Abstract
Introduction
Vaccination is an effective and safe strategy to prevent Human papillomavirus (HPV) infection and related harms. Despite various efforts by French authorities to improve HPV vaccine coverage (VC) these past few years, VC has remained far lower than in most other high-income countries. To improve it, we have coconstructed with stakeholders a school-based and primary care-based multicomponent intervention, and plan to evaluate its effectiveness, efficiency and implementation through a cluster randomised controlled trial (cRCT).
Methods and analysis
This pragmatic cRCT uses an incomplete factorial design to evaluate three components applied alone or in combination: (1) adolescents and parents’ education and motivation at school, using eHealth tools and participatory learning; (2) general practitioners’ training on HPV using motivational interviewing techniques and provision of a decision aid tool; (3) free-of-charge access to vaccination at school. Eligible municipalities (clusters) are located in one of 14 preselected French school districts and must have only one secondary school which enrols at least 2/3 of inhabitants aged 11–14 years. A randomisation stratified by school district and deprivation index allocated 90 municipalities into 6 groups of 15. The expected overall sample size estimate is 41 940 adolescents aged 11–14 years. The primary endpoint is the HPV VC (≥1 dose) among adolescents aged 11–14 years, at 2 months, at the municipality level (data from routine databases). Secondary endpoints include: HPV VC (≥1 dose at 6 and 12 months; and 2 doses at 2, 6 and 12 months); differences in knowledge, attitudes, behaviours, and intention among adolescents, parents and general practitioners between baseline and 2 months after intervention (self-administered questionnaires); incremental cost-effectiveness ratio. Implementation measures include dose, fidelity, adaptations, reached population and satisfaction (activity reports and self-administered questionnaires).
Ethics and dissemination
This protocol was approved by the French Ethics Committee ‘CPP Sud-Est VI’ on 22 December 2020 (ID-RCB: 2020-A02031-38). No individual consent was required for this type of research; all participants were informed of their rights, in particular not to participate or to oppose the collection of data concerning them. Findings will be widely disseminated (conference presentations, reports, factsheets and academic publications).
Trial registration number
Keywords: public health, public health, medical education & training
Strenghts and limitations of this study.
Vaccine coverage is measured using data collected in routine by the national health insurance and vaccination centres, thus avoiding reporting bias.
Few medicoeconomic analyses of interventions on human papillomavirus (HPV) vaccination uptake are available and mostly concern reminder interventions.
Assessing impacts on several determinants of vaccination behaviours will help understand how the intervention may promote behavioural change and HPV vaccine uptake.
Measures of implementation (dose, fidelity, adaptations, reached population and satisfaction) will help stakeholders decide how the intervention may be replicated or generalised at a national level.
Due to feasibility constraints, large French municipalities are not included in the study, and a possible selection bias in the future results cannot be excluded.
Introduction
Human papillomavirus (HPV) infection is the most common viral infection of the reproductive tract, and a major public health issue.1 2 More than 80% of sexually active men and women will acquire HPV by age 45,3 often shortly after the onset of sexual activity.1 Most HPV infections (70%–90%) are asymptomatic and resolve spontaneously.2 However, persistent infections can cause anogenital warts, precancerous lesions of the cervix, vagina, vulva, anus, penis and head and neck, which, if untreated, may sometimes progress to cancers.2 Worldwide, HPV contributed to about 690 000 new cases of cancers in 2018 (ie, 4% of all cancers; women: 620,000; men: 70,000).4 Cervical cancer is by far the most common HPV-related cancer,2 with 7 out of 10 cases caused by two high-risk HPV types (16 and 18).5 It is the fourth most frequent cancer in women worldwide, accounting for 604 127 new cases and 341 831 deaths in 2020 (respectively, 3379 and 1452 in France).6 7
Vaccination is the most effective primary prevention strategy against HPV infection.2 7 It protects against HPV infections, anogenital warts and high-grade precancerous cervical lesions (ie, cervical intraepithelial neoplasia (CIN) 2+ and CIN3+).8–11 After 5–8 years of vaccination, data from 14 countries showed a significant decrease in the prevalence of HPV 16 and 18 infections (−83% among girls aged 13–19 years), anogenital warts diagnoses (−67% and −48% among girls and boys aged 15–19 years, respectively) and CIN2+ (−51% among girls aged 15–19 years), with a greater decrease in countries with both a wider range of targeted age groups and high vaccine coverage (VC).10 More recently, HPV vaccination has been associated with a reduced risk of invasive cervical cancer among Swedish girls/women aged 10–30 years.12 HPV vaccines have an ‘excellent safety profile’ according to the WHO,2 with adverse events generally being non-serious and of short duration. Moreover, a study on postlicensure safety surveillance did not find any association between HPV vaccination and some conditions (eg, autoimmune diseases) that have occurred postvaccination.2
Since 2006, most high-income countries have introduced HPV vaccination in their vaccination schedules for adolescents (for girls only or girls and boys, depending on the country).13 14 In France, HPV vaccination was initially recommended for girls when it was introduced in 2007; in 2021, it was included in the vaccine schedule for all adolescents aged 11–14 years.15 The currently recommended vaccine is the latest nonavalent one (against 6, 11, 16, 18, 31, 33, 45, 52, 58 types) with two injections 6 months apart.15 A catch-up with three injections is possible up to age 19 years. HPV VC varies significantly across high-income countries, from a few percentage points (eg, in Poland, Bulgaria) to 90% (eg, in Norway and Iceland).13 14 Almost 15 years after HPV vaccine introduction in France and despite various efforts by health authorities to improve HPV vaccine uptake,16 17 complete HPV VC remains lower than in most other high-income and European countries,13 14 having been estimated at 23.7% among 16-year girls in 2018.18 In this context, the French Institute for Public Health Research (IReSP) and the theme-based Multi-Organisation Institutes for Cancer and for Public Health (ITMO Cancer and ITMO Public Health) launched in 2018 a national research programme to improve HPV VC among French adolescents. This research programme in epidemiology and social and human sciences is conducted by a consortium of eight French teams (The PrevHPV Consortium—see list in online supplemental table 1) and funded as part of the National Cancer Plan 2014–2019. This programme, called the PrevHPV programme, includes the following three phases.
