Abstract
Background:
Multiple Sclerosis (MS) is a growing global health challenge affecting nearly 3 million people. Progress has been made in the understanding and treatment of MS over the last several decades, but cures remain elusive. The National MS Society is focused on achieving cures for MS.
Objectives:
Cures for MS will be hastened by having a roadmap that describes knowledge gaps, milestones, and research priorities. In this report, we share the Pathways to Cures Research Roadmap and recommendations for strategies to accelerate the development of MS cures.
Methods:
The Roadmap was developed through engagement of scientific thought leaders and people affected by MS from North America and the United Kingdom. It also included the perspectives of over 300 people living with MS and was endorsed by many leading MS organizations.
Results:
The Roadmap consist of three distinct but overlapping cure pathways: (1) stopping the MS disease process, (2) restoring lost function by reversing damage and symptoms, and (3) ending MS through prevention. Better alignment and focus of global resources on high priority research questions are also recommended.
Conclusions:
We hope the Roadmap will inspire greater collaboration and alignment of global resources that accelerate scientific breakthroughs leading to cures for MS.
Keywords: Multiple sclerosis, global health, research priorities, knowledge gaps, patient advocacy
Introduction
Multiple Sclerosis (MS) is a growing global health challenge affecting nearly 3 million people with significant public health and economic impacts. 1 While substantial progress has been made in the development of more than a dozen effective disease-modifying treatments (DMTs) for relapsing forms of MS, we still lack a fundamental understanding of all the pathological processes driving disease, we lack effective treatments for progressive forms of MS, and cures remain elusive. The National Multiple Sclerosis Society is focused on achieving breakthroughs to cures for MS. Progress toward this goal will be hastened by having a roadmap that describes the knowledge gaps, milestones, and research priorities that will lead to cures for everyone living with this condition.
In this report, we share the Society’s Pathways to MS Cures Research Roadmap. The Roadmap was developed with input from scientific experts, health care providers, and people affected by MS from the United States, Canada, and the United Kingdom (Table 1). The Roadmap has also been endorsed by leading MS patient and professional organizations (Table 2). We hope the Roadmap will inspire greater collaboration and alignment of global resources that accelerate scientific breakthroughs leading to cures for MS. Achievement of this ambition will require enhanced engagement of global stakeholders and implementation of a range of new approaches.
Table 1.
Pathways to cures roadmap advisors.
| Name | Location | Role | Contribution |
|---|---|---|---|
| Sergio Baranzini, PhD | University of California, San Francisco | Pathways to Cures Workteam | Provided content expertise and feedback |
| Lisa Barcellos, PhD, MPH | University of California, Berkely | Pathways to Cures Workteam | Provided content expertise and feedback |
| Philip De Jager, MD, PhD | Columbia University, New York | Pathways to Cures Workteam | Provided content expertise and feedback |
| Robin Franklin, PhD | University of Cambridge, Cambridge | Pathways to Cures Workteam | Provided content expertise and feedback |
| Vitorio Gallo, PhD | The Children's National Medical Center, Washington DC | Pathways to Cures Workteam | Provided content expertise and feedback |
| Gavin Giovanonni, MD, PhD | Queen Mary University of London, London | Pathways to Cures Workteam | Provided content expertise and feedback |
| Jennifer Graves, MD, PhD | University of California, San Diego | Pathways to Cures Workteam | Provided content expertise and feedback |
| Amy Lovett-Racke, PhD | Ohio State University Medical Center, Columbus | Pathways to Cures Workteam | Provided content expertise and feedback |
| Kassandra Munger, ScD | Harvard T. H. Chan School of Public Health, Boston | Pathways to Cures Workteam | Provided content expertise and feedback |
| Daniel Ontaneda, MD, PhD | Cleveland Clinic, Cleveland | Pathways to Cures Workteam | Provided content expertise and feedback |
| Michelle Plowman, PhD | Memorial University of Newfoundland, St Johns | Pathways to Cures Workteam | Provided content expertise and feedback |
| Kathryn Smith | KES Business Consulting, LLC, Lyme, CT | Pathways to Cures Workteam | Provided content expertise and feedback |
| Terry Wood, PhD | Rutgers New Jersey Medical School, Newark | Pathways to Cures Workteam | Provided content expertise and feedback |
| Rob Motl, PhD | University of Alabama at Birmingham, Birmingham | Pathways to Cures Workteam and Scientific Advisory Committee | Provided content expertise and feedback |
| Michael Bogdonoff | Retired Partner, Dechert LLP, Philadelphia | Scientific Advisory Committee | Advised on direction and process |
| Peter Calabresi, MD | Johns Hopkins University, Baltimore | Scientific Advisory Committee | Advised on direction and process |
| Cathy Carlson | National Multiple Sclerosis Society, New York | Scientific Advisory Committee | Advised on direction and process |
| Timothy Coetzee, PhD | National Multiple Sclerosis Society, Albany | Scientific Advisory Committee | Advised on direction and process |
| Bruce Cohen, MD | Northwestern University Medical School, Chicago | Scientific Advisory Committee | Advised on direction and process |
| Benjamin Davis | Multiple Sclerosis Society of Canada, Halifax | Scientific Advisory Committee | Advised on direction and process |
| Paula Dore-Duffy, PhD | Wayne State University School of Medicine, Detroit | Scientific Advisory Committee | Advised on direction and process |
| Peter Galligan | Boston | Scientific Advisory Committee | Advised on direction and process |
| Shyam Gidumal | WeWork, New York | Scientific Advisory Committee | Advised on direction and process |
| Joan Goverman, PhD | University of Washington, Seattle | Scientific Advisory Committee | Advised on direction and process |
| Fay Horak, PT, PhD | Oregon Health and Science University, Portland | Scientific Advisory Committee | Advised on direction and process |
| Mary Hughes, MD | Premier Neurology, Greer, SC | Scientific Advisory Committee | Advised on direction and process |
| David Kelleher | 4G Clinical, Boston | Scientific Advisory Committee | Advised on direction and process |
| Karen Lee, PhD | Multiple Sclerosis Society of Canada, Toronto | Scientific Advisory Committee | Advised on direction and process |
| Bill MacNally | Blaine, MN | Scientific Advisory Committee | Advised on direction and process |
| Aaron Miller, MD | Mount Sinai School of Medicine, New York | Scientific Advisory Committee | Advised on direction and process |
| Richard Slifka | Global Petroleum Corporation, Waltham | Scientific Advisory Committee | Advised on direction and process |
| Bari Talente | National Multiple Sclerosis Society, Washington, DC | Scientific Advisory Committee | Advised on direction and process |
| Peter Tarricone | Wells Fargo Insurance Services USA, Inc., Summit, NJ | Scientific Advisory Committee | Advised on direction and process |
| Alan Thompson, MD | University College London, London | Scientific Advisory Committee | Advised on direction and process |
| Bruce Trapp, PhD | Cleveland Clinic, Cleveland | Scientific Advisory Committee | Advised on direction and process |
| Emmanuelle Waubant, MD, PhD | University of California, San Francisco | Scientific Advisory Committee | Advised on direction and process |
| Cyndi Zagieboylo | National Multiple Sclerosis Society, Rochester | Scientific Advisory Committee | Advised on direction and process |
Table 2.
