Abstract
Background
Port-A-Cath systems (PCS) are safe and convenient devices for long-term infusion in patients with malignant tumors. This study retrospectively analyzed the complications from PCS and their management.
Methods
Data of 1695 adults (641 males and 1054 females) with malignant tumors who underwent PCS implantation in our center from January 1, 2009 to December 31, 2019 who had complete follow-up records were collected in this study. The early and late complications and corresponding treatments were studied.
Results
A total of 1716 PCSs were implanted; 21 patients underwent 2 implantations each. The success rate was 100% and no severe complications occurred during implantation. The overall occurrence rate of post-implantation complications was 18.5% (318/1716); 5.5% (94/1716) were early complications and 13.0% (224/1716) were late complications. A total of 451 PCSs were removed, of which 398 were removed due to the end of chemotherapy, while 53 were removed because of complications. A total of 4 deaths occurred from these complications.
Conclusions
The incidence of intra- and post-operative complications is low. In most cases, complications can be effectively controlled without the removal of the PCS and regular follow-up and maintenance are critical.
Keywords: Port-a-cath system (PCS), Complications, Treatments
1. Introduction
A Port-A-Cath system (PCS), also known as a totally implantable venous access device, refers to an intravenous infusion device that can be implanted under the skin and indwelled for a prolonged period. It consists of an injection port and a catheter that is threaded into a vein. PCS can be used to infuse various medications and supplement fluids, transfuse fluids, and collect samples in patients. It helps prevent the pain and discomfort caused by repeated peripheral punctures as it transports drugs directly to the central veins and prevents irritants and corrosives from damaging the peripheral veins. It also provides a permanent passage for intravenous (IV) infusions in patients with cancer. The most common complications include venous thrombosis, infection, and catheter dislocation.1,2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54,2 It is difficult to comprehensively evaluate the complications and treatment methods related to the implantation and long-term use of PCS due to the small sample sizes and short follow-up periods in many clinical studies. This study retrospectively analyzed the data, including the success rate, complications and corresponding treatments, of patients who underwent PCS implantation in our center during the past 10 years.
2. Patients and methods
2.1. Subjects
This retrospective clinical cohort study was approved by the Medical Ethics Committee of the Shenzhen People's Hospital (No. LL-KY-2020444). The requirement for informed consent was waived due to the retrospective nature of the study. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013).
2.2. Patients
A total of 1734 adults with malignant tumors underwent PCS implantation in the Interventional Radiology Department, Shenzhen People's Hospital from January 2009 to December 2019. The patient records were obtained from the hospital medical record system and information about their current status was collected through telephonic interviews. Thirty-nine patients who could not be contacted were excluded. The main indications for PCS were IV chemotherapy, difficult venous access, long-term infusions and prolonged treatment with IV medications.
Data about the patients' age, gender, primary tumor location, PCS type, number of implantations, implantation-related complications and their treatment, implantation results, and PCS removal, were collected. The patients’ follow-up began on the day of implantation and ended on the day of PCS removal, death, or last available record (December 31, 2019). PCS implantation was considered successful when the PCS was functional and was retained in its position. Complications occurring within 2 weeks after implantation were considered early complications, while those that occurred after 2 weeks were considered late complications. The patient details are shown in Table 1.
Table 1.
Patients’ general characteristics and information.
| Number of patients | 1695 |
|---|---|
| Age | Y |
| Mean (SD) | 57.2 (13.6) |
| Range | 19–88 |
| Gender | n (%) |
| Female | 1054 (62.2%) |
| Male | 641 (37.8%) |
| Indications | n (%) |
| Chemotherapy | 1197 (70.6%) |
| Difficult venous access | 248 (14.6%) |
| Parenteral nutrition | 174 (10.3%) |
| Some medications | 76 (4.5%) |
| Primary malignancy | N |
| Colorectal | 781 |
| Breast | 429 |
| Gastric | 127 |
| Pancreatic | 75 |
| Bladder | 63 |
| Lung | 57 |
| Prostate | 38 |
| Sarcoma MSK | 28 |
| Gallbladder | 24 |
| HCC | 20 |
| Testicular | 16 |
| Lymphoma | 12 |
| Esophageal | 6 |
| Ovarian | 5 |
| Small intestine | 5 |
| Kidney | 4 |
| Skin | 3 |
| Brain | 1 |
| Thymoma | 1 |
SD: standard deviation; HCC: hepatocellular carcinoma.
2.3. Perioperative management
The patients underwent chest radiography/color ultrasonography of the subclavian vein and internal jugular vein (to exclude thrombosis), D-dimer test, routine blood tests, and coagulation function tests before implantation. Real-time blood pressure, blood oxygen level, heart rate and electrocardiogram indexes were monitored during surgery. Laboratory data about the total blood count, prothrombin time and partial thromboplastin time (PTT) were obtained within 3 days after surgery.
2.4. Subclavian catheterization
PCS implantation was performed by a qualified senior physician and a resident in an operating room equipped with digital subtraction angiography (DSA). All patients were hospitalized and signed the informed consent form. The PCS was chosen by the physician according to the patient's needs. Tramadol hydrochloride (100 mg, Grunenthal Gmbh, Aachen, Germany) was injected intramuscularly before implantation. The right subclavian vein (SCV) was the preferred venous access for PCS implantation. The left SCV was used only when the puncture was unsuccessful in the right SCV or if imaging showed possible stenosis, compression or occlusion of the right SCV, or a possible thoracic deformity.
The patient was placed in a supine position with the head rotated opposite to the operating side without a pillow. The puncture site was 4.0 cm below the junction of the middle and outer one-thirds of the clavicle and the Seldinger technique was used under local anesthesia. The right SCV was punctured with a 21-G micropuncture kit (Cook, Indiana, USA) and the dilator and catheter sheath were. The indwelling catheter was implanted and fluoroscopy was used to ensure that its proximal end was in a satisfactory position. Local anesthesia was administered to 3–4 cm below the puncture site and a long transverse incision (3–4 cm) was made. Blunt dissection was performed on one side of the incision to create a pocket for the port. The catheter was introduced subcutaneously from the puncture point to the pocket with a tunnel needle. The flat tracheal bifurcation (generally 15–18 cm in length) at the end of the catheter was adjusted, connected to the port after removal of the excess length, and fixed under fluoroscopy. Heparin saline solution (100 U/mL) was used to flush the catheter. The incision was sutured after confirming that there was no leakage.
