Abstract
Introduction
On November 20, 2019, the Sierra Leone International Health Regulations (IHR) National Focal Point was notified of an exported case of Lassa fever in The Netherlands, by a Dutch doctor who previously practiced in a rural hospital in Sierra Leone. This report describes the extent of the outbreak, possible sources of infection, and the outbreak response measures taken.
Methods
Response measures implemented to control the outbreak included coordination across multiple countries and cities, outbreak investigation, active case finding, contact tracing and monitoring, laboratory investigation, and isolation and treatment of cases.
Results
We report a hospital-associated outbreak that resulted in 3 confirmed cases (health workers) and 2 probable cases (patients). The case fatality rate was 60%, whereas the secondary attack rate was 14%. Two cases involved exportations to The Netherlands. Failure to detect the index case and poor adherence to infection prevention and control (IPC) protocols contributed to disease spread. Pregnancy status and nonspecific signs and symptoms of the index case contributed to failure in early case detection.
Conclusions
Rapid activation of national and subnational incident management systems resulted in rapid outbreak control. We recommend regular training for clinicians on surveillance and IPC protocols and strengthening in-country Lassa virus diagnostic capacity.
Keywords: Lassa Fever, Outbreak, Sierra Leone, Hospital-Associated Infection
INTRODUCTION
On November 20, 2019, the Sierra Leone International Health Regulations (IHR) National Focal Point alerted the district medical officer (DMO) in Tonkolili District of a confirmed case of Lassa fever, diagnosed by an expatriate doctor who worked in a rural missionary hospital in Tonkolili district. The doctor had been medically evacuated to The Netherlands on November 19, 2019, where Lassa virus (LASV) was detected by RT-PCR (Overbosch et al., 2020). The doctor eventually died while undergoing treatment in The Netherlands. A second expatriate doctor who was also working in the same mission hospital in Tonkolili was repatriated to The Netherlands on November 23, 2019, and eventually recovered.
Upon receiving the information of the confirmed case in The Netherlands, the DMO in Tonkolili District immediately activated the rapid response team to investigate the incident. As per the national preparedness guidelines, the event was classified as a level 2 threat, and this required activation of the national Emergency Operation Centre (EOC). A national rapid response team from Ministry of Health and Sanitation (MOHS), WHO Sierra Leone, and African Field Epidemiology Network (AFENET) Sierra Leone travelled to the affected hospital, where they joined the district rapid response team in investigating and responding to the outbreak.
This report describes measures used to control the outbreak and the various challenges encountered, including delayed detection and confirmation of Lassa fever, coordination of the response across cities and countries, and contact tracing and monitoring. We also share lessons learned and recommendations for future outbreaks.
Epidemiology of Lassa fever
Lassa fever is a viral zoonotic disease first described in Nigeria in 1969 (Frame et al., 1970) and is endemic to the West African region including Sierra Leone (Sogoba et al., 2012). Mastomys natalensis rodents are the primary reservoirs for Lassa fever, and human infection mainly occurs through ingestion of food contaminated with fecal matter or urine of infected rodents. Consumption of rodents as food is a common practice in some rural parts of Sierra Leone (Bonwitt et al., 2016) and is a known mode of transmission of LASV. Less commonly, human to human transmission of LASV occurs when healthy persons come into contact with bodily fluids of infected persons (Hallam et al., 2018).
Hospital associated infections of LASV are common in Lassa fever-endemic regions (Keane and Gilles, 1977) (Nasidi et al., 1995) and have resulted in exportation of more than 30 cases of Lassa fever to other continents (Macher and Wolfe, 2006) (Kofman et al., 2019). With the ever-increasing interconnectivity among countries, Lassa fever poses a risk not only in endemic countries but also globally. Therefore, early notification, as required by the International Health Regulations (IHR 2005), is crucial in controlling the spread of cases.
Health workers can be exposed to LASV during routine patient care or when undertaking more invasive procedures such as surgery or autopsies (Nasidi et al., 1995). Diagnosis of LASV is complex, requiring a series of tests that are often not readily available in low-resource settings. Limited detection and diagnostic capacity in rural health facilities, including those in Lassa fever-endemic regions, means that health workers are unable to identify patients with potential infectious diseases and institute timely infection prevention and control measures.