Organizations endorsing the Roadmap.
| Organization | Website |
|---|---|
| Americas Committee for Treatment and Research in MS | https://www.actrims.org/ |
| European Committee for Treatment and Research in MS | https://www.ectrims.eu/ |
| Middle East North Africa Committee for Treatment and Research in MS | https://menactrims.com/ |
| Latin American Committee for Research and Treatment in Multiple Sclerosis | https://www.lactrimsweb.org/ |
| Pan Asian Committee for Treatment and Research in MS | https://www.pactrims.org/ |
| International Women in Multiple Sclerosis | https://iwims.world/ |
| Consortium of Multiple Sclerosis Centers | https://www.mscare.org/ |
| European Charcot Foundation | https://www.charcot-ms.org/ |
| International Society of Neuroimmunology | https://www.isniweb.org/ |
| World Federation of Neurology | https://wfneurology.org/ |
| Multiple Sclerosis Society of Canada | https://mssociety.ca/ |
| Multiple Sclerosis Society, United Kingdom | https://www.mssociety.org.uk/ |
| MS Australia | https://www.msaustralia.org.au/ |
| Sclerosi Multipla Associazione Italiana | https://www.aism.it/italian_multiple_sclerosis_society_aism |
| Scleroseforeningen (Denmark) | https://www.scleroseforeningen.dk/ |
| Multiple Sclerosis International Federation | https://www.msif.org/ |
| Accelerated Cure Project for Multiple Sclerosis | https://www.acceleratedcure.org/ |
| MS Views and News | http://www.msviews.org/msviewsandnews4/ |
| Multiple Sclerosis Foundation | https://msfocus.org/ |
| National Stem Cell Foundation | https://nationalstemcellfoundation.org/ |
| United Spinal Association | https://unitedspinal.org/ |
| Multiple Sclerosis Association of America | https://mymsaa.org/ |
MS: multiple sclerosis.
Development of the roadmap
The Roadmap was developed through a consensus building process that included the National MS Society’s Scientific Advisory Committee, National Board of Directors, and the Pathways to Cures Task Force-composed of scientific thought leaders and people affected by MS (Table 1). In addition, the perspectives of over 300 people with MS were obtained and incorporated in the Roadmap through a survey conducted in collaboration with the Accelerated Cure Project for Multiple Sclerosis. This survey established that the definition of a cure was different depending on an individual’s perspective, but the responses could be grouped into three main categories: (1) stopping the MS disease process, (2) restoring lost function by reversing damage and symptoms, and (3) ending MS through prevention. These perspectives are consistent with what we and others have learned through various outreach efforts and largely align with findings of other MS organizations.
The scientific foundations of the Roadmap were developed and refined by the Task Force and Scientific Advisory committees. Subsequently, the Roadmap was endorsed by many leading global MS patient and professional organizations, research funders, and other stakeholders (Table 2). We hope that the Roadmap and the stakeholder endorsements will inspire greater alignment of resources on research that accelerates progress toward scientific breakthroughs that lead to cures for MS. In the following paragraphs, we outline the key objectives, barriers, potential solutions, and recommendations for implementation of strategies to advance each of the pathways in the Roadmap.
The stop pathway
The Roadmap defines stopping MS as achieving a state of no new disease activity or central nervous system (CNS) injury, no worsening of daily living or quality of life, and no change in disease manifestations. By stopping all forms of disease activity and tissue injury, we prevent the accumulation of disability and create a permissive environment for myelin and axonal repair and other pathways that promote restoration of function. The opportunities for stopping MS disease activity span from the sub-clinical to later stages of disease (Figure 1). The Stop pathway includes two major objectives: (1) Early Detection and (2) Precision Medicine.
Figure 1.
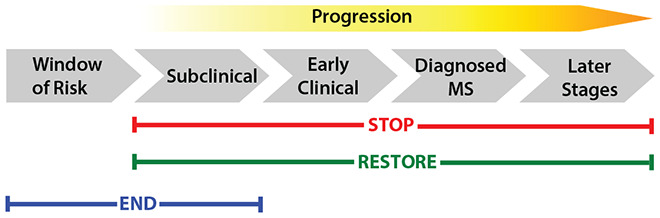
The evolution of MS and opportunities for the discovery of cures.
Current knowledge
Much has been learned about the role of the immune system in MS pathogenesis, aiding the development of more than a dozen effective DMTs that target different cells, mediators, and pathways, with tremendous improvements in the quality of life for people with MS. 2 Most of these therapies directly modulate the adaptive immune system or impact immune cell trafficking. In addition, cell depletion/reconstitution therapies have shown promise in clinical studies of aggressive forms of relapsing MS. 3 Having many treatment options with different mechanisms of action and efficacy, adverse event profiles, and routes of administration creates an opportunity for health care providers to tailor their treatment approach for individuals with MS.
Despite successes in relapsing MS, there are far fewer therapeutic options for people living with progressive forms of MS. The lack of a full understanding of the pathophysiologic mechanisms driving progression is arguably the main reason why there are not better treatment options for this form of disease. Attention is turning to CNS-compartmentalized inflammation as a promising area of study. It is becoming clear that there are both protective and destructive interactions taking place between cells of the immune system and neurons and glia in the CNS. This knowledge is starting to reveal targets for possible interventions. In addition, there is a need for more sensitive and specific endpoints that would facilitate rapid proof-of-concept clinical trials and better animal models that more closely recapitulate the disease course and pathology of progressive MS.
Efforts at biological phenotyping are starting to lead to a better understanding of both relapsing and progressive disease heterogeneity and the identification new therapeutic targets. Recent single cell profiling studies have led to the discovery of multiple populations of peripheral immune cells as well as microglial cells, astrocytes, and oligodendrocytes that may allow more precise interventions to be developed. A recent machine learning study using data derived from thousands of MRI scans obtained from well-controlled clinical trials and cohort studies identified three MRI-defined MS subtypes that are independent of the clinically defined forms of disease. 4 The subtypes predict disability progression and may also have value in predicting treatment responses. While much attention has been focused on stopping MS with DMTs, certain comorbidities such as obesity and smoking have been shown to negatively impact disease progression. 5 Identifying approaches that promote lasting lifestyle changes and address comorbidities are also components of the this pathway.
Early detection
There is growing consensus on the importance of early application of disease-modifying interventions to minimize CNS damage, potentially delay the accumulation of disability, and maximize function. 6 This suggests that an earlier MS diagnosis or the identification of individuals at high risk for a future diagnosis could improve long-term outcomes. As many as 85% of individuals with clinically isolated syndrome (CIS) are diagnosed with MS within 2 years. 7 Early treatment of CIS with interferons,7,8 glatiramer acetate, 9 or teriflunomide 10 has been reported to delay the ascertainment of MS and provide persistent long-term benefits. Identifying features of those who ultimately are diagnosed with MS will improve precision and enable early treatment of the high-risk subset.
