SBP, and triglyceride concentration. However, 79% of patients treated with liraglutide experienced gastrointestinal side effects [70]. Another prospective study with nine subjects demonstrated that a 24-week liraglutide treatment could effectively reduce fat mass in overweight and obese elderly with T2DM [71]. Overall, liraglutide can effectively promote and maintain weight loss in the elderly. However, further studies should assess the effect of semaglutide, a novel weight-loss therapeutic drug.
4.1.3 GLP-1 RAs and osteoporosis
Aging reduces bone strength, thus increasing the risk of fracture (osteoporosis) in the elderly. The imbalance between bone resorption and bone formation during bone remodeling causes osteoporosis. GLP-1 treatment can improve the viability levels of MG-63 and TE-85 osteoblastic cell lines, suggesting that GLP-1 may bind to specific GLP-1 receptors on osteoblasts, thereby promoting bone formation [72]. GLP-1 influences bone metabolism, possibly through ATP-induced c-Fos activation [73]. Moreover, the expression of GLP-1 receptors is increased during the osteogenic differentiation of adipose-derived stem cells (ADSCs) [74]. GLP-1 stimulates the osteoblast differentiation and inhibits adipocyte differentiation in human ADSCs via ERK and Wnt/GSK-3β/β-catenin pathways [75, 76]. GLP-1 promotes the proliferation of human mesenchymal stem cells (hMSCs), reducing apoptosis and preventing their differentiation into adipocytes. Further evidence has shown that MEK and PKC pathways mediate the impacts of GLP-1 on these cells [77]. In addition, GLP-1 RAs may inhibit bone resorption by stimulating calcitonin release from thyroid C-cells [78].
A clinical study showed that exenatide treatment for 44 weeks does not affect BMD and serum markers of bone metabolism [79]. Another two-centered clinical trial reported no effect on BMD or bone turnover markers after 24 weeks of exenatide treatment in newly diagnosed T2DM patients [80]. Furthermore, GLP-1 RAs are not correlated to the increase in bone fracture risk [81]. Similarly, a meta-analysis showed that GLP-1 RAs are not related to fracture risk [82]. The level of serum GLP-1 decrease in older patients with fractures. However, vitamin D is positively correlated with GLP-1, suggesting GLP-1 has a bone-protective effect [83].
4.2 The roles of GLP-1 RAs in aging-related neurodegenerative diseases
Neurodegenerative diseases are caused by the loss of neurons or myelin sheaths, which deteriorate over time, resulting in dysfunction. GLP-1RAs play an essential role in aging-related neurodegenerative diseases.
4.2.1 GLP-1 RAs and AD
AD is characterized by the deficits in cognition, memory, and learning. Emerging evidence shows that GLP-1 RAs are associated with AD. Impaired brain insulin signaling in T2DM is closely related to AD pathogenesis [84]. Exendin-4 can protect against apoptosis and regulate GLP-1/insulin/IGF-1 pathway in middle-aged T2DM rat brains [85]. Liraglutide can significantly increase hippocampal CA1 pyramidal neuron numbers and improve memory function in SAMP8 mice [86]. Liraglutide can also cross the blood-brain barrier (BBB) of 7-month-old APP/PS1 mice, reducing amyloid plaque, decreasing the inflammation response, and increasing young neurons [87]. Similar results have also been obtained in older APP/PS1 mice after three years [88]. The above two studies demonstrate that liraglutide has protective effects in the early and late AD stages. Beside reducing the levels of amyloid-beta (Aβ) protein precursor and Aβ in 3xTg-AD mice brains, exendin-4 can ameliorate Aβ toxicity and oxidative damage at the cellular level [89]. Moreover, exenatide can also significantly reverse transcriptomic alterations underlying brain endothelial cells (ECs) aging at a molecular level [90].
Liraglutide treatment can significantly increase blood-brain glucose metastasis and prevent a decrease in cerebral metabolic rate for glucose (CMRglc) in AD patients compared with placebo [91]. An exploratory analysis suggested that long-term treatment with dulaglutide has beneficial effects on cognitive impairment in patients aged ≥50 years with established or newly diagnosed T2DM [92]. Moreover, a 6-month liraglutide treatment increases CMRglc in AD patients but does not affect Aβ levels and cognitive scores [93]. An 18-month phase II clinical trial showed that exenatide treatment reduces Aβ42 levels in plasma neuronal extracellular vesicles (EVs). However, no significance in cortical thickness and volume, cognitive measures, or biomarkers were found between the exenatide group or placebo [94]. Meanwhile, it is unknown whether liraglutide has neuroprotective benefits in middle-aged persons with AD [95]. A multi-center study with 206 patients randomized to liraglutide is currently underway [96], and better results are highly expected in future research.
4.2.2 GLP-1 RAs and PD
PD is shared among the elderly. Liraglutide and lixisenatide can cross the BBB, thus enhancing neurogenesis [97]. NLY01, a brain-penetrant GLP-1 RA, exerts neuroprotective effects by blocking the microglial-mediated transition of astrocytes into A1 neurotoxic phenotypes in PD mice [98]. Furthermore, GLP-1 has neurotrophic effects of protecting against human neuronal apoptosis at the cellular level [99]. Altogether, these results point to the function of GLP-1 RAs in treating PD.
Clinical evidence suggest that a 12 month-exenatide treatment can significantly improve motor and cognitive functions in PD patients [100]. Moreover, the motor and cognitive advantages could persist for an extended period after exenatide withdrawal [101]. Exenatide also improves practically defined off-medication motor scores in patients with moderate PD. However, it is unknown whether exenatide treatments can alter the course of the underlying PD [102]. A systematic review also suggested the function of exenatide in the improvement of motor impairment for PD patients requires deep research [103]. Hence, exenatide may represent a new therapeutic drug for treating PD, but additional studies are needed to evaluate its long-term effecacy in treating everyday symptoms.
4.3 The roles of GLP-1 RAs in aging-related cardiovascular diseases
Aging represents a crucial risk factor in the occurrence, development and outcome of cardiovascular diseases. GLP-1 RAs can benefit aging-related cardiovascular diseases, such as vascular aging, atherosclerosis, and hypertension.
4.3.1 GLP-1 RAs and vascular aging
Experimental and clinical studies have shown that GLP-1 RAs decrease the risk of adverse cardiovascular events. However, the detailed mechanisms involved are unknown. Vascular aging contributes to the pathology of cardiovascular diseases. GLP-1/exendin-4 can attenuate ROS-induced ECs senescence via an AMP/PKA-dependent pathway [11]. Exendin-4 can also significantly alleviate angiotensin (ANG) II-induced superoxide generation and the subsequent vascular smooth muscle cells (VSMCs) senescence by targeting Rac1 and Nrf2 [104, 105]. Exenatide can ameliorate vascular aging stimulated by a high-fat diet in ApoE-/- mice by modulating inflammation and oxidative stress response [106]. Moreover, exenatide can protect endothelial dysfunction caused by ischemia-reperfusion injury in humans by opening K ATP channels [107]. Moreover, the dipeptidyl peptidase 4 (DPP-4)/GLP-1 axis can prevent vascular aging and maintain ischemia-induced neovascularization in mice [11, 108]. In ApoE-/- mice, increased DPP-4 levels promote diet-associated vascular aging in the presence of chronic stress [109]. Moreover, inhibiting DPP-4 would ameliorate chronic stress-related vascular aging, potentially through the improvements of oxidative stress and vascular inflammation [110]. DPP-4, mediated by the GLP-1/GLP-1R axis, can also regulate chronic stress-associated inflammatory cell production and bone marrow hematopoietic stem cell activation [111]. Overall, these findings imply a regulatory mechanism of GLP-1 RAs in vascular aging and indirectly suggest roles of GLP-1 RAs in managing cardiovascular diseases.
