Abstract
Besides direct bactericidal activity, long-term effectiveness is one of the most important features to consider when developing new drugs for chemotherapy. In this study, we evaluated the ability of rifapentine (RFP), in monotherapy and combination therapy, to completely eradicate a Mycobacterium tuberculosis infection and to prevent relapse posttreatment in a Swiss mouse model. The combination of RFP, isoniazid (INH), and pyrazinamide (PZA) administered daily resulted in an apparent clearance of M. tuberculosis organisms in the lungs and spleens of infected mice after 10 weeks of treatment. However, 3 months after the cessation of therapy, bacterial regrowth occurred in mice treated for a 12-week period, indicating a relapse of infection. In intermittent treatment regimens of RFP in combination with INH and PZA, sterilization was achieved when mice were treated two to five times per week for 9 weeks. Bacterial growth was still observed in the once-weekly treatment group. Our results show that mouse models can predict important parameters for new drugs. We stress the necessity for long-term posttreatment observation in animal models for the routine evaluation of new drugs for antituberculosis chemotherapy.
Presently, one-third of the world’s population is infected with Mycobacterium tuberculosis (23). Therapy for tuberculosis is arduous due to its long duration and multidrug regimens. The current standard regimen of isoniazid (INH), rifampin (RIF), and pyrazinamide (PZA) requires 6 to 9 months of daily treatment. Therapy is now further complicated by the emergence of drug-resistant strains. These phenomena are responsible for the increasing demand for the development of new compounds to treat tuberculosis. One of the factors contributing to drug resistance is poor compliance by patients. Approaches to improve patient compliance include shortening the therapy period or instituting an intermittent treatment regimen, e.g., once- or twice-weekly therapy. With the discovery of the newer rifamycins KRM-1648 (KRM) and rifapentine (RFP), the potential for shortening existing treatment regimens and using intermittent drug regimens was created. These newer rifamycins not only have greater efficacies than RIF but also have longer half-lives (9).
Another problem with antituberculosis therapy is the transition of M. tuberculosis into a dormant or latent state. In a primary infection, the clinical course ranges from benign self-limited disease to progressive dissemination (20). It is thought that during acute primary tuberculosis, when the patient remains untreated, foci harboring latent M. tuberculosis organisms are established in the host (19). The recrudescence of these quiescent foci months to years later can cause active disease. The majority of active tuberculosis cases nowadays arise as a result of the reactivation of latent organisms which survive in the host rather than reinfection (15). On the other hand, the treatment of active tuberculosis with the presently used combination drug regimens will reduce the bacillary burden by a substantial amount. However, a proportion of the tubercle bacilli originally present may shift into dormancy (7). M. tuberculosis can be quiescent in the host for months or years without producing overt disease, and then it can revive and initiate the production of lesions and active tuberculosis (19). Antituberculosis therapy should, therefore, aim for a total sterilization of the infection in the patient with highly effective drugs.
In the last decade, several groups extensively compared the efficacy of the newer rifamycins against M. tuberculosis in vitro (5, 17, 18) and in mouse models (1, 2, 4, 8, 10), and they studied the pharmacokinetics of the different rifamycins (9). However, these studies were primarily short-term and were aimed at evaluating the performance of the drugs given as intermittent therapy. In this study, we compared the efficacies of the newer rifamycins in order to obtain a durable cure in our mouse model. Durable cure is defined here as sterilization without a relapse of M. tuberculosis infection during the observation period (3 months) following drug treatment.
Initially, the activities of the different rifamycins, RIF, KRM, and RFP, administered as single-drug treatments in infected mice were compared. In addition, long-term experiments were performed to establish the length of the treatment period required for a durable cure. In these experiments, infected mice were treated with RIF or RFP combined with INH or INH-PZA. Of significance, we demonstrate the importance of studying the abilities of new drugs to establish a long-term cure without a relapse of infection in an animal model.
MATERIALS AND METHODS
Drugs.