There is emerging evidence that the MS disease process starts many years before it becomes clinically apparent and includes a prodromal phase characterized by non-disease-specific clinical symptoms. 11 Retrospective reviews of medical records and health utilization have uncovered evidence of increased healthcare usage 5 to 10 years before a first clinically evident demyelinating event or MS diagnosis. The types of symptoms reported such as pain, anxiety, and others do not provide the specificity needed for diagnosis but may be reflections of an underlying early disease process. Recent studies also provide evidence of axonal injury occurring years before an MS diagnosis. Longitudinal sampling from a cohort of US military veterans revealed that elevated serum neurofilament light chain (NfL) levels preceded MS diagnosis by 6 years. 12 Some individuals without clinical signs of MS are found to have brain lesions characteristic of MS. These asymptomatic individuals with so-called radiologically isolated syndrome (RIS) are also at an increased risk for an MS diagnosis. 13 More recently, an increased cerebrospinal fluid NfL concentration in RIS has been identified as a risk factor for diagnosis. 14 Not everyone with RIS or CIS will go on to develop an ascertained diagnosis of MS. The earliest phases of MS onset and development of biological markers, health data, and sociological features to help identify onset, define biology-based phenotypes, and improve the diagnostic process are needed. Identification of the prodromal period of MS necessitates a set of diagnostic tools with defined thresholds. There is an opportunity to intervene during this pre-clinical phase of MS and delay, reduce, or perhaps even stop the subsequent development of disability. While the focus of much of this work is on the adult population, we must not lose sight of the importance of addressing issues and opportunities for early detection in the pediatric population.
Precision medicine
MS is a heterogeneous disease, and each person with MS experiences the disease differently. Treatment choice is a personal decision balancing risk and efficacy and may also be influenced by the policies of payers. Early treatment is desirable and has been shown to impact long-term disease trajectory. 7 Research is underway to determine whether an escalation or higher-efficacy first-line treatment approach offers better long-term outcomes. Analysis of lesions over time and space suggests that different immune-effector mechanisms may predominate in individuals at different times. 15
Given that heterogeneity may also exist at the patient level, an evidence-driven approach that could prognosticate outcomes would help frame the full benefits and risks of any specific treatment and help guide the selection of an optimal therapy for a given patient at a given point in time.16,17 In certain settings, nonclinical measures such as serum NfL and new MRI activity can discriminate between treatment and placebo groups, suggesting that monitoring treatment response is possible. 18 Learnings from other disease areas such as oncology, where precision medicine approaches have been incorporated as standard of care, could be helpful. MS clinicians already have experience utilizing precision medicine in clinical practice. The determination of JC virus status prior to and during treatment with natalizumab is an example of precision medicine used to risk-stratify and monitor safety. 19 In addition, MRI is commonly used to track brain lesion activity as part of ongoing disease management. Additional non-invasive biomarkers are needed that will allow the tracking of different aspects of disease activity. Consideration should also be given to how precision medicine tools that improve MS treatment will be implemented by general neurologists and non-MS specialists.
The most advanced fluid biomarker in development is NfL. NfL is a neuronal structural protein released through any cause of neuroaxonal injury and can be monitored with a blood test. Numerous retrospective studies20,21 and prospective analyses of phase 3 trials in relapsing MS 22 suggest that the concentration of NfL in serum, plasma, or cerebrospinal fluid can serve as a useful predictor of disease worsening at the population level. Correlations have been observed for acute disease activity and prediction of subsequent MRI lesion activity, brain volume loss, relapse rate, and worsening of disability. Recent studies on age and sex effects in normal adults show increased and more variable sNfL in subjects over 60 years of age. 22 Understanding normative characteristics for sNfL is essential to enable clinical utility. Other proteins such as glial fibrillary acidic protein (GFAP), released by astrocytes, are also being investigated as potential biomarkers.
Additional imaging and fluid biomarker approaches are needed that will further inform and possibly predict disease course and will allow tracking of neuroinflammation, myelination status, cortical lesions, and the distinct pathologies of relapsing and progressive MS. An improved understanding of genetic and environmental factors that influence disease course is also highly desirable. Data-driven algorithms combining clinical data and known genetic and environmental risk factors with biological and imaging biomarker data may present a pathway to optimized diagnosis, prognosis, disease activity monitoring and response to therapy that will lead us to stopping MS.
Recommendations
The relationship between acute inflammation, compartmentalized inflammation, and neurodegeneration needs to be better understood to allow more precise intervention and the development of new therapeutic approaches (Table 3). Health data and sociological features collected from representative cohorts may help identify earlier onset and defining the variability of MS disease expression biologically may further improve the diagnostic process to allow treatment prior to accumulation of disability. Better biological markers and tools, including improved predictive models, will lead to a better understanding of the biology and heterogeneity of MS. Biomarkers informed by research into disease mechanisms, powered by carefully monitored cohorts with improved outcome measures, high-quality longitudinal samples, and curated data may enable an understanding of the prodromal phase. Finally, better coordination of properly collected longitudinal cohorts that include diverse populations of people with MS will be needed to answer these key epidemiological questions.
Table 3.
Stop pathway recommendations and research priorities.
| Gap | Action | Outcome |
|---|---|---|
| An understanding of mechanisms driving the MS prodrome | Fund research into early detection of MS before accumulation of neurological deficit | Processes contributing to MS risk are clearly defined; therapeutic interventions are implemented at the earliest point in time, leading to improved clinical outcomes |
| Longitudinal biomarker studies | Enhance the impact of cohorts, registries, and repositories Facilitate access and utilization by MS research community Promote best practices in biomarker development and evaluation |
Existing and new biomarkers enable early detection of disease activity |
| Research based framework to select the best therapy for individual patients (e.g., precision medicine) | Promote research to provide clinical validation of multi-modal biomarker approaches to predict response to therapy. Foster collaboration between diverse biomarker fields |
Robust multi-modal biomarkers are fully integrated into clinical practice guidelines to support clinical decisions Partnerships are expanded to develop and implement better tools for precision medicine |
| Therapies for progressive forms/stages of MS | Promote investment in clinical testing of therapeutics that modulate pathways in progressive MS | Putting a stop to both relapsing and progressive injury mechanisms in each individual patient |
MS: multiple sclerosis.
The restore pathway
The Roadmap defines the Restore pathway as reversing symptoms and recovering function to enable full participation in society. While DMTs can limit the occurrence of relapses and in some cases delay disability worsening, they have limited capacity to enhance or restore function. This pathway explores the opportunity to enhance regeneration and remyelination, as well as focus on strategies to reverse symptoms and improve quality of life.
One focus is to integrate the study of pathophysiological mechanisms and their association with functional capacity, as well as rigorously evaluate the potential to enhance neuroplasticity, remyelination, and restoration of function. An integrated approach is needed that enhances remyelination, neural regeneration, and neuroplasticity, while optimizing the extent to which wellness behaviors, rehabilitation, self-care, and exercise promote reversal or diminution of symptoms. The development and improvement of outcomes that can measure or even identify patients who have the necessary substrate for regeneration, as well as the advancement of clinical intervention trials that measure neural recovery and its impact on a person’s life after diagnosis and during disease is critical to enable full participation in society. Opportunities for advancing the Restore Pathway span from the subclinical through later stages of disease, although it is likely that earlier interventions will be more successful (Figure 1). The Restore Pathway includes two main objectives: (1) Regeneration and (2) Restoration of Functional Activity.
