Abstract
Background
The mortality of COVID-19 patients who are admitted to a hospital because of the disease remains high. The implementation of evidence-based treatments can improve the quality of care.
Methods
The new clinical practice guideline is based on publications retrieved by a systematic search in the Medline databases via PubMed and in the Cochrane COVID-19 trial registry, followed by a structured consensus process leading to the adoption of graded recommendations.
Results
Therapeutic anticoagulation can be considered in patients who do not require intensive care and have an elevated risk of thromboembolism (for example, those with D-dimer levels ≥ 2 mg/L). For patients in intensive care, therapeutic anticoagulation has no benefit. For patients with hypoxemic respiratory insufficiency, prone positioning and an early therapy attempt with CPAP/noninvasive ventilation (CPAP, continuous positive airway pressure) or high-flow oxygen therapy is recommended. Patients with IgG-seronegativity and, at most, low-flow oxygen should be treated with SARS-CoV-2-specific monoclonal antibodies (at present, casirivimab and imdevimab). Patients needing no more than low-flow oxygen should additionally be treated with janus kinase (JAK) inhibitors. All patients who need oxygen (low-flow, high-flow, noninvasive ventilation/CPAP, invasive ventilation) should be given systemic corticosteroids. Tocilizumab should be given to patients with a high oxygen requirement and progressively severe COVID-19 disease, but not in combination with JAK inhibitors.
Conclusion
Noninvasive ventilation, high-flow oxygen therapy, prone positioning, and invasive ventilation are important elements of the treatment of hypoxemic patients with COVID-19. A reduction of mortality has been demonstrated for the administration of monoclonal antibodies, JAK inhibitors, corticosteroids, tocilizumab, and therapeutic anticoagulation to specific groups of patients.
The guideline on the intensive care management of patients with COVID-19 (1), first drawn up in March 2020, has—as a “living guideline”—subsequently been updated several times and expanded to cover all areas of in-hospital treatment (2, 3). In the following, particular attention is being paid to treatment recommendations and amendments. The guideline documents, including the long version of the guideline and the evidence report, are available in German at: https://www.awmf.org/leitlinien/detail/ll/113–001LG.html (4).
Methods
This S3-level clinical practice guideline was drawn up in accordance with the criteria of the Association of the Scientific Medical Societies in Germany (AWMF, Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften). The Guideline Development Group is made up of 24 mandate holders from the 16 participating specialist societies as well as one patient representative (eBox). The evidence supporting the recommendations was compiled within the framework of the CEOsys project with funding of the German Network University Medicine, using the digital guideline tool MAGICapp. CEOsys is funded by the German Federal Ministry of Education and Research (BMBF, Bundesministerium für Bildung und Forschung).
eBOX.
Composition of the guideline group (collaborators*)
-
Guideline authors
Prof. Dr. Stefan Kluge, Klinik für Intensivmedizin, Universitätsklinikum Hamburg-Eppendorf, Germany
Prof. Dr. Uwe Janssens*, Klinik für Innere Medizin, St.-Antonius-Hospital Eschweiler, Germany
Prof. Dr. Tobias Welte*, Klinik für Pneumologie, Medizinische Hochschule Hannover, Germany
Prof. Dr. Steffen Weber-Carstens*, Klinik für Anästhesiologie mit Schwerpunkt operative Intensivmedizin, Charité – Universitätsmedizin Berlin, Germany
Prof. Dr. Gereon Schälte*, Klinik für Anästhesiologie, Uniklinik RWTH Aachen, Germany
PD Dr. Christoph D. Spinner*, Klinikum rechts der Isar, Klinik und Poliklinik für Innere Medizin II, Technische Universität München, Fakultät für Medizin, München, Germany
Dr. Jakob J. Malin, Klinik I für Innere Medizin, Klinische Infektiologie, Medizinische Fakultät und Uniklinik Köln, Universität zu Köln, Germany
Prof. Dr. Petra Gastmeier*, Institut für Hygiene und Umweltmedizin, Charité – Universitätsmedizin Berlin, Germany
Prof. Dr. Florian Langer*, Hämostaseologie, II. Medizinische Klinik und Poliklinik, Universitätsklinikum Hamburg-Eppendorf, Germany
