Abstract
Objective
To compare the safety of conventional transarterial chemoembolization (cTACE) vs drug-eluting bead TACE (DEB-TACE) in very early- and early-stage hepatocellular carcinoma (HCC).
Methods
Data of patients with early- and very early-stage HCC treated with cTACE or DEB-TACE were evaluated retrospectively in this study. A total of 40 patients were included, 20 treated with cTACE and 20 with DEB-TACE. The cTACE and DEB-TACE groups were comprised of 80% and 75% males, while there were 20% females in cTACE group and 25% in Deb-TACE group respectively. The mean age of patients in cTACE group was 57.43 + 5.6 years, while it was 56.4 + 5.5 years in DEB-TACE group. All patients had liver status of Child–Pugh Class A and a score ≤ 7 in Child-Pugh class type B in very early- (stage 0) or early-phase (stage A) stages according to the Barcelona Clinic Liver Cancer (BCLC) system.
Results
The Child-Pugh class degradation in the cTACE group was slightly higher than that in the DEB-TACE group. Serious complications like peritumoral parenchymal ischemia were observed in 4 patients in the cTACE group and 5 in the DEB-TACE group. Localized bile duct dilation was seen in 2 patients in the cTACE group and 6 in the DEB-TACE group.
No significant variation in serious complications between the two groups was established in localized bile duct dilatation. Other minor complications noted were liver failure, liver abscess, liver infarction, acute cholecystitis, biliary tree necrosis, and mortality. Further, no substantial variation in tumor response between the groups was reported immediately and 1-year post-procedural assessment. Conversion rate to other treatment modalities such as surgical resection, radiofrequency ablation (RFA), or swap between cTACE and DEB-TACE was substantially higher in the DEB-TACE group (40%) than in the cTACE group (10%) at the 1-year completion period of the study.
Conclusion
In terms of tumor response, the DEB-TACE group showed a better response, to some extent, as an initial therapy for HCC in the early stages as compared to the cTACE group, and DEB-TACE also exhibited better clinical efficacy in patients with HCC.
Keywords: HCC, TACE, cTACE, DEB-TACE
1. Introduction
Hepatocellular carcinoma (HCC) is the most widespread primary hepatic malignancy globally. It is the fifth most prevalent carcinoma worldwide and the third most common cause of cancer-related death. Hepatic fibrosis along with cirrhosis constitutes the most frequent cause that leads to the development of HCC. However, chronic hepatitis C and hepatitis B infections also represent the other key factors that lead to the development of HCC. (see Table 1, Table 2, Table 3, Table 4, Table 5, Fig. 1, Fig. 2)
Table 1.
Distribution based on various parameters.
| Variables | cTACE | DEB-TACE |
|---|---|---|
| Gender | ||
| Male | 16 (80%) | 15 (75%) |
| Female | 4 (20%) | 5 (25%) |
| Total no. of patients | 20 (100%) | 20 (100%) |
| Age (Mean ± SD) | 57.43 ± 5.6 | 56.4 ± 5.5 |
| Liver Disease | ||
| Hepatitis – B | 8 (40%) | 9 (45%) |
| Hepatitis – C | 5 (25%) | 3 (15%) |
| Alcoholic | 7 (35%) | 8 (40%) |
| BCLC Stages | ||
| Very Early (0) | 6 (30%) | 5 (25%) |
| Early (A) | 14 (70%) | 15 (75%) |
| Child PUGH Class | ||
| A | 17 (85%) | 18 (90%) |
| ≤B7 | 3 (15%) | 2 (10%) |
| Size of Hepatocellular Carcinoma | ||
| 1–1.9 cms | 6 (30%) | 5 (25%) |
| 2–2.9 cms | 10 (50%) | 11 (55%) |
| 3–5 cms | 4 (20%) | 4 (20%) |
Table 2.
