Abstract
Background
A key mitigation strategy to the COVID-19 pandemic has been the development and roll-out of vaccines. However, pregnant and lactating people were not included in initial vaccine trials and this population is hesitant to receive the vaccine, despite contrary recommendations from the American College of Obstetrics and Gynecology and the Centers for Disease Control and Prevention. Understanding the reasons behind this hesitancy is vital to promote vaccine uptake.
Methods
We surveyed pregnant people in California from December 2020 to January 2021 (n = 387) to describe cognitions and decision-making regarding COVID-19 vaccination. Using descriptive, regression-based analyses, we examined rates of planned uptake and reasoning among individuals who reported COVID-19 vaccine hesitancy.
Results
Overall, the pregnant Californians that we surveyed were aware of the COVID-19 vaccines. Of 387 participants, 43% reported planning to get the vaccine as soon as possible. The remaining 57% were hesitant: 27% responded that they would not receive the vaccine. Some demographic features did predict more COVID-19 vaccine hesitancy, particularly younger age (AOR = 0.95, p = 0.025) and living in a less urban context (AOR = 0.80, p = 0.041). Essential worker status also was associated with vaccine hesitancy. Having had, or intending to have, a flu vaccine was negatively associated with COVID-19 vaccine hesitancy (p < 0.001). The most commonly reported reason for COVID-19 vaccine hesitancy was “I don’t know enough about the vaccine.” Low levels of self-reported knowledge were highly predictive of hesitancy.
Conclusions
Terms like “vaccine hesitance” and “anti-vax” do not adequately characterize decisions regarding delaying COVID-19 vaccination. Rather, these decisions are largely based on the lack of knowledge about the impacts of vaccination on pregnancy, fetal development, and later child wellbeing. This lack of knowledge should be countered by conversations between individual healthcare providers and their pregnant patients, and better inclusion of pregnant people and children in vaccine trials.
Keywords: COVID-19, SARS-CoV-2, Pregnancy, Vaccination, Vaccine hesitancy, California, Health decision-making
1. Introduction
More than one year into the COVID-19 pandemic there have been over 170 million confirmed cases and nearly 4 million deaths worldwide [1], making it one of the deadliest pandemics in history. A key mitigation strategy has been the rapid development of vaccines, which began in earnest in March 2020 [2]. After completion of Phase 3 trials for multiple vaccines, an emergency use authorization (EUA) for the first COVID vaccine in the United States was granted on December 11, 2020. As of February 2021, at least seven different vaccines have been rolled out worldwide, and the United States Food & Drug Administration (FDA) has granted EUAs for three, which are currently being distributed across the country [3].
Data from Phase 3 trials demonstrated that COVID-19 vaccines are safe and effective at preventing severe disease and death [4], [5]. However, pregnant and lactating people were not included in these trials, so there were no safety data in these populations. Despite their lack of inclusion, the American College of Obstetrics and Gynecology (ACOG) and the Centers for Disease Control and Prevention (CDC) has recommended that pregnant and lactating individuals who are otherwise eligible to receive the vaccine be allowed to access them [6]. ACOG specifically has advised obstetric providers that they should feel confident recommending their patients get the vaccine as soon as it is available to them, but also to weigh the risks and benefits individually on a patient-by-patient basis [7]. This recommendation is consistent with emerging evidence on COVID-19 vaccination in pregnant and lactating people, which is showing that these vaccines are well tolerated, generate robust immunity in recipients, and transfer immunity to neonates via placenta and breastmilk [8]. These positive findings are especially important given that pregnant people are at higher risk than the general population for COVID-19 complications [9], [10].
