Abstract
Background
Patients with SLE have an endothelial dysfunction (ED), which is considered the earliest marker of cardiovascular (CV) disease. Endothelial cell activation induced by proinflammatory cytokines is defined by the endothelial expression of cell-surface adhesion molecules, such as vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1) and E-selectin. The aim of this study was to investigate whether serum endothelial adhesion molecule levels are influenced by blood hydroxychloroquine (HCQ) levels in SLE.
Methods
Consecutive patients with SLE taking a stable dose of HCQ were investigated. At study entry and 6 months later HCQ blood levels were quantified by tandem mass spectrometry. Serum levels of P-selectin, E-selectin, ICAM-1 and VCAM-1 were also measured using a Luminex 200 instrument. Comparison of endothelial soluble adhesion molecules in groups with different HCQ blood levels was performed by t-test.
Results
83 patients with SLE were enrolled. Correlation were demonstrated between mean blood HCQ concentrations and endothelial soluble adhesion molecules (E-selectin, ICAM-1 and VCAM-1). Moreover, serum levels of ICAM-1 and VCAM-1 were significantly lower in the patients with SLE with HCQ blood levels >500 ng/mL (83.67±52.8 ng/mL vs 158.81±125.1 ng/mL and 8.9±2.2 ng/mL vs 10.4±2.3 ng/mL). Serum levels of E-selectin were nearly significantly lower in the patients with SLE with HCQ blood levels >500 ng/mL (64.7±30.2 ng/mL vs 71.6±42.2 ng/mL, p=0.06). No significant difference in concentration of P-selectin was detected.
Conclusions
In the present study, there was a trend towards higher adhesion molecules levels with lower HCQ blood levels in patients with SLE. Further longitudinal studies will determine whether changes in endothelial biomarkers reflect decreased clinical CV events.
Keywords: atherosclerosis, systemic lupus erythematosus, cardiovascular diseases
Key messages.
What is already known about this subject?
Endothelial function appears to be affected by SLE, potentially contributing to the increased cardiovascular (CV) risk observed in these patients.
Endothelial dysfunction in these patients is characterised by increased expression and release of adhesion molecules.
What does this study add?
In this study, therapeutic blood hydroxychloroquine (HCQ) levels were associated with decreased adhesion molecules levels in SLE.
How might this impact on clinical practice or future developments?
These findings further support the previous observations of the potential beneficial effect of HCQ in preventing CV disease in SLE.
Introduction
Despite the improvement in survival over the past decades, SLE is still characterised by increased mortality due to cardiovascular diseases (CVD).1 The high risk of CVD in SLE is only partially explained by an increased prevalence of traditional CV risk factors; SLE-related factors also contributing to the risk.2 SLE-associated systemic inflammation may impair endothelium functionality, leading to atherosclerosis development and progression.3
Endothelial dysfunction (ED) is recognised as an early marker of atherosclerosis and is characterised by the inability of the endothelium to appropriately modulate blood flow due to a reduced bioavailability of endothelium-derived vasodilators, such as nitric oxide and a concomitant increased reactive oxygen species generation.4 Various studies have shown that patients with SLE have an ED.5 In addition to ED, another important feature of atherosclerotic vessels in SLE is endothelial cell activation.6 The expression of adhesion molecules on activated endothelial cells is an important step in the initiation and progression of atherosclerosis. At the beginning of atherosclerotic plaque formation, endothelial cells show increased expression and release of adhesion molecules, including E-selectin, P-selectin, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1). These molecules are released in response to stimuli of inflammatory cytokines and promote cell-cell and cell-extracellular matrix adhesion, leading to foam cell accumulation on the subendothelial space.7 In particular, the endothelial selectins (E-selectin, P-selectin) mediate the initial weak interaction that allows leukocytes to roll along the vessel wall, while adhesion molecules of the immunoglobulin superfamily (ICAM-1 and VCAM-1) on endothelial cells mediate the second tight adhesion. In vitro studies have shown that the serum concentrations of soluble adhesion molecules correlate with the expression of these molecules on the endothelial cell surface.8 Elevated concentrations of circulating soluble adhesion molecules were closely linked to the development of atherosclerosis and predict CV events.8–11 Interestingly, ED is a reversible alteration, thus representing a potentially attractive target for preventive intervention.
