Abstract
Objective
To describe COVID-19 in children and the differences between the two waves.
Methods
The electronic medical records of children younger than 16 y of age with laboratory-confirmed COVID-19 infection between June 1st 2020 and May 31st 2021 at Christian Medical College, Vellore were retrospectively reviewed. Demographic, clinical, and laboratory data were collected on a predesigned case record form and analyzed.
Results
A total of 988 children were diagnosed with confirmed COVID-19 during the study period. Of these, there were 585 children diagnosed during the 1st wave (June 2020–Feb 2021) and 403 children during the 2nd wave (March 2021–May 2021). It was found that loose stools and rash were significantly more frequent during the 1st wave and fever, cough, coryza, heart rate and temperature were significantly more during the 2nd wave. There was no significant difference between the two groups in terms of requirement of oxygen therapy, need for ICU admission, duration of ICU stay or hospital stay, or severity of illness. Mortality was significantly higher during the 2nd wave (0.3% vs. 2%).
Conclusion
The COVID-19 pandemic among children during the 1st and 2nd waves were similar in severity, though there was a higher mortality during the 2nd wave.
Keywords: SARS-CoV-2, MIS-C, RT-PCR, COVID, Remdesivir
Introduction
Coronavirus disease 2019 caused by the novel virus severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) emerged from Wuhan province, China in December 2019 and spread across the world as a pandemic [1]. Its genomic sequences were quite similar to beta coronaviruses discovered in bats. Two other beta coronaviruses which led to previous epidemics affecting more than 10,000 patients with considerable morbidity and mortality were severe acute respiratory syndrome coronavirus (SARS‐CoV), and Middle-east respiratory distress syndrome coronavirus (MERS‐CoV) [2]. The difference in the pathogenicity has been attributed mainly due to protein structure of SARS-CoV-2 virus and the and virus induced pathophysiological process [3]. It is estimated that only 5% of those infected with SARS-CoV and 2% infected with MERS-CoV were in children < 18 y [4]. The COVID-19 outbreak primarily affects the adult population and the severity of the disease is much less in children compared to adults [5]. There is heterogeneous clinical presentation with wide spectrum of severity and mortality rates among children, both being lesser compared to older age groups [6]. Their atypical presentation also becomes a hidden source of infection which could play a role in community transmission [7].
Multiple immunological hypotheses have been proposed for the reasons behind less severe disease in children such as poor expression of ACE2 receptors and Transmembrane serine protease TMPRSS2 in the respiratory epithelial cells in children compared to adults, lower levels of pro-inflammatory cytokines such as IL-6, more effective cytotoxic CD8 T cell activation and good humoral antibody response and “trained immunity” as children’s are exposed to frequent viral infections and vaccinations [8]. Children tend to remain protected despite mutational change in the viral protein and infectivity [9]. The most important difference between the presentation in adults and children is the immune dysregulation that happens with children causing multisystem inflammatory syndrome in children also called MIS-C. It is primarily a post infectious phenomenon [10], that is different from severe acute COVID infection causing considerable morbidity and some mortality among affected children [11].
Host factors play a vital role in disease severity and prior medical comorbid factors such as hemato-oncologic malignancies, immune deficiency syndromes, immune suppressant states and metabolic diseases [5, 12]. In Mexico, where there were high prevalence of childhood obesity and metabolic syndrome and leading to diabetes, chronic kidney disease and cardiovascular risk, higher infection severity was reported in such cohorts [13]. Multiple variants of the SARS-CoV-2 virus have been implicated in the increased transmissibility and pathogenicity since the onset of the pandemic. Four of them viz., B.1.1.7 (alpha), B.1.351 (beta), P.1 (gamma) and B.1.617.2 (delta) have been classified by the WHO as variants of concern [14]. India has witnessed a devastating 2nd wave of COVID-19 which peaked during the first half of May primary driven by B.1.617 lineage variants with the delta variant in particular [15]. It is possible that the clinical characteristics in each of the waves could be different depending on the variants dominating the waves. Hence in this retrospective study, the spectrum of COVID-19 disease among children is described and its characteristics during the 1st and the 2nd waves of the pandemic in India till May 31, 2021 are compared.