4.3.2 GLP-1 RAs and atherosclerosis
Atherosclerosis is common in the elderly. Aging alters the vascular structure and function, such as intimal thickening, inflammation, and lipid deposition, thus accelerating the progression of atherosclerotic diseases [112]. However, liraglutide can inhibit the formation of atherosclerotic plaques and enhance the stability of early atherosclerotic plaques by binding to the GLP-1 receptor [113]. Liraglutide can also ameliorate atherogenesis by reducing serum-advanced glycation end products (AGEs) expression of receptor for advanced glycation end products (RAGE) [114]. DPP-4 inhibition decreases chronic stress-related carotid artery thrombosis, possibly by improving oxidative stress [115]. DPP4 inhibition can also attenuate oxidative stress, plaque inflammation, and proteolysis related with GLP-1-mediated adiponectin production [109]. Monocytes and macrophages, primary immune cells, are involved in the inflammatory processes in the atherosclerotic lesion. GLP-1 RAs induce anti-atherosclerotic effects by reducing monocyte/macrophage accumulation in the arterial vessel, regulating pro-inflammatory mediators, and modulating immune cell phenotypes [116-119]. Moreover, liraglutide enhances bone marrow-derived macrophages in mice and MΦ2 phenotypes in human THP-1 [117]. Liraglutide can also inhibit VSMCs proliferation through AMPK signaling activation and cell cycle regulation, thereby delaying atherosclerosis [120].
A prospective study showed that an 8-month liragluide treatment could reduce triglycerides (TG), low-density lipoprotein-cholesterol (LDL-C), and total cholesterol (TC), but increase high-density lipoprotein cholesterol (HDL-C) in T2DM patients [7]. The treatment can also significantly decrease carotid intima-media thickness (CIMT), a surrogate marker of subclinical atherosclerosis [7, 121]. Liraglutide can significantly reduce LDL-C, TG, and CIMT levels in T2DM and metabolic syndrome [122]. Altogether, these studies indicate that GLP-1 RAs are strongly associated with hyperlipidemia and atherosclerosis, especially in middle-aged and older patients.
4.3.3 GLP-1 RAs and hypertension
Hypertension is ubiquitous in the elderly, and more than half of people aged 45-75 years in China and the United States suffer from hypertension [123]. Liraglutide combined with OADs, such as rosiglitazone, glimepiride, and metformin have antihypertensive effects [124]. Several recent studies have investigated whether GLP-1 RAs alone can exert blood-pressure-lowering effects. Liraglutide can decrease SBP by 1.2 mmHg [8]. In SUSTAIN-6 trial, respectively, the mean SBP in the semaglutide group receiving 0.5 mg and 1.0 mg was 1.3 mm Hg and 2.6 mm Hg lower than the placebo [125]. Similarly, compared with placebo group, the SBP was 2.6 mmHg lower in the oral semaglutide group in the PIONEER 6 trial [126]. Moreover, considerable evidence supports that GLP-1 RAs are also associated with a modest reduction in diastolic blood pressure (DBP) in middle-aged and older patients with T2DM [127, 128].
The principal mechanisms of antihypertensive effect of GLP-1 RAs may consist of the following four fronts. First, GLP-1 RAs promote vasodilation. Chai et al. found that GLP-1 enhances muscle microvascular blood volume (MBV) in overnight-fasted adult male rats by increasing the production of nitric oxide (NO) [129]. Kim et al. also indicated that liraglutide induces atrial natriuretic peptide (ANP) secretion, thereby promoting vasorelaxation [130]. Notably, GLP-1 can directly affect relaxing rat conduit arteries, independently of NO and the endothelium [131]. GLP-1 infusion can regulate vasorelaxation in the brachial artery of middle-aged patients with T2DM and coronary artery disease [132]. Second, GLP-1 RAs facilitate and promote natriuresis. GLP-1 receptor can be expressed on atrial cardiomyocytes. GLP-1 RAs can increase cAMP via activating GLP-1 receptor and mediate ANP release, leading to urine sodium excretion and blood pressure (BP) reduction [130]. GLP-1 RAs may also increase urinary sodium excretion by regulating Na+/H+ exchanger in renal tubules [133, 134]. Third, GLP-1 RAs indirectly control BP through weight loss. Weight loss is correlated with BP reduction in middle-aged and older T2DM patients [135]. Fourth, GLP-1 RAs are involved in the central control of BP. Liraglutide attenuates the progression of hypertension in spontaneously hypertensive rats by activating brainstem dopamine beta-hydroxylase (DBH) neurons and suppressing sympathetic nerve activity [136].
4.4 The roles of GLP-1 RAs in aging-related kidney diseases
Aging alters the kidney structure, such as decreased renal mass and cortical thickness, glomerulosclerosis, glomerular basement membrane (GBM) thickening, interstitial fibrosis, and tubular atrophy [137]. The eGFR declines by about 5%-10% per decade after 30 years [138], one of the most predominant functional changes of renal aging. It is usually difficult to distinguish common kidney diseases in the elderly, such as diabetic kidney disease (DKD), acute kidney injury (AKI), and chronic kidney disease (CKD), which are caused by normal aging or are secondary to the disease development. However, aging is undoubtedly a vital part of the pathogenesis of these kidney diseases in the elderly [137, 139, 140]. GLP-1 RAs have been used by almost all clinical trials for kidney outcomes in the diabetic population, the majority of whom are elderly. Herein, we differatiate between patients with DKD and those with diabetes and CKD.
Accelerated kidney aging promotes DKD progression [139]. Kidney aging in diabetic individuals involves cellular senescence, AGEs accumulation, renin-angiotensin-aldosterone system (RAAS) dysfunction, inflammation, and oxidative stress [139, 141-143]. Moreover, downregulation of anti-aging gene klotho and Sirt1 and the impairment of lysosome-dependent autophagy promote DKD development [139, 144-146]. Several pre-clinical research pointed out that GLP-1 RAs can ameliorate renal injury induced by aging and diabetes by reducing renal inflammation, relieving renal oxidative stress, and inhibiting renal fibrosis [12, 13, 147]. DPP-4 inhibition exerts protective roles on podocyte injury through antioxidant and anti-apoptotic mechanisms [148]. A LEADER trial indicated that liraglutide significantly reduces new-onset severely increased albuminuria compared with placebo. Liraglutide is also associated with lower rates of DKD events than placebo [9]. A small clinical trial conducted in China assessed the renal outcomes of exenatide in T2DM and DKD patients (mean age, 56 years). The inclusion criteria were an eGFR ≥30 mL/min/1.73m2 and 24h urinary albumin excretion rate (UAER) >0.3g/24h. Compared with the control group, exenatide significantly reduced albuminuria after 24 weeks of intervention [149].
Chronic kidney disease mainly affects elderly populations worldwide, and it is a growing concern [150]. AWARD-7 trial investigated the glycemic control and kidney outcomes of dulaglutide in patients with T2DM and moderate-to-severe CKD. Dulaglutide was associated with less eGFR decline than insulin glargine at 52 weeks of follow-up [151]. The post-hoc analysis of SUSTAIN 1-7 trials showed that semaglutide decreases UACR [152]. Cardiovascular trails have shown that GLP-1 RAs can benefit CKD patients [153, 154]. A meta-analysis evaluated seven cardiovascular studies of GLP-1 RAs, stating that GLP-1 RAs treatment can reduce broad composite kidney outcomes, including the sustained decline in eGFR, emergence of new macroalbuminuria, progression to end-stage kidney disease (ESKD), or death from renal causes [154]. GLP-1 RAs reduce the risk of serious renal events compared with other hypoglycemic drugs, such as sulfonylureas and DPP-4 inhibitors [155, 156]. However, GLP-1 is less effective in preventing the progression of kidney diseases than SGLT-2 inhibitors in some cases [157]. The KDIGO (Kidney Disease: Improving Global Outcomes) 2020 clinical practice guidelines recommend that patient preferences, comorbidities, eGFR, and cost should be considered when approaches of choosing hypoglycemic drugs other than SGLT-2 inhibitors and metformin, if needed GLP-1 RAs are preferred, especially for the T2DM and CKD patients with an eGFR <30 ml/min per 1.73 m2 or those treated with dialysis [158]. The findings illustrate that GLP-1 RAs can improve DKD and CKD management.
In people over 60 years, the incidence of community-acquired AKI has increased by more than 3-fold [159]. Risk factors for AKI in this population include aging-related hemodynamic alterations, kidney aging, comorbidities and medications [140]. A few cases of GLP-1 RAs-induced AKI have been reported in middle-aged and elderly patients [160-162]. However, it has been reported that GLP-1 RAs are not correlated to the enhanced risk for AKI. It was found that the AKI rate was similar in liraglutide and placebo groups in LEADER trial [9]. Additionally, exenatide did not raise the incidence of acute renal failure (ARF) [163]. It has also been documented that SGLT2 inhibitors exert a lower risk for AKI than GLP-1 RAs [164]. However, the roles of GLP-1 RAs in AKI development among the elderly have not been fully established.