History of Lassa fever in Sierra Leone
Lassa fever is a recognized public health challenge in Sierra Leone, accounting for up to 10%–15% of all adult admissions, 30% of adult deaths, and 25% of maternal mortality in endemic regions (Price et al., 1988). Since 1976, the Lassa Fever Consortium has supported the Ministry of Health and Sanitation to set up a Lassa fever isolation, diagnosis, and treatment unit at Kenema Government Hospital in the southeastern region of Sierra Leone (Shaffer et al., 2014). A biosafety level 3 laboratory was part of the unit, and samples referred from other parts of Sierra Leone as well as Guinea and Liberia were tested for LASV and other viruses. Although LASV diagnosis and treatment were interrupted during the civil conflict (1991–2002), active case detection resumed in the after the conflict period, revealing a high prevalence (35%) of LASV among suspected patients tested in the Kenema Government Hospital laboratory (Shaffer et al., 2014).
It is highly likely that outbreaks of Lassa fever in Sierra Leone may have gone undetected due to weak public health systems during the conflict period (1991–2002) and also after the conflict period (Shaffer et al., 2014). The unprecedented 2013–2016 Ebola outbreak too had a devastating impact on management of Lassa fever in the region, including significant loss of experienced health care workers in the Kenema Government Hospital, which compromised detection and treatment throughout the protracted crisis and thereafter.
Public health surveillance was weak until 2015 when the country adapted the African regional Integrated Disease Surveillance and Response (IDSR) strategy (World Health Organization, 2010) and rolled out implementation in all public health facilities (Njuguna et al., 2019). Difficulties in case detection as a result of nonspecific symptoms of Lassa fever patients, as well as low access to specialized laboratories, has often led to underestimation of the burden of Lassa fever cases in Sierra Leone. For example, the number of confirmed cases were 15 in 2019, 22 in 2018, 30 in 2017, and 35 in 2016. The positivity rate of suspected cases ranged between 6% and 12% in those 4 years, whereas case fatality rate for admitted cases ranged between 40% and 72%.
Chronology of the Lassa fever outbreak
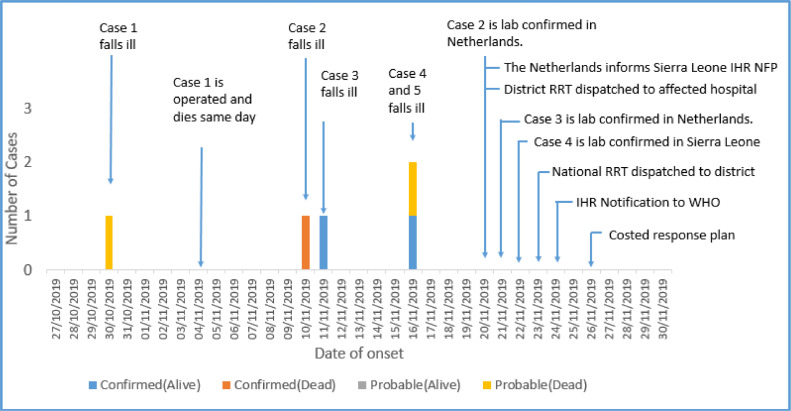
The probable index case (Case 1) was believed to be a 30-year-old pregnant woman who was first seen at Makonthadae Maternal and Child Health Post (MCHP) in Tonkolili District on November 3, 2019, where she presented with complaints of fever, vaginal bleeding, and vomiting. She was referred to Masanga Hospital in Tonkolili district where she underwent a caesarean section on November 4, 2019 (Figure 1). She developed complications and died immediately after surgery despite attempted resuscitation. The surgery was conducted by 2 Dutch doctors who were assisted by a local anesthetist.
Figure 1.
Chronology of Events, Lassa Fever Outbreak, Tonkolili District, Sierra Leone, Nov-Dec 2019. IHR NFP: International Health Regulations National Focal Point; RRT: Rapid Response Team; WHO: World Health Organization.
On November 10, 1 of the surgeons (Dutch Doctor – Case 2) who operated on the index case developed fever, headache, and general malaise, and although the doctor tested negative for malaria, he was treated for malaria and typhoid. A day later (November 11, 2019), his colleague (also an expatriate doctor from The Netherlands - case 3), who assisted during the surgery, also became symptomatic (fever, vomiting, and anorexia). On November 16, 2 additional cases occurred: the anesthetist (case 4) who assisted in the caesarean section and a 33-year-old woman (case 5) who underwent a laparotomy by the same team in the same theatre on November 4, 2019.
