Abstract
To characterize the penetration of moxifloxacin (BAY 12-8039) into peripheral target sites, the present study aimed at measuring unbound moxifloxacin concentrations in the interstitial space fluid by means of microdialysis, an innovative clinical sampling technique. In addition, moxifloxacin concentrations were measured in cantharides-induced skin blisters, saliva, and capillary plasma and compared to total- and free-drug concentrations in venous plasma. For this purpose, 12 healthy volunteers received moxifloxacin in an open randomized crossover fashion either as a single oral dose of 400 mg or as a single intravenous infusion of 400 mg over 60 min. An almost-complete equilibration of the free unbound plasma fraction of moxifloxacin with the interstitial space fluid was observed, with mean area under the concentration-time curve (AUC)interstitial fluid/AUCtotal-plasma ratios ranging from 0.38 to 0.55 and mean AUCinterstitial fluid/AUCfree-plasma ratios ranging from 0.81 to 0.86. The skin blister concentration/plasma concentration ratio reached values above 1.5 after 24 h, indicating a preferential penetration of moxifloxacin into inflamed lesions. The moxifloxacin concentrations in saliva and capillary blood were similar to the corresponding levels in plasma. Our data show that moxifloxacin concentrations attained in the interstitial space fluid in humans and in skin blister fluid following single doses of 400 mg exceed the values for the MIC at which 90% of isolates are inhibited for most clinically relevant bacterial strains, notably including penicillin-resistant Streptococcus pneumoniae. These findings support the use of moxifloxacin for the treatment of soft tissue and respiratory tract infections in humans.
Moxifloxacin (BAY 12-8039; Bayer, Leverkusen, Germany) is a promising new quinolone with a broad antibacterial spectrum and high activity against gram-positive cocci, notably penicillin-resistant Streptococcus pneumoniae and “atypical” organisms, such as mycoplasma and chlamydia (3, 9, 20). For moxifloxacin, as for most other antibiotics, it is expected that, to be clinically effective, plasma drug concentrations should exceed in vitro MICs for the relevant infective agent. However, for most infections, the site of bacterial growth is not the bloodstream but the extravascular spaces of peripheral organs, e.g., the interstitial spaces of soft tissues or the lungs (16). At these sites, drug concentrations are not readily assessable and are influenced by a variety of factors, such as local blood flow, vascular permeability, and the local surface area-to-volume ratio (5). Thus, comparing total plasma antibiotic concentrations to MICs is only valid under the assumption that all the drug present in the intravascular space is pharmacologically active and is freely diffusible to the target site. It was shown, however, that antimicrobial agents can differ considerably with respect to their penetration potential and that pharmacologically active, free (10, 12) drug concentrations at the target site may be substantially lower than corresponding concentrations in plasma (14). Therefore, concentrations in total plasma are of limited use in predicting clinical efficacy, and the measurement of free target tissue concentrations was considered more relevant (4, 5).
Although most pharmacokinetic studies still rely on blood sampling, several experimental approaches are available for quantification of concentrations of antibiotic drugs in tissue (5). A clinical technique that ideally addresses the above-mentioned issues is in vivo tissue microdialysis (6, 11). This innovative technique offers the unique opportunity to measure unbound, i.e., pharmacologically active (10, 12) drug concentrations in the interstitial spaces of relevant target sites (11, 14, 15, 17).
To assess the potential of moxifloxacin to penetrate peripheral target sites, we used microdialysis to measure the kinetics of moxifloxacin in the interstitial space fluid in healthy volunteers. In addition, the time-versus-concentration profile of moxifloxacin was followed in cantharides-induced skin blisters, capillary blood, and saliva and compared to total-drug and free-drug concentrations in venous plasma.
MATERIALS AND METHODS
The study was approved by the local ethics committee. All volunteers and patients were given a detailed description of the study, and their written consent was obtained. The study was performed in accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines of the European Commission.
Healthy volunteers.