The surgical region was routinely disinfected after the suturing. The needle was slightly withdrawn and 10 mL of heparin saline solution was injected to test the PCS. After confirming that the PCS was functional, the needle was fixed and covered with sterile dressing, and its end was connected to a heparin cap. Chest radiography was performed to locate the catheter tip and rule out possible complications, such as pneumothorax.
2.5. PCS maintenance
In principle, the PCS can be used immediately after implantation. Regular postoperative care and maintenance were performed according to the recommended standard protocol by trained senior nursing staff. The needle was replaced every 7 days. Further, the catheter was sealed with heparin sodium after each infusion, rinsed carefully after blood collection, and flushed with heparin saline solution every 4 weeks during the interval between two infusions.
2.6. PCS removal
The PCSs were removed under local anesthesia. They were removed to prevent peripheral or central venous thrombosis or other complications when tumors did not recur after the end of chemotherapy. PCS's were also removed in patients when there were multiple PCS-related infections which were not responsive to antibiotics. The devices were removed as soon as possible when PCS-related bacteremia (especially Pseudomonas septicemia, Staphylococcus aureus sepsis, and fungal sepsis) occurred or infective endocarditis was suspected during echocardiography. The PCS was removed in patients with catheter fracture and displacement as well. For the patients receiving advanced palliative care, the removal of PCS was dependent on their general condition. If removal was necessary because of complications, a new PCS was implanted into the contralateral SCV unless it was contraindicated.
2.7. Statistical analysis
Statistical analysis was performed with SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Descriptive statistics were expressed as frequency, percentage (%), range, mean value ± standard deviation.
3. Results
A total of 1716 PCSs was implanted in our hospital from January 2009 to December 2019 in the 1695 patients who had complete follow-up data. The average time of PCS implantation was 23.6 min (16–90 min); the success rate was 100%. The patients consisted of 641 males and 1054 females, and their average age was 57.2 ± 13.6 years old (19–88). There were 781 cases of colorectal cancer, 429 of breast cancer, 127 of gastric cancer, 75 of pancreatic cancer, 63 of bladder cancer, 57 of lung cancer, and 163 of other tumors. The indications for PCS implantation were chemotherapy (n = 1,197, 70.6%), difficult venous access (n = 248, 14.6%), parenteral nutrition (n = 174, 10.3%), and IV medications (n = 76, 4.5%). Of the 1716 PCSs, 1583 were implanted into the right SCV, and 133 were implanted into the left SCV; 1401 were Bard Access Systems (Bard, Salt Lake City, UT, USA), 63 were B. Braun Interventional Systems (B. Braun, Boulogne-Billancourt, France), and 252 were Medcomp Access Systems (Medical, Harleysville, USA). The mean follow-up period was 18 months (2 days–102 months). Refer to Table 1, Table 2 for details.
Table 2.
Details about port-a-cath system implantation.
| Number of PCSs | 1716 |
|---|---|
| PCS type | n (%) |
| Bard | 1401 (81.6%) |
| Medcomp | 252 (14.7%) |
| B. Braun | 63 (3.7%) |
| Puncture site | n (%) |
| Right SCV | 1583 (92.2%) |
| Left SCV | 133 (7.8%) |
| Operation time | |
| Mean (SD) | 23.6 (4.1)(min) |
| Range | 16–90(min) |
| Follow-up | |
| Mean | 18 months |
| Range | 2 days to 102 months |
| Success rate | 100% |
PCS: port-a-cath system; SCV: subclavian vein; SD: standard deviation.
3.1. Complications
The incidence of postoperative complications was 18.5% (318/1,716, see Table 3). The rates of early and late complications were 5.5% (94/1716) and 13.0% (224/1716), respectively.
Tables 3.
Early and late complications∗
| Early complications | n (%) |
|---|---|
| Pneumothorax | 21 (1.2%) |
| Bleeding | 19 (1.1%) |
| Early pocket infections | 17 (1.0%) |
| Arterial mispuncture | 14 (0.8%) |
| Delayed wound healing | 11 (0.6%) |
| Vascular sheath detachment | 7 (0.4%) |
| Nerve injury | 5 (0.3%) |
| Total | 94/1716 (5.5%) |
| Late complications | n (%) |
| Infections | 67 (3.9%) |
| Catheter-associated infections | 32 (1.9%) |
| Isolated pocket infections | 25 (1.4%) |
| Skin infections at the puncture site | 10 (0.6%) |
| Catheter-related thrombosis | 55 (3.2%) |
| Catheter dislocation | 52 (3.0%) |
| Tip displacement | 21 (1.2%) |
| Fracture | 31 (1.9%) |
| Catheter blockage | 32 (1.9%) |
| Extravasation | 11 (0.6%) |
| Flipped port | 7 (0.4%) |
| Total | 224/1716 (13.0%) |
∗: Complications occurring within 2 weeks after implantation were registered as early complications, while those beyond 2 weeks were as late complications.
The early complications included pneumothorax (n = 21, 1.2%), bleeding (n = 19, 1.1%), early pocket infections (n = 17, 1.0%), arterial mispuncture (n = 14, 0.8%), delayed wound healing (n = 11, 0.6%), vascular sheath detachment (n = 7, 0.4%), and nerve injury (n = 5, 0.3%) (Table 3). No air embolism, early catheter displacement, or PCS dysfunction occurred in these patients.
Among the 21 patients with pneumothorax, 2 underwent closed percutaneous catheter drainage, while 1 needed endotracheal intubation. The other 18 cases received conservative treatment since only a small part of the lung tissue was compressed, and no progression was observed.