PD Dr. Martin Wepler*, Klinik für Anästhesiologie und Intensivmedizin, Universitätsklinikum Ulm, Germany
PD Dr. Michael Westhoff*, Klinik für Pneumologie, Lungenklinik Hemer, Germany
Prof. Dr. Michael Pfeifer*, Klinik und Poliklinik für Innere Medizin II, Universitätsklinikum Regensburg, Germany
Prof. Dr. Klaus F. Rabe*, Abteilung für Pneumologie, LungenClinic Grosshansdorf, Germany
PD Dr. Florian Hoffmann*, Kinderklinik und Kinderpoliklinik im Dr. von Haunerschen Kinderspital, LMU Klinikum München, Germany
Prof. Dr. Bernd W. Böttiger*, Klinik für Anästhesiologie und Operative Intensivmedizin, Medizinische Fakultät und Uniklinik Köln, Universität zu Köln, Germany
Prof. Dr. Julia Weinmann-Menke*, Schwerpunkt Nephrologie, Universitätsmedizin Mainz, Germany
Dr. Alexander Kersten*, Klinik für Kardiologie, Angiologie und Internistische Intensivmedizin (Medizinische Klinik I), Uniklinik RWTH Aachen, Germany
Prof. Dr. Peter Berlit*, Deutsche Gesellschaft für Neurologie, Berlin
Prof. Dr. Marcin Krawczyk*, Klinik für Innere Medizin II, Universitätsklinikum des Saarlandes, Germany
Dr. Wiebke Nehls*, Klinik für Palliativmedizin und Geriatrie, Helios Klinikum Emil von Behring, Germany
Reiner Haase*, Delingsdorf, Germany
Prof. Dr. Oliver J. Müller, Klinik für Innere Medizin III, Universitätsklinikum Schleswig-Holstein, Campus Kiel, Germany
Dr. Monika Nothacker*, AWMF-Institut für Medizinisches Wissensmanagement Marburg/Berlin, Germany
Prof. Dr. Gernot Marx*, Klinik für Operative Intensivmedizin und Intermediate Care, Uniklinik RWTH Aachen, Germany
Prof. Dr. Christian Karagiannidis, Abteilung Pneumologie, Intensiv- und Beatmungsmedizin, Lungenklinik Köln-Merheim, Germany
-
COVID-19 Evidence Ecosystem (CEOsys) Project
Lead:
Dr. Falk Fichtner, PD Dr. Sven Laudi*, Klinik und Poliklinik für Anästhesiologie und Intensivtherapie, Universitätsklinikum Leipzig, Germany
Dr. Miriam Stegemann*, Medizinische Klinik mit Schwerpunkt Infektiologie und Pneumologie der Charité – Universitätsmedizin Berlin, Germany
Dr. Stephanie Weibel*, Klinik und Poliklinik für Anästhesiologie, Intensivmedizin, Notfallmedizin und Schmerztherapie, Universitätsklinikum Würzburg, Germany
Prof. Dr. Nicole Skoetz, Klinik für Innere Medizin I, Medizinische Fakultät und Uniklinik Köln, Universität zu Köln, Germany
-
Participating specialist societies and interest groups
Lead:
German Society of Medical Intensive Care and Emergency Medicine (DGIIN)
German Interdisciplinary Association for Intensive Care Medicine and Emergency Medicine (DIVI)
German Respiratory Society (DGP)
German Society for Infectious Diseases (DGI)
With the collaboration of:
German Society of Anesthesiology and Intensive Care Medicine (DGAI)
German Society for Hygiene and Microbiology (DGHM)
Society of Thrombosis and Haemostasis Research (GTH)
German Society for Pediatric and Adolescent Medicine (DGKJ)
German Resuscitation Counci (GRC)
German Society of Internal Medicine (DGIM)
German Society of Nephrology (DGfN)
German Cardiac Society (DGK)
German Society of Neurology (DGN)
German Society of Gastroenterology, Digestive and Metabolic Diseases (DGVS)
German Society of Palliative Medicine (DGP)
German Society of Angiology (DGA)
Patient representation (individual affected person)
AWMF Institute of Medical Knowledge Management c/o Philipps Universität Marburg/AWMF Berlin, Germany
The literature search was conducted via the Cochrane COVID-19 Study Register which uses the databases Medline, Embase, CENTRAL, ClinicalTrials.gov, WHO International Clinical Trials Registry Platform (ICTRP), the search service Medrxiv, and the blog RetractionWatch as data sources. The last search was conducted on 1 September 2021; however, current publications until 30 October 2021 were taken into account.
Only randomized controlled trials (RCTs) were included to answer the systematically addressed treatment questions. The studies were rated based on their endpoints, using the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach.
The recommendations were graded in three levels according to the AWMF guideline rules:
Strong recommendation = should/should not
Recommendation = ought to / ought not to
Recommendation open = may (be considered)/may (be disregarded).