4. 5 The roles of GLP-1 RAs in musculoskeletal degenerative diseases
Musculoskeletal degenerative diseases, such as osteoporosis, osteoarthritis and sarcopenia are considerable health challenges among the elderly. The protective roles of GLP-1 RAs on osteoporosis have been elaborated in some detail.
GLP-1 receptor levels decreased significantly in rat models of knee osteoarthritis, while liraglutide inhibited the expression of inflammation-related proteins through the PKA/CREB signaling pathway [165]. Liraglutide attenuated cartilage degeneration in rat models [166]. At the cellular level, the GLP-1 receptor mediates the protective effects of liraglutide in chondrocytes, preventing endoplasmic reticulum stress and apoptosis [166]. In addition, liraglutide exerted beneficial effects on human chondrocytes by inhibiting oxidative stress, mitigating inflammatory responses and suppressing extracellular matrix degradation [167]. These results suggest the clinical potential of GLP-1 RAs in treating osteoarthritis.
Sarcopenia is associated with an aging-related progressive decline of muscle mass, quality, strength and function. In C2C12 myoblasts, liraglutide induced myogenesis through GLP-1 receptor and downstream cAMP-dependent pathways [168]. Additionally, the disrupted structure of myofibrillar was restored by liraglutide in some muscle atrophy models [168]. Analogously, exendin-4 and dulaglutide increases muscle mass and function through the suppression of myostatin and muscle atrophic factors and enhancement of myogenic factors [169]. Moreover, a clinical study showed liraglutide treatment was associated with improved skeletal muscle index (SMI) [71]. Nevertheless, current research on the relationship between GLP-1 RAs and sarcopenia are limited.
5. Prospective therapeutic applications
The elderly are susceptible to various diseases, while GLP-1 RAs have multiple pleiotropic effects. GLP-1 RAs have the potential to be used or can be used in the treatment of various aging-related diseases, including T2DM, overweight or obesity, hypertension, hyperlipidemia, atherosclerosis, vascular aging, kidney disease, AD, PD, osteoporosis, osteoarthritis and sarcopenia. In addition, hepatocyte senescence-associated hepatic fat accumulation and steatosis should be evaluated [170]. This is because GLP-1 RAs have shown various benefits in managing non-alcoholic fatty liver disease (NAFLD), alleviating hepatic inflammation, metabolic dysfunction, insulin resistance and lipotoxicity [171-173]. With the continued decline in physical functioning, loss of capacity and socioeconomic status, the elderly are more likely to experience isolation, loneliness, and consequent psychological problems. In this respect, GLP-1 RAs can improve cognitive functions in patients with mood disorders [174]. Therefore, GLP-1 RAs are potential adjunctive antidepressants. However, although GLP-1 RAs are safe and well-tolerated in most cases, given the higher rates of gastrointestinal adverse effects in GLP-1 RAs treated patients, their clinical administrations in elderly patients should be carefully and comprehensively considered. The therapeutic results, pharmacokinetic characteristics, administration dosage, frequency, and duration vary for different types of GLP-1 RAs. It is essential to determine the specific roles of each drug and their combined actions with other agents. Clinical trials should be performed to investigate the roles of GLP-1 RAs in elderly patients with multimorbidities, thus maximizing their efficacies while minimizing their side effects.
6. Perspectives and conclusions
GLP-1 RAs can safely and effectively lower blood sugar levels and reduce weight as a new hypoglycemic or weight loss drug. We have reviewed the diverse mechanisms underlying the protective function of GLP-1 RAs against aging-related diseases, especially in middle-aged and aged adults. Several hallmarks of aging have all been elucidated systematically and comprehensively. We strive to study the molecular, phenotypic, and functional hierarchical domains, establishing the interlaced time and hierarchical relationship of aging. Plenty of clinical evidence mainly focusing on middle-aged and aged adults support the established or potential benefits of GLP-1 RAs on a variety of common aging-related diseases, including metabolic diseases, neurodegenerative diseases, cardiovascular diseases, kidney diseases, and musculoskeletal degenerative diseases. Nevertheless, the complex mechanisms underlying the relationship between GLP-1 RAs and aging-related diseases have not yet been fully identified. Although GLP-1 RAs have great potential for a wide range of human diseases, there are many hurdles to bring them to the clinic. Large-scale studies are needed to verify the safety and efficacies of GLP-1 RAs in decreasing polypharmacy in elderly patients with multimorbidities.
Footnotes
Competing Interests
The authors declare that they have no competing interests.
References
- [1].Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013). The hallmarks of aging. Cell, 153:1194-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Officer A, Thiyagarajan JA, Schneiders ML, Nash P, de la Fuente-Nunez V (2020). Ageism, Healthy Life Expectancy and Population Ageing: How Are They Related? Int J Environ Res Public Health, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ge H, Yang Z, Li X, Liu D, Li Y, Pan Y, et al. (2020). The prevalence and associated factors of metabolic syndrome in Chinese aging population. Sci Rep, 10:20034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nauck MA (2011). Incretin-based therapies for type 2 diabetes mellitus: properties, functions, and clinical implications. Am J Med, 124:S3-18. [DOI] [PubMed] [Google Scholar]
- [5].Onoviran OF, Li D, Toombs Smith S, Raji MA (2019). Effects of glucagon-like peptide 1 receptor agonists on comorbidities in older patients with diabetes mellitus. Ther Adv Chronic Dis, 10:2040622319862691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Davies M, Faerch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, et al. (2021). Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet, 397:971-984. [DOI] [PubMed] [Google Scholar]
- [7].Rizzo M, Chandalia M, Patti AM, Di Bartolo V, Rizvi AA, Montalto G, et al. (2014). Liraglutide decreases carotid intima-media thickness in patients with type 2 diabetes: 8-month prospective pilot study. Cardiovasc Diabetol, 13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. (2016). Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med, 375:311-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Mann JFE, Orsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, et al. (2017). Liraglutide and Renal Outcomes in Type 2 Diabetes. N Engl J Med, 377:839-848. [DOI] [PubMed] [Google Scholar]
- [10].Valencia WM, Botros D, Vera-Nunez M, Dang S (2018). Diabetes Treatment in the Elderly: Incorporating Geriatrics, Technology, and Functional Medicine. Curr Diab Rep, 18:95. [DOI] [PubMed] [Google Scholar]
- [11].Oeseburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, Sillje HH (2010). Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler Thromb Vasc Biol, 30:1407-1414. [DOI] [PubMed] [Google Scholar]
- [12].Hendarto H, Inoguchi T, Maeda Y, Ikeda N, Zheng J, Takei R, et al. (2012). GLP-1 analog liraglutide protects against oxidative stress and albuminuria in streptozotocin-induced diabetic rats via protein kinase A-mediated inhibition of renal NAD(P)H oxidases. Metabolism, 61:1422-1434. [DOI] [PubMed] [Google Scholar]
