Abstract
Growing evidence suggests an increasing significance for the extent of gastrointestinal tract (GIT) dysfunction in Parkinson’s disease (PD). Most patients suffer from GIT symptoms, including dysphagia, sialorrhea, bloating, nausea, vomiting, gastroparesis, and constipation during the disease course. The underlying pathomechanisms of this α-synucleinopathy play an important role in disease development and progression, i.e., early accumulation of Lewy pathology in the enteric and central nervous systems is implicated in pharyngeal discoordination, esophageal and gastric motility/peristalsis impairment, chronic pain, altered intestinal permeability and autonomic dysfunction of the colon, with subsequent constipation. Severe complications, including malnutrition, dehydration, insufficient drug effects, aspiration pneumonia, intestinal obstruction, and megacolon, frequently result in hospitalization. Sophisticated diagnostic tools are now available that permit more detailed examination of specific GIT impairment patterns. Furthermore, novel treatment approaches have been evaluated, although high-level evidence trials are often missing. Finally, the burgeoning literature devoted to the GIT microbiome reveals its importance for neurologists. We review current knowledge about GIT pathoanatomy, pathophysiology, diagnosis, and treatment in PD and provide recommendations for management in daily practice.
Subject terms: Parkinson's disease, Neurodegeneration
Introduction
Thirty years ago, gastrointestinal tract (GIT) symptoms in Parkinson’s disease (PD) played a subordinate role in clinical practice of most neurologists and movement disorders specialists, despite the existence of numerous very early clinical reports and neuropathological findings that clearly provided evidence of relevant GIT involvement1–3. Although GIT dysfunction can precede somatomotor symptoms by up to 20 years4–6 and impact negatively on quality of life7,8, clinicians mainly focused on the ‘classical’ motor symptomatology at that time. It was not until the late 1980s and early 1990s that the number of clinical studies and neuropathology publications about the complex interaction of PD and the GIT begin to increase, thereby, also stimulating the interest of clinical neurologists for this topic9–15. After 2000, the quest for explanations regarding the potential role of the GIT and the peripheral nervous system in the pathogenesis of PD gained momentum owing to the work by the Braak group, among others16–19. Thus, one finds in PubMed for the period 1960–1969 eleven (keywords ‘Parkinson’ and ‘dysphagia’) and four (keywords ‘Parkinson’ and ‘constipation’) publications, from 1970 to 1979 twenty (‘Parkinson’ and ‘dysphagia’) and seven (‘Parkinson’ and ‘constipation’) publications, as opposed to more than ten entries per year beginning in 1992 and 2003, and, alone for the year 2020, 109 articles (‘Parkinson’ and ‘dysphagia’) and 93 (‘Parkinson’ and ‘constipation’) (<parkinson dysphagia - Search Results - PubMed (nih.gov) and< parkinson constipation - Search Results - PubMed (nih.gov)).
Today, GIT research is a promising and still growing field of inquiry and continues to provide neurologists and movement disorders specialists with novel and valuable data that can help to better understand the complexity of PD. At this point, we still have a multitude of jigsaw puzzle pieces that must be carefully pieced together. Periodic review articles are intended to serve as the basis for future work5,6,20,21. Here, we provide an overview of the latest knowledge, hypotheses, and debates about the pathology, pathophysiology, diagnostic methods for oropharyngeal and esophageal affection as well as impairment of the lower GIT, including a summary of current treatment strategies from an interdisciplinary standpoint. These might be helpful for neurologists, speech- and language therapists, and other clinicians in their daily work with PD patients and PD-associated GIT dysfunction.
Anatomy: gastrointestinal tract and associated brain areas: central control of gastrointestinal motility
The entire GIT is one of the major gateways for extrinsic influences upon the human body. It is autonomously innervated by the largest part of the peripheral nervous system, the so-called enteric nervous system (ENS), which contains several hundreds of neurons22 and even more glial cells. Both neurons and glial cell populations have a variety similar to that found in the brain. Neurons consist of motoneurons, secretomotor-, or interneurons that express acetylecholine, nitric oxide synthase, catecholamines, GABA, or a broad range of neuropeptides23–26. Glial cells can be found in at least four different morphologies and chemical codings, expressing S100B, the reactive gliosis marker GFAP, PDGFRα, or proteolipid-protein-127,28. Both neurons and glial cells form complex networks that populate in ganglionic and aganglionic plexus the complete gut wall from esophagus to anus and from serosa to mucosal layer.
The ENS varies significantly along the gut axis, analogue to the distinct functional differences between the individual gut segments. Different neuronal subtypes and glial cells form neuronal circuits that allow the autonomous regulation of gastrointestinal motility29. Although the gut works independently, there is a varying influence of the central nervous system (CNS) via several additional extrinsic inputs, of which the vagus nerve is the largest. The vagus nerve is part of the so-called brain-gut-axis that connects the CNS with the GIT. The brain-gut-axis consists of two main routes between the two organs30. One is based on humoral factors, such as cytokines, hormones, or even bacterial metabolites from the gut microbiome, whereas the other is a hard-wired connection: the vagus nerve. The vagus nerve contains up to 50,000 fibers that run in both directions, the afferent ones being the majority with ~90% of all fibers31. While the afferent fibers deliver information from the gut, the efferent fibers provide parasympathetic motor stimuli that originate in two brainstem nuclei, first the dorsal motor nucleus of the vagus and, second, the ambiguus nucleus, which both contribute to gastrointestinal motility. Additionally to this direct input from the brainstem, there are several routes of influence represented, i.e., by sympathetic fibers from prevertebral ganglia that connect the gut with thoracic segments of the spinal cord29.
The CNS influence upon gastrointestinal motility is dependent on the location. While there is a considerable impact on both esophagus and stomach32, the gut becomes more independent in the small and large intestine, where autonomous reflex circuits control smooth muscle activity, local blood flow, or secretion and absorption along the mucosal barrier. Interestingly, recent studies provide evidence that there are neural connections between the vagal nuclei and various areas of the cortex that influence stomach motility33 and, thus, might be affected in PD, as demonstrated in a rat model34. Especially the latter might explain the top-down gastrointestinal symptoms in PD. Based on the dual-hit hypothesis35, there will also be a bottom-up process, possibly initiated by local inflammation and a compromised mucosal barrier, that allows gut content, including lipopolysaccharides, short chain fatty acids (SCFA) or other bacterial metabolites to enter the gut wall. In PD patients, the mucosal barrier is compromised and corresponding markers, such as calprotectin, can be found in the feces36. There is a vast amount of evidence that the microbiome in PD patients is disturbed37, combined with an alteration of SCFAs38. Recent studies demonstrate the existence of neuronal circuits that monitor the microbiome or its metabolites report to the CNS or lead to modification of the innervation39. These findings open up perspectives for using the gut, its intrinsic nervous system, the mucosal barrier, or the microbiome as therapeutic targets.
Pathology: alpha-synucleinopathy in the GIT of incidental Lewy body disease and Parkinson’s disease
Lewy pathology (LP, Lewy bodies, Lewy neurites) in prodromal PD (at autopsy, incidental Lewy body disease, ILBD40–42) and in sporadic PD occurs throughout the human GIT3,9,43–48. Interpretation of ENS histological slides from intestinal biopsies requires caution because immunocytochemical protocols vary considerably, and α-synuclein immunoreactivity must be distinguished from α-synuclein aggregates (LP) and α-synuclein aggregating species49. As staged cases show, LP exists in the olfactory bulb, spinal cord, peripheral autonomic ganglia, submandibular gland, cardiac nerves, and ENS before it appears in the substantia nigra, pars compacta, and before neuronal loss occurs there17,50–54. The aggregated α-synuclein lesions are not transient.
One of the largest studies examined a wide range of organs from 92 autopsied individuals, including 17 PD, 7 ILBD, and 23 controls46. In pure PD, LP was found in the GIT of 64.7% (11/17), peripheral vagal nerve (pN. X) of 73.3%, and sympathetic trunk of 80% of cases. In ILBD, 14.2% (1/7) showed LP in the GIT and 28.57% in the pN. X. 50% of the ILBD group also displayed LP in the sympathetic trunk. A decreasing ENS rostral-caudal immunostaining gradient was seen, corroborating an earlier report9: The upper GIT, i.e., distal esophagus followed by the stomach, tended to display the highest pathological burdens46; both sites are directly controlled by parasympathetic preganglionic fibers of the vagus nerve31,55,56 (see section “Anatomy: gastrointestinal tract and associated brain areas: central control of gastrointestinal motility” above). Although there were no ‘ENS-only’ (i.e., ‘ENS-first’) cases in the cohort, the authors surmised that “the findings of the present study are not incompatible with a GI entry for PD, ILBD and DLB”46. In a subsequent investigation of pN. X tissue, they concluded that “the results [i.e., LP in 10/18 ILBD and 42/44 PD subjects; no LP in 49 controls] support initiation of Lewy-type alpha-synucleinopathy in the brain, with early, in some cases preclinical, subsequent progression to the peripheral nervous system”57. However, a lack of α-synuclein immunopositivity in the true control (as opposed to ILBD) group is not surprising [see also58] and, in any event, in 55.56% (ILBD) and 95.45% (manifest PD) of cases the pN. X was involved.
The 2010 study may have a selection bias because, to assess the relative LP frequency in the periphery, the ultimate choice of regions for further study was reduced to those with “a greater likelihood to have positive staining”46 rather than shared innervation or neuronal circuitries. In addition, for the majority of their cases, the authors examined only a single slide for each ENS subdivision46. Relevant ENS-related autonomic structures, e.g., the lumbar prevertebral celiac ganglion (sympathetic innervation of the esophagus, stomach, duodenum, pancreas, liver) and spinal cord SPS44, but also the appendix vermiformis and superior mesenteric ganglion47,48,59, were not included.
Results from investigations performed to date on large cohorts show, despite divergent findings from smaller studies60,61, that 0% of ILBD cases displayed LP in the ENS in the absence of brain lesions44–46. Of the prodromal PD cases analyzed by Stockholm et al., 44% (17/39) of subsequent PD patients displayed no ENS LP47, and not even all manifest PD cases from autopsy-based studies had ENS involvement44,46,53,59 see also ref. 62. The heterogeneity of findings obtained from such studies is complicated not only by differences in immunocytochemical staining techniques and study design (cohort sampling size and stratification, retrospective vs. longitudinal), but also by dissection protocols. Borghammer & Van Den Berge point out that the human GIT “measures ~8–10 m at post-mortem and has a geometric surface area of at least 7000 cm2. Thus, many hundreds of microscopy slides are required to rule out… gut pathology with any degree of confidence”63. Similarly, pN. X specimens necessarily come from a very limited portion of the entire nerve44,52,57,58,64, and multiple sections from both vagal trunks would be required to ascertain whether LP is present or absent in a given individual.
The argument that autopsy-based studies report only rare cases of LP in the ENS in the absence of brain LP does not eliminate the possibility of an ENS and/or peripheral nervous system origin for PD (ILBD), or of LP spread from the ENS to the brain16,17,35,62. That a potential anatomical pathway from intrinsic ENS neurons to the pN. X exists, receives support from the fact that the myenteric plexus, epithelial enteroendocrine cells, and preganglionic portions of the N. X express normal α-synuclein65,66. Converging lines of evidence also support the idea of propagation via cell-to-cell transsynaptic transmission of misfolded α-synuclein into recipient cells; there, misfolded α-synuclein can recruit native α-synuclein and become a template for development of pathological aggregates67–69. Formalin-fixed tissue from the stomach is capable of limited to robust seeding in ILBD (5/8 cases), PD (10/12 cases), and controls (2/9)70.
Overexpressed human α-synuclein and human α-synuclein lysates in animal models can mediate cellular dysfunction71,72 as well as pathological spreading (seeding) along the pN. X bidirectionally73–75, and anatomical connectivities73,76–82 make both directionalities conceivable in human α-synucleinopathy (Fig. 1). The latest findings in the appendix vermiformis of 46/48 ILBD cases47,48 indicate that additional ‘conduits’ for such pathological α-synuclein transport could exist (Fig. 1).
