Abstract
Objective
To assess the safety and efficacy of bronchial artery embolization (BAE) for hemoptysis.
Methods and materials
Databases with articles published in English, including Pubmed, Embase, Web of science and Chochrane library, were comprehensively searched to get accurate, up-to-date and sufficient literature about BAE for hemoptysis until March 2020. The technical success rates, immediate control rates, recurrence rates, mortality rates, and total complication rates (minor and major complication rates) extracted from the articles were pooled to estimate and assess the efficacy and safety of BAE using random-effect and fixed-effect models.
Results
21 articles published between 2008 and 2019, which include a total of 2511 patients, were studied to evaluate the safety and efficacy of BAE. The technical success and immediate control rates are 99.9% (95%CI: 99%–100%) and 99.5% (95%CI: 97.8%–99.2%), respectively. This study showed hemoptysis recurrence in 23.7% (95%CI: 18.5%–28.9%) with a mortality rate of 2% (95%CI: 0–3%). Additionally, the assessment of complications revealed a total complication rate of 13.4% (95% CI: 7.6–19.2%), in which 0.2% (95% CI: 0.2–0.4%) were major complications and 10% (95% CI: 4.7–9.6%) were minor complications.
Conclusion
BAE is an effective, safe, and feasible procedure with a low complication rate for hemoptysis patients. However, recurrence of hemoptysis is still at high risk after BAE due to different underlying diseases.
Keywords: BAE, Hemoptysis, Efficacy, Safety
1. Introduction
Hemoptysis1 is defined as the expectoration of blood, alone or in combination with mucus, from the lower respiratory tract. In a cohort study involving 762325 patients, hemoptysis occurred in 4812 patients as an alarm symptom.2 Etiological surveys have shown that the most common causes of hemoptysis are airway infections, bronchial carcinoma/metastases, and bronchiectasis/cystic fibrosis.3 Generally, hemoptysis can be classified as bloody sputum, mild (<10 mL/d), moderate (100–500 mL/d), or massive hemoptysis (>500 mL/d). Massive hemoptysis can be life-threatening because massive bleeding obstructs the airways. Researchers have reported that the mortality rate of severe hemoptysis was over 50% in uncontrolled bleeding4; therefore, the diagnosis and treatment of hemoptysis should be implemented immediately.
Mild or moderate hemoptysis is often treated with vasoactive drugs and conservative treatment measures, whereas massive hemoptysis requires more aggressive management, including bronchoscopy and surgical treatment.5 Bronchoscopy has clinical significance in controlling bleeding and protecting airways, and its immediate massive hemoptysis control rate is nearly 100%.6 However, the hemostatic effect of bronchoscopy usually last for a short time. Therefore, other procedures should be immediately taken to achieve durable hemostasis when bronchoscopy exerts a poor effect on acute bleeding. From another perspective, the therapeutic effects of bronchoscopy might be constrained when the bleeding site is beyond the reach of bronchoscopy. Surgical treatment is often the second-line therapy for hemoptysis, but the mortality rate of patients who received surgery was approximately 40%.7,8 Additionally, patients with poor lung function, bilateral pulmonary lesions, and other comorbidities are not candidates for surgery.
Considering that the bronchial arteries are the culprit vessels of hemoptysis,9 bronchial artery embolization (BAE) was first introduced as a minimally invasive endovascular treatment for hemoptysis in 1973. Since then, BAE has been widely used for hemoptysis. The technique has improved significantly, especially with the emergence of the “superselective” BAE, which improved the immediate bleeding control rate to >70%. In addition, the procedure can be safely performed even in recurrent hemoptysis.10 Severe BAE procedure-related complications are rare.11 At present, BAE is considered an effective method for treating massive or recurrent hemoptysis. An array of clinical trials12, 13, 14, 15 has been conducted to evaluate the clinical effects of the BAE in managing hemoptysis; however, the meta-analysis on this method is still lacking. This meta-analysis aimed to analyze the efficacy and safety of BAE in the treatment of hemoptysis and provide evidence for guidelines.
2. Method and materials
2.1. Study selection
To assess the efficacy and safety of BAE in patients with hemoptysis, two researchers (Zheng and Zhuang) individually searched the online literature databases of PubMed, Embase, Web of Science, and Cochrane Library, in accordance with March 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The search strategies were as follows: bronchial artery embolization (BAE) and hemoptysis. Additional literature was searched and screened manually. The discrepancy concerning article selection was resolved by another author after analysis and discussion. The Institutional Review Board approval or exemption was not necessary for this study due to no original human and animal information.
2.2. Included and excluded criteria
Rigorous literature publication inclusion standards have been established, and only studies that met all of the criteria were included. The inclusion criteria for the studies were as follows: (1) original study is in English or Chinese; (2) the patients presented with massive or moderate hemoptysis; (3) patients with hemoptysis show ineffectiveness after pharmacologic treatment and endoscopy;(4) the patients were treated by embolotherapy; (5) follow-up data was complete and with a follow-up duration of >3 three months; (6) clinical outcomes were reported with the proportions in the article (i.e., technical success, recurrence rate, mortality rate, major complications, and minor complications); and (7) publication date between 2009 and 2020.