RFP was provided by Marion Merrell Dow Pharmaceuticals, Cincinnati, Ohio. KRM was provided by Kaneka Corporation, Osaka, Japan. INH, PZA, RIF, and pyridoxine (PYR) were purchased from Sigma Chemical Co., St. Louis, Mo. KRM, RIF, and RFP were dissolved in dimethyl sulfoxide, with subsequent dilution in distilled water prior to administration. The final concentration of dimethyl sulfoxide in the drug preparations was 0.5%. INH, PYR, and PZA were dissolved in water. Drugs were freshly prepared each morning prior to administration.
Bacterial isolate.
M. tuberculosis ATCC 35801 (strain Erdman) was obtained from the American Type Culture Collection, Manassas, Va. This isolate was used previously in our laboratory for murine model studies (13, 14). MICs were determined in modified 7H10 broth (pH 6.6) (7H10 agar formulation with agar and malachite green omitted) supplemented with 10% Middlebrook oleic acid-albumin-dextrose-catalase (OADC) enrichment (Difco Laboratories, Detroit, Mich.) and 0.05% Tween 80 (3). The MICs of RFP, KRM, RIF, and INH for ATCC 35801 are 0.015, 0.00047, 0.06, and 0.03 μg/ml, respectively.
Medium.
The organism was grown in modified 7H10 broth with 10% OADC enrichment and 0.05% Tween 80 on a rotary shaker at 37°C for 5 to 10 days. The culture suspension was diluted in modified 7H10 broth to yield 100 Klett units per ml (Klett-Summerson colorimeter; Klett Manufacturing, Brooklyn, N.Y.), or approximately 5 × 107 CFU/ml. The inoculum size was verified by plating serial dilutions of the bacterial suspension in triplicate on 7H10 agar plates (BBL Microbiology Systems, Cockeysville, Md.) supplemented with 10% OADC enrichment. Plates were incubated at 37°C in ambient air for 4 weeks prior to the counting of viable M. tuberculosis colonies (CFU).
Infection study.
Five- to seven-week-old outbred female Swiss mice (Charles River, Wilmington, Mass.) were infected intravenously through a caudal vein. Each mouse received approximately 107 viable organisms suspended in 0.2 ml of modified 7H10 broth. Every group consisted of eight mice at each time point unless stated otherwise.
Treatment was started 1 week postinfection. A control group of infected mice was sacrificed at the start of treatment (early control group). A second group of infected but untreated mice was sacrificed 4 weeks after therapy was initiated (late control group). Treatment was given 5 days per week unless stated otherwise. Drugs were administered orally by gavage. Mice receiving a combination treatment were given each drug separately, with the doses administered approximately 4 h apart. In the experiments with INH-PZA, the combination was administered each morning and RIF or RFP was given each afternoon.
Mice were sacrificed by CO2 inhalation. The spleens and right lungs were aseptically removed and ground in a tissue homogenizer. The number of viable organisms was determined as stated above. For long-term treatment experiments, the entire volume of each organ homogenate was plated to determine the number of culturable mycobacteria per organ.
Statistical analysis.
The viable cell counts were converted to logarithms, which were then evaluated by a one- or two-variable analysis of variance. Statistically significant effects from the analyses of variance were further evaluated by Tukey’s honestly significant difference test (11) to make pairwise comparisons among the means.
RESULTS
Comparison of the activities of RIF, KRM, and RFP given as single-drug treatments.
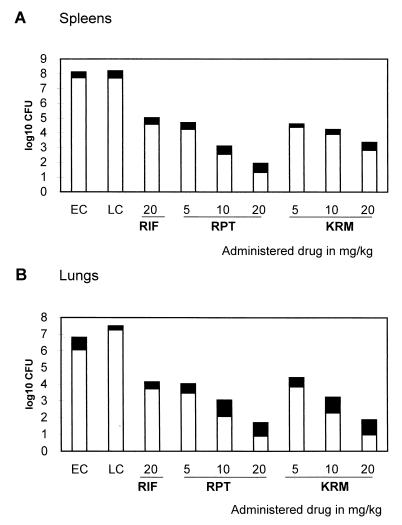
RIF (20 mg/kg of body weight), KRM (5, 10, or 20 mg/kg), or RFP (5, 10, or 20 mg/kg) was administered 5 days per week to female mice which had been infected with 5 × 107 viable mycobacteria. Treatment with each agent for 4 weeks reduced the cell counts in the spleens and lungs significantly compared with those at the initiation of therapy (P < 0.01 for all comparisons) (Fig. 1).