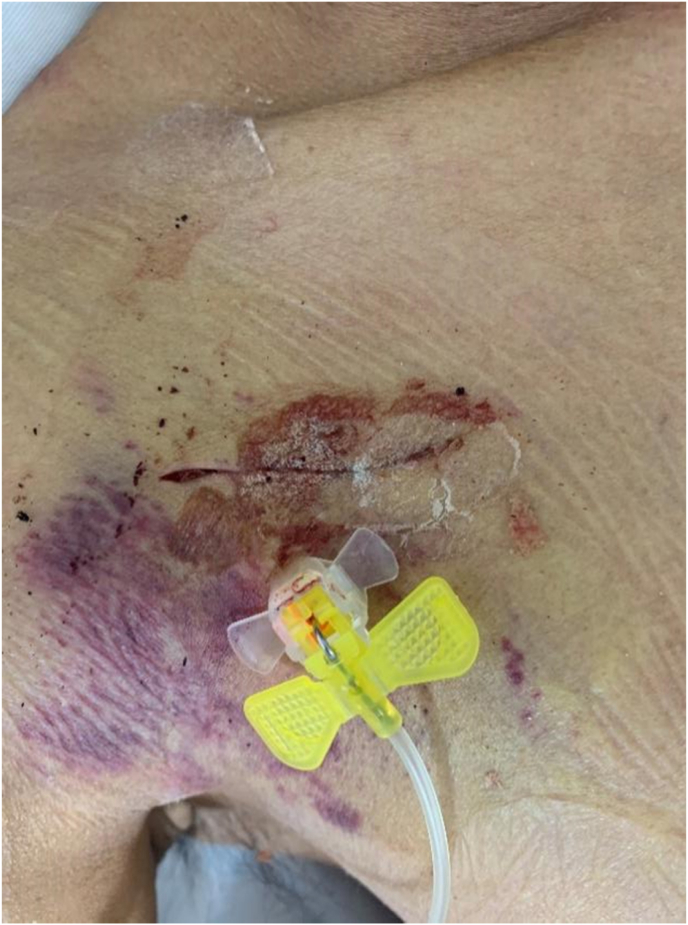
Among the 19 patients who experienced PCS implantation-related bleeding, 10 were taking heparin sodium or aspirin, 4 had severe cirrhosis, 3 had capillary hemorrhage inside the pocket (see Fig. 1), 1 had a low platelet count, and 1 had a prolonged PTT.
Fig. 1.
A 67-year-old male with liver cancer, developing ecchymosis in the area surrounding the pocket on the 3rd day after PCS implantation. Capillary bleeding inside the pocket was considered.
Our evaluation showed that delayed wound healing (n = 11, 0.6%) was not complicated by infections. The wounds were re-sutured in an aseptic environment; no abnormalities were seen when sutures were removed 7 days later.
Among the 17 cases of early pocket infections, 14 patients improved after antibiotic treatment, and 3 patients underwent PCS removal after antibiotic treatment failed and new PCSs were implanted in the contralateral SCV.
Late complications were observed in 13.0% (224/1716) of patients (Table 3). These included infections (skin infections, pocket infections, and catheter-associated bacteremia; n = 67, 3.9%), catheter-related thrombosis (n = 55, 3.2%), catheter dislocation (including fracture and tip displacement; n = 52, 3.0%), catheter blockage (n = 32, 1.9%), extravasation (n = 11, 0.6%), and flipped port (n = 7, 0.4%).
Infections occurred in 67 patients (3.9%) and included catheter-associated bacteremia (n = 32, 1.9%), isolated pocket infections (n = 25, 1.4%), and skin infections at the puncture site (n = 10, 0.6%); associated sepsis was seen in 6 patients. Catheter-associated septicemia was complicated by underlying diseases in the 4 patients who developed septic shock and died after failure of antibiotic treatment. The underlying diseases were diabetes in 1 senior patient, obstructive jaundice and biliary tract infection in 2 middle-aged patients, and an advanced tumor complicated by pneumonia in an elderly patient. The PCS was removed in 4 patients with pocket infections and 7 patients with catheter-associated bacteremia after failure of IV infusion of antibiotics; 3 patients among these 11 were implanted with a new PCS. Pocket infections and catheter-associated bacteremia were alleviated effectively in the other patients after IV infusion of antibiotics. The pathogenic microorganism was Staphylococcus aureus in 80% of patients. Redness and swelling were observed at the puncture site in 10 cases where a butterfly needle was used, which disappeared after regular skin disinfection and anti-inflammatory treatment.
Symptomatic catheter-related thrombosis was observed in 55 cases (3.2%). The symptoms resolved in 49 patients after anticoagulation treatment, 1 developed pulmonary embolisms in a small area that improved after active treatment, and 5 experienced more severe thrombosis after anticoagulation and thrombolytic treatment, and their PCSs were removed.
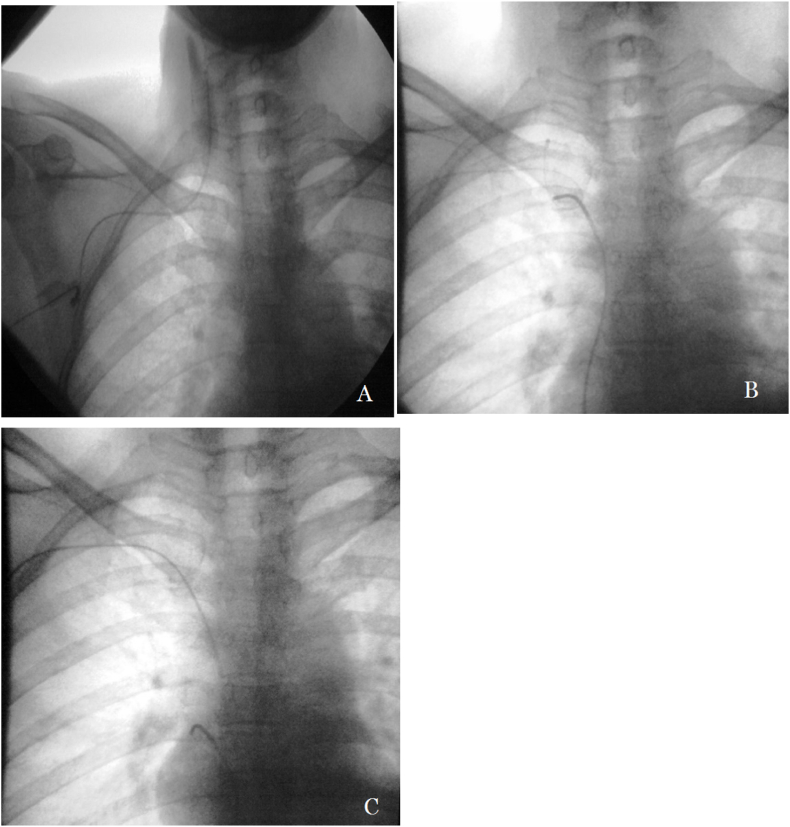
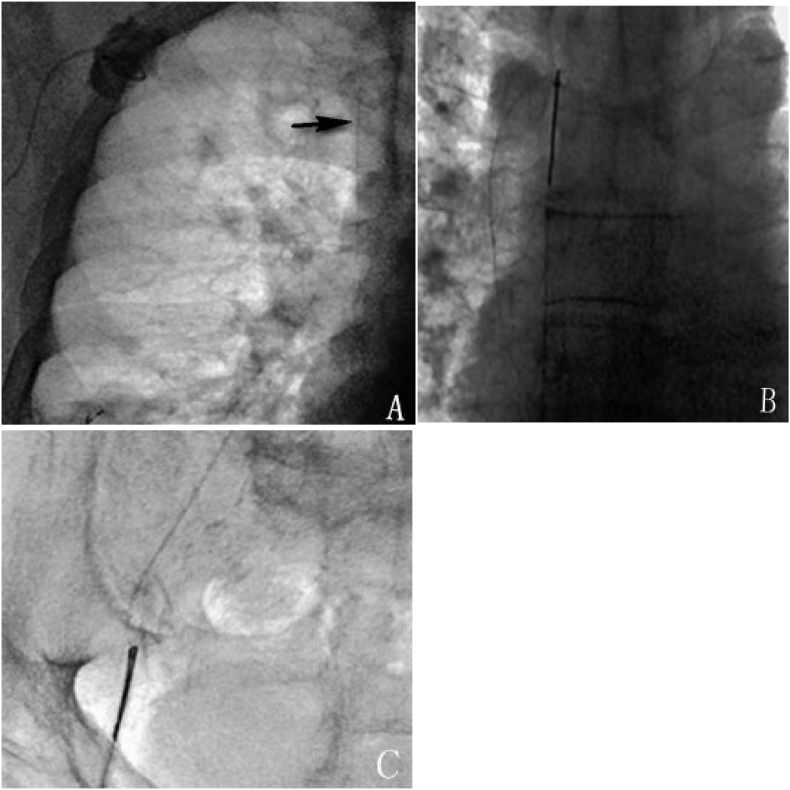
Catheter dislocation occurred in 52 patients (3.0%), which included 21 (1.2%) tip displacements and 31 (1.8%) fractures. Successful percutaneous adjustment or removal was performed in 51 patients using the Cobra catheter, (Cordis, Miami Lakes, USA), pig-tail catheter (Cordis, Miami Lakes, USA), or a retrieving device (see Fig. 2, Fig. 3); the success rate of percutaneous treatment was 98% (51/52). Relocation using both, the catheter and the GooseNeck snare (Microvena, White Bear Lake, MA, USA), failed in 1 case of tip displacement. The patient's PCS was removed entirely and a new one was implanted in the contralateral SCV. PCS in the other 20 cases of tip displacement remained functional after percutaneous adjustment. Percutaneous removal of the migrated catheter was successful in all 31 cases of catheter fracture, and the PCSs were removed simultaneously. The 12 patients who needed to continue chemotherapy and infusion were implanted with new PCSs.
Fig. 2.