Non-pharmacological management of acute hypoxemic respiratory failure
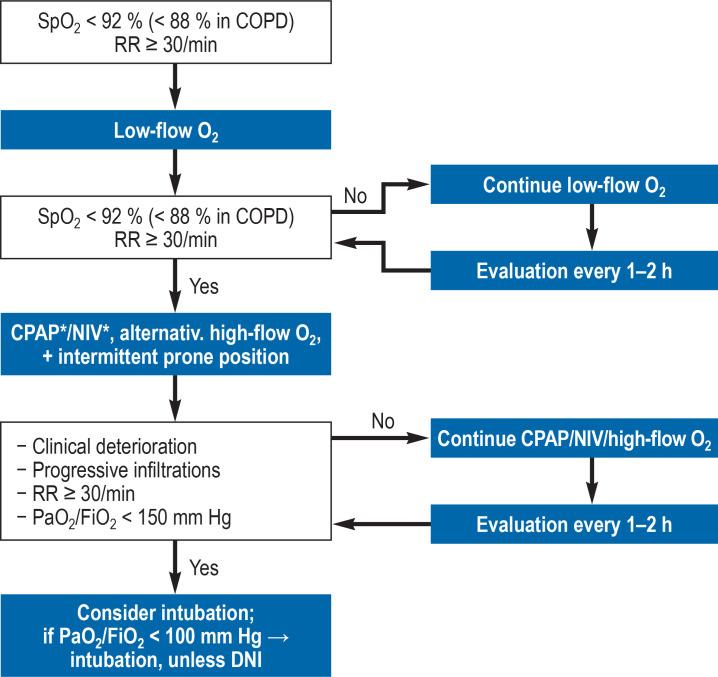
The primary goal is to restore adequate oxygenation; a blood oxygen saturation (SpO2) ≥ 92% ought to be achieved. The first step in the treatment of hypoxemia is the administration of oxygen (figure 1). In case of progressive gas exchange deterioration and increased supplemental oxygen requirement, continuous positive airway pressure (CPAP)/non-invasive ventilation (NIV) or high-flow nasal cannula (HFNC) oxygen therapy is used. During this treatment, patients are continuously monitored and intubation readiness is ensured at all times.
Figure 1.
Possible escalation of device-based therapy in case of acute respiratory insufficiency due to COVID-19 *Initial setting: CPAP (10 mbar); NIV (PEEP 5–10 mbar + 6–10 mbar); target: Vt<9 mL/kg
RR, respiratory rate; O2, oxygen therapy; COPD, chronic obstructive pulmonary disease; CPAP, continuous positive airway pressure; DNI, do not intubate; FiO2, fraction of inspired oxygen; NIV, non-invasive ventilation“ PaO2, arterial partial pressure of oxygen in mm Hg; PEEP, positive end-expiratory pressure;
SpO2, oxygen saturation in the blood; Vt, tidal volume
In a randomized controlled trial comparing HFNC and helmet NIV, the intubation rate was significantly lower in patients treated with helmet NIV (51% versus 30%); however, no significant difference was found in respect to the 28-day interval with respiratory support (primary endpoint; HFNC, NIV, invasive mechanical ventilation) (5). Since there is a lack of further RCTs, comparing the benefits and risks of invasive mechanical ventilation versus CPAP/NIV/HFNC in patients with COVID-19, the recommendations for non-COVID setting are used.
We propose to attempt treatment with HFNC or CPAP/NIV in patients with hypoxemic respiratory failure (PaO2/FiO2 = 100–300 mm Hg) under continuous monitoring and constant intubation readiness. In case of severe hypoxemia (PaO2/FiO2 <150 mm Hg) and respiratory rates >30/min, intubation and invasive mechanical ventilation should be considered; in patients with PaO2/FiO2 of <100 mm Hg, intubation and invasive mechanical ventilation ought to be routinely performed.
In a prospective study, hypoxemic patients with COVID-19 (PaO2/FiO2 ≤ 300 mm Hg) were treated with HFNC (6). The patients were randomly assigned to a group with awake prone positioning or a control group. The primary endpoint (intubation or death within 28 days) occurred in 40% and 46% of patients in the prone positioning group and control group, respectively (relative risk [RR]: 0.86; 95% confidence interval: [0.75; 0.98], p = 0.02). The number of intubations was significantly lower in the prone positioning group (33% versus 40%). Thus, patients receiving high-flow nasal cannula (HFNC) oxygen therapy or CPAP/NIV also ought to be positioned in prone position.
Invasive ventilation and adjuvant measures
Due to the lack of RCTs on mechanical ventilation in patients with COVID-19, here the recommendations are derived from the most recently published guidelines on invasive mechanical ventilation in patients with acute respiratory failure (7, 8). These include recommendations on tidal volume (≤ 6 mL/kg ideal body weight) and end-inspiratory airway pressure (PEI) ≤ 30 cm H2O). As guidance for setting the positive end-expiratory pressure (PEEP) in COVID-19 patients, the low FiO2/PEEP table of the Acute Respiratory Distress Syndrome (ARDS) Network ought to be taken into account.
Patients with ARDS and PaO2/FiO2 <150 mm Hg should be positioned in prone position for at least 16 hours (8). In patients with severe ARDS and refractory hypoxemia (PaO2/FiO2 < 80 and 60 mm Hg, respectively), venovenous (VV) extracorporeal membrane oxygenation is a treatment option to stabilize gas exchange.