A diagnosis of Lassa fever was first confirmed by RT-PCR on November 19 using the samples taken from the Dutch doctor who had been repatriated to The Netherlands for treatment 16 days after the index case had presented at the hospital. Among all cases, the mean age was 33.6 years (SD 1.4) and was lower among female cases (mean 32; SD 1.7 years) than male cases (mean 36; SD 5.6 years). The most common symptoms were fever and vomiting and the case fatality rate was 60% (Table 1).
Table 1.
Characteristics of Probable and Confirmed Lassa Fever Cases, Tonkolili District, Sierra Leone, 2019
| Variable | Frequency (N=5) |
|---|---|
| Sex | |
| Male | 2 |
| Female | 3 |
| Mean age (SD) | 33.6 (1.4) |
| Symptoms | |
| Fever | 5 |
| Vomiting | 3 |
| Diarrhea | 1 |
| General Malaise | 2 |
| Bleeding | 2 |
| Loss of appetite | 1 |
| Laboratory Test | |
| Positive | 3 |
| Negative | 2 |
| Outcome | |
| Alive | 2 |
| Dead | 3 |
| Case Fatality Rate | 60% |
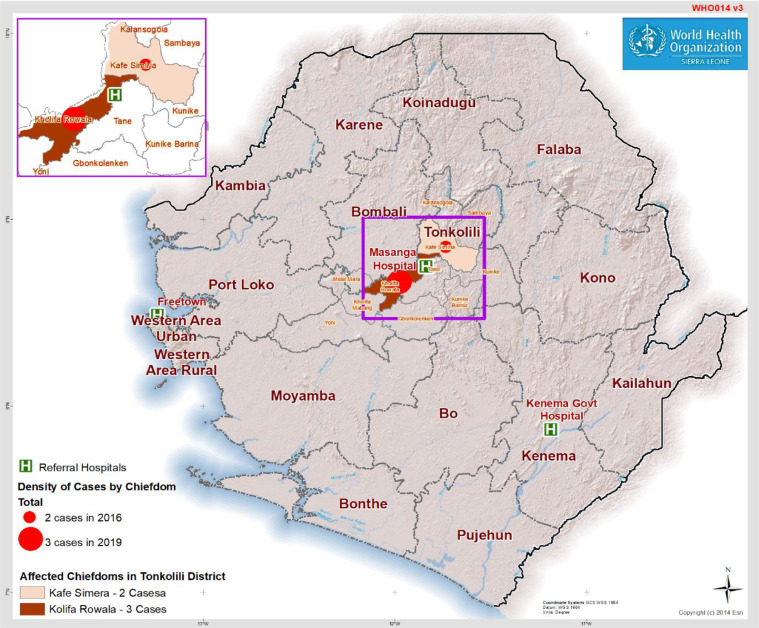
Tonkolili District is located in Northern Sierra Leone and borders the Lassa fever endemic districts to the south, namely Kenema and Bo (Figure 2). Masanga Hospital is among the 108 health facilities that are participating in the IDSR surveillance system in Tonkolili District.
Figure 2.
Map of Sierra Leone showing 16 districts and the location of Lassa fever cases in Tonkolili District from 2016–2019. For 2019, only the 3 laboratory-confirmed cases have been included (probable cases have been excluded).
METHODS
Coordination of the Outbreak Response
On receiving the outbreak notification from The Netherlands, the MOHS responded promptly, initiating an outbreak investigation and activating national and district incidence management systems. The pillar system, adopted since the Ebola outbreak and in line with WHO emergency response framework (World Health Organization, 2017), was adopted in this outbreak response. MOHS relevant departments and multilateral partners were organized into 5 pillars namely: (1) Coordination; (2) Surveillance; (3) Laboratory; (4) Case Management and Infection Prevention and Control (including safe and dignified burial); and (5) Risk Communication and Community Engagement.