Distribution based on PES, liver function changes, pain, and bradycardia.
| cTACE | DEB-TACE | |
|---|---|---|
| Intractable Pain | 18 (90%) | 3 (15%) |
| Bradycardia | 6 (30%) | 0 (0%) |
| Post Embolization Syndrome (PES) | ||
| Incidence | 17 (85%) | 6 (30%) |
| Duration | 3–30 days | 1–15 days |
| Liver Function changes | ||
| AST | 3.5 ± 1.5 | 1.5 ± 0.6 |
| ALT | 3.7 ± 1.5 | 1.6 ± 0.6 |
| Child PUGH class | 25% deterioration | 11% deterioration |
Table 3.
Distribution based on various complications.
| Serious Complications | cTACE | DEB-TACE |
|---|---|---|
| Liver Failure | 1 | 1 |
| Localized bile duct dilation | 2 | 6 |
| Peri tumoral parenchymal ischemia | 4 | 5 |
| Liver Abscess | 1 | 1 |
| Liver Infraction | 1 | 1 |
| Acute Cholecystitis | 1 | 1 |
| Biliary tree necrosis | 1 | 1 |
| Mortality | 1 | 1 |
Table 4.
Target Tumor Response by mRECIST criteria as per APASL guidelines.
| Tumor Response | cTACE |
DEB-TACE |
||||
|---|---|---|---|---|---|---|
| Immediate | 1 yr. | P-value | Immediate | 1 yr. | P-value | |
| Complete response | 81.5 | 79.5 | <0.001 | 77.5 | 77.3 | <0.001 |
| Partial response | 13 | 5.5 | <0.001 | 18.5 | 3.5 | <0.001 |
| Objective response | 95.0 | 85.2 | <0.001 | 96.5 | 81.5 | <0.001 |
| Stable disease | 0 | 5.5 | <0.001 | 0 | 7.0 | <0.001 |
| Progressive disease | 5.5 | 10.0 | <0.001 | 4.0 | 12.0 | <0.001 |
Table 5.
Conversion to another modality.
| cTACE | DEB-TACE | P-Value | |
|---|---|---|---|
| Conversion to another modality | 2 (10%) | 8 (40%) | <0.001 |
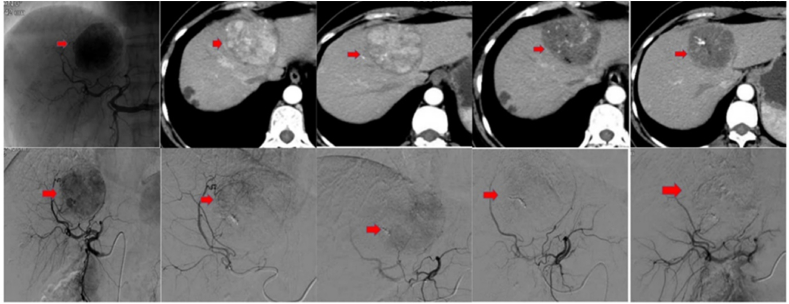
Fig. 1.
CT scans of a patient diagnosed with HCC. A large tumor in the left liver lobe is visible in the top left CT image. The top middle images display residual tumor enhancement in the left liver lobe. Post TACE with doxorubicin 50 mg and CalliSpheres of 300–500 μm are seen in the bottom CT images. No biloma was detected.
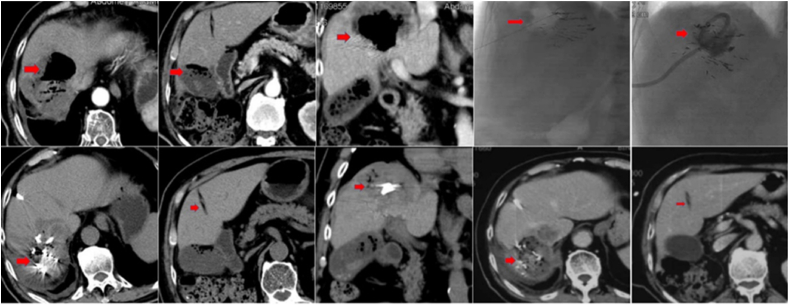
Fig. 2.