Materials and Methods
The outpatient and inpatient electronic medical records of children younger than 16 y of age (as per the hospital policy of children < 16 y being seen in the pediatric department) with laboratory-confirmed COVID-19 infection between June 1st 2020 and May 31st 2021 at Christian Medical College, Vellore were retrospectively reviewed. Diagnosis was based on detection of SARS-CoV-2 virus by real-time polymerase chain reaction (RT-PCR) on nasopharyngeal swab. The study was approved by Institutional Review Board and Ethics Committee.
Demographic, clinical and laboratory data were collected on a predesigned case record form. Clinical variables included presenting symptoms, their duration, comorbidities, physical examination findings and treatment modalities. Laboratory features included haemoglobin, platelet and white cell count, renal and liver function tests, coagulation parameters, and inflammatory markers. Severity of COVID-19 was defined as per World Health Organization (WHO) guidelines [16]. Diagnosis of MIS-C was defined as per WHO criteria [17]. Only children with MIS-C who had a positive RT-PCR were included in the analysis. The outcome parameters included length of hospital stay, need for intensive care management, requirement of oxygen and/or inotropes and mortality. The period till Feb 28, 2021 was considered as wave 1 and the period from March 1, 2021 was considered as wave 2.
The data were analyzed using Statistical Package for Social Sciences for windows (SPSS version 22.0, Chicago, Illinois, USA) and Microsoft Excel. Descriptive statistics were used for representation of data (frequency, mean and standard deviation [SD]). Data not following normal distribution were represented as median and interquartile range (IQR). Groups were compared using chi-square or Fisher exact test for categorical variables and Student-t test for continuous variables. Mann–Whitney U test was used for not normally distributed continuous variables. The p value < 0.05 was considered as significant.
Results
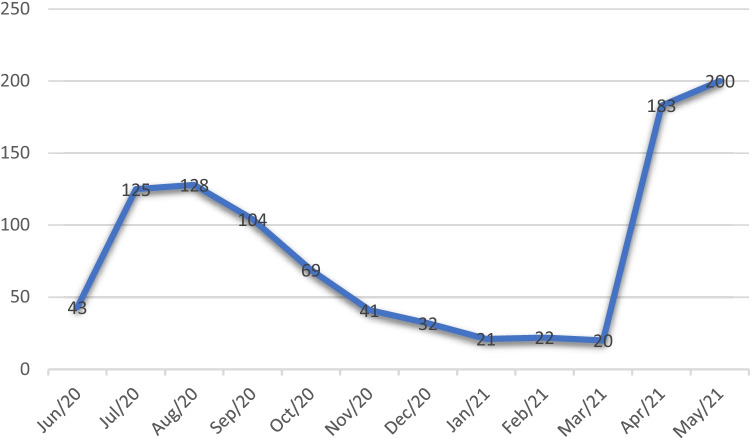
A total of 988 children were diagnosed with confirmed COVID-19 during the study period. Of these, there were 585 children diagnosed during the 1st wave (June 2020–Feb 2021) and 403 children during the 2nd wave (March 2021–May 2021). The distribution of cases during the study period is shown in Fig. 1. The male to female ratio was 1.4:1. The mean age (SD) was 7.8 (5.0) y with 144 (14.6%) infants. Only 302 (30.6%) children were symptomatic at the time of RT-PCR testing and the rest asymptomatic. Almost 90% (883/998) children had asymptomatic or mild disease. The ones who had MIS-C with positive RT-PCR were 14 (1.4%) during the study period. Fever (44.6%) was the most common symptom followed by cough (18.2%) and coryza (11.1%). The commonest abdominal symptoms were nausea and vomiting (8.2%), loose stools (4.8%) and abdominal pain (3.1%). There were 40 (4%) children who presented with seizures, of whom 13 had epilepsy, 14 had simple febrile seizures, 2 had diabetic ketoaciosis, 2 had chronic renal failure, 3 had meningitis and 1 had hemophagocytic lymphohistiocytosis. There were 478 (48.4%) children who had comorbidities. Of those with comorbidities, there were 115 (24.1%) haematological/malignancies, 