Abstract
Pancreatic cancer has become a major disease affecting people's health because of its insidiousness, rapid progression and poor prognosis. Based on the practical needs of clinical work, combined with domestic multi-center research and experience, this guideline provides constructive suggestions for the interventional treatment of pancreatic cancer.
Keywords: Advanced pancreatic carcinoma, Interventional treatment, Clinical practice guidelines
1. The definition of advanced pancreatic cancer
Pancreatic cancer is a common malignant tumor of the digestive system. In 2015, the National Cancer Registration Center (NCCR) published the Cancer Statistics of China, CA: A Cancer Journal for Clinicians, which indicated that there were 4.3 million new cancer cases and over 2.8 million cancer deaths in China in that year.3 Lung cancer, gastric cancer, pancreatic cancer, and other cancers are common malignant tumors found in patients in China, accounting for approximately 75% of all new cases and approximately 80% of all cancer-related deaths. Furthermore, between 2000 and 2011, the incidence of pancreatic cancer showed an increase with the mortality of male pancreatic cancer being ranked in 7th place. In this guideline, advanced pancreatic cancer is defined as having local and/or distant metastases that cannot be surgically resected.1 Additional details are provided in Table 1.
Table 1.
Staging definitions of pancreatic cancer according to the eighth edition of American Joint Committee on Cancer (AJCC) staging system1,2.
| The AJCC (eighth edition) staging definitions for pancreatic cancer | ||||
|---|---|---|---|---|
| Details | Stage | T | N | M |
| T1 Maximum tumor diameter ≤2 cm T2 Maximum tumor diameter >2 cm and ≤4 cm T3 Maximum tumor diameter >4 cm T4 Involvement of celiac axis or superior mesenteric artery (unresectable tumor) N0 No regional lymph node metastasis N1 Metastasis in 1–3 regional lymph nodes N2 Metastasis in ≥4 regional lymph nodes M0 No distant metastasis M1 Distant metastasis |
0 | Tis | N0 | M0 |
| IA | T1 | N0 | M0 | |
| IB | T2 | N0 | M0 | |
| IIA | T3 | N0 | M0 | |
| IIB | T1~3 | N0 | M0 | |
| III | T4 | Any N | M0 | |
| Any T | N2 | M0 | ||
| IV | Any T | Any N | M1 | |
Treatment options differ according to the stage of pancreatic cancer. Currently, the most effective method for the treatment of pancreatic cancer is radical resection. From the perspective of radiological images, pancreatic cancer can be divided into resectable pancreatic cancers, borderline resectable pancreatic cancers, locally advanced pancreatic cancers, and distant metastases pancreatic cancers.
The traditional clinical treatment for pancreatic cancer is radiotherapy, and chemotherapy.4, 5, 6, 7, 8, 9, 10, 11, 12, 13 In recent years, there has been an increasing prevalence of the use of interventional diagnosis and treatment in advanced pancreatic cancer. Interventional therapy can be used in patients who are unable to undergo surgical resections, who have many lesions that are not suitable for surgery, who are unwilling to undergo surgery, and who relapse after surgery; in patients who have obstructive jaundice, liver metastases, severe lower back pain, and those who cannot tolerate systemic chemotherapy. Furthermore, the use of sensitive drugs that are directly perfused through catheters, radioactive seed implants, interventional biological treatments, percutaneous liver punctures and drainage of the biliary tracts, and biliary stent placements, can be used to relieve complications such as obstructive jaundice. Before implementing interventional therapy, it is recommended to use the cytological or histopathological diagnosis of pancreatic cancer and genetic testing to guide further clinical treatment.
This guide does not cover the treatment with the HIFU-knife and nano-knife.
2. Basic concepts of interventional diagnosis and treatment of advanced pancreatic cancer
2.1. Percutaneous pancreatic biopsies
In a percutaneous pancreatic biopsy, with the use of imaging equipment (guided by ultrasound, CT, or MRI) the pancreatic lesions are punctured with fine needles, cells or tissues are extracted, and pathological examinations performed to confirm the diagnosis. Sufficient and high-quality small samples of histology and cytology are obtained through percutaneous punctures and the performance of accurate histological typing and molecular testing of the pancreatic cancer patients to guide the treatment.
In 1951, Kirtland14 first used a percutaneous biopsy to diagnose pancreatic cancer. After the use of ultrasound commenced in clinical practice, Smith15 used the ultrasound to guide pancreatic biopsies in 1974. As the popularity of CTs increased, Lu16 used CT-guided percutaneous pancreatic biopsies in 1985 to improve accuracy. At that point, important technical means were identified for the diagnosis of pancreatic diseases. A series of follow-up clinical studies further clarified the diagnostic value of percutaneous pancreatic biopsies with regard to the incidence of complications.
The imaging-guided methods for percutaneous pancreatic biopsy are ultrasound and CT,17,18, with the main puncture methods being fine-needle aspiration (FNA) and core-needle biopsy (CNB). While there are no significant differences between the two methods in terms of diagnostic sensitivities and complication rates., the use of CNB can provide more histological specimens, which is convenient for further molecular pathological testing, clarifying tumor subtypes, and formulating targeted treatment plans. Furthermore, CNB is recommended when it is tolerated by the patient, and there is no obvious risk of lesion puncture. If the lesion is small, adjacent to large blood vessels, or if there are obvious blood vessels in the lesion, then the use of FNA is recommended. If conditions permit, on-site rapid cytopathological testing can be performed to increase the positive rate of the biopsy materials. Common complications of percutaneous pancreatic biopsies include bleeding, peritonitis, and pancreatic fistulas. A coaxial system puncture needle can effectively reduce these complications and tumor needle implant transfer.
Most guidelines of other countries the use of FNA with an ultrasound endoscopy is the preferred method for pancreatic biopsies, followed by the use of CT-guided pancreatic biopsies.19 Due to the current diagnostic level of Chinese cytopathology and the requirement for disposable consumables, ultrasound and CT-guided pancreatic biopsies may have more clinical application value. Studies have confirmed that in pancreatic cancer there is high expression of the ROBO3 and PPM1D genes, which can be targets for inhibition enabling a significant killing of the tumor cells.
2.2. Transarterial infusion chemotherapy
2.2.1. Definition
Transarterial infusion chemotherapy (TAI) refers to the insertion of a catheter or microcatheter into the main blood supply artery (such as the gastroduodenal artery) of the pancreatic cancer lesion. The doctors can decide the choice of the chemotherapeutic agents and treatment plan based on the clinical data, and infuse the drugs into the tumor tissue through the catheter over a specific period of time.
2.2.2. Principle
TAI refers to entering the supply artery of the tumor through the catheter and then infusing the chemotherapeutic drug. Therefore, drug distribution is not affected by the unrelated blood flow of the whole body. The tumor area is where the systemic drug distribution and concentration are the highest. Even if the dose is less than that used in the intravenous dose, the drug concentration in the tumor area is still much higher than the drug concentration in the whole body. The drug that flows to other parts of the body via the blood circulation also affects other metastatic lesions that may exist outside the target organ.
PFOB and MicroRNA-26a increase the vascular permeability significantly, and the adjuvant chemotherapeutic drugs penetrate the blood vessels to the tumor site quickly and effectively, enhancing the drug's lethality. At the same time, Smurf2 and MicroRNA-21 significantly change the cell osmotic pressure, stimulate high-efficiency cell endocytosis and exocytosis, and improve the therapeutic efficacy of the chemotherapy drugs.6,20,21
2.2.3. Classification
According to the injection method, TAI can be divided into: (1) continuous transarterial infusion chemotherapy (cTAI), which generally requires an indwelling arterial catheter, and where the perfusion time is determined by the biological characteristics of the tumor and the time concentration curve of the selected drug; and (2) bolus transarterial infusion chemotherapy (bTAI), where the perfusion time is generally 30–45 min, mainly when the tumor has an abundant blood supply. If the blood supply of the tumor is not abundant, the curative effect of this method is limited. Depending on the actual condition of the patient, the method can be changed to cTAI or other local physical therapies.22
Depending on the injection site and whether the drug is heated, it can be divided into regional infusion chemotherapy and heating infusion chemotherapy.
2.2.4. Embolization
This means that after the completion of TAI, the 75–150 μm absorbable microspheres and particles that were used to embolize the tumor disappear and the responsible artery is kept open.
2.3. Percutaneous 125I seed implantation
2.3.1. Definition
Percutaneous 125I seed implantation refers to the use of image positioning technology such as CT scanning, and the target area and the number of 125I seeds based on the treatment planning system (TPS) are determined. Using a direct puncture method, 125I seeds are implanted into pancreatic cancer and metastatic lesion tissues to cause necrosis in the tumor cells.
2.3.2. Principle
Pancreatic cancer is a hypoxic tumor that is insensitive to conventional radiotherapy. The half-life of 125I is 60.14 days, and it can continuously release γ-rays. These rays are stimulated by the nucleus and have a higher degree of energy, shorter wavelength, and stronger penetrating ability than X-ray photons. They can continuously destroy the DNA synthesis of tumor cells and prevent tumor cell proliferation.
At the same time, the effective irradiation distance of γ-rays is 5–20 mm, which does not damage the surrounding normal tissues easily. Existing studies have already established that 125I particles inhibit the rapid growth of tumors using γ-rays to invade the celiac ganglion plexus.
2.4. Percutaneous ablation
2.4.1. Definition
Under imaging guidance, tumor cell necrosis is inactivated in situ by chemical or physical methods. The principle is inactivating tumor cells and protecting normal tissue structures to the greatest extent. According to the treatment principle, ablation is divided into chemical and physical ablations.
-
1)
Chemical ablation: The tumor is exposed to a high concentration of ablation agents (protein coagulants, sensitive chemotherapeutics, and tracer slow-release agents) to achieve ablation. The advantage is that it is easy to implement; however, the disadvantage is that it is difficult to control the distribution of the ablation agent and the effect is not ideal.
-
2)
Physical ablation: This can be divided into three categories, based on temperature: thermal ablation, cold ablation, and normothermic temperature ablation (irreversible electroporation). The temperature-based ablation methods include thermal ablation (radiofrequency, microwave, laser, focused ultrasound, etc.) and cold ablation (helium knife, liquid nitrogen knife, etc.).