A 63-year-old male with rectal cancer, experiencing catheter displacement 4 months after PCS implantation. A: X-ray scan showed that the catheter tip was displaced to the right internal jugular vein; B: X-ray scan showed that Cobra Catheter was inserted via the inferior vena cava, and that the displaced catheter was hooked by its tail; C: X-ray scan showed that the catheter tip was relocated to the superior vena cava.
Fig. 3.
A 48-year-old female with lung cancer, experiencing catheter fracture 13 months after PCS implantation. A: Chest radiograph showed catheter fracture in the superior vena cava; B: The floating free end of the catheter was hooked by GooseNeck Snare; C: The broken catheter was taken out with GooseNeck Snare via the femoral artery.
Catheter blockage was observed in 32 patients (1.9%). Recanalization by injecting heparin saline solution through the push-pull injection process was successful in 22 patients; the catheter was recanalized through urokinase thrombolysis in 8 patients. Catheter blockage was not responsive to either of the two recanalization treatments in the other 2 cases and the PCSs were removed accordingly.
Extravasation was seen in 11 patients (0.6%). Among them, 5 developed erythema and edema and no ulcers or necrosis were observed; while the other 6 patients experienced local skin necrosis secondary to extravasation of chemotherapeutic agents. Magnesium sulfate ointment (2%) and hirudoid cream were applied to the necrotic skin and all 6 patients retained their PCSs.
Flipped port occurred in 7 patients (0.4%). The pocket was incised, and the flipped port was adjusted to the right place and fixed. All the adjusted PCSs were functional.
3.2. Complication-related deaths
There were no deaths associated with early complications; 4 deaths were associated with late complications, including of 1 case of suppurative thrombophlebitis caused by E. coli infection, 2 of Staphylococcus aureus sepsis, and 1 of post-chemotherapy febrile neutropenia complicated by septic shock caused by gram negative bacilli. See Table 4 for details.
Table 4.
Reasons for complication-related deaths and PCS removal.
| Complication-related deaths (n) | 4 |
|---|---|
| Staphylococcus aureus sepsis | 2 |
| Suppurative thrombophlebitis | 1 |
| GNB-induced septic shock | 1 |
| PCS removal (n) | 451 |
| End of treatment | 398 |
| Complications | 53 |
| Catheter dislocation | 32 |
| Infections | 14 |
| Venous thrombosis | 5 |
| Catheter blockage | 2 |
| Mean indwelling time (d) | 324 |
| Range (d) | 8–2733 |
PCS: port-a-cath system; GNB: gram negative bacilli.
3.3. PCS removal
A total of 451 PCS were removed, 398 of these due to the end of chemotherapy and 53 due to complications. The mean time of PCS indwelling was 324 days (8–2733 days) in the patients whose PCSs were removed because of implantation-related complications. See Table 4 for details.
4. Discussion
Since Niederhuber3 first proposed subcutaneous PCS implantation in 1982, this mode of implantation has been widely used in the treatment of patients requiring long-term intravenous infusion, such as those with tumors and those needing renal dialysis. PCS implantation was initially performed by surgeons; Morris et al.4 reported the first implantation performed by radiologists with interventional techniques in 1992.
Compared with peripherally inserted central catheters (PICCs), PCS has obvious advantages.5 Statistics show that 5,000,000 PCS are implanted in patients in the United States every year.6 There are about 477,300 patients diagnosed with malignant tumors each year in Germany, of whom 125,790 choose PCSs for chemotherapy.7 Our interventional treatment center has implanted more than 1700 PCSs since it was established in 2009, and the number increases annually. PCS implantation can be done by surgeons, anesthesiologists, and interventional radiologists, and their rates of successful implantation are similar.8,9 In this retrospective study, all PCSs were implanted by two interventional radiologists in an operating room equipped with DSA.