bmjopen-2021-057943supp001.pdf (380.6KB, pdf)
The first ‘diagnostic’ phase (October 2019–March 2021) aimed at exploring knowledge, beliefs, behaviours, practices, barriers, motivations and preferences towards HPV vaccination among four population groups: girls and boys from secondary schools (aged 11–14 years); their parents; staff from schools (eg, teachers, nurses); and general practitioners (GPs). This phase included several quantitative and qualitative surveys, according to a mixed method approach, and manuscripts reporting results are being written (for preliminary results see19 20).
The second ‘coconstruction’ phase (October 2019–June 2021) aimed at designing the multicomponent intervention to improve HPV VC. Three components were identified: adolescents’ and parents’ education and motivation at school (component 1); GPs’ training (component 2); and access to vaccination at school (component 3) (see ‘The three components of the intervention’ section).
The third ‘experimental’ phase, yet to be conducted (November 2021–May 2022), aims at evaluating the effectiveness, efficiency and implementation of the intervention in France, taking into account its multicomponent structure, through an incomplete factorial design and using cluster randomisation (called the PrevHPV study).
The present manuscript describes the protocol of this cluster randomised trial (cRCT) using the ‘Standard Protocol Items: Recommendations for Interventional Trials’ (SPIRIT) statement as a guide21 (see completed SPIRIT checklist in online supplemental table 2).
Methods and analysis
Study organisation
The French National Institute for Health and Medical Research (Inserm) is the sponsor of the PrevHPV study. A scientific and operational committee (called PrevHPV Study Group) is in charge of supervising all scientific aspects and organisational issues occurring during the PrevHPV programme and meets monthly to elaborate, perform and follow the research. This committee comprises the scientific leaders of each of the eight teams involved in the consortium and their staff, and a representative from IReSP and from Inserm.
A steering committee is in charge of supervising the progress of all aspects of the PrevHPV programme, and meets once a year. It comprises the scientific leaders of the eight teams, as well as representatives of the following national institutions: Inserm, IReSP, ITMO Cancer AVIESAN, ITMO Public Health AVIESAN, INCa (French National Cancer Institute), Santé publique France (French Public Health Agency), Ministry of Health, Ministry of National Education, and the Ile-de-France Regional Health Agency.
Patient and public involvement
The three components of the intervention (see ‘The three components of the intervention’ section) are developed using results from our diagnostic phase on target populations’ needs. The public (adolescents, parents, GPs and school staff) is involved in the activities/tools development based on a participatory approach in a coconstruction process. As part of the component 1, educational group sessions on HPV infections and vaccination are delivered to pupils by regular school staff. The public is not involved in the design and the recruitment stage of the study.
Study objectives and endpoints
The primary objective of the PrevHPV study is to evaluate the effectiveness of a multicomponent intervention (components being applied in combination or alone) on the HPV VC among adolescents (girls and boys) aged 11–14 years at the municipality (cluster) level. The corresponding endpoint is the HPV VC (≥1 dose) 2 months after the end of intervention’s implementation (ie, the prevalence of adolescents aged 11–14 years who have received at least one dose of HPV vaccine). HPV VC (≥1 dose) at 6 and 12 months, and HPV VC (two doses) at 2, 6 and 12 months are secondary endpoints (table 1).
Table 1.