The Tonkolili District Emergency Operations Center (DEOC) supported by the Public Health National Emergency Operations Center (PHNEOC) provided a platform for coordination of all outbreak response activities. Daily incident management team (IMT) meetings were held at DEOC and PHNEOC, which convened all MOHS pillar leads, provincial and district representatives of relevant government institutions, and supporting partners. Daily updates of the outbreak situation were provided during the meetings.
IHR Notification and International Coordination
IHR notification was initiated by the national focal point of The Netherlands to Sierra Leone and WHO as required under international health regulations. Subsequently, once this information was received and the outbreak was confirmed in Sierra Leone, the IHR National Focal Point for Sierra Leone also notified WHO. Coordination was complex, as it involved multiple WHO regions (HQ, AFRO, EURO) and country offices, including The Netherlands, Sierra Leone, UK, Germany, and Uganda. Several teleconferences were convened between focal points in all of these offices to ensure prompt information sharing and coordination. This also assisted with contact tracing across the different countries, limiting onward transmission.
Case Finding and Contact Tracing
Suspecting ongoing transmission of Lassa fever in Masanga Hospital, the national and district rapid response team's (RRT's) focused on first identifying additional cases in Masanga hospital and Makonthadae MCHP. All health care workers were interviewed, and symptomatic persons identified were immediately isolated and referred to Kenema Lassa fever Treatment unit. Health workers who reported contact with probable or confirmed cases and their bodily fluids, or materials such as bed sheets were enlisted for 21-day monitoring.
The RRT also interviewed relatives of the deceased and community informants in Mayorroh Village in Kafe Samira Chiefdom, Tonkolili District where the probable index case (case 1) resided and was later buried unsupervised. Household contacts of the 2 deceased women (case 1 and 5) and other community members identified as contacts were screened for symptoms and listed as contacts for monitoring. Contact tracing was also conducted in Mathaman Village, where some relatives of the probable index case resided, many of whom had attended the burial. Contact tracing was also conducted in Freetown, where 1 of the confirmed cases (Dutch Doctor, case 2) had travelled to attend a dinner party and had resided with a family of 4.
We examined the number of contacts per case and calculated the secondary attack rate (proportion of new cases among the contacts). Review of records in 3 other health facilities in Tonkolili District was also conducted to identify potential records of acute viral hemorrhagic fever or suspected Lassa fever cases from October 1, 2019.
A suspected case was defined as any person with gradual onset of 1 or more of the following symptoms: malaise, fever, headache, sore throat, cough, nausea, vomiting, diarrhea, myalgia, chest pain, or hearing loss from 9th October 2019 and a history of contact with a case of Lassa fever or with excreta of rodents. A probable case was defined as one who met the case definition for a suspected case and had an epidemiological link with a laboratory-confirmed case. A confirmed case was defined as a suspected case that was laboratory-confirmed (positive IgM antibody, PCR, or virus isolation).
Contact Monitoring
In total, 81 contacts were identified during 21 days of follow-up in Sierra Leone (Table 2). Approximately 164 contacts were identified in The Netherlands, 20 in the United Kingdom, 5 in Germany, 5 in Denmark, 2 in the USA, and 1 in Uganda. Contacts reported from other countries comprised those who interacted with the 2 Dutch cases in Sierra Leone during evacuation to The Netherlands or during treatment in The Netherlands. In this report, we focused on the contacts that were identified and followed up in Sierra Leone.
Table 2.
Characteristics and Outcomes of Lassa Fever Contacts, Tonkolili District, Sierra Leone, 2019
| No. of Contacts in Sierra Leone | |
|---|---|
| Case 1 | 29 |
| Case 2 | 12 |
| Case 3 | 3 |
| Case 4 | 13 |
| Case 5 | 24 |
| Total No. of Contacts | 81 |
| Average no. of contacts per case | 16 |
| Median age of contacts in years | 31 |
| Sex- Male, No (%) | 36 (44%) |
| District | |
| Tonkolili | 73 (90%) |
| Western Area Urban | 8 (10%) |
| Relationship with Case patient, No. (%) | |
| Relative/household member | 14 (17.3%) |
| Health worker | 4 (4.9%) |
| Patient in the same Health facility | 37 (45.7%) |
| Other | 26 (39.4) |
| Outcome | |
| Completed 21-day Follow-Up, No. (%) | 81 (100%) |
Contacts in Sierra Leone were identified by listing all persons who had been in contact with cases, including household members, friends, hospital workers, and community members. Among the contacts under follow-up in Sierra Leone, median age was 31 years (IQR 19), and 36 contacts (44%) were male. The majority (37; 46%) were patients in the same hospital as the confirmed cases. Among 29 contacts of the index case (case 1), 4 developed signs and symptoms within 1 incubation period (2–21 days); thus, the secondary attack rate was 14%. Besides the 3 confirmed and 2 probable cases, 4 new cases were suspected among the contacts as they developed fever >38⁰C during the 21-day monitoring period. However, LASV was not detected in samples taken from the 4 suspect cases, and they made a full recovery. All other contacts in Sierra Leone completed follow-up and were released after 21 days (Table 2).