Studies were excluded if they met any one of the criteria as follows: (1) review article, systematic review, meta-analysis, comments, discussion, editorials, case reports, animal experiments, and conference papers; (2) duplicate articles reporting the same data; (3) studies without prognostic and survival data; and (4) full-texts were not retrieved, and attempts to contact the author but failed.
2.3. Data extraction
Two investigators (Zheng and Zhuang) independently screened all articles and extracted relevant data from each research based on the established protocol. Any disagreements regarding data extraction were addressed by consensus after discussion. The demographics, characteristics, and primary clinical outcome data were extracted, including author, year, country, number of patients, sex, etiology, technical success rate, immediate control rate, total recurrence rate, major complication rate, minor complication rate, and mortality rate after BAE.
Technical success was defined as successful embolization of targeted arteries, and immediate success was defined as the absence of bleeding within 24 h post-BAE. Total recurrence was defined as post-BAE recurrence of hemoptysis during follow-up, as reported in a previous study. The recurrence mortality rate of hemoptysis was the death rate in recurrent hemoptysis patients. Major complications were defined as unplanned sequelae that may require medical intervention during hospitalization or even death, such as spinal injury, severe diaphragmatic palsy, and other unexpected systematic artery embolization. Minor complications are mild self-limiting symptoms that could be relieved by symptomatic treatment or rest.16 The original data were verified twice.
2.4. Statistical analysis
The technical and immediate control rates, total recurrence rates, major complication rates, minor complication rates, and mortality rates extracted from each original article were pooled to estimate and assess the efficacy and safety of BAE. Pooled statistics are expressed as proportions at 95% confidence intervals (CIs). I2 was used to evaluate the heterogeneity of the included articles. The heterogeneity of included articles was classified into three levels: low (I2<50%), moderate (50% < I2 <75%), and considerable heterogeneity (I2>75%). A random-effect model was used to pool the proportions with I2>50%, whereas a fixed-effect model was applied to articles with low heterogeneity. Publication bias was evaluated by a funnel plot and quantified using the method of Begg's test. Statistical significance was defined at p < 0.05. We used STATA/SE 15.1(StataCorp LLC, Texas, USA) to perform the statistical analysis in this study.
3. Results
3.1. Study identification and selection
A total of 843 articles (PubMed, 174; Embase, 546; Web of Science, 115; and Cochrane Library, 8) were searched according to the PRISMA guidelines (Fig. 1). After excluding duplicate articles, 611 articles were included. We subsequently screened the abstracts of each article and found that 304 articles were reviews, systematic reviews, meta-analyses, comments, animal studies, conference papers, and case reports. A total of 176 articles mismatched the literature inclusion standards for research topics and interventions, and were thus excluded, along with 62 studies with unavailable full-texts. After excluding a total of 542 papers, we read the full text of 69 articles, of which 48 articles were excluded. Finally, 21 full-text articles were included in the study.
Fig. 1.
Flow diagram of literature search and selection.
3.2. Baseline characteristics of included studies
Twenty-one studies12, 13, 14, 15,17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 that included a total of 2511 patients who underwent BAE were included. Table 1 provides basic summaries of these studies. Published between 2008 and 2019, these studies reported that various patients received BAE treatment due to the diverse etiologies of tuberculosis (TB), tumor, etc. Demographic data, namely, age, sex, and number, are also listed. Nineteen articles11, 12, 13, 14, 15,17, 18, 19, 20,22, 23, 24,26, 27, 28, 29, 30, 31 were used to pool the data of the technical success rate, while 16 studies12, 13, 14, 15, 16,18,20,22,24,25,27, 28, 29,31, 32, 33 were used for the evaluation of immediate control of hemoptysis information. We also evaluated 16 studies12, 13, 14, 15, 16,18, 19, 20, 21,23,26,27,29,31,32 to estimate recurrence and mortality after a BAE procedure. Finally, we analyzed major and minor complications secondary to BAE to evaluate its safety.
Table 1.
Demographics characteristics of included studies.