Thromboprophylaxis/ anticoagulation
Thromboembolic events are a common complication of COVID-19. Therefore, all in-patients should receive thromboprophylaxis with low-molecular-weight heparins (LMWHs) (e.g. enoxaparin 4000 IE, dalteparin 5000 IE or tinzaparin 4500 IE). Alternatively, for example in patients with heparin intolerance, fondaparinux may be administered.
Observational studies suggested that standard dosing regimens of LMWH may not be effective enough for VTE prophylaxis. However, in a systematic meta-analysis published in early 2021, which mainly included retrospective observational studies, the VTE rate in patients receiving intermediate-dose thromboprophylaxis was not significantly lower than the VTE rate among patients with standard-dose prophylaxis (9). In the meantime, two prospective randomized trials have been published (10, 11) which allowed to include 763 hospitalized patients in a meta-analysis. No clinical benefit of half-therapeutic-dose anticoagulation could be demonstrated among the predominantly severely ill patients hospitalized for COVID-19; however, an increased rate of severe hemorrhagic events was noted. Thus, the Guideline Development Group makes a weak recommendation against LMWH prophylaxis with half-therapeutic dosing in hospitalized patients. On the other hand, in well-founded individual cases, for example patients with extreme obesity or a history of VTE events, intensified LMWH prophylaxis continues to be a valid treatment option, under careful consideration of the bleeding risk.
Therapeutic-dose anticoagulation in non-intensive care patients
The question of prophylactic versus therapeutic-dose anticoagulation with LMWH was addressed by the ATTACC, ACTIV-4a and REMAP-CAP study groups. They analyzed the data of 2219 hospitalized non-critical-care COVID-19 patients from their randomized open-label studies (12). Therapeutic-dose anticoagulation was associated with an increase in in-hospital survival without organ support by 4% compared to standard thromboprophylaxis. The treatment effect was stronger in patients with higher D-dimer levels. Thus, on a case-by-case basis, therapeutic anticoagulation, preferably with LMWH or unfractionated heparin, may be considered in hospitalized, non-critical-care patients at increased risk (e.g. D-dimers ≥ 2 mg/L) who have a low bleeding risk.
Intensive care patients
The evidence base for intensive care patients comprises two studies on parenteral anticoagulation with 1091 and 20 patients, respectively (13, 14). For the endpoint “occurrence of a thrombotic effect or mortality by day 28“, the effect estimate showed a relative risk of 0.99 [0.86; 1.14], i.e. no significant clinically relevant effect. However, therapeutic anticoagulation reduced the incidence of thrombotic events (RR 0.65 [0.49; 0.85]). The risk of major bleeding was nominally increased, but the increase was not statistically significant (RR 1.63 [0.82; 3.25]). Given the uncertainty of effects on the clinical course in intensive care patients, patients ought not to receive therapeutic-dose anticoagulation without specific indication (e.g. pulmonary embolism).
Drug treatment
Anti-infective therapy and general principles of therapy
Since bacterial co-infection is rare in the initial phase of the disease, antibiotic treatment is not generally recommended for patients with confirmed SARS-CoV-2 infection. If a bacterial pneumonia or a coinfection with extrapulmonary focus is suspected, guideline-adherent diagnosis and empiric antibiotic therapy should be initiated early on (15).
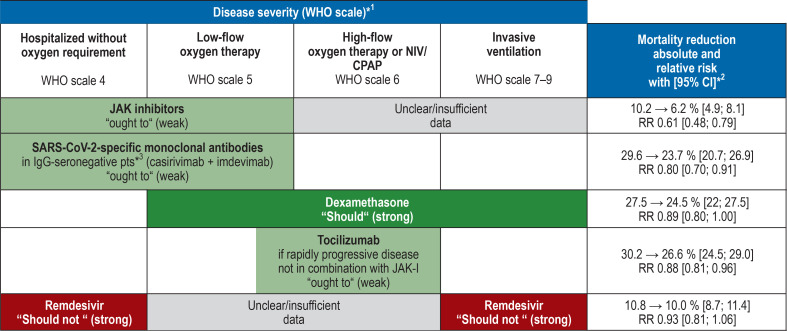
The following recommendations on antiviral and immunomodulatory treatment strategies are summarized in Figure 2 and the Table.
Figure 2.
Overview of the recommendations for drug therapy for COVID-19, according to disease severity
*1 WHO clinical progression scale (34)
*2 Data refer to the overall group of disease severity evaluated in randomized controlled trials (WHO scale). Effects in specific subgroups may differ.
*3 If same-day determination of the serostatus is not possible, patients with inadequate immunization (one vaccination, no vaccination or severe immunosuppression) may be treated with approved antibody drugs or antibody drugs recommended by the European Medicines Agency (EMA) within 72 hours for a maximum of 7 days after symptom onset (expert consensus).