- [13].Jia Y, Zheng Z, Guan M, Zhang Q, Li Y, Wang L, et al. (2018). Exendin-4 ameliorates high glucose-induced fibrosis by inhibiting the secretion of miR-192 from injured renal tubular epithelial cells. Exp Mol Med, 50:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wang T, Yang L, Liang Z, Wang L, Su F, Wang X, et al. (2021). Targeting cellular senescence prevents glucocorticoid-induced bone loss through modulation of the DPP4-GLP-1 axis. Signal Transduct Target Ther, 6:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Moskalev AA, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Yanai H, et al. (2013). The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res Rev, 12:661-684. [DOI] [PubMed] [Google Scholar]
- [16].Blackburn EH, Epel ES, Lin J (2015). Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science, 350:1193-1198. [DOI] [PubMed] [Google Scholar]
- [17].Kubben N, Misteli T (2017). Shared molecular and cellular mechanisms of premature ageing and ageing-associated diseases. Nat Rev Mol Cell Biol, 18:595-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu Y, Weng W, Gao R, Liu Y (2019). New Insights for Cellular and Molecular Mechanisms of Aging and Aging-Related Diseases: Herbal Medicine as Potential Therapeutic Approach. Oxid Med Cell Longev, 2019:4598167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kaushik S, Cuervo AM (2015). Proteostasis and aging. Nat Med, 21:1406-1415. [DOI] [PubMed] [Google Scholar]
- [20].Labbadia J, Morimoto RI (2015). The biology of proteostasis in aging and disease. Annu Rev Biochem, 84:435-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sabath N, Levy-Adam F, Younis A, Rozales K, Meller A, Hadar S, et al. (2020). Cellular proteostasis decline in human senescence. Proc Natl Acad Sci U S A, 117:31902-31913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Salminen A, Kaarniranta K, Kauppinen A (2016). Age-related changes in AMPK activation: Role for AMPK phosphatases and inhibitory phosphorylation by upstream signaling pathways. Ageing Res Rev, 28:15-26. [DOI] [PubMed] [Google Scholar]
- [23].Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, et al. (2012). The sirtuin SIRT6 regulates lifespan in male mice. Nature, 483:218-221. [DOI] [PubMed] [Google Scholar]
- [24].Wang Y, Hekimi S (2015). Mitochondrial dysfunction and longevity in animals: Untangling the knot. Science, 350:1204-1207. [DOI] [PubMed] [Google Scholar]
- [25].Gorgoulis V, Adams PD, Alimonti A, Bennett DC, Bischof O, Bishop C, et al. (2019). Cellular Senescence: Defining a Path Forward. Cell, 179:813-827. [DOI] [PubMed] [Google Scholar]
- [26].Xiao YZ, Yang M, Xiao Y, Guo Q, Huang Y, Li CJ, et al. (2020). Reducing Hypothalamic Stem Cell Senescence Protects against Aging-Associated Physiological Decline. Cell Metab, 31:534-548 e535. [DOI] [PubMed] [Google Scholar]
- [27].Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, et al. (2019). Ageing as a risk factor for neurodegenerative disease. Nat Rev Neurol, 15:565-581. [DOI] [PubMed] [Google Scholar]
- [28].Yang JL, Chen WY, Chen YP, Kuo CY, Chen SD (2016). Activation of GLP-1 Receptor Enhances Neuronal Base Excision Repair via PI3K-AKT-Induced Expression of Apurinic/Apyrimidinic Endonuclease 1. Theranostics, 6:2015-2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ramos H, Bogdanov P, Sampedro J, Huerta J, Simo R, Hernandez C (2020). Beneficial Effects of Glucagon-Like Peptide-1 (GLP-1) in Diabetes-Induced Retinal Abnormalities: Involvement of Oxidative Stress. Antioxidants(Basel), 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shi JX, Huang Q (2018). Glucagonlike peptide1 protects mouse podocytes against high glucoseinduced apoptosis, and suppresses reactive oxygen species production and proinflammatory cytokine secretion, through sirtuin 1 activation in vitro. Mol Med Rep, 18:1789-1797. [DOI] [PubMed] [Google Scholar]
- [31].Xie Y, Zheng J, Li S, Li H, Zhou Y, Zheng W, et al. (2021). GLP-1 improves the neuronal supportive ability of astrocytes in Alzheimer's disease by regulating mitochondrial dysfunction via the cAMP/PKA pathway. Biochem Pharmacol, 188:114578. [DOI] [PubMed] [Google Scholar]
- [32].An J, Zhou Y, Zhang M, Xie Y, Ke S, Liu L, et al. (2019). Exenatide alleviates mitochondrial dysfunction and cognitive impairment in the 5xFAD mouse model of Alzheimer's disease. Behav Brain Res, 370:111932. [DOI] [PubMed] [Google Scholar]
- [33].Zhang L, Tian J, Diao S, Zhang G, Xiao M, Chang D (2020). GLP-1 receptor agonist liraglutide protects cardiomyocytes from IL-1beta-induced metabolic disturbance and mitochondrial dysfunction. Chem Biol Interact, 332:109252. [DOI] [PubMed] [Google Scholar]
- [34].Nuamnaichati N, Mangmool S, Chattipakorn N, Parichatikanond W (2020). Stimulation of GLP-1 Receptor Inhibits Methylglyoxal-Induced Mitochondrial Dysfunctions in H9c2 Cardiomyoblasts: Potential Role of Epac/PI3K/Akt Pathway. Front Pharmacol, 11:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zeng Y, Yang K, Wang F, Zhou L, Hu Y, Tang M, et al. (2016). The glucagon like peptide 1 analogue, exendin-4, attenuates oxidative stress-induced retinal cell death in early diabetic rats through promoting Sirt1 and Sirt3 expression. Exp Eye Res, 151:203-211. [DOI] [PubMed] [Google Scholar]
- [36].Kimura R, Okouchi M, Fujioka H, Ichiyanagi A, Ryuge F, Mizuno T, et al. (2009). Glucagon-like peptide-1 (GLP-1) protects against methylglyoxal-induced PC12 cell apoptosis through the PI3K/Akt/mTOR/GCLc/redox signaling pathway. Neuroscience, 162:1212-1219. [DOI] [PubMed] [Google Scholar]
- [37].Miao XY, Gu ZY, Liu P, Hu Y, Li L, Gong YP, et al. (2013). The human glucagon-like peptide-1 analogue liraglutide regulates pancreatic beta-cell proliferation and apoptosis via an AMPK/mTOR/P70S6K signaling pathway. Peptides, 39:71-79. [DOI] [PubMed] [Google Scholar]
- [38].Ao N, Ma Z, Yang J, Jin S, Zhang K, Luo E, et al. (2020). Liraglutide ameliorates lipotoxicity-induced inflammation through the mTORC1 signalling pathway. Peptides, 133:170375. [DOI] [PubMed] [Google Scholar]
- [39].Sharma S, Mells JE, Fu PP, Saxena NK, Anania FA (2011). GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PLoS One, 6:e25269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Geloneze B, de Oliveira Mda S, Vasques AC, Novaes FS, Pareja JC, Tambascia MA (2014). Impaired incretin secretion and pancreatic dysfunction with older age and diabetes. Metabolism, 63:922-929. [DOI] [PubMed] [Google Scholar]
- [41].Tschen SI, Dhawan S, Gurlo T, Bhushan A (2009). Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes, 58:1312-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Sinclair A, Saeedi P, Kaundal A, Karuranga S, Malanda B, Williams R (2020). Diabetes and global ageing among 65-99-year-old adults: Findings from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract, 162:108078. [DOI] [PubMed] [Google Scholar]
- [43].Pham H, Marathe CS, Phillips LK, Trahair LG, Hatzinikolas S, Huynh L, et al. (2019). Longitudinal Changes in Fasting and Glucose-Stimulated GLP-1 and GIP in Healthy Older Subjects. J Clin Endocrinol Metab, 104:6201-6206. [DOI] [PubMed] [Google Scholar]
- [44].Wang Y, Perfetti R, Greig NH, Holloway HW, DeOre KA, Montrose-Rafizadeh C, et al. (1997). Glucagon-like peptide-1 can reverse the age-related decline in glucose tolerance in rats. J Clin Invest, 99:2883-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].De Ore K, Greig NH, Holloway HW, Wang Y, Perfetti R, Egan JM (1997). The effects of GLP-1 on insulin release in young and old rats in the fasting state and during an intravenous glucose tolerance test. J Gerontol A Biol Sci Med Sci, 52:B245-249. [DOI] [PubMed] [Google Scholar]