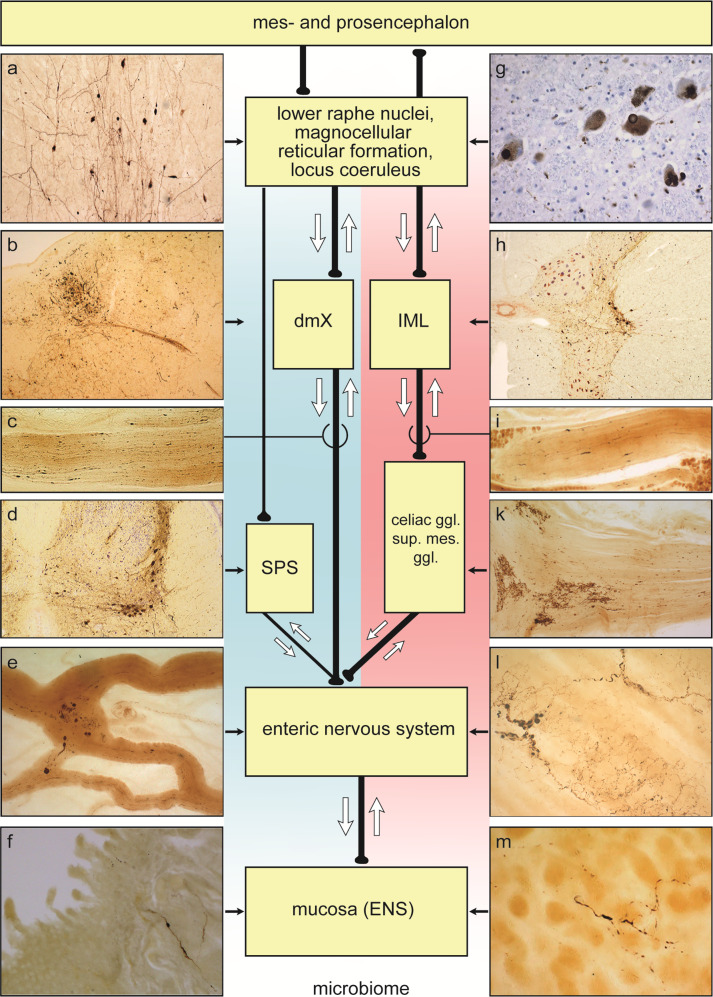
Fig. 1. Diagram showing possible bidirectional (white arrows) parasympathetic (blue background) and sympathetic (pink background) pathways along which pathological α-synuclein propagation in ILBD and PD could occur between the periphery, including the ENS, and the CNS.
Retrograde: parasympathetic (distal esophagus/stomach → pN. X → dorsal motor nucleus of the vagus nerve, dmX); parasympathetic (appendix vermiformis → RIM → pN. X → dmX); parasympathetic (descending colon and further distal → ganglion pelvicum → SPS preganglionic neurons → lower brainstem level-setting nuclei); sympathetic (distal esophagus/stomach → prevertebral celiac ganglion postganglionic neurons → IML preganglionic neurons → lower brainstem level-setting nuclei). Alternatively, anterograde: parasympathetic (dmX → pN. X → distal esophagus/stomach); parasympathetic (lower brainsteim level-setting nuclei → SPS preganglionic neurons → prevertebral postganglionic ganglion pelvicum → descending colon and portions further distal); sympathetic (appendix vermiformis → RIM → prevertebral SMG postganglionic neurons → Nn. splachnici → IML preganglionic neurons → lower brainstem level-setting nuclei).Abbreviations: pN. X peripheral vagus nerve, dmX dorsal motor nucleus of the vagus nerve, IML intermediate mediolateral nucleus, SPS sacral parasympathetic nucleus, RIM root of the small intestine mesentery, sup. mes. ggl. superior mesenteric ganglion. The level-setting nuclei consist of the lower raphe nuclei, magnocellular nucleus of the reticular formation, and locus coeruleus81. The RIM contains parasympathetic and sympathetic fibers innervating the upper GIT extending from the proximal jejunum to the distal ileum, thereby making it another potentially useful structure for neuropathological diagnosis of the existence of LP in the small intestine61,232. Illustrations showing LP in a–m are not to scale: a great raphe nucleus. b dmx and intramedullary N. X. c pN. X at level of the carotid bifurcation. d SPS. e Gastric cardia, Auerbach plexus, tangential section. f Jejunum, Meissner (submucous) plexus, transversal section. g Locus coeruleus. h IML. i Splanchnic nerve at the level of the celiac ganglion. k Celiac ganglion. l Distal esophagus, Auerbach plexus, tangential section. m Gastric cardia, Meissner (submucous) plexus, tangential section. LP within the lamina propria reach the mucosa near gastric glands. Syn-1 immunohistochemistry (BD Biosciences, Eysins, Switzerland) in 100–150 µm sections.
An awareness is gradually emerging that the presence of LP in the GIT and its patterns of progression may well differ between various PD subpopulations and in ILBD48,58,63,83,84. Borghammer & Van Den Berge postulated the existence of a ‘PNS-first Lewy body disorder phenotype’, wherein early pathology in the peripheral autonomic nervous system might spread along retrograde connectivities to nuclei of the lower brainstem that contribute to rapid-eye-sleep (REM) regulation63 (Fig. 1). This intriguing hypothesis, drawing on insights gleaned from idiopathic REM sleep behavioral disorder (RBD) research85–92, could also be tested neuropathologically, provided tissue from RBD patients were still available, in autopsy cohorts staged according to the 2003 PD staging protocol and including early ILBD93–95.
Neurology: upper GIT
Oropharyngeal phase
Prevalence of dysphagia
Oropharyngeal dysphagia is a common and often disabling clinical manifestation in PD. Recent meta-analysis estimates the prevalence of oropharyngeal dysphagia up to 82% during the course of the disease96. However, only 20–40% of patients are aware of their swallowing dysfunction, and only less than 10% report their complaint spontaneously97,98 which might result from early pharyngeal hyposensibility21,99. Recent research has led to the conclusion that oropharyngeal dysphagia is not only a late-stage PD symptom but can occur during any stage of the disease, including the preclinical or prodromal stages96,100,101. Therefore, a comprehensive examination of oropharyngeal swallowing function should be performed regularly, even in early disease stages, when defined clinical predictors (see below) are present102.
Pathophysiology
In contrast to all other parts of the GIT, the oropharynx is not only innervated by the involuntary ENS but is also controlled from voluntarily triggered mechanisms of skeletal muscle movements. Thus, some additional pathophysiological mechanisms play a role that are also relevant for other somatomotor symptoms of PD, such as bradykinesia or tremor21:
Accumulation of Lewy pathology (see section “Pathology: alpha-synucleinopathy in the GIT of incidental Lewy body disease and Parkinson’s disease” above) takes place not only in the substantia nigra but also in various non-dopaminergic swallowing-relevant brainstem and cortical areas.
Putamen and globus pallidus are activated bilaterally during normal swallowing. Therefore, lack of dopamine in the striatum of PD patients may impair this part of the supramedullary swallowing network.
Peripheral mechanisms might be also involved as indicated by α-synuclein deposits in the peripheral sensory and motor nerves of the larynx as well as disease-induced neuromuscular alterations of pharyngeal muscles103–105. Substance P plays an important role in these peripheral oropharyngeal mechanisms.
Substance P (SP)
SP is an ubiquitous neuropeptide in the nervous system, with immunoactive fibers having been detected in the laryngeal nerves, epithelium, and basal membrane of pharyngeal mucosa, especially on the surface of the epiglottis. SP mediates the response to local stimuli in the pharyngeal mucosa and thereby enhances the swallow and cough reflexes106. A reduction of substance P, found in PD patients’ sputum, is postulated to lead to a disturbance of protective reflexes and, ultimately, silent aspiration107. Reduced saliva concentrations of substance P may also be a predictor for the presence of early pharyngeal swallowing dysfunction106. Table 1 provides an overview of PD-related oropharyngeal dysphagia clinical manifestations and postulated pathomechanisms108.
Table 1.
Overview of PD-related oropharyngeal dysphagia clinical manifestations and postulated pathomechanisms.
| Clinical manifestation | Pathomechanisms |
|---|---|
| Prolonged oral transit time: | Dopaminergic + non-dopaminergic (especially Lewy pathology in swallowing cortex?) |
| Premature spillage: | Dopaminergic + non-dopaminergic (Lewy pathology in swallowing cortex?) |
| Delayed swallow reflex: | Dopaminergic + decreased Substance P concentration |
| Prolonged pharyngeal transit time: | Dopaminergic + non-dopaminergic (Lewy pathology in brainstem?) |
| Penetration: | Dopaminergic + non-dopaminergic |
| Aspiration: | Dopaminergic + non-dopaminergic |
| Residue in valleculae: | Primarily dopaminergic |
| Residue in piriform sinus: | Dopaminergic + non-dopaminergic |
| Dysfunction of upper esophageal sphincter: | Primarily non-dopaminergic (Lewy pathology in swallowing centers of medulla oblongata?) |
| Insufficient cough reflex: | Decreased Substance P concentration |
Source108.
Main pathological findings
The main phenotype characteristic of PD-related dysphagia is insufficient pharyngeal bolus clearing, with residues predominant located in the valleculae, in addition to pharyngolaryngeal movement disorders, above all pharyngeal bradykinesia109. Silent aspiration is also a frequent clinical manifestation, even in early stages, but the risk increases with disease duration100. In an analogy to freezing of gait with similar pathophysiologic mechanisms, a recent study described the presence of oropharyngeal freezing resulting in a temporally missing or delayed swallowing reflex110.
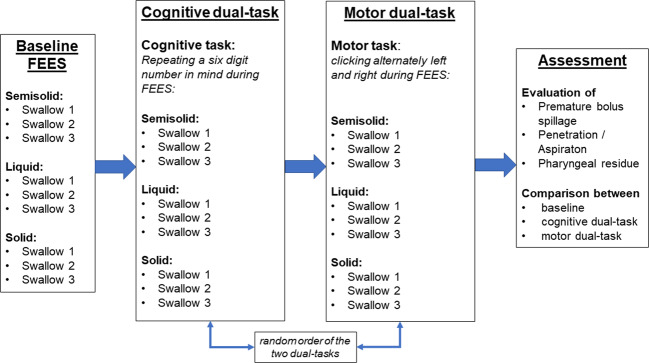
Dual task situations (i.e., cognitive or somatomotor tasks) are challenging to swallowing functional reserve capacities and therefore should be integrated into standard instrumental swallowing evaluations111,112 (Fig. 2). Furthermore, retention of medications in the hypopharynx for long periods of time may account for erratic absorption of levodopa with an insufficient or unpredictable clinical response to oral medication21 (Fig. 3). An association of delayed on-phenomena with pharyngeal residues could have been shown as well113. In a recent study, substantially impaired ability to swallow tablets or capsules was found in 28% (n = 33/118) of patients at all stages of the disease114. Capsules were the easiest to swallow, whereas oval tablets were the most difficult114.
Fig. 2. Dual Task examination algorithm via Flexible Endoscopic Evaluation of Swallowing (FEES) (adapted from ref. 112).
FEES examination protocol including cognitive and motor dual-task for evaluation of swallowing function in PD patients.
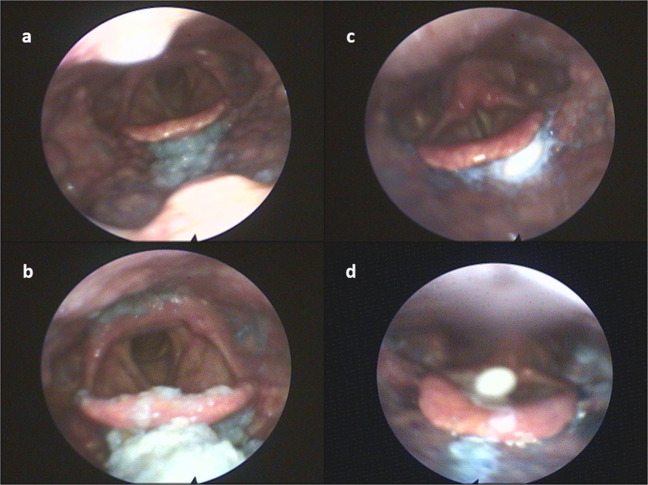
Fig. 3. Examples for pharyngeal residue via FEES.
a Mild residue for solid food located in the valleculae. b Moderate to severe residue for solid food located in the valleculae and piriform sinus with penetration into the laryngeal vestibule. c Tablet residue located in the valleculae. d Tablet penetration.
Clinical predictors
The following clinical conditions have been linked to oropharyngeal dysphagia in PD and can be considered predictors21,115,116:
Hoehn and Yahr stage ≥III
Relevant weight loss
Body Mass Index (BMI) ≤20 kg/m2
Severe drooling or sialorrhea
Dementia
Another major problem for PD patients is drooling117,118. Sialorrhea in PD patients usually does not result from an increased production of saliva but from a reduced spontaneous swallowing rate (48/h vs. 71/h) and/or from oropharyngeal dysphagia with a reduced ability to swallow saliva52. The extent of sialorrhea correlates with the severity of PD-related dysphagia119.