The study population included 13 healthy male volunteers (age, 24 to 36 years; weight, 62 to 96 kg; height, 171 to 191 cm). Each subject underwent a screening examination, including history and physical examination, 12-lead electrocardiogram, complete blood count with differential, urinalysis, urine drug screen, clinical blood chemistry, blood coagulation tests, hepatitis B surface antigen, and human immunodeficiency virus antibody tests. Subjects were excluded if they had taken any prescription medication or over-the-counter drugs within a period of 2 weeks prior to the study. For each study day, volunteers fasted for 12 h prior to the start of the experiments.
Overall study design and plan of trial.
After having validated the suitability of microdialysis for the measurement of the tissue pharmacokinetics of moxifloxacin in a pilot phase with one healthy volunteer who was administered moxifloxacin at a dose of 400 mg orally (p.o.), the study was conducted as a single-center, single-dose, non-placebo-controlled, randomized, open crossover study including 12 healthy young male volunteers. Each volunteer was studied twice and was randomly assigned to receive either one single p.o. dose of 400 mg of moxifloxacin or one single intravenous (i.v.) infusion of 400 mg of moxifloxacin over 60 min on each occasion after an overnight fast. The pharmacokinetics of moxifloxacin were measured in microdialysates of skeletal muscle and subcutaneous adipose tissue, saliva, capillary and venous plasma, and cantharides-induced skin blisters as described below. The 12 volunteers were further randomized into two groups of 6 volunteers each, according to the time period of the microdialysate measurements (group A, microdialysis 0 to 12 h post administration; group B, microdialysis 24 to 36 h post administration). There was a washout phase of at least 1 week between the two treatments. The subjects were hospitalized in the morning before administration of moxifloxacin until 25 h after administration of moxifloxacin in group A and 37 hours after administration in group B.
Sampling of interstitial microdialysis fluid.
The principles of microdialysis have been described in detail previously (6, 11, 14, 17). Briefly, microdialysis is based on sampling of analytes from the interstitial space by means of a semipermeable membrane at the tip of a microdialysis probe. The probe is constantly perfused with a physiological solution (perfusate) at a flow rate of 0.5 to 10 μl/min. Once the probe is implanted in the tissue, substances present in the interstitial fluid at a certain concentration (ctissue) are filtered by diffusion out of the interstitial fluid into the probe, resulting in a concentration (cdialysate) in the perfusion medium. Samples are collected and analyzed. For most analytes, equilibrium between interstitial tissue fluid and the perfusion medium is incomplete; therefore, ctissue is greater than cdialysate. The factor by which the concentrations are interrelated is termed in vivo recovery. Therefore, to obtain absolute interstitial concentrations from dialysate concentrations, microdialysis probes were calibrated for in vivo recovery rates according to a retrodialysis method (17). The principle of this method relies on the assumption that the diffusion process is quantitatively equal in both directions through the semipermeable membrane. Therefore, 0.2% moxifloxacin was added to the perfusate and the disappearance rate through the membrane was taken as the in vivo recovery rate. The in vivo recovery value was calculated as follows: recovery (%) = 100 − (100 · moxifloxacin concentrationdialysate · moxifloxacin concentrationperfusate−1). Microdialysis probes were inserted after cleaning and thorough disinfection of the skin. One dialysis probe was inserted into a medial vastus muscle, and one was inserted into the subcutaneous layer of the thigh by a previously described procedure (14, 17). The microdialysis system was perfused with Ringer’s solution at a flow rate of 1.5 μl/min, except for the in vivo-calibration periods, during which the microdialysis system was perfused with a stock solution containing 0.2% moxifloxacin in Ringer’s solution. After a 30-min baseline perfusion period, the microdialysis probes were calibrated for a period of 30 min followed by a 30-min washout period in group A (before administration); calibration in group B was performed during a period of 30 min after the end of the sampling periods.
Skin blister fluid sampling.