17 (3.6%) respiratory, 20 (4.2%) cardiac abnormalities, 58 (12.1%) neurological, 19 (4%) endocrine/metabolic, 21 (4.4%) renal, 39 (8.2%) gastrointestinal, 7 (1.5%) primary immunodeficiencies and 182 (38.1%) others which included 96 (20.1%) who got tested preoperatively. There were 82 children (8.3%) admitted into intensive care with 20 (2%) of them requiring mechanical ventilation. Oxygen was administered to 112 (11.3%) and inotropes to 61 (6.17%) children. Steroids were administered in 55 (5.5%) children, anticoagulation in 40 (4%), and remdesivir in 2 children (0.2%). There were 10 (1%) deaths during the study period. A summary of the description of the children who died is given in Table 1. Of the 10 children that died, 9 of them had very significant comorbidities: 2 haematological malignancies, 2 primary immunodeficiency, 2 severe cardiac abnormalities, 1 post haematopoietic stem cell transplant who also had Wilson’s disease, 1 aplastic anaemia and a child who had severe failure to thrive, global developmental delay and had presented in septic shock. A 37-d-old infant who died did not have known comorbidities. This infant presented with diarrhea and severe dehydration. Subsequently, the infant developed haemophagocytic lymphohistiocytosis (HLH) and succumbed to the illness. Whether the HLH was primary or secondary could not be ascertained.
Fig. 1.
Monthly distribution of cases over the study period
Table 1.
Details of children with mortality
| Age | Sex | Comorbidities | Duration of hospital stay | Cause of death |
|---|---|---|---|---|
| 14 y | Male | T cell acute lymphoblastic leukemia | 26 d | Respiratory failure and decompensated shock |
| 37 d | Male | Hemophagocytic lymphohistiocytosis | 20 d | Refractory septic shock and pneumonia |
| 10 mo | Male | Wiskott–Aldrich syndrome | 21 d | Severe thrombocytopenia with Intracranial bleed |
| 11 mo | Female | Failure to thrive, global developmental delay | 6 d | Severe hypernatremia/catecholamine-resistant septic shock/MODS |
| 13 y | Male | Post bone marrow transplant for thalassemia, Wilson disease | 52 d | Decompensated chronic liver disease/septic shock |
| 1 y | Female | Complete AV canal defect | 44 d | Severe pulmonary hypertension crisis; post cardiac arrest state |
| 1 y | Female | Aplastic anemia | 4 d | Refractory septic shock; fungal pneumonia |
| 14 y | Male | B Cell acute lymphoblastic leukemia; febrile neutropenia | 22 d | Febrile neutropenia; klebsiella sepsis |
| 6 mo | Female | Dilated cardiomyopathy | 1 d | Decompensated cardiogenic shock/post cardiac arrest state |
| 4 y | Male | Chronic granulomatous disease; severe pneumonia | 8 d | Severe sepsis |
On analysis of the cases diagnosed during the 1st and 2nd waves (Table 2), it was found that loose stools and rash were significantly more frequent during the 1st wave and fever, cough coryza, heart rate and temperature were significantly more during the 2nd wave.There was no significant difference between the two groups in terms of requirement of oxygen therapy, need for ICU admission, duration of ICU stay or hospital stay, or severity of illness. Steroids and anti-coagulants were more frequently used during the 1st wave. Mortality was significantly higher during the 2nd wave (0.3% vs. 2%).
Table 2.