Diagnostic management
Screening-tools
Questionnaires and a specific water swallow test may be used as screening tools:
The Swallowing Disturbance Questionnaire (SDQ) with a sensitivity of 80.5% and a specificity of 81.3% is simple to apply using a score of 15 dysphagia-associated questions to detect PD-related dysphagia120. A score >10 recommends further dysphagia diagnostic (max. score 44.5). Additionally, the patient-rated Radboud Oral Motor Inventory for Parkinson’s disease (ROMP) questionnaire is used for assessment of speech, swallowing, and saliva control121.
The Munich Dysphagia Test—Parkinson’s disease (MDT-PD) with a sensitivity of 82% and a specificity of 71% was designed to detect milder forms of dysphagia without aspiration risk122, although its usefulness as a screening tool for aspiration events is controversial123. A French version is also available124.
The Non-Motor Symptoms Questionnaire (NMS-Quest; Question 3: “difficulty swallowing food or drink or problems with choking”) and the Movement Disorder Society—Unified Parkinson’s disease rating scale (MDS-UPDRS; Question 2.3 of the UPDRS II: “problems swallowing pills or eating meals”) each also include one question about swallowing difficulties125,126. However, in a recent study, NMS and MDS-UPDRS were identified as unreliable tools for detecting previous aspiration127.
Normal water tests that are useful for diagnosing severe dysphagia, e.g., in stroke patients, are not reliable screening tools for PD-related dysphagia when compared with instrumental diagnostic tools115. A detection of a ‘wet voice’ after different bolus consistencies showed a too low sensitivity to be a solid marker of penetration/aspiration in PD128. Therefore, a modified water test was developed to evaluate the stimulability of drinking by using a maximum performance test (maximum swallowing volume <20 mL, maximum swallowing speed <10 mL/s)129. Nonetheless, in a more recent study, swallowing speed was found to be prone to methodological errors and not unsuitable as a screening instrument to predict aspiration in PD patients130.
Instrumental diagnostic tools
Flexible Endoscopic Evaluation of Swallowing (FEES) and Videofluoroscopic Swallowing Study are both considered to be the gold standard for evaluating oropharyngeal dysphagia131,132. These instrumental tools should be applied in cases of unclear and severe PD-associated dysphagia, especially to detect silent aspiration as well as specific dysphagia phenotypes129,133.
Therapeutic management
Over the years, a number of studies have provided evidence-based recommendations for treatment of oropharyngeal dysphagia21,101,134,135. Swallowing therapy, especially as performed by speech and language therapists, and other specific therapeutic options might help to improve oropharyngeal swallowing impairment:
Pharmacotherapy
The effects of dopaminergic medication and levodopa on swallowing function and its role in dysphagia treatment are controversially discussed136–138. Oropharyngeal swallowing parameters with good levodopa-responsiveness could be pharyngeal residue (especially in the valleculae), penetration, and oral as well as pharyngeal transit times139,140. Furthermore, some studies indicate positive effects of the dopamine agonists apomorphine and transdermal rotigotine141–143. As such, an examination whether an improvement in swallowing function could be achieved by increasing or optimizing dopaminergic medication should be performed on a case-by-case basis, i.e., by using the FEES-Levodopa-Test. In this test, three salient parameters (premature spillage, penetration/aspiration events, and residues, each tested with liquid, semisolid, and solid food consistencies) are assessed in off- and on-stage conditions performing a specific score140. A score improvement of >30% indicates levodopa responsiveness of dysphagia140. Subsequently, in such cases, optimizing dopaminergic medication should be considered. Furthermore, levodopa-carbidopa intestinal gel (LCIG) infusion therapy might be capable of alleviating pharyngeal bradykinesia and premature bolus spillage144.
Deep brain stimulation (DBS)
To date, detailed information about the effects of DBS on swallowing function in PD patients remain limited145,146. Using stimulation of the subthalamicus nucleus (STN), low-frequency stimulations (i.e., 60 Hz) may have a beneficial effect on swallowing dysfunction in patients with freezing of gait145,147, whereas high-frequency stimulation might result in beneficial, no, or detrimental effects145. A short-term improvement (lower aspiration rate) was indicated as well but without confirmation in the long-term observation148. Simultaneous STN and substantia nigra (SNr) stimulation seem to have no additional beneficial effect on dysphagia compared with conventional STN stimulation, but swallowing function does not deteriorate as a result149.
Neuromuscular electrical stimulation (NMES)
The use of NMES in PD patients seems to have no measurable benefit150,151. A recent study using new electrode placement methods indicates increased hyoid bone movement and reduced aspiration risks152, but NMES cannot be recommended for PD dysphagia treatment at the present time.
Behavioral swallowing therapy
Because of heterogenous study populations and therapeutic methods as well as different outcome measures, general recommendations for non-pharmacological treatment are difficult to provide135. However, some therapeutic strategies are promising for individual treatment of specific patterns of PD-related dysphagia: Thickened liquids and the chin-tuck maneuver might help to prevent liquid aspiration153,154. The Lee Silverman Voice Treatment (LVST®), originally developed for treatment of PD-associated dysarthria, can also improve swallowing function, although controlled clinical trials are not yet available and the effects are unspecific155. With regard to therapeutic strategies, dual task situations should be avoided in real-life circumstances to focus attention on swallowing performance112. Two larger, randomized placebo-controlled studies showed a positive effect on swallowing safety and efficiency in PD patients who had performed a 4-week- expiratory muscle strength training regimen156,157. Video-assisted swallowing therapy and specific swallowing skill training using surface electromyography might also be helpful for providing biofeedback to patients158,159. In general, every affected PD patient should receive a detailed examination of swallowing disturbance patterns resulting in an individual training program based on available therapeutic methods. The efficacy of the method(s) selected should be confirmed via instrumental testing133.
Treatment of sialorrhea
Parkinson-related sialorrhea can be managed effectively with injections of botulinum toxin A or B into the parotid and submandibular glands160. Another pharmacological treatment option might be the application of the anticholinergic drug glycopyrrolate because it crosses the blood-brain barrier and therefore does not have central anticholinergic side-effects161. In addition, gum chewing also helps to improve PD-related sialorrhea in the short term but without maintaining a long-term effect162.
Esophagogastral phase
Prevalence and main clinical findings
The prevalence of impaired gastric emptying in PD ranges from 70 to 100% and may be present in both early and advanced stages6,163. Major clinical manifestations include nausea, vomiting, early satiety, and postprandial fullness, and these can lead to weight loss, malnutrition, and dehydration164. Furthermore, there is growing evidence for a significant relationship between delayed gastric emptying and levodopa pharmacokinetics leading to drug-response fluctuations with delayed or missed on-phases after medication intake165.
Esophageal motility disorders appear to occur very early and even in premotor stages of PD166,167. A hypotensive peristalsis of the tubular esophagus occurs most frequently and early in the disease course, whereas in later stages diffuse esophageal spasms and multiple contractions may develop167. However, primary opening disorders of the upper esophageal sphincter are rare167.
Diagnostic management
Impaired gastric emptying is defined as >60% retention at 2 h postprandially and/or >10% retention at 4 h after ingestion of a radioactive technetium Tc 99m-labeled solid food168. Other quantitative methods are the use of breath tests with nonradioactive 13C-sodium octanoate bound into a solid meal, or real time visualization by magnetic resonance imaging and electrogastrography164. Because clinical evaluation is difficult, diagnostic examination of esophageal motility disorders nowadays is normally performed by using High Resolution Manometry to detect esophageal alterations169.
Therapeutic management
Therapeutic options for managing PD-associated esophageal motility disorders are rare to date. A pilot study indicates a possible usefulness of botulinum toxin injections for treatment of esophageal spasms, but more evidence is needed170. STN stimulation might also improve esophageal motility171. The use of capsaicin seems to be capable of improving esophageal motility as well as upper esophageal sphincter contraction, and it might be a promising tool for further treatment172,173. In gastroparesis, the increase of levodopa dosage may impair delayed gastric emptying174. Pharmacotherapy options using domperidone might be useful but are said to increase the risk of a long QT syndrome. Recent studies have indicated possible positive effects by using nitzatidine or ghrelin agonists but these require further evaluation6. Benefits from botulinum toxin injection in the pyloric sphincter and possible use of STN-DBS have been reported as well175,176. However, LCIG (with or without entacapone application), subcutaneous apomorphin, and the rotigotine patch are helpful solutions for bypassing the GIT and therefore could be administered in cases of clinically relevant effects of esophageal spasms as well as gastroparesis on somatomotor symptoms.
Summary/Practical algorithm for management
Disturbances of the upper GIT in PD, especially oropharyngeal dysphagia, are complex syndromes that occur early in disease duration but often remain unnoticed until severe complications, such as aspiration pneumonia, develop. Accordingly, standardized and early diagnostic approaches as well as focused treatment of specific dysphagia patterns are required to help affected individuals. Table 2 provides a summary of the most relevant clinical manifestations of upper GIT impairment and feasible treatment approaches.
Table 2.
Summary of most relevant clinical manifestations of upper GIT impairment and feasible treatment approaches.
| Symptom | Pharmacotherapy | Swallowing therapy by Speech language therapists |
|---|---|---|
| Oropharyngeal freezing: |
Increase dose of L-dopa before meal times Amantadine? |
Triggering of swallowing reflex External triggers? |
| Premature spillage: |
Oral bolus control Avoid dual tasks |
|
| Penetration/Aspiration: | Non-oral delivery: patch or pump? |
Protective reflexes Sensory stimulation Supraglottic swallow maneuver Safe food consistencies? PEG? |
| Pharyngeal residues without motor fluctuations: | Individual assessment of L-dopa responsiveness, if positive: Increase dose of L-dopa before meals | Effortful swallow exercise |
| Pharyngeal residues without motor fluctuations: |
Individual assessment of L-dopa responsiveness, if positive: Optimize oral treatment Non-oral delivery: patch or pump? |
Meal times during on state condition Effortful swallow exercise in off state condition |
| Esophageal spasms: |
Non-oral delivery: patch or pump? Botulinum toxin injections into upper esophageal sphincter? |
Protective reflexes Mendelsohn swallow exercise Safe food consistencies? PEG? |
Neurology: lower GIT
Colon
Prevalence of constipation
Since initially being mentioned by James Parkinson, constipation has been considered a very frequent symptom that occurs in up to 80% of PD patients6,14,177–181. As is often the case, constipation is described as the most frequent autonomic symptom14,182,183. Notably, healthy people with constipation complaints, including delayed passage of stools, hard stools, or a sensation of incomplete evacuation, have shown a greater risk for subsequently developing PD184–186. This fits well the neuropathological studies published by Braak and coworkers16,17 (see chapter 3). Constipation is currently considered one of the most relevant early signs of PD, and its frequency seems to be higher than the subjective complaints19,184,185,187–189. The GIT may even play an important role in PD pathogenesis16,80,185 and as a prognostic factor, inasmuch as a significant relationship between constipation severity and progression to dementia has recently been demonstrated190,191. Additional studies are needed to determine whether a similar relationship exists in patients who develop dementia with Lewy bodies (DLB).
Pathophysiology
Medications, reduced physical movement, a reduced muscle tone in the diaphragm and abdominal musculature, and reduced intake of fibers and liquids have been advanced as causes for constipation14. Beginning with the earliest studies and onwards, anticholinergic agents have been particularly related to severe constipating effects, including even the development of a megacolon. Constipation in PD is definitely disease-related189. It was described long before any specific therapy had been found14,177, and many studies of yet untreated patients were able to demonstrate delayed transit6,14. It is much more probable that, in PD patients, a delayed transit plays an intrinsic and prominent role, and that constipation even can be exacerbated by the medical treatment itself14,192.
The causes underlying the delayed transit are most probably degenerative changes involving Lewy pathology located centrally, including the spinal cord intermediolateral nucleus, and peripherally extending from the upper esophagus to the rectum in the Auerbach plexus (myenteric plexus) and Meissner plexus (submucous plexus)3,43,47,53. Additionally, anismus, a failure of relaxation, or involuntary contractions of the anal sphincters during defecation (extremely rare!), can lead to so-called “outlet” constipation14.