Endpoints of the PrevHPV study
| Dimension/measure | Target population | Data sources | Time frame |
| Vaccine coverage (main objective) | |||
| ≥1 dose | Adolescents 11–14 years | Health insurance (SNDS), vaccination centres | M2, M6, M12 |
| 2 doses | Adolescents 11–14 years | Health insurance (SNDS), vaccination centres | M2, M6, M12 |
| Knowledge, beliefs, behaviours, practices, intention towards HPV vaccination (secondary objective 1) | |||
| Items of the KABP-6C questionnaire | Adolescents, parents and GPs | Self-administered online questionnaires | Before intervention, M2 |
| Efficiency (secondary objective 2) | |||
| Incremental cost-effectiveness ratio | Adolescents 11–14 years | Costs of the intervention, Health insurance (SNDS), vaccination centres | M2, M6, M12 |
| Annual cost and health gains of generalising the component(s) at the national level* | Adolescents 11–14 years (whole country) | Costs of the intervention, Health insurance (SNDS), vaccination centres | / |
| Intervention components’ implementation (secondary objective 3) | |||
| Intervention components’ dose and fidelity: activities performed according to the frame of reference for each component, use of tools developed for each component (assessment of the gap between activities/tools planned and activities/ tools really performed/used) | / | Regular activity reports collected on a standardised form during components’ implementation | |
| Reached populations: percentage of target individuals who benefit from (or participate in) activities of each component (assessment of the acceptability of each component) | Adolescents, parents, school staff and GPs | Regular activity reports collected on a standardised form during components’ implementation | |
| Intervention components’ adaptation: components modified to adapt them to the local context/environment of each school/municipality | / | Regular activity reports collected on a standardised form during components’ implementation | |
| Satisfaction of target populations regarding each activity/component and identification of barriers and levers to components’ implementation | Adolescents, parents, schools and vaccination centres’ staff and GPs | Self-administered (paper or online) questionnaires collected at the end of the components’ implementation | |
*Costs associated with generalising effective component(s) at 1 and 5 years will be compared with the corresponding health gains in terms of size of the vaccinated population (1 and 2 doses).
GPs, general practitioners; HPV, human papillomavirus; KABP-6C, Knowledge, attitude, behaviours, practices and six psychological determinants of vaccination intention (Confidence, Complacency, Constraints, Calculation, Collective responsibility and social Conformism); SNDS, Système National des Données de Santé.
Secondary objectives are to evaluate:
(1) The impact of the multicomponent intervention (components being applied in combination or alone) in target populations (adolescents, parents and GPs) on knowledge, beliefs, behaviours and practices towards HPV vaccination, intention to initiate HPV vaccination and psychological determinants of vaccination intention; (2) the efficiency (cost-effectiveness) and the budget impact of the components and components’ combinations that are effective and (3) the implementation of the components of the intervention, and barriers and levers of implementation at both individual and community level.
Endpoints corresponding to secondary objectives are described in table 1.
The three components of the intervention
The intervention comprises three components implemented at a territorial level (a municipality). Components target (1) adolescents aged 11–14 years, who are the target population for HPV vaccination in France, and their parents, who decide whether to vaccinate their child; (2) GPs, who prescribe most HPV vaccines in France,22 and have a fundamental role in patients’ decision-making process towards vaccination.23
Evidence from the literature shows that adolescents’ and parents’ lack of knowledge on HPV infection and vaccine effectiveness and safety are strong barriers to HPV vaccination.23 24 They may also face financial and organisational barriers to HPV vaccination as usual pathway to access vaccination in France is rather complex.14 In general, adolescents and their parents have to take an appointment with a physician to get the vaccine prescription, then go to a community pharmacy to obtain the vaccine, and finally take another appointment with their physician for its administration. Occasionally, individuals may also benefit from vaccination going to hospital vaccination centres, but their geographical accessibility can be difficult. Besides, HPV vaccine is only partially reimbursed by the French national Health Insurance, and some patients may be charged out-of-pocket costs.14
As for GPs, they face difficulties in informing patients on vaccination and need to acquire educational techniques to improve their communication with vaccine hesitant patients.25
Component 1 (‘Adolescents’ and parents’ education and motivation at school’) first includes a webconference on HPV infection and vaccination for parents. Second, adolescents participate during school hours to two educational group sessions on HPV infections and vaccination, using eHealth tools (videos, serious video game) and participatory learning.
Component 2 (‘GPs’ training’) consists of an individual e-learning training session including: (1) an updated information on HPV infection and vaccination; (2) an introduction to the use of motivational interviewing techniques in the field of vaccination and (3) a presentation of the decision aid tool developed as part of the intervention. This tool aims at helping hesitant individuals to take a decision about HPV vaccination and will be provided to GPs who have attended to the training.
Component 3 (‘HPV vaccination at school’) consists of a vaccination day on school premises where health professionals from local vaccination centres initiate HPV vaccination free of charge and without any medical prescription for all eligible adolescents (see ‘Target populations’ section).
Study design and setting
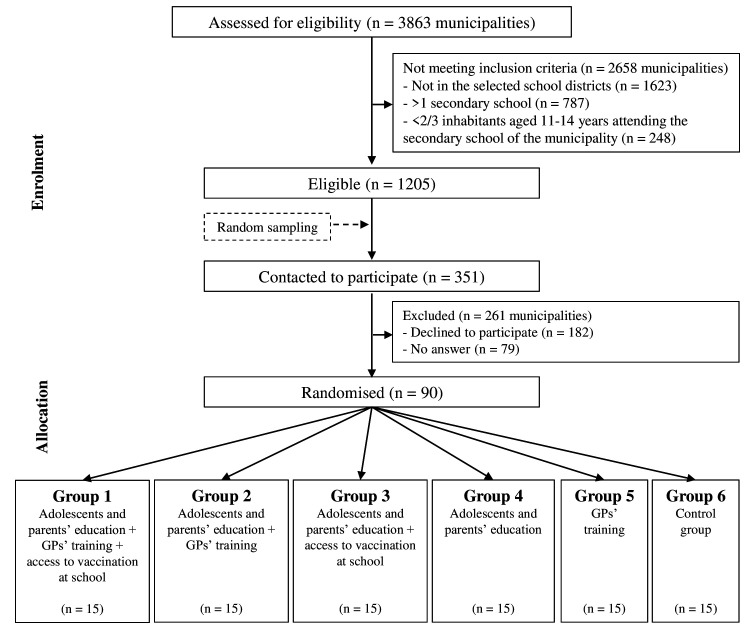
The PrevHPV study is a pragmatic cRCT,26 using an incomplete factorial design. The unit of randomisation (cluster) is the municipality. The factorial design allows to evaluate the multicomponent intervention taking into account that each component could be applied alone or in combination with other(s) component(s); however, it is incomplete because the PrevHPV Study Group considers that access to vaccination at school (component 3) should not be implemented without prior adolescents’ and parents’ education and motivation (component 1). Eventually, 6 groups are compared in this study (figure 1), and we randomly allocated 15 municipalities to each group.