FIG. 1.
Number of viable M. tuberculosis organisms in spleens and lungs of infected mice after once-daily treatment for 5 days per week for 4 weeks with different doses of RIF, RPT (rifapentine), or KRM given as monotherapy. Infected untreated mice from control groups were sacrificed 1 week (early controls [EC]) and 4 weeks (late controls [LC]) after infection. Results are means (open bars) ± standard deviations (solid bars).
Treatment with 10 or 20 mg of RFP or KRM per kg decreased the cell counts significantly more (P < 0.01) than did the 20-mg/kg RIF treatment. The differences in the cell counts between the groups receiving RFP and the ones receiving KRM were significant for the spleens (P < 0.01), but not for the lungs (P > 0.05).
Combination therapy with RIF and INH.
Mice infected with 2 × 107 M. tuberculosis organisms were treated daily with INH (25 mg/kg) and RIF (20 mg/kg) for a maximum of 24 weeks, after which the therapy was discontinued, and the mice were observed during the following 12 weeks for a relapse of infection.
Treatment for 4 weeks with INH or RIF alone or with the INH-RIF combination reduced the cell counts in the spleens and lungs significantly compared with those at the initiation of therapy in the control mice (P < 0.01 for all comparisons), but no significant benefit was observed for INH-RIF versus the drugs given as single agents at that time point (P > 0.05) (Table 1).
TABLE 1.
Number of viable M. tuberculosis organisms in spleens and lungs of infected, INH-RIF-treated micea
| Therapy period (wk) | Log CFU in spleens
|
No. positive (n = 6) | Log CFU in lungs
|
No. positive (n = 6) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | INH | RIF | INH-RIF | Control | INH | RIF | INH-RIF | |||
| 0 | 7.62 ± 0.21 | 5.96 ± 0.30 | ||||||||
| 4 | 3.95 ± 0.38 | 3.79 ± 0.71 | 3.19 ± 0.56 | 6 | 3.11 ± 0.30 | 3.35 ± 0.43 | 2.88 ± 0.43 | 6 | ||
| 12 | 0.26 ± 0.42 | 4 | 0.00 ± 0.00 | 0 | ||||||
| 24 | 0.00 ± 0.00 | 0 | 0.00 ± 0.00 | 0 | ||||||
| 12 + Obs. | 2.56 ± 1.48 | 5 | 1.81 ± 1.66 | 4 | ||||||
| 24 + Obs. | 0.17 ± 0.41 | 1 | 0.00 ± 0.00 | 0 | ||||||
Mice were treated 5 days per week with INH (25 mg/kg) and/or RIF (20 mg/kg), after which the therapy was discontinued and the mice were observed for 12 weeks (Obs.) for relapse of infection. Results are means ± standard deviations. n, number of mice per group.
Twelve weeks of treatment with INH-RIF was not sufficient to obtain a total clearance of bacteria in the spleens; the spleens of four mice out of six still showed small numbers of bacteria (one to nine colonies). A noncultivable state was achieved after 24 weeks of treatment. After an observation period of 3 months, regrowth was noted in all treatment groups. Of note, however, the spleen of only one mouse out of six grew 10 colonies after the 24-week treatment regimen.
Twelve weeks of treatment with INH-RIF yielded a total clearance of bacteria in the lungs. After an observation period of 3 months, no regrowth was noted in the group treated for 24 weeks with INH-RIF.
Combination therapy with RIF, INH, and PZA.
Mice infected with 1.4 × 107 viable organisms were treated daily with INH (25 mg/kg) and RIF (20 mg/kg) for 12 weeks, with or without PZA (150 mg/kg) for the first 8 weeks. INH-PZA was administered each morning, and RIF was given each afternoon. After therapy was discontinued, the mice were observed for 12 weeks for a relapse of infection.
The 12-week treatments with both combination therapies reduced the cell counts in the spleens and lungs significantly compared with those at the initiation of treatment (P < 0.01 for all comparisons) and those at 4 weeks in untreated control mice (P < 0.01 for all comparisons) (Table 2). A total clearance of M. tuberculosis from the spleens or lungs was not achieved in either treatment group.