Cantharides-induced skin blisters are caused by a toxic reaction leading to the formation of a subepidermal blister. To induce skin blisters, cantharides-impregnated plasters were employed as described previously (15). An ointment containing 0.25% cantharidin was prepared by mixing pure cantharidin with ointment base. On the evening before puncturing the blisters (−12 h), eight 1- by 1-cm 0.25% cantharides-impregnated plasters were applied to the abdominal skin of each subject. The patches of polyethylene sheeting were held in place by adhesive tape over 12 h. This led to formation of blisters within 12 h. On the study day, approximately 1 ml of the blister fluid was aspirated into a syringe by puncturing the blister with a fine needle at defined time points. The blister fluid was placed in Eppendorf cups and immediately frozen in an upright position at −80°C.
Sampling of capillary and venous plasma.
For sampling of capillary plasma, two finger pads were pricked with a lancet. Subsequently, blood was taken up with a pipette and transfered to Eppendorf vials for centrifugation. The capillary blood was centrifuged within 10 min for a duration of 5 minutes at 1,600 × g and 5°C. The plasma was then pipetted into polypropylene tubes and immediately frozen in an upright position. For sampling of venous plasma, venous blood was centrifuged within 10 min for a duration of 5 min at 1,600 × g and 5°C and immediately frozen in polypropylene tubes.
Saliva sampling.
The volunteers were requested to chew on cotton rolls for 30 to 45 s at the appropriate sampling times. The cotton rolls were then transferred to plastic tubes (Salivetten, Sarstedt, Germany), which were immediately closed with screw caps to avoid evaporation. Subsequently, the tubes were centrifuged for 5 min at 1,000 × g and 5°C so that at least 0.7 ml of saliva was obtained. The entire device was immediately frozen and stored at −80°C.
Dosage and administration of the study drugs.
Healthy volunteers received moxifloxacin as a single i.v. dose of 400 mg over 60 min or as a single p.o. dose of 400 mg (mean dosage, 5.4 mg/kg of body weight). Each of the 12 volunteers received the study drug according to a randomized crossover design once on two separate study days with a minimum washout period of 7 days.
Analyses. (i) Bioanalysis.
Quantitative determinations of moxifloxacin in all body fluids except microdialysates were carried out by a previously described high-performance liquid chromatography method with fluorescence detection and a limit of detection of 2.5 μg/liter (18). Moxifloxacin concentrations in the microdialysates were measured by capillary zone electrophoresis with laser-induced fluorescence detection (13). To guarantee the validity of the measurements, quality control samples produced from the blank matrix (plasma for plasma, capillary blood, and blister fluid samples; saliva for the saliva samples; and Ringer’s solution for the microdialysates) spiked with known concentrations of the analyte at three concentration levels were analyzed together with the study samples. The accuracy and precision were between 96.2 and 98.6% and 3.6 and 7.6%, respectively, for plasma, capillary blood, and blister fluid and between 99.8 and 106.4% and 4.2 and 12.7% for saliva. They ranged from 96.5 to 101.0 and 5.3 to 7.8% for microdialysates and from 91.2 to 95.9 and 2.7 to 4.5% for urine. This indicates the validity of the bioanalytical data for pharmacokinetic evaluations.
(ii) Determination of the unbound plasma fraction.
Plasma protein binding was determined ex vivo by membrane filtration. Two plasma samples from each subject (one from the p.o. and one from the i.v. administration periods) were subjected to ultrafiltration with a Centricon (Amicon, Switzerland) device. The unbound concentration was measured in the ultrafiltrate by high-performance liquid chromatography (18).
(iii) Calculations and data analysis.
Absolute interstitial fluid concentrations were calculated from dialysate concentrations by the following equation: interstitial fluid concentration = 100 · sample concentration · in vivo recovery value−1. For comparisons between the pharmacokinetic parameters of different compartments, Mann-Whitney U tests were employed, as pharmacokinetic parameters were nonnormally distributed. A value of P < 0.05 was considered the level of significance.
(iv) Pharmacokinetic analysis.