Laboratory Investigation
In total, 5 blood samples from suspected cases in Tonkolili and Freetown were collected and transported to Kenema Government Hospital Lassa Fever Unit Laboratory for testing. The laboratory is 167 kilometers from Tonkolili and 310 kilometers from Freetown. Samples were tested using enzyme linked immunosorbent assays (ELISA) to detect LASV antigen and antibodies (IgM and IgG). Confirmatory testing was performed using real-time polymerase chain reaction (RT-PCR). Laboratory investigations for the 2 Dutch doctors (cases 2 and 3) were conducted in The Netherlands where LASV was detected using RT-PCR. Laboratory investigations were not conducted on the 2 deceased female cases (cases 1 and 5), as they had already been buried when the investigation began, and laboratory specimens could not be obtained.
Of the 5 samples tested for LASV at the Kenema Government Hospital Lassa Fever Laboratory in Sierra Leone, LASV was detected in only 1 sample (Table 3). The median turnaround time from sample collection to arrival and testing was 1 day (24 hours). No LASV antibodies (IgM or IgG) were detected in any of the samples.
Table 3.
Laboratory Results of Suspected Cases in Tonkolili District Tested for Lassa Fever at Kenema Government Hospital, Sierra Leone, November–December, 2019
| Case Description | ELISA Antigen | ELISA IgM | Elisa IgG |
|---|---|---|---|
| Case 4 | Positive | Negative | Negative |
| Suspected Case 6 | Negative | Negative | Negative |
| Suspected Case 7 | Negative | Negative | Negative |
| Suspected Case 8 | Negative | Negative | Negative |
| Suspected Case 9 | Negative | Negative | Negative |
Notes: Case 4 that was positive by Elisa Antigen test was also confirmed by RT-PCR. Probable Cases 1 and 5 had no samples taken; Case 2 and 3 were tested and confirmed in The Netherlands.
Infection, Prevention, and Control
An assessment of infection, prevention, and control (IPC) measures in Masanga Hospital and surrounding health facilities indicated that IPC supplies, including coveralls, face shields, gloves, and aprons were available. Running water and soap, hand washing stations, and chlorine were also available as well as a functional isolation center at Masanga Hospital. However, health workers did not adhere to IPC practices and neglected to use even basic IPC supplies. Moreover, there was a shortage of nurses and cleaners on duty at the time of the assessment. While IPC tools were available for monitoring IPC compliance at point of care, these were not being used.
The IPC structures at district and facility levels were also not functioning. Remedial measures included thorough cleaning and disinfection of the operating theater in Masanga Hospital and the female wards. Capacity building of health workers on IPC in Masanga Hospital and other health facilities in Tonkolili District was conducted. Burial teams, used during the Ebola Outbreak (2014–2016), were reconstituted and received refresher training. However, none of the deceased cases was accorded a safe burial as cases had already been buried by the time Lassa fever was confirmed.
Risk Communication and Social Mobilization
Five days after notification of the outbreak, the MOHS conducted a press briefing on the outbreak response. This provided official, high-level communication on the outbreak while also serving as an opportunity to provide risk communication to the public. Similarly, the district medical officer conducted a media briefing, and social mobilization teams followed up with discussions through the radio, in schools, market places, and health facilities. Radio messages in 6 local languages were aired in local radio stations and information booths were set up in the affected communities to sensitize the general public on recognition of Lassa fever symptoms and appropriate responses.