Hesitancy to receive vaccination and concerns about vaccination, especially during pregnancy, have increased since the mid-1990s. For purposes of this paper, “vaccine hesitance” refers to the general predisposition to avoid getting a vaccine for any reason (including simply needing more information before making a decision). While some hesitance may be related to the newness of the COVID-19 vaccine, anti-vaccination sentiment in general has remained high, per Google page searches, and anti-vaccine reasoning remains a stubborn public health problem even when national and global burden from the COVID-19 pandemic has been so high [11]. The most recent data show suboptimal uptake of recommended vaccines during pregnancy, with just 40% of pregnant people receiving both the influenza and Tdap vaccines in the 2019–20 flu season [12]. For COVID-19 vaccination specifically, Ruiz and Bell (2021) found that 23% of people in a nationally representative US study were unsure about getting the vaccine and 15% were decidedly not going to receive it [13]. The major drivers of hesitancy and refusal included being male, being older, being white, and having less knowledge of vaccines [13]. In a 16-country online survey of pregnant women and mothers of young children that was conducted before the COVID-19 vaccines were authorized, Skjefte et al. [14] found that when given hypothetical information regarding a “highly (90%) effective” vaccine, nearly 70% of pregnant women indicated that they would accept the hypothetical vaccine, although only 52% reported that they would accept the vaccine during their pregnancy. In the US, however, that number was considerably lower with nearly 60% of pregnant women reporting they would not, or would very likely not, get the COVID-19 vaccine despite theoretical high efficacy.
Understanding vaccine hesitancy is critical both to mitigating the COVID-19 pandemic and to preparing for the next global health crisis, which is not a matter of if, but when. Studies show that simply passing on evidence and knowledge does not reduce vaccine hesitancy, and can even backfire, for the general population [15]. Other attitudinal interventions, such as combatting conspiratorial thinking and reactance, are needed to combat the trend of people simply rejecting science in the name of preserving their freedoms or rights [15]. The question remains, however, whether sharing knowledge and evidence is more useful in pregnant populations. The perinatal period is commonly represented as a “teachable moment” where motivation, capability, and opportunity to engage in more healthful behaviors might be increased [16]. Understanding whether information sharing translates into higher vaccination rates, or lower vaccine hesitancy, among pregnant populations with regard to the COVID-19 pandemic remains to be seen.
The purpose of this study was to address some of these gaps in our understanding of vaccine uptake among pregnant people in order to recommend strategies for effectively engaging with pregnant patients on vaccine decision-making. Specifically, we aimed to answer the following research questions:
-
(1)
Were pregnant Californians aware of the new COVID-19 vaccines available during the winter of 2020–2021?
-
(2)
What proportion were planning to get the vaccine as soon as eligible, and what proportion were ‘hesitant’?
-
(3)
Which intra-individual features predicted who was hesitant to get vaccinated in pregnancy and how did these features compare to those identified in studies of non-pregnant, vaccine-hesitant adults?
-
(4)
What were the self-reported reasons for COVID-19 vaccine hesitancy, and how important were each of the reasons to these participants’ decision-making?
-
(5)
Does self-reported knowledge and/or source of information about the newly developed COVID-19 vaccines predict hesitancy?
2. Methods
2.1. Participants
Pregnant Californians were offered a web-based survey beginning two weeks after the first COVID-19 vaccine EUA, from December 24th 2020 to January 27th 2021. Surveys took approximately 30 min to complete and participants were offered a gift card for submitting the survey. We recruited participants using StudyPages, which is a web-based platform that solicits participants using targeted social media campaigns. The recruitment materials and surveys were available in both English and Spanish. Eligibility criteria included: currently pregnant; residing in California; and participant age between the ages of 18 and 45. Informed consent was obtained via web-based survey. Ethics approval was provided prior to the start of the study by University of California, Davis’ Institutional Review Board. All methods, including informed consent procedures, were carried out in accordance with the Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects.
In total, 454 people completed the survey. Participants who attempted to take the survey multiple times, who completed the survey in <10 min, or who did not reach the end of the survey instrument had their data removed prior to analysis to ensure data quality. Additionally, participant age was asked twice, once at the beginning of the survey and once at the end; any participant without matching responses for these two items also was removed prior to analyses. In all, 62 participant surveys were not included in the analytic sample as a result of these quality checks. Sporadic missingness was handled using list-wise deletion. The resulting analytic samples are comprised of 387 participants. See Table 1 for Demographic features of the analytic sample.
Table 1.