Figure 1.
PrevHPV study flow chart of expected number of participating municipalities. GPs, general practitioners; HPV, human papillomavirus.
Two components (1 and 3) are set in secondary schools, whereas component 2 targets GPs practising in private practice in the participating municipalities.
The intervention will be implemented from December 2021 to March 2022 (table 2).
Table 2.
The PrevHPV study schedule of enrolment, intervention’s components and endpoints measurements
| 2021 | 2022 | 2023 | ||||||||||||||||||
| April | May | June | July | August | September | October | November | December | January | February | March | April | May | June | July | August | September | … | March | |
| Enrolment | ||||||||||||||||||||
| Assessment for municipality eligibility | ||||||||||||||||||||
| Randomisation (Group Allocation) | ||||||||||||||||||||
| GPs information and training proposal | ||||||||||||||||||||
| Parents and adolescents information about component(s) performed in their school | ||||||||||||||||||||
| Intervention components | ||||||||||||||||||||
| Adolescents and parents’ education and motivation (groups 1–4) |

|
|||||||||||||||||||
| GPs’ training (groups 1, 2, 5) |

|
|||||||||||||||||||
| HPV vaccination at school (groups 1, 3) |

|
|||||||||||||||||||
| Endpoints measurements | ||||||||||||||||||||
| HPV VC prevalence (groups 1–6) | ||||||||||||||||||||
| KABP-6C adolescents and parents (groups 1–6) | ||||||||||||||||||||
| KABP-6C GPs (groups 1, 2 and 5) | ||||||||||||||||||||
| Costs (groups 1–6) | ||||||||||||||||||||
| Implementation measures (groups 1–5) | ||||||||||||||||||||
GPs, general practitioners; HPV, human papillomavirus; KABP-6C, Knowledge, attitude, behaviours, practices and six psychological determinants of vaccination intention (Confidence, Complacency, Constraints, Calculation, Collective responsibility and social Conformism); VC, vaccine coverage.
Eligibility and allocation of municipalities
Fourteen of the 25 school districts spread over the French territory were first selected by the PrevHPV Study Group together with representatives of the Ministry of National Education to ensure a diversity of geographical, demographic and socioeconomic profiles.
Municipalities were eligible if: (1) they were located in one of the selected school districts; (2) there was only one secondary school (for pupils aged 11–14 years) in the municipality; and (3) at least 2/3 of inhabitants aged 11–14 years attended the municipality’s secondary school. Out of 1205 eligible municipalities, we randomly sampled 351 (see details in online supplemental text 1) and contacted the head of the secondary school located in each municipality by mail and by phone to ask him/her to participate in the study. The first 90 municipalities for which the secondary school agreed to participate were included in the study.
A block randomisation (block size=6) stratified by school district and French deprivation index (see definition in online supplemental table 3) then allocated the 90 municipalities into six groups (group 1–6) of 15 municipalities (figure 1). This randomisation was performed by a senior researcher of the PrevHPV Study Group not involved in the selection process of the municipalities.
Target populations
For adolescents’ and parents’ education and motivation at school (component 1), the target populations of the intervention are: adolescents attending secondary school in the 60 municipalities from groups 1 to 4 (figure 1) and their parents; for GPs’ training (component 2): GPs’ practising in the 45 municipalities from groups 1, 2 and 5; for access to vaccination at school (component 3): adolescents attending secondary schools of the 30 municipalities from groups 1 and 3, never vaccinated against HPV, ≥11 years old, with no contraindication to vaccination, and whose parents have given their written consent to vaccinate their child. Populations included in the statistical analyses are slightly different (see details in online supplemental table 3).
Data collection
HPV VC (at least 1 dose and 2 doses) at 2, 6 and 12 months after components’ implementation in ad hoc groups will be estimated using data from two sources. The first source is the French health insurance database (Système National des Données de Santé, SNDS). Prospectively recorded for all beneficiaries of healthcare in France, the SNDS covers almost the entire French population (67 million inhabitants).27 This database contains individualised and anonymous data on all medical expenditure reimbursements, and most HPV vaccines in France are delivered in community pharmacies and recorded in the SNDS. The second source of data are registries data from vaccination centres which serve participating municipalities (including the number of vaccines delivered as part of component 3), as vaccines administrated by vaccination centres are not recorded in the SNDS. Some characteristics (eg, age, gender, municipality of residence) of each individual who benefits from a medication reimbursement by the French health insurance or a vaccine administration in a vaccination centre are also recorded, allowing us to estimate an HPV VC prevalence per municipality. Total number of inhabitants aged 11–14 years (denominator) will come from the SNDS. Data necessary to calculate HPV VC before the intervention implementation will also be collected to adjust for baseline VC rate by group in the analyses.