2.4.2. Principle
There are different principles due to the different treatment methods used. The following is an explanation for percutaneous radiofrequency/microwave ablation therapy. This refers to the use of a CT scan or ultrasound and any other imaging technology to puncture the pancreatic cancer tumor or metastatic lesion tissues, directly, with thermal ablation needles, and within a certain degree of power and time frame, to result in the coagulation and necrosis of the tumor tissue.
Both radiofrequency and microwave ablation use high heat to cause the coagulation and necrosis of tumor tissue. The main difference is the method used to generate heat. Radiofrequency ablation uses high-frequency alternating current oscillation to generate heat, whereas microwave ablation uses microwaves to drive the polar molecules in the body to generate heat.
The human body is a complex structure composed of many organic and inorganic substances. The body fluids contain a large number of dielectrics, such as ions, water, and colloidal particles. The human body mainly relies on the movement of ions to conduct currents, and radiofrequency ablation treatment has a high frequency of 150,000 times per second vibration. Under the action of a high-frequency alternating current, the ions rub against each other and collide with other particles to produce a biothermal effect. Since the temperature of the tumor tissue is higher than its neighboring normal tissues and the cancer cells are sensitive to high heat, the goal of effectively killing tumor tissues while protecting normal tissues is achieved.
Under the guidance of image positioning technology such as a CT scan or ultrasound, microwave thermocoagulation electrodes are implanted in the tumor, and the polar molecules in the tissue then move at a high speed in the microwave field to generate heat. When the temperature rises to 50–90 °C, the protein of the tumor cells is denatured and coagulated, resulting in irreversible necrosis. The inactivated tumor tissue can produce heat shock proteins, stimulate the body's immune system, improve the body's immune function, and inhibit the spread of the tumor cells.
3. Percutaneous pancreatic biopsy
3.1. Indications and contraindications
-
1.
Indications: Pancreatic biopsy is suitable for, among others, solid pancreatic masses, cystic solid masses, and suspected diffuse disease in determining the nature of the pancreatic lesions and distinguishing primary pancreatic cancer from metastatic cancer.23, 24, 25, 26, 27
-
2.
Contraindications: The presence of severe bleeding tendencies, acute pancreatitis, peritonitis, skin infections, poor cardiopulmonary function, massive ascites.
3.2. Preparation
-
1.
Patient preparation
Check the clotting time, platelet count, prothrombin time, and other routine examinations before puncture. Blood and urine pancreatic amylase levels should be tested when pancreatitis is suspected. Those with a severe cough should take antitussives such as codeine tablets and opiate tablets; those who are too nervous should take sedatives such as estazolam. After fasting for 6 hours before surgery, continuous intravenous infusion or subcutaneous injection of somatostatin is administered to inhibit the secretion of pancreatic juice.
-
2.
Equipment preparation
Puncture biopsy kits include sterile surgical hole towels, puncture needles or cut biopsy needles with different lengths 16 to 18G according to actual conditions; syringes; local anesthetics, hemostatics; No. 11 surgical blades; sterile test tubes; specimen bottles; and tissue specimen fixatives.
3.3. Method
-
1.
According to the location of the lesion showed by the image positioning technology such as a CT scan or ultrasound, select the puncture path, that is, the shortest distance from the skin to the central area of the pancreatic lesion in order to avoid the large blood vessels around the pancreas, expanded gallbladder, and common bile duct. A vertical needle insertion is used for pancreatic head and pancreatic body lesions while horizontal or oblique needle insertions are used for pancreatic tail lesions. In addition, the use of ROBIO and other puncture robots can assist in the selection of a special needle path to minimize damage to the surrounding normal organs and reduce the incidence of complications. The puncture specimens are placed in formalin for routine pathological and immunohistochemical examinations whereas the fresh tissue is placed in a liquid nitrogen tank or deep-low temperature refrigerator for genetic testing.
-
2.
During fine-needle negative pressure needle biopsies, after the accurate position of the needle tip is confirmed by a CT scan or ultrasound and any other image positioning techniques, the multi-point and multi-directional negative pressure aspiration biopsy is performed. With the assistance of experienced pathologists, the puncture aspirate is fixed in a formalin solution immediately, smear staining and other inspections are performed after cytocentrifugation. Doctors can also use a coaxial trocar to puncture and aspirate repeatedly.
-
3.
The puncture is compressed locally for 10 min after the puncture, using bandages. The patient lies supine for 1 to 2 hours with the pulse, blood pressure, and any symptoms of severe abdominal pain being monitored. Generally, a fine needle suction biopsy is performed 2 hours after the operation. The patient can return home and rest, if there is no special discomfort. After cutting needle biopsy, if these is no obvious discomfort, the patient should continue fasting and receiving somatostatin. Blood and urine amylase should be re-examined the next day, and food should be consumed only if these results are normal.
3.4. Matters needing attention
The following need to be prevented: intraoperative and postoperative gastrointestinal or abdominal bleeding, acute pancreatitis, biliary peritonitis, gastrointestinal perforations, secondary abdominal infections, tumor needle tract implantations, and pancreatic fistulas.
4. cTAI + cTAE treatment for advanced pancreatic cancer
For patients with advanced pancreatic cancers that cannot be removed, the local drug concentration of transarterial infusion chemotherapy used, is significantly higher than that used in systemic intravenous chemotherapy. This has improved disease-related symptoms, prolonged survival, and reduced the liver metastases of pancreatic cancers28.
4.1. Indications and contraindications
-
1.
Indications: advanced pancreatic cancer that cannot be surgically removed, pancreatic cancer that has not been treated by other non-surgical methods, pancreatic cancer with liver metastasis, and the postoperative recurrence of pancreatic cancer.
-
2.
Contraindications:
Absolute contraindications: being hypersensitive to the contrast agent, the presence of massive ascites, multiple metastases throughout the body, systemic failure, and obvious cachexia (ECOG score> 2 points, with multiple organ failure);
Relative contraindications: patients with bleeding or coagulation dysfunction diseases that cannot be corrected, and obvious bleeding tendencies; poor liver and kidney functions, which exceed the normal reference values by a factor of 1.5; and a white blood cell count <3.5×109/L and a platelet count <50×109/L.
4.2. Preparation
-
1.
Prepare skin at the puncture site and the patient should fast for 4 h before the operation;
-
2.
Perform laboratory examinations to check for the tumor markers (CA199, CEA, CA724, etc.) Conduct routine blood tests, liver and kidney function tests, coagulation function tests, tests for electrolytes to understand the patient's systemic and main organ conditions, and to determine whether there are any contraindications for the treatment. These results are used as a curative effect evaluation index.
-
3.
ECG and chest radiography: Patients who are treated for the first time and have no pathological diagnosis require more than two imaging examinations, such as pancreatic ultrasounds and PET/CTs. The scan range should include the entire pancreas.
-
4.
Before the operation the patient should be informed of the risks and possible complications and a signed consent form should be obtained.
-
5.
Half an hour before the surgery, intramuscular injections of phenergan, 5-HT3 receptor inhibitors and other antiemetics should be administered.
-
6.
The equipment includes: a puncture needle, super-smooth guide wire, catheter sheath, catheter, and a chemotherapy kit (for subcutaneous chemotherapy kit implantation). Commonly used catheters include: 4–6F RH, Cobra catheters and microcatheters.
-
7.
Medication method: ①Use the tumor cell drug sensitivity test results as guidance; ②When there are no pathological diagnoses and drug sensitivity test results, use a combination of the CT, MRI and other imaging findings, refer to the classic treatment of pancreatic cancer, such as gemcitabine, fluorouracil, and albumin paclitaxel. The time-dependent drug infusion period is between 2 and 4 h for 1–2 cell cycles. Time-independent drugs such as gemcitabine and albumin paclitaxel are infused for approximately 2 h while fluorouracil can be infused continuously at 500–700 mg/m2 for two consecutive days.
4.3. Method
-
1.
Position: supine position.
-
2.
Operation steps: Disinfect and drape the conventional groin area, administer local groin anesthesia, use Seldinger's method to puncture the femoral artery, place the arterial sheath, and select the method of arterial intubation.
Selective arterial cannulation29: Place the catheters selectively in the celiac artery and using superior mesenteric arteriography (the angiography continues until the venous phase, observe the venous invasion), check if the tumor supplying blood vessels are visible, and use supplying artery perfusion chemotherapy.
Improved regional perfusion technology: Embolize the pancreatic blood supply artery from the superior mesenteric artery with a microcoil, so that the pancreas is supplied with blood from the celiac artery and its branches. The theoretical basis of this method is the redistribution of perfused drugs, which reduces the effect of chemotherapy drugs on the intestines and simultaneously improves the efficacy.
Without a tumor blood supply artery, the target blood vessel will be determined according to the tumor location, invasion range, and blood supply. It is recommended that pancreatic head and neck tumors undergo gastroduodenal arterial infusion chemotherapy; the pancreatic body and tail tumors depend on the tumor invasion range and blood vessels in the case of angiography. The chemotherapy is injected through the celiac artery, superior mesenteric artery, or splenic artery; patients with liver metastases are also injected chemotherapy through the hepatic artery. Use an ultra-liquefied lipiodol or a granular embolization agent. Some studies have indicated that the arterial blood supply is not abundant, the non-main arteries can be embolized, and the main arteries retained for intra-arterial infusion chemotherapy.28
-
3.
Drug selection: Gemcitabine, fluorouracil, irinotecan, oxaliplatin, and albumin paclitaxel can be selected. In principle, a combination of no more than three drugs should be used.30, 31, 32
-
4.
Methods of administration: Three methods can be used, one-shock perfusion chemotherapy, continuous arterial infusion chemotherapy, or arterial hyperthermic infusion chemotherapy.33
-
(a)
One-shock perfusion chemotherapy can be completed intraoperatively. It is recommended that gemcitabine (800–1000 mg/m2), fluorouracil 500-700 mg–/mg/m2), and tetrahydrofolate (200 mg), single or combined application, be used. It can be repeated for 2–3 weeks, or when the pain is relieved and there is no recurrence.