| First author (Reference) | Year | Nation | Treatment | No. of patients | Male | Age (Mean) | Etiology |
||
|---|---|---|---|---|---|---|---|---|---|
| Tuberculosis | Tumor | Other | |||||||
| Agmy GM28 | 2013 | Egypt | BAE | 348 | 247 | 45 | 198 | 14 | 136 |
| Chun JY33 | 2010 | UK | BAE | 50 | 24 | 54.9 | 11 | 5 | 34 |
| Baltacioğlu F27 | 2010 | Turkey | BAE | 25 | 19 | 47 | 12 | 4 | 9 |
| Daliri A34 | 2011 | Switzerland | BAE | 28 | 19 | 42 | 2 | 6 | 20 |
| Shin BS22 | 2011 | Korea | BAE | 169 | 129 | 58.58 | 169 | 0 | 0 |
| Dave BR32 | 2011 | USA | BAE | 58 | 28 | 49 | 2 | 16 | 40 |
| Yoo DH20 | 2011 | Korea | BAE | 108 | 66 | 56.2 | 49 | 8 | 51 |
| Bommart S30 | 2012 | France | BAE | 15 | 9 | 62.9 | 0 | 5 | 10 |
| Anuradha C12 | 2012 | India | BAE | 58 | 46 | 43 | 58 | 0 | 0 |
| Shimohira M26 | 2015 | Japan | BAE | 12 | 8 | 64 | 3 | 0 | 9 |
| Lee MK21 | 2015 | Korea | BAE | 852 | 553 | 59.9 | 173 | 64 | 615 |
| Shao H25 | 2015 | China | BAE | 336 | 186 | 57 | 190 | 20 | 126 |
| Mehta AS19 | 2015 | USA | BAE | 26 | 13 | 61.1 | 0 | 26 | 0 |
| Miyano Y17 | 2016 | Japan | BAE | 27 | 23 | 61.7 | 2 | 3 | 22 |
| Pathak V18 | 2016 | USA | BAE | 50 | 30 | 43 | 0 | 4 | 46 |
| Lee H29 | 2017 | Korea | BAE | 26 | 20 | 45 | 0 | 0 | 26 |
| Ayx I13 | 2017 | Germany | BAE | 34 | 25 | 58 | 2 | 19 | 13 |
| Springer DM23 | 2018 | Poland | BAE | 30 | NA | 33 | 0 | 0 | 30 |
| Kucukay F31 | 2018 | Turkey | BAE | 174 | 84 | 39.4 | 18 | 52 | 11 |
| Lee SH15 | 2019 | Korea | BAE | 33 | 15 | 60.0 | 16 | 0 | 17 |
| Shimohira M24 | 2019 | Japan | BAE | 52 | 32 | 70 | 7 | 7 | 38 |
3.3. Technical success rate
Twenty-one of the included studies reported technical success rates ranging from 76.9% to 99.9%. The pooled proportion of technical success rate was 99.9% (95% CI: 99.6–100%; Fig. 2 and Table 2). The heterogeneity analysis showed low heterogeneity in 21 original studies; hence, a fixed-effect model was used (I2 = 44.2%, p = 0.02, Fig. 2 and Table 2).
Fig. 2.
Forest plot for technical success after BAE.
Table 2.
Summary of variables.
| Variables | Study(n) | Event | Total | Proportion | 95%CIs | I2 | p | Model | Publication bias(Begg,s test) |
|---|---|---|---|---|---|---|---|---|---|
| Technical success | 19 | 1294 | 1323 | 0.999 | 0.99–1.00 | 44% | 0.020 | Fixed | <0.01 |
| Immediate control | 16 | 1061 | 1099 | 0.995 | 0.978–0.992 | 41.3% | 0.020 | Fixed | <0.01 |
| Hemoptysis recurrence | 16 | 338 | 1458 | 0.237 | 0.185–0.289 | 73.6% | <0.01 | Random | 0.137 |
| Mortality of recurrence | 21 | 33 | 2511 | 0.002 | 0.000–0.003 | 39% | 0.035 | Fixed | <0.01 |
| Total complication | 9 | 59 | 396 | 0.134 | 0.076–0.192 | 69.1% | 0.001 | Random | 0.076 |
| Major complication | 21 | 14 | 2511 | 0.002 | 0.001–0.004 | 0 | 0.934 | Fixed | 0.291 |
| Minor complication | 8 | 40 | 412 | 0.100 | 0.047–0.096 | 54.3% | 0.032 | Random | 0.004 |
3.4. Immediate control rate
In 16 studies, 1099 patients were referred, and 1061 patients with hemoptysis achieved immediate control of bleeding (Table 2). As shown in Fig. 3, the pooled estimated proportion of immediate control for hemoptysis was 99.5% (95% CI: 97.8–99.2%). Each original study reported that the immediate control rate ranged from 86.0% to approximately 99.5%. The fixed-effect model was utilized due to the relatively low heterogeneity (I2 = 41.3%, p = 0.043).
Fig. 3.
Forest plot for hemoptysis immediate control after BAE.
3.5. Recurrence of hemoptysis
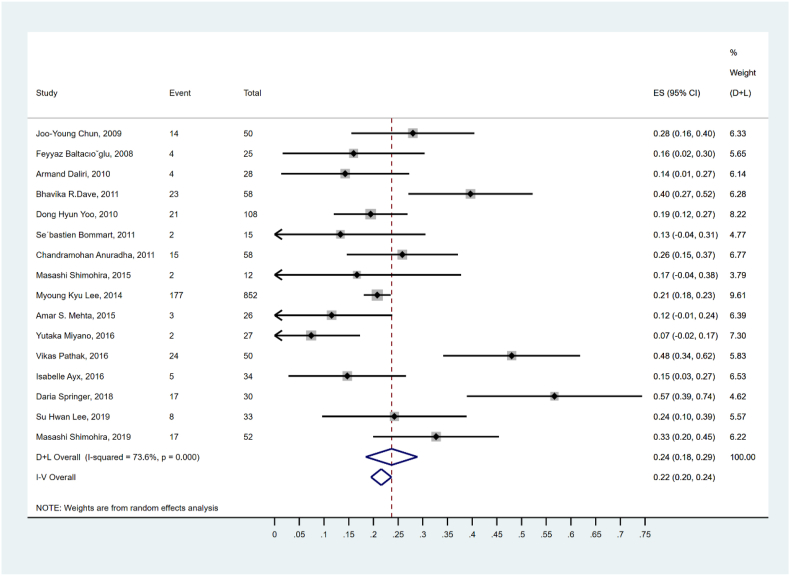
Sixteen studies with a total of 1458 patients reported 338 cases of recurrence of hemoptysis after BAE (Table 2). According to the forest plot (Fig. 4), the pooled second recurrence rate of hemoptysis was 23.7% (95% CI: 18.55–28.9%), ranging from 7.4% to 56.7%. A random-effect model was applied due to moderate heterogeneity (I2 = 73.6%, p < 0.01).