Demographic characteristics, n = 387.
| Characteristic | N | (%) | |
|---|---|---|---|
| Maternal age | |||
| 18–35 | 308 | (71.1) | |
| 35+ | 125 | (28.9) | |
| Participant is essential worker | |||
| Yes | 103 | (23.8) | |
| No | 112 | (25.9) | |
| Not currently employed/no answer | 218 | (50.3) | |
| Ethnicity | |||
| Hispanic | 149 | (34.4) | |
| Not Hispanic | 280 | (64.7) | |
| No answer | 4 | (0.9) | |
| Race | |||
| White | 233 | (53.8) | |
| Black/African American | 20 | (4.6) | |
| Indigenous/First Nations | 4 | (0.9) | |
| Asian | 35 | (8.1) | |
| Pacific Island/Native Hawaiian | 1 | (0.2) | |
| Other race | 37 | (8.6) | |
| Multiracial | 82 | (18.9) | |
| No answer | 21 | (4.9) | |
| Urbanicity | |||
| Rural | 22 | (5.1) | |
| Semi-rural | 51 | (11.8) | |
| Suburban | 191 | (44.1) | |
| Urban | 99 | (22.9) | |
| Major metropolitan | 63 | (14.6) | |
| Parity | |||
| Primipara | 206 | (47.8) | |
| Multipara | 214 | (49.7) | |
| Grand Multipara | 11 | (2.6) | |
2.2. Measures
2.2.1. COVID-19 vaccination awareness and knowledge
Participants were asked about vaccine awareness via the following survey item: “Do you know that there are new COVID-19 vaccines available in the United States?” Responses were recorded as yes/no and were treated dichotomously in analysis. To assess vaccine knowledge, participants were asked: “How much do you know about the new COVID-19 vaccines?” Responses were recorded on a sliding scale from 0 (No information) to 10 (More information than I need). Up to 1 decimal place was allowed in the responses, and responses were treated as continuous. Participants also were asked about where they primarily receive information about the COVID-19/coronavirus vaccine. Participants were asked to drag-and-drop up to 3 sources of information from the following list of 10 sources: My health care provider (e.g., doctor or nurse); Other health care source (e.g., hospital, pharmacy); President’s press conferences; Governor’s press conferences; Other government source (e.g., CDC, department of public health [DPH]); My friends and family; My religious leader or institution; My employer; Other, write in; and I prefer not to answer. These indicators were then collapsed into the following 4 categories: individual healthcare sources (e.g., doctor, nurses, hospital, pharmacy); governmental sources (Governor or President’s press conferences); public health agencies (e.g., CDC, DPH); and their social network (e.g., friends, family, employer, religious leaders). Each source category was treated as dichotomous in analysis.
2.2.2. Demographic characteristics and other intra-individual factors
Participant age was measured in years and treated as continuous. Urbanicity was captured with the following question: “How would you describe the city in which you live?” within 5 categories: 1 – Rural (e.g., town <2,500 people); 2 – Semi-rural (e.g., town more than 2,500 people but <20,000 people); 3 – Suburban (e.g., city or town more than 20,000 people but <250,000 people); 4 – Urban (e.g., city more than 250,000 people but <1,000,000 people); 5 – Major metropolitan area (e.g., city more than 1,000,000 people). Responses were treated as continuous in analysis. Essential worker status, as well as partner essential worker status, was measured with two items: “Are you an essential employee?” and, for those who reported having a cohabitating partner, “Is your partner an essential employee?”. Responses were yes, no, “I prefer not to answer”, and “Not working full time” for each item. Responses were treated as categorical, with yes responses compared to no and not currently working. Ethnicity was measured dichotomously (Hispanic and Non-Hispanic), and race (i.e., social stratification based on minoritization in a white supremacist context) was measured as mutually exclusive categories: white only; Black/African American only; Indigenous/American Indian/Native Alaskan only; Asian/Asian-American/Pacific Islander/Native Hawaiian only; some other race only; and Multiracial. Responses were treated categorically in analysis. Primiparity was measured dichotomously based on whether the participant had given birth to at least one living child. Intention to receive a seasonal influenza vaccine was measured using two items: first, participants were asked “Did you receive a seasonal flu vaccine (the flu shot) during the fall/winter of 2020?” (yes/no/unsure). If the participant responded anything other than ‘yes’, they were asked: “Do you plan to get the seasonal flu vaccine this season?” (yes/no/unsure). Flu vaccine hesitancy was defined as responding negatively to both items (i.e., not having had, nor intending to receive, the flu vaccine this season), and was treated dichotomously in analysis.