Green = recommended drugs; red = not recommended drugs JAK inhibitors, janus kinase inhibitors; NIV, noninvasive ventilation, CPAP, continuous positive airway pressure, CI, confidence interval
Table. Evidence-based negative recommendations for COVID-19 drug therapy.
| Pharmacological intervention | Recommendation for hospitalized patients with COVID-19 | Mortality reduction absolute and relative risk [95% CI] | Quality of evidence (for mortality) |
| Convalescent plasma | Should not | 23.7→ 23.3 % [21.8; 24.9] RR 0.98 [0.92; 1.05] |
High |
| Ivermectin | Should not | 9.6→ 5.8 % [1.3; 24.1] RR 0.6 (0.14−2.51) |
Very low |
| Vitamin D | Should not | Not pooled (heterogeneity) | Very low |
| Azithromycin | Should not | 22.3→ 21.9 % [20.1; 23.6] RR 0.98 [0.9; 1.06] |
High |
| Bamlanivimab monotherapy | Should not | 2.7→ 3.8 % [1.1; 13.0] RR 1.39 [0.4; 4.83] |
Low |
| Anakinra | Should not | 23.6→ 21.9 % [11.1; 43.2] RR 0.93 [0.47; 1.83] |
Moderate |
| Colchicin | Should not | 20.7→ 20.7 % [19.3; 22.4] RR 1 [0.93; 1.08] |
Moderate |
CI, confidence interval; RR, relative risk
Antiviral treatment strategies
Monoclonal neutralizing antibodies
Casirivimab/imdevimab
The recommendation to administer these monoclonal neutralizing antibodies is based on data from a total of 9785 hospitalized patients (16). In patients without detectable SARS-CoV-2 immunoglobulin G (IgG-seronegative), treatment with casirivimab/imdevimab combination therapy reduced the 28-day mortality from 29.6% to 23.7% (RR 0.8 [0.70 0.91]). However, no positive effects on 28-day mortality (primary endpoint) could be demonstrated for the total (IgG-negative and IgG-positive) patient population.
In doses of 600–4000 mg, casirivimab and imdevimab showed good safety and tolerability (17, 18). In sporadic cases, allergic reactions may occur. Given the positive effects on mortality and the disease course in IgG-seronegative patients, the Guideline Development Group makes a weak recommendation for their use in seronegative patients without supplemental oxygen requirement or with low-flow oxygen therapy. Comparing the various doses used in studies (600 mg vs. 1200 mg vs. 4000 mg), the currently available data do not suggest that a relevant dose-response relationship exists. Administration is once by intravenous (IV) infusion at an approved dose or at a dose recommended by the European Medicines Agency (EMA).
Monoclonal antibodies in patients with unknown serostatus
Currently, there is insufficient data available to conclude on the use of SARS-CoV-2-specific monoclonal antibodies (MABs) in hospitalized patients with unknown serostatus (serum anti-SARS-CoV-2 IgG levels). In clinical practice, however, same-day determination of the serostatus has so far often proved impractical. As a rule, MABs should be administered as early as possible after diagnosis and their use should not be delayed by waiting longer than 24 hours for test results. Consequently, if it is not possible to determine the serostatus on the same day, treatment with SARS-CoV-2-specific MABs may be initiated in hospitalized patients with incomplete immunization or with severe immunosuppression and previous SARS-CoV-2 infection within 72 hours, but no longer than 7 days after the onset of symptoms (expert consensus). Administration is once by intravenous (IV) infusion at an approved dose or at a dose recommended by the EMA.
Bamlanivimab monotherapy
The ACTIV3-/TICO study failed to demonstrate a clinical benefit of bamlanivimab in moderately to severely ill patients hospitalized for COVID-19 (19). Bamlanivimab has no in-vitro effectiveness against the currently prevailing delta variant. Thus, the Guideline Development Group makes a strong recommendation against the use of bamlanivimab as a monotherapy in patients hospitalized for COVID-19.
Remdesivir
Based on data from five RCTs with a total of 7452 patients, no significant advantage was found for the use of remdesivir compared to standard therapy with regard to 28-day mortality and other clinical endpoints in the overall group (20). The findings of the individual studies are heterogeneous. In a randomized, double-blind trial including 1062 patients (ACTT-1), treatment with remdesivir for 10 days reduced the time to recovery from 15 days to 10 days (median) (21). However, the largest RCT with 2750 patients (SOLIDARITY) showed no significant advantage with regard to the endpoints mortality, initiation of mechanical ventilation and duration of hospitalization (22). In the most recently published DisCoVeRy study with 857 patients who required oxygen support or had pneumonic infiltrates, treatment with remdesivir was not superior to standard of care (23). In all studies, remdesivir was well tolerated. Remdesivir was associated with less severe adverse events compared to placebo.
Given the lack of clinical benefits, the Guideline Development Group makes a strong recommendation against the use of remdesivir in patients without supplemental oxygen requirement and in patients with invasive ventilation support. Due to currently insufficient data quality with regard to clinical benefits, no recommendation can be made either for or against the use of remdesivir in patients with low-flow/high-flow oxygen therapy or noninvasive ventilation.