Hydroxychloroquine (HCQ) remains the first-line treatment for patients with SLE owing to its safety profile and multiple beneficial effects, including control of disease activity, reduction of damage accrual and improvement of survival.12–15 Moreover, HCQ has demonstrated to reduce serum cholesterol and mean glucose levels.16 17 We recently conducted a long-term study which demonstrated the HCQ-related benefits in the primary thromboprophylaxis in patients with SLE.18 19 A beneficial effect of HCQ on vascular reactivity indices has also been reported in lupus murine models.20 21 Based on these evidences, we hypothesised that HCQ could restore ED. Therefore, the present study was designed to analyse the effect of HCQ blood levels on the concentrations of soluble adhesion molecules (ICAM-1, VCAM-1, E-selectin and P-selectin).
Materials and methods
Study population
Caucasian patients with SLE prospectively followed at our lupus clinic were eligible for inclusion in the study if they met the SLE American College of Rheumatology classification criteria22 and were on treatment with a stable dose of oral HCQ during the previous 6 months. To avoid the effect of active inflammation on ED, we included patients in remission according to the Definitions of Remission in SLE criteria, for at least 1 year.23
The exclusion criteria included other autoimmune diseases, antiphospholipid antibody syndrome, pre-existing CVD and currently taking medications that influence the endothelial function marker level, such as ACE inhibitor or statin. Data collected about each patient from admission to throughout follow-up include basic demographic characteristics (gender, date of birth, age at SLE onset, ethnicity), disease duration, disease manifestations, current and previous therapies, damage accrual and laboratory investigations, family history of vascular disease (ischaemic heart disease, stroke and peripheral vascular disease), menopausal status. At each visit, physical examination, treatment regimen, Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) and SLICC Damage Index (SDI),24 25 adverse events and laboratory testing, including C3 and C4 levels and antidouble-stranded DNA antibodies were recorded.
HCQ extraction from whole blood and definition of non-adherence
Consecutive patients with SLE taking a stable dose of HCQ for at least 6 months were investigated. Patients were prescribed HCQ not exceeding 6.5 mg/kg (maximum 400 mg/day). At study entry (T0) and 6 months later (T6), a blood venous sample was taken to measure whole blood concentration of HCQ. If the patient had to discontinue HCQ use due to retinopathy or intolerance, was excluded. HCQ levels were serially quantified from EDTA whole blood by liquid chromatography-tandem mass spectrometry as previously described.26 A mean HCQ value for each patient was then calculated. This study design was chosen as there are individual variations in HCQ bioavailability that are considered secondary to pharmacokinetic and pharmacodynamics factors, which are as yet poorly understood. The therapeutic range was 500–2000 ng/mL. This was chosen as our therapeutic range based on a review of the available literature. The patients were divided according to their blood level. Levels <100 ng/mL were considered to be consistent with complete non-adherence. Levels of 100–500 ng/mL were considered partially adherent, between 500 and 2000 ng/mL were therapeutic and >2000 ng/mL were considered supratherapeutic.27
Serum marker quantification
We then evaluated the serum levels of adhesion molecules (ICAM-1, VCAM-1, E-selectin and P-selectin). Peripheral blood was obtained at enrolment by venipuncture. Serum was separated by centrifugation, aliquoted and stored at −20°C. All analytes were measured by single or multiple suspension fluorescence-based immunoassay (Merck Millipore, Billerica, Massachusetts, USA) using a Luminex 200 instrument (Luminex, Austin, Texas, USA). Concentrations were expressed as ng/mL.
Statistical analysis
Continuous data were expressed as mean±SD or median with range and compared by the unpaired Student’s t-test or the Mann-Whitney U test as appropriate. Categorical data were analysed by the Fisher’s exact test and the χ2 test, respectively. Correlation analysis between two variables was performed using Spearman’s rank correlation. The grading of correlation coefficients (r) can vary, but for the purposes of this study 0.2–0.3=weak correlation, 0.3–0.7=moderate correlation and 0.7–1.0=strong correlation. For statistical analysis, the patients were divided according to mean HCQ blood levels (between baseline and follow-up). We compared the HCQ groups with respect to serum adhesions markers levels at baseline and assessed significance using t-test. P values <0.05 were considered statistically significant. MedCalc software, V.15.4, was used for all analyses.