Comparison of clinical features, laboratory investigations, treatment, severity of illness, and mortality between wave 1 and wave 2
| Variables | Wave 1 N = 585 |
Wave 2 N = 403 |
p value |
|---|---|---|---|
| Age | |||
| < 1 y (n, %) | 82 (14) | 62 (15.3) | |
| 1–5 y (n, %) | 147 (25.1) | 83 (20.6) | 0.386 |
| 6–10 y (n, %) | 156 (26.7) | 101 (25.1) | |
| 11–16 y (n, %) | 200 (34.2) | 157 (39) | |
| Sex | |||
|
Male (n, %) Female (n, %) |
348 (59.5) 237 (40.5) |
232 (57.61) 171 (42.4) |
0.547 |
| Comorbidities present (n, %) | 289 (49.4%) | 182 (45.2%) | 0.092 |
| Fever (n, %) | 220 (37.6) | 221 (54.8) | < 0.001 |
| Cough (n, %) | 68 (11.6) | 112 (27.8) | < 0.001 |
| Coryzal symptoms (n, %) | 48 (6.2) | 62 (15.4) | < 0.001 |
| Breathing difficulty (n, %) | 30 (5.1) | 32 (7.9) | 0.073 |
| Nausea vomiting (n, %) | 56 (9.6) | 25 (5.7) | 0.058 |
| Loose stools (n, %) | 37 (6.3) | 10 (2.5) | 0.005 |
| Abdominal pain (n, %) | 22 (3.8) | 9 (2.2) | 0.176 |
| Lethargy (n, %) | 2 (0.3%) | 0 | 0.240 |
| Seizures (n, %) | 21 (3.6) | 19 (4.7) | 0.378 |
| Headache (n, %) | 12 (2.1) | 8 (2) | 0.947 |
| Respiratory distress (n, %) | 39 (6.7) | 32 (7.9) | 0.446 |
| Rash (n, %) | 19 (3.2) | 4 (1.0) | 0.021 |
| Hepatomegaly (n, %) | 19 (3.2) | 13 (3.2) | 0.985 |
| Splenomegaly (n, %) | 8 (1.4) | 8 (2.0) | 0.450 |
| Heart rate (mean, SD) |
108.23 (26.183) n = 585 |
113.00 (25.202) n = 403 |
0.004 |
| Respiratory rate (mean, SD) |
29.58 (8.493) n = 585 |
29.89 (7.720) n = 403 |
0.554 |
| Temperature (mean, SD) |
98.919 (1.52) n = 585 |
99.704 (1.71) n = 403 |
< 0.001 |
| Hemoglobin (mean, SD) |
11.28 (2.26) n = 362 |
11.047 (2.17) n = 186 |
0.25 |
| Total count (mean, SD) |
11,349 (19640) n = 336 |
9761 (11381) n = 169 |
0.33 |
| Platelet (mean, SD) |
279,626 (137897) n = 340 |
256,572 (140195) n = 170 |
0.08 |
| Creatinine (mean, SD) |
0.49 (0.62) n = 320 |
0.55 (0.85) n = 164 |
0.37 |
| ALT (median, IQR) |
18 (14,26) n = 254 |
19 (15,35) n = 124 |
0.08 |
| Prothrombin time (mean, SD) |
16.2 (5.4) n = 87 |
17.9 (7.1) n = 61 |
0.1 |
|
Partial thromboplastin time (mean, SD) |
38.7 (12.78) n = 85 |
37 (10) n = 60 |
0.4 |
| Ferritin (mean, SD) |
725 (2311) n = 162 |
1315 (2625) n = 44 |
0.147 |
| D-dimer (mean, SD) |
2409 (4714) n = 179 |
3316 (5124) n = 55 |
0.223 |
|
C-reactive protein (mean, SD) |
38.7 (63.4) n = 145 |
26.1 (50.6) n = 71 |
0.15 |
|
Intravenous fluid administration (n, %) |
129 (22.1%) | 70 (17.4%) | 0.071 |
| Oxygen use | |||
| No oxygen (n, %) | 516 (88.2) | 360 (89.3) | |
| Low flow (n, %) | 11 (1.9) | 5 (1.2) | |
| High flow (n, %) | 46 (7.9) | 24 (6) | 0.441 |
| NIV/CPAP (n, %) | 3 (0.5) | 3 (0.7) | |
| Mechanical ventilation (n, %) | 9 (1.5) | 11 (2.7) | |
| Inotropes (n, %) | 36 (6.2) | 25 (6.2) | 0.975 |
| Antibiotics (n, %) | 164 (28) | 105 (26) | 0.317 |
| Glucocorticoids (n, %) | 44 (7.5) | 11 (2.7) | 0.003 |
| Anticoagulants (n, %) | 31 (5.3) | 8 (2.0) | 0.016 |
| ICU admission (n, %) | 52 (8.9) | 30 (7.4) | 0.48 |
| Duration of ICU stay (mean, SD) |
7 (4) n = 52 |
8 (5) n = 29 |
0.21 |
|
Duration of hospital stay (mean, SD) |
8 (4) n = 434 |
8 (8) n = 122 |
0.52 |
| Severity | |||
| Asymptomatic/mild (%) | 520 (88.9) | 363 (90.1) | |
| Moderate (%) | 18 (3.1) | 9 (2.2) | |
| Severe (%) | 2 (0.3) | 4 (1) | 0.055 |
| Critical (%) | 32 (5.5) | 26 (6.5) | |
| MIS-C (%) | 13 (2.2) | 1 (0.25) | |
| Deaths | 2 (0.3) | 8 (2) | 0.03 |
ALT Alanine amino transferase, CPAP Continuous Positive Airway Pressure, ICU Intensive care unit, MIS-C Multisystem Inflammatory Syndrome in Children, NIV Non Invasive ventilation
Discussion