Diagnostic management
Because constipation can develop into a megacolon, pseudo-obstruction, or volvulus, adequate diagnosis is essential14. Unfortunately, a megacolon usually remains asymptomatic, with the exception of the singular symptom of constipation, although an ileus followed by surgery14 and colon perforation have been described as megalcolon consequences. Surprisingly, despite the existence of severe constipation at the time of presentation, patients seldom report this problem spontaneously, often because of embarrassment, which indicates that it is most probably underdiagnosed189.
A simple method during the diagnostic work-up involves administering radiopaque markers (ROM) (Fig. 4) as the gold standard diagnostic test14: Abdominal X-rays are taken at defined time intervals to identify the retained numbers of ingested ROMs to calculate colonic transit time. Alternatively, a gamma camera can be used to track the movements of radioisotope test meals or capsules at specified time points for quantitative evaluation of scintigraphic colonic transit times193. For orientation, it is also useful to ask patients to eat poppy seed cake and then note when the poppy seeds are excreted.
Fig. 4. Colonic transit time in a 72-year-old male PD patient.
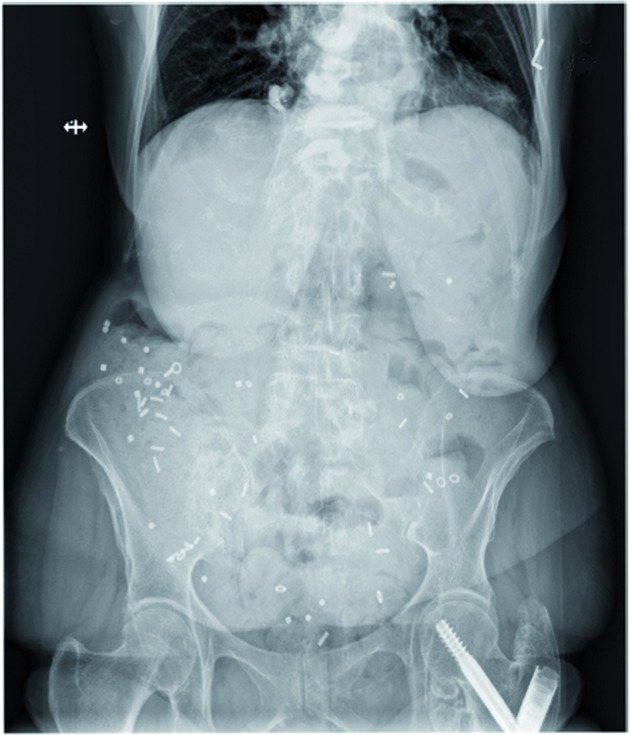
White spots in the entire colon are radiopaque markers (erect, anterior-posterior).
Although there is no gold standard method for the assessment of outlet constipation, defecography is widely preferred. It involves the instillation of barium in the rectum, and subjects are then asked to empty it during recording of a cinematic film194. Anorectal dysfunction can also be assessed by external anal sphincter electromyography, a balloon distension and expulsion test, and anorectal manometry195.
Therapeutic management
At present, there are no specific guidelines for the management of PD-associated constipation available. A fiber-rich diet, psyllium as a bulk laxative, stool softener, and sufficient liquid-intake have high therapeutic value in treating constipation, but regular physical exercise and physical therapy are also advisable178. Exclusion of aggravating factors, such as anticholinergics, should be considered178. Unfortunately, these measures are only useful in mild or moderate cases. In many instances, a colonic transit of more than 7 days is reported, and no improvement of colonic transit can be achieved by the various therapeutic options owing to the upper threshold. In this case, additional medication must be prescibed. An effect of domperidone in the upper GIT has not been shown for constipation178.
Stimulants, such as bisacodyl, sodium picosulfate, and senna are safe and helpful196. In addition, stimulant laxatives and osmotic laxatives are recommended. The best results to date are achieved with macrogol197,198. A disadvantage of lactulose is flatulence14. Positive data are also available for therapies with probiotics and probiotic fibers196,199.
There are still no studies on the effects of modern prokinetic agents, such as serotonin (5-HT4) agonists, e.g., mosapride200. In the meantime, prucaloprid201 has been approved for severe constipation and may be administered to PD patients, although specific studies in this population are still lacking. Several new drugs, including relamorelin (ghrelin agonist)181, and chlorid channel activators, such as linaclotide, lubipprostone, and plenacanatide188,201–203 are in discussion. In rare cases of anismus, we recommend botulinum toxin injections14.
Excursus: the role of the GIT microbiome
The GIT microbiome in PD has been intensively researched in recent years6,30,37,38,204–210. By applying metagenomic and next-generation sequencing procedures, it is now possible to distinguish PD patients from healthy individuals204,210 at a very early disease stage by means of individually altered microbiota204. There may even be a ‘prodromal’ GIT microbiome because a microbial shift has been found, for instance, in patients with RBD211. In one large cohort, reduced GIT microbial diversity in PD patients correlated significantly with greater GIT symptom severity in comparison to controls212, and evidence exists for an ‘enteric pro-inflammatory profile’ in PD213,214. Intestinal dysbiosis and small intestinal bacterial overgrowth in PD patients215 might increase intestinal barrier permeability, thereby triggering excessive stimulation of the innate immune system and systemic inflammation, mechanisms possibly involved in the initiation of α-synuclein deposition216–218. According to this scenario, α-synuclein expression in the GIT would reflect an immune defense mechanism219, which is further supported by the finding that the protein is capable of triggering T cell responses that may also potentiate neurodegeneration220.
At present, interpretation of the available findings is difficult because a great variety of factors can influence the microbial configuration of the GIT. For example, evaluation of the GIT microbiome in patients undergoing treatment for PD is still of limited use, inasmuch as levodopa and other antiparkinson medications act upon the intestinal flora192,206,221, and, at least in a subset of patients, the opposite is also the true222. In addition, particularly for PD, it cannot be clarified retrospectively whether the altered GIT microbiome is the cause or the effect of motility disturbances, such as severe constipation30,207,223, and the association between the microbiome and neuroinflammation in PD still remains unclear63,204,206,224, in part because the cohorts studied to date, with few notable exceptions222,225, have been small204,207. Finally, if the microbiome and its metabolites were to play a key pathogenetic role in PD, then considerable differences should be observable between populations on different continents owing simply to dietary variability, but this has not proved to be the case. Nonetheless, it is imperative, going forward, to examine not only the precise role of the GIT microbiome and the effects a targeted diet and probiotics might have on PD patients196,226,227 but also the potential advantages and adverse side effects associated with fecal microbiotica transplantation228–231.
Final conclusions/practical algorithm for management
Dysfunction of the upper GIT in PD, especially oropharyngeal dysphagia, are complex syndromes occurring early in disease that often remain unnoticed until severe complications, such as aspiration pneumonia, become manifest. In the lower GIT, constipation is a widespread and debilitating symptom with the potential of leading to severe bowel complications and even cognitive dysfunction.
In closing, standardized and early diagnostic approaches together with continuous and long-term treatment are necessary to help patients (Table 2).
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
K.D.T. wishes to thank Ms. Simone Feldengut (immunohistochemistry), Mr. David Ewert (Fig. 1 digital image processing) and the Hans & Ilse Breuer Foundation, Frankfurt am Main (funding). We also acknowledge support from the Open Access Publication Fund of the University of Münster and express our thanks to Prof. Michael Heike, Dortmund (gastroenterologist) for reading the final version of the manuscript.
Author contributions
Concept and design—W.J. and T.W.; original draft preparation—K.-H.S., K.D.T., T.W., I.C., W.J.; editing—K.D.T. Final revised version: all co-authors read and approved the completed version of the manuscript.
Data availability
All data generated or analyzed during this study are included in this published review (see references).
Competing interests
K.-H.S., K.D.T., I.C., T.W., and W.J. declare no current or potential conflicts of interest. I.C. has previously received honoraria from Abbvie, BIAL, STADAPHARM, Georg Thieme Verlag KG, and consultancies from STADAPHARM. T.W. is an advisory board member of AbbVie, UCB, Archimedes, Phagenesis, Zambon, Bial, and Kyowa; he has received honoraria for lectures from Bial, AbbVie, STADA, UCB, Biogen, Licher, Desitin, Pfizer, Zambon, Teva, and Bayer; he also has received grants (investigator-initiated) from UCB, Licher, Abbvie, as well as academic grants from the G-BA Innovation Fund, Deutsche Parkinson-Vereinigung, (dPV), IZKF, and Neuro NRW (Germany). W.J. is a speaker and advisor for Abbie, Bial, Desitin, Licher, Stada, UCB, and Zambon.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: K. Del Tredici, W. H. Jost.
Supplementary information
The online version contains supplementary material available at 10.1038/s41531-022-00295-x.
References
- 1.Eadie MJ, Tyrer JH. Alimentary disorder in parkinsonism. Australas. Ann. Med. 1965;14:13–22. doi: 10.1111/imj.1965.14.1.13. [DOI] [PubMed] [Google Scholar]
- 2.Eadie MJ, Tyrer JH. Radiological abnormalities of the upper part of the alimentary tract in Parkinsonism. Australas. Ann. Med. 1965;14:23–27. doi: 10.1111/imj.1965.14.1.23. [DOI] [PubMed] [Google Scholar]
- 3.den Hartog Jager WA, Bethlem J. The distribution of Lewy bodies in the central and autonomic nervous systems in idiopathic paralysis agitans. J. Neurol. Neurosurg. Psychiatry. 1960;23:283–290. doi: 10.1136/jnnp.23.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coelho M, et al. Late-stage Parkinson’s disease: the Barcelona and Lisbon cohort. J. Neurol. 2010;257:1524–1532. doi: 10.1007/s00415-010-5566-8. [DOI] [PubMed] [Google Scholar]
- 5.Jost WH. Gastrointestinal dysfunction in Parkinson’s disease. J. Neurol. Sci. 2010;289:69–73. doi: 10.1016/j.jns.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Fasano A, Visanji NP, Liu LW, Lang AE, Pfeiffer RF. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015;14:625–639. doi: 10.1016/S1474-4422(15)00007-1. [DOI] [PubMed] [Google Scholar]
- 7.Lubomski M, Rushworth RL, Tisch S. Hospitalisation and comorbidities in Parkinson’s disease: a large Australian retrospective study. J. Neurol. Neurosurg. Psychiatry. 2015;86:324–330. doi: 10.1136/jnnp-2014-307822. [DOI] [PubMed] [Google Scholar]
- 8.Lubomski M, Davis RL, Sue CM. Gastrointestinal dysfunction in Parkinson’s disease. J. Neurol. 2020;267:1377–1388. doi: 10.1007/s00415-020-09723-5. [DOI] [PubMed] [Google Scholar]
- 9.Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta. Neuropathol. 1988;76:217–221. doi: 10.1007/BF00687767. [DOI] [PubMed] [Google Scholar]
- 10.Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta. Neuropathol. 1990;79:581–583. doi: 10.1007/BF00294234. [DOI] [PubMed] [Google Scholar]
- 11.Wakabayashi K, Takahashi H, Ohama E, Takeda S, Ikuta F. Lewy bodies in the visceral autonomic nervous system in Parkinson’s disease. Adv. Neurol. 1993;60:609–612. [PubMed] [Google Scholar]
- 12.Bushmann M, Dobmeyer SM, Leeker L, Perlmutter JS. Swallowing abnormalities and their response to treatment in Parkinson’s disease. Neurology. 1989;39:1309–1314. doi: 10.1212/wnl.39.10.1309. [DOI] [PubMed] [Google Scholar]
- 13.Jost WH, Schimrigk K. Constipation in Parkinson’s disease. Klin. Wochenschr. 1991;69:906–909. doi: 10.1007/BF01798536. [DOI] [PubMed] [Google Scholar]
- 14.Jost WH. Gastrointestinal motility problems in patients with Parkinson’s disease: effects of antiparkinsonian treatment and guidelines for management. Drugs Aging. 1997;10:249–258. doi: 10.2165/00002512-199710040-00002. [DOI] [PubMed] [Google Scholar]
- 15.Edwards L, Quigley EMM, Hofman R, Pfeiffer RF. Gastrointestinal symptoms in Parkinson disease: 18-month follow-up study. Mov. Disord. 1993;8:83–86. doi: 10.1002/mds.870080115. [DOI] [PubMed] [Google Scholar]
- 16.Braak H, Rüb U, Gai WP, Del Tredici K. Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 2003;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 17.Braak H, de Vos RAI, Bohl J, Del Tredici K. Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease related brain pathology. Neurosci. Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 18.Langston JW. The Parkinson’s complex: parkinsonism is just the tip of the iceberg. Ann. Neurol. 2006;59:591–596. doi: 10.1002/ana.20834. [DOI] [PubMed] [Google Scholar]
- 19.Cersósimo MG, et al. Gastrointestinal manifestations in Parkinson’s disease: prevalence and occurrence before motor symptoms. J. Neurol. 2013;260:1332–1338. doi: 10.1007/s00415-012-6801-2. [DOI] [PubMed] [Google Scholar]
- 20.Klingelhoefer L, Reichmann H. Parkinson’s disease and gastrointestinal non motor symptoms: Diagnostic and therapeutic options – a practice guide. J. Parkinsons Dis. 2015;5:647–658. doi: 10.3233/JPD-150574. [DOI] [PubMed] [Google Scholar]
- 21.Suttrup I, Warnecke T. Dysphagia in Parkinson’s disease. Dysphagia. 2016;31:24–32. doi: 10.1007/s00455-015-9671-9. [DOI] [PubMed] [Google Scholar]
- 22.Furness JB, Callaghan BP, Rivera L, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Exp. Med. Biol. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- 23.Jessen KR. GABA and the enteric nervous system. A neurotransmitter function? Mol. Cell Biochem. 1981;38 Spec No:69–76. doi: 10.1007/BF00235689. [DOI] [PubMed] [Google Scholar]
- 24.Hens J, Vanderwinden JM, De Laet MH, Scheuermann DW, Timmermans JP. Morphological and neurochemical identification of enteric neurones with mucosal projections in the human small intestine. J. Neurochem. 2001;76:464–471. doi: 10.1046/j.1471-4159.2001.00032.x. [DOI] [PubMed] [Google Scholar]
- 25.Timmermans JP, Hens J, Adriaensen D. Outer submucous plexus: an intrinsic nerve network involved in both secretory and motility processes in the intestine of large mammals and humans. Anat. Rec. 2001;262:71–78. doi: 10.1002/1097-0185(20010101)262:1<71::AID-AR1012>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 26.Qu ZD, et al. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008;334:147–161. doi: 10.1007/s00441-008-0684-7. [DOI] [PubMed] [Google Scholar]
- 27.Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2012;9:625–632. doi: 10.1038/nrgastro.2012.138. [DOI] [PubMed] [Google Scholar]
- 28.Grundmann D, et al. Enteric glia: S100, GFAP, and beyond. Anat. Rec. 2019;302:1333–1344. doi: 10.1002/ar.24128. [DOI] [PubMed] [Google Scholar]
- 29.Spencer NJ, Hu H. Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 2020;17:338–351. doi: 10.1038/s41575-020-0271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Endres K, Schäfer KH. Influence of commensal microbiota on the enteric nervous system and its role in neurodegenerative diseases. J. Innate Immun. 2018;10:172–180. doi: 10.1159/000488629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beyak, M. J., Bulmer, D., Jiang, W., Keating, W., Grundy, D. Extrinsic Sensory Afferent Nerves Innervating the Gastrointestinal Tract, Physiology of the Gastrointestinal Tract Ch. 25 In Physiology of the Gastrointestinal Tract, 4th ed (eds Leonard R. Johnson). (Academic Press, San Diego, 2006), pages 685–725.