Convalescent plasma
The meta-analysis of nine RCTs with a total of 12 762 patients showed no advantage of treatment with convalescent plasma with regard to 28-day mortality and clinical status, but a trend toward an increased incidence of serious adverse events (24). Thus, hospitalized patients with COVID-19 should not be treated with convalescent plasma.
Azithromycin
Given that a meta-analysis of six RCTs, including a total of 8790 patients, found no benefit of azithromycin treatment, but a trend toward higher rates of serious adverse events, the Guideline Development Group made a strong recommendation against the use of azithromycin as an antiviral therapy in COVID-19 patients (25).
Ivermectin
The meta-analysis of five RCTs with a total of 393 patients showed no beneficial effects on 28-day mortality and clinical status (26). Given its uncertain benefits and its potential toxic effects and interactions with other drugs as well as the risk of improper use of ivermectin, the Guideline Development Group makes a strong recommendation against the use of ivermectin in patients with COVID-19.
Immunomodulatory treatment strategies
Corticosteroids
A meta-analysis based on nine RCTs with a total of 7930 patients found a reduction in mortality up to day 28 in patients treated with systemic corticosteroids. This reduction is not statistically significant in the overall group (reduction from 27.5% to 24.5% (RR 0.89 [0.80; 1.00]) (27). By contrast, the subgroup analysis showed a significant positive effect in patient groups requiring oxygen therapy or any other type of respiratory support (RR 0.86 [0.76; 0.97]). At the same time, there was evidence of increased 28-day mortality in patients without supplemental oxygen requirement (RR 1.27 [1.0; 1.6]) (4).
The most conclusive evidence is available for dexamethasone administered at a dose of 6 mg once a day. The safety and tolerability of corticosteroids is very good. Given its positive effects on mortality in patients who need oxygen support and its good tolerability and availability, the Guideline Development Group makes a strong recommendation for the use of dexamethasone (6 mg orally/intravenously once daily for 10 days) in these patient groups. Patients without supplemental oxygen requirement should not be treated with systemic corticosteroids.
Janus kinase inhibitors
The evidence base for the use of janus kinase (JAK) inhibitors comprises four RCTs with a total of 2888 hospitalized patients treated with baricitinib, tofacitinib or ruxolitinib. All evaluated substances had a positive effect on 28-day mortality (reduction from 10.2% to 6.2% [4.9; 8.1]) in patients without oxygen requirement or with low-flow oxygen therapy (4). In these studies, JAK inhibitors showed a very good safety and tolerability profile. There were less adverse events and serious adverse events than with placebo. Given the proven small mortality benefit and the favorable side-effect profile, the Guideline Development Group makes a weak recommendation for the use of JAK inhibitors in hospitalized patients with COVID-19 without supplemental oxygen requirement or with low-flow oxygen therapy (e.g. baricitinib: 1 × 4 mg/day or tofacitinib 2 × 10 mg/day for a maximum of 14 days or until discharge from hospital). Due to the lack of data, JAK inhibitors should not be used in combination with tocilizumab.
Tocilizumab
Based on nine RCTs with a total of 6482 patients, a favorable effect of tocilizumab on 28-day mortality (reduction from 30.2% to 26.6% [24.5; 29]) and on the progression to invasive ventilation was found (28). A clinically relevant benefit can be derived for oxygen-dependent patients with progressive disease, but not for patients already on invasive ventilation. The RECOVERY trial, which included patients with increased C-reactive protein (CRP) levels of >75 mg/L as a surrogate marker of systemic inflammation (29), has the greatest impact on the positive effect in the evidence analysis. The tolerability profile in these studies was good; there is no evidence of increased serious adverse events to date.
Given the statistically significant, but small absolute effect, a weak recommendation was made for the use of tocilizumab in combination with corticosteroids in patients with rapidly progressive oxygen-dependent disease. There ought to be evidence of systemic inflammation (e.g. significantly increased CRP levels) and an increased supplemental oxygen requirement. Tocilizumab should not be used in patients with COVID-19 who have already been intensively ventilated over a period of more than 24 hours. Tocilizumab should strictly not be administered in cases of severe bacterial or fungal infection. Dosing is based on body weight (BW) (e.g. 600 mg in patients with 65–90 kg BW) and the drug is administered as an intravenous single dose. Concurrent treatment with JAK inhibitors should not be undertaken; no evidence of the efficacy and safety of a sequential treatment is available.
Anakinra
The evidence base for the use of anakinra comprises two RCTs with a total of 738 patients on low-flow oxygen therapy (30, 31). The systematic analysis found no positive effects on clinically relevant endpoints. There is no evidence of an increase in adverse events; however, prolonged use is known to be associated especially with severe secondary infections and blood cell count abnormalities. Given the uncertain benefit of anakinra in the treatment of COVID-19 and potentially harmful effects, the Guideline Development Group makes a strong recommendation against the use of anakinra.