Since the start of the COVID-19 pandemic, India has witnessed two waves with considerable morbidity and mortality during the second wave. The present study is a description of COVID-19 disease in children and in which the differences between the two waves are compared, which has not been reported in India so far. The present data is till May 31, 2021 which was just after the peak of the 2nd wave [16]. It was found that there was no significant difference between the spectrum of severity of COVID-19 among children during the two waves. Nearly 90% of the cases were asymptomatic or had mild symptoms during both waves. However, the number of deaths during the second wave in the present study were significantly higher, even though most deaths were due to severe underlying comorbid conditions. Though there is little information from India on the comparison between the two waves, the higher fatality rate in the present study during the 2nd wave is in contrast to estimated infection fatality rate (IFR) in India of 0.46% for wave 1 and 0.18% for wave 2 after accounting for underreporting of deaths (estimating the wave 1 and wave 2 infection fatality rates from SARS-CoV-2 in India) [18].
Lower severity of disease and mortality have been reported with successive waves in other countries which had their second wave much earlier than in India [19, 20]. The mortality rate of 1% in the present study is lower than the reported mortality rate of 1.18% in the whole of India as on 31st May, 2021. Nine out of the 10 deaths in this study were children who had very severe comorbidities. Since the authors' hospital is a multispeciality tertiary care center, almost 50% of the children had comorbidities and this referral bias would have contributed to a higher death rate and the actual death rate among children would be much lower.
The most common symptoms were fever, cough and coryza which is similar to a large meta-analysis on 7780 children by Hoang et al. [21]. Seizures were present in 4% of the children. Though neurological manifestations of COVID-19 have been described, it is often headache, dizziness, impaired consciousness and acute cerebrovascular events. Seizures have been reported in 0.5% of COVID-19 patients in China. Simple febrile seizures which was the most common cause (35%) in the present study has been reported anecdotally in children [22]. Epilepsy syndromes which was the next frequent cause of seizure (32.5%) has also been described anecdotally to be associated with COVID-19 [23]. The use of glucocorticoids in the present study was in 5.5% of children, which was higher than the 4.1% glucocorticoid use in large systematic review with 7780 patients [21]. In the present study, the only antiviral drug used was remdesivir which was used only in 2 children who had a good outcome. Its use in adults has shown to decrease median recovery time from 15 to 10 d but not mortality [24]. There are little clinical trial data on its use in children and anecdotal reports have shown some benefit [25].
Almost 90% of the children had asymptomatic or mild disease. This is similar to the US database where 11.7% of their children required admission [26]. Need for admission was not analyzed or compared in the present study, as the criteria for admission differed based on the address of the patient and the existing district administrative protocols during different months. In some months children were required to be admitted for isolation purposes, even if they were asymptomatic. There were 82 (8.3%) children who required ICU admission. This is similar to the study from Madrid where 10% of their children required ICU admissions [27]. There was a higher proportion of infants requiring ICU admission (20.7% vs. 14%) similar to the study from China where more a higher proportion of infants had severe and critical disease [12]. Thirty percent of the deaths in the present cohort were in the infant age group. MIS-C was diagnosed in only 1.4% of the patients in the present study. This estimate is low because only children who had a positive SARS-CoV-2 RT-PCR, which is positive in 13%–69% of the patients diagnosed with MIS-C, were included [28]. Also, higher number of children were diagnosed with MISC in the 1st wave compared to the 2nd wave (2.2% vs. 0.25%). This difference is likely because the peak for MIS-C usually follows the peak for COVID-19 cases by 2–4 wk [29, 30] since it is hypothesized to be a post infectious syndrome that is distinct from COVID-19 [11]. The present study included the whole of the first peak, but only covered a part of the second peak and the study was stopped 1–2 wk after the peak of the 2nd wave. It is likely that there would have been more children with MIS-C in the following weeks.