- 32.Travagli RA, Anselmi L. Vagal neurocircuitry and its influence on gastric motility. Nat. Rev. Gastroenterol. Hepatol. 2016;13:389–401. doi: 10.1038/nrgastro.2016.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levinthal DJ, Strick PL. Multiple areas of the cerebral cortex influence the stomach. Proc. Natl Acad. Sci. USA. 2020;117:13078–13083. doi: 10.1073/pnas.2002737117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anselmi L, Toti L, Bove C, Hampton J, Travagli RA. A nigro-vagal pathway controls gastric motility and is affected in a rat model of parkinsonism. Gastroenterology. 2017;153:1581–1593. doi: 10.1053/j.gastro.2017.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: a dual hit hypothesis. Neuropathol. Appl. Neurobiol. 2007;33:599–614. doi: 10.1111/j.1365-2990.2007.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwiertz A, et al. Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat. Disord. 2018;50:104–107. doi: 10.1016/j.parkreldis.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 37.Boertjen M, Pereira PAB, Aho VTE, Scheperjans F. Increasing comparability and utility of gut microbiome studies in Parkinson’s disease: A systematic review. J. Parkinsons Dis. 2019;9:S297–S312. doi: 10.3233/JPD-191711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Unger MM, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 39.Muller PA, et al. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature. 2020;583:441–446. doi: 10.1038/s41586-020-2474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dickson DW, et al. Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol. 2008;115:437–444. doi: 10.1007/s00401-008-0345-7. [DOI] [PubMed] [Google Scholar]
- 41.Markesbery WR, Jicha GA, Liu H, Schmitt FA. Lewy body pathology in normal elderly subjects. J. Neuropathol. Exp. Neurol. 2009;68:816–822. doi: 10.1097/NEN.0b013e3181ac10a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hyman BT, et al. National Institute on Aging-Alzheimer’s association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 2012;8:1–13. doi: 10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kupsky WJ, Grimes MM, Sweeting J, Bertsch R, Cote LJ. Parkinson’s disease and megacolon: concentric hyaline inclusions (Lewy bodies) in enteric ganglion cells. Neurology. 1987;37:1253–1255. doi: 10.1212/wnl.37.7.1253. [DOI] [PubMed] [Google Scholar]
- 44.Bloch A, Probst A, Bissig H, Adams H, Tolnay M. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neurobiol. Appl Neurol. 2006;32:284–295. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 45.Probst A, Bloch A, Tolnay M. New insights into the pathology of Parkinson’s disease: does the peripheral autonomic nervous system become central? Eur. J. Neurol. 2008;15:1–4. doi: 10.1111/j.1468-1331.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- 46.Beach TG, et al. Multi-organ distribution of phosphorylated α-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stokholm MG, Danielsen HK, Hamilton-Dutoit SJ, Borghammer P. Pathological α-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Ann. Neurol. 2016;79:940–949. doi: 10.1002/ana.24648. [DOI] [PubMed] [Google Scholar]
- 48.Killinger BA, et al. The vermiform appendix impacts the risk of developing Parkinson’s disease. Sci. Transl. Med. 2018;10:eaar5280. doi: 10.1126/scitranslmed.aar5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shannon K, Vanden Berghe P. The enteric nervous system in PD: gateway, bystander victim, or source of solutions. Cell Tiss. Res. 2018;373:313–326. doi: 10.1007/s00441-018-2856-4. [DOI] [PubMed] [Google Scholar]
- 50.Braak H, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 51.Del Tredici K, Rüb U, de Vos RAI, Bohl JRE, Braak H. Where does Parkinson disease pathology begin in the brain? J. Neuropathol. Exp. Neurol. 2002;61:413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- 52.Del Tredici K, Hawkes CH, Ghebremedhin E, Braak H. Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson’s disease. Acta. Neuropathol. 2010;119:703–713. doi: 10.1007/s00401-010-0665-2. [DOI] [PubMed] [Google Scholar]
- 53.Del Tredici K, Braak H. Spinal cord lesions in sporadic Parkinson’s disease. Acta. Neuropathol. 2012;124:643–664. doi: 10.1007/s00401-012-1028-y. [DOI] [PubMed] [Google Scholar]
- 54.Ghebremedhin E, Del Tredici K, Langston JW, Braak H. Diminished tyrosine hydroxylase immunoreactivity in the cardiac conduction system and myocardium in Parkinson’s disease: an anatomical study. Acta. Neuropathol. 2009;118:777–784. doi: 10.1007/s00401-009-0596-y. [DOI] [PubMed] [Google Scholar]
- 55.Hopkins DA, Bieger D, de Vente J, Steinbusch HWM. Vagal efferent projections: viscerotopy, neurochemistry and effects of vagotomy. Prog. Brain Res. 1996;107:79–96. doi: 10.1016/s0079-6123(08)61859-2. [DOI] [PubMed] [Google Scholar]
- 56.Goyal RK, Hirano I. The enteric nervous system. N. Engl. J. Med. 1996;334:1106–1115. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- 57.Lack and relative lack of vagus nerve alpha-synuclein pathology in an autopsy series of 49 normal elderly and 18 with incidental Lewy body disease. American Association of Neuropathologists, Inc. Abstracts of the 93rd Annual Meeting June 8–11, 2017 Garden Grove, CA. J. Neuropathol. Exp. Neurol.76, Abstract 152 (2017). https://academic.oup.com/jnen/article/76/6/491/3832872.
- 58.Beach TG, et al. Vagus nerve and stomach synucleinopathy in Parkinson’s disease, incidental Lewy body disease, and normal elderly subjects: Evidence against the “body-first” hypothesis. J. Parkinsons Dis. 2021;11:1833–1843. doi: 10.3233/JPD-212733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gelpi E, et al. Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov. Disord. 2014;29:1010–1018. doi: 10.1002/mds.25776. [DOI] [PubMed] [Google Scholar]
- 60.Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH. Is alpha-synuclein in the colon a biomarker for premotor 19. Parkinson’s disease? Evidence from 3 cases. Mov. Disord. 2012;27:716–719. doi: 10.1002/mds.25020. [DOI] [PubMed] [Google Scholar]
- 61.Ito S, et al. Alpha-synuclein immunohistochemistry of gastrointestinal and biliary surgical specimens for diagnosis of Lewy body disease. Int J. Clin. Exp. Pathol. 2014;15:1714–1723. [PMC free article] [PubMed] [Google Scholar]
- 62.Borghammer P. How does Parkinson’s disease begin? Perspectives on neuroanatomical pathways, prions, and histology. Mov. Disord. 2018;33:48–57. doi: 10.1002/mds.27138. [DOI] [PubMed] [Google Scholar]
- 63.Borghammer P, Van Den Berge N. Brain-first versus gut-first Parkinson’s disease: a hypothesis. J. Parkinsons Dis. 2019;9:S281–S295. doi: 10.3233/JPD-191721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Del Tredici K, Braak H. A not entirely benign procedure: progression of Parkinson’s disease. Acta. Neuropathol. 2008;115:379–384. doi: 10.1007/s00401-008-0355-5. [DOI] [PubMed] [Google Scholar]
- 65.Phillips RJ, Walter GC, Wilder SL, Baronowsky EA, Powley TL. Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: autonomic pathway implicated in Parkinson’s disease? Neuroscience. 2008;153:733–750. doi: 10.1016/j.neuroscience.2008.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chandra R, Hiniker A, Kuo YM, Nussbaum RL, Liddle RA. α-Synuclein in gut endocrine cells and its implications for Parkinson’s disease. JCI Insight. 2017;2:e92295. doi: 10.1172/jci.insight.92295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Desplats P, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl Acad. Sci. USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.George S, Rey NL, Reichenbach N, Steiner JA, Brundin P. α-Synuclein: the long distance runner. Brain Pathol. 2013;23:350–357. doi: 10.1111/bpa.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goedert M, Masuda-Suzukake M, Falcon B. Like prions: the propagation of aggregated tau and α-synuclein in neurodegeneration. Brain. 2017;140:266–278. doi: 10.1093/brain/aww230. [DOI] [PubMed] [Google Scholar]
- 70.Fenyi A, et al. Seeding propensity and characteristics of pathogenic αSyn assemblies in formalin-fixed human tissue from the enteric nervous system, olfactory bulb, and brainstem in cases staged for Parkinson’s disease. Cells. 2021;10:139. doi: 10.3390/cells10010139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Recasens A, et al. Lewy body extracts from Parkinson disease brains trigger α-synuclein pathology in neurodegeneration in mice and monkeys. Ann. Neurol. 2014;75:351–362. doi: 10.1002/ana.24066. [DOI] [PubMed] [Google Scholar]
- 72.Chen F, et al. α-Synuclein aggregation in the olfactory bulb induces olfactory deficits by perturbing granule cells and granular-mitral synaptic transmission. NPJ Parkinsons Dis. 2021;7:144. doi: 10.1038/s41531-021-00259-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ulusoy A, et al. Caudo-rostral brain spreading of α-synuclein through vagal connections. EMBO Mol. Med. 2013;5:1119–1127. doi: 10.1002/emmm.201302475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ulusoy A, et al. Brain-to-stomach transfer of α-synuclein via vagal preganglionic projections. Acta. Neuropathol. 2017;133:381–393. doi: 10.1007/s00401-016-1661-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Den Berge N, et al. Evidence for bidirectional and trans-synaptic parasympathetic and sympathetic propagation of alpha-synuclein in rats. Acta. Neuropathol. 2019;138:535–550. doi: 10.1007/s00401-019-02040-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Holmqvist S, et al. Melki R. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta. Neuropathol. 2014;128:805–820. doi: 10.1007/s00401-014-1343-6. [DOI] [PubMed] [Google Scholar]
- 77.Jackson RG. Anatomy of the vagus nerves in the region of the lower esophagus and the stomach. Anat. Rec. 1949;103:1–18. doi: 10.1002/ar.1091030102. [DOI] [PubMed] [Google Scholar]
- 78.Travagli RA, Hermann GE, Browning KN, Rogers RC. Brainstem circuits regulating gastric function. Ann. Rev. Physiol. 2006;68:279–305. doi: 10.1146/annurev.physiol.68.040504.094635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Braak H, Del Tredici K. Invited article: Nervous system pathology in sporadic Parkinson’s disease. Neurology. 2008;70:1916–1925. doi: 10.1212/01.wnl.0000312279.49272.9f. [DOI] [PubMed] [Google Scholar]
- 80.Braak H, Del Tredici K. Potential pathways of abnormal tau and α-synuclein dissemination in sporadic Alzheimer’s and Parkinson’s diseases. Cold Spring Harb. Perspect. Biol. 2016;8:pii: a023630. doi: 10.1101/cshperspect.a023630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Del Tredici K, Braak H. Sporadic Parkinson’s disease: development and distribution of α-synuclein pathology. Neuropathol. Appl. Neurobiol. 2016;42:33–50. doi: 10.1111/nan.12298. [DOI] [PubMed] [Google Scholar]