TABLE 2.
Number of viable M. tuberculosis organisms in spleens and lungs of infected, INH-RIF-PZA-treated micea
| Treatment period (wk) | Log CFU in spleens
|
Log CFU in lungs
|
||||
|---|---|---|---|---|---|---|
| Control | INH-RIF (no. positive [n = 7]) | INH-RIF-PZA (no. positive [n = 7]) | Control | INH-RIF (no. positive [n = 7]) | INH-RIF-PZA (no. positive [n = 7]) | |
| 0 | 7.86 ± 0.13 | 7.95 ± 0.39 | ||||
| 4 | 6.42 ± 0.82 | 7.59 ± 1.43 | ||||
| 12 | 0.34 ± 0.41 (7) | 0.52 ± 0.30 (6) | 0.50 ± 0.49 (7) | 0.55 ± 0.44 (6) | ||
| 12 + Obs. | 3.57 ± 0.88 (7) | 3.25 ± 1.30 (7) | 3.79 ± 0.41 (7) | 2.70 ± 0.86 (7) | ||
Mice were treated 5 days per week with INH (25 mg/kg) and RIF (20 mg/kg), with or without PZA (150 mg/kg). After therapy was discontinued, mice were observed for 12 weeks (Obs.) for relapse of infection. Results are means ± standard deviations. n, number of mice per group.
PZA did not significantly improve the bactericidal activity of INH-RIF (P > 0.05), nor did it influence the regrowth of M. tuberculosis after the 12-week observation phase (P > 0.05).
Combination therapy with RFP, INH, and PZA.
Female mice infected with 1.4 × 107 M. tuberculosis organisms were treated daily with INH (25 mg/kg), PZA (150 mg/kg), and RFP (20 mg/kg) for 6, 8, 10, and 12 weeks. After therapy was discontinued, the mice were observed for 12 weeks for a relapse of infection.
Treatment for 6 weeks with RFP-INH-PZA reduced the cell counts in the spleens and lungs significantly compared with those at the initiation of therapy (P < 0.01 for all comparisons) and those at 8 weeks in untreated control mice (P < 0.01 for all comparisons) (Table 3).
TABLE 3.
Number of viable M. tuberculosis organisms in spleens and lungs of infected, INH-PZA-RFP-treated micea
| Therapy period (wk) | Log CFU in spleens
|
Log CFU in lungs
|
||||
|---|---|---|---|---|---|---|
| Control | INH-RFP-PZA
|
Control | INH-RFP-PZA
|
|||
| Therapy (no. positive/no. tested) | Therapy + Obs. (no. positive/no. tested) | Therapy (no. positive/no. tested) | Therapy + Obs. (no. positive/no. tested) | |||
| 0 | 7.56 ± 0.66 | 7.45 ± 0.24 | ||||
| 6 | 0.20 ± 0.26 (5/7) | 3.73 ± 0.59 (8/8) | 0.85 ± 0.82 (5/7) | 3.17 ± 1.33 (8/8) | ||
| 8 | 6.37 ± 0.81 | 0.00 ± 0.00 (0/7) | 2.79 ± 0.42 (8/8) | 8.11 ± 0.78 | 0.11 ± 0.20 (5/7) | 3.10 ± 0.47 (8/8) |
| 10 | 0.00 ± 0.00 (0/7) | 0.90 ± 1.55 (3/8) | 0.00 ± 0.00 (0/8) | 0.83 ± 1.42 (3/8) | ||
| 12 | 0.00 ± 0.00 (0/7) | 0.95 ± 1.63 (3/8) | 0.00 ± 0.00 (0/8) | 0.55 ± 1.07 (3/8) | ||
Mice were treated 5 days per week with INH (25 mg/kg), PZA (150 mg/kg), and RFP (20 mg/kg). After therapy was discontinued, mice were observed for 12 weeks (Obs.) to study the relapse of infection. Results are means ± standard deviations.
After 8 weeks of treatment, no bacterial growth was detected in the spleens, while the lungs of two animals out of eight were totally cleared of bacteria. After 10 weeks of treatment, a noncultivable state was achieved for the lungs.