COVID-19 Vaccine Hesitancy and Reasons for Hesitancy. Participants were asked “Do you plan to receive the new COVID-19 vaccine when it is available for you?” (yes/no/unsure). Those who responded ‘no’ and ‘unsure’ were then prompted to identify the sources of their hesitancy with the following item: “We would like to know the reasons you do not plan to receive the COVID-19 vaccine. For each statement, please mark how much you agree, and how important it is in your decision to NOT receive the COVID-19 vaccine.” There were 9 possible reasons for vaccine hesitancy provided to the respondents, as well as an option for “Some other reason (please enter other reason below)”, with open-ended response type provided. See Table 2 for the wording of provided reasons. Endorsement (i.e., how much do you agree with the following statement) was measured using a Likert-type scale from 1 – Strongly Disagree to 4 – Strongly Agree. Importance (i.e., how important is this to you?) was also measured using a Likert-type scale with the following anchors: 1 – Not at all important; 2 – A little important; 3 – Somewhat important; 4 – Very important; and 5 – The most important reason. Responses for both endorsement and importance were treated continuously in analysis.
Table 2.
Reasons for COVID-19 Vaccine Hesitancy among Pregnant Californians.
| Average Endorsement | Average Importance | |
|---|---|---|
| (1–4) | (1–5) | |
| Reason for Hesitancy | Strong Disagree to Strong Agree | Not at all to Most Important |
| I don't know enough about the vaccine | 3.1 | 4.3 |
| Vaccine is not safe | 2.3 | 3.7 |
| Some other reason | 2.7 | 3.6 |
| Vaccine is not effective | 2.0 | 3.3 |
| COVID-19 isn't a serious illness | 1.4 | 3.1 |
| Others should get the vaccine, but I should not | 2.3 | 3.0 |
| I do not trust the vaccine makers | 2.0 | 2.8 |
| I do not want authorities telling me what to do | 1.9 | 2.5 |
| Immunizations are not good for anyone | 1.5 | 2.5 |
| Fear of needles or injections | 1.6 | 2.0 |
2.3. Data analysis
First, we described the proportion of survey participants who were aware of the newly developed COVID-19 vaccines, as well as the proportion of the sample that was hesitant to receive the new vaccines when available (research questions 1 and 2). Next, we examined which individual-level factors predicted COVID-19 vaccine hesitancy in our sample. Demographic features were examined together in a multivariate ordinary-least squares (OLS) regression model. Essential worker status and flu-vaccine hesitancy were examined as predictors in their own individual models using unadjusted analyses, with p-values set to 0.05 significance (research question 3). Finally, we described reasons for COVID-19 vaccine hesitancy, including descriptions of both endorsement and importance of 9 closed-ended reasons and descriptive counts of open-ended reasons for those participants which supplied an additional reason for hesitancy (research question 4). We also explored whether knowledge about, and sources of information about, the newly developed vaccines predicted vaccine hesitancy using multivariate logistic regression models and unadjusted ANOVA and analyses to explore additional predictive models that are outside of the primary research questions (research question 5).
3. Results
Research Question 1. Overwhelmingly, the pregnant Californians that we surveyed were aware of the newly developed COVID-19 vaccines at the time of the survey (98.7%).
Research Question 2. Of the 387 participants, 167 (43%) reported planning to get the vaccine as soon as it is offered to them. The remaining 57% were hesitant: 104 (27%) responded that they would not receive the vaccine as soon as it is offered, and the remaining 116 (30%) were unsure.