Fig. 4.
Forest plot for hemoptysis recurrence after BAE.
3.6. Mortality of hemoptysis after BAE
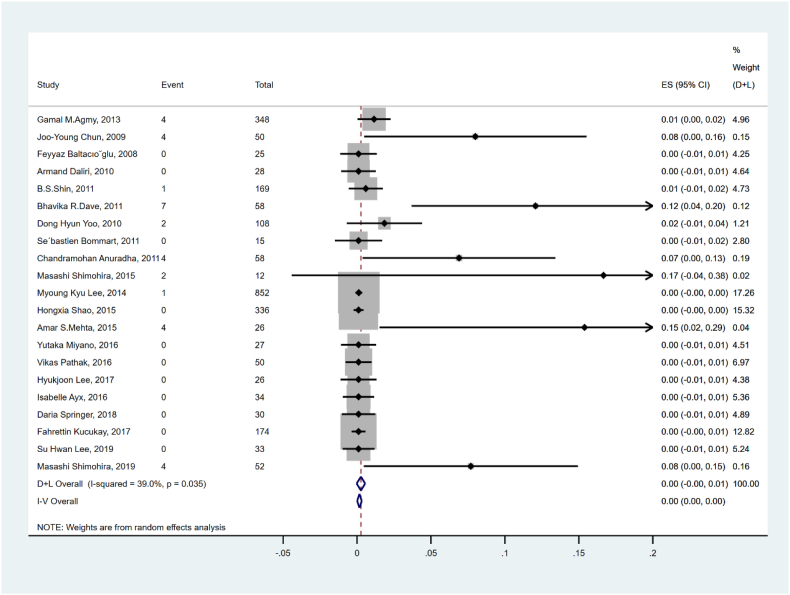
The mortality associated with hemoptysis recurrence is shown in Fig. 5 and Table 2. Twenty-one studies and 2511 patients were summed up and analyzed, and the pooled rate of mortality of recurrence was 0.2% (95% CI: 0.0–0.3%) with low heterogeneity (I2 = 39.0%, p = 0.035).
Fig. 5.
Forest plot for mortality of hemoptysis recurrence.
3.7. Complications
Fifty-nine of 396 cases with complications were reported in nine studies (Table 2). Studies showed that 0.02% (95% CI: 7.6–19.2, Fig. 6) of the patients who received bronchial or non-bronchial artery embolotherapy had complications. The random-effect model was utilized due to moderate heterogeneity (I2 = 69.1%, p = 0.001).
Fig. 6.
Forest plot for total complication after BAE.
The major and minor complications are listed in Table 2.
Major complications occurred after BAE in 59 patients in 21 studies (0.2%, 95% CI: 0.2–0.4%, Fig. 7). After the heterogeneity test was performed, the I2 was zero (p = 0.934); hence, a fixed-effect model was used. Forty of 412 patients had minor complications in eight studies (Fig. 8). The pooled rate of minor complications was 10% (95% CI: 4.7–9.6%), and the random-effect models were used due to moderate heterogeneity (I2 = 54.3%, p = 0.032).
Fig. 7.
Forest plot for major complication after BAE.
Fig. 8.
Forest plot for minor complication after BAE.
3.8. Publication bias
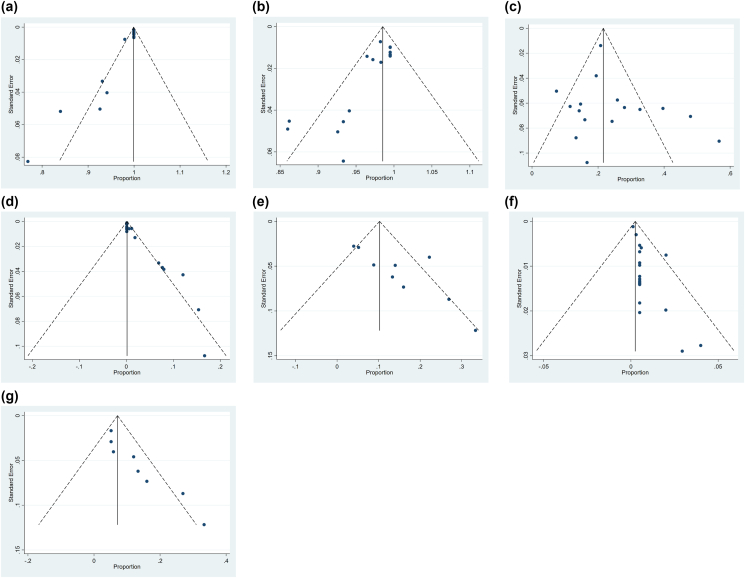
The Begger's test and funnel plot were used to evaluate for publication bias. Results showed that publication bias was detected in technical success (p < 0.01, Table 2 and Fig. 7A), immediate control (p < 0.01, Table 2 and Fig. 7B), recurrence mortality (p < 0.01, Table 2 and Fig. 7D), and minor complications (p = 0.004, Table 2 and Fig. 7G). The nonparametric trim-and-fill method was used to identify and correct for funnel plot asymmetry secondary to publication bias. After using this method, the pooled value of technical success (99.9%, 95% CI: 99.6–100%) and immediate control (99.5%, 95% CI: 97.2–99.2%) did not change, which showed robust statistical power. The recurrence mortality rate was 2% (95% CI: 0–3%) in the fixed-effects model, with four studies added in the future. The minor complication rate was 7.1% (95% CI: 11.9–12.3%) in the random effects model, with three studies which need to be added in the future. All funnel plots are shown in Fig. 9.