Self-administered online questionnaires will be distributed among adolescents attending participating schools, their parents, and GPs located in included municipalities to collect data for the first secondary objective (tables 1 and 2), before and after the components’ implementation in ad hoc groups. Changes in knowledge, beliefs, behaviours and practices, intention to initiate HPV vaccination as well as psychological determinants of vaccination intention based on the ‘6C’ model (Confidence, Complacency, Constraints, Calculation, Collective responsibility and social Conformism)28–30 will be assessed using online KABP-6C questionnaires for adolescents (in-class participation) and parents, linking preassessments and postassessments by anonymous identifiers. Basic demographic and socioeconomic characteristics will also be collected (eg, gender, age, parents’ educational level, and, for GPs, years of experience, type of practice) for each target population.
Indicators have been defined to assess the resources (human, material, financial) consumed for the conception and implementation of each component,31 tools used and activities realised, and populations reached by different activities. Data to calculate these indicators will be regularly collected during the study period by the professionals involved (eg, PrevHPV staff, schools’ staff, GPs, vaccination centres’ staff) through activity reports questionnaires (tables 1 and 2).
Satisfaction of target populations and involved professionals regarding each activity/tool, as well as barriers identified and levers we may use to implement components will be assessed using self-administered paper or online questionnaires filled out at the end of the implementation phase in groups 1–5.
The scientific leaders of the PrevHPV consortium will have access to the final study dataset.
Sample size
For the sample size calculation, we have retained the hypothesis that all adolescents living in a municipality attend the secondary school of this municipality, and used the average number of pupils per secondary school (466, with a coefficient of variation about 0.5, according to data from the Ministry of National Education) as the mean cluster size. The HPV VC (≥1 dose) among all French adolescents is estimated to be at around 8% specifically in the age group of 11–14 years for the two genders,18 knowing that it is close to 0% in boys, for whom it was not included in vaccine schedule until 2021.
Considering an intraclass correlation of 0.05, a sample of 15 municipalities per group would be sufficient to detect an increase of 10 percentage points in the VC between two groups, with a 90% power and a 5% α risk.
We, therefore, included 90 municipalities, that is, 15 per group, in the PrevHPV study. This corresponds to an expected sample of 41 940 adolescents aged 11–14 years.
Statistical analyses
The PrevHPV Study Group defined a statistical analyses plan. Briefly, it includes the following procedures:
A description of the main sociodemographic characteristics (of GPs, of adolescents/parents at the secondary school and municipality levels) overall and per group.
A calculation of the HPV VC prevalence (≥1 dose and 2 doses) among adolescents aged 11–14 years at baseline, 2, 6 and 12 months, in each municipality and in each of the six groups.
A comparison of HPV VC at different times between groups using a linear model including fixed effects (one per component and interactions between components), adjusted for baseline VC. Units of analysis will be municipalities. Subgroup analyses according the adolescents’ gender and the municipalities’ deprivation index will be performed using interaction terms.
For the first secondary objective, scores of knowledge, beliefs, practices and psychological determinants of vaccination intention will be calculated per municipality and per group before and after the intervention, along with the differences between the two measures. The percentage of target individuals (adolescents, parents, GPs) who change positively towards intention/vaccination (ie, unvaccinated people who had no intention to get vaccinated at baseline but who either intend to get vaccinated or initiate the vaccination after the intervention) by municipalities and by groups will also be estimated. The impact of each component and their combination on these variables will then be assessed using a multilevel model that takes into account the hierarchical structure of the data (individuals nested in schools), adjusted for relevant characteristics identified in step (1). The cost-effectiveness analyses will be performed according the French Health High Authority guidelines for economic evaluations32 from an all-payers perspective, with a time horizon of 2 months after the end of the intervention, with secondary analyses at 6 and 12 months. Only direct costs will be considered (costs of component(s), vaccines and medical consultations). The effectiveness criterion will be the difference in HPV VC prevalence (≥1 dose) between baseline and 2 months after intervention. An incremental cost-effectiveness ratio will then be calculated to estimate the incremental cost per increase of 10 percentage points in the VC prevalence for each component as compared with controls and for the component(s) combined to build an efficiency frontier. Deterministic and probabilistic sensitivity analyses will evaluate the robustness of the results. A budgetary impact analysis will then assess the costs associated with generalising effective component(s) at 1 and 5 years, which will be compared with the corresponding health gains in terms of size of the vaccinated population (1 and 2 doses). The time horizon will be too short to assess the impact on cancers and deaths prevented.
All analyses will be performed in intention to treat, using SAS V.9.4 or a future version (SAS Institute), R or STATA V.15.1 or a future version.