Research Question 3. We found that some demographic features did predict more COVID-19 vaccine hesitancy, particularly younger participant age (AOR = 0.95, p = 0.025) and living in a less urban context (AOR = 0.80, p = 0.041). Other demographic features (race, ethnicity, primiparity) did not predict hesitancy. See Table 3 for the fully adjusted demographic model results.
Table 3.
Association between COVID-19 vaccine hesitancy and demographic factors (n = 387).
| β | (Std. Err.) | p-value | Sig. | ||
|---|---|---|---|---|---|
| Participant age | 0.95 | (0.02) | 0.025 | * | |
| Participant race | |||||
| Black/African American only | 1.04 | (0.54) | 0.942 | ||
| Indigenous/First Nations only | 0.95 | (1.40) | 0.973 | ||
| Asian/Asian-American/Pacific Islander only | 1.09 | (0.41) | 0.812 | ||
| Some other race only | 0.89 | (0.35) | 0.765 | ||
| Biracial/Multiracial | 1.36 | (0.42) | 0.325 | ||
| Hispanic ethnicity | 1.09 | (0.29) | 0.748 | ||
| Primiparity | 0.89 | (0.21) | 0.624 | ||
| Urbanicity | 0.80 | (0.09) | 0.041 | * | |
Note: Based on logistic regression in a fully adjusted model.
*p < 0.05; **p < 0.01; ***p < 0.001.
Essential worker status also was associated with vaccine hesitancy: unadjusted analyses showed 64% of those not currently working were hesitant to get the vaccine, 56% of essential workers were hesitant, and 44% of non-essential workers were hesitant (p = 0.01). There was a statistical trend toward those with partners who are essential workers being more hesitant as well: 61% of respondents with essential-worker partners were hesitant, compared to 51% of those with non-essential-worker partners (p = 0.052). To explore this non-statistically significant but also counterintuitive finding, we tested whether results might have been due to actual infection rates of COVID-19 in the previous year. We found this to indeed be a plausible explanation: 12% of non-essential workers had tested positive for COVID-19 at some point, while 18% of essential workers had tested positive ( = 9.76, p = 0.045). Further, there was a statistical trend toward those with a history of COVID-19 infection reporting more hesitance to get the vaccine (65%) than those without a history of COVID infection (53%; p = 0.080).
Having had, or intending to have, a flu vaccine was also strongly negatively associated with COVID-19 vaccine hesitancy: 78 participants indicated that they had not gotten, nor were they intending to receive, a seasonal flu vaccine. Of those 78, 90% (70) were also COVID vaccine hesitant. Meanwhile, of the 287 participants who had either gotten, or intended to get, a flu shot, only 46% (132) were COVID vaccine hesitant ( = 47.50, p < 0.001).
Research Question 4. The most reported reason for COVID-19 vaccine hesitancy was “I don’t know enough about the vaccine”. On the 1–4 Likert-type endorsement scale, the average endorsement was between 3 – Agree and 4 – Strongly Agree. Furthermore, 63% of the participants who responded to that item indicated that this reason was the most important reason for their hesitancy (5 on the 1–5 scale). See Table 2 for descriptive statistics on both endorsement and importance of the various closed-ended hesitancy response choices.
Participants also were permitted to write in another reason for their hesitancy; 77 participants chose to respond to this option. Once again, by far the most common response was uncertainty, lack of knowledge, or discomfort with the extent to which the new vaccines had been tested on pregnant people. For example, common open-ended responses included phrases such as: “Unaware of the vaccine effects on pregnant women and unborn child”, “I do not wish to get the vaccine while pregnant”, “Don’t know if it’s recommended to pregnant women”, and “Very limited data in pregnancy/breastfeeding”.
Research Question 5. Self-reported knowledge was highly predictive of hesitancy; in adjusted models (controlling for age, primiparity, race, ethnicity, and urbanicity), each unit increase in self-reported knowledge was associated with a 18% reduction in hesitancy (AOR = 0.82, p < 0.001).