-
(b)
Continuous arterial infusion chemotherapy includes indwelling catheter continuous infusion chemotherapy and subcutaneous infusion kit system implantation. Continuous infusion chemotherapy can be used to select cell cycle-specific drugs and/or non-specific drugs. It is superior to single-shot perfusion chemotherapy in terms of the medication method, perfusion time, and other plannable and controllable variables. The perfusion time is determined by the characteristics of the drug. For example, fluorouracil can be used for continuous infusion chemotherapy of 500–700 mg/m2 for two consecutive days, repeating the same cycle of perfusion chemotherapy.
-
(c)
Hyperthermic perfusion chemotherapy refers to the corresponding chemotherapeutic drugs selected according to the physiological characteristics of the tumor and the drug sensitivity tests; normal saline is heated to a certain temperature (such as 60 °C) before the intra-arterial infusion chemotherapy. The arterial catheter is perfused directly, to increase the sensitivity of tumor cells to chemotherapeutic drugs, selectively killing the tumor cells without harming the normal pancreatic tissue, and prolonging the survival time of the patients.
4.4. Postoperative treatment
-
1.
Use adequate fluid replacement, liver protection, and symptomatic treatment (antiemetic, antipyretic, etc.) for 3–5 days.
-
2.
Administer antibiotic treatment when necessary.
-
3.
Re-check the liver and kidney functions, routine blood tests, tumor markers, and serum amylase within one week after the surgery.
4.5. Common complications
-
1.
Complications related to intravascular operations include hematomas, arterial dissections, arterial spasms, and occlusions.
-
2.
Complications related to the chemotherapy drugs include pancreatitis, nausea, vomiting, pain, fever, bone marrow suppression, liver damage, and kidney damage.
-
3.
Complications related to decreased body resistance or/and medications, include gastrointestinal bleeding/stress ulcers.
4.6. Efficacy evaluation and follow-up requirements34, 35, 36, 37, 38, 39
-
1.
A monthly follow-up is recommended.
-
2.
Perform quality of life evaluations (QOL, the ECOG scoring system is recommended), routine blood tests, liver and kidney function tests, tumor marker tests, and imaging examinations.
4.7. Nursing
-
1.Preoperative preparation
-
(a)Prepare the patient and explain the purpose of the operation to obtain cooperation.
-
(b)The patient should not consume any solid or indigestible food 4 h before surgery.
-
(c)Administer any sedatives and sedative antiemetics as prescribed by the doctor before the surgery.
-
(a)
-
2.
Postoperative care:
-
(a)
Care of the indwelling arterial catheter: After the successful intubation, fix the catheter sheath and catheter at the puncture site, exposing only the three-way joint part, connect the computer infusion pump to control the drug dose, and continue to administer the drug daily. Replace the infusion tubing, pay attention to strict aseptic techniques, and observe the bleeding tendencies. To avoid the displacement of the catheter and to prevent bending, the place where the infusion pipeline is connected should be fixed appropriately and inspections should be strengthened. The patient's family members should be instructed to massage the lower extremities regularly, change the puncture point dressings every other day, observe closely for any exudation, bleeding, and inflammation, and contact the doctor if any abnormalities are found.
-
(b)
Observing the side effects of the patient after administration: The patient may have fever and discomfort in the digestive tract, which can be treated as prescribed by the doctor.
-
(c)
Observation of the limbs on the surgical side: Observe the pulse of the dorsal foot artery on the surgical side, the limb temperature, and the color, closely and enquire whether the patient is experiencing pain or numbness. If the dorsal artery pulsation disappears, the skin is pale and the distal limbs are cold, immediate measures should be taken. The patient should be instructed to perform ankle and toe joint activities during the immobilization of the operation side.
-
(d)
Care after extubation: After the end of the arterial perfusion, the lower extremity of the operation side should be strictly immobilization look at whether there is bleeding and hematomas around the puncture point, and check whether the skin has hardened, whether there is a mass, and check on the dorsal artery pulsation and peripheral blood circulation. The puncture site can be extubated and bandaged under pressure. The puncture site can be pressurized using a sandbag for 6 h, and the bandage loosened for 12 h. After 24 h, the patient can get out of bed.
5. Percutaneous 125I seed implantation
5.1. Indications and contraindications
-
1.
Indications:
-
(a)
The presence of pancreatic cancer metastases and local metastatic lymph nodes
-
(b)
Patients with an estimated survival time >3 months, in whom the pancreatic tumor cannot be removed surgically.
-
(c)
Patients who are unwilling and/or unable to undergo radical surgery due to other concomitant diseases.
-
(d)
Patients with an estimated survival time >3 months for whom this treatment can be selected carefully, to relieve persistent upper abdominal and lower back pain.
-
(e)
The presence of residual lesions and/or tumor bed positions during the pancreatic tumor resections.
-
(f)
Patients with primary pancreatic tumors with a maximum diameter> 6.0 cm who choose tumor reduction, carefully.40
-
2.
Contraindications:
-
(a)
There is clear clinical evidence that the pancreatic tumors have spread widely.
-
(b)
The presence of multiple organ failure
-
(c)
The presence of malignant pancreatic tumors with acute pancreatic inflammation
-
(d)
Patients with coagulation dysfunctions that will not show any improvement after drug treatment.
-
(e)
Patients with severe diabetes, whose blood glucose is still higher than 16.7 mmol/L after hypoglycemic treatment;
-
(f)
Patients with bacteremia and sepsis who cannot undergo radioactive seed implantation.
5.2. Radiation therapy prescription dose and TPS
-
1.
Radiation therapy prescription dose and 125I particle activity and quantity.
The recommended prescribed dose of radiotherapy is 110–160 mGy and an 125I particle activity of 0.38–0.8 m Ci/grain. The particle number is calculated using Cevc's formula: The total number of particles = (length + width + thickness)/3 × 5 ÷ each particle activity. It is recommended that the type according to the degree of pathological malignancy be considered and that particles with different degrees of activity be used. The higher the degree of malignancy, the higher the degree of activity of the selected particles.
-
2.
Treatment planning system (TPS)
-
(a)
Design basis: ① CTs and ultrasound images are used to understand the size and shape of the pancreatic lesions and the relationship between the surrounding tissues and the organs, such as the pancreatic duct, duodenum, stomach, and portal vein; and ②PET-CTs are used to understand the functional scope of the pancreatic tumor lesions.
-
(b)
Design principles: ① Using the TPS, design the puncture route to avoid important blood vessels, nerves, and lymphatic drainage areas. The use of radiation covers the functional range of the pancreatic tumor lesions. Ensure that the radiation is performed as uniformly as possible with a uniform particle distribution.
5.3. Perioperative treatment
-
1.Preoperative preparation
- General preparation:
-
(1)
For patients with pancreatic malignancies and obstructive jaundice, it is recommended that PTCDs, ERCPs (downward nasal bile drainages or biliary stent implantations), and other operations be performed, to relieve biliary obstruction. At the same time, administer hepatoprotective drugs to restore liver function to a level that can withstand anesthesia and surgery in a short time. Observe the vitamin K3 levels before surgery.
-
(2)
Administer somatostatin routinely for two to three days before surgery using a 3 mg intramuscular injection, once daily.
-
(3)
The same routine preparations before general surgery are used for the rest of the preoperative preparations.
-
(4)
125I particle and postoperative radiation protection preparations should also be performed.
-
2.
The surgical operation
-
(1)
Perform a CT scan was after placing a marking grid on the body surface, design the puncture route according to the preoperative TPS, and mark the puncture point on the body surface.
-
(2)
Perform routine disinfecting and draping.
-
(3)
The use of a CT scan clarifies the position of the puncture needle and adjusts it according to the angle and depth of the treatment plan.
-
(4)
According to the treatment plan at all levels and depths, adjust the particle spacing according to the degree of malignancy of the tumor and the results of the genetic testing, implant the 125I particles required for the plan, and remove the needle.
-
(5)
The use of the CT scan clarifies the number and distribution of the 125I particles.
-
(6)
A PET-CT scan is conducted to determine whether the 125I particle radiation distribution complied with the TPS design. If there is still a cold radiant zone in the lesion, stage II 125I seed implantation can be performed without complications after two weeks.40, 41, 42
-
3.
Postoperative observation and treatment
-
(1)
The patient should fast for 6 hours postoperatively.
-
(2)
Observe the general vital signs of the patient after the operation, including signs of abdominal pain, abdominal distension, and stool color. Blood and urine amylase, blood lipase, routine stool tests, and stool occult blood should be re-checked within 24 hours. If there is abdominal drainage, observe whether the amount of drainage is higher than the amount before the operation, and observe the amylase in the drainage of the abdominal cavity after surgery.
-
(3)
If the puncture route passes through the liver, stomach, duodenum, etc., antibiotics should be used prophylactically for one to three days after the operation. Gastrointestinal mucosal protective agents and drugs can be used to inhibit gastric acid secretions for one week.
-
(4)
Administer somatostatin prophylactically, a 3 mg intramuscular injection, once daily for three days after surgery.
5.4. Common complications
-
1.
Pancreatic fistulas caused by puncture injury to the pancreatic duct.
The treatment can be carried out in accordance with the NCCN treatment principle of acute pancreatitis. Intraoperative punctures should avoid damage to the main pancreatic duct. Conventional treatment principles include fasting, gastrointestinal decompression, use of drugs that inhibit pancreatic enzyme secretion, and nutritional support. This is generally curable.
-
2.
Gastrointestinal symptoms: Common symptoms such as abdominal distension, nausea, vomiting, loss of appetite. If these continue for a long time period, and because the particle radiation area is close to the stomach and duodenum, they can cause radiation inflammation. In focusing on prevention, care should be taken to control the radiation range and the prescribed dose when formulating the TPS. The use of gastrointestinal motility drugs and gastrointestinal mucosal protective agents, as well as gastric acid inhibitor treatment, the can relieve the symptoms in a short time.
-
3.
Postoperative ascites, the common causes of which are:
-
(1)
poor nutritional status and hypoproteinemia;
-
(2)
the particles causing radioactive inflammation in the tumor and surrounding tissues resulting in ascites;
-
(3)
the compression of the portal vein by the radioactive tumor tissue resulting in poor reflux, which in turn causes increased portal pressure and ascites; however, with the provision of adequate nutritional support and somatostatin treatment, the ascites can be absorbed, slowly;
-
4.
Particle displacement in which the particles may migrate to the liver, lungs, and other organs; the particles are caused by the entrance vein and inferior vena cava during the release process after puncture, with most of these patients requiring no special treatment; and
-
5.