Results
Demographic, clinical and laboratory features of patients
A total of 83 consecutive patients with SLE fulfilled the inclusion criteria and was enrolled in the study after giving written informed consent. Clinical and therapeutic features of the patients enrolled are summarised in table 1.
Table 1.
Clinical, demographic and laboratory features of 83 patients enrolled*
| All patients (n=83) | HCQ <100 ng/mL (n=24) | HCQ ≥100 ng/mL (n=59) | P value | |
| Sex, female | 79 (95) | 22 (92) | 57 (97) | 0.575 |
| Age, years mean±SD | 41±11 | 39±11 | 42±11 | 0.772 |
| Disease duration, years median (range) | 15 (2–37) | 17 (2–29) | 13 (2–37) | 0.692 |
| SLICC Damage Index median (range) | 0 (0–3) | 0 (0–1) | 0 (0–3) | 0.385 |
| Current smokers | 31 (37) | 9 (37) | 22 (38) | 0.816 |
| Body mass index (kg/m2) mean±SD | 25±5 | 24±4 | 26±5 | 0.828 |
| Estimated creatinine clearance, mL/min mean±SD | 89±24 | 94±22 | 86±24 | 0.129 |
| HCQ prescribed dose/weight (mg/kg) mean±SD | 5.3±1.2 | 5.4±1.2 | 5.4±1.2 | 0.71 |
| HCQ ng/mL median (range) | 327 (0–4003) | 0 (0–99) | 546.1 (101–4003) | <0.0001 |
| DCQ ng/mL median (range) | 47 (0–650) | 0 (0–11) | 79 (11–650) | <0.0001 |
| Additional treatment | ||||
| Immunosuppressors | 23 (28) | 13 (54) | 10 (17) | 0.001 |
| Azathioprine | 10 (12) | 5 (22) | 5 (8) | |
| Mycophenolate mofetil | 6 (7) | 3 (12) | 3 (5) | |
| Methotrexate | 5 (6) | 4 (17) | 1 (2) | |
| Ciclosporin | 2 (2) | 1 (4) | 1 (2) | |
| Glucocorticoids | 40 (48) | 11 (46) | 29 (52) | 0.974 |
*If not otherwise specified, the values are the number (%) of patients.
DCQ, whole blood concentration of desethylchloroquine; HCQ, whole blood concentration of hydroxychloroquine; SLICC, Systemic Lupus International Collaborating Clinics.
They were Caucasian, mostly female (95%), with a mean age of 41 years, a median disease duration of 15 years (range 2–37 years) and a median SDI of 0 (range 0–3). They were treated with HCQ prescribed at a stable oral dose of 400 or 200 mg/day for at least 6 months (the mean±SD daily HCQ dose was 346±72). The mean±SD dose of HCQ per weight prescribed was 5.3±1.2 mg/kg. The median HCQ blood concentration was 327 ng/mL (range 0–4003 ng/mL). There was no correlation between the prescribed HCQ dose in mg/kg (through the range of clinical use of 4.5–6.5 mg/kg/day) and the HCQ blood levels (r=−0.08, p>0.05). We stratified patients according to their blood HCQ levels. Among the 83 patients, 24 (29%) had values indicative of poor therapeutic adherence, reflected by at least HCQ blood levels ≤100 ng/mL or undetectable blood HCQ concentration. Additional 37 (44%) patients showed HCQ concentration <500 ng/mL in at least a visit. The proportion of patients with mean HCQ levels 100–500 (subtherapeutic) and >500 ng/mL (therapeutic) was 30% and 41%, respectively. There were no statistically significant differences seen in demographic and clinical features between patients with therapeutic levels and non-adherents, except for immunosuppressants use (table 1). Among the 59 patients with mean whole HCQ blood levels in therapeutic range, 12 patients had a mean level >1.000 ng/mL. Despite appropriate weight-based dosing, a patient was above the therapeutic range (>2000 ng/mL).