There are many veins that can be used for PCS implantation, and the most common ones are the internal jugular vein and SCV.10,11 SCV is the preferred vein for all elective implantations since implantation via SCV has better cosmetic results and it does not limit patients’ physical activities.12 It is reported that PCS implantation via SCV has a shorter operation time and a lower risk of infections associated with long-term use.13, 14, 15 All PCS were implanted via SCV with the anatomic-landmark-guided technique in this study, since ultrasound-guided implantation into the SCV is more complicated due to the anatomic position and other issues. Using anatomic landmarks rather than ultrasound to guide PCS implantation is a standard procedure in some medical institutions.11,16 Mudan et al.11 and Brass et al.17 reported that the two guiding techniques had similar rates of complications. A systematic retrospective meta-analysis by Orci et al.18 showed that the initial success rate of implantation through percutaneous SCV puncture was higher than that of implantation with venotomy. Orci et al. also reported in the same study that there were no significant differences in the risk of pneumothorax, hematoma, or infections, while pneumothorax only occurred in the patients who underwent implantation through percutaneous SCV puncture.
Recent retrospective studies have reported incidences of intraoperative and postoperative complications that range from 1.4% to 11.1% and from 6.5% to 17.1%, respectively.11,19,20 Intraoperative and postoperative complications are mainly dependent on the patient's condition, implantation approach, and local vascular anatomy. In this study, the incidences of intraoperative early and late complications are 5.5% (94/1716) and 13.0% (224/1716), respectively, which is consistent with previous reports.
The incidence of pneumothorax in this study was 1.2%, which is similar to that in previous reports.21 The lowest incidence was reported by Tsai et al.22 who found that only 0.3% of the 1848 patients who underwent PCS implantation performed by a surgeon with Seldinger technique guided by anatomical landmarks developed pneumothorax. Mudan et al.11 reported 12 cases (1.2%) of pneumothorax among the 1000 patients on whom implantation was performed with ultrasound via SCV. In this study, 3 of the 21 patients with pneumothorax developed tension pneumothorax. One of the 3 patients underwent endotracheal intubation, while the other 2 underwent closed percutaneous catheter drainage. Conservative treatment was administered to the other patients since only a small part of their lung tissue was compressed and no progression was observed. All the 18 patients got better after conservative treatment. In this study, 1.1% (19/1716) of the patients had local bleeding after implantation, which is consistent with a previous study which reported an incidence of 0.5–1.6%.23 The PCS in all the cases of bleeding were functional after symptomatic treatment.
One of the early complications in this study was pocket infections. Pocket infections occurred in 17 patients, among whom 8 had diabetes and 6 had a low white blood cell count (1.5 × 109/L - 3.0 × 109/L). Two patients developed fever 2–3 days after implantation; 1 tested positive for Staphylococcus aureus, while the other tested positive for Staphylococcus epidermidis. One patient had no fever, but local erythema and pain, and microbiological examination of wound exudate found the presence of Staphylococcus epidermidis in the wound. Altogether, 14 cases of pocket infections resolved after antibiotic treatment. PCS were removed from the other 3 patients after antibiotic treatment failure. Mudan et al.11 reported that the patients were injected with a prophylactic dose of amoxicillin (1.2 g) and that no infections occurred after implantation. Three of the 1716 PCSs were removed due to early pocket infections. The use of prophylactic antibiotics is generally not recommended in PCS implantation, since there is no evidence that it can decrease the incidence of pocket infections.24,25
There were 14 cases (0.8%) of arterial mispuncture, 11 (0.6%) of delayed wound healing and 7 (0.4%) of vascular sheath detachment during the perioperative period. No serious complications were observed after symptomatic treatment. The small number of complications can be attributed to various factors, such as preoperative evaluation, imaging equipment, standardized surgical techniques, and a specialized team.26 Ipsilateral brachial plexus injury was a rare early complication (n = 5, 0.3%), which is consistent with the reported incidence of nerve injury of 0.1–0.3%.27
Late complications are the main reason for PCS removal.28 There were 224 PCS-associated late complications, of which infections were a large part. There were 67 cases (3.9%) of PCS-related infections, which is similar to the incidence of 4.8–8.8% reported previously.29,30 Since some patients were in a stable condition and did not show signs of sepsis, their PCSs were not removed and they were administered antibiotic treatment for 14–21 consecutive days. At the same time, samples of their peripheral blood and blood samples from inside the catheter were collected for culturing. PCSs should be removed immediately when sepsis and infections are not under control. It is difficult to obtain diagnostic evidence of bacteremia through laboratory tests (cultures) because the sensitivity of these tests is low.31,32 The best parameter is clinical evidence of improvement obtained through monitoring.
In this study, catheter-associated bacteremia was the most common type of PCS-related infection. Catheter-associated bacteremia occurred in 32 patients. The pathogenic microorganism was Staphylococcus aureus in most of the patients. PCS was removed from 7 patients accordingly. There were 4 patients who had underlying diseases and died of septic shock after antibiotic treatment failure. According to previous literature,33,34 PCS should be removed from the patients developing catheter-associated bacteremia as soon as possible to avoid severe complications such as severe sepsis, suppurative thrombophlebitis, infective endocarditis, or blood infections, when the bacteremia is not responsive to antibacterial treatment for over 72 h, or is caused by Staphylococcus aureus, Pseudomonas aeruginosa, fungi, or mycobacteria.
Local infections included pocket infections and skin infections at the puncture site in this study, the incidences of which were 1.4% (25/1716) and 0.6% (10/1716), respectively. This is generally consistent with the results of previous reports.35,36 PCSs were removed from 4 cases of pocket infections after antibiotic treatment failure. Pocket infections and skin infections were alleviated effectively in the other patients after antibiotics and symptomatic treatment.