Current knowledge
Regeneration
Remyelination requires myelin producing oligodendrocytes that create new myelin sheaths in the CNS. The brain generates oligodendrocytes from oligodendrocyte precursor cells (OPCs) throughout life, but the efficiency of remyelination declines with age. Strategies for promoting remyelination by restoring a youthful milieu in the CNS and targeting CNS-endogenous cells with remyelination-enhancing therapies hold much promise. 23 Mechanisms that underlie remyelination failure in MS are not fully understood and are thought to occur through a combination of inhibitory factors. Recent evidence suggests that inhibition from secreted factors released by both infiltrating immune cells and resident glia play a role in suppressing remyelination. In addition, certain oligodendroglia subtypes may also negatively impact remyelination. 24 While much attention has been focused on OPCs, recent studies suggest that adult oligodendrocytes can also participate in remyelination. 25
Removing impediments to myelin repair, stimulating endogenous OPC differentiation, and transplanting cells with the potential to promote repair 26 provide opportunities for immune modulation, neuroprotection, or repair in people with MS. Further studies are needed to focus on the cell biology of remyelination and evaluate emerging molecular pathways that could be leveraged for repair therapies.
The use of animal models such as experimental autoimmune encephalomyelitis, cuprizone and lysolecithin have strengths as well as limitations, and need to be optimized, or new tools need to be developed, to better represent MS. Most DMTs for MS target inflammatory processes, yet we know there is an urgent need for therapies that provide neuroprotection and/or promote axonal growth and/or remyelination in the setting of an inflammatory or non-inflammatory tissue environment. Clarifying the functional heterogeneity of OPCs, the remyelinating capacity of mature oligodendrocytes, the role of aging, and the roles of other neural cells in repair offer promising opportunities to expose additional new targets for regeneration.
Promoting neuroprotection, synaptic plasticity, and strategies to limit neurodegeneration are also promising approaches for reducing disability and restoring function in MS. Studies of neuroprotection and synaptic plasticity have primarily involved rodent models and show considerable involvement of neural networks of the hippocampus, basal ganglia, and cerebellum. 27 Pathology studies in MS show significant declines in the number of synapses in the hippocampus, as well as receptors and molecules involved in synaptic plasticity and glutamate neurotransmission. 28 Recent work shows that CNS inflammation affects synaptic transmission and that immune-mediated alterations to synaptic plasticity may be a contributing factor to the pathogenesis of MS-related cognitive impairment; reversing any of these areas could offer functional benefits.29,30 Understanding how to protect neurons and why some clusters of neurons are more resilient than others provide new opportunities for therapeutic approaches.
Restoration of functional activity
Accurately evaluating disease progression and disability is important for understanding the biology of regeneration, testing therapeutic approaches, guiding treatment, and informing personalized care. Imaging measures have expanded substantially and have proved to offer a quantitative and objective way to evaluate MS disease progression but have limited ability to track myelin changes over time in the brain or spinal cord. 31 Brain imaging methods such as magnetization transfer imaging and diffusion tensor imaging offer opportunities to evaluate the evolution of acute white matter lesions, whereas other methods such as myelin water imaging, susceptibility-weighted imaging and positron emission tomography allow for the evaluation of chronic white matter lesions. 32 Studies are needed that target remyelination more precisely and develop better imaging tools that specifically measure changes in myelination.
To different extents, imaging measures have been shown to relate broadly to disability; 33 however, these studies have almost entirely focused on the Expanded Disability Status Scale (EDSS) as the measure of disability. The EDSS is a rating scale that assesses overall disability, placing a greater emphasis on walking function over other symptoms such as spasticity, fatigue, cognitive dysfunction, or hand dysfunction. 34 Impairment-based outcome measures that detect disability worsening and provide specific information about the impairment can be used to tailor treatment interventions for each person based on the specific symptom or based on patient-reported feedback, as in the Fatigue Severity Scale. While these clinical outcome measures can describe and, in some cases, predict disability, they are not sensitive enough to detect either early disease progression or the results of regeneration. Emerging technologies using remote monitoring wearable devices may offer insights into early detection of disease worsening and/or progression as well as responses to regenerative therapies. Biomarkers that relate to the disease process, or symptoms could also be important tools for managing MS. Development of appropriate outcome measures that singularly or in combination quantify neural regeneration, identify patients who have the necessary substrate for regeneration and are associated with specific measures of impairment would improve clinical decision making and expedite the study of clinical interventions.
Clinical trials are already underway exploring pharmaceutical approaches and cell-based therapies to facilitate remyelination and neural repair. Up to now, none of these trials have provided definitive positive results, highlighting deficits in both measurement tools and validated targets. A phase 2b, multi-arm trial of three putative neuroprotective drugs 35 failed to provide evidence for neuroprotection in patients with secondary progressive MS; this trial followed mixed results from two highly anticipated clinical trials interrogating the remyelinating effects of anti-LINGO antibodies in optic neuritis and relapsing-remitting MS. 36 Data from early clinical trials evaluating the safety and efficacy of autologous mesenchymal stem cells delivered intrathecally reported improvements in physical abilities, vision, and cognition along with a decrease in inflammatory biomarkers.37,38 Data are needed from larger studies to provide additional evidence.
Tools to screen compounds that promote remyelination 39 also provide promise for identifying new therapies. High-throughput screening resulted in the first randomized clinical trial to show evidence of remyelination in MS using clemastine fumarate. 40 Other high-throughput screening approaches have identified molecules that enhance the formation of oligodendrocytes and ultimately remyelination. 41 Future screens should also look for compounds that promote remyelination in potentially inhibitory environments. 42 Ongoing clinical trials of Bruton tyrosine kinase inhibitors (BTK) 43 and early phase trials of new drugs exploring novel pathways that block neurite growth inhibition 44 provide promising avenues for limiting MS progression. Clinical trials using biologic outcomes sensitive to regeneration and behavioral markers sensitive to functional recovery are critical components for optimizing recovery and guiding clinical care.
Studies have shown that in MS, exercise is safe, can improve strength, cardiorespiratory fitness, walking, symptomatic fatigue, and cognition, and overall is an effective symptomatic treatment in MS. 45 Clinical trials have begun to evaluate combining exercise with other symptomatic treatments such as cognitive rehabilitation and/or medications, with positive results. 45 The effects of exercise in modifying the disease or even reducing the risk of MS is also being evaluated. 46 Exercise studies provide preliminary evidence of the potential impact of exercise on neuroprotection and regeneration in animal models and humans.46,47 Studies of cardiac rehabilitation provide a powerful example of how rehabilitation can improve quality of life and drive recovery. This evidence highlights the perspective that long-term and large-scale human studies in MS can be tailored to assess and measure the neuroprotective and neurodegenerative benefits of exercise and other rehabilitation interventions.
There are a variety of rehabilitation strategies to support preventive, restorative, compensatory, and maintenance strategies to address symptoms of MS. Balance and gait dysfunction are a leading concern for people with MS, with increasingly pronounced impairments in persons with progressive MS. 48 The evidence supporting rehabilitative strategies is growing but varies in methodological quality and is largely confined to small cohorts with mixed phenotypes of MS included, making translation difficult. 49 Wearable technology has emerged as a useful tool to collect long-term data assessing function in the real-world setting. 50 Further research is needed to develop effective rehabilitation approaches incorporating appropriate study design and outcome measurement and evaluating type and intensity of interventions. Integrating mechanistic studies and rehabilitation approaches through novel collaborations can inform and expand our understanding of regeneration and rehabilitation and their impact on each other.