The primary pharmacokinetic parameters (time to maximum concentration of drug in serum [Tmax], area under the curve [AUC], maximum concentration of drug in serum [Cmax], and half-life [t1/2]) were calculated by model independent noncompartmental analysis. For calculation of plasma-to-interstitium transfer rates, the data for seven data sets from microdialysis experiments (group A; p.o. administration) were fitted according to a two-compartment model for plasma values, employing a commercially available computer program (Topfit 2.0; Gustav Fischer, New York, N.Y.). Data for peripheral-compartment values were fitted according to a one-compartment model, and the transfer rate constant from the central to the peripheral compartment (k12) was calculated as described previously (14). Areas under the curve from 0 to 12 h (AUC0–12 [in nanograms · hour per milliliter]) for individual drugs were determined for plasma and peripheral compartments according to the trapezoidal rule. The following penetration ratios for tissues were determined: AUCperipheral-compartment/AUCtotal-plasma ratio, the concentrationperipheral-compartment/concentrationplasma ratio, and the AUCperipheral-compartment/AUCfree-plasma ratio.
RESULTS
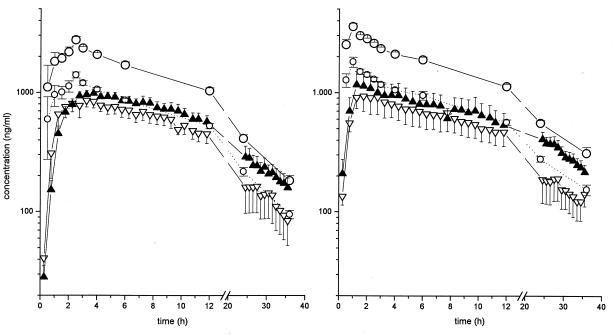
The results of experiments in which probes were inserted simultaneously into the medial vastus muscle and into the subcutaneous adipose tissue of healthy volunteers show that interstitial-target-site drug concentrations and AUC values were clearly below corresponding concentrations in plasma (P < 0.004 [Table 1 and Fig. 1]). However, taking plasma protein binding values of 52% ± 8% (standard deviation [SD]; range, 40 to 72%) into account, an almost-complete equilibration of the unbound plasma drug fraction with the interstitial space fluid could be observed (Fig. 1). This is also indicated by an interstitial/total-plasma concentration ratio of 0.38 to 0.55 (see Fig. 3). In particular, the AUCmuscle/AUCtotal-plasma ratio was 0.55 ± 0.12 (SD), the AUCsubcutis/AUCtotal-plasma ratio was 0.38 ± 0.09, the AUCmuscle/AUCfree-plasma ratio was 0.86 ± 0.17, and the AUCsubcutis/AUCfree-plasma ratio was 0.81 ± 0.19. The time course in subcutaneous adipose tissue closely resembled the time course in skeletal muscle, although subcutaneous concentrations were consistently somewhat lower (Fig. 1), which may be explained by a slightly higher local blood flow in skeletal muscle. As indicated by the transfer rate constants, k12, which were 3.38 ± 3.45 min−1 and 3.38 ± 3.45 min−1 for muscle and subcutaneous adipose tissue, respectively, moxifloxacin rapidly distributes from the plasma to the relevant target sites. The mean residual time, i.e., the mean time a molecule resides in the respective compartment, was 12.97 ± 4.61 min for plasma, 11.70 ± 2.60 min for subcutaneous adipose tissue, and 15.46 ± 2.43 min for skeletal muscle.
TABLE 1.