CT scans of a patient with HCC. The patient developed an acute infection with biloma four days after TACE (doxorubicin 50 mg and CalliSpheres of 100–300 μm). Post external draining and antibiotic treatment, the patient's health status stabilized. The top left CT images show the forming of a biloma. The top right CT images display biloma external drainage. The biloma has almost disappeared in the bottom left CT images that were taken one day after surgery. The CT pictures on the bottom right display tumor necrosis but no biloma. The patient's condition was stable.
Nearly 500,000 cases of HCC are diagnosed each year. In the arterial phase, typical HCCs are hypervascular, whereas, in the portal venous phase, they exhibit washout. Due to a lack of arterial blood flow, hypovascular HCCs only show minimal improvement. Unresectable HCC can be treated by transarterial chemoembolization (TACE).1
For more than 40 years, traditional or, now more commonly known as, conventional transarterial chemoembolization (cTACE), which uses Lipiodol as a drug vehicle to administer chemotherapeutic agents, has been used as a non-surgical or minimally invasive treatment for HCC.2,3
Drug-eluting bead TACE (DEB-TACE) has been commonly used in the West to replace cTACE. DEB-TACE is used to overcome shortcomings of the cTACE. First, the complicated emulsion formed between Lipiodol and the anticancer agent used in cTACE is not held by a true chemical bond; as a consequence, the components split after a brief period of time, allowing efflux of a significant volume of chemotherapeutic medication into the systemic circulation, resulting in systemic side effects and reduced local anti-cancer efficacy. Second, Lipiodol is a very small embolic droplet at the capillary level.4 A Lipiodol-anticancer emulsion stimulates micro-capillary damage to the peri-biliary plexus, resulting in permanent complications such as progressive biliary infection, peri-biliary biloma, portal vein thrombosis, liver infarction, liver abscess, and even death from liver failure and sepsis in the majority of patients.5 Third, embolization materials such as gelatin sponge particles or polyvinyl alcohol particles are used to impede arterial blood supply in cTACE. Compact distal embolization, on the other hand, is difficult to achieve due to its unusual shape and variable size.6
DEB-TACE is a variation of TACE in which doxorubicin DEBs are used as the embolizing product. Since DEBs are costly and permanently embolize the vessel, super selective delivery of the beads into tumor vessels is needed to avoid damage or incomplete embolization of the tumor. When compared to cTACE, DEB-TACE allows for higher drug concentrations within the target tumor and lower systemic concentrations.7 As a result, DEB TACE can help to mitigate drug-related side effects including post-embolization syndrome.8,9
2. Materials and methods
2.1. Design
The study design was approved by the institutional ethics committee of Nanjing First Hospital affiliated to Nanjing Medical University. Written informed consent was obtained from all the patients prior to the diagnostic and therapeutic procedures.
3. Objective
To compare the efficacy of cTACE vs DEB-TACE in patients with early-stage HCC.
3.1. Patient selection
Using a controlled-match procedure, we retrospectively evaluated and compared the use of cTACE and DEB-TACE for the treatment of HCC.
Patient demographics, as well as the presence of any underlying liver disease, tumor staging details, liver function data, and information on tumor size and tumor markers were recorded accordingly from the electronic medical data system of the hospital.
Per the Barcelona Clinic Liver Cancer (BCLC) staging scheme, all the patients were classified as very early (stage 0) or early (stage A). Child–Pugh class type A or ≤ Child-Pugh score of 7 in Child-Pugh class type B liver status was observed in all patients.
3.1.1. Inclusion criteria
The following were the inclusion criteria:
HCC diagnosed clinically or histologically with no prior treatment.
A single HCC tumor.
Child-Pugh class score ≤ 7 in Child-Pugh class type B.
A maximum tumor diameter of 1–5 cm and segmental involvement.