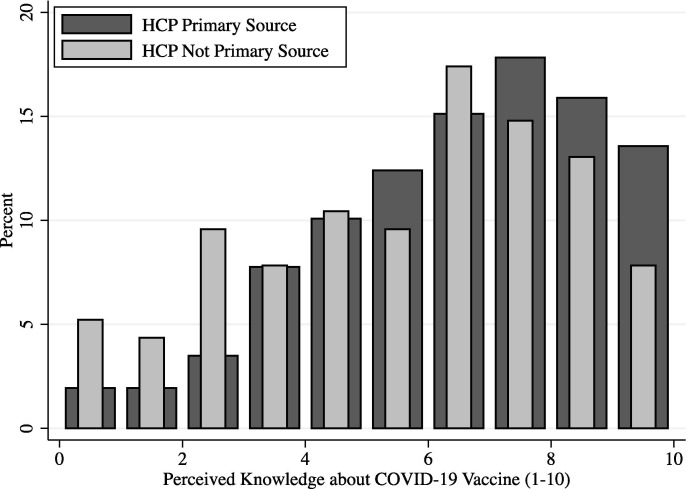
The primary source of COVID-19 vaccine information did not predict vaccine hesitancy in adjusted or unadjusted models. However, source of vaccine information was predictive of self-reported knowledge about the vaccines: unadjusted ANOVA models showed that only individual healthcare providers as information sources on COVID vaccines were predictive of more vaccine knowledge (p = 0.042). Other sources of information – including public health agencies, government sources, and the participants’ social network – were not predictive of vaccine knowledge. Fig. 1 shows the distribution of self-reported knowledge of the vaccine (1 – No information to 10 – More information than I need) for those who reported primarily receiving COVID vaccine information from their individual healthcare providers as compared to those who did not primarily receive COVID-19 vaccine information from their healthcare providers.
Fig. 1.
Distribution of Perceived Knowledge about COVID-19 Vaccines, by Primary Source of COVID-19 Vaccination Information. The distribution of self-reported knowledge of the vaccine is displayed from 1 – No information to 10 – More information than I need for those who reported primarily receiving COVID vaccine information from their individual healthcare providers (HCP Primary Source) as compared to those who did not primarily receive COVID-19 vaccine information from their healthcare providers (HCP Not Primary Source).
4. Discussion
We surveyed pregnant Californians regarding their knowledge of COVID-19 vaccines, their vaccination intentions, reasons for vaccine hesitancy, and associated individual and family characteristics. Unlike studies of COVID-19 vaccination intentions in the general population [13], [14], few demographic characteristics were associated and younger, rather than older, pregnant people reported more hesitance. Younger individuals in our study also were less likely to engage in related health-promoting behaviors, including receiving a flu shot and COVID-19 mitigation strategies such as mask wearing and physical distancing. Respondents who lived in more rural areas and who were not working full-time also reported more vaccine hesitancy. These findings are not surprising. Individuals who may be isolating at home and living in an area with low population density likely feel more comfortable delaying vaccination because they perceive lower risk for COVID-19 infection. Race, ethnicity, and primiparity were not associated with vaccine hesitancy in this sample. Given the lower rates of COVID-19 vaccination among people of color in general [17], pregnant people of color should be a focus of vaccination efforts. Likewise, although previous studies have suggested that primiparity is a main factor associated with vaccine hesitancy [18], at least among this sample of pregnant Californians, this is not a barrier and should be presented thoughtfully in discussions of prenatal vaccine options.
Interestingly, essential worker status, and to a lesser extent, the essential worker status of a respondent’s cohabitating partner, predicted more vaccine hesitancy within our sample. This finding was surprising and counter to those from Ruiz and Bell (2021), who found that individuals who perceived themselves as more vulnerable to infection were less hesitant. Additional analyses to better understand this finding did show that essential workers were more likely to have already contracted COVID-19. Thus, these individuals may perceive themselves to have acquired “natural immunity” and not in need of the vaccine. Future research should investigate attitudes associated with COVID-19 vaccine intentions among individuals with previous infections, and, given data that suggest vaccine-conferred immunity is more robust and longer lasting, educational efforts to improve vaccine uptake among previously infected individuals should be a focus of public health vaccination efforts.