Ethics and dissemination
This study was granted approval by the French Ethics Committee ‘Comité de Protection des Personnes—CPP Sud-Est VI’ on 22 December 2020 (ID-RCB: 2020-A02031-38). No individual consent was required for this type of research; all participants (adolescents, parents of adolescents and GPs) were informed of their rights, in particular not to participate or to oppose the collection of data concerning them (see information sheets in online supplemental text 2).
Findings of this study will be widely disseminated through conference presentations, reports, factsheets and academic publications and generalisation will be further discussed.
Discussion
The PrevHPV study is a pragmatic cluster randomised controlled study included in a major national research programme supported by the French health authorities. Conducted by a multidisciplinary consortium, it aims at evaluating the effectiveness, efficiency and implementation of a multicomponent school-based and primary care-based intervention on HPV vaccine uptake among French adolescents, taking into account the constraints of the environment in which intervention is implemented. This study has several strengths. First, it measures main endpoints (VC) using data collected in routine by the national health insurance and vaccination centres. These data are more reliable than self-reported ones and avoid reporting bias.33 Second, we designed the intervention using results from our diagnostic phase on target populations’ needs, and used a participatory approach in a co-construction process involving adolescents, parents and GPs in the activities/tools development.34 This approach is recommended to enhance the feasibility, effectiveness and acceptability of health interventions.35 We should also acknowledge some limits. We assess VC at the municipality level which is the smallest geographical scale available in routine SNDS databases. As a result, inclusion of municipalities with more than one secondary school would have required that all schools in that municipality accept to participate. To ensure the study feasibility, we limited the study to municipalities with only one secondary school, and thus excluded all large French municipalities. Thus we cannot exclude a possible selection bias in our future results, but a French study using the SNDS database found that HPV vaccine uptake did not vary significantly according to the number of inhabitants in a municipality after adjustment for individual and other area level characteristics (eg, deprivation index, density of gynaecologists).36
The factorial design of this study will provide results on the effectiveness of each of the three components, applied alone or in combination with the other(s). It will add to the small number of studies that compared the effectiveness of different kind of strategies to promote vaccination, as categorised by the Community Guide23 37 38: interventions to increase community demand for vaccination; provider/system care-based interventions; interventions to enhance access to vaccination services. Our study will provide a wide range of other results, including efficiency (cost-effectiveness), when very few economic evaluations of interventions about HPV vaccination are available, and mostly concern reminder/recall interventions.38 Data on implementation (dose, fidelity, adaptations, reach and satisfaction of target populations) are also critical information for stakeholders to help them decide how the intervention may be replicated39 and possibly generalised at a national level.
To gain understanding of how the intervention may promote behaviour change and HPV vaccine uptake among adolescents, we will assess the impact of the intervention on several determinants of vaccination behaviours and intention, among adolescents, theirs parents and GPs. Exploring causal pathways between intervention activities/tools and outcomes may help understand how these effects may be replicated by similar future intervention.39
Finally, the design of the PrevHPV study allows participation of municipalities with different deprivation levels and a balanced allocation between the study’s groups. We plan to assess in exploratory objectives whether and how results vary according to deprivation levels and the impact of the intervention on social inequalities in HPV vaccine uptake. Thus, this study will contribute to pay greater attention to equity in implementation science.40
Supplementary Material
Acknowledgments
The authors would like to thank the representants of the following institutions who participate to the steering committee of the research programme: Inserm, IReSP, ITMO Cancer AVIESAN, ITMO Public Health AVIESAN, INCa (the French National Cancer Institute), the French Public Health Agency (Santé publique France), Ministry of Health, Ministry of National Education, especially Dr Brigitte Moltrecht from the Direction générale de l'enseignement scolaire, and the Ile-de-France Regional Health Agency. They also want to thank all head chiefs, physicians, nurses of the fourteen school districts included in the study, and all staff of the secondary schools which accepted to participate to the PrevHPV study.
Footnotes
Collaborators: The PrevHPV Study group includes the authors of the present manuscript and: For the team 1: Nelly Agrinier, Estelle Fall, Céline Pulcini; for the team 2: Sébastien Bruel, Marie Ecollan, Dragos-Paul Hagiu, Josselin Le Bel, Henri Partouche, Juliette Pinot, Louise Rossignol, Arthur Tron, Minghui Zuo; for the team 3: Gaëlle Vareilles, Julie Bros, Catherine Juneau; for the team 4: Marion Branchereau; for the team 5: Elisabeth Botelho-Nevers, Géraldine Jambon, Florian Jeanleboeuf, Julie Kalecinski, Christine Lasset, Laetitia Marie Dit Asse; for the team 7: Jonathan Sicsic, Jocelyn Raude, Sandra Chyderiotis, Damien Oudin-Doglioni, Anne-Sophie Barret, Isabelle Bonmarin, Daniel Levy-Bruhl; Clémence Castagnet (Inserm), and Mélanie Simony (IReSP).
Contributors: MM, BG, KC and NT developed the study protocol with input from AB, SB, AG-B, AG, SG, A-SLD-B and JM; ABG led the development of the adolescents and parents’ education and motivation component; SG led the development of the general practitioners’ training component; A-SLD-B led the development of access to vaccination at school component; SB facilitated the partnership between the teams of the consortium, and with the steering committee; AB and NT drafted the first version of the manuscript and all authors provided comments and feedback for improvement the manuscript. All authors approved the final version of the manuscript and are responsible for their contributions.