- [46].Irwin N, McClean PL, Harriott P, Flatt PR (2007). Beneficial effects of sub-chronic activation of glucagon-like peptide-1 (GLP-1) receptors on deterioration of glucose homeostasis and insulin secretion in aging mice. Exp Gerontol, 42:296-300. [DOI] [PubMed] [Google Scholar]
- [47].Perfetti R, Zhou J, Doyle ME, Egan JM (2000). Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology, 141:4600-4605. [DOI] [PubMed] [Google Scholar]
- [48].Dai C, Hang Y, Shostak A, Poffenberger G, Hart N, Prasad N, et al. (2017). Age-dependent human beta cell proliferation induced by glucagon-like peptide 1 and calcineurin signaling. J Clin Invest, 127:3835-3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Meneilly GS, Veldhuis JD, Elahi D (2005). Deconvolution analysis of rapid insulin pulses before and after six weeks of continuous subcutaneous administration of glucagon-like peptide-1 in elderly patients with type 2 diabetes. J Clin Endocrinol Metab, 90:6251-6256. [DOI] [PubMed] [Google Scholar]
- [50].Raccah D, Miossec P, Esposito V, Niemoeller E, Cho M, Gerich J (2015). Efficacy and safety of lixisenatide in elderly (>/=65 years old) and very elderly (>/=75 years old) patients with type 2 diabetes: an analysis from the GetGoal phase III programme. Diabetes Metab Res Rev, 31:204-211. [DOI] [PubMed] [Google Scholar]
- [51].Hanefeld M, Berria R, Lin J, Aronson R, Darmon P, Evans M, et al. (2014). Lixisenatide treatment for older patients with type 2 diabetes mellitus uncontrolled on oral antidiabetics: meta-analysis of five randomized controlled trials. Adv Ther, 31:861-872. [DOI] [PubMed] [Google Scholar]
- [52].Dailey GE, Dex TA, Roberts M, Liu M, Meneilly GS (2019). Efficacy and safety of lixisenatide as add-on therapy to basal insulin in older adults with type 2 diabetes in the GetGoal-O Study. J Diabetes, 11:971-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mosenzon O, Miller EM, Warren ML (2020). Oral semaglutide in patients with type 2 diabetes and cardiovascular disease, renal impairment, or other comorbidities, and in older patients. Postgrad Med, 132:37-47. [DOI] [PubMed] [Google Scholar]
- [54].Patorno E, Pawar A, Bessette LG, Kim DH, Dave C, Glynn RJ, et al. (2021). Comparative Effectiveness and Safety of Sodium-Glucose Cotransporter 2 Inhibitors Versus Glucagon-Like Peptide 1 Receptor Agonists in Older Adults. Diabetes Care, 44:826-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Karagiannis T, Tsapas A, Athanasiadou E, Avgerinos I, Liakos A, Matthews DR, et al. (2021). GLP-1 receptor agonists and SGLT2 inhibitors for older people with type 2 diabetes: A systematic review and meta-analysis. Diabetes Res Clin Pract, 174:108737. [DOI] [PubMed] [Google Scholar]
- [56].Carretero Gomez J, Arevalo Lorido JC, Gomez Huelgas R, Garcia de Lucas D, Mateos Polo L, Varela Aguilar JM, et al. (2019). Combination Therapy With Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors in Older Patients With Type 2 Diabetes: A Real-World Evidence Study. Can J Diabetes, 43:186-192. [DOI] [PubMed] [Google Scholar]
- [57].JafariNasabian P, Inglis JE, Reilly W, Kelly OJ, Ilich JZ (2017). Aging human body: changes in bone, muscle and body fat with consequent changes in nutrient intake. J Endocrinol, 234:R37-R51. [DOI] [PubMed] [Google Scholar]
- [58].Chia CW, Egan JM, Ferrucci L (2018). Age-Related Changes in Glucose Metabolism, Hyperglycemia, and Cardiovascular Risk. Circ Res, 123:886-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].St-Onge MP, Gallagher D (2010). Body composition changes with aging: the cause or the result of alterations in metabolic rate and macronutrient oxidation? Nutrition, 26:152-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lonnqvist F, Nyberg B, Wahrenberg H, Arner P (1990). Catecholamine-induced lipolysis in adipose tissue of the elderly. J Clin Invest, 85:1614-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, et al. (1996). A role for glucagon-like peptide-1 in the central regulation of feeding. Nature, 379:69-72. [DOI] [PubMed] [Google Scholar]
- [62].Farr OM, Sofopoulos M, Tsoukas MA, Dincer F, Thakkar B, Sahin-Efe A, et al. (2016). GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo-controlled trial. Diabetologia, 59:954-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Meier JJ (2012). GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol, 8:728-742. [DOI] [PubMed] [Google Scholar]
- [64].Halawi H, Khemani D, Eckert D, O'Neill J, Kadouh H, Grothe K, et al. (2017). Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol Hepatol, 2:890-899. [DOI] [PubMed] [Google Scholar]
- [65].van Can J, Sloth B, Jensen CB, Flint A, Blaak EE, Saris WH (2014). Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int J Obes (Lond), 38:784-793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Xu F, Lin B, Zheng X, Chen Z, Cao H, Xu H, et al. (2016). GLP-1 receptor agonist promotes brown remodelling in mouse white adipose tissue through SIRT1. Diabetologia, 59:1059-1069. [DOI] [PubMed] [Google Scholar]
- [67].Nuffer WA, Trujillo JM (2015). Liraglutide: A New Option for the Treatment of Obesity. Pharmacotherapy, 35:926-934. [DOI] [PubMed] [Google Scholar]
- [68].Mullard A (2021). 2020 FDA drug approvals. Nat Rev Drug Discov, 20:85-90. [DOI] [PubMed] [Google Scholar]
- [69].Kushner RF, Calanna S, Davies M, Dicker D, Garvey WT, Goldman B, et al. (2020). Semaglutide 2.4 mg for the Treatment of Obesity: Key Elements of the STEP Trials 1 to 5. Obesity (Silver Spring), 28:1050-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Kim SH, Abbasi F, Lamendola C, Liu A, Ariel D, Schaaf P, et al. (2013). Benefits of liraglutide treatment in overweight and obese older individuals with prediabetes. Diabetes Care, 36:3276-3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Perna S, Guido D, Bologna C, Solerte SB, Guerriero F, Isu A, et al. (2016). Liraglutide and obesity in elderly: efficacy in fat loss and safety in order to prevent sarcopenia. A perspective case series study. Aging Clin Exp Res, 28:1251-1257. [DOI] [PubMed] [Google Scholar]
- [72].Pacheco-Pantoja EL, Ranganath LR, Gallagher JA, Wilson PJ, Fraser WD (2011). Receptors and effects of gut hormones in three osteoblastic cell lines. BMC Physiol, 11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Pacheco-Pantoja EL, Dillon JP, Wilson PJ, Fraser WD, Gallagher JA (2016). c-Fos induction by gut hormones and extracellular ATP in osteoblastic-like cell lines. Purinergic Signal, 12:647-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Jeon YK, Bae MJ, Kim JI, Kim JH, Choi SJ, Kwon SK, et al. (2014). Expression of Glucagon-Like Peptide 1 Receptor during Osteogenic Differentiation of Adipose-Derived Stem Cells. Endocrinol Metab (Seoul), 29:567-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lee HM, Joo BS, Lee CH, Kim HY, Ock JH, Lee YS (2015). Effect of Glucagon-like Peptide-1 on the Differentiation of Adipose-derived Stem Cells into Osteoblasts and Adipocytes. J Menopausal Med, 21:93-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Li Y, Fu H, Wang H, Luo S, Wang L, Chen J, et al. (2020). GLP-1 promotes osteogenic differentiation of human ADSCs via the Wnt/GSK-3beta/beta-catenin pathway. Mol Cell Endocrinol, 515:110921. [DOI] [PubMed] [Google Scholar]
- [77].Sanz C, Vazquez P, Blazquez C, Barrio PA, Alvarez Mdel M, Blazquez E (2010). Signaling and biological effects of glucagon-like peptide 1 on the differentiation of mesenchymal stem cells from human bone marrow. Am J Physiol Endocrinol Metab, 298:E634-643. [DOI] [PubMed] [Google Scholar]