Pharmacokinetic dataa
| Administration | Site | AUC (μg · h · ml−1) | Cmax (μg · ml−1) | Tmax (h) | t1/2 (h) | Clearance (liter · h−1) |
|---|---|---|---|---|---|---|
| I.v. | Plasma | 22.9 ± 11.1 | 3.7 ± 0.7 | 1.0 ± 0.2 | 14.3 ± 2.4 | 2.3 ± 0.4 |
| Muscle | 9.5 ± 5.9* | 1.2 ± 0.8 | 1.8 ± 0.6 | |||
| Subcutis | 7.9 ± 4.6* | 1.0 ± 0.5 | 2.0 ± 1.0 | |||
| Saliva | 21.4 ± 5.0 | 5.1 ± 1.4 | 1.0 ± 0.1 | 14.9 ± 2.7 | ||
| Capillary | 22.1 ± 2.0 | 4.2 ± 0.4 | 1.0 ± 0.0 | 12.3 ± 2.1 | ||
| Blister | 16.7 ± 4.1 | 1.7 ± 0.3 | 5.6 ± 4.5 | |||
| P.o. | Plasma | 19.8 ± 1.5 | 3.2 ± 0.6 | 1.6 ± 0.8 | 14.3 ± 2.0 | 2.4 ± 0.4 |
| Muscle | 8.5 ± 2.0* | 0.9 ± 0.2 | 3.2 ± 1.4 | 10.2 ± 1.7 | ||
| Subcutis | 8.0 ± 2.1* | 0.9 ± 0.2 | 2.4 ± 1.7 | 7.5 ± 2.6 | ||
| Saliva | 17.6 ± 2.7 | 3.6 ± 1.0 | 1.7 ± 0.7 | 14.3 ± 3.2 | ||
| Capillary | 18.7 ± 3.1 | 2.7 ± 0.8 | 2.5 ± 3.3 | 12.0 ± 2.2 | ||
| Blister | 12.3 ± 3.3* | 1.6 ± 0.2 | 8.0 ± 3.7 |
Pharmacokinetic parameters for venous plasma (0 to 12 h), skeletal muscle and subcutaneous adipose interstitial fluid (0 to 12 h), saliva (0 to 12 h), capillary plasma (0 to 12 h), and cantharides-induced skin blister fluid (0 to 12 h) following administration of moxifloxacin (single i.v. dose of 400 mg over 60 min or p.o. dose of 400-mg tablet) in healthy volunteers (n = 12). The results are presented as means ± SD. *, P < 0.05 versus AUCplasma.
FIG. 1.
Profiles of time versus total-drug (open circles, solid line) and free-drug (open circles, dotted line) concentrations in plasma and interstitial fluid for muscle (solid triangles) and subcutaneous adipose tissue (open triangles) following administration of moxifloxacin (single p.o. dose of 400 mg [left panel] or single i.v. dose of 400 mg over 60 min [right panel]) in healthy volunteers (n = 12). The results are presented as means ± standard errors.
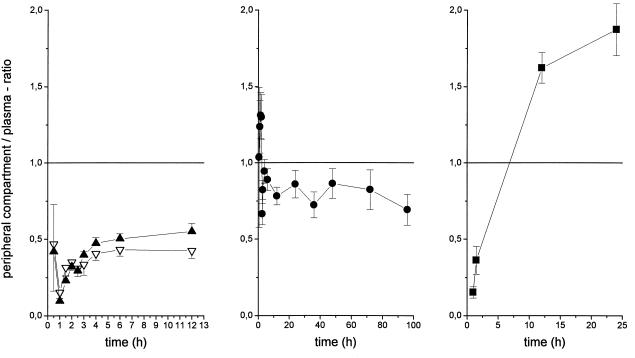
FIG. 3.
Time course of the interstitial fluid of muscle (solid triangles) or subcutaneous adipose tissue (open triangles), saliva (solid circles), and cantharides-induced skin blister fluid (solid squares) drug concentration/plasma drug concentration ratios of moxifloxacin for the experiments shown in Fig. 1 (left panel). The results are presented as means ± standard errors.
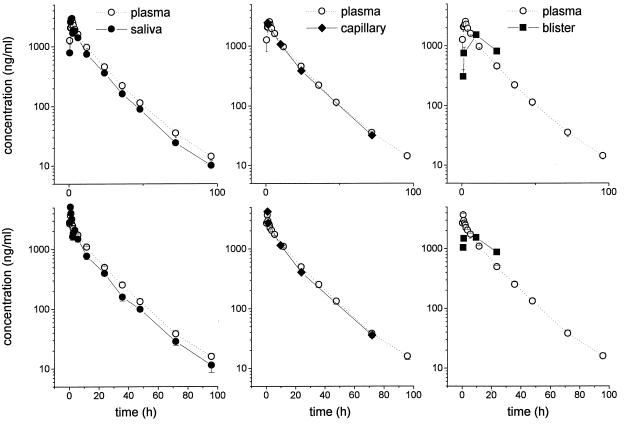
Moxifloxacin concentrations in saliva and capillary plasma closely reflected the corresponding concentrations in venous plasma, and there was no significant difference in the AUC values (Table 1). The time-versus-concentration profile of moxifloxacin in saliva, capillary blood, and skin blister fluid is shown in Fig. 2, and the corresponding concentrationperipheral-compartment/ concentrationplasma ratios are shown in Fig. 3. In particular, the AUCsaliva/AUCplasma ratio, the AUCcapillary blood/AUCplasma ratio, and the AUCskin blister/AUCplasma ratio were 0.83 ± 0.20, 0.95 ± 0.11, and 0.64 ± 0.21, respectively. Pharmacokinetic data for interstitial space fluid in skeletal muscle and subcutaneous adipose layer, saliva, capillary blood, cantharides-induced skin blister fluid, and plasma are given in Table 1.