Recommendations
Growing evidence suggests that neuro-regeneration and restoration of function are possible in MS. Mechanisms underlying the eventual failure of repair are not fully understood in MS, thus limiting generalizability and application to clinical trials. Preserving and repairing myelin is likely to be one of the best ways to prevent neurodegeneration (Table 4). Translation of knowledge from basic mechanisms to functional impact is needed to optimize treatment, manage symptoms, and ultimately restore function for people with MS. In sum, it is important to build the knowledge base integrating mechanisms with rehabilitation so that they inform one another and drive breakthroughs for restoring functional activity.
Table 4.
Restore pathway recommendations and research priorities.
| Gap | Action | Outcome |
|---|---|---|
| Physiologic mechanisms involved in regeneration and repair | Design and conduct studies to understand the role of aging, sex, genetics and other factors associated with regeneration | Identification of new targets for promoting myelin repair |
| MS specific outcome measures (biologic, imaging and clinical) that are sensitive to regeneration and/or functional recovery | Develop consensus around the identification of outcome measures Design and conduct research to identify outcomes that: Can detect and measure myelin regeneration Can detect meaningful recovery of function Are associated with both regeneration and meaningful recovery of function |
Identification of outcomes that can be used in clinical trials to test pharmacologic and rehabilitation interventions, and once approved can be used to guide use of therapies in clinical practice |
| Trial design, that fosters the development of rehabilitation and wellness interventions | Advance guidance of clinical trial design Facilitate and fund clinical intervention trials that target functional recovery, symptom management, rehabilitation, or wellness strategies |
Clinical intervention trials are implemented and evidence from those trials are used for the development of clinical guidelines |
| Standard outcomes across clinical trials | Promote the use of standardized of outcomes across rehabilitation and wellness clinical trials Develop consensus around standard outcomes to remotely measure and monitor functional recovery |
Existing and new outcomes measures are identified and used for clinical trials |
MS: multiple sclerosis.
The end pathway
The Roadmap defines the End Pathway as no new cases of disease. There is a growing appreciation that along with some other autoimmune and neurological conditions, MS may be preventable. One of the objectives of the End pathway is to prevent MS in the general population, commonly referred to as primary prevention. Primary prevention of MS will require population-based public health initiatives that reduce or eliminate exposure to putative risk factors and perhaps could also involve more targeted measures among individuals considered to be at high risk for developing MS (e.g. first degree family members). The second objective of the End pathway focuses on identifying MS in its earliest (prodromal) stages to delay or prevent onset of classical clinical manifestations, defined as secondary prevention. Some of the approaches for achieving secondary prevention overlap with the early detection approaches described in the Stop pathway. Opportunities for preventing MS precede exposures to environmental risk factors and extend through the subclinical stages of disease (Figure 1).
Current knowledge
Primary prevention
The goal of primary prevention is to prevent MS in the general population before it occurs by limiting exposure to modifiable MS risk factors. The cause of MS is not yet known, but progress has been made in identifying contributing factors and biological pathways that increase the risk of developing MS. Environmental risk factors such as low serum levels of vitamin D, 51 adolescent obesity, 52 tobacco smoking, 53 infection with Epstein Barr virus (EBV) and in particular, symptomatic primary EBV infection,54,55 while not yet proven to be causal, have been consistently associated with an increase in MS risk.
A family history of MS is among the strongest risk factors, and more than 230 common gene variants have been identified that contribute to MS risk, with the strongest being multiple risk alleles in the major histocompatibility complex.56,57 The genetics and environmental exposures driving MS risk have mostly been studied in adult Caucasian populations. There is a strong need to determine whether these same factors are driving the risk for MS in other racial and ethnic groups. Furthermore, since the latency between exposure to MS risk factors and the onset of MS is shorter in pediatric MS, studying risk factors in this population could also reveal important insights.
Even in the absence of full knowledge of the cause of MS, strategies for preventing MS may be achievable in the next few years. Compelling evidence currently exists to support preventive, near-term, public health approaches such as vitamin D supplementation, 55 childhood obesity prevention, 58 and EBV vaccination.59,60 A better understanding of all factors and their interactions that can trigger MS, as well as cooperation and buy-in by public health agencies and policy makers to the concept of MS as a preventable disease are needed. Public health initiatives such as these are likely to also help prevent other disorders and could more effectively be advanced by collaboration and coordination with other disease-specific advocacy organizations. It is also worth considering whether higher-risk primary prevention strategies could be deployed for those with a greater risk for developing MS. Substantial gaps also exist in our understanding of MS risk in non-European populations and addressing these gaps will need to be prioritized so that prevention strategies can be developed that benefit diverse populations of people with MS.
Secondary prevention
The goal of secondary prevention is to identify individuals in whom the biologic processes driving the disease have begun, but in whom classical clinical manifestations have yet to emerge. With this knowledge, one could intervene during the pre-clinical and/or prodromal stages of MS, including in asymptomatic people with radiological findings highly suggestive of MS. Because secondary prevention interventions are likely to have greater risks and side effects, it would be ideal to identify individuals at the highest risk for MS for whom early intervention is most likely to be beneficial.
Prodromal periods are recognized in other autoimmune and neurodegenerative conditions like type-1 diabetes, rheumatoid arthritis, Alzheimer’s disease, and Parkinson’s disease, and trials testing interventions designed to delay or perhaps prevent the onset of clinical disease in some of these conditions are underway. 61 Evidence supporting an MS prodrome is also emerging.62,63 Biomarkers like serum NfL are being studied that could help identify individuals in the prodromal stage of MS. It is likely that a Bayesian approach to estimating risk for developing MS that incorporates clinical, radiological, and laboratory data could be developed and deployed that would establish the MS prodromal period with enough confidence that a low-to-moderate risk disease-modifying approach could be used to treat MS in the very earliest stages with significantly improved outcomes. As our knowledge of the prodromal period of disease improves, it might also be possible to deploy high efficacy or even induction therapies that could re-establish tolerance to CNS antigens and prevent MS from occurring in the first place.
Recommendations
Accelerating research that leads to a better understanding of all the factors that contribute to the risk for MS in all populations, including environmental exposures, the microbiome, social determinants of health, and genetics and epigenetics, as well as the interactions among them that may increase risk will help get us closer to realizing primary prevention (Table 5). The cost-effectiveness of some public health initiatives for preventing MS may need to be proven to convince policy makers of their value.
Table 5.