- [78].Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, et al. (2010). Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology, 151:1473-1486. [DOI] [PubMed] [Google Scholar]
- [79].Bunck MC, Eliasson B, Corner A, Heine RJ, Shaginian RM, Taskinen MR, et al. (2011). Exenatide treatment did not affect bone mineral density despite body weight reduction in patients with type 2 diabetes. Diabetes Obes Metab, 13:374-377. [DOI] [PubMed] [Google Scholar]
- [80].Li R, Xu W, Luo S, Xu H, Tong G, Zeng L, et al. (2015). Effect of exenatide, insulin and pioglitazone on bone metabolism in patients with newly diagnosed type 2 diabetes. Acta Diabetol, 52:1083-1091. [DOI] [PubMed] [Google Scholar]
- [81].Fralick M, Kim SC, Schneeweiss S, Kim D, Redelmeier DA, Patorno E (2019). Fracture Risk After Initiation of Use of Canagliflozin: A Cohort Study. Ann Intern Med, 170:155-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Mabilleau G, Mieczkowska A, Chappard D (2014). Use of glucagon-like peptide-1 receptor agonists and bone fractures: a meta-analysis of randomized clinical trials. J Diabetes, 6:260-266. [DOI] [PubMed] [Google Scholar]
- [83].Pazarci O, Dogan HO, Kilinc S, Camurcu Y (2020). Evaluation of Serum Glucagon-Like Peptide 1 and Vitamin D Levels in Elderly Patients with Bone Fractures. Med Princ Pract, 29:219-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Chow HM, Shi M, Cheng A, Gao Y, Chen G, Song X, et al. (2019). Age-related hyperinsulinemia leads to insulin resistance in neurons and cell-cycle-induced senescence. Nat Neurosci, 22:1806-1819. [DOI] [PubMed] [Google Scholar]
- [85].Candeias E, Sebastiao I, Cardoso S, Carvalho C, Santos MS, Oliveira CR, et al. (2018). Brain GLP-1/IGF-1 Signaling and Autophagy Mediate Exendin-4 Protection Against Apoptosis in Type 2 Diabetic Rats. Mol Neurobiol, 55:4030-4050. [DOI] [PubMed] [Google Scholar]
- [86].Hansen HH, Fabricius K, Barkholt P, Niehoff ML, Morley JE, Jelsing J, et al. (2015). The GLP-1 Receptor Agonist Liraglutide Improves Memory Function and Increases Hippocampal CA1 Neuronal Numbers in a Senescence-Accelerated Mouse Model of Alzheimer's Disease. J Alzheimers Dis, 46:877-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].McClean PL, Parthsarathy V, Faivre E, Holscher C (2011). The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. J Neurosci, 31:6587-6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].McClean PL, Holscher C (2014). Liraglutide can reverse memory impairment, synaptic loss and reduce plaque load in aged APP/PS1 mice, a model of Alzheimer's disease. Neuropharmacology, 76 Pt A:57-67. [DOI] [PubMed] [Google Scholar]
- [89].Li Y, Duffy KB, Ottinger MA, Ray B, Bailey JA, Holloway HW, et al. (2010). GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. J Alzheimers Dis, 19:1205-1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zhao L, Li Z, Vong JSL, Chen X, Lai HM, Yan LYC, et al. (2020). Pharmacologically reversible zonation-dependent endothelial cell transcriptomic changes with neurodegenerative disease associations in the aged brain. Nat Commun, 11:4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Gejl M, Brock B, Egefjord L, Vang K, Rungby J, Gjedde A (2017). Blood-Brain Glucose Transfer in Alzheimer's disease: Effect of GLP-1 Analog Treatment. Sci Rep, 7:17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Cukierman-Yaffe T, Gerstein HC, Colhoun HM, Diaz R, Garcia-Perez LE, Lakshmanan M, et al. (2020). Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial. Lancet Neurol, 19:582-590. [DOI] [PubMed] [Google Scholar]
- [93].Gejl M, Gjedde A, Egefjord L, Moller A, Hansen SB, Vang K, et al. (2016). In Alzheimer's Disease, 6-Month Treatment with GLP-1 Analog Prevents Decline of Brain Glucose Metabolism: Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Front Aging Neurosci, 8:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mullins RJ, Mustapic M, Chia CW, Carlson O, Gulyani S, Tran J, et al. (2019). A Pilot Study of Exenatide Actions in Alzheimer's Disease. Curr Alzheimer Res, 16:741-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Watson KT, Wroolie TE, Tong G, Foland-Ross LC, Frangou S, Singh M, et al. (2019). Neural correlates of liraglutide effects in persons at risk for Alzheimer's disease. Behav Brain Res, 356:271-278. [DOI] [PubMed] [Google Scholar]
- [96].Femminella GD, Frangou E, Love SB, Busza G, Holmes C, Ritchie C, et al. (2019). Evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer's disease: study protocol for a randomised controlled trial (ELAD study). Trials, 20:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Hunter K, Holscher C (2012). Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci, 13:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Yun SP, Kam TI, Panicker N, Kim S, Oh Y, Park JS, et al. (2018). Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson's disease. Nat Med, 24:931-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Li Y, Tweedie D, Mattson MP, Holloway HW, Greig NH (2010). Enhancing the GLP-1 receptor signaling pathway leads to proliferation and neuroprotection in human neuroblastoma cells. J Neurochem, 113:1621-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, et al. (2013). Exenatide and the treatment of patients with Parkinson's disease. J Clin Invest, 123:2730-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Kahan J, Ell P, et al. (2014). Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson's disease. J Parkinsons Dis, 4:337-344. [DOI] [PubMed] [Google Scholar]
- [102].Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, et al. (2017). Exenatide once weekly versus placebo in Parkinson's disease: a randomised, double-blind, placebo-controlled trial. Lancet, 390:1664-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Mulvaney CA, Duarte GS, Handley J, Evans DJ, Menon S, Wyse R, et al. (2020). GLP-1 receptor agonists for Parkinson's disease. Cochrane Database Syst Rev, 7:CD012990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zhao L, Li AQ, Zhou TF, Zhang MQ, Qin XM (2014). Exendin-4 alleviates angiotensin II-induced senescence in vascular smooth muscle cells by inhibiting Rac1 activation via a cAMP/PKA-dependent pathway. Am J Physiol Cell Physiol, 307:C1130-1141. [DOI] [PubMed] [Google Scholar]
- [105].Zhou T, Zhang M, Zhao L, Li A, Qin X (2016). Activation of Nrf2 contributes to the protective effect of Exendin-4 against angiotensin II-induced vascular smooth muscle cell senescence. Am J Physiol Cell Physiol, 311:C572-C582. [DOI] [PubMed] [Google Scholar]
- [106].Yang G, Lei Y, Inoue A, Piao L, Hu L, Jiang H, et al. (2017). Exenatide mitigated diet-induced vascular aging and atherosclerotic plaque growth in ApoE-deficient mice under chronic stress. Atherosclerosis, 264:1-10. [DOI] [PubMed] [Google Scholar]
- [107].Ha SJ, Kim W, Woo JS, Kim JB, Kim SJ, Kim WS, et al. (2012). Preventive effects of exenatide on endothelial dysfunction induced by ischemia-reperfusion injury via KATP channels. Arterioscler Thromb Vasc Biol, 32:474-480. [DOI] [PubMed] [Google Scholar]
- [108].Piao L, Zhao G, Zhu E, Inoue A, Shibata R, Lei Y, et al. (2017). Chronic Psychological Stress Accelerates Vascular Senescence and Impairs Ischemia-Induced Neovascularization: The Role of Dipeptidyl Peptidase-4/Glucagon-Like Peptide-1-Adiponectin Axis. J Am Heart Assoc, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Lei Y, Yang G, Hu L, Piao L, Inoue A, Jiang H, et al. (2017). Increased dipeptidyl peptidase-4 accelerates diet-related vascular aging and atherosclerosis in ApoE-deficient mice under chronic stress. Int J Cardiol, 243:413-420. [DOI] [PubMed] [Google Scholar]