Other treatment strategies
Vitamin D3
Based on two RCTs with a total of 313 patients, no benefits with regard to patient-relevant endpoints, such as mortality and need for invasive ventilation, for found (32). Against this background, the Guideline Development Group makes a strong recommendation against the use of vitamin D3.
Colchicine
Based on three RCTs with a total of 11 517 patients, no evidence of a favorable effect on patient-relevant endpoints, such as 28-day mortality or clinical status, was found (33). For this reason, the Guideline Development Group makes a strong recommendation against the use of colchicine.
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
Clinical guidelines are not peer-reviewed in the Deutsche Ärzteblatt, as well as in many other journals, because clinical (S3) guidelines are texts which have already been repeatedly evaluated, discussed and broadly consented by experts (peers).
Footnotes
Conflict of interest
Prof. Kluge received research funding from Ambu, Daiichi Sankyo, ETView Ltd, Fisher & Paykel, Pfizer and Xenios and Cytosorbents. He received lecture fees and reimbursement of travel expenses from Astra, C.R. Bard, Baxter, Biotest, Cytosorbents, Daiichi Sankyo, Fresenius, Medical Care, Gilead, Mitsubishi Tanabe Pharma, MSD, Pfizer, Philips, and Zoll. He received consultancy fees (advisory board) from Bayer, Fresenius, Gilead, MSD and Pfizer and fees for expert opinions from Gilead and Pfizer. He is a board member of the German Society of Internal Medicine and Emergency Medicine and Member of the Presidium of the German Interdisciplinary Association for Intensive Care and Emergency Medicine.
Dr. Malin received consultancy fees from Maple Health Group and Atriva Therapeutics GmbH in context of COVID-19, reimbursement of congress fees and/or travel expenses from Gilead Sciences, ViiV Healthcare and Correvio Pharma as well as institutional research funding (immediately from the employer) from the German Center for Infection Research (DZIF) and the U.S. National Institutes of Health (NIH).
Prof. Müller received research funding von Novartis and Rheacell as well as lecture fees from Bayer, BMS, Pfizer, Servier, Novartis and consultancy fees from Bayer and BMS.He is member of the board of the German Society of Angiology (DGA).
Prof. Karagiannidis received consultancy fees from Bayer and Xenios. He is Head of the German Interdisciplinary Association for Intensive and Emergency Medicine (DIVI) Intensive Care Register, President of DGIIN and member of the COVRIIN expert group at the Robert Koch Institute (RKI).
Dr. Fichtner und Prof. Skoetz received institutional research funding from the German Federal Ministery of Education and Research (BMBF) within the framework of funding of the COVID-19 Evidence Ecosystem (CEOsys) in the German Network University Medicine (NUM) 2020–2021 NaFoUniMed COVID-19 1KX2021. Dr. Fichtner further received a lecture fee from KRH Klinikum Siloah, Hannover, Germany. He is member of the coordination group S3-level clinical practice guideline “Mechanical Ventilation and Extracorporeal Membrane Oxygenation in Acute Respiratory Insufficiency“ AWMF reg. no. 001–021.
References
- 1.Kluge S, Janssens U, Welte T, Weber-Carstens S, Marx G, Karagiannidis C. [Recommendations for critically ill patients with COVID-19] Med Klin Intensivmed Notfmed. 2020;115:175–177. doi: 10.1007/s00063-020-00674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malin JJ, Spinner CD, Janssens U, et al. Key summary of German national treatment guidance for hospitalized COVID-19 patients: Key pharmacologic recommendations from a national German living guideline using an Evidence to Decision Framework (last updated 1705.2021) Infection. 2021:1–14. doi: 10.1007/s15010-021-01645-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kluge S, Janssens U, Spinner CD, Pfeifer M, Marx G, Karagiannidis C. Clinical practice guideline: Recommendations on in-hospital treatment of patients with COVID-19. Dtsch Arztebl Int. 2021;118:1–7. doi: 10.3238/arztebl.m2021.0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.AWMF. S3-Leitlinie: Empfehlungen zur stationären Therapie von Patienten mit COVID-19 - Living Guideline (Stand 10/2021). Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften e.V. (AWMF) https://www.awmf.org/leitlinien/detail/ll/113-001LG.html (last accessed on 2 November 2021) [Google Scholar]
- 5.Grieco DL, Menga LS, Cesarano M, et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: The HENIVOT randomized clinical trial. JAMA. 2021;325:1731–1743. doi: 10.1001/jama.2021.4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrmann S, Li J, Ibarra-Estrada M, et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med. 2021 doi: 10.1016/S2213-2600(21)00356-8. S2213 2600(21)00356-8.Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alhazzani W, Evans L, Alshamsi F, et al. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: First Update. Crit Care Med. 2021;49:e219–e234. doi: 10.1097/CCM.0000000000004899. [DOI] [PubMed] [Google Scholar]
- 8.Deutsche Gesellschaft für Anästhesiologie & Intensivmedizin. S3-Leitlinie: Invasive Beatmung und Einsatz extrakorporaler Verfahren bei akuter respiratorischer Insuffizienz. www.awmf.org. 2017 (last accessed on 2 November 2021) [Google Scholar]