End pathway recommendations and research priorities.
| Gaps | Actions | Outcomes |
|---|---|---|
| Full knowledge of MS risk factors that are necessary and sufficient to cause MS and the time frame for exposure | Convene experts to develop a blueprint for accelerating research of risk factors Promote knowledge generation of MS risk factors by research funders |
Development of approaches to reduce the risk of MS are developed and validated |
| Availability of public health interventions that reduce or eliminate exposures to MS risk factors | Partner with other advocacy groups to advocate for testing of interventions that prevent disease similar to MS Test interventions with the strongest potential to reduce or eliminate the risk for MS (e.g., EBV vaccine) |
Identification and deployment of public health strategies that reduce the risk for MS in the general population |
| A complete understanding of the genetic and epigenetic contributions to MS risk and etiology | Build on the progress made by the International MS Genetics Consortium and others to identify the complete genetic/epigenetic risk for MS Focus on understanding the genetic basis of disease heterogeneity Develop a better understanding of gene environment interactions |
New approaches for prevention and treatment of MS that reduce the burden of disease |
| A full understanding of the early pathological pathways that lead to initiation of MS | Coordinate global resources to accelerate progress on elucidating the pathways contributing to the initiation of MS Emphasize studies of pediatric onset MS |
New approaches for prevention and treatment of MS that reduce the burden of disease |
| Fluid/imaging/clinical indicators that identify people at high risk for developing MS | Promote research of biomarkers and clinical indicators of MS risk Integrate fluid, imaging, clinical data into a risk staging algorithm |
Development of tools for MS risk staging |
| Identification/implementation of interventions that prevent onset of MS in the high-risk population | Accelerate research of interventions that could prevent the onset of MS Support the clinical development of interventions that could delay or prevent onset of MS |
Develop and deploy strategies that reduce or eliminate the risk for MS in the high-risk population |
MS: multiple sclerosis; EBV: Epstein Barr virus.
Biomarkers that indicate risk should be identified and made widely available. Although the presence of multiple biomarkers may increase the accuracy of risk detection, the practicality of detection in an individual will need to be considered. A better understanding of the age at which risk factors act and when prevention interventions should begin will facilitate intervention. More information is needed about how to identify high-risk individuals, stratify risk, and select interventions that are tiered according to the strength of risk. Interventions should balance risk/benefit and be stratified according to the degree of an individual’s risk, ranging from low-risk, long-term strategies such as vitamin D supplementation, dietary approaches, and vaccination against EBV, to higher-risk strategies such as immune-modulatory therapy. More evidence is needed for the causative role of known risk factors. Most of what is known about MS risk factors has been derived from largely white populations, leaving a gap in understanding how risk factors may differ across other racial or ethnic groups that will need to be addressed so that prevention strategies can be developed that benefit everyone at risk for MS.
Conclusion
Tremendous progress has been made in understanding of the pathogenesis and treatment of MS since the Institute of Medicine published their strategic review of MS research in 2001. 64 This progress has led to the development of numerous DMTs and improved quality of life for many people with MS. Furthermore, it has led to optimism that we are close to breakthroughs that will lead to cures for MS. The Pathways to Cures Roadmap includes carefully considered recommendations of a large group of leaders in MS research and clinical care, as well as people affected by MS from North America and the United Kingdom. We hope this report will inspire a heightened sense of urgency among research funders to support research that leads to cures for MS. We also look forward to engaging with the key stakeholders in the MS movement to help promote improved coordination of global research efforts focused on answering the key questions that will lead to cures.
Research breakthroughs leading to MS cures will require strategic investments in research priorities and increased multidisciplinary collaboration. We are hopeful that the Roadmap could be a starting point for a dialogue with our fellow MS organizations that leads to improved coordination and optimization of MS research investments. Over the last decade, we have made significant progress toward better global collaboration through efforts like the International Progressive MS Alliance and the Patient Reported Outcomes in MS initiative. We encourage MS research funders and advocates to build on this progress by seeking more opportunities to collaborate, align, and leverage their collective investments on research that addresses areas of high opportunity identified in the Roadmap. This could potentially be accomplished through improved data sharing among global research funders and the development of platforms to encourage prioritization, coordination, and leveraging of research investments (e.g. the EU-funded MULTI-ACT project).
The lack of diversity in the MS research workforce and in clinical research studies is a critical issue limiting the generalizability of research breakthroughs and the translation of these breakthroughs for everyone with MS, including underrepresented groups. To achieve cures for everyone with MS, we encourage all stakeholders in the MS movement to implement strategies to increase participation of underrepresented groups in the MS research workforce and clinical studies. 64 Improved engagement of these groups will lead to improvements in the quality of scientific data, facilitate the discovery of important efficacy and safety information and help to identify population specific differences in disease course and treatments and accelerate the development of cures for everyone with MS.
It will also be important to update the Roadmap on a regular basis to reflect advances in our understanding of the Pathways and to account for the development of new technologies and approaches. We propose that a biennial meeting of global MS stakeholders that reviews progress on the Pathways to Cures milestones and updates the Roadmap to reflect contemporary knowledge of MS be organized in collaboration with other MS organizations. A regular meeting could also serve as a platform for data sharing between MS advocacy and research funding organizations and be a venue for developing better coordination of MS research activities that accelerate progress toward badly need cures for MS.
Finding cures for MS has taken much longer than initially anticipated, and while significant obstacles remain, we are optimistic that (1) a passionate and committed global research community, (2) a growing spirit of international collaboration and coordination of resources, (3) a highly motivated and talented research workforce, and (4) a dedicated and well-organized network of activists will speed development of MS cures. We look forward to partnering with other global stakeholders in the MS movement to make the hopes and dreams of people with MS around the world come true.
Acknowledgments
The National MS Society is deeply grateful to Jim and Kathleen Skinner for their $10 million lead investor gift to fund Pathways to MS Cures. The authors would like to acknowledge Howard Weiner who first introduced the concept of the three cures for MS. Medical writing assistance provided by Kristine De La Torre, PhD and Cathy Carlson.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Bruce Bebo, Mark Allegretta, Douglas Landsman, Kathy Zackowski, Fiona Brabazon, Walter Kostich, and Timothy Coetzee are employees of the National MS Society and have no other relevant conflicts to disclose. Alexander Ng has no relevant conflicts to disclose. Ruth Ann Marrie is a co-investigator on a study funded by Roche and Biogen (no funds to Dr. Marrie or her institution). Kelly Monk has no relevant conflicts to disclose. Amit Bar-Or has received research grants and consulting fees from Biogen, grants, and consulting fees from Genetech/Roche, consulting fees from GlaxoSmithKlein, research grants and consulting fees from Merck/EMD Serono, consulting fees from Medimmune, research grants and consulting fees from Novartis, consulting fees from Celgene/Receptos, consulting fees from Sanofi-Genzyme, consulting fees from Atara Biotherapeutics, and Jansen/Actelion. None of these disclosures are related to this work. Caroline Whitacre has no relevant conflicts to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Activities supporting the development of the Roadmap were funded by the National Multiple Sclerosis Society.
ORCID iDs: Kathy M Zackowski  https://orcid.org/0000-0002-2399-5704
https://orcid.org/0000-0002-2399-5704
Fiona Brabazon  https://orcid.org/0000-0003-1642-808X
https://orcid.org/0000-0003-1642-808X
Timothy Coetzee  https://orcid.org/0000-0002-3031-7549
https://orcid.org/0000-0002-3031-7549
Alexander Victor Ng  https://orcid.org/0000-0002-0742-9825
https://orcid.org/0000-0002-0742-9825
Ruth Ann Marrie  https://orcid.org/0000-0002-1855-5595
https://orcid.org/0000-0002-1855-5595
Contributor Information
Bruce F Bebo, Jr, National Multiple Sclerosis Society 733 3rd Ave New York, NY 10017 USA.