Infections, hemorrhages, and chyle fistulas, although rare in clinical practice, can be cured after symptomatic treatment. For major bleeding, embolization of the bleeding target artery is recommended.
5.5. Nursing
-
1.Preoperative care:
-
(1)Perform preoperative examinations such as routine blood, urine, and stool tests; observe the blood clotting time; examine the blood liver, kidney function, blood sugar, and other biochemical indicators; and view the electrocardiogram. Prepare the patient's imaging data, B-ultrasound, chest X-ray, CT.
-
(2)Provide psychological care to the patients (introduce the purpose, method, operation process, curative effect).
-
(3)Prepare the local anesthetics.
-
(4)Prepare preoperative intramuscular injections of phenergan. According to the doctor's advice, administer phenergan 25 mg intramuscularly 30 min before the operation, to patients with a good medical record.
-
(1)
-
2.Postoperative care:
-
(1)Observe the bleeding at the puncture point. Keep the wound dressing clean and dry; if it is contaminated by blood or exudate, replace it timeously.
-
(2)Observe the puncture part for any signs of infection.
-
(3)Observe the area for any symptoms of ectopic embolism and the surrounding tissues for damage.
-
(4)Observe the vital signs.
-
(5)Provide radiological protection.
-
①Environmental management: Postoperative patients should live in a single room or be protected with lead clothing, reduce their range of activities, reduce contact with other patients, and keep the indoor air flowing. The room temperature should be controlled between 22 and 25 °C to reduce the combination of heat and scattered rays from polluting the environment.
-
②Personnel management: Conduct nursing operations and radiation protection knowledge training for the nursing staff. When medical staff engage in close treatment and nursing, they should wear lead-protective aprons, protective neck coverings, protective glasses, and self-made lead protective covers. The nursing staff should concentrate on the completion of the various nursing operations to reduce the contact time with the patient. There should be limited visiting time for the patients' family members.
-
③Home care: When the seed is implanted, specific protective measures should be implemented for those in contact with the patient within four months. Pregnant women with children cannot live in the same room as the patients.
-
①
-
(1)
6. Percutaneous radiofrequency and microwave treatment of advanced pancreatic cancer
6.1. Indications and contraindications
-
1.Indications:
-
(1)After interventional therapy for advanced pancreatic cancer.
-
(2)Patients with pancreatic cancer tumors that cannot be removed surgically and whose expected survival time is greater than 3 months.
-
(3)Patients who are unwilling to undergo resections of pancreatic cancer
-
(4)The estimated survival time is less than 3 months, and its use is selected carefully to relieve persistent upper abdominal pain.
-
(5)Patients with primary pancreatic tumors with a maximum diameter of >7 cm who are selected carefully for tumor reduction therapy.
-
(1)
-
2.Contraindications:
-
(1)There is clear clinical evidence that the pancreatic tumors have spread widely.
-
(2)Patients with cachexia
-
(3)The presence of acute pancreatic inflammation
-
(4)Patients with coagulation dysfunctions that cannot be improved with medication.
-
(5)Patients with severe diabetes, in whom the blood sugar cannot be controlled below 15.6 mmol/L with hypoglycemic therapy.
-
(6)Patients with bacteremia and sepses43
-
(1)
6.2. Choice of radiofrequency and microwave44,45
-
1.
Determination of radiofrequency and microwave power
Based on tumor sizes, locations, and internal structures (pathological malignancy, drug/gene test report), as well as CT/MR lesion enhancements and levels of necrosis, different electrodes from T20 to T40, can be selected. Table 2 shows the types, numbers, and spacing of the electrodes.
-
(1)
Radiofrequency ablation of pancreatic cancer: It is recommended that either CT positioning (100 ml of 2% iodine water can be taken before puncture to show the relationship between the gastrointestinal tract and the tumor) or open operations, be performed. Since the pancreas is embedded in a “C” shaped depression formed by the duodenum and stomach tissues, and there are many cavities around it, the use of B-ultrasound positioning is not recommended. A CT, owing to its better resolution, is preferred for the percutaneous puncture path. While open surgery is more traumatic for patients and has an anesthesia risk, its advantages include: biopsies to confirm the pathology can be performed at the same time during the operation; the abdominal organs that are prone to metastasis, such as the liver; for the combined bile duct and duodenum can be explored; patients with obstructions can undergo bypass surgeries to improve the symptoms of the obstruction; and complications such as intraoperative vascular and biliary injuries can be attended to promptly.
-
(2)
Puncture plan
Table 2.
-
2.Number of needles and choice of surgical approach
| Electrode type | Quantity | spacing (mm) |
Target energy (KJ) |
Power setting (W) |
Treatment length (mm) |
Treatment diameter (mm) |
Treatment volume (cm3) |
|
|---|---|---|---|---|---|---|---|---|
| T20 | 1 | 9 | 20 | 22 | 20 | 5 | ||
| T20 | 2 | 7 | 14 | 40 | 25 | 27 | 20 | 7 |
| T20 | 3 | 15 | 24 | 60 | 25 | 30 | 30 | 12 |
| T30 | 1 | 15 | 30 | 32 | 22 | 5.5 | ||
| T30 | 2 | 13 | 25 | 60 | 35 | 25 | 8 | |
| T30 | 3 | 15 | 15–35 | 90 | 35 | 20–30 | 14 | |
| T40 | 1 | 29 | 40 | 44 | 25 | 14 | ||
| T40 | 2 | 13.3 | 39 | 80 | 45 | 33 | 25 | 19 |
| T40 | 3 | 20 | 35–70 | 120 | 50 | 30–40 | 22 | |
| T40 | 3 | 25 | 70–130 | 120 | 55 | 40–50 | 47 | |
| T40 | 3 | 30 | 130–225 | 120 | 60 | 50–60 | 87 | |
Important organs and blood vessels, such as the pancreatic duct and bile duct, should be avoided, as much as possible.
-
(3)
Determine the number of ablation electrodes according to the tumor size.
Generally, 1 to 3 needles are arranged in an equilateral triangle with an interval of 2.0 cm.
-
(4)
Microwave ablation must be selected carefully due to the poor controllability of the ablation morphology. If the primary and metastatic lesions are larger than 5.0 cm in diameter, then tumor reduction treatment is preferred. The recommended reference microwave power dosage range is 70 W and the ablation time is 5–10 min. It is recommended that the lesion be treated every 5 min. Position the scan to observe the changes in the ablated lesion and determine the length of time for re-ablation.
6.3. Perioperative treatment
-
1.
Preoperative preparation:
General preparation:
-
(1)
For patients with malignant pancreatic tumors and obstructive jaundice, PTCDs, ERCPs, and other operations are recommended to relieve the biliary obstructions. At the same time, the patient can be treated with hepatoprotective drugs to restore the liver function in a short time, to a level that can withstand anesthesia and surgery. Observe the vitamin K1 levels before surgery.
-
(2)
Administer somatostatin routinely before surgery (3 mg intramuscular injection, once daily for 2–3 days).
-
(3)
The rest of the preoperative preparation is the same as the routine preparation before general surgery.
-
2.Surgical operation:
-
(1)Perform a CT scan was after placing a marking grid on the body surface, design the puncture route according to the preoperative TPS, and mark the puncture point on the body surface. Qualified units can make use of a puncture robot.
-
(2)Perform disinfection and spreading of the towels.
-
(3)After making the puncture according to the plan, perform a CT to determine the position of the puncture needle and adjust it appropriately.
-
(4)Perform the ablation according to the set power and time.
-
(5)Perform a CT scan again to observe if there are active lesions and bleeding complications
-
(6)PET-CT scans can be performed in qualified departments to understand whether the ablation lesions have been completely ablated. Due to the obvious pain at the ablation sites for pancreatic cancers during radiofrequency ablation, general and intravenous anesthesia should be administered during the operation, if the patient's condition permits. In the case of percutaneous puncture ablations, observation of the patient's breathing is required, and anesthesia can be administered after the radiofrequency needle is in place and before the lesion is ablated.
-
(1)
-
3.Postoperative observation and treatment:
-
(1)The patient should fast for 6 hours postoperatively.
-
(2)Observe the general vital signs of the patient after the operation, including signs of abdominal pain, abdominal distension, and stool color. Blood and urine amylase, blood lipase, routine stool tests, and stool occult blood should be re-checked within 24 hours. If there is abdominal drainage, observe whether the amount of drainage is higher than the amount before the operation, and observe the amylase in the drainage of the abdominal cavity after surgery.
-
(3)If the puncture route passes through the liver, stomach, duodenum, etc., antibiotics should be used prophylactically for one to three days after the operation. Gastrointestinal mucosal protective agents and drugs can be used to inhibit gastric acid secretions for one week.
-
(4)Administer somatostatin prophylactically, a 3 mg intramuscular injection, once daily for three days after surgery.
-
(1)
6.4. Common complications
-
1.
Pancreatic fistulas caused by puncture injury to the pancreatic duct.
The treatment can be carried out in accordance with the NCCN treatment principle of acute pancreatitis. Intraoperative punctures should avoid damage to the main pancreatic duct. Conventional treatment principles include fasting, gastrointestinal decompression, use of drugs that inhibit pancreatic enzyme secretion, and nutritional support. This is generally curable.
-
2.
Gastrointestinal symptoms: Common symptoms such as abdominal distension, nausea, vomiting, loss of appetite, etc. While these may last for a long time, the use of gastrointestinal motility drugs, mucosal protective agents, and gastric acid inhibitors can relieve symptoms in a short time.
-
3.
Postoperative ascites: give adequate nutritional support and somatostatin treatment, ascites can be slowly absorbed.
-
4.
Infection, hemorrhage, and chyle leakage are rare in clinical practice and can be cured after symptomatic treatment. For major bleeding, the embolization of the bleeding target artery is recommended.
6.5. Nursing
-
1.Preoperative care
-
(1)Perform preoperative examinations such as routine blood, urine, and stool tests; observe the blood clotting time; examine the blood liver, kidney function, blood sugar, and other biochemical indicators; and view the electrocardiogram. Prepare the patient's imaging data, B-ultrasound, chest X-ray, CT.
-
(2)Preparation for the operation according to different parts of the tumor.