FIG. 2.
Time versus plasma and saliva, capillary blood, and cantharides-induced skin blister fluid drug concentration profiles following administration of moxifloxacin (single p.o. dose of 400 mg [upper panel] or single i.v. dose of 400 mg over 60 min [lower panel] in healthy volunteers (n = 12). The results are presented as means ± standard errors.
DISCUSSION
Moxifloxacin is a highly effective antimicrobial agent in vitro (3, 9, 20, 21), and it was shown in vivo that plasma moxifloxacin concentrations exceed the in vitro MIC90s for most relevant bacteria (19). Thus, by relating concentrations in plasma to in vitro MICs, it may be concluded that moxifloxacin is a highly effective drug for the treatment of various bacterial infections, in particular, soft tissue and respiratory tract infections. However, while it is generally agreed that the plasma antibiotic concentration should be above the MIC for an infective agent, it is also generally accepted that most infections to be treated by quinolones occur in the interstitial spaces of peripheral organs (16), which limits the use of concentrations in plasma to predict clinical efficacy (4). The present study, therefore, aimed at measuring moxifloxacin concentrations in relevant peripheral target sites, i.e., in the interstitial space fluid of soft tissues, by microdialysis, a clinical technique which allows for the in vivo measurement of interstitial free-drug concentrations (11, 14, 17).
A main finding of the present study was that interstitial moxifloxacxin concentrations were only about 50% of corresponding concentrations in plasma following both i.v. and p.o. administration, a finding which is also compatible with our protein binding data. However, we could not confirm previous findings of quinolone tissue concentration/plasma concentration ratios of >1 obtained by tissue biopsy (2). As shown previously, however, biopsy data may clearly overestimate target site concentrations for drugs that accumulate in the intracellular space and may underestimate effect site concentrations of drugs that equilibrate exclusively with the interstitial space, like beta-lactams (1, 14). Since microdialysis selectively mirrors the unbound interstitial concentrations, it may be better suited for the assessment of target site penetration than other methods (15).
Our present pharmacokinetic experiments provide evidence that, considering a MIC90 of 0.12 mg/liter for Staphylococcus aureus and Streptococcus pyogenes (3, 21) and of <1 for most members of the family enterobacteriaceae (21), the administration of single doses of 400 mg of moxifloxacin leads to peripheral target site free-drug concentrations clearly exceeding the MICs for most relevant pathogens throughout the dosing interval (7). However, MICs may not be the ideal surrogate for the assessment of clinical antimicrobial efficacy. For quinolones, the Cmax/MIC ratio is considered the most relevant pharmacokinetic surrogate marker which also proved to be predictive of bacterial eradication (8). Although 99% killing can be obtained by quinolones at a low ratio, i.e., 3 for ciprofloxacin, bacterial regrowth and development of bacterial resistance may occur unless higher ratios, i.e., 8 for ciprofloxacin, are reached (8). In our experiments Cmax/MIC ratios for methicillin-susceptible S. aureus of approximately 30 and 8 to 10 were attained for plasma levels and interstitial space fluid, respectively. As shown previously (14), Cmax/MIC ratios may differ significantly between the central and the peripheral compartments. Based on the observation that at concentrations of 0.48 to 0.96 mg/liter Cmax/MIC ratios of 8 are achieved for streptococci (9), our data corroborate the view that moxifloxacin may be a highly effective antimicrobial agent, in particular for the treatment of soft tissue and respiratory tract infections.