Contact tracing teams faced resistance from the community and relatives of the deceased at the beginning of the outbreak response and cooperation only improved through the involvement of community health workers and local leaders. Although attempts were made to ensure that all contacts, including health care workers in Masanga Hospital, underwent psychosocial counselling; only 2 nurses trained in counselling were available at the time. Therefore, it is likely that these counselling services were inadequate.
DISCUSSION
Following IHR notification of a Lassa fever case in The Netherlands on November 20, 2021, which originated in Sierra Leone, a cluster of 5 Lassa fever cases were reported in Tonkolili district from October 30–November 16, 2021. The likely source of the outbreak was exposure to blood and body fluids of the probable index case during an obstetric surgical operation. It is likely that a breech in IPC protocols, including failure to use appropriate personal protective equipment and incomplete decontamination of surgical theater and instruments, resulted in the subsequent transmission to 4 additional cases. This hypothesis is supported by the occurrence of all secondary cases within 1 incubation period of the surgical operation and the lack of other cases among contacts of the index case. Subsequent cleaning and disinfection of the theater and hospital wards, as well as refresher training on IPC among health workers contributed to controlling the spread of the outbreak.
Hospital associated outbreaks of Lassa fever, without recognized outbreaks in the community, have been reported in West Africa (Bowen et al., 1975) (Macher and Wolfe, 2006) (Nasidi et al., 1995). It is likely that subclinical illness in the majority of individuals infected with LASV may mask ongoing community transmission, as only severe cases seek treatment at health facilities. Poor IPC practices, including reuse of injection cannulas were common in the 1970s, at the time when Lassa Fever was first recognized (Nasidi et al., 1995). Globally, advances in clinical practice have resulted in improved compliance to IPC protocols; although, this is often not translated to low-resource settings with weak health systems.
Recognizing the role of poor IPC in the high incidence of HAI during the West Africa Ebola Virus Disease Outbreak (2014–2016), there has been extensive investment in IPC programs across the 3 most affected countries (Kilmarx et al., 2014 Pathmanathan et al., 2014;). In Sierra Leone, this involved considerable investments in IPC both during the Ebola outbreak and in the subsequent years, including establishment of a national IPC coordinating unit within the MOHS (Ershova et al., 2018), provision of IPC supplies, and capacity building of healthcare workers. Despite this, IPC compliance among health care workers’ remains suboptimal in Sierra Leone, as demonstrated by this Lassa fever outbreak.
Circulation of pathogens such as Marburg, Ebola, and LASVs in animal populations in Sierra Leone and neighboring countries (Bonwitt et al., 2016 Fichet-Calvet and Rogers, 2009; Goldstein et al., 2018; Mylne et al., 2015;) present the threat of zoonotic disease spilling over to human populations. Health workers in Sierra Leone should be trained and educated on these threats to ensure that they can both identify cases at health care facilities and also take the necessary measures to protect themselves.
Despite Lassa fever being endemic in parts of Sierra Leone, clinicians attending to the index case, including the 2 Dutch doctors, did not suspect Lassa fever. Moreover, the cluster of illness that followed also went undetected until the diagnosis of the index case in The Netherlands and notification through IHR mechanisms. This points to a low index of suspicion for Lassa fever among clinicians and low surveillance capacity. In Sierra Leone, Lassa fever is often a differential diagnosis and is usually only suspected in patients who do not respond to treatment for common tropical diseases, such as malaria and typhoid. Pregnancy may have biased the clinicians towards an obstetric diagnosis of antepartum or postpartum hemorrhage because it is often difficult to distinguish hemorrhagic phenomenon due to viral causes from that resulting from obstetric complications (19). Lassa Fever is known to cause more severe disease in individuals with underlying medical conditions, especially pregnant women (Branco et al., 2011 Jamieson et al., 2006; Price et al., 1988; White et al., 2002;); and this was consistent with the clinical presentation of the index case.
Historically, Lassa fever is not common in Tonkolili District, with the last confirmed cases reported in 2016. Notably, the detection of this outbreak at Masanga Hospital suggests an ongoing presence of LASV in the rodent population with potential for spill-over to humans in the community. Caution is needed when interpreting the low burden of LASV in Tonkolili, as challenges in case detection and diagnosis of LASV, as demonstrated by this event, may result in low number of samples tested and inability to confirm outbreaks.