Mark Allegretta, National Multiple Sclerosis Society 733 3rd Ave New York, NY 10017 USA.
Douglas Landsman, National Multiple Sclerosis Society 733 3rd Ave New York, NY 10017 USA.
Kathy M Zackowski, National Multiple Sclerosis Society 733 3rd Ave New York, NY 10017 USA.
Fiona Brabazon, National Multiple Sclerosis Society 733 3rd Ave New York, NY 10017 USA.
Walter A Kostich, National Multiple Sclerosis Society 733 3rd Ave New York, NY 10017 USA.
Timothy Coetzee, National Multiple Sclerosis Society 733 3rd Ave New York, NY 10017 USA.
Alexander Victor Ng, Department of Physical Therapy, Marquette University, Milwaukee, WI, USA.
Ruth Ann Marrie, Department of Internal Medicine (Neurology), University of Manitoba, Winnipeg, MB, Canada.
Kelly R Monk, Vollum Institute, Oregon Health & Science University, Portland, OR, USA.
Amit Bar-Or, Center for Neuroinflammation and Neurotherapeutics, Multiple Sclerosis Division, Department of Neurology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA.
Caroline C Whitacre, National Multiple Sclerosis Society 733 3rd Ave New York, NY 10017 USA.
References
- 1. Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult Scler 2020; 26(14): 1816–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bar-Or A, Li R. Cellular immunology of relapsing multiple sclerosis: Interactions, checks, and balances. Lancet Neurol 2021; 20(6): 470–483. [DOI] [PubMed] [Google Scholar]
- 3. Bose G, Thebault SDX, Atkins HL, et al. Does resetting the immune system fix multiple sclerosis. Can J Neurol Sci 2020; 47(1): 1–10. [DOI] [PubMed] [Google Scholar]
- 4. Eshaghi A, Young AL, Wijeratne PA, et al. Identifying multiple sclerosis subtypes using unsupervised machine learning and MRI data. Nat Commun 2021; 12: 210408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marrie RA. Comorbidity in multiple sclerosis: Past, present and future. Clin Invest Med 2019; 42: E5–E12. [DOI] [PubMed] [Google Scholar]
- 6. Giovannoni G, Butzkueven H, Dhib-Jalbut S, et al. Brain health: Time matters in multiple sclerosis. Mult Scler Relat Disord 2016; 9(Suppl. 1): S5–S48. [DOI] [PubMed] [Google Scholar]
- 7. Kappos L, Polman CH, Freedman MS, et al. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology 2006; 67: 1242–1249. [DOI] [PubMed] [Google Scholar]
- 8. Hartung HP, Graf J, Kremer D. Long-term follow-up of multiple sclerosis studies and outcomes from early treatment of clinically isolated syndrome in the BENEFIT 11 study. J Neurol 2020; 267(2): 308–316. [DOI] [PubMed] [Google Scholar]
- 9. Comi G, Martinelli V, Rodegher M, et al. Effect of glatiramer acetate on conversion to clinically definite multiple sclerosis in patients with clinically isolated syndrome (PreCISe study): A randomised, double-blind, placebo-controlled trial. Lancet 2009; 374: 1503–1511. [DOI] [PubMed] [Google Scholar]
- 10. Miller AE, Wolinsky JS, Kappos L, et al. Oral teriflunomide for patients with a first clinical episode suggestive of multiple sclerosis (TOPIC): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol 2014; 13(10): 977–986. [DOI] [PubMed] [Google Scholar]
- 11. Tremlett H, Marrie RA. The multiple sclerosis prodrome: Emerging evidence, challenges, and opportunities. Mult Scler 2021; 27(1): 6–12. [DOI] [PubMed] [Google Scholar]
- 12. Bjornevik K, Munger KL, Cortese M, et al. Serum neurofilament light chain levels in patients with presymptomatic multiple sclerosis. JAMA Neurol 2020; 77: 58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lebrun-Frenay C, Kantarci O, Siva A, et al. Radiologically isolated syndrome: 10-year risk estimate of a clinical event. Ann Neurol 2020; 88(2): 407–417. [DOI] [PubMed] [Google Scholar]
- 14. Matute-Blanch C, Villar LM, Álvarez-Cermeño JC, et al. Neurofilament light chain and oligoclonal bands are prognostic biomarkers in radiologically isolated syndrome. Brain 2018; 141: 1085–1093. [DOI] [PubMed] [Google Scholar]
- 15. Metz I, Weigand SD, Popescu BF, et al. Pathologic heterogeneity persists in early active multiple sclerosis lesions. Ann Neurol 2014; 75(5): 728–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chitnis T, Prat A. A roadmap to precision medicine for multiple sclerosis. Mult Scler 2020; 26(5): 522–532. [DOI] [PubMed] [Google Scholar]
- 17. Rotstein D, Montalban X. Reaching an evidence-based prognosis for personalized treatment of multiple sclerosis. Nat Rev Neurol 2019; 15(5): 287–300. [DOI] [PubMed] [Google Scholar]
- 18. Calabresi PA, Kappos L, Giovannoni G, et al. Measuring treatment response to advance precision medicine for multiple sclerosis. Ann Clin Transl Neurol 2021; 8(11): 2166–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Plavina T, Subramanyam M, Bloomgren G, et al. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol 2014; 76(6): 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Disanto G, Barro C, Benkert P, et al. Serum Neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017; 81: 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cantó E, Barro C, Zhao C, et al. Association between serum neurofilament light chain levels and long-term disease course among patients with multiple sclerosis followed up for 12 years. JAMA Neurol 2019; 76: 1359–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019; 92: e1007–e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ruckh JM, Zhao JW, Shadrach JL, et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 2012; 10: 96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kirby L, Jin J, Cardona JG, et al. Oligodendrocyte precursor cells present antigen and are cytotoxic targets in inflammatory demyelination. Nat Commun 2019; 10: 190831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duncan ID, Radcliff AB, Heidari M, et al. The adult oligodendrocyte can participate in remyelination. Proc Natl Acad Sci U S A 2018; 115: E11807–E11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Scolding NJ, Pasquini M, Reingold SC, et al. Cell-based therapeutic strategies for multiple sclerosis. Brain 2017; 140: 2776–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Di Filippo M, Portaccio E, Mancini A, et al. Multiple sclerosis and cognition: Synaptic failure and network dysfunction. Nat Rev Neurosci 2018; 19(10): 599–609. [DOI] [PubMed] [Google Scholar]
- 28. Michailidou I, Willems JG, Kooi EJ, et al. Complement C1q-C3-associated synaptic changes in multiple sclerosis hippocampus. Ann Neurol 2015; 77(6): 1007–1026. [DOI] [PubMed] [Google Scholar]
- 29. Maynard ME, Redell JB, Zhao J, et al. Sarm1 loss reduces axonal damage and improves cognitive outcome after repetitive mild closed head injury. Exp Neurol 2020; 327: 113207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geisler S, Huang SX, Strickland A, et al. Gene therapy targeting SARM1 blocks pathological axon degeneration in mice. J Exp Med 2019; 216: 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lubetzki C, Zalc B, Williams A, et al. Remyelination in multiple sclerosis: From basic science to clinical translation. Lancet Neurol 2020; 19(8): 678–688. [DOI] [PubMed] [Google Scholar]