3.1.2. Exclusion criteria
The following exclusion criteria were used:
Infiltrative/pedunculated/cirrhotomimetic morphology.
Bile duct invasion, vascular invasion, hepatofugal portal flow.
Total bilirubin level 3 mg/dL.
Performance status ≥ 1.
Prior to TACE, angiography of the superior mesenteric artery and celiac trunk (or common hepatic artery) was performed using a 5-Fr angiographic catheter, with access via the common femoral artery approach under local anesthesia, to assess portal vein patency and preservation of hepatoportal traffic, map the hepatic arterial anatomy, and locate tumor feeding arteries of the HCC.
In cases where there were numerous tumor feeding arteries, segmental arteries were catheterized selectively, while in smaller lesions with a subsegmental tumor feeding artery, the subsegmental branch artery was super selected accordingly. Feeding arteries were catheterized selectively or super selectively using a microcatheter and a suitable microguide wire.
cTACE was conducted with a combination of Lipiodol and doxorubicin, followed by the use of gelatin sponge particle for embolization of the feeding artery using a Progreat Microcatheter (150cm/2.8Fr) via femoral access. Lipiodol and doxorubicin were used at maximum doses of 10 mL and 50 mg, respectively.
For DEB-TACE, a limit of 75–150 mg of doxorubicin per bottle, filled in two bottles of DEB, was used for DEB beads, HepaSphere or CalliSpheres. 100–300 μm sized beads were selected for small HCCs with low vascularity.
The major differences between the cTACE and DEB-TACE methods were as follows: During cTACE, post embolization syndrome (PES), changes in liver function, complications, and assessment of target tumor response were observed, while severe pain and bradycardia during TACE, and PES were observed accordingly.
All patients were followed up with computed tomography (CT) or magnetic resonance imaging (MRI) after 1 month of TACE, and then at 3-month, 6-month, and 1-year intervals, until residual tumor or local tumor recurrence was noted in them.
4. Results
A total of 40 patients were included in our study, 20 who were treated with cTACE and 20 with DEB-TACE.
The target tumor response was calculated using the APASL criteria.
All statistical analyses of the collected data were performed with SPSS software (version 17.0, SPSS, Chicago, Illinois).
The cTACE group comprised 80% males, while the DEB-TACE group had 75% males, while females were 20% in cTACE group and 25% in Deb-TACE group respectively.
The mean age of the patients in the cTACE group was 57.43 + 5.6 years; in DEB-TACE group, it was 56.4 + 5.5 years.
In cTACE group, according to BCLC stages, 70% of the patients were diagnosed with Early-stage disease (A) and 30% with Very Early-stage disease (0); in DEB-TACE group, 75% of the patients were diagnosed with Early-stage disease (A) and 25% with Very Early-stage disease (0), respectively.
Child PUGH Class A was seen in 85% of patients in the cTACE group and in 90% of patients in the DEB-TACE group and <B7 class was seen in 15% of patients in the cTACE group and in 10% of patients in the DEB-TACE group.
During the TACE procedure, the cTACE group had a slightly higher rate of severe intractable pain (90%) than the DEB-TACE group (15%).
Immediately after the treatment, the frequency of PES was slightly higher in the cTACE group than in the DEB-TACE group.
The cTACE group had a slightly higher acute post-TACE increase in liver enzyme levels (AST) (ALT) than the DEB-TACE group. The mean SD elevation of liver enzyme levels (AST and ALT) in the cTACE group was 3.5 ± 1.5-fold and 3.7 ± 1.5-fold, respectively. The mean SD elevation (AST and ALT) for the DEB-TACE group, on the other hand, was 1.5 ± 0.6-fold and 1.6 ± 0.6-fold, respectively.
Child-Pugh class deterioration was also slightly higher in the cTACE group than in the DEB-TACE group during the follow-up period. Child-Pugh class deterioration was seen in 25% of patients in the cTACE group, compared to 11% patients in the DEB-TACE group.