- 82.Orimo S, Ghebremedhin E, Gelpi E. Peripheral and central autonomic nervous system: does the sympathetic or parasympathetic nervous system bear the brunt of the pathology during the course of sporadic PD? Cell Tiss. Res. 2018;373:267–286. doi: 10.1007/s00441-018-2851-9. [DOI] [PubMed] [Google Scholar]
- 83.Breen DP, Halliday GM, Lang AE. Gut-brain axis and the spread of α-synuclein pathology: vagal highway or dead end? Mov. Disord. 2019;34:307–316. doi: 10.1002/mds.27556. [DOI] [PubMed] [Google Scholar]
- 84.Horsager J, et al. Brain-first versus body-first Parkinson’s disease: a multimodal imaging case-control study. Brain. 2020;143:3077–3088. doi: 10.1093/brain/awaa238. [DOI] [PubMed] [Google Scholar]
- 85.Boeve BF, et al. Pathophysiology of REM sleep behavior disorder and relevance to neurodegenerative disease. Brain. 2007;130:2770–2788. doi: 10.1093/brain/awm056. [DOI] [PubMed] [Google Scholar]
- 86.Boeve BF, et al. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep. Med. 2013;14:754–762. doi: 10.1016/j.sleep.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iranzo A, Toloso E, Gelpi E. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behavior disorder: an observational cohort study. Lancet Neurol. 2013;12:443–453. doi: 10.1016/S1474-4422(13)70056-5. [DOI] [PubMed] [Google Scholar]
- 88.Sprenger FS, et al. Enteric nervous system α-synuclein immunoreactivity in idiopathic REM sleep behavior disorder. Neurology. 2015;85:1761–1768. doi: 10.1212/WNL.0000000000002126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ehrminger M, et al. The coeruleus/subcoeruleus complex in idiopathic rapid eye movement sleep behaviour disorder. Brain. 2016;139:1180–1188. doi: 10.1093/brain/aww006. [DOI] [PubMed] [Google Scholar]
- 90.Vilas D, et al. Assessment of α-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study. Lancet Neurol. 2016;15:708–718. doi: 10.1016/S1474-4422(16)00080-6. [DOI] [PubMed] [Google Scholar]
- 91.Knudson K, et al. In-vivo staging of pathology in REM sleep behaviour disorder: a multimodality imaging case-control study. Lancet Neurol. 2018;17:618–628. doi: 10.1016/S1474-4422(18)30162-5. [DOI] [PubMed] [Google Scholar]
- 92.Rees RN, Noyce AJ, Schrag A. The prodromes of Parkinson’s disease. Eur. J. Neurosci. 2019;49:320–327. doi: 10.1111/ejn.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van de Berg WD, et al. Patterns of alpha-synuclein pathology in incidental cases and clinical subtypes of Parkinson’s disease. Parkinsonsim Relat. Disord. 2012;18:S28–S30. doi: 10.1016/S1353-8020(11)70011-6. [DOI] [PubMed] [Google Scholar]
- 94.Coughlin DG, et al. Most cases with Lewy pathology in a population-based cohort adhere to the Braak progression pattern but ‘failure to fit’ is highly dependent on staging system applied. Parkinsonism Relat. Disord. 2019;64:124–131. doi: 10.1016/j.parkreldis.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jellinger K. Is Braak staging valid for all types of Parkinson’s disease. J. Neural Transm. 2019;126:423–431. doi: 10.1007/s00702-018-1898-9. [DOI] [PubMed] [Google Scholar]
- 96.Kalf J, de Swart BJ, Bloem BR, Munneke M. Prevalence of oropoharyngeal dysphagia in Parkinson’s disease: A meta-analysis. Parkinsonism Relat. Disord. 2012;18:311–315. doi: 10.1016/j.parkreldis.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 97.Bushmann M, Dobmeyer SM, Leeker L, Perlmutter JS. Swallowing abnormalities and their responses to treatment in Parkinson’s disease. Neurology. 1989;39:1309–1314. doi: 10.1212/wnl.39.10.1309. [DOI] [PubMed] [Google Scholar]
- 98.Bird M, Woodward MC, Gibson EM, Phyland DJ, Fonda D. Asymptomatic swallowing disorders in elderly patients with Parkinson’s disease: A description of findings on clinical examination and videofluoroscopy in sixteen patients. Age Ageing. 1994;23:251–254. doi: 10.1093/ageing/23.3.251. [DOI] [PubMed] [Google Scholar]
- 99.Hammer MJ, Murphy CA, Abrams TM. Airway somatosensory deficits and dysphagia in Parkinson’s disease. J. Parkinson Dis. 2013;3:39–44. doi: 10.3233/JPD-120161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pflug C. Critical dysphagia is common in Parkinson disease and occurs even in early stages: A prospective cohort study. Dysphagia. 2018;33:41–50. doi: 10.1007/s00455-017-9831-1. [DOI] [PubMed] [Google Scholar]
- 101.Patel B, et al. A comprehensive review of the diagnosis and treatment of Parkinson’s disease dysphagia and aspiration. Exp. Rev. Gastroenterol. Hepatol. 2020;14:411–424. doi: 10.1080/17474124.2020.1769475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Burgos R, et al. ESPEN guidelines clinical nutrition in neurology. Clin. Nutr. 2018;37:354–396. doi: 10.1016/j.clnu.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 103.Mu L, et al. Altered pharyngeal muscles in Parkinson disease. J. Neuropathol. Exp. Neurol. 2012;71:520–530. doi: 10.1097/NEN.0b013e318258381b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mu L, et al. Alpha-synuclein pathology and axonal degeneration of the peripheral motor nerves innervating pharyngeal muscles in Parkinson’s disease. J. Neuropathol. Exp. Neurol. 2013;72:119–129. doi: 10.1097/NEN.0b013e3182801cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mu L, et al. Parkinson’s disease affects peripheral sensory nerves in the pharynx. J. Neuropathol. Exp. Neurol. 2013;72:614-623-38. doi: 10.1097/NEN.0b013e3182965886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schröder JB, et al. Substance P saliva reduction predicts pharyngeal dysphagia in Parkinson’s disease. Front. Neurol. 2019;10:386. doi: 10.3389/fneur.2019.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Troche MS, Brandimore AE, Okun MS, Davenport PW, Hegland KW. Decreased cough sensitivity and aspiration in Parkinson disease. Chest. 2014;146:1294–1299. doi: 10.1378/chest.14-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Warnecke, T., Dziewas, R. & Langmore, S. Neurogenic dysphagia. Originally published in German: Neurogene Dysphagien: Diagnostik und Therapie (W. Kohlhammer Verlag, Stuttgart, 2013; 2nd extended and rev. ed. 2018) (Springer, Cham, 2021).
- 109.Warnecke T, et al. Neurogenic dysphagia: A systematic review and proposal of a classification system. Neurology. 2021;96:e876–e889. doi: 10.1212/WNL.0000000000011350. [DOI] [PubMed] [Google Scholar]
- 110.Labeit B, et al. Oropharyngeal freezing and its relation to dysphagia—An analogy to freezing of gait. Parkinsonism Relat. Disord. 2020;75:1–6. doi: 10.1016/j.parkreldis.2020.05.002. [DOI] [PubMed] [Google Scholar]
- 111.Troche MS, Okun MS, Rosenbek JC, Altmann LJ, Sapienza CM. Attentional resource allocation and swallowing safety in Parkinson’s disease: a dual task study. Parkinsonism Relat. Disord. 2014;20:439–443. doi: 10.1016/j.parkreldis.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Labeit B, et al. Effect of cognitive and motor dual-task on oropharyngeal swallowing in Parkinson’s disease. Eur. J. Neurol. 2021;28:754–762. doi: 10.1111/ene.14603. [DOI] [PubMed] [Google Scholar]
- 113.Fukae J, et al. Impact of residual drug in the pharynx on the delayed-on phenomenon in Parkinson’s disease patients. Mov. Disord. Clin. Pr. 2020;7:273–278. doi: 10.1002/mdc3.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buhmann C, et al. Pill swallowing in Parkinson’s disease: a prospective study based on flexible endoscopic evaluation of swallowing. Parkinsonism Relat. Disord. 2019;62:51–56. doi: 10.1016/j.parkreldis.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 115.Lam K, et al. Simple clinical tests may predict severe oropharyngeal dysphagia in Parkinson’s disease. Mov. Disord. 2007;22:640–644. doi: 10.1002/mds.21362. [DOI] [PubMed] [Google Scholar]
- 116.Cereda E, et al. Swallowing disturbances in Parkinson’s disease: a multivariate analysis of contributing factors. Parkinsonism Relat. Disord. 2014;20:1382–1387. doi: 10.1016/j.parkreldis.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 117.Cersósimo MG, et al. Hyposialorrhea as an early manifestation of Parkinson disease. Auton. Neurosci. 2009;150:150–151. doi: 10.1016/j.autneu.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 118.Nienstedt JC, et al. Drooling is no early sign of dysphagia in Parkinson’s disease. Neurogastroenterol. Motil. 2018;30:e13259. doi: 10.1111/nmo.13259. [DOI] [PubMed] [Google Scholar]
- 119.Nóbrega AC, Rodrigues B, Melo A. Silent aspiration in Parkinson’s disease patients with diurnal sialorrhea. Clin. Neurol. Neurosurg. 2008;110:117–119. doi: 10.1016/j.clineuro.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 120.Manor Y, Giladi N, Cohen A, Fliss DM, Cohen JT. Validation of a swallowing disturbance questionnaire for detecting dysphagia in patients with Parkinson’s disease. Mov. Disord. 2007;22:1917–1921. doi: 10.1002/mds.21625. [DOI] [PubMed] [Google Scholar]
- 121.Kalf JG, et al. Reproducibility and validity of patient-related assessment of speech, swallowing, and saliva control in Parkinson’s disease. Arch. Phys. Med Rehabil. 2011;92:1152–1158. doi: 10.1016/j.apmr.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 122.Simons JA, et al. Development and validation of a new screening questionnaire for dysphagia in early stages of Parkinson’s disease. Parkinsonism Relat. Disord. 2014;20:992–998. doi: 10.1016/j.parkreldis.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 123.Buhmann C, et al. Is the Munich dysphagia Test—Parkinson’s disease (MDT-PD) a valid screening tool for patients at risk of aspiration? Parkinsonism Relat. Disord. 2019;61:138–143. doi: 10.1016/j.parkreldis.2018.10.031. [DOI] [PubMed] [Google Scholar]
- 124.Simons JA, et al. Multilingual validation of the first French version of Munich Dysphagia Test—Parkinson’s disease (MDT-PD) in the Luxembourg Parkinson’s study. Front Neurol. 2019;10:1180. doi: 10.3389/fneur.2019.01180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chaudhuri KR, Healy DG, Schapira AH, National Institute for Clinical Excellence. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5:235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 126.Goetz CG, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov. Disord. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 127.Nienstedt J, et al. Predictive clinical factors for penetration and aspiration in Parkinson’s disease. Neurogastroenterol. Motil. 2019;31:e13524. doi: 10.1111/nmo.13524. [DOI] [PubMed] [Google Scholar]
- 128.Sampaio M, Argolo N, Melo A, Nóbrega AC. Wet voice as a sign of penetration/aspiration in Parkinson’s disease: does testing material matter? Dysphagia. 2014;29:610–615. doi: 10.1007/s00455-014-9552-7. [DOI] [PubMed] [Google Scholar]
- 129.Kalf, H. et al. Guidelines for speech-language therapy in Parkinson’s disease. Nijmegen, the Netherlands/Miami (FL). (ParkinsonNet/NPF, U.S.A., 2011). https://www.parkinsonnet.com/discipline/speech-and-language.