Fig. 9.
(a) Funnel plot for technical success; (b) Funnel plot for immediate control; (c) Funnel plot recurrence of hemoptysis; (d) Funnel plot recurrence mortality; (e) Funnel plot total complication; (f) Funnel plot major complication; (g) Funnel pot minor complication.
4. Discussion
BAE is a significant intervention which plays a vital role in hemoptysis-associated diseases. Additionally, its application allows more time to treat the underlying disease of massive hemoptysis. Our study focuses on the efficacy and safety of BAE to evaluate its availability and aid clinicians in practice through a review of articles on the management of abrupt hemoptysis. The technical and immediate control rates were 99.9% (95% CI: 99–100%) and 99.5% (95% CI: 97.2–99.2%) in our study, respectively. We also found recurrence in 23.7% (95% CI: 18.9–28.9%) of post-BAE hemoptysis patients, of whom 0.2% died. The assessment of complications revealed a total complication rate of 13.4% (95% CI: 7.6–19.2%), in which 0.2% (95% CI: 0.2–0.4%) were major complications and 10% (95% CI: 4.7–9.6%) were minor complications.
Most patients with hemoptysis achieved BAE technical success and immediate control of bleeding. Immediate bleeding control was observed in 95% of cases, and hemoptysis was controlled within a month using BAE in 90% of patients. Failed hemostasis for BAE is usually due to non-bronchial arterial bleeding, although it generally presents in only a small group of patients with hemoptysis.12Hemoptysis gradually becomes common because of more and more cases of tuberculosis and bronchiectasis. In addition, it should be emphasized that advanced embolization materials significantly improve the safety and efficacy of the procedure and control the rate of hemoptysis. Generally, four embolization materials are available for embolization of hemoptysis-associated arteries.34 The most conventional embolization material is gelatin sponge, which is widely used by interventionists globally when hemoptysis occurs. This may be attributed to its low cost and ease of use during emergencies. However, due to its absorbability in human tissues, it is relatively temporary, which increases the risk of recurrent bleeding. To overcome this limitation, some mid- or long-term embolization materials, such as polyvinyl alcohol (PVA) and N-Butyl-2-Cyanoacrylate Glue (NBCA), are gradually being used as alternatives. They significantly improve the technical success and immediate control of hemoptysis due to their high availability and convenience. The use of coils in BAE also improved the immediate bleeding control rate, in combination with superselective embolization. Coils are a permanent embolization agent with a low recanalization rate and a high thrombogenicity. Once released to targeted vessels, coils instantly form thrombi when in contact with blood, achieving good hemostasis. However, for inexperienced interventionists, problems arise with the use of coils. Firstly, the targeted bleeding artery becomes tiny and distorted due to the underlying disease erosion and drastic reduction in local blood volume, making superselective embolization difficult to achieve. Secondly, traditional coils are still widely used in hemoptysis therapy, and these coils are often difficult to recycle if released once. Thirdly, there have been several discussions on the occlusion of the proximal artery following the use of coils, which would, in turn, complicate the embolization of the distal part of the same artery in case of rebleeding. Moreover, interlocking detached coils is now a viable option, in which doctors can adjust and reposition the guiding catheter with detached coils to find a suitable location for immediate bleeding control. A Japanese study reported that short coils entwined with long coils made it easy to control bleeding, with a 92.6% likelihood of immediate success.17 Currently, detached coils are relatively used less in the treatment of hemoptysis due to their cost-effectiveness for superselective embolization.