Serious complications seen were peritumoral parenchymal ischemia in 4 patients in the cTACE group and in 5 in the DEB-TACE group. Localized bile duct dilation was seen in 2 patients in the cTACE group and in 6 in the DEB-TACE group.
There was no substantial variation between the two modalities in the description of both treatment as an initial recovery option both in the immediate and the 1-year evaluation. The immediate complete response rates were 82% and 78%, respectively, while the immediate objective response rates were 95% and 97%, respectively, in the cTACE and DEB-TACE groups. The target response rates at 1 year were 85% and 82% in the cTACE and DEB-TACE groups. At the 1-year follow-up, similar rates were found for the number of patients with stable disease and progressive disease in the two groups.
The conversion rate to other treatment modalities such as surgical resection, RFA, or swap between cTACE and DEB-TACE, was substantially higher in the DEB-TACE group (40%) than in the cTACE group (10%) at the 1-year completion period of the study.
5. Discussion
DEB-TACE was shown to have a more robust pharmacokinetics profile than cTACE in clinical trials.8, 9, 10, 11 An earlier study comparing cTACE and DEB-TACE found that problems arising as a result of the treatment, such as anticancer drug effusion and hepatic dysfunction, were less common in the DEB-TACE group. Furthermore, DEB-TACE led to a higher target response rate and disease prevention rate for HCC than cTACE.12
Procedure stability and clinical outcome are also important considerations to remember when comparing cTACE and DEB-TACE. Currently, DEB-TACE has a higher level of procedural safety and patient compliance than cTACE13; however, the efficacy is still debatable.14
We assume that the disparity in safety concerns, such as extreme intractable pain and bradycardia during the treatment, PES, liver function improvements, and severe complications, stems from the fundamental differences in the chemo-vehicles and embolic materials used in cTACE and DEB-TACE. The smaller chemo-laden Lipiodol droplets, which are an emulsion of the anticancer agent and Lipiodol, cause inflammation to the peri-biliary plexus and liver capsule during cTACE, resulting in extreme ischemic discomfort. Blood pressure control and bradycardia care may also be required in some cases. Pain is rare with DEB-TACE, which is usually ambiguous and tolerable, and vital signs remain stable during the procedure.
Both cTACE and DEB-TACE are embolic procedures could cause liver function and PES to deteriorate. However, by comparing cTACE to DEB-TACE, the majority of studies and meta-analyses have found slightly greater variations in liver function profiles and the prevalence of PES with cTACE.15 This finding is consistent with pharmacokinetic profiles studied in preclinical research.8,9
In terms of clinical results, it is difficult to compare the evidence since, even with a limited viable subset, most of the patients were converted to other treatment modalities during follow-up based on hospital policies or the particular clinician's discretion irrespective of whether there was local tumor recurrence. Upon recurrence, nearly all patients were converted to other treatments, such as surgery, RFA, adjunctive external beam radiation therapy, conversion from DEB-TACE to cTACE, and systemic chemotherapy or molecular targeted therapy.
Owing to the small size of the chemo-Lipiodol emulsion, which can be delivered even to fine collaterals or injured arteries, cTACE can be repeated as many times as necessary. If local recurrence of less successful cTACE happens again from certain collaterals or damaged arteries, conversion to other therapies such as surgery or RFA should be considered accordingly. In comparison, there seems to be a weakness in the scope of repeat procedures for DEB-TACE. Since DEB microspheres are persistent embolic products, they deprive the native arteries of blood supply and cause fine collateral formation; therefore, big DEB microspheres cannot be delivered to fine collaterals or damaged arteries. Hence, cTACE or RFA should be used in such situations. As a result, even though DEB-TACE is used as the initial procedure, comparison of the true effects on health of the two modalities is complicated since the same treatment is rarely continued in patients.