- [110].Xin M, Jin X, Cui X, Jin C, Piao L, Wan Y, et al. (2019). Dipeptidyl peptidase-4 inhibition prevents vascular aging in mice under chronic stress: Modulation of oxidative stress and inflammation. Chem Biol Interact, 314:108842. [DOI] [PubMed] [Google Scholar]
- [111].Zhu E, Hu L, Wu H, Piao L, Zhao G, Inoue A, et al. (2017). Dipeptidyl Peptidase-4 Regulates Hematopoietic Stem Cell Activation in Response to Chronic Stress. J Am Heart Assoc, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Fajemiroye JO, da Cunha LC, Saavedra-Rodriguez R, Rodrigues KL, Naves LM, Mourao AA, et al. (2018). Aging-Induced Biological Changes and Cardiovascular Diseases. Biomed Res Int, 2018:7156435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Gaspari T, Welungoda I, Widdop RE, Simpson RW, Dear AE (2013). The GLP-1 receptor agonist liraglutide inhibits progression of vascular disease via effects on atherogenesis, plaque stability and endothelial function in an ApoE(-/-) mouse model. Diab Vasc Dis Res, 10:353-360. [DOI] [PubMed] [Google Scholar]
- [114].Li P, Tang Z, Wang L, Feng B (2017). Glucagon-like peptide-1 analogue liraglutide ameliorates atherogenesis via inhibiting advanced glycation end product-induced receptor for advanced glycosylation end product expression in apolipoprotein-E deficient mice. Mol Med Rep, 16:3421-3426. [DOI] [PubMed] [Google Scholar]
- [115].Jin X, Jin C, Nakamura K, Jin T, Xin M, Wan Y, et al. (2020). Increased dipeptidyl peptidase-4 accelerates chronic stress-related thrombosis in a mouse carotid artery model. J Hypertens, 38:1504-1513. [DOI] [PubMed] [Google Scholar]
- [116].Arakawa M, Mita T, Azuma K, Ebato C, Goto H, Nomiyama T, et al. (2010). Inhibition of monocyte adhesion to endothelial cells and attenuation of atherosclerotic lesion by a glucagon-like peptide-1 receptor agonist, exendin-4. Diabetes, 59:1030-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Bruen R, Curley S, Kajani S, Crean D, O'Reilly ME, Lucitt MB, et al. (2017). Liraglutide dictates macrophage phenotype in apolipoprotein E null mice during early atherosclerosis. Cardiovasc Diabetol, 16:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Bruen R, Curley S, Kajani S, Lynch G, O'Reilly ME, Dillon ET, et al. (2019). Liraglutide Attenuates Preestablished Atherosclerosis in Apolipoprotein E-Deficient Mice via Regulation of Immune Cell Phenotypes and Proinflammatory Mediators. J Pharmacol Exp Ther, 370:447-458. [DOI] [PubMed] [Google Scholar]
- [119].Vinue A, Navarro J, Herrero-Cervera A, Garcia-Cubas M, Andres-Blasco I, Martinez-Hervas S, et al. (2017). The GLP-1 analogue lixisenatide decreases atherosclerosis in insulin-resistant mice by modulating macrophage phenotype. Diabetologia, 60:1801-1812. [DOI] [PubMed] [Google Scholar]
- [120].Jojima T, Uchida K, Akimoto K, Tomotsune T, Yanagi K, Iijima T, et al. (2017). Liraglutide, a GLP-1 receptor agonist, inhibits vascular smooth muscle cell proliferation by enhancing AMP-activated protein kinase and cell cycle regulation, and delays atherosclerosis in ApoE deficient mice. Atherosclerosis, 261:44-51. [DOI] [PubMed] [Google Scholar]
- [121].Rizvi AA, Patti AM, Giglio RV, Nikolic D, Amato A, Al-Busaidi N, et al. (2015). Liraglutide improves carotid intima-media thickness in patients with type 2 diabetes and non-alcoholic fatty liver disease: an 8-month prospective pilot study. Expert Opin Biol Ther, 15:1391-1397. [DOI] [PubMed] [Google Scholar]
- [122].Rizzo M, Rizvi AA, Patti AM, Nikolic D, Giglio RV, Castellino G, et al. (2016). Liraglutide improves metabolic parameters and carotid intima-media thickness in diabetic patients with the metabolic syndrome: an 18-month prospective study. Cardiovasc Diabetol, 15:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Khera R, Lu Y, Lu J, Saxena A, Nasir K, Jiang L, et al. (2018). Impact of 2017 ACC/AHA guidelines on prevalence of hypertension and eligibility for antihypertensive treatment in United States and China: nationally representative cross sectional study. BMJ, 362:k2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Gallwitz B, Vaag A, Falahati A, Madsbad S (2010). Adding liraglutide to oral antidiabetic drug therapy: onset of treatment effects over time. Int J Clin Pract, 64:267-276. [DOI] [PubMed] [Google Scholar]
- [125].Marso SP, Holst AG, Vilsboll T (2017). Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med, 376:891-892. [DOI] [PubMed] [Google Scholar]
- [126].Husain M, Birkenfeld AL, Donsmark M, Dungan K, Eliaschewitz FG, Franco DR, et al. (2019). Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med, 381:841-851. [DOI] [PubMed] [Google Scholar]
- [127].Wang B, Zhong J, Lin H, Zhao Z, Yan Z, He H, et al. (2013). Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide: a meta-analysis of clinical trials. Diabetes Obes Metab, 15:737-749. [DOI] [PubMed] [Google Scholar]
- [128].Sun F, Wu S, Guo S, Yu K, Yang Z, Li L, et al. (2015). Impact of GLP-1 receptor agonists on blood pressure, heart rate and hypertension among patients with type 2 diabetes: A systematic review and network meta-analysis. Diabetes Res Clin Pract, 110:26-37. [DOI] [PubMed] [Google Scholar]
- [129].Chai W, Dong Z, Wang N, Wang W, Tao L, Cao W, et al. (2012). Glucagon-like peptide 1 recruits microvasculature and increases glucose use in muscle via a nitric oxide-dependent mechanism. Diabetes, 61:888-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, et al. (2013). GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med, 19:567-575. [DOI] [PubMed] [Google Scholar]
- [131].Nystrom T, Gonon AT, Sjoholm A, Pernow J (2005). Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul Pept, 125:173-177. [DOI] [PubMed] [Google Scholar]
- [132].Nystrom T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, Ahren B, et al. (2004). Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab, 287:E1209-1215. [DOI] [PubMed] [Google Scholar]
- [133].Crajoinas RO, Oricchio FT, Pessoa TD, Pacheco BP, Lessa LM, Malnic G, et al. (2011). Mechanisms mediating the diuretic and natriuretic actions of the incretin hormone glucagon-like peptide-1. Am J Physiol Renal Physiol, 301:F355-363. [DOI] [PubMed] [Google Scholar]
- [134].Carraro-Lacroix LR, Malnic G, Girardi AC (2009). Regulation of Na+/H+ exchanger NHE3 by glucagon-like peptide 1 receptor agonist exendin-4 in renal proximal tubule cells. Am J Physiol Renal Physiol, 297:F1647-1655. [DOI] [PubMed] [Google Scholar]
- [135].Hu M, Cai X, Yang W, Zhang S, Nie L, Ji L (2020). Effect of Hemoglobin A1c Reduction or Weight Reduction on Blood Pressure in Glucagon-Like Peptide-1 Receptor Agonist and Sodium-Glucose Cotransporter-2 Inhibitor Treatment in Type 2 Diabetes Mellitus: A Meta-Analysis. J Am Heart Assoc, 9:e015323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Katsurada K, Nakata M, Saito T, Zhang B, Maejima Y, Nandi SS, et al. (2019). Central Glucagon-like Peptide-1 Receptor Signaling via Brainstem Catecholamine Neurons Counteracts Hypertension in Spontaneously Hypertensive Rats. Sci Rep, 9:12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Chou YH, Chen YM (2021). Aging and Renal Disease: Old Questions for New Challenges. Aging Dis, 12:515-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Glassock RJ, Rule AD (2016). Aging and the Kidneys: Anatomy, Physiology and Consequences for Defining Chronic Kidney Disease. Nephron, 134:25-29. [DOI] [PubMed] [Google Scholar]