There are some limitations in the present study. First, the study is from a single center, which is a multispeciality tertiary care center, and hence, is prone to referral bias. It is likely that there would have been a more severe end of the spectrum of COVID-19 disease and more comorbidities. Secondly, the study spanned the entire first wave, but did not include the entire 2nd wave. It is possible that there may be some differences in the severity of illness, especially MIS-C, if the entire 2nd wave had been included. Thirdly, sequencing data was not there to determine the variants that were responsible for the two waves at the authors' center.
Conclusion
The COVID-19 pandemic among children during the 1st and 2nd waves were similar in severity, though there was a higher mortality during the 2nd wave.
Acknowledgements
The authors wish to thank Mr. Balamurugan who helped with data collection.
Authors' Contributions
LGM, WR: Concept, study design, revision and final approval of manuscript; MTP: Data collection and writing of initial draft; UG, AP, RJR, JC, DDA: Revision of manuscript; All authors approved the manuscript. WR will act as the guarantor for this paper.
Declarations
Ethics Approval
The study was approved by the Institutional Review Board and Ethics Committee of Christian Medical College, Vellore. IRB min no: 12757 dated 1.5.2020.
Conflict of Interest
None.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yasuhara J, Kuno T, Takagi H, Sumitomo N. Clinical characteristics of COVID-19 in children: A systematic review. Pediatr Pulmonol. 2020;55:2565–2575. doi: 10.1002/ppul.24991. [DOI] [PubMed] [Google Scholar]
- 2.Perikleous E, Tsalkidis A, Bush A, Paraskakis E. Coronavirus global pandemic: An overview of current findings among pediatric patients. Pediatr Pulmonol. 2020;55:3252–67. [DOI] [PMC free article] [PubMed]
- 3.Bayesheva D, Boranbayeva R, Turdalina B, et al. COVID-19 in the paediatric population of Kazakhstan. PaediatrInt Child Health. 2021;41:76–82. doi: 10.1080/20469047.2020.1857101. [DOI] [PubMed] [Google Scholar]
- 4.Iannarella R, Lattanzi C, Cannata G, et al. Coronavirus infections in children: from SARS and MERS to COVID-19, a narrative review of epidemiological and clinical features. Acta Biomed. 2020;91:e2020032. [DOI] [PMC free article] [PubMed]
- 5.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. International COVID-19 PICU Collaborative. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174:868–73. [DOI] [PMC free article] [PubMed]
- 6.CDC COVID-19 Response Team. Coronavirus disease in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2019;2020(69):422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jahangir M, Nawaz M, Nanjiani D, Siddiqui MS. Clinical manifestations and outcomes of COVID-19 in the paediatric population: a systematic review. Hong Kong Med J. 2021;27:35–45. doi: 10.12809/hkmj208646. [DOI] [PubMed] [Google Scholar]
- 8.Lingappan K, Karmouty-Quintana H, Davies J, Akkanti B, Harting MT. Understanding the age divide in COVID-19: why are children overwhelmingly spared? Am J Physiol Lung Cell Mol Physiol. 2020;319:L39–44. doi: 10.1152/ajplung.00183.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahase E. Covid-19: What have we learnt about the new variant in the UK? BMJ. 2020;371:m4944. [DOI] [PubMed]
- 10.Yonker LM, Neilan AM, Bartsch Y, et al. Pediatric severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): clinical presentation, infectivity, and immune responses. J Pediatr. 2020;227:45–52. doi: 10.1016/j.jpeds.2020.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feldstein LR, Tenforde MW, Friedman KG, et al. Overcoming COVID-19 Investigators. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325:1074–87. [DOI] [PMC free article] [PubMed]
- 12.Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145:e20200702. [DOI] [PubMed]
- 13.Rivas-Ruiz R, Roy-Garcia IA, Ureña-Wong K, et al. Factors associated with death in children with COVID-19 in Mexico. Gac Med Mex. 2021;156:516–522. doi: 10.24875/GMM.M21000478. [DOI] [PubMed] [Google Scholar]
- 14.World Heath Organization.Tracking SARS-CoV-2 variants.World Heath Organization. Available at: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/. Accessed on 13 Aug 2021.