- 9.Patell R, Chiasakul T, Bauer E, Zwicker JI. Pharmacologic thromboprophylaxis and thrombosis in hospitalized patients with COVID-19: a pooled analysis. Thromb Haemost. 2021;121:76–85. doi: 10.1055/s-0040-1721664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sadeghipour P, Talasaz AH, Rashidi F, et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: The INSPIRATION randomized clinical trial. Jama. 2021;325:1620–1630. doi: 10.1001/jama.2021.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perepu US, Chambers I, Wahab A, et al. Standard prophylactic versus intermediate dose enoxaparin in adults with severe COVID-19: a multi-center, open-label, randomized controlled trial. J Thromb Haemost. 2021;19:2225–2234. doi: 10.1111/jth.15450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawler PR, Goligher EC, Berger JS, et al. Therapeutic anticoagulation with heparin in noncritically Ill patients with Covid-19. N Engl J Med. 2021;385:790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemos ACB, do Espírito Santo DA, Salvetti MC, et al. Therapeutic versus prophylactic anticoagulation for severe COVID-19: a randomized phase II clinical trial (HESACOVID) Thromb Res. 2020;196:359–366. doi: 10.1016/j.thromres.2020.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goligher EC, Bradbury CA, McVerry BJ, et al. Therapeutic anticoagulation with heparin in critically Ill patients with Covid-19. N Engl J Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–1247. doi: 10.1007/s00134-021-06506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kreuzberger N, Hirsch C, Chai KL, et al. SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19. Cochrane Database Syst Rev. 2021;9 doi: 10.1002/14651858.CD013825.pub2. CD013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinreich DM, Sivapalasingam S, Norton T, et al. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O‘Brien MP, Forleo-Neto E, Musser BJ, et al. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N Engl J Med. 2021;385:1184–1195. doi: 10.1056/NEJMoa2109682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lundgren JD, Grund B, Barkauskas CE, et al. A neutralizing monoclonal antibody for hospitalized patients with Covid-19. N Engl J Med. 2021;384:905–914. doi: 10.1056/NEJMoa2033130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ansems K, Grundeis F, Dahms K, et al. Remdesivir for the treatment of COVID--19. Cochrane Database Syst Rev. 2021;8 doi: 10.1002/14651858.CD014962. CD014962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the Treatment of Covid-19—final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan H, Peto R, Henao-Restrepo AM, et al. Repurposed antiviral drugs for Covid-19—Interim WHO solidarity trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ader F, Bouscambert-Duchamp M, Hites M, et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00485-0. S1473 3099(21)00485-0. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piechotta V, Chai KL, Valk SJ, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2020;7 doi: 10.1002/14651858.CD013600.pub2. CD013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popp M, Stegemann M, Riemer M, et al. Antibiotics for the treatment of COVID-19. Cochrane Database Syst Rev. 2021;10 doi: 10.1002/14651858.CD015025. CD015025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Popp M, Stegemann M, Metzendorf MI, et al. Ivermectin for preventing and treating COVID-19. Cochrane Database Syst Rev. 2021;7 doi: 10.1002/14651858.CD015017.pub2. CD015017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wagner C, Griesel M, Mikolajewska A, et al. Systemic corticosteroids for the treatment of COVID-19. Cochrane Database Syst Rev. 2021;8 doi: 10.1002/14651858.CD014963. CD014963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosn L, Chaimani A, Evrenoglou T, et al. Interleukin-6 blocking agents for treating COVID-19: a living systematic review. Cochrane Database Syst Rev. 2021;3 doi: 10.1002/14651858.CD013881. CD013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tharaux P, Pialoux G, Pavot A, Mariette X, Hermine O, Resche-Rigon M. Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respir Med. 2021;9:295–304. doi: 10.1016/S2213-2600(20)30556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyriazopoulou E, Poulakou G, Milionis H, et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: a double-blind, randomized controlled phase 3 trial. Nat Med. 2021;27:1752–1760. doi: 10.1038/s41591-021-01499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murai IH, Fernandes AL, Sales LP, et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. 2021;325:1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mikolajewska A, Fischer AL, Piechotta V, et al. Colchicine for the treatment of COVID--19. Cochrane Database Syst Rev. 2021;10 doi: 10.1002/14651858.CD015045. CD015045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical researchLancet Infect Dis 2020. Lancet Infect Dis. 2020;20:e192–e197. doi: 10.1016/S1473-3099(20)30483-7. [DOI] [PMC free article] [PubMed] [Google Scholar]