It is important to note that almost everyone who took our survey was aware that COVID-19 vaccines were available, but few reported being knowledgeable about them. As an apparent result of this lack of knowledge, more than half of the pregnant people surveyed were hesitant to be vaccinated themselves. Refusing the influenza vaccination was highly predictive of COVID-19 vaccination hesitancy. However, many of those who had received, or intended to receive, the influenza vaccine also remained hesitant to receive a COVID vaccine. Thus, it is not simply that these individuals were hesitant to receive any vaccine while pregnant, but rather, the lack of knowledge about the COVID-19 vaccine specifically, and particularly how the vaccine affects pregnant and lactating people and their children, was likely the primary driver of hesitance for these individuals. Further, at the time of this survey, public health agencies and medical professionals had yet to offer consistent guidance on the COVID-19 vaccine for pregnant and lactating people. Given that the mRNA vaccine technology is perceived as novel, as well as the fact that the Phase 3 trials on which the EUAs were granted systematically omitted pregnant and lactating people, this may be a reasonable decision for the pregnant person. However, it also demonstrates that a top priority moving forward is to generate and disseminate knowledge about these new vaccines for pregnant, lactating, and neonatal populations. Importantly, we found that the pregnant people in our survey trusted individual healthcare providers, including doctors, nurses, and pharmacists, to provide them vaccine information. Moving forward, public health agencies should work collaboratively with individual providers to help them disseminate vaccine information to this vulnerable population, including providing talking points, sample brochures and pamphlets that can be customized to the practice, and resource lists. It is also interesting to note that individual providers were most important for pregnant people’s vaccination decision-making, however, our previous study on COVID-19 mitigation behaviors found that public health agencies were most important for pregnant people’s COVID-related behavior changes (e.g., mask wearing, physical distancing). Future studies should investigate the best pathways for disseminating health messaging as it pertains to changing and/or encouraging individual health behaviors in a public health context. Lastly, it may be important to tailor information and education based on what appear to be three sub-types of vaccine decision-makers: (1) those who are generally vaccine hesitant; (2) those who are vaccine hesitant when new vaccines or recommendations emerge; and (3) those who are generally vaccine confident. Our study suggests that a one-size-fits-all approach likely is insufficient to address major public health challenges.
Lastly, our study demonstrates that understanding nuance in “vaccine hesitancy” is important to promoting vaccination. In the literature, vaccine hesitancy is often considered synonymous with “anti-vaccination.” However, that view does not consider the individual circumstances of the decision-maker and whether the decision not to vaccinate reflects an appropriate risk–benefit analysis. For example, someone who can work exclusively from home and whose other household members also can remain at home, as well as who is masking and practicing physical distancing, is at very low risk of contracting COVID-19. Thus, the decision to not vaccinate can be considered a reasonable choice given the lack of data on the vaccine in pregnant people. To label these individuals as “anti-vax” or “anti-science,” or to assume that they have institutional mistrust or some other barrier, is systemically biased and does not respect the agency of the individual. This is especially critical when pregnant people are trying to make evidence-based decisions, but they are having to do so without good evidence, as from participation of pregnant people in the Phase 3 trials. Moving forward, it is critical that we start developing strategies for including pregnant people into vaccine trials from the outset. Now is the time to develop systems to include pregnant people in trials, so that we are prepared to respond when the next pandemic emerges.
Our findings should be considered within the context of its limitations. First, data were from California and findings may not apply to pregnant people in other states or countries. Second, given this was an anonymous survey, respondents who were not pregnant or otherwise ineligible may have falsified answers to obtain the gift card. Although we included several quality checks within the survey (e.g., asking age twice in different sections of the survey), some data may not be from pregnant people. Despite these limitations, our study provides important information about COVID vaccination in pregnancy, which remains a critical public health issue.