- 15.Kumar A, Asghar A, Dwivedi P, et al. Second wave of COVID-19 in India could be predicted with genomic surveillance of SARS-CoV-2 variants coupled with epidemiological data: A tool for future. medRxiv. 2021. 10.1101/2021.06.09.21258612
- 16.World Health Organization. Coronavirus disease (COVID-19) pandemic. World Health Organization. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed on 13 Aug 2021.
- 17.World Health Organization. Multisystem inflammatory syndrome in children and adolescents temporally related to COVID-19. World Health Organization. Available at: https://www.who.int/news-room/commentaries/detail/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19. Accessed on 13 Aug 2021.
- 18.Purkayastha S, Kundu R, Bhaduri R, et al. Estimating the wave 1 and wave 2 infection fatality rates from SARS-CoV-2 in India. BMC Res Notes. 2021;14:262. doi: 10.1186/s13104-021-05652-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan G, Yang Z, Lin Q, Zhao S, Yang L, He D. Decreased case fatality rate of COVID-19 in the second wave: A study in 53 countries or regions. Transbound Emerg Dis. 2021;68:213–215. doi: 10.1111/tbed.13819. [DOI] [PubMed] [Google Scholar]
- 20.Iftimie S, López-Azcona AF, Vallverdú I, et al. First and second waves of coronavirus disease-19: A comparative study in hospitalized patients in Reus, Spain. PLoS ONE. 2021;16:e0248029. [DOI] [PMC free article] [PubMed]
- 21.Hoang A, Chorath K, Moreira A, et al. COVID-19 in 7780 pediatric patients: A systematic review. E Clinical Medicine. 2020;24:100433. [DOI] [PMC free article] [PubMed]
- 22.Dewiyanti L, Sumarni N, Lie JD, et al. Children with COVID-19 who manifest febrile seizure. Case Rep Med. 2021;2021:9992073. doi: 10.1155/2021/9992073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.García-Howard M, Herranz-Aguirre M, Moreno-Galarraga L, et al. Case report: benign infantile seizures temporally associated with COVID-19. Front Pediatr. 2020;8:507. doi: 10.3389/fped.2020.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beigel JH, Tomashek KM, Dodd LE, et al. ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383:1813–26. [DOI] [PMC free article] [PubMed]
- 25.Méndez-Echevarría A, Pérez-Martínez A, Gonzalez Del Valle L, et al. Compassionate use of remdesivir in children with COVID-19. Eur J Pediatr. 2021;180:1317–1322. doi: 10.1007/s00431-020-03876-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preston LE, Chevinsky JR, Kompaniyets L, et al. Characteristics and disease severity of US children and adolescents diagnosed with COVID-19. JAMA Netw Open. 2021;4:e215298. [DOI] [PMC free article] [PubMed]
- 27.Tagarro A, Epalza C, Santos M, et al. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr. 2020. 10.1001/jamapediatrics.2020.1346 [DOI] [PMC free article] [PubMed]
- 28.Abrams JY, Godfred-Cato SE, Oster ME, et al. Multisystem inflammatory syndrome in children associated with severe acute respiratory syndrome coronavirus 2: a systematic review. J Pediatr. 2020;226:45–54. doi: 10.1016/j.jpeds.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dufort EM, Koumans EH, Chow EJ, et al. Multisystem inflammatory syndrome in children in New York state. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 202l;383:334–46. [DOI] [PMC free article] [PubMed]