After an observation period of 3 months, regrowth was noted in all treatment groups. For the 10- and 12-week treatment groups, no bacteria were cultured from the lungs and spleens from five out of eight mice.
Intermittent dosage of RFP.
Mice infected with 6 × 106 M. tuberculosis organisms were treated for 9 weeks either five times per week with INH (25 mg/kg) and RFP (20 mg/kg) or one or two times weekly with INH (75 mg/kg), PYR (10 mg/kg), and RFP (20 mg/kg). The administration of the drugs was done with or without PZA (150 mg/kg).
After 9 weeks of INH-RFP treatment once weekly, with or without PZA, the lungs and spleens of four out of eight mice were cleared of mycobacteria (Table 4). PZA did significantly improve the bactericidal activity of INH-RFP given once weekly (P < 0.05). For all the groups in which INH-RFP was given two or five times per week for 9 weeks, a total clearance of mycobacteria was observed in both the spleen and the lungs.
TABLE 4.
Number of viable M. tuberculosis organisms in spleens and lungs of infected mice treated with RFP intermittentlya
| Treatment period (wk) | Dosage regimen (no. of times/wk) | Log CFU in spleens
|
Log CFU in lungs
|
||||
|---|---|---|---|---|---|---|---|
| Control | INH-RFP (no. positive [n = 8]) | INH-RFP-PZA (no. positive [n = 8]) | Control | INH-RFP (no. positive [n = 8]) | INH-RFP-PZA (no. positive [n = 8]) | ||
| 0 | 7.35 ± 0.25 | 5.89 ± 0.15 | |||||
| 4 | 5.37 ± 0.40 | 6.05 ± 0.28 | |||||
| 9 | 5 | 0.00 ± 0.00 (0) | 0.00 ± 0.00 (0) | 0.00 ± 0.00 (0) | 0.00 ± 0.00 (0) | ||
| 9 | 2 | 0.00 ± 0.00 (0) | 0.00 ± 0.00 (0) | 0.00 ± 0.00 (0) | 0.00 ± 0.00 (0) | ||
| 9 | 1 | 1.70 ± 0.50 (8) | 0.89 ± 0.65 (8) | 0.27 ± 0.29 (4) | 0.08 ± 0.21 (4) | ||
Mice were treated one, two, or five times per week with INH-RFP, with or without PZA (150 mg/kg). Daily doses of INH (25 mg/kg) and RFP (20 mg/kg) were administered, as were intermittent doses of INH (75 mg/kg), PYR (10 mg/kg), and RFP (20 mg/kg). Results are means ± standard deviations. n, number of mice per group.
DISCUSSION
One of the reasons that antituberculosis therapy remains problematic is the transition of M. tuberculosis, in certain situations, into a dormant state. Current antimycobacterial agents are most effective against actively dividing organisms. Dormant bacteria are defined, in the context of this publication, as viable, nonreplicating bacteria which are nonculturable in the laboratory on standard media and have the potential to reactivate upon the cessation of drug treatment (6, 7, 22). Since there are no surrogate markers for the detection of dormant bacteria at this time, we cannot distinguish whether the drug treatment totally cleared the infection in the test subject or partially reduced the bacillary burden in which a proportion of the tubercle bacilli shifted into dormancy. Drug treatment should, therefore, aim at the complete eradication of bacteria in order to avoid an eventual relapse of the infection.
With the discovery of the newer rifamycins KRM and RFP, more efficient drug therapy became possible. Several groups evaluated the newer rifamycins in animal models (1, 2, 4, 8, 10), and our results confirm their observations. Our laboratory was the first to evaluate the ability of the newer rifamycins, KRM previously (12, 13) and RFP in this study, to be used in combination therapy to completely eradicate M. tuberculosis infection and to prevent relapse posttreatment.
We initially compared the activities of RIF, KRM, and RFP as single agents in a 4-week daily treatment regimen. Treatment with RFP or KRM reduced the bacterial burden of the infected mice significantly better (P < 0.01) than treatment with the same dose of RIF. Differences in the cell counts between the groups receiving RFP and KRM were significant only for the spleens (P < 0.01). In our mouse model, RFP seemed to exert a significantly greater bactericidal effect in the spleens than did KRM when each was administered as a single agent in a short-term experiment.