Funding: The study is conducted with the support of IReSP and with financial support from ITMO Cancer AVIESAN (Alliance Nationale pour les Sciences de la Vie et de la Santé/ National Alliance for Life Sciences & Health) within the framework of the Cancer Plan 2014–2019.
Disclaimer: The ITMO Cancer AVIESAN had no role in the design of the study and in writing the manuscript; it will have no role in the collection, analysis, and interpretation of data.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
The PrevHPV Study group:
Nelly Agrinier, Estelle Fall, Céline Pulcini, Sébastien Bruel, Marie Ecollan, Dragos-Paul Hagiu, Josselin Le Bel, Henri Partouche, Juliette Pinot, Louise Rossignol, Arthur Tron, Minghui Zuo, Gaëlle Vareilles, Julie Bros, Catherine Juneau, Marion Branchereau, Elisabeth Botelho-Nevers, Géraldine Jambon, Florian Jeanleboeuf, Julie Kalecinski, Christine Lasset, Laetitia Marie DitAsse, Jonathan Sicsic, Jocelyn Raude, Sandra Chyderiotis, Damien Oudin-Doglioni, Anne-Sophie Barret, Isabelle Bonmarin, Daniel Levy-Bruhl, Clémence Castagnet, and Mélanie Simony
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. World Health Organisation . Human papillomavirus (HPV) and cervical cancer, 2022. Available: https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
- 2. World Health Organisation . Human papillomavirus vaccines: who position paper, may 2017. Weekly epidemiological record 2017;2017:241–68. [Google Scholar]
- 3. Chesson HW, Dunne EF, Hariri S, et al. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex Transm Dis 2014;41:660–4. 10.1097/OLQ.0000000000000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Martel C, Georges D, Bray F, et al. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health 2020;8:e180–90. 10.1016/S2214-109X(19)30488-7 [DOI] [PubMed] [Google Scholar]
- 5. de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010;11:1048–56. 10.1016/S1470-2045(10)70230-8 [DOI] [PubMed] [Google Scholar]
- 6. International Agency for Research on Cancer, World Health Organization . The Global Cancer Observatory - World. Source: Globocan 2020, 2021. Available: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf
- 7. International Agency for Research on Cancer, World Health Organization . The Global Cancer Observatory - France. Source: Globocan 2020, 2021. Available: https://gco.iarc.fr/today/data/factsheets/populations/250-france-fact-sheets.pdf
- 8. Arbyn M, Xu L, Simoens C, et al. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev 2018;5:CD009069. 10.1002/14651858.CD009069.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bergman H, Buckley BS, Villanueva G, et al. Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV-related disease in females and males. Cochrane Database Syst Rev 2019;2019:CD013479. 10.1002/14651858.CD013479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drolet M, Bénard Élodie, Pérez N, et al. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet 2019;394:497–509. 10.1016/S0140-6736(19)30298-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garland SM, Kjaer SK, Muñoz N, et al. Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of 10 years of real-world experience. Clin Infect Dis 2016;63:519–27. 10.1093/cid/ciw354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lei J, Ploner A, Elfström KM, et al. HPV vaccination and the risk of invasive cervical cancer. N Engl J Med 2020;383:1340–8. 10.1056/NEJMoa1917338 [DOI] [PubMed] [Google Scholar]
- 13. Bruni L, Saura-Lázaro A, Montoliu A, et al. HPV vaccination introduction worldwide and who and UNICEF estimates of national HPV immunization coverage 2010-2019. Prev Med 2021;144:106399. 10.1016/j.ypmed.2020.106399 [DOI] [PubMed] [Google Scholar]
- 14. Nguyen-Huu N-H, Thilly N, Derrough T, et al. Human papillomavirus vaccination coverage, policies, and practical implementation across Europe. Vaccine 2020;38:1315–31. 10.1016/j.vaccine.2019.11.081 [DOI] [PubMed] [Google Scholar]
- 15. Ministère des solidarités et de la santé . Calendrier des vaccinations et recommandations vaccinales, 2021, 2021. Available: https://solidarites-sante.gouv.fr/IMG/pdf/calendrier_vaccinal_090721.pdf
- 16. Institut National Du Cancer . Le plan cancer 2009-2013. available:. Available: https://www.e-cancer.fr/Institut-national-du-cancer/Strategie-de-lutte-contre-les-cancers-en-France/Les-Plans-cancer
- 17. Haute Autorité de Santé (HAS) . Cancer du col de l’utérus : une meilleure couverture vaccinale et un dépistage renforcé restent la priorité, 2017. Available: https://www.has-sante.fr/jcms/c_2797450/fr/cancer-du-col-de-l-uterus-une-meilleure-couverture-vaccinale-et-un-depistage-renforce-restent-la-priorite
- 18. Fonteneau L, Barret AS, Lévy-Bruhl D. Evolution de la couverture vaccinale du vaccin contre le papillomavirus en France - 2008-2018. Bull Epidémiol Hebd 2019;22:424–30 http://beh.santepubliquefrance.fr/beh/2019/22-23/pdf/2019_22-23_3.pdf [Google Scholar]
- 19. Bruel S, Rakoto Z, Agrinier N. French health students’ knowledge about human papilloma virus infections and vaccine: it is time to fill the gaps. In: Abstract book 2020 European Congress of clinical microbiology and infectious diseases, 2020. https://markterfolg.de/ESCMID/Abstractbook2020.pdf [Google Scholar]