Serum adhesion molecules and relationship with HCQ blood levels
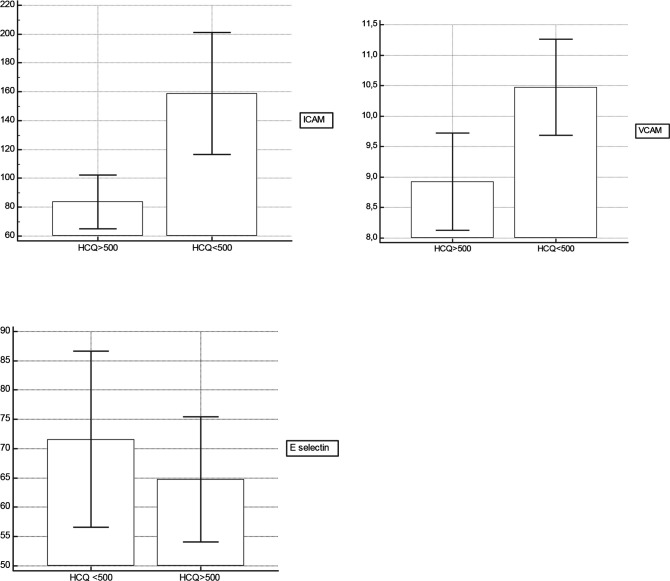
All investigated factors (ICAM-1, VCAM-1, P-selectin, E-selectin) were detectable in sera from patients with SLE. In this regard, correlations between mean HCQ blood levels and serum adhesion molecules were performed. The analysis of the whole cohort showed that correlation coefficients ranged from −0.28 (p=0.03) for E-selectin to −0.38 (p=0.005) for ICAM and to −0.45 (p=0.001) for VCAM. Since these results can be influenced by the non-therapeutic HCQ whole blood levels found in non-adherent patients (HCQ whole blood levels ≤100 ng/mL), we excluded such patients from the analysis. The analysis of the remaining 59 patients showed that correlation coefficients ranged from −0.26 (p=0.04) for E-selectin to −0.36 (p=0.005) for ICAM and to −0.41 (p=0.001) for VCAM. Likewise, no correlation between serum P-selectin and mean blood levels of HCQ were found (data not shown). We then compared each HCQ group with respect to serum adhesion molecules levels (figure 1). Serum levels of ICAM-1 and VCAM-1 were significantly lower in the patients with SLE with mean HCQ blood levels >500 ng/mL (respectively: 83.67±52.8 ng/mL (65.2–102.1) vs 158.81±125.1 ng/mL (116.4–201.1), p=0.001 and 10.4±2.3 ng/mL (9.6–11.2) vs 8.9±2.2 ng/mL (8.1–9.7), p=0.006). Serum levels of E-selectin were nearly significantly lower in the patients with SLE with mean HCQ blood levels >500 ng/mL (64.7±30.2 ng/mL (54.1–75.4) vs 71.6±42.2 ng/mL (56.6–86.5), p=0.06). No significant difference in concentration of P-selectin was detected in patients with SLE.
Figure 1.
Mean of soluble intercellular adhesion molecule-1 (ICAM-1), soluble vascular cell adhesion molecule-1 (VCAM-1) and E-selectin levels, with difference between the two hydroxychloroquine (HCQ) groups (mean HCQ blood levels >500 ng/mL or <500 ng/mL).
Disease duration, sex, age, body mass index, antiphopholipid antibodies (aPL), postmenopausal status did not correlate the serum profile of the investigated soluble factors in patients with SLE.
Discussion
To our knowledge, this is the first study to evaluate the effect of HCQ on serum biomarkers of endothelial activation in patients with SLE. HCQ represents a milestone in the treatment of patients with SLE. In recent years, HCQ therapy has been associated with positive effect on traditional CV risk factors and a reduction of CV morbidity and mortality in SLE.18 19 Moreover, maintaining an average HCQ whole blood level >1.068 ng/mL significantly reduced the risk of thrombosis in SLE, based on data from 739 patients of the John Hopkins cohort.28 However, it was still unclear if the protective CV effect of HCQ is an indirect result of better control of disease activity or a direct effect of HCQ on the endothelium. It is known that after activation by proinflammatory cytokines, adhesion molecules are shed from the surface of endothelial cells. For this reason, these soluble adhesion molecules may be useful as markers of endothelial activation.8 This study shows that higher mean HCQ whole blood levels were associated with lower levels of soluble adhesion molecules. In particular, we found lower serum levels of E-selectin, soluble VCAM-1 (sVCAM-1) and soluble ICAM-1 (sICAM-1) in patients with mean HCQ blood levels in therapeutic range.