Based on the Cornell model, in which a 12-week multidrug therapy was followed by an observation period of another 12 weeks (16), RFP treatment was compared to RIF treatment in combination with INH and PZA. The treatment regimen of 12 weeks with RIF-INH did not result in a complete eradication of M. tuberculosis organisms, while 24 weeks of treatment yielded a noncultivable state. After an observation period of 3 months, however, a relapse of infection was noted in all treatment groups. The addition of PZA to RIF-INH did not significantly improve these results. The administration of RFP in combination with INH-PZA for 10 weeks resulted in a temporary clearance of mycobacteria in the spleens and lungs. After an observation period of 3 months, regrowth was noted in all treatment groups (6, 8, 10, and 12 weeks), although not all mice receiving the 12-week regimen showed this relapse and the mice which did had only a low number of bacteria in the spleens and lungs. These combination therapy experiments showed the greater ability of RFP than RIF to achieve the sterilization of organs and eventually lead to a durable cure. This promising activity of RFP should allow for a significant shortening of the duration of therapy for tuberculosis.
These newly obtained RFP data were compared with previous results obtained in our laboratory from a similar experiment performed with KRM-INH (12). In the previous study the activity of KRM, either alone or in combination with INH, was compared with the activities of INH, RIF, and RIF-INH in the Swiss mouse model. Although this experiment was not performed in parallel with the present study, drug efficacies were tested in our established Swiss mouse model in both cases and bacterial counts were compared to those in a RIF group as an internal control. A slight benefit of RFP treatment over KRM treatment was seen in the initial phase. In the present study, a significant difference between the groups receiving RFP and those receiving KRM in a 4-week regimen could be observed in the cell counts from the spleens. However, an advantage of KRM became apparent in a later phase of treatment and became even more pronounced during the observation phase. KRM-INH treatment resulted in an apparent sterilization of the organs after 6 weeks of treatment. A durable cure of at least 6 months was achieved after a 12-week KRM-INH treatment period. For RFP-INH-PZA treatment, as described above, an apparent clearance of organisms was achieved after 10 weeks of treatment; however, a 12-week treatment regimen yielded modest regrowth 3 months after the cessation of therapy. In conclusion, the direct bactericidal efficacies of RFP and KRM appeared to be very similar in short-term in vivo experiments. However, KRM-INH had a significantly higher activity than RFP-INH with regard to achieving a durable cure. These data show the importance of long-term experiments in establishing differences between the efficacies of new drug candidates.
RFP, approved by the U.S. Food and Drug Administration in 1998, is the first new antituberculosis drug approved in more than a decade. Because of the long half-life of RFP, it is thought that the administration of RFP daily at 5 to 10 mg/kg for 6 weeks in humans might lead to an accumulation of this agent (discussed in reference 9). For this reason, together with the problem of patient compliance with a daily treatment regimen, RFP was tested primarily in animal models and clinical trials with intermittent dosing regimens. Daily treatment with RFP at 5 mg/kg for 12 weeks is unlikely to improve the compliance of patients treated with daily doses of RIF at 10 mg/kg for the same period (discussed in reference 9). The drug was approved on the basis of a randomized clinical trial conducted by Hoechst Marion Roussel, Kansas City, Mo. The study was divided into two phases based on dosing frequency. In the first part of the trial, half the patients received RFP-INH-PZA-ethambutol for 60 days while the other half received RIF-INH-PZA-ethambutol for the same length of time. During this intensive phase of treatment, all drugs were administered daily except for RFP, which was given twice a week. During the second phase, patients receiving RFP continued to be treated with this compound, combined with INH, once weekly for up to 120 days. Patients receiving RIF continued to receive this drug in combination with INH twice weekly for up to 120 days. Study results showed that 87% of the patients belonging to the RFP-treated group became culture negative, while 81% of the patients from the RIF-treated group became culture negative. During long-term follow-up, 10% of the patients treated with RFP relapsed, compared to only 5% in the RIF-treated group. Even more worrisome are the results of a 60-patient human immunodeficiency virus (HIV)-tuberculosis study conducted by the Centers for Disease Control and Prevention, in which the continuation-phase treatment with once-weekly doses of RFP and INH was compared to treatment with twice-weekly doses of RIF and INH (21). Five of 30 HIV-infected patients randomly selected to take RFP relapsed after the completion of treatment, compared to three who relapsed after taking RIF. After four of these five RFP-treated patients developed resistance to rifamycin class drugs, compared to none of the patients in the RIF group, the enrollment of HIV+ patients in the study was closed (21). The higher relapse rate in the clinical studies with RFP versus standard RIF-based therapy is expected to be compensated for by greater compliance. Nevertheless, these clinical trials again underscore the importance of investigating the ability of drug regimens to achieve the long-term sterilization of M. tuberculosis in the host.