- 20. Chyderiotis S, Sicsic J, Raude J, et al. Optimising HPV vaccination communication to adolescents: a discrete choice experiment. Vaccine 2021;39:3916–25. 10.1016/j.vaccine.2021.05.061 [DOI] [PubMed] [Google Scholar]
- 21. Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. Spirit 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ganry O, Bernin-Mereau A-S, Gignon M, et al. Human papillomavirus vaccines in Picardy, France: coverage and correlation with socioeconomic factors. Rev Epidemiol Sante Publique 2013;61:447–54. 10.1016/j.respe.2013.04.005 [DOI] [PubMed] [Google Scholar]
- 23. Rodriguez SA, Mullen PD, Lopez DM, et al. Factors associated with adolescent HPV vaccination in the U.S.: a systematic review of reviews and multilevel framework to inform intervention development. Prev Med 2020;131:105968. 10.1016/j.ypmed.2019.105968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Karafillakis E, Simas C, Jarrett C, et al. HPV vaccination in a context of public mistrust and uncertainty: a systematic literature review of determinants of HPV vaccine hesitancy in Europe. Hum Vaccin Immunother 2019;15:1615–27. 10.1080/21645515.2018.1564436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bouchez M, Ward JK, Bocquier A, et al. Physicians' decision processes about the HPV vaccine: a qualitative study. Vaccine 2021;39:521–8. 10.1016/j.vaccine.2020.12.019 [DOI] [PubMed] [Google Scholar]
- 26. Loudon K, Treweek S, Sullivan F, et al. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ 2015;350:h2147. 10.1136/bmj.h2147 [DOI] [PubMed] [Google Scholar]
- 27. Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions: from the système national d'information interrégimes de l'Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev Epidemiol Sante Publique 2017;65 Suppl 4:S149–67. 10.1016/j.respe.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 28. MacDonald NE, SAGE Working Group on Vaccine Hesitancy . Vaccine hesitancy: definition, scope and determinants. Vaccine 2015;33:4161–4. 10.1016/j.vaccine.2015.04.036 [DOI] [PubMed] [Google Scholar]
- 29. Betsch C, Schmid P, Heinemeier D, et al. Beyond confidence: development of a measure assessing the 5C psychological antecedents of vaccination. PLoS One 2018;13:e0208601. 10.1371/journal.pone.0208601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seanehia J, Treibich C, Holmberg C, et al. Quantifying population preferences around vaccination against severe but rare diseases: a conjoint analysis among French university students, 2016. Vaccine 2017;35:2676–84. 10.1016/j.vaccine.2017.03.086 [DOI] [PubMed] [Google Scholar]
- 31. Briançon S, Bonsergent E, Agrinier N, et al. PRALIMAP: study protocol for a high school-based, factorial cluster randomised interventional trial of three overweight and obesity prevention strategies. Trials 2010;11:119. 10.1186/1745-6215-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haute Autorité de Santé (HAS) . Guide méthodologique - choix méthodologiques pour l’évaluation économique la HAS, 2011. Available: https://www.has-sante.fr/upload/docs/application/pdf/2011-11/guide_methodo_vf.pdf
- 33. Suarez L, Simpson DM, Smith DR. Errors and correlates in parental recall of child immunizations: effects on vaccination coverage estimates. Pediatrics 1997;99:e3. 10.1542/peds.99.5.e3 [DOI] [PubMed] [Google Scholar]
- 34. Leite S. The basics about community based research. Advances in Social Sciences Research Journal 2019;6:178–83. [Google Scholar]
- 35. Bleijenberg N, de Man-van Ginkel JM, Trappenburg JCA, et al. Increasing value and reducing waste by optimizing the development of complex interventions: enriching the development phase of the medical Research Council (MRC) framework. Int J Nurs Stud 2018;79:86–93. 10.1016/j.ijnurstu.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 36. Blondel C, Barret A, Pelat C. Influence des facteurs socioéconomiques sur la vaccination contre les infections HPV chez les adolescentes en France. Bull Epidémiol Hebd 2019:441–50. [Google Scholar]
- 37. Walling EB, Benzoni N, Dornfeld J, et al. Interventions to improve HPV vaccine uptake: a systematic review. Pediatrics 2016;138. 10.1542/peds.2015-3863. [Epub ahead of print: 13 06 2016]. [DOI] [PubMed] [Google Scholar]
- 38. Smulian EA, Mitchell KR, Stokley S. Interventions to increase HPV vaccination coverage: a systematic review. Hum Vaccin Immunother 2016;12:1566–88. 10.1080/21645515.2015.1125055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Moore GF, Audrey S, Barker M, et al. Process evaluation of complex interventions: medical Research Council guidance. BMJ 2015;350:h1258. 10.1136/bmj.h1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brownson RC, Kumanyika SK, Kreuter MW, et al. Implementation science should give higher priority to health equity. Implementation Sci 2021;16:28. 10.1186/s13012-021-01097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-057943supp001.pdf (380.6KB, pdf)