We compared and summarized recurrence data from the literature on BAE. The pooled total recurrence rate was 23.7%, and the pooled mortality rate from hemoptysis recurrence was 2%. However, it should be emphasized that several studies have shown highly variable recurrence rates. Lee et al.21 reported that only one of 26 patients (4%) experienced a recurrence of hemoptysis after BAE. However, another Korean study on TB-associated hemoptysis reported a different result, showing that 52% (88/169) of TB patients experienced recurrent hemoptysis after BAE.22 There are three main reasons for the variable recurrence rates in available literature. First, different vascular embolization degrees account for the uneven recurrence proportions. Usually, incomplete embolization results in early recurrence in patients with hemoptysis. In emergencies, operators often have very limited time to locate and embolize the target artery. Large arteries are not hard to detected and embolized easily, whereas some small bleeding arteries cannot be detected initially, which may lead to massive hemoptysis or recurrence. Moreover, BAE is usually performed in tertiary referral centers, and patients suffering from hemoptysis have already been injected with positive vasoactive agents to control bleeding prior to transfer to a higher-level hospital. Interventionists may also miss some culprit vessels. These vessels cause suspended bleeding due to vasoconstriction from the administration of vasoconstrictors. In addition, the variation in recurrence rates from the literature may be attributed to divergent underlying diseases, irregular treatment, late treatment, and progression of respiratory diseases. TB, invasive pulmonary aspergillosis, bronchiectasis, and lung tumor are the top four etiologies of hemoptysis.18,22,35 To reduce hemoptysis recurrence, the treatment should be based on its specific etiology and not on the subjective clinical experience of the doctor. Most cases of hemoptysis recurrence were due to the progression of the primary disease when the lesion erodes into the artery. Neglecting the treatment of underlying diseases (such as anti-infection or anti-tumor agents) contribute to the recurrence of hemoptysis. Therefore, the appropriate treatment for hemoptysis and the primary disease improves the safety and efficacy of BAE. Considering the different etiologies of hemoptysis, early and timely therapy of mild hemoptysis through BAE could prevent the erosion of pulmonary tissues and progression to massive hemoptysis. Achieving sustained hemoptysis and primary disease control will decrease hemoptysis recurrence and improve the quality of life of the patient.
In our study, the low hemoptysis recurrence mortality in this patient population is consistent with published data.35,36 Some studies have reported that hemoptysis recurrence results from incomplete embolization and undetected bleeding in non-bronchial arteries, which occurs 1–6 months later. Contrarily, the progression of the underlying disease may account for the rebleeding when hemoptysis occurs 6–12 months after BAE.37 In particular, the mortality after BAE was associated with the progression of primary diseases (e.g., TB, tumor, and COPD), rather than BAE-associated adverse events.
Most bleeding arteries originate from the bronchial or intercostal arteries with high blood pressure; thus, the recurrent bleeding in these blood vessels after BAE are often severe compared with the less frequent pulmonary artery hemorrhage with relatively low blood pressure.
To achieve high success and low recurrence, a systematic and thorough search for culprit vessels and complete embolization should be performed. This is an effective method for interventions. The majority of recurrent hemoptysis patients would receive second-line intervention therapy using BAE in case of spontaneous recurrence or if they were not candidates for surgery. In the study, only 2% of patients underwent surgical treatment. The patients also had a high acceptance of the procedures, which can also demonstrate their safety and repeatability.
The pooled total complication rate for BAE was 13.4%, in which 10% were minor complications and 2% were major complications. Compared with surgical treatment for hemoptysis, BAE has a lower complication rate and fewer adverse events. The most severe complication of BAE is spinal artery embolization, but it is rarely reported in relevant studies. It should be noted that paralysis-related adverse events occurred during erroneous embolization of the spinal artery by clinicians. This major complication is attributed to unclear spinal artery imaging on a fluorescent screen and reflux of a liquid embolization agent. Meanwhile, minor complications are common, and the symptoms are mild. Most minor complications are self-limiting, such as fever, chest pain, cough, dysphagia, back pain, and lower limb muscle weakness, which typically resolve within a week. Back pain was the most common symptom due to peripheral nerve stimulation from the embolization agent and a transient vascular embolization.38 Fevers, mostly absorption fevers, are also common after BAE due to hypersensitivity to the contrast agent. Other minor complications, such as dysphagia and lower limb muscle weakness, are rare and transient. Superselective and meticulous BAE are required to achieve high therapeutic efficacy during the perioperative period. Additionally, alleviating discomfort from minor complications and assisting the patient's recovery to their previous quality of life are of utmost importance.
This study has several limitations. First, a major limitation is the effect of bias on this meta-analysis due to the non-randomized controlled study designs and retrospective nature of most of the included studies. Second, the small population sizes of the included studies may have influenced the accuracy of the pooled values. Third, the heterogeneity of some pooled values (e.g., hemoptysis recurrence and complications) remained high, which indicates that large randomized control trials should be performed in the future. Fourth, the consensus standard has not been unified for BAE, and the included studies have different definitions of key terms such as recurrence and complications, to which the heterogeneity of this meta-analysis can be attributed. Fifth, this study may have overestimated or underestimated the efficacy and safety of BAE; thus, further studies involving different races and populations are needed.
5. Conclusion
This study demonstrated that BAE is a safe, effective, and feasible intervention for hemoptysis through the analysis of its technical success rate, immediate hemoptysis control, mortality, recurrence, and complications. It is also an efficacious means to control moderate or massive hemoptysis resulting from diverse etiologies for TB, bronchiectasis, tumor, and vascular malformation. It is particularly beneficial for some severe hemoptysis patients in the acute phase, even in shock, who are not candidates for bronchoscopy and surgery for the management of the underlying cause of hemoptysis, such as a pulmonary mass. Thus, BAE may be a crucial bridging intervention for massive hemoptysis patients and allow more time for other treatment options during emergencies.
Declaration of interests
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
Contributor Information
Zhiyuan Zheng, Email: 20111320006@fudan.edu.cn.
Zhiquan Zhuang, Email: 19211320003@fudan.edu.cn.
Wen Zhang, Email: zhang.wen2@zs-hospital.sh.cn.