Testing RFP in long-term animal models could have predicted the relapse of infection after drug treatment. In addition, the use of RFP in clinical trials with an intermittent treatment regimen could have been improved with prior extensive testing in animal models. We evaluated RFP in our mouse model with an intermittent dosing regimen in combination with INH or INH-PZA given once, twice, or five times a week. We found remarkable differences in drug efficacy after once- or twice-weekly administration of the drug. After a 9-week period of treatment, the bactericidal activity in mice treated two or five times weekly led to complete sterilization, whereas bacterial growth was still observed after once-weekly treatment. We can infer from these experiments that sterilization of the bacterial burden in the mouse requires a combination treatment with RFP at least twice per week.
New drugs need to be tested extensively in animal models in order to establish important treatment parameters prior to clinical trials. Although the pathophysiology of tuberculosis in mice is different than that in humans, mice remain the best and most economical option for initial chemotherapy evaluation. The reactivation of M. tuberculosis infection can be modeled in a mouse and should be part of the routine evaluation of new compounds. Of significance, the importance of long-term observation after drug treatments in in vivo models must be stressed, based on our data.
ACKNOWLEDGMENTS
We acknowledge the technical assistance provided by M. S. DeStefano.
This study was supported in part by the NCDDG-OI program, cooperative agreement U19-AI40972 with NIAID, and a grant from Marion Merrell Dow Pharmaceuticals.
REFERENCES
- 1.Brooks J V, Orme I M. Evaluation of once-weekly therapy for tuberculosis using isoniazid plus rifamycins in the mouse aerosol infection model. Antimicrob Agents Chemother. 1998;42:3047–3048. doi: 10.1128/aac.42.11.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapuis L, Ji B, Truffot-Pernot C, O’Brien R J, Raviglione M C, Grosset J H. Preventive therapy of tuberculosis with rifapentine in immunocompetent and nude mice. Am J Respir Crit Care Med. 1994;150:1355–1362. doi: 10.1164/ajrccm.150.5.7952564. [DOI] [PubMed] [Google Scholar]
- 3.Cynamon M H. Comparative in vitro activities of MDL 473, rifampin, and ansamycin against Mycobacterium intracellulare. Antimicrob Agents Chemother. 1985;28:440–441. doi: 10.1128/aac.28.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhillon J, Dickinson J M, Guy J A, Ng T K, Mitchison D A. Activity of two long-acting rifamycins, rifapentine and FCE 22807, in experimental murine tuberculosis. Tuber Lung Dis. 1992;73:116–123. doi: 10.1016/0962-8479(92)90066-S. [DOI] [PubMed] [Google Scholar]
- 5.Dhillon J, Mitchison D A. Activity in vitro of rifabutin, FCE 22807, rifapentine, and rifampin against Mycobacterium microti and M. tuberculosis and their penetration into mouse peritoneal macrophages. Am Rev Respir Dis. 1992;145:212–214. doi: 10.1164/ajrccm/145.1.212. [DOI] [PubMed] [Google Scholar]
- 6.Dixon B. Viable but nonculturable. ASM News. 1998;64:372–373. [Google Scholar]
- 7.Gangadharam P R J. Mycobacterial dormancy. Tuber Lung Dis. 1995;76:477–479. doi: 10.1016/0962-8479(95)90521-9. [DOI] [PubMed] [Google Scholar]
- 8.Grosset J, Lounis N, Truffot-Pernot C, O’Brien R J, Raviglione M C, Ji B. Once-weekly rifapentine-containing regimens for treatment of tuberculosis in mice. Am J Respir Crit Care Med. 1998;157:1436–1440. doi: 10.1164/ajrccm.157.5.9709072. [DOI] [PubMed] [Google Scholar]