- 130.Pflug C, Niessen A, Buhmann C, Bihler M. Swallowing speed is no adequate predictor of aspiration in Parkinson’s disease. Neurogastroenterol. Motil. 2019;31:e13713. doi: 10.1111/nmo.13713. [DOI] [PubMed] [Google Scholar]
- 131.Logemann JA. Evaluation and treatment of swallowing disorders. 2nd ed. Austin: Pro-Ed; 1998. [Google Scholar]
- 132.Langmore SE. Evaluation of oropharyngeal dysphagia: which diagnostic tool is superior? Curr. Opin. Otolaryngnol Head. Neck Surg. 2003;11:485–489. doi: 10.1097/00020840-200312000-00014. [DOI] [PubMed] [Google Scholar]
- 133.Dziewas, R. et al. German guideline for neurogenic dysphagia (2020) https://dgn.org/wp-content/uploads/2013/01/030111_LL_Neurogene_Dysphagie_2020.pdf.
- 134.Ciucci MR, et al. Early identification and treatment of communication and swallowing deficits in Parkinson disease. Semin Speech Lang. 2013;34:185–202. doi: 10.1055/s-0033-1358367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Van Hooren MRA, et al. Treatment effects for dysphagia in Parkinson’s disease: a systematic review. Parkinsonism Relat. Disord. 2014;20:800–807. doi: 10.1016/j.parkreldis.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 136.Melo A, Monteiro L. Swallowing improvement after levodopa treatment in idiopathic Parkinson’s disease: lack of evidence. Parkinsonsim Relat. Disord. 2013;19:279–281. doi: 10.1016/j.parkreldis.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 137.Sutton J. Dysphagia in Parkinson’s disease is responsive to levodopa. Parkinsonism Relat. Disord. 2013;19:282–284. doi: 10.1016/j.parkreldis.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 138.Chang MC, Park JS, Lee BJ, Park D. Effectiveness of pharmacologic treatment for dysphagia in Parkinson’s disease: a narrative review. Neurological Sci. 2021;42:513–519. doi: 10.1007/s10072-020-04865-w. [DOI] [PubMed] [Google Scholar]
- 139.Monte F, da Silva-Júnior FP, Braga-Neto P, Nobre e Souza MA, de Bruin VM. Swallowing abnormalities and dyskinesia in Parkinson’s disease. Mov. Disord. 2005;20:457–462. doi: 10.1002/mds.20342. [DOI] [PubMed] [Google Scholar]
- 140.Warnecke T, et al. Levodopa responsiveness of dysphagia in advanced Parkinson’s disease and reliability testing of the FEES-Levodopa-test. Parkinsonism Relat. Disord. 2016;28:100–106. doi: 10.1016/j.parkreldis.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 141.Hirano M, et al. Rotigotine transdermal patch improves swallowing in dysphagic patients with Parkinson’s disease. Dysphagia. 2015;30:452–456. doi: 10.1007/s00455-015-9622-5. [DOI] [PubMed] [Google Scholar]
- 142.Hirano M, et al. Effects of the rotigotine transdermal patch versus oral levodopa on swallowing in patients with Parkinson’s disease. J. Neurol. Sci. 2019;15:404–405. doi: 10.1016/j.jns.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 143.Torti M, Bravi D, Vacca L, Stocchi F. Are all dopamine agonists essentially the same? Drugs. 2019;79:693–703. doi: 10.1007/s40265-019-01103-2. [DOI] [PubMed] [Google Scholar]
- 144.Labeit B, et al. Effect of intestinal levodopa-carbidopa infusion on pharyngeal dysphagia: results from a retrospective pilot study in patients with Parkinson’s disease. Parkinsons Dis. 2020;11:4260501. doi: 10.1155/2020/4260501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yu H, Takahashi K, Bloom L, Quaynor SD, Xie T. Effect on Deep Brain Stimulation on swallowing function: A systematic review. Front Neurol. 2020;11:547. doi: 10.3389/fneur.2020.00547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Chang, M. C., Park, J. S., Lee, B. J., Park, D. The effect of deep brain stimulation on swallowing function in Parkinson’s disease: A narrative review. Dysphagia. 10.1007/s00455-020-10214-y (2021). [DOI] [PubMed]
- 147.Xie T, et al. Low-frequency stimulation of STN-DBS reduces aspiration and freezing of gait in patients with PD. Neurology. 2015;84:415–420. doi: 10.1212/WNL.0000000000001184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xie T, et al. Long-term effect of low frequency stimulation of STN on dysphagia, freezing of gait and other motor symptoms in PD. J. Neurol. Neurosurg. Psychiatry. 2018;89:989–994. doi: 10.1136/jnnp-2018-318060. [DOI] [PubMed] [Google Scholar]
- 149.Pflug C, et al. Impact of simultanous subthalamic and nigral stimulation on dysphagia in Parkinson’s disease. Ann. Clin. Transl. Neurol. 2020;7:628–638. doi: 10.1002/acn3.51027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Heijnen BJ, Speyer R, Baijens LW, Bogaardt HC. Neuromuscular electrical stimulation versus traditional therapy in patients with Parkinson’s disease and oropharyngeal dysphagia: effects on quality of life. Dysphagia. 2012;27:336–345. doi: 10.1007/s00455-011-9371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Baijens LW, et al. Surface electrical stimulation in dysphagic Parkinson patients: a randomized clinical trial. Laryngoscope. 2013;123:E38–E44. doi: 10.1002/lary.24119. [DOI] [PubMed] [Google Scholar]
- 152.Park JS, Oh DH, Hwang NK, Lee JH. Effects of neuromuscular electrical stimulation in patients with Parkinson’s disease and dysphagia: A randomized, single-blind, placebo-controlled trial. NeuroRehab. 2018;42:457–463. doi: 10.3233/NRE-172306. [DOI] [PubMed] [Google Scholar]
- 153.Logemann JA, et al. A randomized study of three interventions for aspiration of thin liquids in patients with dementia or Parkinson’s disease. J. Speech Lang. Hear Res. 2008;51:173–183. doi: 10.1044/1092-4388(2008/013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ayres AL, Jotz GP, Rieder CR, Olchik MR. Benefit from the chin-down maneuver in the swallowing performance ans self-perception of Parkinson’s disease patients. Parkinsons Dis. 2017;2017:e7460343. doi: 10.1155/2017/7460343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sharkawi AE, et al. Swallowing and voice effects on Lee Silverman Voice. J. Neurol. Neurosurg. Psychiatry. 2002;72:31–37. doi: 10.1136/jnnp.72.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Troche MS, et al. Aspiration and swallowing in Parkinson’s disease and rehabilitation with EMST: a randomized trial. Neurology. 2010;75:1912–1919. doi: 10.1212/WNL.0b013e3181fef115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Claus I, et al. Expiratory muscle strength training for therapy of pharyngeal dysphagia in Parkinson’s disease. Mov. Disord. 2021;36:1815–1824. doi: 10.1002/mds.28552. [DOI] [PubMed] [Google Scholar]
- 158.Manor Y, Mootanah R, Freud D, Giladi N, Cohen JT. Video-assisted swallowing therapy for patients with Parkinson’s disease. Parkinsonism Relat. Disord. 2013;19:207–211. doi: 10.1016/j.parkreldis.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 159.Athukorala RP, Jones RD, Sella O, Huckabee ML. Skill training for swallowing rehabilitation in patients with Parkinson’s disease. Arch. Phys. Med. Rehabil. 2014;95:1374–1382. doi: 10.1016/j.apmr.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 160.Jost WH, et al. SIAXI: Placebo-controlled, randomized, double-blind study of incobotulinumtoxin A for sialorrhea. Neurology. 2019;92:e1982–e1991. doi: 10.1212/WNL.0000000000007368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Arbouw MEL, et al. Glycopyrrolate for sialorrhea in Parkinson’s disease: a randomized, double-blind, crossover trial. Neurology. 2010;74:1203–1207. doi: 10.1212/WNL.0b013e3181d8c1b7. [DOI] [PubMed] [Google Scholar]
- 162.South AR, Somers SM, Jog MS. Gum chewing improves swallow frequency and latency in Parkinson patients: a preliminary study. Neurology. 2010;74:1198–1202. doi: 10.1212/WNL.0b013e3181d9002b. [DOI] [PubMed] [Google Scholar]
- 163.Marrinan S, Emmanuel AV, Burn DJ. Delayed gastric emptying in Parkinson’s disease. Mov. Disord. 2014;29:23–32. doi: 10.1002/mds.25708. [DOI] [PubMed] [Google Scholar]
- 164.Mukherjee A, Biswas A, Das SK. Gut dysfunction in Parkinson’s disease. World J. Gastroenterol. 2016;22:5742–5752. doi: 10.3748/wjg.v22.i25.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Doi H, et al. Plasma levodopa peak delay and impaired gastric emptying in Parkinson’s disease. J. Neurol. Sci. 2012;319:86–88. doi: 10.1016/j.jns.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 166.Sung HY, et al. The prevalence and patterns of pharyngoesophageal dysmotility in patients with early stage Parkinson’s disease. Mov. Disord. 2010;25:2361–2368. doi: 10.1002/mds.23290. [DOI] [PubMed] [Google Scholar]
- 167.Suttrup I, et al. Esophageal dysfunction in different stages of Parkinson’s disease. Neurogastroenterol. Motil. 2017;29:e12915. doi: 10.1111/nmo.12915. [DOI] [PubMed] [Google Scholar]
- 168.Pasricha PJ, Parkman HP. Gastroparesis: definitions and diagnosis. Gastroenterol. Clin. North Am. 2015;44:1–7. doi: 10.1016/j.gtc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 169.Kahrilas PJ, et al. Smout AJ, The Chicago classification of esophageal motility disorders, v3.0. Neurogastroenterol. Motil. 2015;27:160–174. doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Triadafilopoulos G, Gandhy R, Barlow C. Pilot cohort study of endoscopic botulinum neurotoxin injection in Parkinson’s disease. Parkinsonism Relat. Disord. 2017;44:33–37. doi: 10.1016/j.parkreldis.2017.08.020. [DOI] [PubMed] [Google Scholar]
- 171.Derrey S, et al. Impact of deep brain stimulation on pharyngo-esophageal motility: a randomized cross-over study. Neurogastroenterol. Motil. 2015;27:1214–1222. doi: 10.1111/nmo.12607. [DOI] [PubMed] [Google Scholar]
- 172.Alvarez-Berdugo D, et al. A comparative study on the therapeutic effect of TRPV1, TRPA1, and TRPM8 agonists on swallowing dysfunction associated with aging and neurological diseases. Neurogastroenterol. Motil. 2018;30:e13185. doi: 10.1111/nmo.13185. [DOI] [PubMed] [Google Scholar]
- 173.Suntrup-Krueger S, et al. Effect of capsaicionoids on neurophysiological, biochemical and mechanical parameters of swallowing function. Neurotherapeutics. 2021;18:1360–1370. doi: 10.1007/s13311-020-00996-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Heetun ZS, Quigley EM. Gastroparesis and Parkinson’s disease: a systematic review. Parkinsonism Relat. Disord. 2012;18:433–440. doi: 10.1016/j.parkreldis.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 175.Gil RA, Hwynn N, Fabian T, Joseph S, Fernandez HH. Botulinum toxin type A for the treatment of gastroparesis in Parkinson’s disease patients. Parkinsonism Relat. Disord. 2011;17:285–287. doi: 10.1016/j.parkreldis.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 176.Arai F, et al. Subthalamic deep brain stimulation can improve gastric emptying in Parkinson’s disease. Brain. 2012;135:1478–1485. doi: 10.1093/brain/aws086. [DOI] [PubMed] [Google Scholar]
- 177.Parkinson J. An essay on the shaking palsy. London: Sherwood, Neely, and Jones; 1817. [Google Scholar]
- 178.Jost WH. An update on the recognition and treatment of autonomic symptoms in Parkinson’s disease. Exp. Rev. Neurother. 2017;17:791–799. doi: 10.1080/14737175.2017.1345307. [DOI] [PubMed] [Google Scholar]
- 179.Adler CH, Beach TG. Neuropathological basis of nonmotor manifestations of Parkinson’s disease. Mov. Disord. 2016;31:1114–1119. doi: 10.1002/mds.26605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Knudsen K, Krogh K, Østergaard K, Borghammer P. Constipation in Parkinson’s disease: subjective symptoms, objective markers, and new perspectives. Mov. Disord. 2017;32:94–105. doi: 10.1002/mds.26866. [DOI] [PubMed] [Google Scholar]