Zhiping Yan, Email: yan.zhiping@zs-hospital.sh.cn.
Xiaolin Wang, Email: wang.xiaolin@zs-hospital.sh.cn.
References
- 1.Davidson K., Shojaee S. Managing massive hemoptysis. Chest. 2020;157:77–88. doi: 10.1016/j.chest.2019.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Jones R., Charlton J., Latinovic R., et al. Alarm symptoms and identification of non-cancer diagnoses in primary care: cohort study. BMJ. 2009;339:b3094. doi: 10.1136/bmj.b3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ittrich H., Bockhorn M., Klose H., et al. The diagnosis and treatment of hemoptysis. Dtsch Arztebl Int. 2017;114:371–381. doi: 10.3238/arztebl.2017.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corey R., Hla K.M. Major and massive hemoptysis: reassessment of conservative management. Am J Med Sci. 1987;294:301–309. doi: 10.1097/00000441-198711000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Parrot A., Tavolaro S., Voiriot G., et al. Management of severe hemoptysis. Expet Rev Respir Med. 2018;12:817–829. doi: 10.1080/17476348.2018.1507737. [DOI] [PubMed] [Google Scholar]
- 6.Valipour A., Kreuzer A., Koller H., et al. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest. 2005;127:2113–2118. doi: 10.1378/chest.127.6.2113. [DOI] [PubMed] [Google Scholar]
- 7.Garzon A.A., Gourin A. Surgical management of massive hemoptysis. A ten-year experience. Ann Surg. 1978;187:267–271. doi: 10.1097/00000658-197803000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gourin A., Garzon A.A. Operative treatment of massive hemoptysis. Ann Thorac Surg. 1974;18:52–60. doi: 10.1016/s0003-4975(10)65717-7. [DOI] [PubMed] [Google Scholar]
- 9.Rémy J., Arnaud A., Fardou H., et al. Treatment of hemoptysis by embolization of bronchial arteries. Radiology. 1977;122:33–37. doi: 10.1148/122.1.33. [DOI] [PubMed] [Google Scholar]
- 10.Panda A., Bhalla A.S., Goyal A. Bronchial artery embolization in hemoptysis: a systematic review. Diagn Interv Radiol. 2017;23:307–317. doi: 10.5152/dir.2017.16454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun J.Y., Morgan R., Belli A.M. Radiological management of hemoptysis: a comprehensive review of diagnostic imaging and bronchial arterial embolization. Cardiovasc Intervent Radiol. 2010;33:240–250. doi: 10.1007/s00270-009-9788-z. [DOI] [PubMed] [Google Scholar]
- 12.Anuradha C., Shyamkumar N.K., Vinu M., et al. Outcomes of bronchial artery embolization for life-threatening hemoptysis due to tuberculosis and post-tuberculosis sequelae. Diagn Interv Radiol. 2012;18:96–101. doi: 10.4261/1305-3825.DIR.3876-11.2. [DOI] [PubMed] [Google Scholar]
- 13.Ayx I., Muller-Wille R., Wohlgemuth W.A., et al. Treatment of acute hemoptysis by bronchial artery embolization with the liquid embolic agent ethylene vinyl alcohol copolymer. J Vasc Intervent Radiol. 2017;28:825–831. doi: 10.1016/j.jvir.2016.12.1226. [DOI] [PubMed] [Google Scholar]
- 14.Daliri A., Probst N.H., Jobst B., et al. Bronchial artery embolization in patients with hemoptysis including follow-up. Acta Radiol. 2011;52:143–147. doi: 10.1258/ar.2010.100302. [DOI] [PubMed] [Google Scholar]
- 15.Lee S.H., Lee J.H., Chang J.H., et al. Hemoptysis requiring bronchial artery embolization in patients with nontuberculous mycobacterial lung disease. BMC Pulm Med. 2019;19:117. doi: 10.1186/s12890-019-0881-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Angle J.F., Siddiqi N.H., Wallace M.J., et al. Quality improvement guidelines for percutaneous transcatheter embolization: society of interventional radiology standards of practice committee. J Vasc Intervent Radiol. 2010;21:1479–1486. doi: 10.1016/j.jvir.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Miyano Y., Kanzaki M., Obara T., et al. Fabricated bronchial artery embolization for hemoptysis using a coil. Asian Cardiovasc Thorac Ann. 2016;24:445–449. doi: 10.1177/0218492316643843. [DOI] [PubMed] [Google Scholar]
- 18.Pathak V., Stavas J.M., Ford H.J., et al. Long-term outcomes of the bronchial artery embolization are diagnosis dependent. Lung India. 2016;33:3–8. doi: 10.4103/0970-2113.173059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehta A.S., Ahmed O., Jilani D., et al. Bronchial artery embolization for malignant hemoptysis: a single institutional experience. J Thorac Dis. 2015;7:1406–1413. doi: 10.3978/j.issn.2072-1439.2015.07.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoo D.H., Yoon C.J., Kang S.G., et al. Bronchial and nonbronchial systemic artery embolization in patients with major hemoptysis: safety and efficacy of N-butyl cyanoacrylate. AJR Am J Roentgenol. 2011;196:W199–W204. doi: 10.2214/AJR.10.4763. [DOI] [PubMed] [Google Scholar]