Catheter-related thrombi include small thrombi at the catheter tip and large catheter venous thrombi. When small thrombi are present at the catheter tip, no blood can be withdrawn while infusion is normal, while large catheter venous thrombi are by venous thrombosis. Clinical experience has shown that catheter-tip thrombi are usually benign, and that the actual incidence is much higher than the reported because they do not present with obvious clinical symptoms. The best way to prevent catheter-related thrombosis is to ensure that PCS maintenance is performed by well-trained nursing staff, while further research is needed to explore the effect of the routine use of anticoagulants, such as warfarin.37 Minassian et al.38 reported that prophylactic use of low-dose oral anticoagulants could reduce the incidence of such complications. In this series, the patients did not take prophylactic anticoagulants. Systematic anticoagulation therapy was only administered in clinically suspected cases of catheter-related thrombosis with heparin sodium as the preferred anticoagulant. Local thrombolysis with urokinase was administered when necessary. There were 55 cases (3.2%) of symptomatic catheter-related thrombosis in this study; the symptoms were effectively resolved in 49 of them after anticoagulation, while thrombosis became more severe in 5 cases after anticoagulation and thrombolysis and their PCSs were removed. One patient had pulmonary embolisms in a small area, but no signs of shock, such as decreased blood oxygen level or blood pressure. The patient showed signs of improvement after active treatment.
There were 52 cases (3.0%) of catheter dislocation, including 21 (1.2%) of tip displacement and 31 (1.8%) of fracture, which included 14 (14/31, 45%) cases of pinch-off syndrome and 17 (17/31, 55%) of detachment of the port from the proximal end of the catheter. Several studies have shown that the incidence of catheter dislocation is only 0–4.1%,30,39, 40, 41, 42, 43, 44 however, it can lead to death and severe complications.45, 46, 47, 48 Therefore, once this occurs, immediate intervention should be carried out. Percutaneous adjustment or removal of the PCS catheter with the GooseNeck Snare or a catheter is a standardized technique.49,50 The abnormally positioned catheters were successfully adjusted or removed in 51 patients using a Cobra Catheter, Pig-tail Catheter or GooseNeck Snare. Both catheters and GooseNeck Snare failed in relocating the catheter tip in 1 patient and the PCS was removed. Therefore, the success rate of percutaneous adjustment or removal was 98% (51/52).
Catheter blockage, another common complication of long-term PCS indwelling, may be caused by thrombosis, fibrous cap formation, or adhesion of the catheter lumen to the venous wall. It was reported that the incidence of catheter blockage was 3.2–21.5%.30,51 There were 32 cases (1.9%) of catheter blockage in this study, therefore, the incidence is slightly lower than previously reported. This complication was mainly characterized by low infusion speed, inability to withdraw blood, or infusion failure in the present study. The blocked catheters were recanalized in 30 patients (93.8%) after they were pressure-rinsed by injecting diluted heparin saline solution via the port with a 10 mL syringe using the "push-pull" injection technique. PCSs were removed in the other 2 patients after recanalization with heparin saline solution and urokinase failed. Ten milliliter syringes have the best performance in pressure-rinsing, because they can strike a good balance between the pressure on the catheter and the volume of the catheter.52
Extravasation occurred in 11 patients (0.6%) as a late complication. Extravasation, especially that of chemotherapeutic agents, can cause severe complications.53 Therefore, once it is observed, infusion should be stopped immediately, and fluids leaking into local regions should be evacuated as much as possible. In clinical practice, the standardized procedures should be followed strictly when selecting an appropriate needle and puncture technique. Besides, the operator should puncture the center, avoid puncturing the site used previously, and fix the needle properly.
Flipped port was a rare late complication in this study (n = 7, 0.4%). The pocket was incised and the port was relocated to the right place and fixed in confirmed cases. It was associated with loose subcutaneous tissues, a large pocket for the port, poor fixation and excessive arm movements. It was characterized by unsuccessful puncture, and the diagnosis could be confirmed through radiographs.
5. Conclusions
The overall rate of complications was 18.5% in this study, which is consistent with previous reports.54 In most cases, complications can be effectively controlled through active clinical treatment without removing PCS. The 10-year follow-up results show that PCSs are being increasingly accepted by patients and physicians. PCSs are safe, convenient and effective for long-term use if (a) they are implanted using standardized techniques and properly maintained, (b) complications are dealt with immediately and properly. We believe that the incidence of complications can be further reduced through the joint efforts of a specialized team consisting of physicians and nurses.
Conflicts of interest and source of funding
There are no conflicts of interest. It has received honoraria from Key-Area Research and Development Program of Guangdong Province, China (2020B010165004).
Contributions
Yong Li: Conceptualization, Methodology, Software, Data curation, Writing- Original draft preparation, Visualization, Investigation; Jianxi Guo: Conceptualization, Methodology, Software, Data curation, Writing- Original draft preparation, Visualization, Investigation, Writing- Reviewing and Editing; Yanfang Zhang: Validation, Writing- Reviewing and Editing; Jian Kong: Conceptualization, Methodology, Data curation, Writing- Original draft preparation, Visualization, Investigation, Supervision, Validation, Writing- Reviewing and Editing;
References
- 1.Yildizeli B., Laçin T., Batirel H.F., et al. Complications and management of long-term central venous access catheters and ports. J Vasc Access. 2004;5:174–178. doi: 10.1177/112972980400500407. [DOI] [PubMed] [Google Scholar]
- 2.Biffi R., Orsi F., Pozzi S., et al. Best choice of central venous insertion site for the prevention of catheter-related complications in adult patients who need cancer therapy: a randomized trial. Ann Oncol. 2009;20:935–940. doi: 10.1093/annonc/mdn701. [DOI] [PubMed] [Google Scholar]