- 181.Parkinson Study Group. A randomized trial of relamorelin for constipation in Parkinson’s disease (MOVE-PD): Trial results and lessons learned. Parkinsonism Relat. Disord. 2017;37:101–105. doi: 10.1016/j.parkreldis.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 182.Jost WH. Autonome Regulationsstörungen beim Parkinson Syndrom [Disorders of autonomic regulation in Parkinson syndrome] Fortschr. Neurol. Psychiatr. 1995;63:194–205. doi: 10.1055/s-2007-996616. [DOI] [PubMed] [Google Scholar]
- 183.Barone P, et al. The PRIAMO study: A multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov. Disord. 2009;24:1641–1649. doi: 10.1002/mds.22643. [DOI] [PubMed] [Google Scholar]
- 184.Abbott RD, et al. Frequency of bowel movements and the future risk of Parkinson’s disease. Neurology. 2001;57:456–464. doi: 10.1212/wnl.57.3.456. [DOI] [PubMed] [Google Scholar]
- 185.Stirpe P, Hoffman M, Badiali D, Colosimo C. Constipation: an emerging risk factor for Parkinson’s disease? Eur. J. Neurol. 2016;23:1606–1613. doi: 10.1111/ene.13082. [DOI] [PubMed] [Google Scholar]
- 186.Svensson E, Henderson VW, Borghammer P, Horváth-Puhó E, Sørensen HT. Constipation and risk of Parkinson’s disease: A Danish population-based cohort study. Parkinsonism Relat. Disord. 2016;28:18–22. doi: 10.1016/j.parkreldis.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 187.Pierantozzi M, et al. Helicobacter pylori eradication and l-dopa absorption in patients with PD and motor fluctuations. Neurology. 2006;66:1824–1829. doi: 10.1212/01.wnl.0000221672.01272.ba. [DOI] [PubMed] [Google Scholar]
- 188.Savica R, et al. Medical records documentation of constipation preceding Parkinson disease: A case-control study. Neurology. 2009;73:1752–1758. doi: 10.1212/WNL.0b013e3181c34af5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gage H, et al. Correlates of constipation in people with Parkinson’s. Parkinsonism Relat. Disord. 2011;17:106–111. doi: 10.1016/j.parkreldis.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 190.Camacho M, et al. Early constipation predicts faster dementia onset in Parkinson’s disease. NPJ Parkinsons Dis. 2021;7:45. doi: 10.1038/s41531-021-00191-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.García Roca, L. et al. COPPADIS Study Group. Constipation predicts cognitive decline in Parkinson’s disease: results from the COPPADIS Cohort at 2-year follow-up and comparison with a control group. J. Parkinsons Dis. 10.3233/JPD-212868 (2021). [DOI] [PubMed]
- 192.Kenna JE, et al. Characterization of gastrointestinal symptom type and severity in Parkinson’s disease: A case-control study in an Australian cohort. Mov. Disord. Clin. Pr. 2021;8:245–253. doi: 10.1002/mdc3.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lundin E, et al. Segmental colonic transit studies: Comparison of a radiological and a scintigraphic method. Colorectal Dis. 2007;9:344–351. doi: 10.1111/j.1463-1318.2006.01153.x. [DOI] [PubMed] [Google Scholar]
- 194.Agachan F, Pfeifer J, Wexner SD. Defecography and proctography. Results of 744 patients. Dis. Colon Rectum. 1996;39:899–905. doi: 10.1007/BF02053989. [DOI] [PubMed] [Google Scholar]
- 195.Jost WH, Schrank B, Herold A, Leiß O. Functional outlet obstruction: Anismus, spastic pelvic floor syndrome, and dyscoordination of the voluntary sphincter muscles. Scand. J. Gastroenterol. 1999;34:449–453. doi: 10.1080/003655299750026146. [DOI] [PubMed] [Google Scholar]
- 196.Barichella M, et al. Probiotics and prebiotic fiber for constipation associated with PD. Neurology. 2016;87:1274–1280. doi: 10.1212/WNL.0000000000003127. [DOI] [PubMed] [Google Scholar]
- 197.Zangaglia R, et al. Macrogol for the treatment of constipation in Parkinson’s disease. A randomized placebo-controlled study. Mov. Disord. 2007;22:1239–1244. doi: 10.1002/mds.21243. [DOI] [PubMed] [Google Scholar]
- 198.Zesiewicz TA, et al. Practice parameter: treatment of nonmotor symptoms of Parkinson disease: report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2010;74:924–931. doi: 10.1212/WNL.0b013e3181d55f24. [DOI] [PubMed] [Google Scholar]
- 199.Tan AH, et al. Probiotics for constipation in Parkinson disease: A randomized placebo-controlled study. Neurology. 2021;96:e772–e782. doi: 10.1212/WNL.0000000000010998. [DOI] [PubMed] [Google Scholar]
- 200.Liu Z, et al. Mosapride citrate, a novel 5-HT4 agonist and partial 5-HT3 antagonist, ameliorates constipation in parkinsonian patients. Mov. Disord. 2005;20:680–686. doi: 10.1002/mds.20387. [DOI] [PubMed] [Google Scholar]
- 201.Freitas ME, Alqaraawi A, Lang AE, Liu LWC. Linaclotide and prucalopride for management of constipation in patients with parkinsonism. Mov. Disord. Clin. Pr. 2018;5:218–220. doi: 10.1002/mdc3.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bassotti G, Usai Satta P, Bellini M. Plecanatide for the treatment of chronic idiopathic constipation in adult patients. Expert Rev. Clin. Pharm. 2019;12:1019–1026. doi: 10.1080/17512433.2019.1670057. [DOI] [PubMed] [Google Scholar]
- 203.Ondo WG, et al. Placebo-controlled trial of lubiprostone for constipation associated with Parkinson disease. Neurology. 2012;78:1650–1654. doi: 10.1212/WNL.0b013e3182574f28. [DOI] [PubMed] [Google Scholar]
- 204.Bedarf JR, et al. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients. Genome Med. 2017;9:39. doi: 10.1186/s13073-017-0428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Pietrucci D, et al. Dysbiosis of gut microbiota in a selected population of Parkinson’s patients. Parkinsonism Relat. Disord. 2019;65:124–130. doi: 10.1016/j.parkreldis.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 206.Weis S, et al. Effect of Parkinson’s disease and related medications on the composition of the fecal bacterial microbiota. NPJ Parkinsons Dis. 2019;5:28. doi: 10.1038/s41531-019-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chapelet G, Leclair-Visonneau L, Clairembault T, Neunlist M, Derkinderen P. Can the gut be the missing piece in uncovering PD pathogenesis? Parkinsonism Relat. Disord. 2020;59:26–31. doi: 10.1016/j.parkreldis.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 208.Cryan JF, O’Riordan KJ, Sandhu K, Peterson V, Dinan TG. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19:179–194. doi: 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- 209.Metta, V. et al. Gastrointestinal dysfunction in Parkinson’s disease: molecular pathology and implications of gut microbiome, probiotics, and fecal microbiota transplantation. J. Neurol.10.1007/s00415-021-10567-w (2021). [DOI] [PubMed]
- 210.Weis S, et al. Association between Parkinson’s disease and the faecal eukaryotic microbiota. NPJ Parkinsons Dis. 2021;7:101. doi: 10.1038/s41531-021-00244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Heintz-Buschart A, et al. The nasal and gut microbiome in Parkinson’s disease and idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. 2018;33:88–98. doi: 10.1002/mds.27105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Does the truth lie within the gut? Investigating the gut microbiome in an Australian cohort of Parkinson’s disease patients. American Academy of Neurology, Abstracts of the 72nd Annual Meeting April 25-May 1, 2020 Toronto. Ontario. Neurology94, Abstract 1438, https://n.neurology.org/content/94/15_Supplement/1438 (2020)..
- 213.Devos D, et al. Colonic inflammation in Parkinson’s disease. Neurobiol. Dis. 2013;50:42–48. doi: 10.1016/j.nbd.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 214.Nielsen SD, Pearson NM, Seidler K. The link between the gut microbiota and Parkinson’s disease: A systematic mechanism review with focus on α-synuclein transport. Brain Res. 2021;1769:147609. doi: 10.1016/j.brainres.2021.147609. [DOI] [PubMed] [Google Scholar]
- 215.Hill-Burns EM, et al. Parkinson’s disease and Parkinson’s disease medications have distinct signatures of the gut microbiome. Mov. Disord. 2017;32:739–749. doi: 10.1002/mds.26942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Forsyth CB, et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One. 2011;6:e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.de Vos WM, de Vos EA. Role of the intestinal microbiome in health and disease: from correlation to causation. Nutr. Rev. 2012;70:S45–S56. doi: 10.1111/j.1753-4887.2012.00505.x. [DOI] [PubMed] [Google Scholar]
- 218.Visanji NP, Brooks PL, Hazrati LN, Lang AE. The prion hypothesis in Parkinson’s disease: Braak to the future. Acta Neuropathol. Commun. 2013;1:2. doi: 10.1186/2051-5960-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Stolzenberg E, et al. A role for neuronal alpha-synuclein in gastrointestinal immunity. J. Innate Immun. 2017;9:456–463. doi: 10.1159/000477990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sulzer D, et al. T cells from patients with Parkinson’s disease recognize α-synuclein peptides. Nature. 2017;546:656–661. doi: 10.1038/nature22815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.van Kessel SP, et al. Gut bacterial tyrosine decarboxylases restrict levels of levodopa in the treatment of Parkinson’s disease. Nat. Commun. 2019;10:310. doi: 10.1038/s41467-019-08294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.van Kessel SP, Auvinen P, Scheperjans F, El Aidy S. Gut bacterial tyrosine decarboxylase associates with clinical variables in a longitudinal cohort study of Parkinson’s disease. NPJ Parkinsons Dis. 2021;7:115. doi: 10.1038/s41531-021-00260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Keshavarzian A, Engen P, Bonvegna S, Cilia R. The gut microbiome in Parkinson’s disease: A culprit or a bystander? Prog. Brain Res. 2020;252:357–450. doi: 10.1016/bs.pbr.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 224.Seguella L, Sarnelli G, Esposito G. Leaky gut, dysbiosis, and enteric glia activation: the trilogy behind the intestinal origin of Parkinson’s disease. Neural Regen. Res. 2020;15:1037–1038. doi: 10.4103/1673-5374.270308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gorecki AM, et al. Single nucleotide polymorphisms associated with gut homeostasis influence risk and age-at-onset of Parkinson’s disease. Front. Aging Neurosci. 2020;12:603849. doi: 10.3389/fnagi.2020.603849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bedarf JR, et al. Das Darm-Mikrobiom bei der Parkinson-Krankheit [The gut microbiome in Parkinson’s disease] Nervenarzt. 2019;90:160–166. doi: 10.1007/s00115-018-0601-6. [DOI] [PubMed] [Google Scholar]
- 227.Jackson A, et al. Diet in Parkinson’s disease: critical role for the microbiome. Front. Neurol. 2019;10:1245. doi: 10.3389/fneur.2019.01245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sampson TR, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell. 2017;167:1469–1480. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Vendrik KEW, et al. Fecal microbiota transplantation in neurological disorders. Front. Cell Infect. Microbiol. 2020;10:98. doi: 10.3389/fcimb.2020.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Segal A, Zlotnik Y, Moyal-Atias K, Abuhasira R, Ifergane G. Fecal microbiota transplant as a potential treatment for Parkinson’s disease—A case series. Clin. Neurol. Neurosurg. 2021;207:106791. doi: 10.1016/j.clineuro.2021.106791. [DOI] [PubMed] [Google Scholar]
- 231.Fecal Microbiota Transplantation for Parkinson’s Disease. https://clinicaltrials.gov/ct2/show/NCT03808389. Accessed 31 Dec 2021.
- 232.Okino Y, et al. Root of the small-bowel mesentery: correlative anatomy and CT features of pathologic conditions. Radiographics. 2001;21:1475–1490. doi: 10.1148/radiographics.21.6.g01nv121475. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published review (see references).