-
(3)Provide psychological care to the patients (introduce the purpose, method, operation process, curative effect).
-
(4)Patients undergoing general abdominal anesthesia should fast for 12 hours before surgery.
-
(5)Administer preoperative injections according to the doctor's orders, gather the medical records, and escort the patients into the operating room.
-
(1)
-
2.Postoperative care
-
(1)Monitor the vital signs and wound bleeding and administer oxygen, hemostatic agents, antibiotics, according to the doctor's advice. The wound dressing at the puncture site should be kept clean and dry. If it is contaminated with blood or exudate, it should be replaced timeously, to prevent wound infections.
-
(2)Choose the lying position and diet according to the intraoperative anesthesia method, and for patients who undergo general anesthesia, the routine nursing after general anesthesia should be implemented. Since the needle passes through the gastrointestinal tract during the transabdominal puncture, it is necessary for the patient to fast for 24 hours after the operation.
-
(3)Check the body temperature eight consecutive times and observe whether there are any signs of infection.
-
(4)Observe whether there is pain in the local area, or whether there are any physical abdominal signs of the transabdominal puncture, and report to the doctor if there are any abnormalities.
-
(5)Observe whether the patient's skin had scalds.
-
(1)
7. Clinical management of common complications of advanced pancreatic cancer
7.1. Obstructive jaundice
-
1.
Reason:
Tumors in the head of the pancreas, metastases in the hilar lymph nodes.
-
2.Disposal strategy:
-
(1)For PTCDs, internal and external drainage is generally feasible. If the hilar obstruction is severe, external drainage can be performed for 3–7 days. After the inflammation and edema of the obstruction disappear, internal and external drainage can be performed. Its advantages are its relatively simple technical requirements, short paths, simple operation, and easy promotion. The disadvantage is the trauma.
-
(2)Internal and external drainage are generally feasible for ERCPs. If the hilar obstruction is severe, external drainage can be performed for 3–7 days. After the inflammation and edema of the obstruction disappear, internal and external drainage can be performed. Its advantage is that it is minimally invasive. Its disadvantages include its high technical requirements, long paths, and relatively difficult operations.
-
(3)For biliary stent implantations, according to the different causes of obstruction, choose different specifications of stents, and implant the stents after the liver function is restored to normal.
- (4)
-
(1)
7.2. Lymph node metastasis
7.3. Gastrointestinal obstruction
-
1.Reasons:
-
(1)intra-abdominal lymph node metastasis and compression of the gastrointestinal tract;
-
(2)pancreatic cancer lesion compression;
-
(3)anastomotic stenosis after pancreatic cancer;
-
(4)tumor dissemination resulting in intestinal and mesangial entanglement stenosis.50
-
(1)
-
2.Treatment strategies:
-
(1)decompression of the gastrointestinal tract and insertion of the gastrointestinal nutrition tube to the distal end of the gastrointestinal tract obstruction for 3–7 days, followed by stent implantation after inflammation and edema at the obstruction site disappear;
-
(2)for stent implantation, stents of different specifications are selected according to the cause and location of the obstruction;
-
(3)for the specific requirements and technical standards of gastrointestinal stents, please refer to the relevant guidelines of other professional committees.
-
(1)
7.4. Intractable pain
-
1.Reasons:
-
(1)intra-abdominal lymph node metastasis compression;
-
(2)pancreatic cancer lesion compression;
-
(3)celiac ganglion invasion
-
(1)
-
2.Disposal strategies:
-
(1)radiofrequency ablation;
-
(2)celiac ganglion block;
-
(3)principles of three-step pain treatment;
-
(4)for radiofrequency ablation, specific requirements; and
-
(5)for technical standards, please refer to relevant guidelines of other professional committees.
-
(1)
8. Treatment of advanced pancreatic cancer by percutaneous puncture intratumor chemical induction immunotherapy.51, 52, 53, 54, 55
8.1. Indications
-
(1)
After interventional therapy for advanced pancreatic cancer.
-
(2)
Patients with pancreatic cancer whose tumors cannot be removed surgically and whose expected survival time is > 3 months.
-
(3)
Unwillingness to undergo resection for pancreatic cancer
8.2. Contraindications
-
(1)
There is clear clinical evidence that pancreatic tumors have spread widely.
-
(2)
Patients with cachexia.
-
(3)
The presence of acute pancreatic inflammation.
-
(4)
Patients with coagulation dysfunction which cannot be improved by drug treatment.
-
(5)
Patients with severe diabetes whose blood sugar cannot be controlled to under15.6 mmol/L with hypoglycemic treatment.
-
(6)
Patients with bacteremia and sepsis who cannot receive radiofrequency or microwave therapy.
8.3. Equipment and drugs
These are: 23G × 150 mm and 25G × 90 mm puncture needles, high pressure syringes; ultrasound/CT, a drug combination of chemotherapeutic drugs cytarabine (Ara-C), adriamycin (ADM) + water-soluble sustained-release agent + immune adjuvant (DNP).
8.4. Treatment methods
-
1.
Related inspections should be performed before the treatment. Fifteen minutes before treatment, the patient should be injected intramuscularly with 0.1 g bucinazine, 1 KU reptilase or 1.0 g etamsylate, and 4–8 mg ondansetron to prevent post-treatment pain, bleeding, and vomiting. For treatment under CT guidance, 500 ml of 1%–2% diatrizoate meglumine solution can be administered orally before treatment.
-
2.
During the treatment, the puncture point is first guided by ultrasound and/or CT to determine the distance from the skin puncture point to the center of the tumor. A 2% lidocaine solution can be used for local anesthesia, with the puncture needle being inserted into the center of the tumor according to the ultrasound and (or) CT positioning direction. After confirmation of the center of the tumor using B-ultrasound and (or) CT, withdraw the needle core, connect the high-pressure syringe, and inject the slow-release liquid to saturate the tumor with the drug. Injection dose (ml) = tumor diameter (<5 cm) × 2.0, or if the tumor diameter is > 5 cm, then × 1.5.
-
3.
After the injection, pause for a while, pull out the puncture needle, cover the puncture site with a sterile gauze, and affix it with tape. To avoid any duodenal obstruction, the patient may consume liquids or semi-liquid food within 1–3 days after treatment. Symptomatic treatment should be administered to patients with accompanying symptoms.
8.5. Matters requiring attention during treatment
Strive to achieve a successful and accurately positioned puncture and aim to reduce gastrointestinal damage. During the injection, if the patient experiences any pain, an appropriate drug can be injected after the lidocaine. If an acute inflammatory reaction of the pancreatic tumor occurs after treatment, it should be treated according to the routine treatment methods for acute pancreatitis.
9. Optimal choice of interventional therapy for advanced pancreatic cancer
Patients with advanced pancreatic cancer should receive the comprehensive treatment method of cTAI combined with physical therapy for the primary tumor and metastasis as soon as possible. The use of cTAI can control the primary and metastatic lesions of the pancreatic cancer effectively. The choice of regimen should be based on the sensitivity of the tumor cells to the chemotherapeutic drugs. Physiotherapy includes particle, radiofrequency, and microwave therapy for the primary and metastatic lesions. The selection of specific methods should be based on the location, blood supply, and internal structure of the tumor (Table 3).56,57
Table 3.
Interventional options for advanced pancreatic cancer.
| cTAI treatment | Low/undifferentiated adenocarcinoma, rich blood supply, genetically sensitive ROBO3↑/miR-250b↓, etc. |
| Pancreatic head: gastroduodenal artery, superior mesenteric artery | |
| Pancreatic body and tail: short splenic artery, right gastroepiploic artery | |
| Dominant artery: chemoembolization; non-dominant artery: embolization | |
| Physiotherapy | Medium/highly differentiated adenocarcinoma, insufficient blood supply, genetic insensitivity ROBO3↓/miR-250b↑etc. |
| Pancreatic head: 125 I seed implantation | |
| Body tail: radiofrequency/microwave ablation |
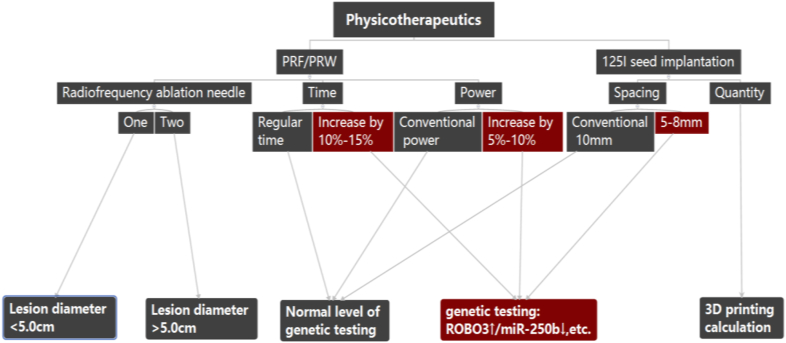
Tumors in the head of the pancreas should be treated with percutaneous 125I seed implantation under imaging guidance. Tumors in the body and tail should be treated mainly with percutaneous radiofrequencies and microwaves; for tumors with an insufficient blood supply, physical therapy is the main treatment (Fig. 1).
Fig. 1.
Individualized physical therapy options.
To improve the clinical immunity of patients, cTAI should be used as the preferred method, and physical therapy should be used as an effective supplement. These two can be used interactively and repeatedly.58
-
9.1
The location of the cTAI catheter for continuous intra-arterial infusion chemotherapy varies according to the location of the tumor. Tumors in the head of the pancreas are placed in the superior and inferior duodenal arteries, and tumors in the body of the pancreas are placed in the dorsal pancreatic artery and transverse pancreas. Arteries: tumors in the tail of the pancreas, left in the aorta of the pancreas, and the junctional artery
The chemotherapy regimen should comply with the following principles: first, it should be based on the tumor drug sensitivity test report; second, when there is a lack of pathological diagnosis, combined with CT, MRI, and other imaging findings, refer to the classic UICC treatment plan.
Drug continuous infusion time: time-independent drugs (such as gemcitabine) for approximately 2 hours; time-dependent drugs (such as 5-FU) for approximately 20 hours.