- 3.Niederhuber J.E., Ensminger W., Gyves J.W., et al. Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery. 1982;92:706–712. [PubMed] [Google Scholar]
- 4.Morris S.L., Jaques P.F., Mauro M.A. Radiology-assisted placement of implantable subcutaneous infusion ports for long-term venous access. Radiology. 1992;184:149–151. doi: 10.1148/radiology.184.1.1609072. [DOI] [PubMed] [Google Scholar]
- 5.Gonsalves C.F., Eschelman D.J., Sullivan K.L., et al. Incidence of central vein stenosis and occlusion following upper extremity PICC and port placement. Cardiovasc Intervent Radiol. 2003;26:123–127. doi: 10.1007/s00270-002-2628-z. [DOI] [PubMed] [Google Scholar]
- 6.Silberzweig J.E., Sacks D., Khorsandi A.S., et al. Reporting standards for central venous access. Technology Assessment Committee. J Vasc Intervent Radiol. 2000;11:391–400. doi: 10.1016/s1051-0443(07)61435-3. [DOI] [PubMed] [Google Scholar]
- 7.Klaiber U., Grummich K., Jensen K., et al. Closed cannulation of subclavian vein vs open cut-down of cephalic vein for totally implantable venous access port (TIVAP) implantation: protocol for a systematic review and proportional meta-analysis of perioperative and postoperative complications. Syst Rev. 2015;4:53. doi: 10.1186/s13643-015-0043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozyuvaci E., Kutlu F. Totally implantable venous access devices via subclavian vein: a retrospective study of 368 oncology patients. Adv Ther. 2006;23:574–581. doi: 10.1007/BF02850046. [DOI] [PubMed] [Google Scholar]
- 9.Teichgräber U.K., Pfitzmann R., Hofmann H.A. Central venous port systems as an integral part of chemotherapy. Dtsch Arztebl Int. 2011;108:147–153. doi: 10.3238/arztebl.2011.0147. ; quiz 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taxbro K., Berg S., Hammarskjöld F., et al. A prospective observational study on 249 subcutaneous central vein access ports in a Swedish county hospital. Acta Oncol. 2013;52:893–901. doi: 10.3109/0284186X.2013.770601. [DOI] [PubMed] [Google Scholar]
- 11.Mudan S., Giakoustidis A., Morrison D., et al. 1000 Port-A-Cath ® placements by subclavian vein approach: single surgeon experience. World J Surg. 2015;39:328–334. doi: 10.1007/s00268-014-2802-x. [DOI] [PubMed] [Google Scholar]
- 12.Capaccioli L., Nistri M., Distante V., et al. Posizionamento e gestione dei sistemi per accesso venoso centrale a lungo termine: contributo della diagnostica per immagini [Insertion and management of long-term central venous devices: role of radiologic imaging techniques] Radiol Med. 1998;96:369–374. Italian. [PubMed] [Google Scholar]
- 13.Ruesch S., Walder B., Tramèr M.R. Complications of central venous catheters: internal jugular versus subclavian access--a systematic review. Crit Care Med. 2002;30:454–460. doi: 10.1097/00003246-200202000-00031. [DOI] [PubMed] [Google Scholar]
- 14.Timsit J.F. What is the best site for central venous catheter insertion in critically ill patients? Crit Care. 2003;7:397–399. doi: 10.1186/cc2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreidieh F.Y., Moukadem H.A., El Saghir N.S. Overview, prevention and management of chemotherapy extravasation. World J Clin Oncol. 2016;7:87–97. doi: 10.5306/wjco.v7.i1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orsi F., Grasso R.F., Arnaldi P., et al. Ultrasound guided versus direct vein puncture in central venous port placement. J Vasc Access. 2000;1:73–77. doi: 10.1177/112972980000100209. [DOI] [PubMed] [Google Scholar]
- 17.Brass P., Hellmich M., Kolodziej L., et al. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst Rev. 2015;1:CD011447. doi: 10.1002/14651858.CD011447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orci L.A., Meier R.P., Morel P., et al. Systematic review and meta-analysis of percutaneous subclavian vein puncture versus surgical venous cutdown for the insertion of a totally implantable venous access device. Br J Surg. 2014;101:8–16. doi: 10.1002/bjs.9276. [DOI] [PubMed] [Google Scholar]
- 19.Ignatov A., Hoffman O., Smith B., et al. An 11-year retrospective study of totally implanted central venous access ports: complications and patient satisfaction. Eur J Surg Oncol. 2009;35:241–246. doi: 10.1016/j.ejso.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 20.Matsushima H., Adachi T., Iwata T., et al. Analysis of the outcomes in central venous access port implantation performed by residents via the internal jugular vein and subclavian vein. J Surg Educ. 2017;74:443–449. doi: 10.1016/j.jsurg.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Lenz H., Myre K., Draegni T., et al. A five-year data report of long-term central venous catheters focusing on early complications. Anesthesiol Res Pract. 2019;2019:6769506. doi: 10.1155/2019/6769506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai Y.F., Ku Y.H., Chen S.W., et al. Right- and left-subclavian vein port-a-cath systems: comparison of complications. Eur Surg Res. 2012;49:66–72. doi: 10.1159/000339308. [DOI] [PubMed] [Google Scholar]
- 23.Eisen L.A., Narasimhan M., Berger J.S., et al. Mechanical complications of central venous catheters. J Intensive Care Med. 2006;21:40–46. doi: 10.1177/0885066605280884. [DOI] [PubMed] [Google Scholar]
- 24.Ranson M.R., Oppenheim B.A., Jackson A., et al. Double-blind placebo controlled study of vancomycin prophylaxis for central venous catheter insertion in cancer patients. J Hosp Infect. 1990;15:95–102. doi: 10.1016/0195-6701(90)90025-j. [DOI] [PubMed] [Google Scholar]
- 25.Jaeger K., Osthaus A., Heine J., et al. Efficacy of a benzalkonium chloride-impregnated central venous catheter to prevent catheter-associated infection in cancer patients. Chemotherapy. 2001;47:50–55. doi: 10.1159/000048501. [DOI] [PubMed] [Google Scholar]
- 26.Wolosker N., Yazbek G., Nishinari K., et al. Totally implantable venous catheters for chemotherapy: experience in 500 patients. Sao Paulo Med J. 2004;122:147–151. doi: 10.1590/S1516-31802004000400003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grannan K.J., Taylor P.H. Early and late complications of totally implantable venous access devices. J Surg Oncol. 1990;44:52–54. doi: 10.1002/jso.2930440112. [DOI] [PubMed] [Google Scholar]