In our study, the role of HCQ in improving ED was not linked to a better control of the disease, as disease activity in both groups did not differ significantly. The present data confirm in humans the previous demonstration of the direct effect of HCQ in lupus mouse models on endothelium,20 where endothelium-dependent vasodilator responses were normalised by HCQ. Data from studies previously published in Rheumatoid Arthritis (RA) patients revealed similar results.29
Moreover, in this study we confirmed the previous findings that adherence to medications is a problem in patients with SLE.30–33 Our analysis demonstrates that up to 30% of our patients with inactive SLE did not take their HCQ as prescribed. Previous studies have shown that the rate of non-adherence assessed by HCQ blood measurement is highly variable, ranging from 7% up to 83%.33 34 We pointed out that 24 (29%) had undetectable blood HCQ levels and additional 37 (44%) patients showed a HCQ concentration <500 ng/mL in a visit, although it is possible that in this group there may be individuals who, due to genetic differences in HCQ metabolism, are adherent but achieve lower blood concentrations. In our study, the rate of non-adherent patients decreased at the second sampling as only about 10% of patients had very low levels of blood HCQ. This is similar to the reported literature in SLE and monitoring HCQ blood levels can be important to improve medication adherence in patients with lupus.34 35 Moreover, in our study there was no correlation between the prescribed HCQ dose and the HCQ blood levels. Our data clearly indicate the need for personalised HCQ dosing approaches beyond empirical dosing recommendation through routinely HCQ blood level measurements.
Our study has several limitations. The endothelial biomarkers that we used were in circulating form, which have less important biological function than the form that is present on the surface of endothelial cells. Moreover, the ED improvement was characterised by lowering of E-selectin, sVCAM-1 and sICAM-1 levels in HCQ group in therapeutic range. However, these results were not confirmed prospectively and were not followed by a significant decrease in P-selectin levels. However, E-selectin may be more specific in reflecting the atherosclerotic burden compared with P-selectin, as is expressed exclusively by activated vascular endothelium, whereas P-selectin also mediates platelet-neutrophil interactions.36 Another limitation is that the targeted threshold HCQ levels differed between studies. Some studies used <200 ng/mL as the threshold HCQ blood level to identify severe non-adherence in patients treated with 400 mg/day of HCQ.31 We used a cut-off of 100 ng/mL, which is more stringent to exclude any possible interference with factors known to influence HCQ blood concentration. Similarly, other studies used HCQ levels >500 ng/mL as a therapeutic threshold.33 Additional limitations include the single site and small sample size.
In conclusion, our study showed significant reduction in endothelial biomarkers in patients with SLE with therapeutic HCQ blood levels but there is no proof of causality and further research is needed to find molecular mechanisms that can explain if and how HCQ can improve ED in patients with SLE.
Footnotes
Contributors: SF conceived and planned the experiments. AB carried out the experiments. All authors contributed to the interpretation of the results. SF took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript. SF is the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available on reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s)
Ethics approval
Participants gave written informed consent and the protocol was approved by the local Ethics Committee (University of Campania Luigi Vanvitelli ID9619).