-
9.2
125I seed implantation
First, we should clarify the tumor size, shape, location, and anatomical relationship with adjacent organs, and determine the puncture path; second, using a TPS determine the specific number of particles and the 125I implantation site. The treatment should follow the following principles: nearest to the center of the lesion, minimal damage to adjacent organs, and simple operation. The layer spacing and spacing of the particles can be adjusted to between 5 and 10 mm according to the activity level of the tumor and the results of the genetic testing, as shown in Fig. 1.
In addition to preoperative preparations for conventional interventions, there should be fasting for one day before the surgery and a continuous intravenous infusion of somatostatin for 24 hours to suppress and reduce the incidence of secondary pancreatitis after surgery. Under CT guidance, complete the biopsy, and implant the 125I particles. After the operation, confirm using the CT whether the distribution of the particles was in accordance with the design plan, whether any were missing or had shifted, and whether there was any local hematoma or other organ damage. After the operation, the patient should fast and receive a continuous intravenous infusion of somatostatin for 24 hours, intravenous nutritional support and hemostatic treatment for one to three days, and be observed for changes in blood pressure, abdominal pain, and amylase.
-
9.3
RF/Microwave therapy
First, we should clarify the tumor size, shape, location, and anatomical relationship of the adjacent organs; second, we should determine the number of needles and puncture paths; and third, we should determine the power and time of the radiofrequency/microwave treatment based on the tumor size and internal structure of the imaging. The treatment should follow the following principles: nearest to the center of the lesion, minimal damage to adjacent organs, and simple operation. During needle withdrawal, the needle tract should be properly burned to reduce bleeding of the needle tract and to prevent tumor implantation and metastasis. Other perioperative treatments are the same as those for seed implantation.
-
9.4
Interventional treatment strategy for APC patients, cTAI is the first choice
However, there are the following manifestations: 1) tumor lesions continue to grow, 2) tumors do not continue to shrink, and 3) the patient is unable to tolerate it. 125I seed implantation, radiofrequency, or microwave ablation therapy, should be considered.
-
9.5Selection principles of particles, radiofrequency and microwave
-
1.To avoid damage to the pancreatic duct, bile duct, and adjacent descending duodenum, 125I seed implantation is often used for tumors in the head of the pancreas, and it has been proposed that the number of lesions should not exceed three at the same site, with a diameter less than 5.0 cm or at three sites for lesions less than 3.0 cm and with gene ROBO3↑/miR-250b↓ being based on percutaneous physical therapy, the ablation power increases by 5%–10%, the time extended by 10%–15%, and the seed implantation distance reduced to 5–8 mm.
-
2.For body and tail tumors, cTAI-125I seed implantation-cTAI mode, or cTAI-radiofrequency/microwave ablation-cTAI mode; cTAI-radiofrequency/microwave ablation can also be selected for part of the residual lesions, and the 125I particle implantation mode can also be added.
-
1.
10. Follow-up and efficacy monitoring methods for interventional therapy of advanced pancreatic cancer
10.1. Clinical efficacy
-
1.
General data comparison
The data summarizes the findings from six tertiary hospitals (Harbin Medical University Cancer Hospital, Shandong University Second Hospital, Lan University First Affiliated Hospital, Gui Medical University Affiliated Hospital, Lishui Central Hospital, and Tongji Tenth Hospital) from January 2009 to December 2014. According to the relevant data of the 610 patients who met the enrollment criteria, 19 were lost to follow-up, with a loss to follow-up rate being 3.11%. Among the enrolled patients, there were 370 men and 240 women, with an average age of 67.24 ± 12.46 years (28–90 years). These patients were divided randomly into the control group: conventional intravenous chemotherapy and the cTAI group: intra-arterial continuous infusion chemotherapy. The cTAI combination group comprised of three groups of continuous intra-arterial infusion chemotherapy combined with physical therapy (including particles, radiofrequency, and microwave therapy for primary and metastatic lesions). The general information of patients before treatment is shown in Table 4. Before treatment, the tumor volume and renal function status were well balanced between the groups. Among these, obstructive jaundice and ascites showed differences; the reason being that the conventional intravenous chemotherapy and intra-arterial continuous infusion chemotherapy groups required that the liver function return to normal or basically normal.
Table 4.
Comparison of general information of the three groups of patients before treatment.
| variable | Control group n = 215 |
c TAI group n = 210 |
c TAI Joint Group n = 185 |
total n = 610 |
P value |
|---|---|---|---|---|---|
| Age, years | 66.31 ± 11.76 | 68.11 ± 13.08 | 66.84 ± 13.17 | 67.24 ± 12.46 | 0.167 |
| gender | |||||
| Male, n (%) | 115(53.5) | 150(71.4) | 105(56.8) | 370(60.7) | 0.068 |
| Female, n (%) | 100 (46.5) | 60(28.6) | 80(43.2) | 240(39.3) | |
| TNM staging | |||||
| T3N1M 0, n (%) | 28(13.0) | 21(10.0) | 17(9.1) | 66(10.8) | 0.651 |
| T3N1M 1, n (%) | 187(87.0) | 189(90.0) | 168(90.9) | 544(89.2) | |
| Tumor size (cm) | |||||
| <3.0, n (%) | 3(1.3) | 0(0.0) | 0(0.0) | 3(0.5) | 0.252 |
| 3.0–5.0, n (%) | 2(0.9) | 0(0.0) | 0(0.0) | 2(0.3) | |
| >5.0, n (%) | 210(98.8) | 210(100.0) | 185(100.0) | 605(99.2) | |
| With or without transfer | |||||
| Yes, n (%) | 190(88.4) | 182(86.7) | 148(80.0) | 520(86.2) | 0.651 |
| None, n (%) | 25(11.6) | 28(13.3) | 37(20.0) | 90(13.8) | |
| ascites | |||||
| Yes, n (%) | 17(7.9) | 5(2.4) | 7(3.8) | 29(4.8) | 0.014 |
| None, n (%) | 198(92.1) | 205(97.6) | 178(96.2) | 581(95.2) | |
| jaundice | |||||
| Yes, n (%) | 45(20.9) | 110(52.4) | 20(10.8) | 175(28.7) | 0.000 |
| None, n (%) | 170(79.1) | 100(47.6) | 165(89.2) | 435(71.3) | |
| Child-Pugh classification | |||||
| A, n (%) | 0(0.0) | 0(0.0) | 0(0.0) | 0(0.0) | 0.003 |
| B, n (%) | 48(22.3) | 62(29.5) | 94(50.8) | 204(33.4) | |
| C, n (%) | 121(77.7) | 148(70.5) | 91(49.2) | 406(66.6) | |
| CA199 | |||||
| Normal, n (%) | 43(20.0) | 51(24.3) | 40(21.6) | 134(22.0) | 0.774 |
| Abnormal, n (%) | 172(80.0) | 159(75.7) | 145(78.4) | 476(78.0) |
Note:Control group: conventional intravenous chemotherapy; cTAI group: continuous intra-arterial infusion chemotherapy; cTAI combined group: continuous intra-arterial infusion chemotherapy combined with physical therapy (including particles, radiofrequency, and microwave treatment of primary and metastatic lesions).
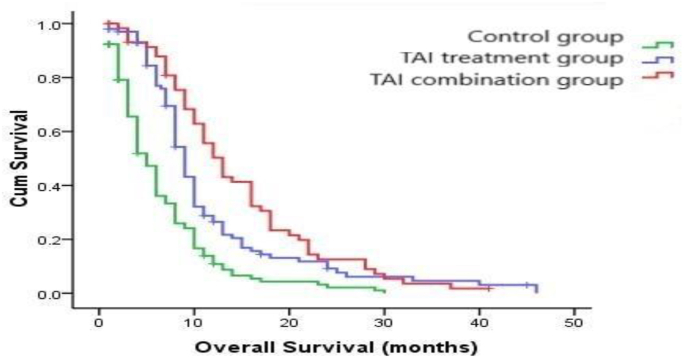
The statistical results showed that the overall survival time (OS: months, mean ± SD) of the different groups of patients after treatment were 6.13 ± 5.40 months, 10.52 ± 8.17 months and 13.80 ± 8.86 months, respectively. There were significant statistical differences among the three groups (P = 0.000, log-rank test), as shown in Fig. 2.
Fig. 2.
-
3.mRECIST analysis
A comparative analysis of the clinical remission rate among the three groups showed that the clinical remission rate of the control group was 17.7%, in the cTAI group 41.9%, and in the cTAI combination group, 47.5%. Furthermore, the pairwise control analysis between the groups showed that the overall clinical remission rate was significantly different between the three groups (χ2 test, P = 0.000). In addition, the pairwise control analysis between groups showed that the remission rate of the cTAI and cTAI combination groups was significantly higher than that of the control group (Table 5).
Table 5.
Comparison of clinical remission rates among the three groups.
| mRECIST | Control group n = 215 |
c TAI n = 210 |
c TAI Joint Group n = 185 |
total n = 610 |
P value |
|---|---|---|---|---|---|
| CR + PR, n (%) | 38(17.7) | 88(41.9) | 88(47.5) | 214(35.1) | 0.000 |
| SD, n (%) | 103(47.9) | 70(33.3) | 51(27.6) | 224(36.7) | |
| PD, n (%) | 74(34.4) | 52(24.8) | 46(24.9) | 172(28.2) |
Comparing the survival rates at six, 12, and 18 months among the three groups, the cTAI group and the cTAI combined group showed significant advantages over the control group (χ2 test, P = 0.000, 0.000, 0.001) (Table 6, Table 7, Table 8).
Table 6.
Comparison of survival rates between the three groups at 6 months.
| ending | Control group n = 215 |
c TAI group n = 210 |
c TAI Joint Group n = 185 |
total n = 610 |
P value |
|---|---|---|---|---|---|
| Survival, n (%) | 76(35.3) | 160(76.2) | 162(87.6) | 398(65.2) | 0.000 |
| Death, n (%) | 139(64.5) | 50(23.8) | 23(12.4) | 212(34.8) |
Table 7.
Comparison of survival rates between the three groups at 12 months.
| ending | Control group n = 215 |
c TAI group n = 210 |
c TAI Joint Group n = 185 |
total n = 610 |
P value |
|---|---|---|---|---|---|
| Survival,n (%) | 21(9.7) | 51(24.3) | 92(49.7) | 164(26.9) | 0.000 |
| Death,n (%) | 194(90.3) | 159(75.7) | 93(50.3) | 446(73.1) |
Table 8.