- 28.Ballarini C., Intra M., Pisani Ceretti A., et al. Complications of subcutaneous infusion port in the general oncology population. Oncology. 1999;56:97–102. doi: 10.1159/000011947. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz R.E., Groeger J.S., Coit D.G. Subcutaneously implanted central venous access devices in cancer patients: a prospective analysis. Cancer. 1997;79:1635–1640. [PubMed] [Google Scholar]
- 30.Kock H.J., Pietsch M., Krause U., et al. Implantable vascular access systems: experience in 1500 patients with totally implanted central venous port systems. World J Surg. 1998;22:12–16. doi: 10.1007/s002689900342. [DOI] [PubMed] [Google Scholar]
- 31.Blot F., Nitenberg G., Chachaty E., et al. Diagnosis of catheter-related bacteraemia: a prospective comparison of the time to positivity of hub-blood versus peripheral-blood cultures. Lancet. 1999;354:1071–1077. doi: 10.1016/s0140-6736(98)11134-0. [DOI] [PubMed] [Google Scholar]
- 32.Hanna H.A., Raad I. Blood products: a significant risk factor for long-term catheter-related bloodstream infections in cancer patients. Infect Control Hosp Epidemiol. 2001;22:165–166. doi: 10.1086/501885. [DOI] [PubMed] [Google Scholar]
- 33.Mermel L.A., Farr B.M., Sherertz R.J., et al. Guidelines for the management of intravascular catheter-related infections. Clin Infect Dis. 2001;32:1249–1272. doi: 10.1086/320001. [DOI] [PubMed] [Google Scholar]
- 34.Mermel L.A., Allon M., Bouza E., et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Biffi R., Pozzi S., Agazzi A., et al. Use of totally implantable central venous access ports for high-dose chemotherapy and peripheral blood stem cell transplantation: results of a monocentre series of 376 patients. Ann Oncol. 2004;15:296–300. doi: 10.1093/annonc/mdh049. [DOI] [PubMed] [Google Scholar]
- 36.Dal Molin A., Rasero L., Guerretta L., et al. The late complications of totally implantable central venous access ports: the results from an Italian multicenter prospective observation study. Eur J Oncol Nurs. 2011;15:377–381. doi: 10.1016/j.ejon.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Koonings P.P., Given F.T., Jr. Long-term experience with a totally implanted catheter system in gynecologic oncologic patients. J Am Coll Surg. 1994;178:164–166. [PubMed] [Google Scholar]
- 38.Minassian V.A., Sood A.K., Lowe P., et al. Longterm central venous access in gynecologic cancer patients. J Am Coll Surg. 2000;191:403–409. doi: 10.1016/s1072-7515(00)00690-6. [DOI] [PubMed] [Google Scholar]
- 39.Poorter R.L., Lauw F.N., Bemelman W.A., et al. Complications of an implantable venous access device (Port-a-Cath) during intermittent continuous infusion of chemotherapy. Eur J Cancer. 1996;32A:2262–2266. doi: 10.1016/s0959-8049(96)00274-2. [DOI] [PubMed] [Google Scholar]
- 40.Biffi R., de Braud F., Orsi F., et al. Totally implantable central venous access ports for long-term chemotherapy. A prospective study analyzing complications and costs of 333 devices with a minimum follow-up of 180 days. Ann Oncol. 1998;9:767–773. doi: 10.1023/a:1008392423469. [DOI] [PubMed] [Google Scholar]
- 41.Charvát J., Linke Z., Horáèková M., et al. Implantation of central venous ports with catheter insertion via the right internal jugular vein in oncology patients: single center experience. Support Care Cancer. 2006;14:1162–1165. doi: 10.1007/s00520-006-0073-2. [DOI] [PubMed] [Google Scholar]
- 42.Cheng C.C., Tsai T.N., Yang C.C., et al. Percutaneous retrieval of dislodged totally implantable central venous access system in 92 cases: experience in a single hospital. Eur J Radiol. 2009;69:346–350. doi: 10.1016/j.ejrad.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 43.Wang P.C., Liang H.L., Wu T.H., et al. Percutaneous retrieval of dislodged central venous port catheter: experience of 25 patients in a single institute. Acta Radiol. 2009;50:15–20. doi: 10.1080/02841850802524493. [DOI] [PubMed] [Google Scholar]
- 44.Narducci F., Jean-Laurent M., Boulanger L., et al. Totally implantable venous access port systems and risk factors for complications: a one-year prospective study in a cancer centre. Eur J Surg Oncol. 2011;37:913–918. doi: 10.1016/j.ejso.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 45.isher R.G., Ferreyro R. Evaluation of current techniques for nonsurgical removal of intravascular iatrogenic foreign bodies. AJR Am J Roentgenol. 1978;130:541–548. doi: 10.2214/ajr.130.3.541. [DOI] [PubMed] [Google Scholar]
- 46.Winston C.B., Wechsler R.J., Kane M. Vertebral vein migration of a long-term central venous access catheter: a cause of brachial plexopathy. J Thorac Imag. 1994;9:98–100. doi: 10.1097/00005382-199421000-00008. [DOI] [PubMed] [Google Scholar]
- 47.Collin G.R., Ahmadinejad A.S., Misse E. Spontaneous migration of subcutaneous central venous catheters. Am Surg. 1997;63:322–326. [PubMed] [Google Scholar]
- 48.Groebli Y., Wuthrich P., Tschantz P., et al. Une complication rare des accès veineux permanents: laminage, fracture et embolisation du catheter [A rare complication of permanent venous access: constriction, fracture and embolization of the catheter] Swiss Surg. 1998;4:141–145. French. [PubMed] [Google Scholar]
- 49.Thomas J., Sinclair-Smith B., Bloomfield D., et al. Non-surgical retrieval of a broken segment of steel spring guide from the right atrium and inferior vena cava. Circulation. 1964;30:106–108. doi: 10.1161/01.cir.30.1.106. [DOI] [PubMed] [Google Scholar]
- 50.Gebauer B., Teichgräber U.K., Podrabsky P., et al. Radiological interventions for correction of central venous port catheter migrations. Cardiovasc Intervent Radiol. 2007;30:216–221. doi: 10.1007/s00270-006-0218-1. [DOI] [PubMed] [Google Scholar]
- 51.Strum S., McDermed J., Korn A., et al. Improved methods for venous access: the Port-A-Cath, a totally implanted catheter system. J Clin Oncol. 1986;4:596–603. doi: 10.1200/JCO.1986.4.4.596. [DOI] [PubMed] [Google Scholar]
- 52.Barbetakis N., Asteriou C., Kleontas A., et al. Totally implantable central venous access ports. Analysis of 700 cases. J Surg Oncol. 2011;104:654–656. doi: 10.1002/jso.21990. [DOI] [PubMed] [Google Scholar]
- 53.Schrijvers D.L. Extravasation: a dreaded complication of chemotherapy. Ann Oncol. 2003;14(Suppl 3):iii26–30. doi: 10.1093/annonc/mdg744. [DOI] [PubMed] [Google Scholar]
- 54.Voog E., Campion L., du Rusquec P., et al. Totally implantable venous access ports: a prospective long-term study of early and late complications in adult patients with cancer. Support Care Cancer. 2018;26:81–89. doi: 10.1007/s00520-017-3816-3. [DOI] [PubMed] [Google Scholar]