- 32. Oh J, Ontaneda D, Azevedo C, et al. Imaging outcome measures of neuroprotection and repair in MS: A consensus statement from NAIMS. Neurology 2019; 92: 519–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lambe J, Fitzgerald KC, Murphy OC, et al. Association of spectral-domain OCT with long-term disability worsening in multiple sclerosis. Neurology 2021; 96: e2058–e2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Simmons SB, Schippling S, Giovannoni G, et al. Predicting disability worsening in relapsing and progressive multiple sclerosis. Curr Opin Neurol 2021; 34: 312–321. [DOI] [PubMed] [Google Scholar]
- 35. Chataway J, De Angelis F, Connick P, et al. Efficacy of three neuroprotective drugs in secondary progressive multiple sclerosis (MS-SMART): A phase 2b, multiarm, double-blind, randomised placebo-controlled trial. Lancet Neurol 2020; 19(3): 214–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cadavid D, Mellion M, Hupperts R, et al. Safety and efficacy of opicinumab in patients with relapsing multiple sclerosis (SYNERGY): A randomised, placebo-controlled, phase 2 trial. Lancet Neurol 2019; 18(9): 845–856. [DOI] [PubMed] [Google Scholar]
- 37. Petrou P, Kassis I, Levin N, et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain 2020; 143: 3574–3588. [DOI] [PubMed] [Google Scholar]
- 38. Harris VK, Stark JW, Yang S, et al. Mesenchymal stem cell-derived neural progenitors in progressive MS: Two-year follow-up of a phase I study. Neurol Neuroimmunol Neuroinflamm 2021; 8(1): e928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mei F, Fancy SPJ, Shen YA, et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat Med 2014; 20(8): 954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Green AJ, Gelfand JM, Cree BA, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): A randomised, controlled, double-blind, crossover trial. Lancet 2017; 390: 2481–2489. [DOI] [PubMed] [Google Scholar]
- 41. Hubler Z, Allimuthu D, Bederman I, et al. Accumulation of 8,9-unsaturated sterols drives oligodendrocyte formation and remyelination. Nature 2018; 560(7718): 372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Petersen MA, Tognatta R, Meyer-Franke A, et al. BMP receptor blockade overcomes extrinsic inhibition of remyelination and restores neurovascular homeostasis. Brain 2021; 144: 2291–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martin E, Aigrot MS, Grenningloh R, et al. Bruton’s tyrosine kinase inhibition promotes myelin repair. Brain Plast 2020; 5: 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mothe AJ, Tassew NG, Shabanzadeh AP, et al. RGMa inhibition with human monoclonal antibodies promotes regeneration, plasticity and repair, and attenuates neuropathic pain after spinal cord injury. Sci Rep 2017; 7: 170907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lozinski BM, Yong VW. Exercise and the brain in multiple sclerosis. Mult Scler. Epub ahead of print 30 October 2020. DOI: 10.1177/1352458520969099. [DOI] [PubMed] [Google Scholar]
- 46. Dalgas U, Langeskov-Christensen M, Stenager E, et al. Exercise as medicine in multiple sclerosis-time for a paradigm shift: Preventive, symptomatic, and disease-modifying aspects and perspectives. Curr Neurol Neurosci Rep 2019; 19: 191114. [DOI] [PubMed] [Google Scholar]
- 47. Prosperini L, Di Filippo M. Beyond clinical changes: Rehabilitation-induced neuroplasticity in MS. Mult Scler 2019; 25(10): 1348–1362. [DOI] [PubMed] [Google Scholar]
- 48. Feys P, Bibby BM, Baert I, et al. Walking capacity and ability are more impaired in progressive compared to relapsing type of multiple sclerosis. Eur J Phys Rehabil Med 2015; 51(2): 207–210. [PubMed] [Google Scholar]
- 49. Khan F, Amatya B. Rehabilitation in multiple sclerosis: A systematic review of systematic reviews. Arch Phys Med Rehabil 2017; 98(2): 353–367. [DOI] [PubMed] [Google Scholar]
- 50. Brichetto G, Pedullà L, Podda J, et al. Beyond center-based testing: Understanding and improving functioning with wearable technology in MS. Mult Scler 2019; 25(10): 1402–1411. [DOI] [PubMed] [Google Scholar]
- 51. Pierrot-Deseilligny C, Souberbielle JC. Vitamin D and multiple sclerosis: An update. Mult Scler Relat Disord 2017; 14: 35–45. [DOI] [PubMed] [Google Scholar]
- 52. Gianfrancesco MA, Barcellos LF. Obesity and multiple sclerosis susceptibility: A review. J Neurol Neuromedicine 2016; 1(7): 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Degelman ML, Herman KM. Smoking and multiple sclerosis: A systematic review and meta-analysis using the Bradford Hill criteria for causation. Mult Scler Relat Disord 2017; 17: 207–216. [DOI] [PubMed] [Google Scholar]
- 54. Guan Y, Jakimovski D, Ramanathan M, et al. The role of Epstein-Barr virus in multiple sclerosis: From molecular pathophysiology to in vivo imaging. Neural Regen Res 2019; 14: 373–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ascherio A, Munger KL. Epidemiology of multiple sclerosis: From risk factors to prevention—An update. Semin Neurol 2016; 36(2): 103–114. [DOI] [PubMed] [Google Scholar]
- 56. Sawcer S, Ban M, Maranian M, et al. A high-density screen for linkage in multiple sclerosis. Am J Hum Genet 2005; 77(3): 454–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. International Multiple Sclerosis Genetics Consortium. Multiple sclerosis genomic map implicates peripheral immune cells microglia in susceptibility. Science 2019; 365(6460): eaav7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Alfredsson L, Olsson T. Lifestyle and Environmental Factors in Multiple Sclerosis. Cold Spring Harb Perspect Med 2019; 9: a028944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cohen JI. Vaccine development for Epstein-Barr virus. Adv Exp Med Biol 2018; 1045: 477–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ascherio A, Munger KL, Lünemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol 2012; 8: 602–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Herold KC, Bundy BN, Long SA, et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N Engl J Med 2019; 381: 603–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cortese M, Riise T, Bjørnevik K, et al. Preclinical disease activity in multiple sclerosis: A prospective study of cognitive performance prior to first symptom. Ann Neurol 2016; 80(4): 616–624. [DOI] [PubMed] [Google Scholar]
- 63. Zhao Y, Wijnands JMA, Högg T, et al. Interrogation of the multiple sclerosis prodrome using high-dimensional health data. Neuroepidemiology 2020; 54(2): 140–147. [DOI] [PubMed] [Google Scholar]
- 64. Institute of Medicine (US) Committee on Multiple Sclerosis: Current Status and Strategies for the Future. Multiple sclerosis: Current status and strategies for the future (eds JE Joy, RB Johnston Jr.). Washington, DC: National Academies Press (US), National Academy of Sciences, 2001. [PubMed] [Google Scholar]