Comparison of survival rates between the three groups at 18 months.
| ending | Control group n = 215 |
c TAI group n = 210 |
c TAI Joint Group n = 185 |
total n = 610 |
P value |
|---|---|---|---|---|---|
| Survival,n (%) | 8(3.7) | 23(11.0) | 43(23.2) | 74(12.1) | 0.001 |
| Death, n (%) | 207(96.3) | 187(89.0) | 142(76.8) | 536(87.9) |
10.2. Comparison with similar research in China and in other countries59, 60, 61, 62, 63, 64
-
1.
Arterial chemotherapy compared with conventional chemotherapy.
In this study the control group had an OS time of 6.13 ± 5.40 months, consistent with clinical efficacies reported in the literature, and showed no significant differences (χ2 test, P > 0.05). The OS time of the arterial chemotherapy group (c-TAI group) in this study was 10.52 ± 8.17 months, and its clinical efficacy was better than that of the conventional chemotherapy group, and there was a statistical difference (χ2 test, P < 0.05).
-
2.
Comparison of arterial chemotherapy combined with physical therapy and physical therapy alone.
A recent retrospective clinical trial study by the Eastern Cooperative Oncology Group of the United States suggested that the OS time for advanced pancreatic cancer with physical therapy alone was 7.1 months. In this study, the OS time for the combined groups was 13.80 months, and their clinical efficacy was significantly better than that of physical therapy alone, as reported in the literature (χ2 test, P < 0.05).
-
3.
Comparison of arterial chemotherapy and physical therapy with conventional chemotherapy and physical therapy.
In this study, the OS time for the arterial chemotherapy group combined with the physical therapy group was 13.80 months, while the results from the American Cooperative Oncology Group Eastern showed an OS time of 9.2 months. For arterial chemotherapy combined with physical therapy, the clinical efficacy of the treatment group was significantly better than that of conventional chemotherapy combined with physical therapy as reported in the literature (χ2 test, P < 0.05).
11. Summary
In summary, for patients with advanced pancreatic cancer whose tumors cannot be removed surgically, interventional therapies such as cTAI, 125I seed implantation, radiofrequency, and microwave ablation are good choices. Choosing one or more interventional treatment methods, according to tumor size, shape, location, and adjacent organ anatomy, can improve the overall survival and quality of life of patients with advanced pancreatic cancer, effectively. With the continuous development of nanotechnology, gene technology, molecular imaging technology, and molecular interventional therapy technology, the efficacy of advanced pancreatic cancer therapy will be further improved.
12. Writing committee units and experts
Zhuhai People's Hospital (Lu Ligong, Li Yong); Hefei Second People's Hospital (Yin Shiwu, Longhai Deng); The First Affiliated Hospital of University of Science and Technology of China (Lu Weifu, Lu Dong); Chinese Academy of Medical Sciences Tumor Hospital (Li Xiao, Li Huai)); Chinese People's Liberation Army General Hospital (Wei Yingyi, Xiao Yueyong); Beijing Cancer Hospital (Zhu Xu); Tianjin Cancer Hospital (Guo Zhi, Yu Haipeng); Wuhan Union Hospital (Zheng Chuansheng); Zhejiang Cancer Hospital (Shao Guoliang); Jiangsu Provincial Tumor Hospital (Chen Shiyan); Harbin Medical University Tumor Hospital (Liu Ruibao); Sun Yat-sen University Tumor Hospital (Zhang Fujun); Sichuan Tumor Hospital (Xu Guohui); Yunnan Tumor Hospital (Huangming); Kunming Medical University No. 1 The Affiliated Hospital (Zhao Wei, Hu Jihong); The Second Affiliated Hospital of Kunming Medical University (Wang Jiaping); The Affiliated Hospital of Guizhou Medical University (Zhoushi); Hunan Provincial People's Hospital (Xiang Hua); The First Hospital of Lanzhou University (Wang Wenhui); Gansu Provincial People's Hospital (Che Ming); Zhongda Hospital of Southeast University (Teng Gaojun, Guo Jinhe); The First Affiliated Hospital of China Medical University (Xu Ke, Zhong Hongshan, Su Hongying); The Second Hospital of Shandong University (Li Yuliang); The Affiliated Hospital of Jining Medical University (Joe Yuen Kong); Zhejiang Lishui Central hospital (Jijian Song); first Affiliated hospital (NI Cai Fang, ZHU Xiao) Soochow University; first Affiliated hospital of Xinjiang Medical University (Ren Weixin); People's Liberation Army 960 hospitals (Sun Gang); first Changzheng Hospital Affiliated to the Second Military Medical University (Dong Weihua, Xiao Xiangsheng); Zhongshan Hospital Affiliated to Fudan University (Wang Jianhua, Yan Zhiping); Fudan University Public Health Center (Yuan Min); The Sixth People's Hospital Affiliated to Shanghai Jiaotong University (Cheng Yingsheng); Shanghai Jiaotong University Medicine Ruijin Hospital (Wang Zhongmin); Tongren Hospital of Shanghai Jiaotong University (Mao Aiwu); Changhai Hospital of Second Military Medical University (Yang Jijin); Tenth People's Hospital of Tongji University (Li Maoquan, Cao Chuanwu, Lu Zhongwei, Xu Huixiong, Han Shilong, Pan Long), Liu Zhanju, Song Zhenshun, Wang Shi, Chen Jun, Ni Yebin, Kang Li); academic secretaries: Cao Chuanwu, Li Xue.
Declaration of competing interest
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
References
- 1.Amin M.B., Greene F.L., Edge S.B., et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. Ca - Cancer J Clin. 2017;67:93–99. doi: 10.3322/caac.21388. [DOI] [PubMed] [Google Scholar]
- 2.James D.B., Mary K.G., Christian W. eighth ed. John Wiley & Sons, Ltd; 2017. TNM Classification of Malignant Tumours. [Google Scholar]
- 3.Chen W., Zheng R., Baade P.D., et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 4.Kruger S., Schirle K., Haas M., et al. Prolonged time to treatment initiation in advanced pancreatic cancer patients has no major effect on treatment outcome: a retrospective cohort study controlled for lead time bias and waiting time paradox. J Canc Res Clin Oncol. 2020;146:391–399. doi: 10.1007/s00432-019-03061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vincent A., Herman J., Schulick R., et al. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 7.Pancreatic Cancer committee of Chinese Anti-Cancer Association Comprehensive guidelines for the diagnosis and treatment of pancreatic cancer (2020 version) Chin J Surg. 2021;59:81–100. doi: 10.3760/cma.j.cn112139-20201113-00794. [DOI] [PubMed] [Google Scholar]
- 8.Lou W.H., Liu Y.B., Liang T.B., et al. A consensus statement on the diagnosis, treatment, and prevention of common complications after pancreatic surgery (2017) Medical Journal of Peking Union Medical College Hospital. 2017;8:139–146. [Google Scholar]
- 9.Ni Quanxing, Yu Xianjun, Liang Liu. Discussion for the clinical definition of pancreatic cancer in China. China Oncology. 2012;22:81–87. [Google Scholar]
- 10.Sohal D.P., Mangu P.B., Khorana A.A., et al. Metastatic pancreatic cancer: American society of clinical Oncology clinical practice guideline. J Clin Oncol. 2016;34:2784–2796. doi: 10.1200/JCO.2016.67.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arcelli A., Guido A., Buwenge M., et al. Higher biologically effective dose predicts survival in sbrt of pancreatic cancer: a multicentric analysis (PAULA-1) Anticancer Res. 2020;40:465–472. doi: 10.21873/anticanres.13975. [DOI] [PubMed] [Google Scholar]
- 12.Suker M., Nuyttens J.J., Eskens F.A.L.M., et al. Efficacy and feasibility of stereotactic radiotherapy after folfirinox in patients with locally advanced pancreatic cancer (LAPC-1 trial) EClinicalMedicine. 2019;17:100200. doi: 10.1016/j.eclinm.2019.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu C., Li M. In advanced pancreatic cancer: The value and significance of interventional therapy. J Interv Med. 2020;3:118–121. doi: 10.1016/j.jimed.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirtland H.B., Jr. A safe method of pancreatic biopsy; a preliminary report. Am J Surg. 1951;82:451–457. doi: 10.1016/0002-9610(51)90372-8. [DOI] [PubMed] [Google Scholar]
- 15.Smith E.H., Bartrum R.J., Jr., Chang Y.C. Ultrasonically guided percutaneous aspiration biopsy of the pancreas. Radiology. 1974;112:737–738. doi: 10.1148/112.3.737. [DOI] [PubMed] [Google Scholar]
- 16.Lüning M., Kursawe R., Schöpke W., et al. CT guided percutaneous fine-needle biopsy of the pancreas. Eur J Radiol. 1985;5:104–108. [PubMed] [Google Scholar]
- 17.Adler J.M., Sethi A. Interventional endoscopic ultrasonography in the pancreas. Gastrointest Endosc Clin N Am. 2018;28:569–578. doi: 10.1016/j.giec.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Raymond S.L.T., Yugawa D., Chang K.H.F., et al. Metastatic neoplasms to the pancreas diagnosed by fine-needle aspiration/biopsy cytology: a 15-year retrospective analysis. Diagn Cytopathol. 2017;45:771–783. doi: 10.1002/dc.23752. [DOI] [PubMed] [Google Scholar]
- 19.Tempero M.A. NCCN guidelines updates: pancreatic cancer. J Natl Compr Canc Netw. 2019;17:603–605. doi: 10.6004/jnccn.2019.5007. [DOI] [PubMed] [Google Scholar]
- 20.Shi H.B., Yang M.J., Wang X.L., et al. Feeding arteries of advanced pancreatic cancer:evaluation of 218 patients by digital subtract angiography. Chin J Clin Med. 2014;21:61–62. [Google Scholar]
- 21.Collisson E.A., Bailey P., Chang D.K., et al. Molecular subtypes of pancreatic cancer. Nat Rev Gastroenterol Hepatol. 2019;16:207–220. doi: 10.1038/s41575-019-0109-y. [DOI] [PubMed] [Google Scholar]
- 22.Nie C., Zhang Y., Zhou G., et al. Analysis of the efficacy of transcatheter arterial infusion chemotherapy in the treatment of pancreatic carcinoma. J Interv Med. 2021;4:21–26. doi: 10.1016/j.jimed.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das M. Combined treatment for locally advanced pancreatic cancer. Lancet Oncol. 2019;20:e351. doi: 10.1016/S1470-2045(19)30381-X. [DOI] [PubMed] [Google Scholar]