5. Conclusions
To address the COVID-19 pandemic effectively, vaccination efforts will require that a vast majority of the population is vaccinated. Understanding hesitancy – and the factors underlying that hesitancy – is crucial in this effort. Given that pregnant people are at higher risk for complications of COVID-19, it is crucial that we generate and disseminate data on the safety and efficacy of these vaccines for pregnant people, as well as develop strategies for including pregnant people in future vaccine trials. We found that among pregnant people in California, there was nearly universal awareness of newly developed vaccines, but that this population is hesitant to receive the vaccine largely due to lack of knowledge about the newly developed vaccines. Healthcare providers working one-on-one with pregnant patients appear to be uniquely situated to boost vaccine knowledge, and therefore acceptance, for this vulnerable population.
Funding
Funding for the current study was provided by Hatch Project #CA-D-HCE-2582-H, Addressing Multifactorial Influences on Pregnancy Outcomes to Promote Health Equity. Funding for Dr. Whipps was provided by 5R01NR017659 (Simmons PI).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.https://covid19.who.int/ [accessed 6/9/2021].
- 2.Corey L., Mascola J.R., Fauci A.S., Collins F.S. A strategic approach to COVID-19 vaccine R&D. Science. 2020;368:948–950. doi: 10.1126/science.abc5312. [DOI] [PubMed] [Google Scholar]
- 3.https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines [accessed 6/9/2021].
- 4.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Creech C.B., Walker S.C., Samuels R.J. SARS-CoV-2 Vaccines. JAMA. 2021;325:1318–1320. doi: 10.1001/jama.2021.3199. [DOI] [PubMed] [Google Scholar]
- 6.https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/vaccinating-pregnant-and-lactating-patients-against-covid-19 [accessed 6/9/2021].
- 7.Rasmussen S.A., Kelley C.F., Horton J.P., Jamieson D.J. Coronavirus Disease 2019 (COVID-19) Vaccines and Pregnancy: What Obstetricians Need to Know. Obstet Gynecol. 2021;137:408–414. doi: 10.1097/AOG.0000000000004290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray K.J., Bordt E.A., Atyeo C., Deriso E., Akinwunmi B., Young N., et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2021.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allotey J., Stallings E., Bonet M., Yap M., Chatterjee S., Kew T., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith V., Seo D., Warty R., Payne O., Salih M., Chin K.L., et al. Maternal and neonatal outcomes associated with COVID-19 infection: A systematic review. PLoS ONE. 2020;15 doi: 10.1371/journal.pone.0234187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pullan S., Dey M. Vaccine hesitancy and anti-vaccination in the time of COVID-19: A Google Trends analysis. Vaccine. 2021;39:1877–1881. doi: 10.1016/j.vaccine.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Razzaghi H., Kahn K.E., Black C.L., Lindley M.C., Jatlaoui T.C., Fiebelkorn A.P., et al. Influenza and Tdap Vaccination Coverage Among Pregnant Women - United States, April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1391–1397. doi: 10.15585/mmwr.mm6939a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz J.B., Bell R.A. Predictors of intention to vaccinate against COVID-19: Results of a nationwide survey. Vaccine. 2021;39:1080–1086. doi: 10.1016/j.vaccine.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skjefte M., Ngirbabul M., Akeju O., Escudero D., Hernandez-Diaz S., Wyszynski D.F., et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36:197–211. doi: 10.1007/s10654-021-00728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hornsey M.J., Harris E.A., Fielding K.S. The psychological roots of anti-vaccination attitudes: A 24-nation investigation. Health Psychol. 2018;37:307–315. doi: 10.1037/hea0000586. [DOI] [PubMed] [Google Scholar]
- 16.Olander E.K., Darwin Z.J., Atkinson L., Smith D.M., Gardner B. Beyond the 'teachable moment' – A conceptual analysis of women's perinatal behaviour change. Women Birth. 2016;29:e67–e71. doi: 10.1016/j.wombi.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Reverby S.M. Racism, disease, and vaccine refusal: People of color are dying for access to COVID-19 vaccines. PLoS Biol. 2021;19 doi: 10.1371/journal.pbio.3001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosso A., Massimi A., Adamo G., Baccolini V., Pitini E., Vacchio M.R., et al. A systematic review of factors influencing pregnant women’s future vaccination choices. Eur J Pub Health. 2019;29 [Google Scholar]