Moxifloxacin concentrations in saliva and capillary plasma closely reflected corresponding concentrations in venous plasma, and there was no significant difference in the key pharmacokinetic parameters. However, concentrations in saliva, like concentrations in capillary plasma, initially exceeded the corresponding concentrations in venous plasma, a finding that was previously described for different drugs and which may be explained by active drug transport across the salivary epithelium (15). An important limitation of the present measurements is the fact that they reflect only drug penetration into physiological compartments, which may not necessarily reflect relevant pathological situations, e.g., penetration into inflamed tissues. This is also highlighted by the results obtained for cantharides-induced skin blister measurements in our study. Cantharides-induced skin blister fluid was shown to represent an inflammatory environment, and measurement of drug concentrations in this compartment may therefore mirror pathological conditions in infected tissue. In our experiments, there was an increase in the concentration in skin blister/concentration in plasma ratio after an initial lag time, with a ratio of approximately 1.8 after 24 h. This finding is in agreement with previous studies of quinolones and supports the concept that moxifloxacin, like other fluoroquinolones, may accumulate in phagocytes and thereby in inflammatory lesions (1). These results, which due to the small sample size may only be viewed as estimations, might, however, be explained by the fact that skin blister fluid is an inflammatory exudate (15) and some of the observed increase may be due to binding of moxifloxacin to blister proteins, i.e., by an increase in a pharmacologically inactive drug fraction (14, 15). These results, which could provide evidence for a preferential target site distribution of moxifloxacin, warrant further studies of moxifloxacin penetration, e.g., in soft tissue infections.
In conclusion, we have shown that after administration of moxifloxacin at single doses of 400 mg, peripheral target site concentrations are attained which exceed the MICs for most relevant pathogens throughout the dosing interval. These findings provide evidence that moxifloxacin may qualify as a rational choice for the treatment of soft tissue and respiratory tract infections in humans.
REFERENCES
- 1.Baldwin D R, Honeybourne D, Wise R. Pulmonary disposition of antimicrobial agents: in vivo observations and clinical relevance. Antimicrob Agents Chemother. 1992;36:1176–1180. doi: 10.1128/aac.36.6.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cakmakci M, Gossweiler L, Schilling J, Schlumpf R, Geroulanos S. Penetration of fleroxacin into human lung, muscle, and fat tissue. Drugs Exp Clin Res. 1992;18:299–302. [PubMed] [Google Scholar]
- 3.Dalhoff A, Petersen U, Endermann R. In vitro activity of BAY 12-8039, a new 8-methoxyquinolone. Chemotherapy. 1996;42:410–425. doi: 10.1159/000239474. [DOI] [PubMed] [Google Scholar]
- 4.Derendorf H. Pharmacokinetic evaluation of beta-lactam antibiotics. J Antimicrob Chemother. 1989;24:407–413. doi: 10.1093/jac/24.3.407. [DOI] [PubMed] [Google Scholar]
- 5.Eichler H G, Müller M. Drug distribution—the forgotten relative of clinical pharmacokinetics. Clin Pharmacokinet. 1998;34:95–99. doi: 10.2165/00003088-199834020-00001. [DOI] [PubMed] [Google Scholar]
- 6.Elmquist W F, Sawchuk R J. Application of microdialysis in pharmacokinetic studies. Pharm Res. 1997;14:267–288. doi: 10.1023/a:1012081501464. [DOI] [PubMed] [Google Scholar]