- 21.Lee M.K., Kim S.H., Yong S.J., et al. Moderate hemoptysis: recurrent hemoptysis and mortality according to bronchial artery embolization. Clin Res J. 2015;9:53–64. doi: 10.1111/crj.12104. [DOI] [PubMed] [Google Scholar]
- 22.Shin B.S., Jeon G.S., Lee S.A., et al. Bronchial artery embolisation for the management of haemoptysis in patients with pulmonary tuberculosis. Int J Tubercul Lung Dis. 2011;15:1093–1098. doi: 10.5588/ijtld.10.0659. [DOI] [PubMed] [Google Scholar]
- 23.Springer D.M., Cofta S., Juszkat R., et al. The effectiveness of bronchial artery embolisation in patients with haemoptysis. Adv Respir Med. 2018;86:220–226. doi: 10.5603/ARM.2018.0035. [DOI] [PubMed] [Google Scholar]
- 24.Shimohira M., Ohta K., Nagai K., et al. Bronchial arterial embolization using a gelatin sponge for hemoptysis from pulmonary aspergilloma: comparison with other pulmonary diseases. Emerg Radiol. 2019;26:501–506. doi: 10.1007/s10140-019-01695-y. [DOI] [PubMed] [Google Scholar]
- 25.Shao H., Wu J., Wu Q., et al. Bronchial artery embolization for hemoptysis: a retrospective observational study of 344 patients. Chin Med J (Engl). 2015;128:58–62. doi: 10.4103/0366-6999.147811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimohira M., Hashimoto T., Abematsu S., et al. Triaxial system in bronchial arterial embolization for haemoptysis using N-butyl-2-cyanoacrylate. Br J Radiol. 2015;88 doi: 10.1259/bjr.20150265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baltacioğlu F., Cimşit N.C., Bostanci K., et al. Transarterial microcatheter glue embolization of the bronchial artery for life-threatening hemoptysis: technical and clinical results. Eur J Radiol. 2010;73:380–384. doi: 10.1016/j.ejrad.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Agmy G.M., Wafy S.M., Mohamed S.A.A., et al. Bronchial and nonbronchial systemic artery embolization in management of hemoptysis: experience with 348 patients. ISRN Vascular Medicine. 2013;2013:1–7. [Google Scholar]
- 29.Lee H., Yoon C.J., Seong N.J., et al. Cryptogenic hemoptysis: effectiveness of bronchial artery embolization using N-butyl cyanoacrylate. J Vasc Intervent Radiol. 2017;28:1161–1166. doi: 10.1016/j.jvir.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 30.Bommart S., Bourdin A., Giroux M.F., et al. Transarterial ethylene vinyl alcohol copolymer visualization and penetration after embolization of life-threatening hemoptysis: technical and clinical outcomes. Cardiovasc Intervent Radiol. 2012;35:668–675. doi: 10.1007/s00270-011-0270-3. [DOI] [PubMed] [Google Scholar]
- 31.Kucukay F., Topcuoglu O.M., Alpar A., et al. Bronchial artery embolization with large sized (700–900 μm) Tris-acryl Microspheres (Embosphere) for massive hemoptysis: long-term results (clinical research) Cardiovasc Intervent Radiol. 2018;41:225–230. doi: 10.1007/s00270-017-1818-7. [DOI] [PubMed] [Google Scholar]
- 32.Dave B.R., Sharma A., Kalva S.P., et al. Nine-year single-center experience with transcatheter arterial embolization for hemoptysis: Medium-term outcomes. Vasc Endovasc Surg. 2011;45:258–268. doi: 10.1177/1538574410395036. [DOI] [PubMed] [Google Scholar]
- 33.Chun J.Y., Belli A.M. Immediate and long-term outcomes of bronchial and non-bronchial systemic artery embolisation for the management of haemoptysis. Eur Radiol. 2010;20:558–565. doi: 10.1007/s00330-009-1591-3. [DOI] [PubMed] [Google Scholar]
- 34.Daliri A., Probst N.H., Jobst B., et al. Bronchial artery embolization in patients with hemoptysis including follow-up. Acta Radiol. 2011;52:143–147. doi: 10.1258/ar.2010.100302. [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa H., Hara M., Ryuge M., et al. Efficacy and safety of super selective bronchial artery coil embolisation for haemoptysis: a single-centre retrospective observational study. BMJ Open. 2017;7 doi: 10.1136/bmjopen-2016-014805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Syha R., Benz T., Hetzel J., et al. Bronchial artery embolization in hemoptysis: 10-year survival and recurrence-free survival in benign and malignant etiologies - a retrospective study. Röfo. 2016;188:1061–1066. doi: 10.1055/s-0042-112227. [DOI] [PubMed] [Google Scholar]
- 37.Davis P.B., Di Sant'Agnese P.A. Diagnosis and treatment of cystic fibrosis. An update. Chest. 1984;85:802–809. doi: 10.1016/s0012-3692(16)62421-2. [DOI] [PubMed] [Google Scholar]
- 38.Haponik E.F., Fein A., Chin R. Managing life-threatening hemoptysis: has anything really changed? Chest. 2000;118:1431–1435. doi: 10.1378/chest.118.5.1431. [DOI] [PubMed] [Google Scholar]