- [139].Guo J, Zheng HJ, Zhang W, Lou W, Xia C, Han XT, et al. (2020). Accelerated Kidney Aging in Diabetes Mellitus. Oxid Med Cell Longev, 2020:1234059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Chang-Panesso M (2021). Acute kidney injury and aging. Pediatr Nephrol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Jang IA, Kim EN, Lim JH, Kim MY, Ban TH, Yoon HE, et al. (2018). Effects of Resveratrol on the Renin-Angiotensin System in the Aging Kidney. Nutrients, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Nass N, Bartling B, Navarrete Santos A, Scheubel RJ, Borgermann J, Silber RE, et al. (2007). Advanced glycation end products, diabetes and ageing. Z Gerontol Geriatr, 40:349-356. [DOI] [PubMed] [Google Scholar]
- [143].Liochev SI (2013). Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med, 60:1-4. [DOI] [PubMed] [Google Scholar]
- [144].Chuang PY, Cai W, Li X, Fang L, Xu J, Yacoub R, et al. (2017). Reduction in podocyte SIRT1 accelerates kidney injury in aging mice. Am J Physiol Renal Physiol, 313:F621-F628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Kim SS, Song SH, Kim IJ, Lee EY, Lee SM, Chung CH, et al. (2016). Decreased plasma alpha-Klotho predict progression of nephropathy with type 2 diabetic patients. J Diabetes Complications, 30:887-892. [DOI] [PubMed] [Google Scholar]
- [146].Rubinsztein DC, Marino G, Kroemer G (2011). Autophagy and aging. Cell, 146:682-695. [DOI] [PubMed] [Google Scholar]
- [147].Xu WW, Guan MP, Zheng ZJ, Gao F, Zeng YM, Qin Y, et al. (2014). Exendin-4 alleviates high glucose-induced rat mesangial cell dysfunction through the AMPK pathway. Cell Physiol Biochem, 33:423-432. [DOI] [PubMed] [Google Scholar]
- [148].Moon JY, Woo JS, Seo JW, Lee A, Kim DJ, Kim YG, et al. (2016). The Dose-Dependent Organ-Specific Effects of a Dipeptidyl Peptidase-4 Inhibitor on Cardiovascular Complications in a Model of Type 2 Diabetes. PLoS One, 11:e0150745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Wang X, Zhang H, Zhang Q, Guan M, Sheng S, Mo W, et al. (2020). Exenatide and Renal Outcomes in Patients with Type 2 Diabetes and Diabetic Kidney Disease. Am J Nephrol, 51:806-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Fraser SDS, Roderick PJ (2019). Kidney disease in the Global Burden of Disease Study 2017. Nat Rev Nephrol, 15:193-194. [DOI] [PubMed] [Google Scholar]
- [151].Tuttle KR, Lakshmanan MC, Rayner B, Busch RS, Zimmermann AG, Woodward DB, et al. (2018). Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicentre, open-label, randomised trial. Lancet Diabetes Endocrinol, 6:605-617. [DOI] [PubMed] [Google Scholar]
- [152].Mann JFE, Hansen T, Idorn T, Leiter LA, Marso SP, Rossing P, et al. (2020). Effects of once-weekly subcutaneous semaglutide on kidney function and safety in patients with type 2 diabetes: a post-hoc analysis of the SUSTAIN 1-7 randomised controlled trials. Lancet Diabetes Endocrinol, 8:880-893. [DOI] [PubMed] [Google Scholar]
- [153].Gerstein HC, Colhoun HM, Dagenais GR, Diaz R, Lakshmanan M, Pais P, et al. (2019). Dulaglutide and renal outcomes in type 2 diabetes: an exploratory analysis of the REWIND randomised, placebo-controlled trial. Lancet, 394:131-138. [DOI] [PubMed] [Google Scholar]
- [154].Kristensen SL, Rorth R, Jhund PS, Docherty KF, Sattar N, Preiss D, et al. (2019). Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol, 7:776-785. [DOI] [PubMed] [Google Scholar]
- [155].Pasternak B, Wintzell V, Eliasson B, Svensson AM, Franzen S, Gudbjornsdottir S, et al. (2020). Use of Glucagon-Like Peptide 1 Receptor Agonists and Risk of Serious Renal Events: Scandinavian Cohort Study. Diabetes Care, 43:1326-1335. [DOI] [PubMed] [Google Scholar]
- [156].Xie Y, Bowe B, Gibson AK, McGill JB, Maddukuri G, Yan Y, et al. (2020). Comparative Effectiveness of SGLT2 Inhibitors, GLP-1 Receptor Agonists, DPP-4 Inhibitors, and Sulfonylureas on Risk of Kidney Outcomes: Emulation of a Target Trial Using Health Care Databases. Diabetes Care, 43:2859-2869. [DOI] [PubMed] [Google Scholar]
- [157].Zelniker TA, Wiviott SD, Raz I, Im K, Goodrich EL, Furtado RHM, et al. (2019). Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation, 139:2022-2031. [DOI] [PubMed] [Google Scholar]
- [158].Kidney Disease: Improving Global Outcomes Diabetes Work G (2020). KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int, 98:S1-S115. [DOI] [PubMed] [Google Scholar]
- [159].Feest TG, Round A, Hamad S (1993). Incidence of severe acute renal failure in adults: results of a community based study. BMJ, 306:481-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Kaakeh Y, Kanjee S, Boone K, Sutton J (2012). Liraglutide-induced acute kidney injury. Pharmacotherapy, 32:e7-11. [DOI] [PubMed] [Google Scholar]
- [161].Dubois-Laforgue D, Boutboul D, Levy DJ, Joly D, Timsit J (2014). Severe acute renal failure in patients treated with glucagon-like peptide-1 receptor agonists. Diabetes Res Clin Pract, 103:e53-55. [DOI] [PubMed] [Google Scholar]
- [162].Sharma T, Paixao R, Villabona C (2019). GLP-1 agonist associated acute kidney injury: A case report and review. Diabetes Metab, 45:489-491. [DOI] [PubMed] [Google Scholar]
- [163].Pendergrass M, Fenton C, Haffner SM, Chen W (2012). Exenatide and sitagliptin are not associated with increased risk of acute renal failure: a retrospective claims analysis. Diabetes Obes Metab, 14:596-600. [DOI] [PubMed] [Google Scholar]
- [164].Zhao M, Sun S, Huang Z, Wang T, Tang H (2020). Network Meta-Analysis of Novel Glucose-Lowering Drugs on Risk of Acute Kidney Injury. Clin J Am Soc Nephrol, 16:70-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Que Q, Guo X, Zhan L, Chen S, Zhang Z, Ni X, et al. (2019). The GLP-1 agonist, liraglutide, ameliorates inflammation through the activation of the PKA/CREB pathway in a rat model of knee osteoarthritis. J Inflamm (Lond), 16:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Chen J, Xie JJ, Shi KS, Gu YT, Wu CC, Xuan J, et al. (2018). Glucagon-like peptide-1 receptor regulates endoplasmic reticulum stress-induced apoptosis and the associated inflammatory response in chondrocytes and the progression of osteoarthritis in rat. Cell Death Dis, 9:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Mei J, Sun J, Wu J, Zheng X (2019). Liraglutide suppresses TNF-alpha-induced degradation of extracellular matrix in human chondrocytes: a therapeutic implication in osteoarthritis. Am J Transl Res, 11:4800-4808. [PMC free article] [PubMed] [Google Scholar]
- [168].Gurjar AA, Kushwaha S, Chattopadhyay S, Das N, Pal S, China SP, et al. (2020). Long acting GLP-1 analog liraglutide ameliorates skeletal muscle atrophy in rodents. Metabolism, 103:154044. [DOI] [PubMed] [Google Scholar]
- [169].Hong Y, Lee JH, Jeong KW, Choi CS, Jun HS (2019). Amelioration of muscle wasting by glucagon-like peptide-1 receptor agonist in muscle atrophy. J Cachexia Sarcopenia Muscle, 10:903-918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, et al. (2017). Cellular senescence drives age-dependent hepatic steatosis. Nat Commun, 8:15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Armstrong MJ, Hull D, Guo K, Barton D, Hazlehurst JM, Gathercole LL, et al. (2016). Glucagon-like peptide 1 decreases lipotoxicity in non-alcoholic steatohepatitis. J Hepatol, 64:399-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. (2021). A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med, 384:1113-1124. [DOI] [PubMed] [Google Scholar]
- [173].Petit JM, Verges B (2017). GLP-1 receptor agonists in NAFLD. Diabetes Metab, 43 Suppl 1:2S28-22S33. [DOI] [PubMed] [Google Scholar]
- [174].Mansur RB, Ahmed J, Cha DS, Woldeyohannes HO, Subramaniapillai M, Lovshin J, et al. (2017). Liraglutide promotes improvements in objective measures of cognitive dysfunction in individuals with mood disorders: A pilot, open-label study. J Affect Disord, 207:114-120. [DOI] [PubMed] [Google Scholar]