- 7.Goldstein E J, Citron D M, Hudspeth M, Gerardo S H, Merriam C V. In vitro activity of Bay 12-8039, a new 8-methoxyquinolone, compared to the activities of 11 other oral antimicrobial agents against 390 aerobic and anaerobic bacteria isolated from human and animal bite wound skin and soft tissue infections in humans. Antimicrob Agents Chemother. 1997;41:1552–1557. doi: 10.1128/aac.41.7.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hyatt J M, McKinnon P S, Zimmer G S, Schentag J J. The importance of pharmacokinetic/pharmacodynamic surrogate markers to outcome. Clin Pharmacokinet. 1995;28:143–160. doi: 10.2165/00003088-199528020-00005. [DOI] [PubMed] [Google Scholar]
- 9.Klugman K P, Capper T. Concentration-dependent killing of antibiotic-resistant pneumococci by the methoxyquinolone moxifloxacin. J Antimicrob Chemother. 1997;40:797–802. doi: 10.1093/jac/40.6.797. [DOI] [PubMed] [Google Scholar]
- 10.Kunin C M, Craig W A, Kornguth M, Monson R. Influence of binding on the pharmacologic activity of antibiotics. Ann NY Acad Sci. 1973;226:214–224. doi: 10.1111/j.1749-6632.1973.tb20483.x. [DOI] [PubMed] [Google Scholar]
- 11.Lönnroth P, Jansson P A, Smith U. A microdialysis method allowing characterization of interstitial water space in humans. Am J Physiol. 1987;16:E228–E231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- 12.Merrikin D J, Briant J, Rolison G N. Effect of protein binding on antibiotic activity in vivo. J Antimicrob Chemother. 1983;11:233–238. doi: 10.1093/jac/11.3.233. [DOI] [PubMed] [Google Scholar]
- 13.Möller J G, Staß H, Heinig R, Blaschke G. Capillary electrophoresis with laser induced fluorescence: a routine method to determine moxifloxacin in human body fluids in very small sample volumes. J Chromatogr. 1998;716:325–334. doi: 10.1016/s0378-4347(98)00302-8. [DOI] [PubMed] [Google Scholar]
- 14.Müller M, Haag O, Burgdorff T, Georgopoulos A, Weninger W, Jansen B, Stanek G, Pehamberger H, Agneter E, Eichler H G. Characterization of peripheral compartment kinetics of antibiotics by in vivo microdialysis in humans. Antimicrob Agents Chemother. 1996;40:2703–2709. doi: 10.1128/aac.40.12.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller M, Brunner M, Schmid R, Putz E M, Schmiedberger A, Wallner I, Eichler H G. Comparison of three different experimental methods for the assessment of peripheral compartment pharmacokinetics in humans. Life Sci. 1998;62:PL227–PL234. doi: 10.1016/s0024-3205(98)00071-x. [DOI] [PubMed] [Google Scholar]
- 16.Ryan D M. Pharmacokinetics of antibiotics in natural and experimental superficial compartments in animals and humans. J Antimicrob Chemother. 1993;31(Suppl. D):1–16. doi: 10.1093/jac/31.suppl_d.1. [DOI] [PubMed] [Google Scholar]
- 17.Stahle L, Arner P, Ungerstedt U. Drug distribution studies with microdialysis. III:Extracellular concentration of caffeine in adipose tissue in man. Life Sci. 1991;49:1853–1858. doi: 10.1016/0024-3205(91)90488-w. [DOI] [PubMed] [Google Scholar]
- 18.Stass H, Dalhoff A. Determination of BAY 12-8039, a new 8-methoxyquinolone, in human body fluids by high-performance liquid chromatography with fluorescence detection using on-column focusing. J Chromatogr B. 1997;702:163–174. doi: 10.1016/s0378-4347(97)00371-x. [DOI] [PubMed] [Google Scholar]
- 19.Stass H, Dalhoff A, Kubitza D, Schuhly U. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob Agents Chemother. 1998;42:2060–2065. doi: 10.1128/aac.42.8.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Visalli M A, Jacobs M R, Appelbaum P C. Antipneumococcal activity of BAY 12-8039, a new quinolone, compared with activities of three other quinolones and four oral beta-lactams. Antimicrob Agents Chemother. 1997;41:2786–2789. doi: 10.1128/aac.41.12.2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodcock J M, Andrews J M, Boswell F J, Brenwald N P, Wise R. In vitro activity of BAY 12-8039, a new fluoroquinolone. Antimicrob Agents Chemother. 1997;41:101–106. doi: 10.1128/aac.41.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]