References
- 1.Bartels CM, Buhr KA, Goldberg JW, et al. Mortality and cardiovascular burden of systemic lupus erythematosus in a US population-based cohort. J Rheumatol 2014;41:680–7. 10.3899/jrheum.130874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum 2001;44:2331–7. [DOI] [PubMed] [Google Scholar]
- 3.Mauro D, Nerviani A. Endothelial dysfunction in systemic lupus erythematosus: pathogenesis, assessment and therapeutic opportunities. Rev Recent Clin Trials 2018;13:192–8. 10.2174/1574887113666180314091831 [DOI] [PubMed] [Google Scholar]
- 4.Pennathur S, Heinecke JW. Oxidative stress and endothelial dysfunction in vascular disease. Curr Diab Rep 2007;7:257–64. 10.1007/s11892-007-0041-3 [DOI] [PubMed] [Google Scholar]
- 5.Mak A, Kow NY, Schwarz H, et al. Endothelial dysfunction in systemic lupus erythematosus - a case-control study and an updated meta-analysis and meta-regression. Sci Rep 2017;7:7320. 10.1038/s41598-017-07574-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atehortúa L, Rojas M, Vásquez G, et al. Endothelial activation and injury by microparticles in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther 2019;21:34. 10.1186/s13075-018-1796-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies MJ, Gordon JL, Gearing AJ, et al. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol 1993;171:223–9. 10.1002/path.1711710311 [DOI] [PubMed] [Google Scholar]
- 8.Leeuwenberg JF, Smeets EF, Neefjes JJ, et al. E-Selectin and intercellular adhesion molecule-1 are released by activated human endothelial cells in vitro. Immunology 1992;77:543–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Pradhan AD, Rifai N, Ridker PM. Soluble intercellular adhesion molecule-1, soluble vascular adhesion molecule-1, and the development of symptomatic peripheral arterial disease in men. Circulation 2002;106:820–5. 10.1161/01.CIR.0000025636.03561.EE [DOI] [PubMed] [Google Scholar]
- 10.Ridker PM, Hennekens CH, Roitman-Johnson B, et al. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet 1998;351:88–92. 10.1016/S0140-6736(97)09032-6 [DOI] [PubMed] [Google Scholar]
- 11.Dessein PH, Joffe BI, Singh S. Biomarkers of endothelial dysfunction, cardiovascular risk factors and atherosclerosis in rheumatoid arthritis. Arthritis Res Ther 2005;7:R634–43. 10.1186/ar1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, et al. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 2010;69:20–8. 10.1136/ard.2008.101766 [DOI] [PubMed] [Google Scholar]
- 13.Willis R, Seif AM, McGwin G, et al. Effect of hydroxychloroquine treatment on pro-inflammatory cytokines and disease activity in SLE patients: data from LUMINA (LXXV), a multiethnic US cohort. Lupus 2012;21:830–5. 10.1177/0961203312437270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akhavan PS, Su J, Lou W, et al. The early protective effect of hydroxychloroquine on the risk of cumulative damage in patients with systemic lupus erythematosus. J Rheumatol 2013;40:831–41. 10.3899/jrheum.120572 [DOI] [PubMed] [Google Scholar]
- 15.Pons-Estel GJ, Alarcón GS, González LA, et al. Possible protective effect of hydroxychloroquine on delaying the occurrence of integument damage in lupus: LXXI, data from a multiethnic cohort. Arthritis Care Res 2010;62:393–400. 10.1002/acr.20097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wallace DJ, Metzger AL, Stecher VJ, et al. Cholesterol-Lowering effect of hydroxychloroquine in patients with rheumatic disease: reversal of deleterious effects of steroids on lipids. Am J Med 1990;89:322–6. 10.1016/0002-9343(90)90345-E [DOI] [PubMed] [Google Scholar]
- 17.Petri M. Hydroxychloroquine use in the Baltimore lupus cohort: effects on lipids, glucose and thrombosis. Lupus 1996;5 Suppl 1:16–22. 10.1177/0961203396005001051 [DOI] [PubMed] [Google Scholar]
- 18.Fasano S, Pierro L, Pantano I, et al. Longterm hydroxychloroquine therapy and low-dose aspirin may have an additive effectiveness in the primary prevention of cardiovascular events in patients with systemic lupus erythematosus. J Rheumatol 2017;44:1032–8. 10.3899/jrheum.161351 [DOI] [PubMed] [Google Scholar]
- 19.Fasano S, Margiotta DP, Navarini L, et al. Primary prevention of cardiovascular disease in patients with systemic lupus erythematosus: case series and literature review. Lupus 2017;26:1463–72. 10.1177/0961203317722847 [DOI] [PubMed] [Google Scholar]