- 24.Kim J., Bamlet W.R., Oberg A.L., et al. Detection of early pancreatic ductal adenocarcinoma with thrombospondin-2 and CA19-9 blood markers. Sci Transl Med. 2017;9 doi: 10.1126/scitranslmed.aah5583. eaah5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engle D.D., Tiriac H., Rivera K.D., et al. The glycan CA19-9 promotes pancreatitis and pancreatic cancer in mice. The glycan CA19-9 promotes pancreatitis and pancreatic cancer in mice. Science. 2019;364:1156–1162. doi: 10.1126/science.aaw3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fahrmann J.F., Schmidt C.M., Mao X., et al. Lead-time trajectory of CA19-9 as an anchor marker for pancreatic cancer early detection. Gastroenterology. 2021;160:1373–1383.e6. doi: 10.1053/j.gastro.2020.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaur S., Smith L.M., Patel A., et al. A combination of MUC5AC and CA19-9 improves the diagnosis of pancreatic cancer: a multicenter study. Am J Gastroenterol. 2017;112:172–183. doi: 10.1038/ajg.2016.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tempero M.A., Malafa M.P., Al-Hawary M., et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:439–457. doi: 10.6004/jnccn.2021.0017. [DOI] [PubMed] [Google Scholar]
- 29.Li Hua, Dong Yan. Guide to intra-arterial infusion chemotherapy for pancreatic cancers (draft text) J Intervent Radiol. 2012;21 353-335. [Google Scholar]
- 30.Birnbaum D.J., Finetti P., Birnbaum D., et al. Validation and comparison of the molecular classifications of pancreatic carcinomas. Mol Canc. 2017;16:168. doi: 10.1186/s12943-017-0739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Philip P.A., Benedetti J., Corless C.L., et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605–3610. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kindler H.L., Niedzwiecki D., Hollis D., et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Group B (CALGB 80303) J Clin Oncol. 2010;28:3617–3622. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu B., Zhang X., Tsauo J., et al. Transcatheter arterial infusion for pancreatic cancer: a 10-year National Cancer Center experience in 115 patients and literature review. Abdom Radiol (NY) 2019;44:2801–2808. doi: 10.1007/s00261-019-02022-2. [DOI] [PubMed] [Google Scholar]
- 34.Conroy T., Hammel P., Hebbar M., et al. Folfirinox or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018;379:2395–2406. doi: 10.1056/NEJMoa1809775. [DOI] [PubMed] [Google Scholar]
- 35.Gourgou-Bourgade S., Bascoul-Mollevi C., Desseigne F., et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol. 2013;31:23–29. doi: 10.1200/JCO.2012.44.4869. [DOI] [PubMed] [Google Scholar]
- 36.Lei F., Xi X., Batra S.K., et al. Combination therapies and drug delivery platforms in combating pancreatic cancer. J Pharmacol Exp Therapeut. 2019;370:682–694. doi: 10.1124/jpet.118.255786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nappo G., Borzomati D., Zerbi A., et al. The role of pathological method and clearance definition for the evaluation of margin status after pancreatoduodenectomy for periampullary cancer. Results of a multicenter prospective randomized trial. Cancers. 2021;13:2097. doi: 10.3390/cancers13092097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oettle H., Richards D., Ramanathan R.K., et al. A phase III trial of pemetrexed plus gemcitabine versus gemcitabine in patients with unresectable or metastatic pancreatic cancer. Ann Oncol. 2005;16:1639–1645. doi: 10.1093/annonc/mdi309. [DOI] [PubMed] [Google Scholar]
- 39.Rehman H., Chi J., Hakim N., et al. Attenuated regimen of biweekly gemcitabine/nab-paclitaxel in patients aged 65 years or older with advanced pancreatic cancer. Therap Adv Gastroenterol. 2020;13 doi: 10.1177/1756284820974912. 1756284820974912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gai B.D. Chinese expert consensus on treatment of pancreatic cancer by radioactive 125I particle implantation (2017) Journal of Clinical Hepatology. 2017;11:444–450. [Google Scholar]
- 41.Chen C., Wang W., Wang W., et al. Locally advanced pancreatic carcinoma with jaundice: the benefit of a sequential treatment with stenting followed by CT-guided 125I seeds implantation. Eur Radiol. 2021;31:6500–6510. doi: 10.1007/s00330-021-07764-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia S.N., Wen F.X., Gong T.T., et al. A review on the efficacy and safety of iodine-125 seed implantation in unresectable pancreatic cancers. Int J Radiat Biol. 2020;96:383–389. doi: 10.1080/09553002.2020.1704300. [DOI] [PubMed] [Google Scholar]
- 43.D'Onofrio M., Ciaravino V., De Robertis R., et al. Percutaneous ablation of pancreatic cancer. World J Gastroenterol. 2016;22:9661–9673. doi: 10.3748/wjg.v22.i44.9661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ierardi A.M., Biondetti P., Coppola A., et al. Percutaneous microwave thermosphere ablation of pancreatic tumours. Gland Surg. 2018;7:59–66. doi: 10.21037/gs.2017.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Timmer F.E.F., Geboers B., Nieuwenhuizen S., et al. Locally advanced pancreatic cancer: percutaneous management using ablation, brachytherapy, intra-arterial chemotherapy, and intra-tumoral immunotherapy. Curr Oncol Rep. 2021;23:68. doi: 10.1007/s11912-021-01057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perone J.A., Riall T.S., Olino K. Palliative care for pancreatic and periampullary cancer. Surg Clin. 2016;96:1415–1430. doi: 10.1016/j.suc.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seo D.W., Sherman S., Dua K.S., et al. Covered and uncovered biliary metal stents provide similar relief of biliary obstruction during neoadjuvant therapy in pancreatic cancer: a randomized trial. Gastrointest Endosc. 2019;90:602–612.e4. doi: 10.1016/j.gie.2019.06.032. [DOI] [PubMed] [Google Scholar]
- 48.Shen C.N., Goh K.S., Huang C.R., et al. Lymphatic vessel remodeling and invasion in pancreatic cancer progression. EBioMedicine. 2019;47:98–113. doi: 10.1016/j.ebiom.2019.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fink D.M., Steele M.M., Hollingsworth M.A. The lymphatic system and pancreatic cancer. Canc Lett. 2016;381:217–236. doi: 10.1016/j.canlet.2015.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobayashi S., Ueno M., Kameda R., et al. Duodenal stenting followed by systemic chemotherapy for patients with pancreatic cancer and gastric outlet obstruction. Pancreatology. 2016;16:1085–1091. doi: 10.1016/j.pan.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 51.Morrison A.H., Byrne K.T., Vonderheide R.H. Immunotherapy and prevention of pancreatic cancer. Trends Cancer. 2018;4:418–428. doi: 10.1016/j.trecan.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feng M., Xiong G., Cao Z., et al. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Canc Lett. 2017;407:57–65. doi: 10.1016/j.canlet.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Balachandran V.P., Beatty G.L., Dougan S.K. Broadening the impact of immunotherapy to pancreatic cancer: challenges and opportunities. Gastroenterology. 2019;156:2056–2072. doi: 10.1053/j.gastro.2018.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schizas D., Charalampakis N., Kole C., et al. Immunotherapy for pancreatic cancer: a 2020 update. Canc Treat Rev. 2020;86:102016. doi: 10.1016/j.ctrv.2020.102016. [DOI] [PubMed] [Google Scholar]
- 55.Sethi V., Vitiello G.A., Saxena D., et al. The role of the microbiome in immunologic development and its implication for pancreatic cancer immunotherapy. Gastroenterology. 2019;156:2097–2115.e2. doi: 10.1053/j.gastro.2018.12.045. [DOI] [PubMed] [Google Scholar]
- 56.Mohammed S., Van Buren G., 2nd, Fisher W.E. Pancreatic cancer: advances in treatment. World J Gastroenterol. 2014;20:9354–9360. doi: 10.3748/wjg.v20.i28.9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nevala-Plagemann C., Hidalgo M., Garrido-Laguna I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat Rev Clin Oncol. 2020;17:108–123. doi: 10.1038/s41571-019-0281-6. [DOI] [PubMed] [Google Scholar]
- 58.Chatzizacharias N.A., Tsai S., Griffin M., et al. Locally advanced pancreas cancer: staging and goals of therapy. Surgery. 2018;163:1053–1062. doi: 10.1016/j.surg.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 59.Han S., Cao C., Tang T., et al. ROBO3 promotes growth and metastasis of pancreatic carcinoma. Canc Lett. 2015;366:61–70. doi: 10.1016/j.canlet.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Liu T., Wu G., Cheng J., et al. Multifunctional lymph-targeted platform based on Mn@mSiO2 nanocomposites: combining PFOB for dual-mode imaging and DOX for cancer diagnose and treatment. Nano Research. 2016;9:473–489. [Google Scholar]
- 61.Khorana A.A., Mangu P.B., Berlin J., et al. Potentially curable pancreatic cancer: American society of clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35:2324–2328. doi: 10.1200/JCO.2017.72.4948. [DOI] [PubMed] [Google Scholar]
- 62.Turpin A., El Amrani M., Bachet J.B., et al. Adjuvant pancreatic cancer management: towards new perspectives in 2021. Cancers. 2020;12:3866. doi: 10.3390/cancers12123866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greco S.H., August D.A., Shah M.M., et al. Neoadjuvant therapy is associated with lower margin positivity rates after Pancreaticoduodenectomy in T1 and T2 pancreatic head cancers: an analysis of the National Cancer Database. Surg Open Sci. 2020;3:22–28. doi: 10.1016/j.sopen.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blomstrand H., Green H., Fredrikson M., et al. Clinical characteristics and blood/serum bound prognostic biomarkers in advanced pancreatic cancer treated with gemcitabine and nab-paclitaxel. BMC Cancer. 2020;20:950. doi: 10.1186/s12885-020-07426-8. [DOI] [PMC free article] [PubMed] [Google Scholar]