6. Conclusion
TACE remains a viable option for standard of care in the treatment of HCC. Despite several advances in TACE techniques, radiological response evaluation, and patient selection for TACE, there is room for improvement with regard to therapeutic efficacy. To compensate for the limitations of cTACE, DEB-TACE was introduced as a procedure capable of providing more continual and tumor-selective drug administration and permanent embolization, which enables local administration of high doses of anti-cancer agents to the tumor without an increase in systemic levels of the drugs. DEB-TACE, as seen through various outcomes of the studies mentioned above, presented superior—or at least parallel—outcomes compared to cTACE in terms of better clinical efficacy and patient safety profile in patients with HCC. As regards tumor response and procedural safety for initial therapy, DEB-TACE in the very early or early stage of HCC is certainly better than cTACE. However, a long-term comparative analysis of the variations between the two tumor control modalities and clinical results remains challenging. Since the morphological gradations of HCC and the status of clinical liver function are very diverse, it is prudent to understand the benefits and shortcomings of cTACE and DEB-TACE and to select the correct form of care for each patient or even the same patient.
These conclusions favour the use of DEB-TACE in the treatment of HCC, and they may be expandable to more advanced-stage HCC in the future. This may be achieved by conducting further clinical trials testing and comparing the outcomes of DEB-TACE and cTACE in well-selected patients. Although the results of contemporary studies demonstrate a slight favour toward DEB-TACE in terms of efficacy and tumor regression, further studies are needed to obtain a clearer insight into their efficiency, and a model needs to be formulated to ensure their implementation and achieve better results in the management of patients with intermediate- and advanced-stage HCC.
Declaration of competing interest
We all the authors of this article hereby declare no conflict of interest.
References
- 1.Bray F., Ferlay J., Soerjomataram I., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Llovet J.M., Real M.I., Montaña X., et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 3.Lo C.M., Ngan H., Tso W.K., et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 4.Cammà C., Schepis F., Orlando A., et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 5.Llovet J.M., Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 6.Tu J., Jia Z., Ying X., et al. The incidence and outcome of major complication following conventional TAE/TACE for hepatocellular carcinoma. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nouri Y.M., Kim J.H., Yoon H.K., et al. Update on transarterial chemoembolization with drug-eluting microspheres for hepatocellular carcinoma. Korean J Radiol. 2019;20:34–49. doi: 10.3348/kjr.2018.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong K., Khwaja A., Liapi E., et al. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006;12:2563–2567. doi: 10.1158/1078-0432.CCR-05-2225. [DOI] [PubMed] [Google Scholar]
- 9.Lee K.H., Liapi E.A., Cornell C., et al. Doxorubicin-loaded QuadraSphere microspheres: plasma pharmacokinetics and intratumoral drug concentration in an animal model of liver cancer. Cardiovasc Intervent Radiol. 2010;33:576–582. doi: 10.1007/s00270-010-9794-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varela M., Real M.I., Burrel M., et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46:474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y.S., Ou M.C., Tsai Y.S., et al. Transarterial chemoembolization using gelatin sponges or microspheres plus lipiodol-doxorubicin versus doxorubicin-loaded beads for the treatment of hepatocellular carcinoma. Korean J Radiol. 2015;16:125–132. doi: 10.3348/kjr.2015.16.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lammer J., Malagari K., Vogl T., et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33:41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woo H.Y., Heo J. Transarterial chemoembolization using drug eluting beads for the treatment of hepatocellular carcinoma: now and future. Clin Mol Hepatol. 2015;21:344–348. doi: 10.3350/cmh.2015.21.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Facciorusso A., Mariani L., Sposito C., et al. Drug-eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2016;31:645–653. doi: 10.1111/jgh.13147. [DOI] [PubMed] [Google Scholar]
- 15.Razi Murtuza, Gu Jianping, He Xu, et al. Conventional versus drug-eluting bead transarterial chemoembolization: a better option for treatment of unresectable hepatocellular carcinoma. J Interv Med. 2021;4:11–14. doi: 10.1016/j.jimed.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]