Due to the prompt activation of pre-existing emergency preparedness and response coordination structures, which ensured smooth coordination of the outbreak response at national and district level, this outbreak had only 5 cases in total. Significant investment has been made in building IHR core capacities in Sierra Leone since the Ebola outbreak ended in 2016. The country has, therefore, made great milestones in strengthening IHR capacities, which have also been instrumental in the COVID-19 pandemic response.
CONCLUSION
This outbreak highlighted many of the challenges faced by low- and middle-income countries when responding to infectious disease threats, including delayed detection, weak diagnostics capacity, poor IPC compliance, and also pertinent issues during the current COVID-19 pandemic. In addition, it underscores the importance of IHR notification in limiting the spread of infectious disease threats across countries and regions in an increasingly interconnected world; this is also underscored by the COVID-19 pandemic. We recommend regular sensitization of clinicians on disease surveillance and adherence of health care workers to IPC protocols as well as periodic, independent audits of IPC compliance. Diagnostic capacity for Lassa fever in Sierra Leone needs to be expanded by increasing the number of laboratories capable of diagnosing LASV and ensuring that a functional sample referral system is in place. Development of rapid diagnostic tests should be accelerated to improve access to screening even in remote health facilities. The contribution of Lassa fever to maternal mortality, which remains persistently high in Sierra Leone, should be investigated through national laboratory-based surveillance.
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Acknowledgments
Author Contributions
All authors listed in this report made substantial contributions in the planning, design, and implementation of the work described herein. CN wrote the first draft of the manuscript, and all co-authors made critical reviews. All listed co-authors approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Authors’ note
The findings and conclusions in this paper are the authors’ and do not represent the official position of the Sierra Leone Ministry of Health and Sanitation nor the World Health Organization (WHO)
Funding Source
The World Health Organization provided financial support to the Ministry of Health and Sanitation for the Lassa Fever outbreak investigations and response that provided data for this report.
Ethical Approval and Consent for Publication
Consent to publish this work was obtained from the Ministry of Health and Sanitation, Sierra Leone. Ethical approval was not sought or obtained from an ethical review board, as it is not required for routine outbreak response activities undertaken by the Ministry of Health and Sanitation. Patient consent for publication was not required as no personal-level data have been included.
Acknowledgments
We sincerely thank the staff of the Ministry of Health and Sanitation, the Tonkolili District Health Management Team, African Field Epidemiology Network, Masanga Hospital, and all others who took part in the outbreak investigation and response.
REFERENCES
- Bonwitt J, Kelly AH, Ansumana R, Agbla S, Sahr F, Saez AM, et al. Rat-atouille: A Mixed Method Study to Characterize Rodent Hunting and Consumption in the Context of Lassa Fever. Ecohealth. 2016;13:234–247. doi: 10.1007/s10393-016-1098-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen GS, Tomori O, Wulff H, Casals J, Noonan A, Downs WG. Lassa fever in Onitsha, East Central State, Nigeria, in 1974. Bull World Health Organ. 1975;52:599–604. [PMC free article] [PubMed] [Google Scholar]
- Branco LM, Boisen ML, Andersen KG, Grove JN, Moses LM, Muncy IJ, et al. Erratum: Lassa hemorrhagic fever in a late term pregnancy from northern Sierra Leone with a positive maternal outcome: Case report (Virology Journal (2011) 8 (480) DOI:10.1186/1743-422X-8-480) Virol J. 2011;8:1–14. doi: 10.1186/1743-422X-8-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ershova K, Savin I, Kurdyumova N, Wong D, Danilov G, Shifrin M, et al. Implementing an infection control and prevention program decreases the incidence of healthcare-associated infections and antibiotic resistance in a Russian neuro-ICU. Antimicrob Resist Infect Control. 2018;7:1–11. doi: 10.1186/s13756-018-0383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichet-Calvet E, Rogers DJ. Risk maps of lassa fever in West Africa. PLoS Negl Trop Dis. 2009;3 doi: 10.1371/journal.pntd.0000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame JD, Baldwin JM, Gocke DJ, Troup JM. Lassa fever, a new virus disease of man from West Africa. I. Clinical description and pathological findings. Am J Trop Med Hyg. 1970;19:670–676. doi: 10.4269/ajtmh.1970.19.670. [DOI] [PubMed] [Google Scholar]