- 9.Ji B, Truffot-Pernot C, Lacroix C, Raviglione M C, O’Brien R J, Olliaro P, Roscigno G, Grosset J. Effectiveness of rifampin, rifabutin, and rifapentine for preventive therapy of tuberculosis in mice. Am Rev Respir Dis. 1993;148:1541–1546. doi: 10.1164/ajrccm/148.6_Pt_1.1541. [DOI] [PubMed] [Google Scholar]
- 10.Kelly B P, Furney S K, Jessen M T, Orme I M. Low-dose aerosol infection model for testing drugs for efficacy against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1996;40:2809–2812. doi: 10.1128/aac.40.12.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirk R E. Experimental design: procedures for the behavioral sciences. Belmont, Calif: Brooks-Cole Publishing Co.; 1986. [Google Scholar]
- 12.Klemens S P, Cynamon M H. Activity of KRM-1648 in combination with isoniazid against Mycobacterium tuberculosis in a murine model. Antimicrob Agents Chemother. 1996;40:298–301. doi: 10.1128/aac.40.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klemens S P, Grossi M A, Cynamon M H. Activity of KRM-1648, a new benzoxazinorifamycin, against Mycobacterium tuberculosis in a murine model. Antimicrob Agents Chemother. 1994;38:2245–2248. doi: 10.1128/aac.38.10.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klemens S P, Sharpe C A, Rogge M C, Cynamon M H. Activity of levofloxacin in a murine model of tuberculosis. Antimicrob Agents Chemother. 1994;38:1476–1479. doi: 10.1128/aac.38.7.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle. 1991;72:1–6. doi: 10.1016/0041-3879(91)90017-m. [DOI] [PubMed] [Google Scholar]
- 16.McCune R M, Feldmann F M, Lambert H P, McDermott W. Microbial persistence. I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J Exp Med. 1966;123:445–468. doi: 10.1084/jem.123.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moghazeh S L, Pan X, Arain T, Stover C K, Musser J M, Kreiswirth B N. Comparative antimycobacterial activities of rifampin, rifapentine, and KRM-1648 against a collection of rifampin-resistant Mycobacterium tuberculosis isolates with known rpoB mutations. Antimicrob Agents Chemother. 1996;40:2655–2657. doi: 10.1128/aac.40.11.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mor N, Simon B, Mezo N, Heifets L. Comparison of activities of rifapentine and rifampin against Mycobacterium tuberculosis residing in human macrophages. Antimicrob Agents Chemother. 1995;39:2073–2077. doi: 10.1128/aac.39.9.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stead W W. Pathogenesis of a first episode of chronic pulmonary tuberculosis in man: recrudescence of residuals of the primary infection or exogenous reinfection? Am Rev Respir Dis. 1967;95:729–745. doi: 10.1164/arrd.1967.95.5.729. [DOI] [PubMed] [Google Scholar]
- 20.Stead W W, Kerby G R, Schleuter D P, Jordahl C W. The clinical spectrum of primary tuberculosis in adults. Confusion with reinfection in the pathogenesis of chronic tuberculosis. Ann Intern Med. 1968;68:731–745. doi: 10.7326/0003-4819-68-4-731. [DOI] [PubMed] [Google Scholar]
- 21.Vernon A, Khan A, Bozeman L, Wang Y C. Update on US Public Health Service (USPHS) study 22: a trial of once weekly isoniazid (INH) & rifapentine (RFP) in the continuation phase of TB treatment. Am J Respir Crit Care Med. 1998;157:A467. [Google Scholar]
- 22.Wayne L G. Dormancy of Mycobacterium tuberculosis and latency of disease. Eur J Clin Microbiol Infect Dis. 1994;13:908–914. doi: 10.1007/BF02111491. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. Treatment of tuberculosis: guidelines for national programmes, 2nd ed. World Health Organization publication no. TB/97.220. Geneva, Switzerland: World Health Organization; 1997. [Google Scholar]