- 20.Gómez-Guzmán M, Jiménez R, Romero M, et al. Chronic hydroxychloroquine improves endothelial dysfunction and protects kidney in a mouse model of systemic lupus erythematosus. Hypertension 2014;64:330–7. 10.1161/HYPERTENSIONAHA.114.03587 [DOI] [PubMed] [Google Scholar]
- 21.Virdis A, Tani C, Duranti E, et al. Early treatment with hydroxychloroquine prevents the development of endothelial dysfunction in a murine model of systemic lupus erythematosus. Arthritis Res Ther 2015;17:277. 10.1186/s13075-015-0790-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hochberg MC. Updating the American College of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. 10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 23.van Vollenhoven RF, Bertsias G, Doria A, et al. 2021 DORIS definition of remission in SLE: final recommendations from an international Task force. Lupus Sci Med 2021;8:e000538. 10.1136/lupus-2021-000538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002;29:288–91. [PubMed] [Google Scholar]
- 25.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the systemic lupus international collaborating Clinics/American College of rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996;39:363–9. 10.1002/art.1780390303 [DOI] [PubMed] [Google Scholar]
- 26.Iudici M, Pantano I, Fasano S, et al. Health status and concomitant prescription of immunosuppressants are risk factors for hydroxychloroquine non-adherence in systemic lupus patients with prolonged inactive disease. Lupus 2018;27:265–72. 10.1177/0961203317717631 [DOI] [PubMed] [Google Scholar]
- 27.Mok CC, Penn HJ, Chan KL, et al. Hydroxychloroquine serum concentrations and flares of systemic lupus erythematosus: a longitudinal cohort analysis. Arthritis Care Res 2016;68:1295–302. 10.1002/acr.22837 [DOI] [PubMed] [Google Scholar]
- 28.Petri M, Konig MF, Li J, et al. Association of higher hydroxychloroquine blood levels with reduced thrombosis risk in systemic lupus erythematosus. Arthritis Rheumatol 2021;73:997–1004. 10.1002/art.41621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hidayat R, et al. The effect of hydroxychloroquine on endothelial dysfunction in patients with rheumatoid arthritis: a double blind randomized clinical trial. International Journal of Clinical Rheumatology 2019;14:2. [Google Scholar]
- 30.Costedoat-Chalumeau N, Amoura Z, Hulot J-S, et al. Low blood concentration of hydroxychloroquine is a marker for and predictor of disease exacerbations in patients with systemic lupus erythematosus. Arthritis Rheum 2006;54:3284–90. 10.1002/art.22156 [DOI] [PubMed] [Google Scholar]
- 31.Costedoat-Chalumeau N, Amoura Z, Hulot J-S, et al. Very low blood hydroxychloroquine concentration as an objective marker of poor adherence to treatment of systemic lupus erythematosus. Ann Rheum Dis 2007;66:821–4. 10.1136/ard.2006.067835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costedoat-Chalumeau N, Houssiau F, Izmirly P, et al. A prospective international study on adherence to treatment in 305 patients with flaring SLE: assessment by drug levels and self-administered questionnaires. Clin Pharmacol Ther 2019;106:374–82. 10.1002/cpt.1194 [DOI] [PubMed] [Google Scholar]
- 33.Garg S, Unnithan R, Hansen KE, et al. Clinical Significance of Monitoring Hydroxychloroquine Levels in Patients With Systemic Lupus Erythematosus: A Systematic Review and Meta-Analysis. Arthritis Care Res 2021;73:707–16. 10.1002/acr.24155 [DOI] [PubMed] [Google Scholar]
- 34.Durcan L, Clarke WA, Magder LS, et al. Hydroxychloroquine blood levels in systemic lupus erythematosus: Clarifying dosing controversies and improving adherence. J Rheumatol 2015;42:2092–7. 10.3899/jrheum.150379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petri M, Elkhalifa M, Li J, et al. Hydroxychloroquine blood levels predict hydroxychloroquine retinopathy. Arthritis Rheumatol 2020;72:448–53. 10.1002/art.41121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gonzalez-Gay MA, Garcia-Unzueta MT, De Matias JM, et al. Influence of anti-TNF-alpha infliximab therapy on adhesion molecules associated with atherogenesis in patients with rheumatoid arthritis. Clin Exp Rheumatol 2006;24:373–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available on reasonable request.