- Goldstein T, Anthony SJ, Gbakima A, Bird BH, Bangura J, Tremeau-Bravard A, et al. The discovery of Bombali virus adds further support for bats as hosts of ebolaviruses. Nat Microbiol. 2018;3:1084–1089. doi: 10.1038/s41564-018-0227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallam HJ, Hallam S, Rodriguez SE, Barrett ADT, Beasley DWC, Chua A, et al. Baseline mapping of Lassa fever virology, epidemiology and vaccine research and development review-article. Npj Vaccines. 2018;3 doi: 10.1038/s41541-018-0049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson DJ, Theiler RN, Rasmussen SA. Emerging infections and pregnancy. Emerg Infect Dis. 2006;12:1638–1643. doi: 10.3201/eid1211.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane E, Gilles HM. Lassa fever in Panguma Hospital, Sierra Leone, 1973–6. Br Med J. 1977;1:1399–1402. doi: 10.1136/bmj.1.6073.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmarx PH, Clarke KR, Dietz PM, Hamel MJ, Husain F, McFadden JD, et al. Ebola virus disease in health care workers — Sierra Leone, 2014. Morb Mortal Wkly Rep. 2014;63:1167–1171. [PMC free article] [PubMed] [Google Scholar]
- Kofman A, Choi MJ, Rollin PE. Lassa fever in travelers from West Africa, 1969–2016. Emerg Infect Dis. 2019;25:236–239. doi: 10.3201/eid2502.180836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macher AM, Wolfe MS. Historical Lassa fever reports and 30-year clinical update. Emerg Infect Dis. 2006;12:835–837. doi: 10.3201/eid1205.050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylne AQN, Pigott DM, Longbottom J, Shearer F, Duda KA, Messina JP, et al. Mapping the zoonotic niche of Lassa fever in Africa. Trans R Soc Trop Med Hyg. 2015;109:483–492. doi: 10.1093/trstmh/trv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasidi A, Fakile Y, Hutwagner L, Mccormick JB. Review ofcases of nosocomial Lassa fever in Nigeria : the high price ofpoor medical practice. 1995;311:1993–1995. doi: 10.1136/bmj.311.7009.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njuguna C, Jambai A, Chimbaru A, Nordstrom A, Conteh R, Latt A, et al. Revitalization of integrated disease surveillance and response in Sierra Leone post Ebola virus disease outbreak. BMC Public Health. 2019;19:1–11. doi: 10.1186/s12889-019-6636-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbosch F, de Boer M, Veldkamp KE, Ellerbroek P, Bleeker-Rovers CP, Goorhuis B, et al. Public health response to two imported, epidemiologically related cases of Lassa fever in the Netherlands (ex Sierra Leone), November 2019. Eurosurveillance. 2020;25:6–10. doi: 10.2807/1560-7917.ES.2020.25.15.2000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathmanathan I, O'connor KA, Adams ML, Rao CY, Kilmarx PH, Park BJ, et al. Rapid assessment of Ebola infection prevention and control needs — Six districts, Sierra Leone, October 2014. Morb Mortal Wkly Rep. 2014;63:1172–1174. [PMC free article] [PubMed] [Google Scholar]
- Price ME, Fisher-hoch SP, Craven RB, Mccormick JB. A prospective study of maternal and fetal outcome in acute Lassa fever infection during pregnancy. Br Med J. 1988;297:3–6. doi: 10.1136/bmj.297.6648.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer JG, Grant DS, Schieffelin JS, Boisen ML, Goba A, Hartnett JN, et al. Lassa Fever in Post-Conflict Sierra Leone. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0002748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogoba N, Feldmann H, Safronetz D. Lassa Fever in West Africa: Evidence for an Expanded Region of Endemicity. Zoonoses Public Health. 2012;59:43–47. doi: 10.1111/j.1863-2378.2012.01469.x. [DOI] [PubMed] [Google Scholar]
- White SR, Henretig FM, Dukes RG. Medical management of vulnerable populations and co-morbid conditions of victims of bioterrorism. Emerg Med Clin North Am. 2002;20:365–392. doi: 10.1016/S0733-8627(01)00006-2. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Emergency Response Framework, Second Edition. 2017.
- World Health Organization. Technical Guidelines for Integrated Disease Surveillance and Response in the African Region 2010:1–416.